Abstract
We have previously shown that microvascular smooth muscle activates CD4+ T lymphocytes in sterile co-culture, presents antigen, and produces inflammatory cytokines. Adoptive transfer of lymphocytes co-cultured with syngeneic smooth muscle cells to healthy recipient mice results in vasculitic lesions predominantly in postcapillary venules. The present study assessed the pathogenic role of immunoglobulin and B cells in a murine model of vasculitis. Here, we show that transferred B cells, including plasmablast cells, accumulated, persisted, and proliferated in lung and secondary lymphoid organs of recipient mice. The induction of vasculitis was accompanied by production of IgM and IgG2a autoantibodies specific for vascular smooth muscle intracellular antigens. Circulating immunoglobulin had a pathogenic role in this vasculitis model, because the disease could be induced by transfer of serum from vasculitic mice to untreated animals but not by transfer of serum depleted of anti-smooth muscle autoantibodies. Additionally, the pathogenic mechanisms triggered by the transfer of vasculitogenic serum were dependent on T lymphocytes because both wild-type and B cell-deficient mice developed the disease after serum transfer, whereas RAG2-deficient mice did not. Thus, immunoglobulin and cell-mediated pathways work in concert to produce vasculitis in this model.
Vasculitides are a heterogeneous group of clinical disorders delineated by the common feature of perivascular inflammation and damage to blood vessel walls (vasculitis). Of yet unknown etiology and uncertain pathogenesis, these syndromes may become life threatening due to obliteration of vessel lumens, eventually resulting in organ failure. Adding to their seriousness are the difficulties in diagnosis and assessment of disease activity.1,2 To date, both the impact of harmful environmental factors and an as yet unidentified genetic susceptibility are factors believed to result in autoimmune reactions leading to vascular inflammation.3,4
The initial site in inflammation of small- and medium-size vessels is the media, usually in the presence of morphologically intact endothelium and apparently unaffected external elastic lamina. Later on, the inflammatory lesions evolve to include the adventitia, with development of vascular fibrosis and thromboses, followed by tissue necrosis and vessel rupture.2 This sequence of events suggests that the subendothelial structures may be the early targets of an autoimmune attack in vasculitis. To evaluate this hypothesis, a murine model of vasculitis has been developed in which microvasculature-derived smooth muscle (SM) cells are tested for their capacity to interact with leukocytes and contribute to inflammatory reactions.5–9 In this model, naïve mouse splenocytes, cultured for 1 week in the presence of syngeneic vascular SM cells, induce vasculitis after adoptive transfer into syngeneic hosts. Vasculitic lesions affect venules, especially in the lung, but also in liver, skeletal muscle, kidney, and other organs of recipient mice with 20% of mice showing severe pathology (blood vessel occlusion, granuloma-like formations).9,10 Although T-cell activation and skewage of the TCR repertoire in the presence of SM cells and in organs affected by vasculitis was documented in previous work,6,10,11 it has remained unclear whether vasculitis is provoked solely by the activated T lymphocytes, or if other factors contribute equally to the pathology in this particular model. For this study we hypothesized that B lymphocytes and autoantibodies may possibly play a role in the pathogenesis of vasculitis in the described experimental model.
Antibodies directed to ubiquitous self-antigens are a common finding in all vasculitides. Although they are primarily considered as diagnostic markers, they are assumed to mediate multiple pathogenic reactions resulting in inflammation and extensive tissue damage in the late course of these diseases. In conditions associated with primary systemic vasculitis, the autoantibodies show restricted specificities, being directed against neutrophilic and monocytic antigens12,13—anti-proteinase 3 (PR3), anti-myeloperoxidase—and against the vascular wall. The latter are commonly targeted to endothelium14–16 and vascular SM.17,18 Several in vivo studies performed on idiotypic networks indicated that human anti-PR3 antibodies are strongly pathogenic and human anti-endothelial cell autoantibodies are weakly pathogenic after injection into mice.4,19–21 Recently, compelling in vivo experimental evidence has established the pathogenicity of autoantibodies directed against murine myeloperoxidase in an animal model of crescentic glomerulonephritis and small-vessel vasculitis.22 To date, no reports are available on the pathogenicity of anti-SM antibodies in vasculitis. In the present study, we aimed to determine whether induction of vasculitis by adoptive transfer of SM-stimulated lymphocytes is followed by the production of autoantibodies targeted to blood vessel wall SM cells and if these antibodies have a pathogenic role. Furthermore, we sought to delineate the mechanisms mediated by pathogenic immunoglobulin in the development of vasculitis.
Materials and Methods
Mice
BALB/c mice, B-cell-deficient mice (JhD), and recombination activating gene 2-deficient mice (RAG2−/−) (6 to12 weeks old) on BALB/c background (Taconic, Germantown, NY) were housed in specific pathogen-free conditions in the Animal Research Facility of Middleton Veterans Hospital (Madison, WI). The experimental animal protocols were approved by the Animal Research Committee of the Middleton Veterans Hospital and the Animal Care Committee of the University of Wisconsin.
Flow Cytometry
Cells were analyzed by four-color flow cytometry (FACSCalibur using Cell Quest 3.3 software; BD Biosciences, Franklin Lakes, NJ). Labeled antibodies CYCR-anti-CD4 (clone L3T4), phycoerythrin (PE)-anti-CD8a (clone 53-6.7), PE- or CYCR-anti-CD45R/B220 (hybridoma clone 6B2), PE-anti-CD138, PE anti CD38, APC-labeled streptavidin and PE-labeled F(ab′)2 of goat anti-mouse IgM (H+L), were all purchased from Pharmingen (San Diego, CA). Fluorescein isothiocyanate (FITC)-anti-CD45R/B220 (clone 6B2), biotin-anti-CD23 (clone B3B4), and CY5-anti-CD11b (Mac-1) were purified and labeled in our laboratory. Nonspecific Fc binding of monoclonal antibodies was prevented by co-incubation with purified anti-CD32/CD16 (2.4G2) at 2 μg/ml.
Cell Cultures
SM cells from microvasculature were isolated from BALB/c mice as previously described11,23 and were 97% pure as confirmed by immunofluorescence FACS (fluorescence-activated cell sorting) analysis using FITC anti-SM α-actin monoclonal antibody (Sigma, St. Louis, MO). As controls, cultured adherent cells (BALB/c astrocytic cultures and the BALB/c fibroblast cell line 3T3; American Type Culture Collection, Manassas, VA) were used. Cells were maintained in sterile culture for maximum 10 passages in complete Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (BioWhittaker, Walkersville, MD), containing 4 mmol/L l-Glu, 10 mmol/L sodium pyruvate, and 50 μmol/L β-mercaptoethanol. For confocal microscopy cells were grown on Lab-Tek 8-well Permanox slides (Nalge Nunc, Naperville, IL).
Stimulation of Naïve Splenocytes by Co-Culture with SM
Freshly isolated splenocytes from naïve mice were co-cultured for 6 days under sterile conditions with irradiated syngeneic SM cell monolayers in a 10:1 ratio of splenocytes to SM cells. Floating cells were then harvested from the adherent cell monolayer by mild pipetting, washed in sterile Hanks’ balanced salt solution, and resuspended at 4 × 107 cells/ml shortly before being transferred to recipient mice. Cell viability was estimated with Trypan Blue (0.2% w/v).
Murine Model of Vasculitis
Adoptive transfer of SM-stimulated splenocytes into sex-matched syngeneic mice (7 to 10 × 106 cells/mouse) was performed by tail vein injection. After 1 week, blood was collected under anesthesia by heart puncture, mice were euthanized, and organs (lung, liver, kidney) were fixed in buffered 10% formalin and paraffin-embedded. Four 50-μm-spaced sections were stained with hematoxylin and eosin and evaluated by light microscopy independently and in a blinded manner by a pathologist and an immunologist for the presence of vasculitic lesions. Lesions were defined by inflammation and disruption of blood vessel wall, accompanied by transmural cuffing with three layers or more of infiltrated leukocytes and presence of leukocytes adherent to, or passing through, the endothelium. Scoring of vasculitis incidence was achieved on lung sections by reporting the number of vessels bearing vasculitic lesions, out of a total number of 300 small blood vessels, counted over a surface of 30 to 50 mm2 (cross sections of three separate lung lobes). Negative control animals (that did not develop vasculitis) were mice injected either with SM cells alone (5 × 106 cells); with SM cells (5 × 106 cells) homogenized in complete Freund’s adjuvant (v/v); with SM culture medium (2 × 100 μl); with SM/spleen co-culture medium (2 × 100 μl), or with fresh isolated splenocytes (107cells).
Cell Proliferation
Cells were labeled at 2.5 μmol/L 5-(and 6) carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 5 minutes at 37°C, isolated by Lympholyte M (Cedarlane, Hornby, Ontario, Canada) gradient separation, and washed promptly three times with sterile Hanks’ balanced salt solution before adoptive transfer to syngeneic mice. Mice were euthanized at 1, 3, 7, and 10 days after adoptive transfer and cell suspensions isolated from peripheral blood, lung, spleen, axillary and inguinal lymph nodes were analyzed by FACS for follow-up of CFSE-tagged transferred cells.
Serum Transfers
Pooled serum samples obtained from vasculitic mice, and pooled serum samples from healthy controls were filtered (0.22 μm), prewarmed at 37°C, and transferred to syngeneic recipient mice by two intravenous injections of 100 μl each, given at 2-day intervals. Alternatively, two intraperitoneal injections of 250 μl of serum each were given. The efficacy of serum transfer was assessed by immunofluorescence microscopy detection of anti-SM-specific IgM antibodies in the recipient mice. Vasculitis incidence was scored as described above 7 days after the last serum injection.
Antibody Depletion
Another batch of pooled sera from vasculitic mice was used for depletion of SM-binding antibodies. Serum (1.5 ml) was incubated with formaldehyde-fixed and phosphate-buffered saline (PBS)-washed SM cells (1.5 × 108 cells) for 2 hours at 4°C, recovering 1.2 ml of serum after centrifugation. Alternatively, 1.5 ml of serum was absorbed in identical conditions on control cells (mouse primary astrocytic cultures, 1.5 × 108 cells). Serum was then 0.22-μm filtered and tested for decreased IgM binding to SM cells by immunofluorescence microscopy and flow cytometry, before being transferred to recipient mice.
Enzyme-Linked Immunosorbent Assay (ELISA)
Serum Ig levels were determined by indirect ELISA; 96-well plates were coated with goat anti-mouse Ig classes and subclasses (Sigma). After blocking, 1:100 diluted serum was added in duplicate wells. Bound serum Ig was detected with alkaline phosphatase-labeled anti-murine Ig (Sigma) followed by methylumbelliferyl-phosphate dicyclohexylammonium (MUP DCA) salt (Molecular Probes) substrate breakdown. Fluorescence was measured at 358-nm excitation/450-nm emission in a fluorescence plate reader. Standard curves were established with internal reference-positive sera. Serum Ig reacting with vasculitis-associated autoantigens was assayed by direct ELISA. The wells were coated with 1.5 μg/ml of purified antigen (either proteinase 3, PR3; myeloperoxidase; or ribonucleoproteins, RNP/Sm; all diagnostic grade and purchased from AroTec Diagnostics Ltd., Wellington, New Zealand). Triplicates of individual mouse serum samples diluted 1:10 were tested. The Ig detection was performed as described above. Assay linearity was probed on standard curves of serum from 8-month-old CD32-deficient mice. All values obtained for vasculitic mice were compared with values from normal healthy mice, and statistical significance was evaluated by Student’s t-test.
Immunofluorescence
Formaldehyde-fixed and 0.1% saponin-permeabilized SM cells or healthy mouse frozen-tissue sections were incubated with pooled vasculitic sera at different dilutions for 60 minutes. Slides were washed with PBS/bovine serum albumin 0.1% and stained with FITC goat anti-mouse IgM or with FITC goat anti-mouse balanced IgG1, 2a, 2b, and 3. Binding of Ig isotypes was assayed using goat anti-mouse IgG subclass-specific antibodies (Sigma) and stained with FITC donkey IgG anti-goat Ig. Fluorochrome-labeled antibodies were purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). Serum binding to cell surface markers was assessed on suspensions of live cells kept on ice in Hanks’ balanced salt solution/bovine serum albumin 1% and NaN3 0.1% using the same antibodies and analyzed by flow cytometry. Slides were viewed under a Noran Odyssey XL scanning laser confocal microscope (Prairie Technologies, Middleton, WI), through oil-immersion objectives.
Immunohistochemistry
The binding of serum antibodies was tested on 3.7% formaldehyde-fixed tissue sections of organs (liver, spleen, lung, kidney, skin, brain, skeletal muscle, heart, diaphragm, intestine) sampled from healthy control mice. Dewaxed sections were blocked, and then incubated with either vasculitic or normal serum, as described above. Horseradish peroxidase-labeled goat anti-mouse Ig (Boehringer Mannheim Biochemicals, Indianapolis, IN) was added, then developed with diaminobenzidine substrate (Sigma). Slides were lightly counterstained with hematoxylin.
Results
Splenocytes Cultured with Syngeneic SM Induce Vasculitic Lesions after Adoptive Transfer into Mice
Our results confirmed previous findings9,10 asserting that splenic mouse lymphocytes, cultured for 1 week in the presence of syngeneic SM cells, induced vasculitis after adoptive transfer into syngeneic hosts. Thus, of the 24 BALB/c animals that received splenocytes co-cultured with SM cells, 13 mice developed vasculitic lesions in the lungs and liver, 5 showing granuloma-like inflammation of small blood vessels in the lung. Control experiments also confirmed previous data9 because none of the 6 control mice injected with 107 naïve splenocytes and only 1 of 12 control mice injected with SM cells alone was found to have vascular inflammation in the lungs. We thereafter used this vasculitis model in assessing the humoral pathways contributing to vascular inflammation.
Autoantibodies Specific for Vascular SM Are Present in the Serum of Vasculitic Mice
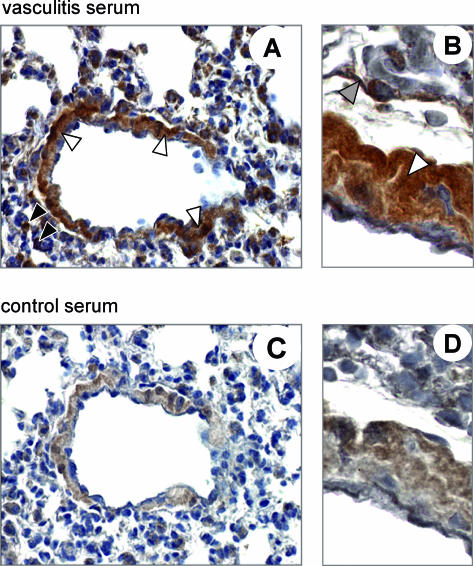
Serum collected from mice in which vasculitis was induced was assayed for the presence of antibodies reacting with self-tissue. Immunoglobulin (Ig) from all these mice reacted only with mural SM of small- and medium-size blood vessels on sections of normal mouse tissue (lung, brain, skin, and heart). In the lung, Ig from vasculitic serum bound also to alveolar capillary pericyte-appearing cells and to some extent to extracellular matrix in the adventitia of medium-size vessels (Figure 1). Serum IgM and IgG evidenced a perinuclear-speckled pattern of binding on purified cultured microvascular SM cells (Figure 2A) but no reactivity with chromatin or with cell surface molecules (Figure 2, A and B). Serum from vasculitic mice did not react with other cultured cells (3T3 fibroblast cell line, astrocyte primary cultures, either live or permeabilized) or with freshly isolated leukocytes (splenic or peripheral blood lymphocytes; resting or thioglycolate-activated peritoneal macrophages; resting and activated peritoneal or peripheral blood polymorphonuclear cells) as determined by microscopy and flow cytometry. With regard to other autoantibodies often associated with vasculitis in patients, ELISA screening of the sera from vasculitic mice showed no increase in anti-PR3 or anti-RNP/Sm reactivity but an increase (not statistically significant) in anti-myeloperoxidase antibodies (data not shown). These results suggest that although other autoantibodies may be present in the circulation of the vasculitic mice, the anti-SM antibodies represent the main class of autoantibodies in this model.
Figure 1.
Serum from vasculitic mice contains autoantibodies reacting with small- and medium-size blood vessels. Consecutive paraffin sections of normal mouse lung were used to evaluate the binding of serum Ig from vasculitic mice (top row), versus serum Ig from control healthy mice (bottom row). Binding to SM of vessel walls (light arrows), capillary pericytes (dark arrows), and to extracellular matrix (shaded arrow) was evidenced by horseradish peroxidase immunohistochemistry. Counterstain: hematoxylin. Original magnifications: ×100 (A, C); ×400 (B, D).
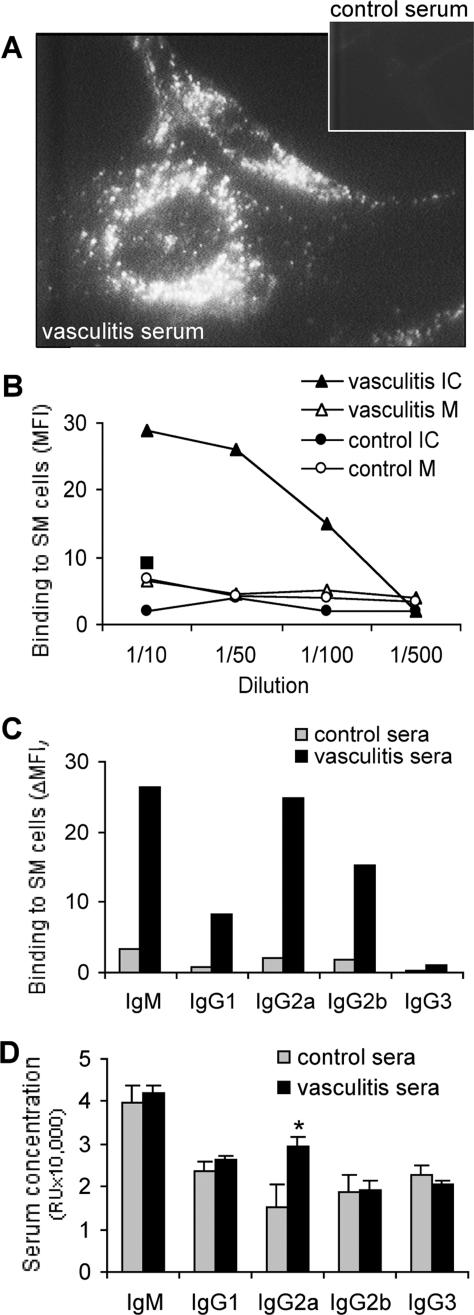
Figure 2.
Immunofluorescence staining shows binding of antibodies from vasculitic mice to microvasculature-derived SM cells. A: Staining of microvasculature-derived SM cells indicates the specificity of IgM from vasculitic serum for intracellular structures (inset: IgM from control serum). B: FACS-determined titer of serum IgM binding to membrane (M) of live SM cells, or intracellular (IC) to permeabilized SM cells. Mean fluorescence intensity (MFI) was used as a measure of the binding of serum IgM to SM cells. The square indicates the MFI corresponding to serum IgM of negative control mice injected with SM cells and complete Freund’s adjuvant (v/v). C: FACS-determined binding of serum IgM and of IgG isotypes to permeabilized SM cells. MFI values were corrected by subtraction of autofluorescence (ΔMFI). D: ELISA determination of total IgM and total IgG isotype concentration in the serum of healthy or vasculitic mice. In A to C, results shown are representative of at least three separate determinations using a pool of 17 vasculitic sera, and as control a pool of 12 normal sera; D shows averages ± SD of 12 individual vasculitic and 12 normal serum samples, *P < 0.05 (Student’s t-test). Original magnification, ×1000.
Antibodies of the IgG class reacting with isolated microvascular SM cells were essentially of IgG2a and IgG2b isotypes (Figure 2C). Total IgG2a serum concentration increased almost twofold in mice 7 days after vasculitis induction (Figure 2D), whereas other classes/subclasses of Ig did not show significant variations. There is a strong positive correlation (coefficient, 0.87) between the serum IgG2a concentration and the binding to vascular SM cells, for individual mice sera (n = 12), suggesting that a major part of IgG2a in the vasculitic mice are anti-SM cell autoantibodies. This absence of polyclonal B-cell activation and the prevalence of an IgG2a response suggest that a Th1-polarized response rather than a Th2,24 or a T-independent antigen-driven one, would be involved in the humoral manifestations associated with vasculitis in this model. Replacement of fetal bovine serum with mouse serum in control tissue cultures did not affect the antibody binding to SM cells, excluding the possibility of cross-reactivity between vasculitic serum and bovine antigens. Control mice injected with SM alone did not develop anti-SM antibodies.
B Cells Exhibit an Activated Phenotype after Co-Culture with SM Cells and Proliferate after Adoptive Transfer
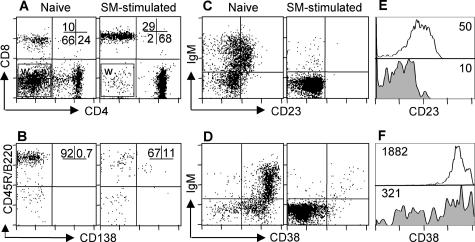
The presence of autoantibodies in mice that received SM-stimulated splenocytes suggests that some B cells get primed during culture of splenocytes with SM. To determine the activation status and composition of the pathogenic cell populations after the culture with SM, we performed four-color FACS analysis of the cells. The results showed that after SM stimulation, on average, 70% of the cells were CD4+ and more than 25% were CD8a+ lymphocytes (Figure 3A), whereas less than 5% were CD45R/B220+ B lymphocytes (Figure 3, A and B). On average, 10% of the IgM+ CD45R/B220+ CD4− CD8a− lymphocytes were CD45R/B220lo expressing CD138 (syndecan-1) molecules (Figure 3B), a phenotype suggesting they were not differentiated plasma cells but B cells committed to a plasmablast phenotype. Additionally, the majority of the B cells appeared to be primed cells, as evidenced by the decrease in their surface expression of the differentiation markers CD23 and CD38 (Figure 3, C and D).
Figure 3.
Phenotype of SM-stimulated lymphocytes, as compared with naïve spleen, before adoptive transfer to syngeneic mice. Immunofluorescence FACS analysis shows: A: preferential survival of CD4+ and CD8+ T lymphocytes after co-culture with SM cells (numbers indicate percentage of total lymphocytes). Analysis window (W) contains the non-CD4/CD8 lymphocytes further illustrated in B. B: Expression of CD138 on B lymphocytes (numbers are percentage of cells CD138+ and CD138− of CD45R/B220+ non-CD4/CD8 cells). C–F: Decrease in the expression level of B-cell differentiation markers CD23 (C, E) and CD38 (D, F) on the surface of lymphocytes after SM stimulation, as compared with naïve splenic lymphocytes. In E and F, numbers represent mean fluorescence intensity of the two markers on CD45R/B220+ B cells, either naïve (open histogram) or after SM stimulation (filled histogram).
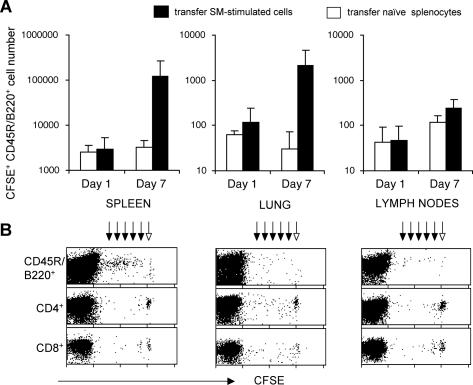
To track the localization and persistence of the pathogenic SM-stimulated lymphocytes in the recipient mice after vasculitis induction, cells tagged with CFSE were used for the adoptive transfer. Seven days after the transfer, the number of CFSE+ CD45R/B220+ B lymphocytes among host CFSE− B cells recovered from the lung and spleen was up to two orders of magnitude higher in the mice receiving SM-stimulated lymphocyte transfer, than that observed in mice receiving naïve green cells (summarized in Figure 4A). Analysis of the mean fluorescence intensity of the transferred CFSE+ cells showed proliferation of B lymphocytes in the spleen of recipient mice during vasculitis induction (Figure 4B), in contrast to control naïve cell transfers that did not show proliferation. Control co-injection of fluorescence-tagged naïve splenocytes together with SM cells did not result in accumulation and/or proliferation of B cells in lung or lymphoid organs of recipient mice, indicating that SM cells had no mitogenic effect on B cells after injection (data not shown). Priming of B lymphocytes during culture with SM and their further expansion after adoptive transfer are likely to determine the anti-SM antibody production in vasculitic mice.
Figure 4.
B lymphocytes accumulate and proliferate in spleen and lung after vasculitis induction. Mice received intravenously 7 × 106 CFSE-tagged splenocytes, either naïve (n = 6 mice, open bars) or SM-stimulated (n = 6 mice, filled bars). Presence of transferred cells was assessed by flow cytometry 1 and 7 days after transfer. A: Depicted is the number of CFSE+ B lymphocytes recovered per mouse organ (spleen, lung, one axillary lymph node), calculated from the percentage of CFSE+ B cells (CD45R/B220+ CD4− CD8−) in the entire cell population analyzed by FACS and reported to the yield of cells recovered from the whole organ. Data presented are average of absolute B-cell numbers ± SD from three experiments performed. B: Decline of the CFSE fluorescence intensity of the SM-stimulated cells, measured by flow cytometry 7 days after adoptive transfer, reflects discrete reduction in dye content of daughter cells after cell division (dark arrows) from original level (light arrow), in lymphocyte populations from each analyzed organ. Presented are FACS dot plots from one experiment of three performed with similar results.
Anti-SM Antibodies from Vasculitic Mice Are Pathogenic
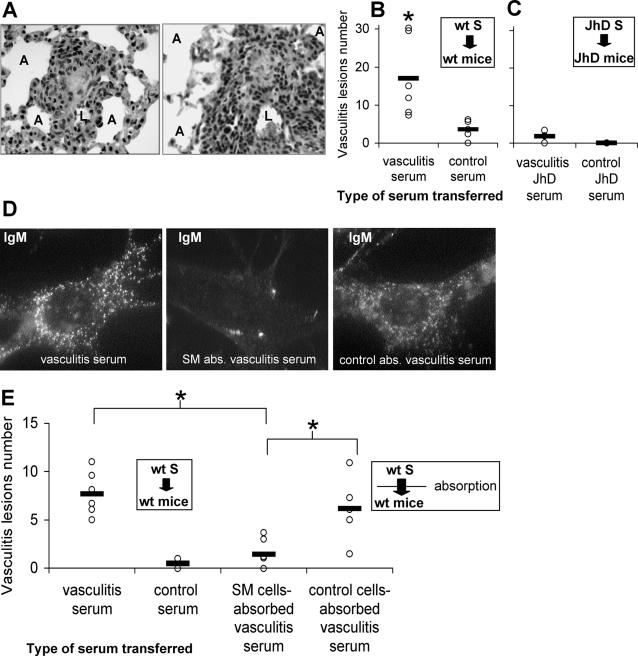
We hypothesized that serum antibodies reacting with vascular SM might be involved in the pathogenesis of vasculitis. Indeed, passive transfer of serum from vasculitic mice to normal healthy syngeneic controls resulted in vasculitis by day 7 after transfer (Figure 5, A and B) in all six mice injected. Histopathological analysis of the lung indicated numerous foci of periarteriolar infiltration, and a high incidence of vasculitic lesions after the intravenous transfer of serum. Intraperitoneal administration of vasculitic serum in three other mice resulted in the same pathological outcome (not shown).
Figure 5.
Serum transfer experiments show that anti-SM cell antibodies from vasculitic mice are pathogenic. Mice received intravenously pooled serum collected from either vasculitic mice or from healthy control mice. A: H&E staining of 4-μm paraffin sections of lung 7 days after vasculitic serum transfer, revealing granulomatous-like inflammation (left) and florid vasculitis with infiltration of leukocytes and vessel wall destruction (right). B and C: Individual scores (each circle represents a mouse) and the average vasculitis incidence (horizontal bar) in each experimental group (n = 6 mice per group, results from three experiments are presented). B: Incidence of vasculitis in wt BALB/c mice after transfer of serum collected from wt BALB/c mice with vasculitis, compared with transfer of wt control serum. C: In the absence of Ig, incidence of vasculitis in JhD mice after transfer of serum collected from JhD mice with vasculitis, as compared with JhD control serum. D: Immunofluorescence staining showing the reactivity of IgM from wt BALB/c vasculitic serum with permeabilized SM cells, in the original (vasculitis serum) and after serum Ig depletion on SM cells (SM abs. vasculitis serum) or on non-SM control cells (control abs. vasculitis serum). E: Incidence of vasculitis in wt BALB/c mice after transfer of wt BALB/c vasculitic serum previously absorbed on SM cells to deplete specific Ig, as compared with original vasculitis serum, control serum, and non-SM control cell-absorbed vasculitis serum (n = 6 mice per group, results from two experiments are presented); *P < 0.05 (Student’s t-test). Original magnifications: ×200 (A); ×1000 (D).
Pathogenicity of serum could indicate the presence of pathogenic autoantibodies, free or in immune complexes, in the circulation of vasculitic mice. Alternatively, there could be soluble factors in the serum, other than immunoglobulin, capable of initiating vascular inflammation in recipient mice. To address these issues we performed experiments under identical conditions as those described above, but in the absence of immunoglobulin. Mice deficient in mature B cells (JhD strain, on BALB/c background) were used, both as donors and as recipients in vasculitis induction, and in subsequent serum transfers. In contrast to the results obtained in wild-type (wt) BALB/c mice, transfer of serum that lacked immunoglobulin collected from vasculitic B-cell-deficient mice (n = 12) failed to induce the disease in B-cell-deficient recipients (Figure 5C). This result implies that the immunoglobulin may be the pathogenic component in the serum transfer-based model of vasculitis in the wt mice. Additionally, this suggests that any other soluble factor, present in the serum of vasculitic mice, would not be equivalent in importance when compared to the pathogenic role of antibodies in development of vasculitis in this model.
To confirm this finding, but also to discriminate between the role of free anti-SM antibodies and circulating immune complexes, serum collected from vasculitic wt mice was absorbed on formaldehyde-fixed SM cells, resulting in reduction of anti-SM antibodies (Figure 5D). Subsequent intravenous transfer of SM-absorbed vasculitic serum failed to induce vasculitis or perivascular infiltrates in recipient mice (Figure 5E). The absorption of anti-SM antibodies was cell-specific because it did not occur when using non-SM control cells (Figure 5D). In control mice, nonabsorbed vasculitic serum but also vasculitic serum absorbed on non-SM control cells induced vasculitis after transfer (Figure 5E). This indicates that free immunoglobulin specific for SM antigens was pathogenic after transfer of vasculitic serum to mice, rather than circulating immune complexes containing autoantibodies.
Anti-SM Antibodies Need T Cells to Cause Vascular Inflammation
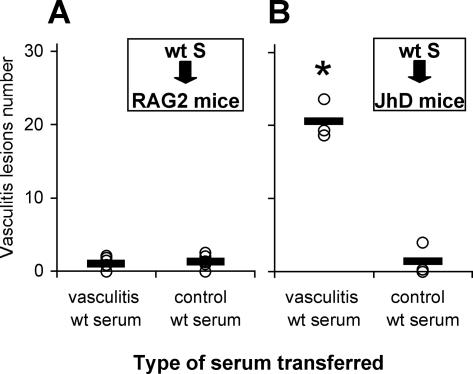
To gain insight into the pathogenic mechanisms triggered by the transfer of vasculitic serum, mice deficient in B cells (JhD) and mice deficient in both B and T cells (RAG2−/−) were used as serum recipients. Vasculitic serum was collected from wt BALB/c mice 7 days after vasculitis induction and used for passive transfer to RAG2−/− mice on BALB/c background. Surprisingly, none of the six RAG2−/− mice that received wt vasculitic serum transfer developed vasculitis, which was also the case for the six control RAG2−/− mice injected with wt normal mouse serum (Figure 6A). Because RAG2−/− mice are capable of developing antibody-mediated pathogenic reactions22,25 after serum transfer, we hypothesized that it was the lack of T cells that impaired the pathogenicity of vasculitic serum in these mice. To check this hypothesis, we performed passive serum transfers to JhD mice deficient in B cells, which are capable of mounting nonproliferative T-cell responses.26 All of the B-cell-deficient mice that received wt vasculitic serum developed vasculitis 7 days after the serum transfer (Figure 6B), whereas the control littermates that received normal serum did not. These results show that T lymphocytes have an essential role in mediating the pathogenicity of vasculitic serum containing anti-SM antibodies.
Figure 6.
T lymphocytes are necessary for pathogenicity of vasculitic serum. Serum collected and pooled from wt BALB/c mice with vasculitis was transferred to either RAG2−/− or JhD mice intravenously. A: Incidence of vasculitis in RAG2−/− BALB/c mice after transfer of wt BALB/c vasculitic serum, as compared to transfer of control healthy mouse serum (n = 6 mice per group). B: Incidence of vasculitis in JhD BALB/c mice after transfer of wt BALB/c vasculitic serum, as compared to transfer of control healthy mouse serum (n = 3 mice per group). Each circle represents the vasculitis incidence for one recipient mouse; the horizontal bar depicts the average incidence of vasculitis lesions in a particular experimental group of mice; *P < 0.05 (Student’s t-test). Results from three experiments are presented.
Discussion
The present results show that the development of small-vessel vasculitic lesions in experimental mice is accompanied by the presence of pathogenic antibodies reactive with vascular SM cells and by the in vivo persistence and expansion of activated, potentially pathogenic, B cells. The pathogenic role of anti-SM antibodies in vasculitis induction is supported by the following three observations. The transfer of serum from BALB/c mice with vasculitis to healthy syngeneic recipients resulted in vasculitis, whereas transfer of normal control serum had no effect. Next, transfer of serum lacking Ig, collected from B-cell-deficient mice with vasculitis, did not induce vasculitis lesions in syngeneic B-cell-deficient recipients. Finally, vasculitic serum lost its pathogenicity only after depletion of specific Ig by absorption on SM cells, signifying that it is the Ig anti-SM that is pathogenic after transfer to healthy mouse recipients.
The pathogenic anti-SM antibodies recognized intracellular antigens (Figure 2), which is also the case for human anti-SM antibodies from vasculitis patients,17,18 thus indicating that other pathogenic mechanisms that result in damage to vascular cells would need to take place first, in order for B cells to get exposed to the self antigen and be primed. In this experimental model, exposure of autoreactive B cells to SM antigens occurred most probably during the in vitro co-culture with SM cells. This fact is supported by the lowered CD38 and CD23 expression and the presence of CD138 on the surface of IgM+ CD45R/B220+ CD4− CD8− cells before adoptive transfer, and by the absence of B-cell proliferation in vitro,10,11 a phenotype consistent with cell differentiation to plasmablasts.27,28 Additionally, CFSE tagging showed that it is only after the adoptive transfer that these cells became subject to signals triggering their expansion, a prerequisite in the differentiation of plasmablasts to plasma cells.28
The generation of antibodies selectively targeted to intracellular SM antigens and not to cell surface proteins in vasculitic mice was surprising, but several explanations may be considered. Recent studies in transgenic mice expressing an intracellular sequestered variant of hen egg lysozyme versus a membrane-bound hen egg lysozyme indicate that intracellular sequestration of an antigen is sufficient to convert the tolerogenic self-antigen to a strong autoimmunogen.29 In that system, B cells reacting with the cell-surface hen egg lysozyme were subject to elimination or receptor editing. However, the intracellular hen egg lysozyme antigen expression positively selected antigen-binding B cells to differentiate into large numbers of IgM autoantibody-secreting plasma cells. The same concept is emphasized in a study of autoantibody patterns in spontaneous autoimmune and wt mice that calls attention to the fact that natural IgM antibodies are particularly reactive with intracellular self antigens, and that autoreactive IgG antibodies reacting with intracellular antigens are associated with the generation of autoimmunity.30
An alternative possibility is that autoantibodies directed to intracellular self antigens occur as a result of relocalization,31 or enzymatic modification of these antigens,32 mainly after apoptosis progression.33 Although a number of SM cells undergo apoptosis during the co-culture with splenocytes, the distribution and alteration of the SM autoantigens on apoptotic cells has not been determined. Nevertheless, apoptosis by itself does not determine the generation of pathogenic autoreactive B cells in culture because previous findings show that vasculitis may be induced in mice after lymphocyte stimulation with vascular SM cells,9,10 vascular endothelial cells,34 but not with skeletal muscle cells9 or fibroblasts.34 Other key factors in the generation of anti-SM autoantibodies may be the presence or absence of co-stimulatory signals during antigen encounter. Besides secreting cytokines involved in lymphocyte survival,35 SM cells release complement components, including component C3,36 potentially reactive with select antigens present in the SM co-culture. Subsequent binding of C3b-complexes to complement receptors (CR1/2) expressed on B lymphocytes may lower the reactivity threshold of B cells toward T-cell-dependent antigens.37,38
With regard to the pathogenesis of autoimmune diseases, three B-cell-mediated mechanisms are postulated so far: 1) autoantibodies generate an excess of circulating immune complexes, and their subsequent deposition may trigger complement activation, local inflammation, and tissue damage;2 2) autoantibodies targeted to surface molecules of myeloid cells may directly activate them, even in the absence of T cells;22 3) activated autoreactive B cells may prime autoreactive T cells, even in the absence of circulating antibody.39,40 Our findings suggest that an additional mechanism may be taken into consideration, in which autoantibodies may exert their pathogenic function only with the assistance of T lymphocytes. We bring evidence that in this experimental model of vasculitis, antibody mediated vascular damage required T cells, since wt and JhD mice developed the disease after vasculitic wt serum transfer, whereas RAG2−/− mice did not. One possible explanation could be that IgG autoantibodies mediated the uptake of the opsonized SM antigen by antigen-presenting cells bearing activating Fc gamma receptors (FcR), such as macrophages and dendritic cells, for cross-presentation to T cells.41–43 Because the nature of the receptor (namely FcR) that mediates the antigen internalization determines the selection of epitopes to be presented to T lymphocytes,44 IgG autoantibodies that were generated in vasculitic mice may have determined the cross-presentation of an atypical epitope of a SM antigen to T lymphocytes. This same epitope, then, would not be cross-presented by the same antigen-presenting cells in the context of free antigen uptake, or after endocytosis of IgM-antigen immune complexes.45 Future studies addressing the contribution of FcR in antigen presentation and repertoire of activated T cells in this model would be needed to further elucidate the pathogenic mechanisms mediated by autoantibodies in vasculitis. This holds true also in the light of novel hypotheses on the role of autoantigen complementarity in the etiology and pathogenesis of autoimmune disease.46,47
In conclusion, the present study argues for the parallel action of immunoglobulin and cell-mediated pathways in the pathogenesis of vasculitis in this model. Our results show that serum autoantibodies directed to vascular vessel wall (primarily SM) are pathogenic and that the mechanisms that mediate the pathogenicity of the anti-SM antibodies in this model critically depend on T cells.
Acknowledgments
We thank Shin Il Kim for FACSCalibur operation and Satoshi Kinoshita for paraffin block processing.
Footnotes
Address reprint requests to M.N. Hart, Department of Pathology and Laboratory Medicine, 6152 MSC, University of Wisconsin, 1300 University Ave., Madison, WI 53706. E-mail: mnhart@wisc.edu.
Supported by the National Institutes of Health (grant R01HL48658).
References
- Jayne D. Evidence-based treatment of systemic vasculitis. Rheumatology. 2000;39:585–595. doi: 10.1093/rheumatology/39.6.585. [DOI] [PubMed] [Google Scholar]
- Katz P. Vasculitis. Austen KF, Frank MM, Atkinson JP, Cantor H, editors. Baltimore: Lippincott Williams and Wilkins,; Samter’s Immunologic Diseases. (ed 6) 2001:pp 560–570. [Google Scholar]
- Morahan G, Morel L. Genetics of autoimmune diseases in humans and in animal models. Curr Opin Immunol. 2002;14:803–811. doi: 10.1016/s0952-7915(02)00401-6. [DOI] [PubMed] [Google Scholar]
- Specks U. Are animal models of vasculitis suitable tools. Curr Opin Rheumatol. 2000;12:11–19. doi: 10.1097/00002281-200001000-00003. [DOI] [PubMed] [Google Scholar]
- Hart MN, Waldschmidt MM, Hanley-Hyde JM, Moore SA, Kemp JD, Schelper RL. Brain microvascular smooth muscle expresses class II antigens. J Immunol. 1987;138:2960–2963. [PubMed] [Google Scholar]
- Fabry Z, Sandor M, Gajewski TF, Herlein JA, Waldschmidt MM, Lynch RG, Hart MN. Differential activation of Th1 and Th2 CD4+ cells by murine brain microvessel endothelial cells and smooth muscle/pericytes. J Immunol. 1993;151:38–47. [PubMed] [Google Scholar]
- Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- Hart MN, Fabry Z, Waldschmidt M, Sandor M. Lymphocyte interacting adhesion molecules on brain microvascular cells. Mol Immunol. 1990;27:1355–1359. doi: 10.1016/0161-5890(90)90043-y. [DOI] [PubMed] [Google Scholar]
- Hart MN, Tassell SK, Sadewasser KL, Schelper RL, Moore SA. Autoimmune vasculitis resulting from in vitro immunization of lymphocytes to smooth muscle. Am J Pathol. 1985;119:448–455. [PMC free article] [PubMed] [Google Scholar]
- Swanson BJ, Baiu DC, Sandor M, Fabry Z, Hart MN. A small population of vasculitogenic T cells expands and has skewed T cell receptor usage after culture with syngeneic smooth muscle cells. J Autoimmun. 2003;20:125–133. doi: 10.1016/s0896-8411(02)00113-0. [DOI] [PubMed] [Google Scholar]
- Fabry Z, Waldschmidt MM, Van Dyk L, Moore SA, Hart MN. Activation of CD4+ lymphocytes by syngeneic brain microvascular smooth muscle cells. J Immunol. 1990;145:1099–1104. [PubMed] [Google Scholar]
- Savage CO, Harper L, Holland M. New findings in pathogenesis of anti-neutrophil cytoplasm antibody-associated vasculitis. Curr Opin Rheumatol. 2002;14:15–22. doi: 10.1097/00002281-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Beliveau A, Dagenais P, Menard HA. Finding a valid model for antineutrophil cytoplasmic antibody-related vasculitis. Arthritis Rheum. 1997;40:986–987. doi: 10.1002/art.1780400534. [DOI] [PubMed] [Google Scholar]
- Bordron A, Revelen R, D’Arbonneau F, Dueymes M, Renaudineau Y, Jamin C, Youinou P. Functional heterogeneity of anti-endothelial cell antibodies. Clin Exp Immunol. 2001;124:492–501. doi: 10.1046/j.1365-2249.2001.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum E, Blank M, Cohen S, Afek A, Kopolovic J, Meroni PL, Youinou P, Shoenfeld Y. The role of anti-endothelial cell antibodies in Kawasaki disease—in vitro and in vivo studies. Clin Exp Immunol. 2002;130:233–240. doi: 10.1046/j.1365-2249.2002.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Chung HS, Kim HS, Oh SH, Ha MK, Baik JH, Lee S, Bang D. Human alpha-enolase from endothelial cells as a target antigen of anti-endothelial cell antibody in Behcet’s disease. Arthritis Rheum. 2003;48:2025–2035. doi: 10.1002/art.11074. [DOI] [PubMed] [Google Scholar]
- Cunningham MW, Meissner HC, Heuser JS, Pietra BA, Kurahara DK, Leung DY. Anti-human cardiac myosin autoantibodies in Kawasaki syndrome. J Immunol. 1999;163:1060–1065. [PubMed] [Google Scholar]
- Suzuki H, Muragaki Y, Uemura S, Takeuchi T, Minami T, Shibuta S, Ohshima A, Yoshikawa N. Detection of auto-antibodies against a 70 kDa protein derived from vascular smooth muscle cells in patients with Kawasaki disease. Eur J Pediatr. 2002;161:324–329. doi: 10.1007/s00431-002-0943-7. [DOI] [PubMed] [Google Scholar]
- Damianovich M, Gilburd B, George J, Del Papa N, Afek A, Goldberg I, Kopolovic Y, Roth D, Barkai G, Meroni PL, Shoenfeld Y. Pathogenic role of anti-endothelial cell antibodies in vasculitis. An idiotypic experimental model. J Immunol. 1996;156:4946–4951. [PubMed] [Google Scholar]
- Bordron A, Dueymes M, Levy Y, Jamin C, Ziporen L, Piette JC, Shoenfeld Y, Youinou P. Anti-endothelial cell antibody binding makes negatively charged phospholipids accessible to antiphospholipid antibodies. Arthritis Rheum. 1998;41:1738–1747. doi: 10.1002/1529-0131(199810)41:10<1738::AID-ART6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Krause I, Blank M, Shoenfeld Y. The induction of experimental vascular diseases by immunization with pathogenic autoantibodies. Clin Exp Rheumatol. 2000;18:257–261. [PubMed] [Google Scholar]
- Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Strauch AR, Yoder EJ, Rubenstein PA, Hart MN. Cerebral microvascular smooth muscle in tissue culture. In Vitro. 1984;20:512–520. doi: 10.1007/BF02619625. [DOI] [PubMed] [Google Scholar]
- Tomer Y, Barak V, Gilburd B, Shoenfeld Y. Cytokines in experimental autoimmune vasculitis: evidence for a Th2 type response. Clin Exp Rheumatol. 1999;17:521–526. [PubMed] [Google Scholar]
- Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Carding S, Jones B, Murray J, Portoles P, Rasmussen R, Rojo J, Saizawa K, West J, Bottomly K. CD4+ T cells: specificity and function. Immunol Rev. 1988;101:39–80. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- Sze DM, Toellner KM, Garcia de, Vinuesa C, Taylor DR, MacLennan IC. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry H, Jones M, Vaux DJ, Roberts IS, Cornall RJ. The cellular location of self-antigen determines the positive and negative selection of autoreactive B cells. J Exp Med. 2003;198:1415–1425. doi: 10.1084/jem.20030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR. Autoantibody patterns in diabetes-prone NOD mice and in standard C57BL/6 mice. J Autoimmun. 2001;17:191–197. doi: 10.1006/jaut.2001.0544. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz PJ, Gensler TJ, Anderson P. Death, autoantigen modifications, and tolerance. Arthritis Res. 2000;2:101–114. doi: 10.1186/ar75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauova L, Gilburd B, Zurgil N, Blank M, Guegas LL, Brickman CM, Cebecauer L, Deutsch M, Wiik A, Shoenfeld Y. Induction of biologically active antineutrophil cytoplasmic antibodies by immunization with human apoptotic polymorphonuclear leukocytes. Clin Immunol. 2002;103:69–78. doi: 10.1006/clim.2002.5194. [DOI] [PubMed] [Google Scholar]
- Hart MN, Sadewasser KL, Cancilla PA, DeBault LE. Experimental autoimmune type of vasculitis resulting from activation of mouse lymphocytes to cultured endothelium. Lab Invest. 1983;48:419–427. [PubMed] [Google Scholar]
- Wuttge DM, Eriksson P, Sirsjo A, Hansson GK, Stemme S. Expression of interleukin-15 in mouse and human atherosclerotic lesions. Am J Pathol. 2001;159:417–423. doi: 10.1016/S0002-9440(10)61712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Nagasawa K, Tsukamoto H, Horiuchi T, Nishizaka H, Ikeda K, Niho Y. Production of the third and fourth component of complement (C3, C4) by smooth muscle cells. Immunology. 1996;89:183–188. doi: 10.1046/j.1365-2567.1996.d01-725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongini PKA, Vilenski MA, Highet P, Inman JK. The affinity threshold for human B cell activation via the antigen receptor complex is reduced upon coligation of the antigen receptor with CD21 (CR2). J Immunol. 1997;159:3782–3791. [PubMed] [Google Scholar]
- Baiu DC, Prechl J, Tchorbanov A, Molina HD, Erdei A, Sulica A, Capel PJ, Hazenbos WL. Modulation of the humoral immune response by antibody-mediated antigen targeting to complement receptors and Fc receptors. J Immunol. 1999;162:3125–3130. [PubMed] [Google Scholar]
- Lin RH, Mamula MJ, Hardin JA, Janeway CA., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991;173:1433–1439. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioan-Facsinay A, de Kimpe SJ, Hellwig SM, van Lent PL, Hofhuis FM, van Ojik HH, Sedlik C, da Silveira SA, Gerber J, de Jong YF, Roozendaal R, Aarden LA, van den Berg WB, Saito T, Mosser D, Amigorena S, Izui S, van Ommen GJ, van Vugt M, van de Winkel JG, Verbeek JS. Fcγ RI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daeron M, van de Winkel JG, Verbeek JS. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fcγ RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, Ezekowitz A, Carroll MC, Brenner M, Weissleder R, Verbeek JS, Duchatelle V, Degott C, Benoist C, Mathis D. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Amigorena S, Lankar D, Briken V, Gapin L. Type II and III receptors for immunoglobulin G (IgG) control the presentation of different T cell epitopes from single IgG-complexed antigens. J Exp Med. 1998;187:505–515. doi: 10.1084/jem.187.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol. 2005;6:107–113. doi: 10.1038/ni1147. [DOI] [PubMed] [Google Scholar]
- Pendergraft WF, III, Preston GA, Shah RR, Tropsha A, Carter CW, Jr, Jennette JC, Falk RJ. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat Med. 2004;10:72–79. doi: 10.1038/nm968. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y. The idiotypic network in autoimmunity: antibodies that bind antibodies that bind antibodies. Nat Med. 2004;10:17–18. doi: 10.1038/nm0104-17. [DOI] [PubMed] [Google Scholar]