Abstract
Hormonal therapy (androgen ablation and/or inhibition of androgen action) is the treatment of choice for advanced prostate cancer. After an initial response in most patients, tumors invariably progress to an androgen-independent state. It is unclear how prostate cancer cells proliferate without androgen. Recent studies suggest that interleukin-8 may promote androgen-independent proliferation, but the source of interleukin-8 in the prostate is unknown. Using immunohistochemistry, we show that interleukin-8 was expressed by the neuroendocrine tumor cells in human prostate cancer tissue. Expression of the interleukin-8 receptor CXCR1 was negative or low in benign prostatic tissue and was frequently increased in malignant cells of high-grade prostatic intraepithelial neoplasia and prostate cancer; however, CXCR1 was not detected in the neuroendocrine tumor cells, suggesting a paracrine mechanism by which interleukin-8 produced by neuroendocrine tumor cells stimulates androgen-independent proliferation of prostate cancer. Neuroendocrine tumor cells expressed another type of interleukin-8 receptor, CXCR2, suggesting an autocrine mechanism by which interleukin-8 regulates the differentiation or function of the neuroendocrine cells. These results, combined with previous reports that neuroendocrine differentiation is induced by hormonal therapy, suggest that neuroendocrine cells play an important role in promoting androgen-independent growth of prostate cancer through interleukin-8 signaling.
The treatment of choice for advanced and metastatic prostate cancer (PC) is hormonal therapy, which includes androgen ablation and/or blockage of androgen action. Most patients respond well to this treatment initially but the disease eventually recurs, and they enter the hormone-refractory stage. At this stage, the tumor cells proliferate in the absence of the androgen, but the mechanism is unknown. Various hypotheses have been proposed, including amplification of the androgen receptor (AR) gene,1 increased AR protein stability with hypersensitivity to low androgen,2 AR mutation,3 and activation of mutant AR by different ligands.4 Antiandrogens may activate the AR,5,6 particularly with alterations of AR co-activators.7–9 Anti-androgens may also activate AR-independent signaling pathways to promote androgen-independent growth.10
Growth factors and cytokines may also activate the AR,11–13 and of these interleukin (IL)-8 has been implicated in the pathogenesis of androgen-independent growth of PC. IL-8 was initially identified as a regulator of leukocyte recruitment and trafficking.14,15 In patients with PC, serum IL-8 levels increase with progression of the disease.16,17 The PC cell line, PC3, expresses and secretes IL-818,19 and also expresses the IL-8 receptors CXCR1 and CXCR2.19 IL-8 is a mitogenic20 and angiogenic factor.21 PC cell line LNCaP does not express IL-8, but selection of the cells in androgen-deprived media led to the emergence of a cell line that produces IL-8 and is more tumorigenic than the parental cells.22 A recent study showed that IL-8 promotes androgen-independent growth and migration of LNCaP cells,23 suggesting that IL-8 may facilitate transition of PC to an androgen-independent state.
Although there is strong evidence linking IL-8 to the androgen-independent growth of PC, the source of IL-8 in PC is unclear. In benign prostatic tissue, there are three types of epithelial cells, namely, luminal secretory cells, basal cells, and neuroendocrine (NE) cells. In PC, there are two types of cells: the secretory-type tumor cells, which are the majority of the tumor cells; and scattered NE cells, which comprise a minor component of the tumor. The number of NE cells increases after hormonal therapy, particularly when the PC is in the androgen-independent stage and it has been hypothesized that these NE tumor cells may contribute to androgen-independent growth of PC.24,25 In this study we examined the expression of IL-8 and its receptors in PC, particularly their relationship to the NE and non-NE compartments of PC.
Materials and Methods
Construction of Tissue Microarrays Containing Benign and Malignant Prostatic Tissue
The construction of anonymized prostatic tissue microarrays was approved by the University of Rochester’s Research Subjects Review Board. Archival prostatectomy cases (from 2002 to 2003) in the Department of Pathology at the University of Rochester Medical Center were reviewed and 80 cases selected. One or more cores were taken from areas of normal prostatic tissue (N, 50 cores), benign prostatic hyperplasia (BPH, 82 cores), high-grade prostatic intraepithelial neoplasia (PIN, 35 cores), low-grade adenocarcinoma (LGPC; Gleason grades 2 and 3, 104 cores) and high-grade adenocarcinoma (HGPC; Gleason grades 4 and 5, 82 cores).
Tissue cores from the areas chosen were removed from their paraffin blocks and constructed into two arrays using a manual tissue arrayer (Beecher Instruments, Sun Prairie, WI). One array consisted of cores of N, BPH, and PIN and the other contained cores of LGPC and HGPC. Each core was 0.6 mm in diameter, 2.0 mm in height, and spaced by digital micrometry so that the center of one core was ∼1.0 mm from the center of its neighboring cores. Once the core transfer process was completed, the arrays were incubated for 6 minutes at 64°C to facilitate fusion between the paraffin within cores and the paraffin surrounding them. A section from each array was stained with routine hematoxylin and eosin method and additional sections prepared for immunohistochemical analysis.
Immunohistochemical Study
Immunohistochemical study was performed on formalin-fixed, paraffin-embedded tissue sections using a rabbit polyclonal antibody against IL-8 (catalog no. AHC0881, used at 1:600; Biosource Int., Camarillo, CA), a mouse monoclonal antibody against IL-8 receptor CXCR1 (catalog no. 555937, used at 1:800; BD Biosciences, San Diego, CA), a mouse monoclonal antibody against IL-8 receptor CXCR2 (catalog no. 555932, used at 1:50; BD Biosciences), and a mouse monoclonal antibody against chromogranin A (clone 2H10, used at 1:1000; Chemicon Int. Inc., Temecula, CA). Paraffin-embedded tissues were sectioned at 5 μm thickness and floated on distilled water at 45°C. Sections were mounted on chemically charged slides, dried at room temperature until opaque and placed in the oven at 57°C overnight. Sections were deparaffinized according to established procedures and quenched with 3% hydrogen peroxide for 6 minutes. They were then cleared in running water followed by TBS (50 mmol/L Tris-hydrogen chloride, 150 mmol/L sodium chloride, and 0.05% Tween 20 at pH 7.6).
Antigen unmasking was performed by one of the following methods: for CXCR1, sections were digested with 0.1% pepsin (Sigma-Aldrich, St. Louis, MO) for 10 minutes at 37°C; for IL-8, heat-retrieval was performed with citrate buffer, pH 6.1 (DakoCytomation, Carpinteria, CA) in the Borg Decloaking Chamber (Biocare Medical, Walnut Creek, CA) at 120 to 123°C and 18 to 21 psi for 6 minutes; for CXCR2 and chromogranin A, antigen retrieval was performed with preheated (95 to 99°C) citrate buffer, pH 6.1 (DakoCytomation) in a Black and Decker steamer (model HS800; Shelton, CT) for 30 minutes followed by a 15-minute cool down period. Slides were then rinsed with Tris-buffered saline for 5 minutes and mounted in the DAKO autostainer. Slides were covered with fresh Tris-buffered saline to prevent drying of sections during mounting. The sections were incubated with the primary antibodies at room temperature for 60 minutes (CXCR2) or 45 minutes (chromogranin A). For IL-8 and CXCR1, the sections were incubated with the respective primary antibody overnight at 4°C in a humid chamber. The sections were then incubated for 30 minutes with the link antibody (rabbit or mouse)-labeled polymer-horseradish peroxidase (Envision Plus System, DakoCytomation). Slides were developed with AEC+ (DakoCytomation) for 10 minutes, rinsed in running distilled water, counterstained in Modified Mayer’s Hematoxylin, blued in 0.3% ammonia water followed by a tap water rinse. Slides were mounted using an aqueous media and viewed with a light microscope.
Tissue cores with less than 50% of the original tissue left on the slides after immunohistochemistry were not used for the scoring of the stains. In cores that remained intact after staining, the intensity of staining (0 to 3+) and the percentage of positively stained cells in the benign and malignant epithelial cells of the prostatic tissue were recorded. For chromogranin A, IL-8, and CXCR2, any cell with a staining intensity of 2+ or 3+ was considered a positive cell and a core was considered positive with any positive cell. For CXCR1, staining in all cores from the same case was examined and combined to derive the average percentage of positive staining. A case was considered positive only when the staining intensity was 2+ or more in more than 10% of the epithelial cells, while cases with less than 10% staining in the epithelial cells or staining intensity below 2+ were recorded as negative, following the scoring method of HercepTest, which is widely used for the evaluation of Her2-Neu overexpression in breast cancer.26 Fisher’s exact test was performed to compare the statistical significance of the staining results among different groups.
Results
IL-8 Is Produced by NE Cells of the Prostate
To study the expression of IL-8 in PC, microarrays containing benign and malignant prostatic tissue cores were stained with a polyclonal antibody to IL-8. After elimination of cores with significant tissue loss during staining, 49 cores of normal prostate, 76 cores of BPH, 30 cores of PIN, 101 cores of LGCA, and 79 cores HGCA were examined. In ∼70% of the cores of PIN, LGCA, and HGCA, scattered individual cells or cell nests stained strongly, typical of the NE cells of the prostate; while no staining was seen in the remaining cases. In benign prostatic tissue (normal + BPH), ∼40% of the cores contained positive cells, also seen as individual cells or small nests. To confirm that it is the NE cells that produce IL-8 in the prostate, immediately adjacent sections were prepared from the tissue microarrays. The first section was stained with a monoclonal antibody against chromogranin A, a widely used marker of NE cells of prostate,27 and the second section stained with a polyclonal anti-IL-8 antibody and the two sections compared. In 78% of the cores that contained NE cells, the two sections showed an identical staining pattern in that cells that were positive for chromogranin A were also positive for IL-8 (Figure 1). Therefore, NE cells are the producers of IL-8 in human prostate, which is true in both benign prostate and PC. In the remaining cores the positive cells (either for chromogranin-A or IL-8) seen in one section were not seen in the adjacent section. To confirm that this was due to uneven sectioning of those cells in the two sections, additional continuous sections were stained so that a chromogranin A-stained slide was flanked by two IL-8-stained slides and vice versa. Examination of these slides showed 100% correspondence of chromogranin A and IL-8 staining in the adjacent sections of the prostate tissue microarrays, confirming that all IL-8-positive cells were NE cells.
Figure 1.
IL-8 is produced by NE cells of the prostate. Adjacent sections from the prostate tissue microarray were prepared. A and B were adjacent sections from one core and C and D were adjacent sections from another core. The first sections (A, C) were stained with a monoclonal anti-chromogranin A antibody to highlight the NE cells and the next sections were stained with a polyclonal antibody to IL-8 (B, D). The NE cells of PC (brown-stained cells in A and C) are positive for the expression of IL-8 (brown-stained cells in B and D). Immunohistochemical staining. Original magnifications, ×400.
IL-8 Receptor CXCR1 Is Overexpressed in Malignant Cells of PC
Two types of IL-8 receptors, CXCR1 and CXCR2,15 have been identified in humans. Since previous experiments demonstrated that IL-8 is expressed by NE cells of PC, it was important to determine whether IL-8 receptors are expressed in PC cells. To study if CXCR1 is expressed in the prostate, the prostate tissue microarrays were stained with a monoclonal antibody against CXCR1 and the results are summarized in Table 1. Benign and malignant prostatic epithelial cells showed very different staining patterns. Epithelial cells in benign prostatic tissue (normal and BPH) showed negative to weak staining with few positive cases, whereas in malignant epithelial cells of PIN, LGPC, and HGPC, the staining was typically strong and diffuse (Figure 2). Positive staining of the epithelial cells was seen in 12.2% cases of N, 0% cases of BPH (positive staining in all cases including N and BPH, 4.7%), 33.3% of PIN, 71% cases of LGPC, and 81% cases of HGPC (positive staining in all PCs including LGPC and HGPC, 74.8%). Fisher’s exact test showed statistically significant differences in the staining between benign prostate (including N and BPH) and PIN (P < 0.001), between benign prostate (including N and BPH) and PCs (including LGPC and HGPC) (P < 0.001) and between PIN and carcinomas (including LGPC and HGPC) (P < 0.005). The staining difference between LGPC and HGPC was not statistically significant.
Table 1.
Immunohistochemical Study of Prostate Tissue Microarray for the Expression of CXCR1
| Total cores (cases) scored | Negative cores (cases) | Positive cores (cases) | % positive cases | |
|---|---|---|---|---|
| Normal (N) | 44 (41) | 38 (36) | 6 (5) | 12.2 |
| BPH | 77 (65) | 77 (65) | 0 (0) | 0 |
| Benign (N + BPH) | 121 (106) | 115 (101) | 6 (5) | 4.7 |
| PIN | 32 (21) | 18 (14) | 14 (7) | 33.3 |
| LGCA | 103 (69) | 38 (20) | 65 (49) | 71 |
| HGCA | 80 (42) | 27 (8) | 53 (34) | 81 |
| PC (LG+HG) | 183 (111) | 65 (28) | 118 (111) | 74.8 |
Figure 2.
CXCR1 is overexpressed in malignant cells of PC. The prostatic tissue microarray was stained with a monoclonal anti-CXCR1 antibody. Expression of CXCR1 is negative in benign epithelial cells (A) while its expression in PIN (B) and PC (C) was significantly increased. D was taken from an area with both benign prostatic glands (large arrows) and cancerous glands (small arrows) showing increased expression of CXCR1 in PC. Immunohistochemical staining. Original magnifications, ×400 (A–C); ×200 (D).
The IL-8 Receptor CXCR1 Is Expressed in Non-NE Tumor Cells, Not the NE Tumor Cells of PC
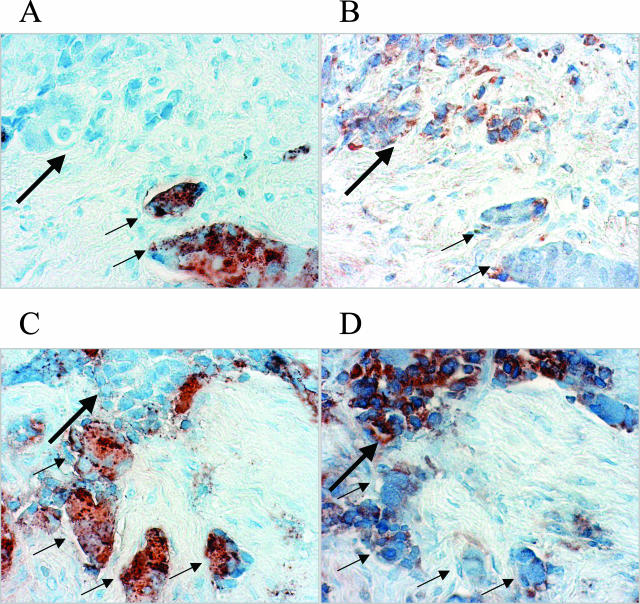
The previous experiments demonstrated that IL-8 receptor CXCR1 is overexpressed in malignant prostatic epithelial cells in comparison to benign cells. As discussed above, PC is composed of NE tumor cells and non-NE secretory-type tumor cells. In most tumors, because NE cells were rare and the staining for CXCR1 was diffuse, it was unclear whether CXCR1 was expressed in the NE tumor cells. To address this question, we focused on tissue cores from cancer cases with extensive NE differentiation. Adjacent sections from the tissue microarrays were stained with monoclonal antibodies for chromogranin A and CXCR1, respectively. As shown in Figure 3, tumor cells that were positive for chromogranin A were negative for CXCR1 whereas those that were negative for chromogranin A were positive for CXCR1. Therefore, only the non-NE tumor cells of PC, not the NE tumor cells, express CXCR1.
Figure 3.
CXCR1 is expressed in non-NE tumor cells, not the NE tumor cells of PC. Adjacent sections from the prostate tissue microarray were prepared. A and B were adjacent sections from one core and C and D were adjacent sections from another core. The first sections (A, C) were stained with a monoclonal antibody for chromogranin A and the next sections (B, D) were stained with a monoclonal antibody for CXCR1. NE tumor cells (brown-stained cells in A and C, small arrows) are negative for CXCR1 (cells in B and D that do not show brown staining, small arrows). The non-NE tumor cells (cells in A and C that do not show brown staining, large arrows) are positive for CXCR1 (brown-stained cells in B and D, large arrows). Immunohistochemical staining. Original magnifications, ×400.
The IL-8 Receptor CXCR2 Is Expressed in NE Tumor Cells of PC
We next studied the expression of CXCR2, the other type of IL-8 receptor, in benign and malignant epithelial cells of PC using a monoclonal anti-CXCR2 antibody. Adjacent sections from the tissue microarrays were stained with monoclonal antibodies against chromogranin A and CXCR2, respectively. A total of 47 cores of N, 76 cores of BPH, 35 cores of PIN, 100 cores of LGCA, and 80 cores of HGCA were examined after staining. Scattered individual cells or small nests of cells stained strongly for both markers. Comparison of the two sections showed that the same cells were positive for both markers (Figure 4). Thus, like IL-8, CXCR2 was expressed only in NE cells of the prostate. Multiple consecutive sections stained with the two antibodies alternately also showed 100% correspondence of the cells stained positively with the two antibodies.
Figure 4.
CXCR2 is only expressed in NE tumor cells of PC. Adjacent sections from the prostate tissue microarray were prepared. A and B were adjacent sections from one core and C and D were adjacent sections from another core. The first sections (A, C) were stained with a monoclonal anti-chromogranin A antibody to highlight the NE cells and the next sections (B, D) were stained with a monoclonal antibody to CXCR2. The NE cells of PC (brown-stained cells in A and C) are positive for the expression of CXCR2 (brown-stained cells in B and D). Immunohistochemical staining. Original magnifications, ×400.
Discussion
The emergence of androgen-independence (hormone refractory state) in tumors of patients receiving hormonal therapy for PC is the common path of disease progression and remains a major obstacle for therapy. Thus, understanding the mechanism by which tumor cells grow without androgen is essential for the design of future treatment strategies aimed at prevention of cancer progression. Aberrant AR signaling has been the focus of study for many researchers. Recently, however, a growing body of evidence has indicated that NE differentiation may be one of the mechanisms contributing to androgen-independent proliferation of PC.24,25 All PCs have some NE differentiation.28 NE differentiation increases in high-grade/high-stage tumors29,30 and particularly in androgen-deprived31 and androgen-independent tumors.32 NE differentiation is considered a prognostic factor in androgen-independent PC.25,33,34
The function of NE differentiation in PC has been extensively studied. NE products increase proliferation and invasiveness of PC cell lines35 and may confer apoptosis resistance to the adjacent non-NE epithelial cancer cells.36 NE cells are the major producers of vascular endothelial growth factor in PC37 and the degree of NE differentiation correlates with neovascularization.38 It is thus hypothesized that hormonal therapy leads to increased number and activity of NE cells, which promote androgen-independent growth of the non-NE cancer cells in a paracrine manner.25
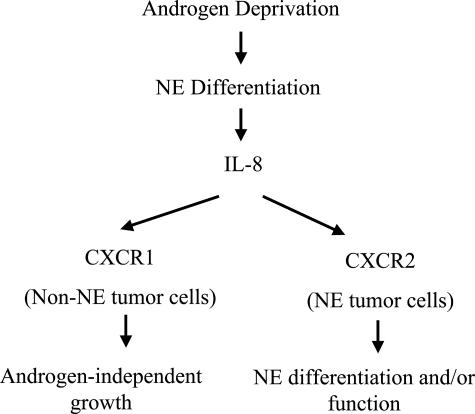
Results from the current study provide a novel mechanism by which NE cells in the prostate may promote androgen-independent growth of the neighboring non-NE tumors cells. IL-8 is a mitogenic20 and angiogenic21 factor for PC and contributes to the migration and invasion of PC3 cells.18 A recent study has shown that IL-8 may promote androgen-independent growth of PC.23 Here we demonstrate that NE cells are the source of IL-8 in PC. Although the IL-8 receptor CXCR1 is rarely expressed in benign epithelial cells, its expression is increased in PIN and further increased in invasive tumor, suggesting a paracrine mechanism whereby IL-8 produced by the NE tumor cells may promote the proliferation of the non-NE tumor cells in the absence of androgen (Figure 5).
Figure 5.
A model of the function of NE differentiation and IL-8 and its receptors in promoting androgen-independent growth of PC. In this model, androgen deprivation induces NE differentiation of PC. The NE tumor cells produce IL-8, which acts on CXCR1 in the non-NE tumor cells to promote androgen-independent growth. The IL-8 also acts on CXCR2 in the NE tumor cells to regulate NE differentiation and/or function.
The exclusive expression of IL-8 in NE cells of the prostate is a very important finding, particularly in relation to androgen-independent growth of PC because IL-8 is considered to play an important role in this process. An equally important finding is the overexpression of CXCR1 in tumor cells of PC, which may be an essential component of tumorigenesis. To compare the level of expression of CXCR1 in different cells, we adopted the semiquantitative scoring method of HercepTest. Although an imperfect method, this scoring method has been widely adopted and proven useful in studying the expression of Her2-Neu, another cell surface receptor, in breast cancer, as well as in many other published studies involving immunohistochemistry. Because our study shows a stepwise increase in the expression of CXCR1 from benign cells to PIN to invasive tumor, it will be very important to study the molecular mechanisms that regulate the expression of CXCR1 in the epithelial cells of the prostate, especially its potential role during the gradual transition from benign glands to PIN to invasive PC.
CXCR2 is the other type of receptor for IL-8. Both CXCR1 and CXCR2 are G-protein-coupled receptors that bind IL-8 with high affinity and they share significant sequence homology.39 The two IL-8 receptors are expressed in a wide range of cell types, including T cells, monocytes, monocyte-like cell lines, melanoma cells, synovial fibroblasts, neutrophils, and HL-60 and THP-1 myeloid precursor cell lines.40–43 Neutrophils can use both receptors to promote chemotaxis, and both receptors mediate IL-8-stimulated chemotaxis in transfected Jurkat cells.44,45 Interestingly, CXCR2, but not CXCR1, is expressed on Purkinje cells in human brain, in fetal neurons, and in a human neuronal cell line, hNT.46,47 CXCR2 in these cells is functional, because IL-8 binds with high affinity and induces the migration of hNT neurons.46,47 Recently, human fetal quiescent astrocytes have also been shown to express low amounts of CXCR2.47 IL-8 increases rat hippocampal neuronal survival in vitro,48 and astrocytes produce large amounts of IL-8 under proinflammatory conditions or lower amounts under noninflammatory conditions.49,50 These reports suggest that IL-8, working through CXCR2, may play an important role in neuronal function. Our demonstration of the exclusive expression of CXCR2 in NE tumor cells, but not the non-NE tumor cells of PC, is consistent with the concept that IL-8 produced by the NE cells of the prostate may regulate the function of NE cells via an autocrine mechanism through CXCR2 (Figure 5).
In cultured PC3 cells, experiments using neutralizing antibodies suggested that CXCR2 plays an important role in IL-8-stimulated invasion and metastasis, although CXCR1 may also be important.19 Our results, however, suggest that CXCR1 may be the receptor directly involved in the effector function of IL-8 produced by NE cells in promoting androgen-independent proliferation of PC because CXCR1, not CXCR2, is expressed in the non-NE tumor cells, the major cell type of PC. We hypothesize that the interaction of IL-8 with CXCR2 in the prostate is important for NE differentiation or the function of NE cells, indirectly contributing to the androgen-independent growth of PC.
We found no difference in the expression of IL-8 and its receptors between benign and malignant NE cells. Like the NE cells in PC, those in benign prostate also express IL-8 and CXCR2, but not CXCR1 (data not shown). The significance of IL-8 production by the benign NE cells is unclear, because the benign non-NE epithelial cells generally do not express IL-8 receptors. Because the benign NE cells express both IL-8 and CXCR2, there again may be an autocrine mechanism involved in either NE differentiation or the function of NE cells of the benign prostate.
In rare PC cores of our microarrays, there were focal areas of inflammation and the inflammatory cells stained strongly for IL-8, CXCR1, and CXCR2 (data not shown). A potential relationship between chronic inflammation and PC has been proposed.51 Thus it is possible that inflammatory cells may also play a role in prostate carcinogenesis through local IL-8 production. It is conceivable that both the NE cells and the inflammatory cells may contribute to the increased serum IL-8 levels seen in patients with advanced PC.
Footnotes
Address reprint requests to Jiaoti Huang, M.D., Ph.D., Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, 601 Elmwood Ave., Box 626, Rochester, NY 14642. E-mail: jiaoti_huang@urmc.rochester.edu.
Support by the University of Rochester.
References
- Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–2898. [PubMed] [Google Scholar]
- Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60:132–139. doi: 10.1016/s0090-4295(02)01593-5. [DOI] [PubMed] [Google Scholar]
- Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, Gumerlock PH, DeVere White RW, Pretlow TG, Harris SE, Wilson EM, Mohler JL, French FS. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11:450–459. doi: 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- Wilding G, Chen M, Gelmann EP. Aberrant response in vitro of hormone-responsive prostate cancer cells to antiandrogens. Prostate. 1989;14:103–115. doi: 10.1002/pros.2990140204. [DOI] [PubMed] [Google Scholar]
- Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Yeh S, Kang HY, Inui S, Chang HC, Mizokami A, Chang C. Cloning and characterization of androgen receptor coactivator, ARA55, in human prostate. J Biol Chem. 1999;274:8316–8321. doi: 10.1074/jbc.274.12.8316. [DOI] [PubMed] [Google Scholar]
- Yeh S, Kang HY, Miyamoto H, Nishimura K, Chang HC, Ting HJ, Rahman M, Lin HK, Fujimoto N, Hu YC, Mizokami A, Huang KE, Chang C. Differential induction of androgen receptor transactivation by different androgen receptor coactivators in human prostate cancer DU145 cells. Endocrine. 1999;11:195–202. doi: 10.1385/endo:11:2:195. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Yeh S, Wilding G, Chang C. Promotion of agonist activity of antiandrogens by the androgen receptor coactivator, ARA70, in human prostate cancer DU145 cells. Proc Natl Acad Sci USA. 1998;95:7379–7384. doi: 10.1073/pnas.95.13.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YF, Lin WJ, Huang J, Messing EM, Chan FL, Wilding G, Chang C. Activation of mitogen-activated protein kinase pathway by the antiandrogen hydroxyflutamide in androgen receptor-negative prostate cancer cells. Cancer Res. 2002;62:6039–6044. [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor and epidermal growth factor. Eur Urol. 1995;27(Suppl 2):45–47. doi: 10.1159/000475232. [DOI] [PubMed] [Google Scholar]
- Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- Matsushima K, Baldwin ET, Mukaida N. Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem Immunol. 1992;51:236–265. [PubMed] [Google Scholar]
- Onuffer JJ, Horuk R. Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol Sci. 2002;23:459–467. doi: 10.1016/s0165-6147(02)02064-3. [DOI] [PubMed] [Google Scholar]
- Veltri RW, Miller MC, Zhao G, Ng A, Marley GM, Wright GL, Jr, Vessella RL, Ralph D. Interleukin-8 serum levels in patients with benign prostatic hyperplasia and prostate cancer. Urology. 1999;53:139–147. doi: 10.1016/s0090-4295(98)00455-5. [DOI] [PubMed] [Google Scholar]
- Lehrer S, Diamond EJ, Mamkine B, Stone NN, Stock RG. Serum interleukin-8 is elevated in men with prostate cancer and bone metastases. Technol Cancer Res Treat. 2004;3:411–412. doi: 10.1177/153303460400300501. [DOI] [PubMed] [Google Scholar]
- Moore BB, Arenberg DA, Stoy K, Morgan T, Addison CL, Morris SB, Glass M, Wilke C, Xue YY, Sitterding S, Kunkel SL, Burdick MD, Strieter RM. Distinct CXC chemokines mediate tumorigenicity of prostate cancer cells. Am J Pathol. 1999;154:1503–1512. doi: 10.1016/S0002-9440(10)65404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiland J, Furcht LT, McCarthy JB. CXC-chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate. 1999;41:78–88. doi: 10.1002/(sici)1097-0045(19991001)41:2<78::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, Dinney CP. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6:2104–2119. [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Patel BJ, Pantuck AJ, Zisman A, Tsui KH, Paik SH, Caliliw R, Sheriff S, Wu L, deKernion JB, Tso CL, Belldegrun AS. CL1-GFP: an androgen independent metastatic tumor model for prostate cancer. J Urol. 2000;164:1420–1425. [PubMed] [Google Scholar]
- Lee LF, Louie MC, Desai SJ, Yang J, Chen HW, Evans CP, Kung HJ. Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene. 2004;23:2197–2205. doi: 10.1038/sj.onc.1207344. [DOI] [PubMed] [Google Scholar]
- di Sant’Agnese PA. Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer. 1992;70:254–268. doi: 10.1002/1097-0142(19920701)70:1+<254::aid-cncr2820701312>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Huang J, di Sant’Agnese PA. Neuroendocrine differentiation in prostate cancer: an overview. Lamberts SWJ, Dogliotti L, editors. Bristol: BioScientifica Ltd.,; Advances in OncologyThe Expanding Role of Actreotide I. 2002:pp 243–262. [Google Scholar]
- Rhodes A, Jasani B, Anderson E, Dodson AR, Balaton AJ. Evaluation of HER-2/neu immunohistochemical assay sensitivity and scoring on formalin-fixed and paraffin-processed cell lines and breast tumors: a comparative study involving results from laboratories in 21 countries. Am J Clin Pathol. 2002;118:408–417. doi: 10.1309/97WN-W6UX-XJWT-02H2. [DOI] [PubMed] [Google Scholar]
- Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol. 2005;47:147–155. doi: 10.1016/j.eururo.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Abrahamsson PA, Wadstrom LB, Alumets J, Falkmer S, Grimelius L. Peptide-hormone- and serotonin-immunoreactive tumour cells in carcinoma of the prostate. Pathol Res Pract. 1987;182:298–307. doi: 10.1016/S0344-0338(87)80065-1. [DOI] [PubMed] [Google Scholar]
- Abrahamsson PA, Falkmer S, Falt K, Grimelius L. The course of neuroendocrine differentiation in prostatic carcinomas. An immunohistochemical study testing chromogranin A as an “endocrine marker.”. Pathol Res Pract. 1989;185:373–380. doi: 10.1016/S0344-0338(89)80016-0. [DOI] [PubMed] [Google Scholar]
- Bohrer MH, Schmoll J. Immunohistochemical and morphometric studies on neuroendocrine differentiation of prostate carcinomas. Verh Dtsch Ges Pathol. 1993;77:107–110. [PubMed] [Google Scholar]
- Ahlgren G, Pedersen K, Lundberg S, Aus G, Hugosson J, Abrahamsson PA. Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate. 2000;42:274–279. doi: 10.1002/(sici)1097-0045(20000301)42:4<274::aid-pros4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Jiborn T, Bjartell A, Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 1998;51:585–589. doi: 10.1016/s0090-4295(97)00684-5. [DOI] [PubMed] [Google Scholar]
- Abrahamsson PA. Neuroendocrine differentiation and hormone-refractory prostate cancer. Prostate Suppl. 1996;6:3–8. doi: 10.1002/(sici)1097-0045(1996)6+<3::aid-pros2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- di Sant’Agnese PA. Neuroendocrine differentiation in human prostatic carcinoma. Hum Pathol. 1992;23:287–296. doi: 10.1016/0046-8177(92)90110-o. [DOI] [PubMed] [Google Scholar]
- Hoosein NM, Logothetis CJ, Chung LW. Differential effects of peptide hormones bombesin, vasoactive intestinal polypeptide and somatostatin analog RC-160 on the invasive capacity of human prostatic carcinoma cells. J Urol. 1993;149:1209–1213. doi: 10.1016/s0022-5347(17)36349-8. [DOI] [PubMed] [Google Scholar]
- Segal NH, Cohen RJ, Haffejee Z, Savage N. BCL-2 proto-oncogene expression in prostate cancer and its relationship to the prostatic neuroendocrine cell. Arch Pathol Lab Med. 1994;118:616–618. [PubMed] [Google Scholar]
- Harper ME, Glynne-Jones E, Goddard L, Thurston VJ, Griffiths K. Vascular endothelial growth factor (VEGF) expression in prostatic tumours and its relationship to neuroendocrine cells. Br J Cancer. 1996;74:910–916. doi: 10.1038/bjc.1996.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobholz R, Bohrer MH, Siegsmund M, Junemann KP, Bleyl U, Woenckhaus M. Correlation between neovascularisation and neuroendocrine differentiation in prostatic carcinoma. Pathol Res Pract. 2000;196:277–284. doi: 10.1016/S0344-0338(00)80056-4. [DOI] [PubMed] [Google Scholar]
- Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12:313–335. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Amento EP, Bauer EA, Horuk R. Melanoma growth-stimulatory activity/GRO decreases collagen expression by human fibroblasts. Regulation by C-X-C but not C-C cytokines. J Biol Chem. 1993;268:1338–1342. [PubMed] [Google Scholar]
- Moser B, Barella L, Mattei S, Schumacher C, Boulay F, Colombo MP, Baggiolini M. Expression of transcripts for two interleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J. 1993;294:285–292. doi: 10.1042/bj2940285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Delgado MB, Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur J Immunol. 1998;28:164–170. doi: 10.1002/(SICI)1521-4141(199801)28:01<164::AID-IMMU164>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Both interleukin-8 receptors independently mediate chemotaxis. Jurkat cells transfected with IL-8R1 or IL-8R2 migrate in response to IL-8, GRO alpha and NAP-2. FEBS Lett. 1994;341:187–192. doi: 10.1016/0014-5793(94)80454-0. [DOI] [PubMed] [Google Scholar]
- Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158:2882–2890. [PubMed] [Google Scholar]
- Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- Araujo DM, Cotman CW. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- Desbaillets I, Diserens AC, Tribolet N, Hamou MF, Van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. 1997;186:1201–1212. doi: 10.1084/jem.186.8.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J Urol. 2004;171:S36–S40. doi: 10.1097/01.ju.0000108131.43160.77. [DOI] [PubMed] [Google Scholar]