Abstract
Tumor embolism occurs in 30 to 50% of all cases of cardiac myxoma, but the causes are still uncertain. Matrix metalloproteinases (MMPs) are proteolytic enzymes that degrade the extracellular matrix (ECM) and play a crucial role in plaque instability and aortic aneurysm development, in addition to cancer and heart failure. To determine whether MMP activity contributes to tumor embolism, we examined 27 left atrium-sided myxomas, 10 of which showed clinical signs of peripheral embolism. Immunohistochemistry (in all cases) and Western blotting, and in situ and in-gel zymography (in four embolic and six nonembolic consecutive tumors) demonstrated higher expression and activity of MT1-MMP, pro-MMP-2, and pro-MMP-9 in embolic myxomas, whereas pro-MMP-1, MMP-3, and TIMP-1 levels were similar to those of nonembolic tumors. Reverse transcriptase-polymerase chain reaction demonstrated that increased MMP activity was due, at least in part, to increased transcription and that TIMP-2 transcripts increased in embolic myxomas. In vitro, embolic tumor cells retained higher MT1-MMP and pro-MMP-2 levels in basal conditions and after stimulation with interleukin-1β and interleukin-6. Increased MMP synthesis and release correlated with enhanced ECM degradation products containing glycosaminoglycan chains in embolic myxoma tissue. Our results strongly suggest that MMP overexpression may contribute to an excessive degradation of tumor ECM and increase the risk of embolism in cardiac myxomas.
Myxomas are the most frequent primary cardiac neoplasms, accounting for 50% of all tumors.1–3 Although all cardiac chambers may be involved, myxomas usually arise from the interatrium septum and most frequently involve the left side.1–4 Biatrial or ventricular localizations are rare.1,2,5 Diagnosis is easily performed with echocardiography2 but patients frequently present only general symptoms or are asymptomatic.1–3 Myxomas are considered tumors with an endocardial origin,2 whose name derives from their prevalent myxoid extracellular matrix (ECM) rich in proteoglycans.1 Chondroitin-6-sulfate, hyaluronic acid, and chondroitin-4-sulfate represent more than 90% of tumor glycosaminoglycans (GAGs) and are abundant in myxoma tissues.6 Cardiac myxomas are benign tumors that are unable to infiltrate the myocardium or give rise to metastases.1,2,7 Nevertheless, they are considered clinically malignant tumors because of their susceptibility to embolize to distant organs.1–3,8 As a matter of fact, clinical signs of tumor embolism represent the primary manifestation in 30 to 50% of cases and, when surgical removal of the embolus is possible, diagnosis of myxoma is confirmed by histological examination.1–5,8 Because most myxomas are left atrium-located, emboli prevalently involve peripheral districts, in particular cerebral arteries, including the retinal artery.1–3,8 Echocardiographic polypoid and irregular macroscopic aspects,3,5 changes in the composition of the myxomatous matrix,6 as well as the autocrine production of interleukin (IL)-69 have been considered, but the real causes of embolism in cardiac myxomas are still unknown. Matrix metalloproteinases (MMPs) are a large family of zinc-dependent proteolytic enzymes that are responsible for ECM remodeling in both normal and pathological processes.10–15 Many MMPs are released into the extracellular milieu in a proenzyme state with affinity to specific ECM proteins.10,16 MT-MMPs are a recently described class of membrane-bound MMPs17 that undergo intracellular activation18 and are proteolytically active once inserted into the cell membrane. In most neoplasms, MMPs are variably secreted according to histogenesis and the degree of malignancy.13–15 The deregulated activity of MMPs has also been reported in congestive heart failure and myxomatous heart valves.17–21 The present study aims to evaluate MMP activity in cardiac myxomas with the hypothesis of its possible role in the pathogenesis of tumor embolism.
Materials and Methods
Samples and Histology
Twenty-seven cardiac myxomas were obtained from surgical pathology material throughout the last 5 years. Ten patients showed as first symptom, a classical sign of tumor embolism;1–3 among these, nine presented transient or permanent neurological symptoms and in three, magnetic resonance imaging confirmed the presence of cerebral foci of abnormal signal. In one case, echocardiographs were consistent with an acute myocardial infarction. After transthoracic or transesophageal echocardiographic diagnosis, tumors were surgically removed. In four embolic and six nonembolic consecutive tumors (case nos. 1 to 10), after intraoperatory diagnosis, freshly excised small samples were also frozen in isopentane, cooled in liquid nitrogen, and stored at −80°C or used for in vitro studies (Table 1). Control left ventricular myocardial tissue was obtained from the autopsy of a 32-year-old man who died from a cerebral aneurysm. The protocol of the study was approved by the local ethical human research committee. Hematoxylin and eosin-, Alcian blue-, periodic acid-Schiff-, and Masson’s trichrome-stained sections were used for morphological and histochemical studies by two independent researchers without knowledge of data regarding tumors. Intervariability was less than 5%. Cellularity (cell number/mm2) was expressed by the mean value of 10 fields at ×400.
Table 1.
Clinical Characteristics of Cardiac Myxomas
| Case no. | Sex | Age (years) | Clinical presentation | Embolism | Type of surface | Size (cm) | Weight (g) |
|---|---|---|---|---|---|---|---|
| 1 | Male | 55 | Dyspnea | No | Smoothened | 6 × 5 | 43.5 |
| 2 | Female | 69 | Asymptomatic | No | Smoothened | 7.7 × 3.5 × 5.9 | 159 |
| 3 | Female | 48 | Dyspnea | No | Smoothened | 5.5 × 4.9 | 58 |
| 4 | Male | 49 | Dyspnea | No | Smoothened | 4 × 5 | 29 |
| 5 | Female | 59 | Thoracic pain | No | Smoothened | 3.4 × 2 | 9 |
| 6 | Female | 65 | Asymptomatic | No | Smoothened | 2.5 × 3 | 5.4 |
| 7 | Male | 48 | T.I.A | Yes | Irregular | 5.5 × 4 | 32 |
| 8 | Male | 19 | T.I.A | Yes | Irregular | 5 × 4 × 3 | 43.5 |
| 9 | Female | 53 | T.I.A | Yes | Irregular | 4 × 2.5 | 14.5 |
| 10 | Male | 43 | T.I.A., dyspnea, weight loss | Yes | Irregular | 8 × 4 | 92.8 |
| 11 | Female | 52 | Ictus | Yes | Irregular | 6 × 5 | 65 |
| 12 | Female | 52 | Non-Q infarction | Yes | Irregular | 2 × 3 | 8 |
| 13 | Male | 74 | T.I.A | Yes | Irregular | 3.2 × 4 | 18.5 |
| 14 | Female | 79 | T.I.A | Yes | Irregular | 6.5 × 5 × 3 | 70 |
| 15 | Female | 52 | T.I.A | Yes | Irregular | 3 × 1.5 × 1 | 3.3 |
| 16 | Male | 41 | T.I.A | Yes | Irregular | 1.5 × 2 | 2 |
| 17 | Female | 63 | Dyspnea | No | Smoothened | 3.5 × 3.5 | 17.8 |
| 18 | Female | 47 | Dyspnea | No | Irregular | 2.5 × 1 | 3.6 |
| 19 | Female | 62 | Dyspnea | No | Smoothened | 5 × 3 | 21.8 |
| 20 | Female | 68 | Thoracic pain | No | Smoothened | 5 × 4 × 3 | 43.5 |
| 21 | Female | 85 | Dyspnea | No | Smoothened | 6.5 × 5.5 × 4 | 103.6 |
| 22 | Female | 46 | Dyspnea | No | Smoothened | 5 × 2 | 14 |
| 23 | Female | 78 | Dyspnea | No | Irregular | 1.5 × 1 | 1.1 |
| 24 | Female | 78 | Dyspnea | No | Smoothened | 2.5 × 2 | 3.6 |
| 25 | Female | 59 | Dyspnea | No | Smoothened | 8 × 6 × 5 | 174 |
| 26 | Female | 59 | Cardiac failure | No | Smoothened | 4 × 3 × 3 | 26.1 |
| 27 | Female | 59 | Dyspnea | No | Smoothened | 4.2 × 2.5 | 15 |
Immunohistochemistry
Tumor serial sections from archival paraffin-embedded tissue blocks were placed on SuperFrost Plus slides (Menzel-Gläser, Braunschweig, Germany) and baked overnight at 60°C, deparaffinized in xylene, and rehydrated in graded concentrations of ethanol. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide-methanol at room temperature. Slides were rehydrated in phosphate-buffered saline. Antigen retrieval was performed by incubating sections in 10 mmol/L sodium citrate buffer (pH 6.0) at 98°C for 30 minutes for MMP-1, MMP-2, MMP-3, MT1-MMP, TIMP-2, and Ki-67 and for 10 minutes at 37°C with 0.5% trypsin in Tris buffer, 0.05 mol/L, pH 7.6, for MMP-9. Nonspecific antibody binding was blocked by incubation with normal goat serum (1:20 in bovine serum albumin 5%; Ylem, Avezzano, Italy) for 30 minutes at room temperature. The optimal dilutions for each primary antibody were found to be 1:15 for mouse monoclonal anti-MMP-1, -2, and -3 (Calbiochem, Darmstadt, Germany), 1:25 for mouse monoclonal anti-Ki-67 (Ylem); 1:100 for mouse monoclonal anti-TIMP-2, rabbit polyclonal anti-MT1-MMP, and anti-TIMP-4; 1:200 for rabbit polyclonal anti-MMP-9 (Neomarkers, Fremont, CA). Incubation time was 30 minutes for polyclonal and 1 hour for monoclonal antibodies at room temperature, except for Ki-67, which was incubated overnight at 4°C. Slides were then incubated with two different biotin-labeled secondary antibodies, followed by a streptavidin-horseradish peroxidase conjugate. Bound antibody was revealed with the use of the substrate 3,3′-diaminobenzidine. Positive (high-grade mammary carcinoma) and negative controls (nonspecific IgG, 5 μg/ml, instead of primary antibody and absence of secondary antibody) were included with each batch of sections to confirm the consistency of the analysis. MMP immunostaining was graded as follows: 0, complete absence of staining; 1, weak in <50% of cells; 2, weak in >50% or strong in <10% of cells; 3, strong in 10 to 50% of cells; 4, strong in >50% of myxoma cells. Semiquantitative analysis was done by two observers without knowledge of the tumor group or antibodies. The results were expressed as mean number of 10 high-power fields.
Western Blot Analysis
MMP content in tumor tissue and cultured myxoma cells was detected by Western blot analysis. Frozen tumor specimens were minced with a mortar and pestle at liquid nitrogen temperature and homogenized by sonication in reducing Laemmli loading buffer.22 Cells from cultures were trypsinized, centrifuged, suspended in loading buffer (100 μl/106 cells), sonicated, and boiled for 3 minutes. Protein content was determined by the Bradford assay.23 Proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (10% polyacrylamide) and transferred to nitrocellulose membranes (0.45 μm; Schleicher and Schuell).24 The following primary antibodies were used: mouse monoclonal anti-MMP-1, anti-MMP-2, anti-MMP-3 (Calbiochem), and rabbit polyclonal anti-TIMP-2, anti-MMP-9, anti-MT1-MMP (Neomarkers), and anti-total actin (provided by Prof. G. Gabbiani, Geneva, Switzerland); anti-mouse and anti-rabbit peroxidases (Nordic) were used as secondary antibodies. Detection was performed with ECL detection reagents (Amersham Life Sciences, Little Chalfont, UK). Western blot intensity was calculated for each band by densitometry using Fluor-S Max Multi Imager (Bio-Rad Laboratories, Hercules, CA); results were expressed in arbitrary units (uncalibrated optical density, uOD)25 in relationship to control protein (total actin).
To verify if ECM degradation was present in embolic myxomas, we investigated tumor tissue by 4 to 18% gradient SDS-PAGE under reducing conditions and Western blotting using an anti-GAG monoclonal antibody reacting with an O-linked oligosaccharide moiety of proteoglycans, in particular chondroitin sulfate chains (clone 2D2, 1:150; US Biological, Swampscott, MA) and total actin as control; densitometric examination of the bands corresponding to GAG-containing degradation fragments of less than ∼100 kd26 was performed as reported above. In addition, 1 ml of supernatant from embolic and nonembolic myxoma cell serum-deprived cultures was added to 30 μg of previously freeze-thawed and finely minced bovine nasal cartilage27 and incubated at 37°C for 24 hours with ethylenediamine tetraacetic acid (EDTA). After centrifugation, 50 μl of digestion volume were separated by SDS-PAGE (8% polyacrylamide) and release of GAG-containing fragments was revealed and quantified after Western blotting. All experiments were repeated in triplicate.
In Situ Zymography
In situ zymography was performed as reported by Oh and colleagues.28 Briefly, 6-μm-thick frozen sections were incubated with 0.1 mg/ml fluorescein isothiocyanate-labeled DQ gelatin (Molecular Probes, Eugene, OR) in 1× reaction buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 5 mmol/L CaCl2, 0.2 mmol/L NaN3, pH 7.6) overnight at 35°C. At the end of the incubation period and without fixation or washes, gelatinolytic MMP activity evidenced by enhanced green color was photographed under a fluorescence microscope (Eclipse E600; Nikon, Tokyo, Japan) by a digital camera (DMX1200F; Nikon). No gelatinolytic activity was detected when sections were incubated in the same buffer at 4°C or in the presence of 20 mmol/L EDTA, a known inhibitor of MMPs, at 35°C.
In-Gel Zymography
MMP-2 and MMP-9 (gelatinase A and B) levels were also investigated by in-gel zymography. Frozen tumor tissue fragments minced at liquid nitrogen temperature and cultured cells were collected and lysed in lysis buffer (150 mmol/L NaCl, 10 mmol/L KCl, 150 mmol/L Na2HPO4, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 0.02% NaN3) for 1 hour at 4°C. Protein content was determined by the Bradford assay.22 Conditioned media were collected and concentrated using Centricon YM-10 concentrators (Amicon Millipore, Bedford, MA). Samples (15 and 25 μg for tissues and cells, respectively) were diluted in 20 μl and separated by SDS-PAGE in 8% polyacrylamide gels containing 0.1% gelatin under nonreducing conditions, washed first in renaturing buffer (2.7% Triton X-100), then in a developing buffer (50 mmol/L Tris HCl, 200 mmol/L NaCl, 10 mmol/L CaCl2, pH 7.5) for 30 minutes, and left in the same buffer overnight at 37°C. Gels were stained with Coomassie brilliant blue R-250 and destained with 5% methanol and 7% acetic acid. When appropriate, the developing buffer contained 20 mmol/L EDTA or 1 mmol/L phenylmethyl sulfonyl fluoride, a known inhibitor of serine proteases. A semiquantitative analysis of gelatinase activity was performed in triplicate by densitometric methods and intensity for each band expressed in uOD.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Trizol (Invitrogen, Carlsbad, CA) was used to extract RNA from tumor cells and tissues. First-strand complementary DNA was synthesized from 2 μg of total RNA with random primers (Promega, Madison, WI). The following primers were used: β2-microglobulin, 5′CTT GTC TT TCA GCA AGG ACT GG (sense) and 5′CCT CCA TGA TGC TGC TTA CAT GTC (anti-sense); MMP-1, 5′GAT CAT CGG GAC AAC TCT CCT (sense) and 5′TCC GGG TAG AAG GGA TTT GTG (anti-sense);29 MMP-2, 5′CTG ACA TTG ACC TTG GCA CC (sense) and 5′TAG CCA GTC GGA TTT GAT GC (anti-sense);30 MMP-3, 5′GAA CAA TGG ACA AAG GAT ACA ACA (sense) and 5′TTC TTC AAA AAC AGC ATC AAT CTT (anti-sense);31 MMP-9, 5′CGC TAC CAC CTC GAA CTT TG (sense) and 5′GCC ATT CAC GTC GTC CTT AT (anti-sense);32 TIMP-2, 5′TGC AGC TGC TCC CCG GTG CAC (sense) and 5′TTA TGG GTC CTC GAT GTC GAG (anti-sense);33 TIMP-4, 5′AAT CTC CAG TGA GAA GGT AGT TCC (sense) and 5′CGA TGT CAA CAA ACT CCT TCC TGA (anti-sense);34 MT1-MMP, 5′ ATC TGT GAC GGG AAC TTT GAC (sense) and 5′ACC TTC AGC TTC TGG TTG TTG (anti-sense).33 A semiquantitative analysis of RT-PCR was performed in triplicate by densitometric methods and intensity for each band expressed in uOD; β2-microglobulin was used as control gene.
Cell Culture
Myxoma cells were extracted ex vivo by enzymatic digestion with collagenase (Sigma-Aldrich, St. Louis, MO) and grown in RPMI medium with 20% fetal bovine serum at 37°C in a 5% CO2 atmosphere. After differential trypsinization and morphological confirmation, myxoma cells (5 × 103/cm2) at passage 2 were treated in serum-free conditions with IL-1β (5 ng/ml, Sigma) and IL-6 (7.5 ng/ml, Sigma); the medium was replaced after 24 hours. Cells and supernatants were collected after 24 and 48 hours.
Statistical Analysis
The categorical scores obtained from the morphological study were compared in embolic and nonembolic tumor groups using χ2 test analysis. MMP expression differences among clinicopathological groups were analyzed by Student’s t-test and one-way analysis of variance. All calculations were performed with the statistical analysis software (SPSS) computer program. Results were expressed as mean ± SEM and the differences were considered statistically significant for value of P < 0.05.
Results
Clinicopathological Findings
Clinical findings are reported in Table 1. The mean age of patients with embolic and nonembolic myxomas was 51.3 ± 5.2 and 61.7 ± 2.7 years, but this difference was not statistically significant (P < 0.065). The same was observed comparing tumors classified according to their macroscopic aspect, ie, irregular (53.2 ± 4.9) and smoothened myxomas (61.6 ± 2.7; P < 0.13). Tumor tissue fragmentation was the result of routine surgical procedures in 40% of embolic and none of nonembolic myxomas. As reported in Table 2, all embolic myxomas showed a villous or irregular surface and the absence of areas with superficial collagenization; among nonembolic tumors, only two (11.8%) showed an irregular surface and 64.7% the presence of superficial collagenization. Tumor cellularity did not significantly differ when comparing embolic and nonembolic myxomas (P < 0.072). No significant difference was present when comparing the remaining morphological features typical of cardiac myxomas, in particular the presence of inflammatory cell infiltrates (Table 2), according to previously reported data.1–4
Table 2.
Main Histological Features in Embolic and Nonembolic Cardiac Myxomas
| Embolic myxomas (n = 10) | Nonembolic myxomas (n = 17) | P value | |
|---|---|---|---|
| Irregular surface (%) | 100 | 11.8 | <0.001 |
| Superficial collagenization (%) | 10 | 64.7 | <0.02 |
| Myxomatous areas (>50%) | 80 | 41.2 | n.s. |
| Ki-67-positive cells (>5%) | 10 | 5.9 | n.s. |
| Calcification (%) | 0 | 11.8 | n.s. |
| Small superficial thrombi (%) | 60 | 35.3 | n.s. |
| Extramedullary hematopoiesis (%) | 10 | 11.8 | n.s. |
| Cellularity (cells/mm2) | 2577 ± 162 | 2150 ± 150 | n.s. |
n.s., not significant.
MMP Overexpression in Embolic Myxomas in Vivo
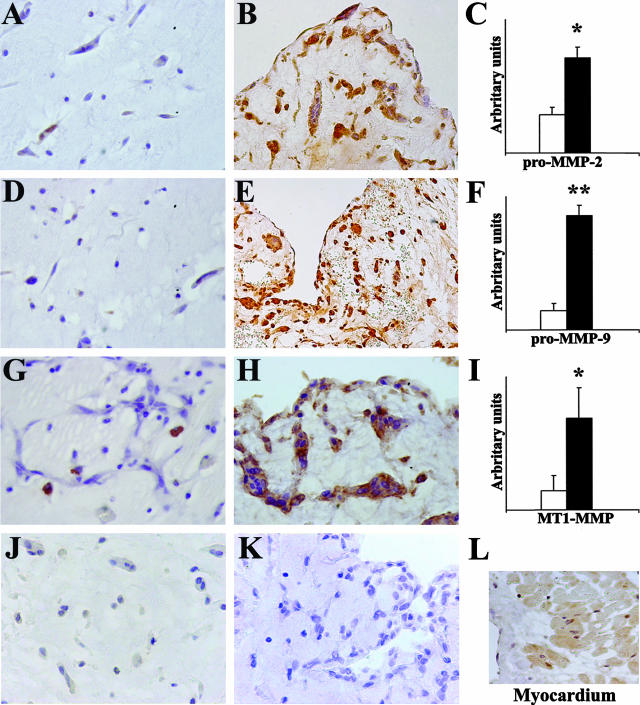
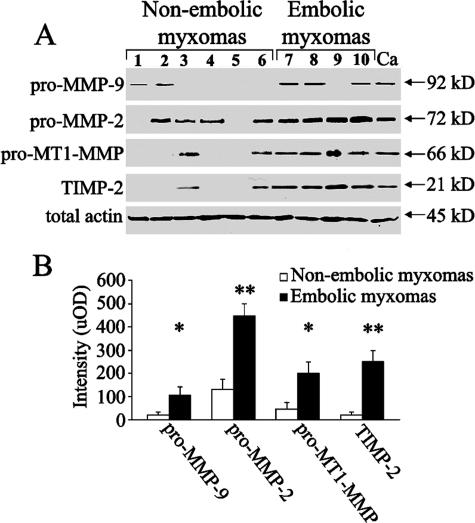
Immunohistochemical results showed that embolic myxoma expressed higher levels of catabolic enzymes than nonembolic myxomas (Figure 1). Myxoma cells appeared markedly positive, whereas myxoid stroma was less positive for pro-MMP-2 and -9, suggesting an extracellular diffusion after secretion, and negative for MT1-MMP (Figure 1). A semiquantitative analysis showed that pro-MMP-2, MMP-9, and MT1-MMP levels in embolic myxomas (grades 2.3 ± 0.4, 1.5 ± 0.4, and 2.4 ± 0.3, respectively) were significantly higher than those in nonembolic myxomas (grades 0.5 ± 0.14, 0.5 ± 0.15, and 0.5 ± 0.1; P < 0.02, P < 0.001, and P < 0.02, respectively). Pro-MMP-1 and pro-MMP-3 expression was generally low and no significant differences were observed comparing embolic and nonembolic myxomas. TIMP-4 immunostaining was negative in embolic and nonembolic myxomas (Figure 1, J and K) and positive in control myocardium (Figure 1L). Evaluation of the myxomas classified according to clinical and macroscopic features confirmed the increased MMP-2, MT1-MMP (P < 0.001) and MMP-9 (P < 0.005) expression in embolic irregular compared to nonembolic smoothened and nonembolic irregular tumors. No significant differences were present comparing nonembolic smoothened and nonembolic irregular tumors. MMP levels were also documented by Western blot analysis (Figure 2A). The densitometric blot analysis (Figure 2B) showed that although there was a discrete individual variability, pro-MMP-9, pro-MMP-2, pro-MT1-MMP, and TIMP-2 content was greater in embolic as compared to nonembolic myxomas (P < 0.05, P < 0.002, P < 0.05, and P < 0.002, respectively).
Figure 1.
Immunohistochemical evaluation of MMP expression in cardiac myxomas. Embolic myxomas (n = 10) show a high expression of pro-MMP-2, pro-MMP-9, and MT1-MMP (B, E, and H, respectively) whereas in nonembolic myxomas (n = 17) their expression is low or absent (A, D, and G, respectively); TIMP-4 immunostaining is negative in embolic (J) and nonembolic myxomas (K) and positive in control myocardium (L). In C, F, and I, semiquantitative evaluation of immunohistochemical staining expressed in arbitrary units (as reported in Materials and Methods) confirms the higher MMP expression in embolic (▪) compared to nonembolic myxomas (□); results are given as means ± SEM; *P < 0.02 and **P < 0.001 versus nonembolic myxomas. Original magnifications: ×200 (E, L); ×250 (B); ×400 (A, D, G, H, J, K).
Figure 2.
MMP expression in cardiac myxomas evaluated by Western blot analysis. A: Pro-MMP-2, pro-MMP-9, pro-MT1-MMP, and TIMP-2 content is increased in embolic as compared to nonembolic myxomas. B: Densitometric blot analysis in uOD values confirms that MMP expression is increased in embolic as compared to nonembolic myxomas; results are given as means ± SEM; *P < 0.05 and **P < 0.002 versus nonembolic myxomas Ca, control mammary carcinoma.
In-Gel and In Situ Zymography
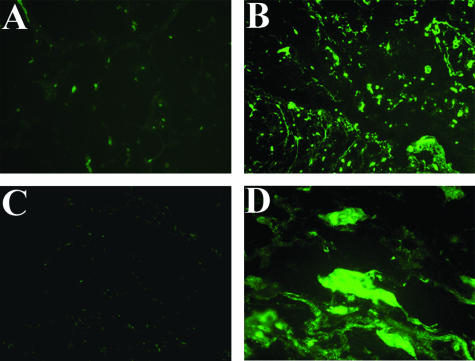
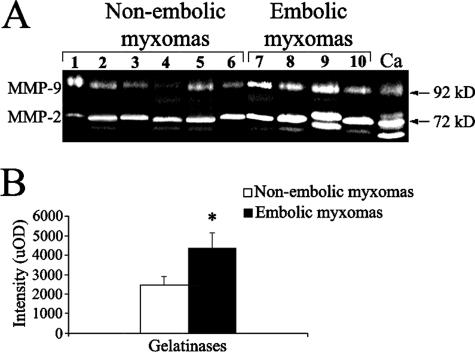
To localize the sites of net gelatinolytic activity at tumor tissue level, we examined gelatin degradation beneath cryostatic sections. This method allows active forms to be detected and overrides inhibitory potential of endogenous inhibitors.28 Densitometric evaluation of in situ zymography (Figure 3) showed that gelatin degradation was more marked in embolic myxoma tissue (64,680 ± 6047) compared to nonembolic tumors (2530 ± 473, P < 0.01) and somehow more evident in superficial compared to deeper tumor portions (not shown). In-gel zymography (Figure 4A) confirmed the increase of gelatinases in embolic compared to nonembolic myxomas (P < 0.05; Figure 4B). Gelatinase activity was inhibited by metal chelator EDTA but not by phenylmethyl sulfonyl fluoride (not shown), confirming their identity as gelatinases.
Figure 3.
Gelatinolytic activity in cardiac myxomas evaluated by in situ zymography. Overall gelatinolytic activity is lower in nonembolic myxoma tissue (A) than in embolic tumor (B), as demonstrated by increased fluorescence of the quenched fluorescein isothiocyanate-labeled substrate. C: Negative control of embolic myxoma tissue in the presence of 20 mmol/L EDTA. D: A detail of embolic myxoma tissue showing the green positive staining of cells and interstitium. Original magnifications: ×100 (A–C); ×600 (D).
Figure 4.
Gelatinolytic activity in cardiac myxomas evaluated by in-gel zymography. A: Gelatinolytic activity is greater in embolic than in nonembolic myxomas. B: Densitometric analysis in uOD values of gelatinase activity; results are given as means ± SEM; *P < 0.05 versus nonembolic myxomas. Ca, control mammary carcinoma.
RT-PCR
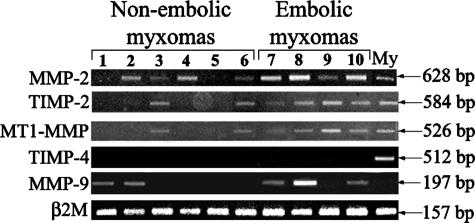
RT-PCR (Figure 5) showed that embolic myxomas expressed higher levels of MT1-MMP, MMP-2, and TIMP-2 transcripts than those of nonembolic myxomas. In fact, semiquantitative analysis showed that levels of MMP-2, TIMP-2, and MT1-MMP transcripts in embolic myxomas (626 ± 115, 727 ± 88, and 616 ± 81) were significantly higher than those of nonembolic myxomas (247 ± 92, 169 ± 107, and 138 ± 87; P < 0.03, P < 0.01, and P < 0.01, respectively). The MMP-9 transcript level was increased in embolic myxomas but the difference was not statistically significant for the high variability observed. No TIMP-4 transcripts were observed in any of the examined myxomas but they were present in control myocardium.
Figure 5.
Evaluation of MMP transcripts in cardiac myxomas. RT-PCR shows increased transcript products for MMP-2, MT1-MMP, and TIMP-2 in embolic compared to nonembolic myxomas. My, control myocardium.
Myxoma Cells and MMPs in Vitro
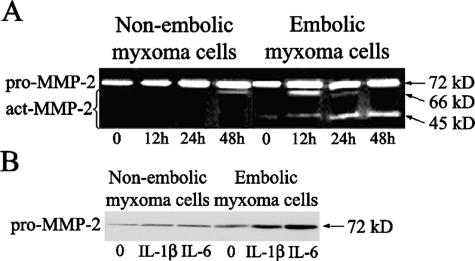
In-gel zymography and Western blot analysis showed that MMP-2 was the prevalent gelatinase secreted by myxoma cells in vitro. In-gel zymography (Figure 6A) showed the gelatinase A increase in cells from embolic (903 ± 232), compared to nonembolic myxomas (141 ± 141, P < 0.02), cultured in the presence of IL-1β, with no changes in cell morphology. Gelatinase activity increase was also observed in supernatants from embolic compared to nonembolic myxoma cell cultures (not shown). Densitometric evaluation of Western blots (Figure 6B) confirmed that nonembolic myxoma cells maintained lower levels of MMPs, in particular MMP-2, in basal conditions (11.1 ± 1.1) and also after 24 hours of stimulation with IL-1β and IL-6 (46 ± 5 and 58 ± 4.6) compared to embolic tumor cells (96 ± 7.2, 193 ± 8.1, and 296 ± 20.2, respectively; P < 0.001).
Figure 6.
Changes in MMP-2 expression in cardiac myxoma cells in response to cytokine stimulation at different times in vitro. A: In-gel zymography shows that IL-1β (5 μg/ml) increases MMP-2 active products (act-MMP-2) more in embolic myxoma cells than in those from nonembolic tumors. The 45-kd band represents a further cleavage product of the 66-kd active form. B: Western blot analysis shows that embolic myxoma cells express more pro-MMP-2 compared to nonembolic tumors, in basal conditions (20% FBS), and in the presence of stimulation with IL-1β (5 μg/ml) and IL-6 (7.5 μg/ml) for 24 hours.
MMPs and Tumor ECM Degradation
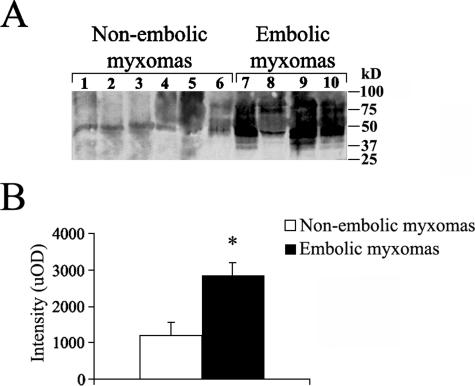
To verify if degradation increases in embolic myxomas, we investigated the presence of proteolytic ECM fragments containing GAG chains in tumor tissue by Western blotting. Densitometric analysis (Figure 7) demonstrated an increase of intensity in bands less than ∼100 kd as compared to nonembolic tumors (1194 ± 373) in embolic myxomas (2854 ± 340, P < 0.02). In addition, densitometric analysis after Western blotting (not shown) of supernatants showed that the delivering of ECM degradation products containing GAG chains of embolic tumor cell cultures (1301 ± 108) was greater than that obtained from those of nonembolic tumor cells (520 ± 132, P < 0.01), indicating that increased MMP activity in embolic myxomas was maintained in vitro.
Figure 7.
ECM degradation in cardiac myxoma tissue examined by Western blotting analysis. A: In embolic cardiac myxoma tissues, GAG-containing proteoglycan fragments of less than ∼100 kd are observed and compared to nonembolic tumors. B: Densitometric blot analysis in uOD shows that a greater accumulation of GAG-containing proteoglycan fragments occurs in embolic myxomas. Results are given as means ± SEM; *P < 0.02 versus nonembolic myxomas.
Discussion
Main Findings and Clinicopathological Considerations
The major new finding of this study is that embolic cardiac myxomas show higher MMP expression and activity compared to nonembolic tumors, and this associated to the presence of increased tumor ECM degradation products containing GAG chains. To investigate MMPs, we used immunohistochemistry in paraffin-embedded tissue sections from a large series of myxomas and Western blot analysis, RT-PCR and zymography in a smaller number of consecutive tumors. Our results strongly support, for the first time, an involvement of MMPs in cardiac myxoma embolism. Systemic embolism from tumor fragments originating from the tumor mass is a common complication of left atrial myxomas,2–4,8 so differential diagnosis of peripheral embolism should always include cardiac myxoma.2,3,5 Embolism has been related to the echocardiographic aspect (pedunculated, with a mobile and lobulated surface) of cardiac myxomas compared to the smoothened profile of nonembolic tumors.5 These findings are the ultrasonographic counterpart of the macroscopic and microscopic irregular surface we found in embolic myxomas, in accordance with that previously reported by Burke and Virmani.3 In the two nonembolic irregular myxomas, we found MMP expression did not significantly differ from nonembolic smoothened tumors. In any case, the finding of a limited number of nonembolic irregular tumors in ours as well as in other series3 suggests that embolism is not the natural consequence of the different macroscopic appearance. It is well recognized that increased MMP activity may induce a distortion of the balance between matrix synthesis and degradation, resulting in a dramatic alteration of the mechanical properties of tumor tissue.35–37 As a matter of fact, the increased MMP activity was associated to the finding of tumor tissue fragmentation in 40% of embolic myxomas and none of the nonembolic tumors during routine surgical procedures. ECM is not a static structure but rather a dynamic entity playing a fundamental role in tissue adaptation to mechanical stress.10 ECM turnover depends on the balance between the synthesis and degradation of the molecules composing the three-dimensional network.36 We documented an increase of tumor ECM degradation products containing GAG chains. Although the nature of proteoglycans and their possible assembly in cardiac myxomas is not known, their presence was previously documented by histochemical and ultrastructural methods.38 Proteoglycans are complex macromolecules with a variable proteic core and one or multiple and different GAG chains attached to the core protein.39 Chondroitin-6-sulfate, hyaluronic acid, and chondroitin-4-sulfate are the most abundant GAGs of cardiac myxomas.6 It has also been suggested that the changes in GAGs synthesized by myxomatous cells may favor the friability of cardiac myxomas,6 but no data concerning analysis of embolic tumors were supplied to support this hypothesis. MMPs degrade proteoglycans and generate GAG-containing degradation fragments of variable molecular weight according to the MMP type.26,27 We demonstrated that in embolic myxomas accumulation of ECM degradation products containing GAG chains was greater than in nonembolic myxomas. All together, our results strongly suggest that increased MMP levels and activity contribute to ECM degradation and may also influence the overall cardiac myxoma tissue morphology.
MMP Expression in Cardiac Myxomas in Vivo
We found an increase of MMP-2, MMP-9, and MT1-MMP expression and activity in embolic myxomas. MMPs are a large family of zinc-dependent proteolytic enzymes that are responsible for ECM remodeling in both normal and pathological processes,10–15 in particular in tumor-directed tissue remodeling.37 Among MMPs, MMP-2 activity has been reported to be fundamental in ECM remodeling and three-dimensional tissue organization.40 MMP-2 is the member of the secreted MMP family that most specifically degrades proteoglycans and mucopolysaccharides.16 Unlike other secreted MMPs, MMP-2 activation does not occur through proteolysis by serine proteases but rather through a cell-surface-associated MMP activator.41 Several membrane-bound MMPs have more recently been identified and MT1-MMP represents the prototypical form of secreted MMP activator.42 MT1-MMP contains a recognition site for pro-MMP-2 and MMP-9, which are consequently processed and activated.17,18 In addition, MT1-MMP also degrades ECM fibrillar collagen, glycoproteins, and proteoglycans.17 Moreover, MT1-MMP forms a complex with TIMP-2 that specifically enhances gelatinase activation.43
Mechanisms Regulating MMP Synthesis in Myxoma Cells
Although fascinating, in vivo data alone did not allow us to clarify if increased MMP activity in embolic myxomas is an intrinsic feature of tumor cells or, alternatively, it is related to an increased susceptibility to locally delivered cytokines. To this aim, we demonstrated that cells from embolic myxomas retained a higher MT1-MMP, MMP-2, and TIMP-2 basal level in vitro, confirming that a positive balance between productive and inhibitory activities is reached in embolic tumors. In addition, embolic myxoma cells treated with IL-1β and IL-6 also showed a greater increase of MMP activity than those from nonembolic tumors. These results indicate an increased intrinsic susceptibility of embolic myxoma cells to inflammatory stimuli that may further support MMP overexpression. In normal conditions, MMPs are produced by inflammatory and resident cells.18,20,37 Cytokines and growth factors including IL-1, platelet-derived growth factor, and tumor necrosis factor-α have been shown to induce or stimulate MMP synthesis, whereas others such as tumor growth factor-β1 have inhibitory effects.18,36,37 Inflammatory infiltration is normally observed in cardiac myxomas, although its degree was similar in embolic and nonembolic myxomas, according to what was previously reported.3 Increased susceptibility to locally delivered cytokines may further induce MMP overexpression and favor embolic events by increasing the friability of tumor ECM. It has been previously reported that embolic myxomas constitutively express high levels of IL-6.9 Autocrine production of IL-6 may represent one of the mechanisms responsible for the excessive MMP synthesis in embolic myxomas. Increased MMP delivery in the medium of embolic myxoma cultures also suggests that the increase of MMP plasma levels may represent an additional peripheral marker to identify patients at risk of embolism among those cases in which echocardiographic criteria are not characteristic.5
An increased tumor cell susceptibility to microenviromental stimuli may also explain the different behavior of myxomas originating in the left and right atrium, respectively. In their reported series, Burke and Virmani3 observed 18 right atrial myxomas and none with embolic symptoms, whereas embolic left atrial tumors were 25.9% of all cases, a statistic similar to that of our present series. Pulmonary embolism in myxoma from right cardiac chambers is extremely rare, although sometimes lethal.2 It is likely that hemodynamic factors such as high-pressure value and turbulence in left chambers play a role in embolism of cardiac myxomas. Higher arterial pressure levels in left cardiac chambers are likely to act as a breaking-out factor of tumor embolism. In addition to an increased risk of mechanical rupture, MMP synthesis modulation can be also hypothesized. The alteration of hemodynamic microenvironment is among the major drivers of MMP-mediated cardiovascular remodeling.44 Tissue forces caused by arterial pressure are likely to induce deposition of proteoglycans in superficial pseudoaneurysms45 and may also favor gelatinase-mediated tissue remodeling. In fact, mechanical strain modulates gelatinase production and leads to enhanced ECM degradation in vitro.46,47 As for the increased MMP expression we observed after cytokine stimulation, a different hemodynamic condition may stimulate MMP production in susceptible myxoma cells and accelerate embolic events.
Study Limitations
Although embolism from tumor fragments originating from the atrial myxoma is widely recognized,2–4,8 we could not exclude that a myxoma-associated thrombus detachment may also be responsible for clinical events, in particular transient ischemic attack frequently observed in patients with cardiac myxoma.8 It is also documented that MMP overexpression promotes intravascular thrombus formation48 and causes structural disruption of the arterial wall, which triggers thrombosis.44 We microscopically detected the presence of small thrombi at the surface of a certain percentage of cardiac myxomas, but there was no significant difference in their frequency comparing embolic and nonembolic tumors. It is likely that MMP overexpression may also favor small superficial thrombus detachment and contribute to an early clinical manifestation of cardiac myxoma. In any case, all these concepts strengthen the possible role of MMP overexpression in determining clinical behavior of cardiac myxomas.
Conclusions
Embolic myxomas showed increased MMP-2, MMP-9, and MT1-MMP levels and activity compared to nonembolic tumors; this was associated with an increase of ECM tumor degradation products containing GAG chains. Our findings strongly suggest that MMPs may contribute to tumor ECM remodeling and increase the risk of embolism in cardiac myxomas.
Acknowledgments
We thank Prof. M. Coletta for assistance in tumor ECM remodeling, Prof. S. Bottini for statistical analysis, Prof. M. Federici for in-gel zymography, S. Cappelli and A. Colantoni for technical help, and M. Bonta for language revision.
Footnotes
Address reprint requests to Prof. Augusto Orlandi Anatomic Pathology Institute, Department of Biopathology, Tor Vergata University of Rome Via Montpellier 1, Rome, Italy. E-mail: orlandi@uniroma2.it.
Partially supported by a grant from a joint project with “Spedali Civili of Brescia, Italy (protocol 20906055).
References
- Burke AP, Virmani R. Washington: Armed Forced Institute of Pathology,; Atlas of Tumor Pathology, fasc 16. (ed 3) 1996:pp 21–46. [Google Scholar]
- Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610–1617. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- Burke AP, Virmani R. Cardiac myxoma. A clinicopathologic study. Am J Clin Pathol. 1993;100:671–680. doi: 10.1093/ajcp/100.6.671. [DOI] [PubMed] [Google Scholar]
- Johansson L. Histogenesis of cardiac myxomas. An immunohistochemical study of 19 cases, including one with glandular structures, and review of the literature. Arch Pathol Lab Med. 1989;113:735–741. [PubMed] [Google Scholar]
- Ha JW, Kang WC, Chung N, Chang BC, Rim SJ, Kwon JW, Jang Y, Shim WH, Cho SY, Kim SS, Cho SH. Echocardiographic and morphologic characteristics of left atrial myxoma and their relation to systemic embolism. Am J Cardiol. 1999;83:1579–1582. doi: 10.1016/s0002-9149(99)00156-3. [DOI] [PubMed] [Google Scholar]
- Negishi M, Sakamoto H, Sakamaki T, Ishikawa O, Kanda T, Tamura J, Kurabayashi M, Nagai R. Disaccharide analysis of glycosaminoglycans synthesized by cardiac myxoma cells in tumor tissues and in cell culture. Life Sci. 2003;73:849–856. doi: 10.1016/s0024-3205(03)00359-x. [DOI] [PubMed] [Google Scholar]
- Kairemo KJ, Blomqvist CP, Miettinen M. Cardiac myxomas. N Engl J Med. 1996;334:1407–1408. [PubMed] [Google Scholar]
- Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–172. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Parissis JT, Mentzikof D, Georgopoulou M, Gikopoulos M, Kanapitsas A, Merkouris K, Kefalas C. Correlation of interleukin-6 gene expression to immunologic features in patients with cardiac myxomas. J Interferon Cytokine Res. 1996;16:589–593. doi: 10.1089/jir.1996.16.589. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996;148:1345–1350. [PMC free article] [PubMed] [Google Scholar]
- Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107:789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- Linask KK. Regulation of heart morphology: current molecular and cellular perspectives on the coordinated emergence of cardiac form and function. Birth Defects Res Part C Embryo Today. 2003;69:14–24. doi: 10.1002/bdrc.10004. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi T, Okada Y. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev. 2003;22:145–152. doi: 10.1023/a:1023039230052. [DOI] [PubMed] [Google Scholar]
- Singer CF, Kronsteiner N, Marton E, Kubista M, Cullen KJ, Hirtenlehner K, Seifert M, Kubista E. MMP-2 and MMP-9 expression in breast cancer-derived human fibroblasts is differentially regulated by stromal-epithelial interactions. Breast Cancer Res Treat. 2002;72:69–77. doi: 10.1023/a:1014918512569. [DOI] [PubMed] [Google Scholar]
- Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995;77:863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- Hernandez-Barrantes S, Bernardo M, Toth M, Fridman R. Regulation of membrane type-matrix metalloproteinases. Semin Cancer Biol. 2002;12:131–138. doi: 10.1006/scbi.2001.0421. [DOI] [PubMed] [Google Scholar]
- Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- Soini Y, Satta J, Maatta M, Autio-Harmainen H. Expression of MMP2, MMP9, MT1-MMP, TIMP1, and TIMP2 mRNA in valvular lesions of the heart. J Pathol. 2001;194:225–231. doi: 10.1002/path.850. [DOI] [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res. 1998;82:482–495. doi: 10.1161/01.res.82.4.482. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi A, Francesconi A, Cocchia D, Corsini A, Spagnoli LG. Phenotypic heterogeneity influences apoptotic susceptibility to retinoic acid and cis-platinum of rat arterial smooth muscle cells in vitro: implications for the evolution of experimental intimal thickening. Arterioscler Thromb Vasc Biol. 2001;21:1118–1123. doi: 10.1161/hq0701.092144. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, Miura R, Yamaguchi Y, Okada Y. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem. 2000;275:38885–38890. doi: 10.1074/jbc.M003875200. [DOI] [PubMed] [Google Scholar]
- Arner EC, Decicco CP, Cherney R, Tortorella MD. Cleavage of native cartilage aggrecan by neutrophil collagenase (MMP-8) is distinct from endogenous cleavage by aggrecanase. J Biol Chem. 1997;272:9294–9299. doi: 10.1074/jbc.272.14.9294. [DOI] [PubMed] [Google Scholar]
- Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben PM, Brogley MA, Sun Y, Cheung HS. Molecular mechanism of the induction of metalloproteinases 1 and 3 in human fibroblasts by basic calcium phosphate crystals. Role of calcium-dependent protein kinase C alpha. J Biol Chem. 2002;277:15190–15198. doi: 10.1074/jbc.M200278200. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Sellers RG, Wiehr C, Melamud O, Pei D, Peehl DM. Expression of matrix metalloproteinase-2 and -9 and their inhibitors, tissue inhibitor of metalloproteinase-1 and -2, in primary cultures of human prostatic stromal and epithelial cells. J Cell Physiol. 2002;191:208–216. doi: 10.1002/jcp.10092. [DOI] [PubMed] [Google Scholar]
- Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003;89:556–562. doi: 10.1002/jcb.10534. [DOI] [PubMed] [Google Scholar]
- Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002;67:889–894. doi: 10.1095/biolreprod.102.005116. [DOI] [PubMed] [Google Scholar]
- Chung HW, Lee JY, Moon HS, Hur SE, Park MH, Wen Y, Polan ML. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil Steril. 2002;78:787–795. doi: 10.1016/s0015-0282(02)03322-8. [DOI] [PubMed] [Google Scholar]
- Groft LL, Muzik H, Rewcastle NB, Johnston RN, Knauper V, Lafleur MA, Forsyth PA, Edwards DR. Differential expression and localization of TIMP-1 and TIMP-4 in human gliomas. Br J Cancer. 2001;85:55–63. doi: 10.1054/bjoc.2001.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G, O’Byrne KJ. Matrix metalloproteinases and cancer. Anticancer Res. 2001;21:4207–4219. [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- Lam RM, Hawkins ET, Roszka J. Cardiac myxoma: histochemical and ultrastructural localization of glycosaminoglycans and proteoglycans. Ultrastruct Pathol. 1984;6:69–81. doi: 10.3109/01913128409016666. [DOI] [PubMed] [Google Scholar]
- Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–870. [PubMed] [Google Scholar]
- Brown LM, Fox HL, Hazen SA, LaNoue KF, Rannels SR, Lynch CJ. Role of the matrixin MMP-2 in multicellular organization of adipocytes cultured in basement membrane components. Am J Physiol. 1997;272:937–949. doi: 10.1152/ajpcell.1997.272.3.C937. [DOI] [PubMed] [Google Scholar]
- Strongin AY, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell-surface activation of 72 kDa type IV gelatinase. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumor cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- Burke AP, Jarvelainen H, Kolodgie FD, Goel A, Wight TN, Virmani R. Superficial pseudoaneurysms: clinicopathologic aspects and involvement of extracellular matrix proteoglycans. Mod Pathol. 2004;17:482–488. doi: 10.1038/modpathol.3800060. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Magid R, Johnson C, Nerem RM, Galis ZS. Uniaxial strain upregulates matrix-degrading enzymes produced by human vascular smooth muscle cells. Am J Physiol. 2003;284:H1778–H1784. doi: 10.1152/ajpheart.00494.2002. [DOI] [PubMed] [Google Scholar]
- Lee RT, Yamamoto C, Feng Y, Potter-Perigo S, Briggs WH, Landschulz KT, Turi TG, Thompson JF, Libby P, Wight TN. Mechanical strain induces specific changes in the synthesis and organization of proteoglycans by vascular smooth muscle cells. J Biol Chem. 2001;276:13847–13851. doi: 10.1074/jbc.M010556200. [DOI] [PubMed] [Google Scholar]
- Morishige K, Shimokawa H, Matsumoto Y, Eto Y, Uwatoku T, Abe K, Sueishi K, Takeshita A. Overexpression of matrix metalloproteinase-9 promotes intravascular thrombus formation in porcine coronary arteries in vivo. Cardiovasc Res. 2003;57:572–585. doi: 10.1016/s0008-6363(02)00710-1. [DOI] [PubMed] [Google Scholar]