Abstract
Despite the potentially protective effects of estrogen on bone and cardiovascular tissue as well as against kidney diseases, its effects on diabetic nephropathy are unknown. Here, we examined the therapeutic effectiveness of 17β-estradiol and raloxifene, a selective estrogen receptor modulator, for preventing functional and histological alterations in the kidneys of db/db mice, a model of type 2 diabetes. In the first experiment, ovariectomized female db/db mice were treated with 17β-estradiol for 8 weeks. The treatment significantly ameliorated albuminuria, attenuated weight gain, and reduced hyperglycemia in diabetic ovariectomized db/db mice. Histologically, the increases in mesangial area and the accumulation of fibronectin were significantly inhibited by 17β-estradiol. In the second experiment, mice were administered vehicle or raloxifene hydrochloride (3 mg/kg/day) for 8 weeks. Raloxifene significantly reduced mesangial expansion and fibronectin accumulation in db/db mice, but in contrast to 17β-estradiol, it failed to affect body weight or hyperglycemia. An in vitro experiment further demonstrated that raloxifene inhibited transforming growth factor β-1-induced fibronectin transcription and AP-1 activity. Thus, our findings suggest that raloxifene, which lacks the harmful effects of estrogen, is useful for the treatment of diabetic nephropathy.
Female gender is associated with a slower progression of chronic kidney disease to end-stage renal failure.1 Furthermore, premenopausal diabetic women have a lower risk of developing end-stage renal disease compared with age-matched diabetic males, and this protection is lost after menopause.2 A population-based study also demonstrated that male gender is associated with the development of diabetic nephropathy.3 These clinical studies, therefore, indicate that estrogen-like effects may potentially prevent the progression of diabetic nephropathy. Indeed, experimental results also suggest that estrogen may have favorable effects on the progression of renal diseases in uninephrectomized and hypertensive rats.4,5 In mesangial cell cultures, estrogens reduce proliferation and collagen synthesis.6,7 In contrast, it is reported that the incidence of glomerulosclerosis is increased in ovariectomized diabetic rats treated with estrogen.8 In female obese Zucker rats, it has been shown that estrogen accelerates the development of renal disease.9 Moreover, in Otsuka Long-Evans Tokushima Fatty rats, estrogen also contributes to the development of glomerulosclerosis.10 Given these inconsistent results, it is unclear whether estrogen replacement therapy can protect against the development of diabetic nephropathy.
Recent large clinical studies have indicated that the usefulness of estrogen is limited because of its harmful effects such as carcinogenesis of the mamma.11 Thus, selective estrogen receptor modulators (SERMs), which bind to the estrogen receptor and modulate estrogen receptor-mediated gene transcription, are clinically applied to treat all stages of hormone-responsive breast cancer. Raloxifene hydrochloride (RLX) is a SERM with a benzothiophene structure that has been approved for the prevention and treatment of osteoporosis in postmenopausal women.12 SERMs specifically behave in certain tissue not only as estrogen agonists but also as antiestrogens. In addition to the beneficial effect on bone, RLX has favorable effects on the cardiovascular system.13,14 Interestingly, it is also reported that RLX suppresses mesangial cell collagen synthesis in vitro,15 suggesting that RLX may be useful for the treatment of kidney diseases. Based on these observations, we performed the present study to clarify the separate effects of 17β-estradiol and RLX on functional and histological alterations in the kidneys of db/db mice, a model of type 2 diabetes.
Materials and Methods
Animals
Female C57BLKsJ-db/db mice and their age-matched non-diabetic db/m littermates were purchased from Japan Clea (Tokyo, Japan). All animals were housed in box cages under standard conditions and had free access to water and standard chow. The Shiga University of Medical Science Animal Care Committee approved all experiments.
Experimental Protocol
In the first experiment, 10-week-old female db/db mice were randomly divided into the following three groups: sham-operated (Sham) (n = 10), ovariectomized (OVX) (n = 10), and OVX and estradiol treated (OVX+E) (n = 10). Bilateral ovariectomy or sham surgery via dorsal approach was performed, and estrogen or placebo treatment was started at 10 weeks of age. Sixty-day-release pellet (Innovative Research of America, Sarasota, FL), which releases 17β-estradiol at the dose of 8.3 μg/day, was implanted in the OVX+E group. Placebo pellet was implanted in the Sham and OVX groups. All pellets were inserted subcutaneously via a small incision on the nape of the neck.
At 18 weeks of age, body weights and blood glucose levels were measured, and 24-hour urine samples were collected and stored frozen at −80°C until analysis. Blood pressure was measured with a tail cuff sphygmomanometer. Mice were deeply anesthetized by an intraperitoneal injection of 50 mg/kg pentobarbital sodium. After perfusion with ice-cold phosphate-buffered saline (0.1 mol/L, pH 7.4), the right kidney was fixed with 4% paraformaldehyde in phosphate-buffered saline. The kidneys were sectioned in a plane perpendicular to the long axis and were embedded in paraffin for morphometric and immunohistochemical analysis. Total RNA was extracted from the renal cortex of the left kidney. Urinary albumin excretion was measured with a mouse-specific sandwich enzyme-linked immunosorbent assay system (Albuwell; Exocell, Philadelphia, PA).
In the second experiment, 10-week-old female db/db mice were treated subcutaneously with either vehicle (sesame oil) or RLX (3 mg/kg/day) for 8 weeks (n = 10). Samples were collected and mice were sacrificed as described above.
Glucose Tolerance Test and Insulin Tolerance Test
Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed at the age of 14 weeks (n = 3). For GTTs, mice were fasted overnight for 14 hours followed by intraperitoneal glucose injection (1 g/kg body weight). Blood glucose was measured by using tail blood collected at 0, 15, 30, 60, and 120 minutes after the injection. For ITTs, mice were injected with human regular insulin (Novolin R; Novo Nordisk, Clayton, NC) at 0.75 U/kg of body weight intraperitoneally after a 6-hour fast, and blood glucose was measured at 0, 15, 30, and 60 minutes.
Morphological Investigations
For the morphometric analysis, sections were stained with periodic acid-Schiff (PAS) as previously described.16 From each animal of the experimental groups, 20 glomeruli cut at their vascular poles were used for the morphometric analysis. The extent of the mesangial matrix (defined as mesangial area) was determined by assessing the PAS-positive and nuclei-free area in the mesangium. The glomerular area was traced along the outline of the capillary loop using a computer-assisted color image analyzer (LUZEX F; Nikon, Tokyo, Japan).
Immunohistochemical staining was performed with fibronectin-specific polyclonal anti-mouse fibronectin antibody (A852/R5H; Biogenesis, Poole, United Kingdom). To evaluate the immunostaining for fibronectin, a total of 20 randomly chosen glomeruli per mouse were graded as follows: 0, staining absent to 5%; 1, 5 to 25%; 2, 25 to 50%; 3, 50 to 75%; and 4, >75%.17
Extraction of Total RNA and Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Real-time RT-PCR was used to quantify renal fibronectin transcript levels in experimental animals. Briefly, RNA was extracted with TRIzol (Invitrogen Co., Carlsbad, CA), and total RNA (4 μg) was reverse transcribed with Superscript II (Invitrogen). Real-time quantitative PCR was performed using fluorescence dye SYBR Green I (Roche Molecular Biochemicals, Mannheim, Germany) and LightCycler (Roche). The following PCR primers sets were designed: fibronectin forward, GCAAGCCAGTTTCCATCAAT, and fibronectin reverse, CATTTTTGGGAGTGGTGGTCA; and β-actin forward, CTGAGAGGGAAA-TCGTGCGT, and β-actin reverse, AGGGACTTCCTG-TAACCACT, for a two-step PCR protocol. In the first part, polymerase was activated for 10 minutes at 95°C. During the second part, the target region was amplified (45 cycles: 10 seconds, 95°C; 10 seconds, 55°C; and 5 seconds, 72°C). β-actin was used as a house-keeping gene.
Cell Culture, Transfection, and Luciferase Assay
SV40-transformed murine mesangial cells (American Type Culture Collection, Rockville, MD) were grown in Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal bovine serum and antibiotics. All experiments were performed in Dulbecco’s modified Eagle’s medium containing 0.2% fatty-acid-free bovine serum albumin. Subconfluent mesangial cells on a six-well plate (Falcon, Franklin Lakes, NJ) were transfected with 0.8 μg of pGL2F1900 containing the −1908-bp 5′ flanking region of the rat fibronectin gene fused to the luciferase reporter gene18 or the 3XAP-1 luciferase reporter construct (Stratagene, La Jolla, CA), using LipofectAMINE-2000 reagents (Gibco-BRL, Rockville, MD). The β-galactosidase-containing pCMVβ vector (Clontech, Palo Alto, CA) was used to control for transfection efficiency.
Twenty-four hours after transfection, the cells were preincubated for 1 hour in media containing RLX. The cells were then co-incubated with transforming growth factor-β1 (TGF-β1) (R&D System, Minneapolis, MN) for an additional 24 hours. The cells were harvested in 200 μl of a reporter lysis buffer (Promega, Madison, WI). Twenty-microliter aliquots of extracts were used to measure luciferase activity by Luciferase Assay System (Promega) and a luminometer (AutoLUMIcounter Nu1422ES; Nition, Tokyo, Japan). Luciferase activity was normalized to β-galactosidase activity.
Statistical Analysis
Results are expressed as mean ± SE. Comparison among groups was performed by one-way analysis of variance, followed by Bonferroni’s test. P values of < 0.05 were defined as statistically significant.
Results
Effects of 17β-Estradiol on Body Weight, Blood Glucose Level, Kidney Weight, and Urinary Albumin Excretion
At the beginning of the study, there were no significant differences in body weight and blood glucose level among the Sham, OVX, and OVX+E groups (data not shown). In animals supplemented with 17β-estradiol, the mean circulating concentration of 17β-estradiol at the end of the experiment was 793.2 pmol/L. In the db/db mice in the Sham and OVX groups, the levels of 17β-estradiol were 121.5 pmol/L and 125.8 pmol/L, respectively, whereas that in the db/m mice was 183.9 pmol/L.
As shown in Table 1, body weight was increased in db/db mice as compared with non-diabetic, db/m mice at the age of 18 weeks. In the Sham and OVX groups, the weight gains at the end of the experiments were 37.4 and 29.7%, respectively, whereas in the OVX+E group, it was 12.5%. Blood glucose levels were significantly higher in the Sham (15.8 ± 0.97 mmol/L) and OVX (22.0 ± 1.95 mmol/L) groups than in the db/m mice (7.0 ± 0.23 mmol/L), whereas hyperglycemia was normalized in the OVX+E group (6.9 ± 0.39 mmol/L). Food intake was reduced in the OVX+E group (3.4 ± 0.13 g/day) compared with those in the Sham (4.4 ± 0.39 g/day) and OVX (5.5 ± 0.23 g/day) groups. To gain insights into the mechanism of action of 17β-estradiol, we examined GTTs and ITTs. The area under the curve for blood glucose from 0 to 60 minutes after glucose administration was decreased in the OVX+E group (13,347.5 ± 1946.3 mg/dl/min) compared with the Sham (31,942.5 ± 1781.7 mg/dl/min) and OVX (27,862.5 ± 2471.1 mg/dl/min) groups (P < 0.01, Sham or OVX versus OVX+E). The decreases of blood glucose levels at 60 minutes after insulin challenge in the Sham, OVX, and OVX+E groups were 78.9 ± 1.7%, 78.5 ± 3.7%, and 24.1 ± 2.3% of the initial values, respectively (P < 0.01, Sham or OVX versus OVX+E). Kidney weight was greater in the db/db mice than in the db/m mice, and there were no significant differences among the Sham, OVX, and OVX+E groups. Mean blood pressure in db/db mice was elevated compared with that in db/m mice, and it was not affected by 17β-estradiol treatment.
Table 1.
Characteristics of Experimental Animals at the Age of 18 Weeks
| db/db, Sham | db/db, OVX | db/db, OVX+E | db/m | |
|---|---|---|---|---|
| Body weight (g) | 60.0 ± 1.26* | 56.6 ± 1.19* | 48.8 ± 1.56*†‡ | 21.6 ± 0.52 |
| Blood glucose (mmol/L) | 15.8 ± 0.97* | 22.0 ± 1.95*† | 6.9 ± 0.39†‡ | 7.0 ± 0.23 |
| Kidney weight (g) | 0.39 ± 0.01* | 0.38 ± 0.02* | 0.35 ± 0.02* | 0.27 ± 0.01 |
| Mean BP (mmHg) | 91.9 ± 2.2* | 97.0 ± 1.9* | 92.5 ± 2.6* | 83.0 ± 2.7 |
Data are expressed as means ± SE (n = 10).
P < 0.05 versusdb/m mice.
P < 0.05 versusSham.
P < 0.05 versusOVX.
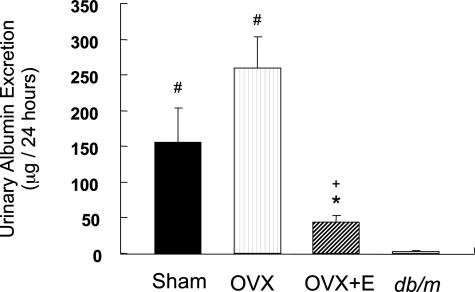
Urinary albumin excretions in the Sham (156.1 ± 47.2 μg/24 hours) and OVX (259.5 ± 43.4 μg/24 hours) groups were increased compared with that in db/m mice (3.26 ± 0.76 μg/24 hours), and it was significantly decreased in the OVX+E group (44.0 ± 9.54 μg/24 hours) compared with the Sham or OVX group (Figure 1).
Figure 1.
17β-Estradiol suppresses urinary albumin excretion in db/db mice. Twenty-four-hour urine samples were collected using metabolic cages. Urinary albumin excretion was measured with a mouse-specific sandwich enzyme-linked immunosorbent assay system. Data are expressed as means ± SE (n = 10). #P < 0.05 versus db/m mice, *P < 0.05 versus Sham group, +P < 0.05 versus OVX group.
Effects of 17β-Estradiol on Mesangial Expansion and Fibronectin Expression
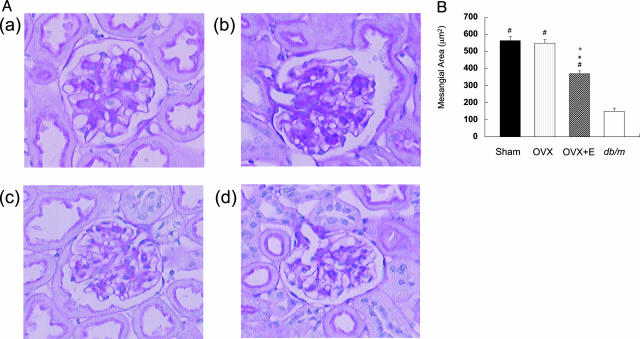
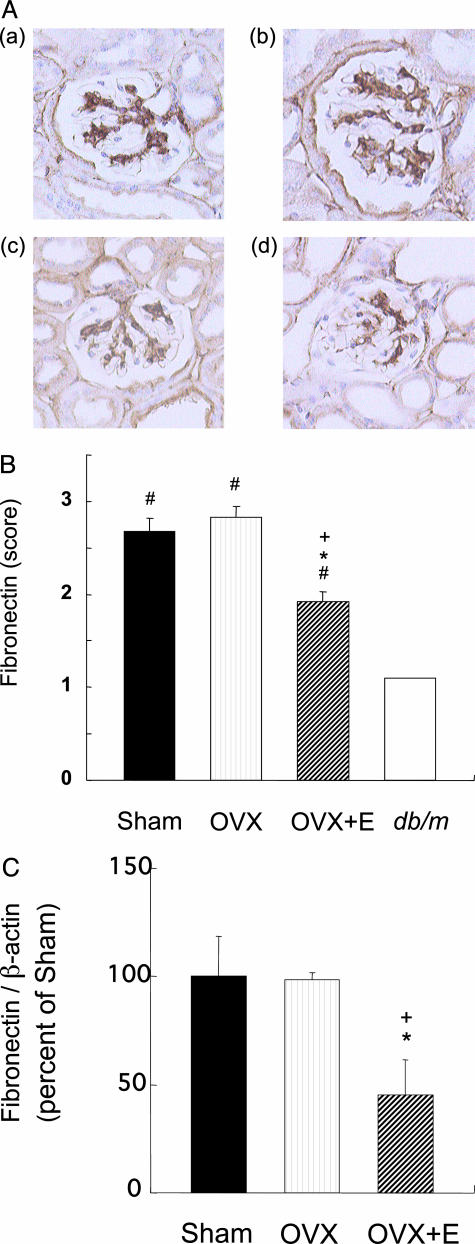
The mesangial areas in the Sham (562.7 ± 21.9 μm2) and OVX (546.9 ± 21.3 μm2) groups were larger than in the db/m mice (148.2 ± 19.6 μm2), but the mesangial expansion in db/db mice was significantly ameliorated by the 17β-estradiol treatment (368.5 ± 16.8 μm2) (Figure 2). There was no significant change in glomerular surface area in the Sham, OVX, and OVX+E groups (data not shown). Immunohistochemical staining revealed an increase in fibronectin deposition in the glomeruli of mice in the Sham and OVX groups compared with that in the db/m mice, but the intense fibronectin staining was attenuated by 17β-estradiol treatment (Figure 3, A and B). Renal expression of fibronectin mRNA in the OVX+E group was significantly decreased compared with the Sham or OVX group (Figure 3C).
Figure 2.
17β-Estradiol suppresses mesangial expansion in db/db mice. A: Representative microphotographs of glomeruli from db/db mice in the Sham (a), OVX (b), and OVX+E (c) groups and from a db/m mouse (d) are shown. Original magnifications, ×400. B: Quantitative results for mesangial areas from 20 glomeruli per mouse. Data are expressed as means ± SE (n = 10). #P < 0.01 versus db/m mice, *P < 0.01 versus Sham group, +P < 0.01 versus OVX group.
Figure 3.
17β-Estradiol suppresses fibronectin expression in db/db mice. A: Representative results for immunoreactive expression of renal fibronectin from db/db mice in the Sham (a), OVX (b), and OVX+E (c) groups and from a db/m mouse (d) are shown. Original magnifications, ×400. B: The semiquantitative fibronectin scores for 20 glomeruli per mouse. Data are expressed as means ± SE (n = 10). #P < 0.01 versus db/m mice, *P < 0.01 versus Sham group, +P < 0.01 versus OVX group. C: Renal expression of fibronectin mRNA in the Sham, OVX, and OVX+E groups. Fibronectin and β-actin mRNA levels are quantified by real-time reverse transcription-PCR method. Data are expressed as means ± SE (n = 5). *P < 0.05 versus Sham group, +P < 0.05 versus OVX group.
Effects of RLX on Body Weight, Blood Glucose Level, Kidney Weight, and Urinary Albumin Excretion
We next examined whether RLX affects metabolic control and renal damage in db/db mice. There was no significant difference in body weight, blood glucose level, or kidney weight between the vehicle and RLX groups (Table 2). Food intake in the RLX group was similar to that in the vehicle control. Mean blood pressure measured at the age of 18 weeks was not affected by RLX treatment. Urinary albumin excretion was somewhat decreased in the RLX group (172.0 ± 36.8 μg/24 hours) compared with the vehicle control group (217.2 ± 45.5 μg/24 hours), but the difference was not significant.
Table 2.
Characteristics of Vehicle- and RLX-Treated db/db Mice at the Age of 18 Weeks
| db/db, Vehicle | db/db, RLX | |
|---|---|---|
| Body weight (g) | 55.5 ± 1.28 | 54.5 ± 1.13 |
| Blood glucose (mmol/L) | 22.3 ± 2.19 | 19.3 ± 2.61 |
| Left kidney weight (g) | 0.188 ± 0.005 | 0.184 ± 0.005 |
| Mean BP (mmHg) | 91.9 ± 2.2 | 88.7 ± 3.1 |
Data are expressed as means ± SE (n = 10).
Effects of RLX on Mesangial Expansion and Fibronectin Expression
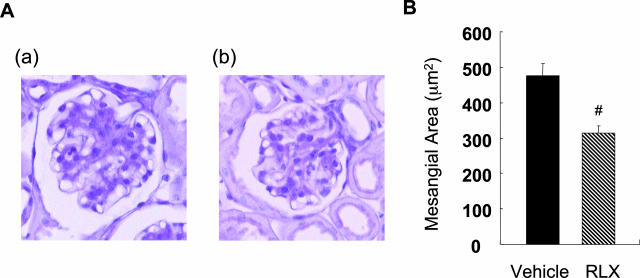
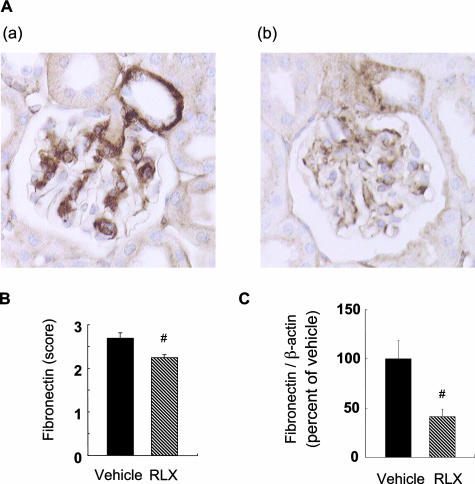
PAS staining of the renal tissue revealed that the mesangial area was smaller in the RLX group (314.4 ± 20.5 μm2) than in the vehicle control group (476.2 ± 35.9 μm2) (Figure 4). Immunohistochemical staining of fibronectin in glomeruli also revealed less deposition in the former group (Figure 5, A and B). Renal expression of fibronectin mRNA in the RLX group was significantly decreased compared with the vehicle group (Figure 5C).
Figure 4.
Raloxifene suppresses mesangial expansion in db/db mice. A: Representative photomicrographs of glomeruli from vehicle-treated (a) and RLX-treated (b) db/db mice are shown. Original magnifications, ×400. B: Quantitative results for 20 glomeruli per mouse. Data are expressed as means ± SE (n = 10). #P < 0.01 versus vehicle-treated control group.
Figure 5.
Raloxifene suppresses fibronectin deposition in db/db mice. A: Representative photomicrographs of glomeruli from vehicle-treated (a) and RLX-treated (b) db/db mice are shown. Original magnifications, ×400. B: Fibronectin score for 20 glomeruli per mouse. Data are expressed as means ± SE (n = 10). #P < 0.05 versus vehicle-treated control group. C: Renal expression of fibronectin mRNA in the vehicle and RLX groups. Fibronectin and β-actin mRNA levels are quantified by real-time RT-PCR method. Data are expressed as means ± SE (n = 5). #P < 0.05 versus vehicle-treated control group.
Effect of RLX on TGF-β1-Induced Fibronectin Expression
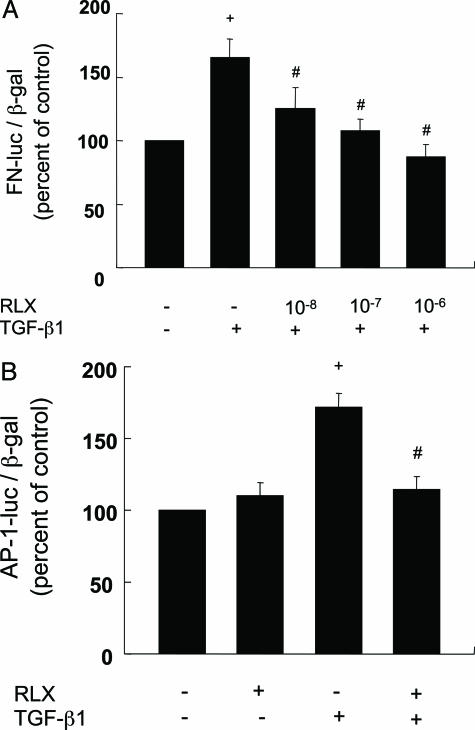
To explore the molecular mechanism by which RLX inhibits fibronectin expression, we examined fibronectin promoter activity in cultured mesangial cells. TGF-β1 stimulated the activity in the mesangial cells. RLX significantly inhibited the TGF-β1-induced fibronectin promoter activity in a dose-dependent manner (Figure 6A). In addition, RLX suppressed TGF-β1-stimulated AP-1 activity in mesangial cells (Figure 6B), indicating that RLX inhibited fibronectin expression via an AP-1-dependent mechanism.
Figure 6.
A: Raloxifene suppresses TGF-β1-stimulated fibronectin gene transcription in mesangial cells. Cells were transfected with 0.8 μg of pGL2F 1900 along with 0.2 μg of pCMVβ. After 24 hours, cells were preincubated with or without RLX (10−8-10−6 mol/L) for 1 hour and then stimulated by TGF-β1 (2.5 ng/ml). After an additional 24 hours of stimulation, luciferase and β-galactosidase activities were measured. Results are means ± SE of four independent experiments. +P < 0.01 versus control, #P < 0.05 versus stimulation with TGF-β1 alone. B: RLX suppresses TGF-β1-stimulated AP-1 gene transcription in mesangial cells. Cells were transfected with 0.8 μg of 3XAP-1-Luc along with 0.2 μg of pCMVβ. After 24 hours, cells were preincubated with or without RLX (10−6 mol/L) for 1 hour. Cells were then stimulated by TGF-β1 (2.5 ng/ml). +P < 0.01 versus control, #P < 0.01 versus stimulation with TGF-β1 alone.
Discussion
Epidemiological studies have implicated male gender as a risk factor for persistent proteinuria in type 2 diabetes and as associated with increased prevalence of microalbuminuria in type 1 diabetes.3,19 Thus, estrogen could potentially protect against the progression of diabetic nephropathy, although apparent benefits and risks have generated debate and confusion among researchers. In this study, we demonstrated that the treatment with 17β-estradiol or RLX ameliorated albuminuria, mesangial expansion, and accumulation of extracellular matrix in diabetic db/db mice.
In corroboration of our findings, several in vitro studies have confirmed an antifibrotic effect of estrogen in renal cells. 17β-Estradiol inhibits the proliferation of mesangial cells, suppresses the synthesis of collagen types I and IV,7,20 and increases the production of matrix metalloproteinases-2 and -9.21,22 TGF-β plays a key role in the development of diabetic nephropathy, especially by stimulating extracellular matrix proteins.23 Interestingly, crosstalk between TGF-β and estrogen has recently been reported: 17β-estradiol has been shown to reverse TGF-β1-induced collagen type IV expression in mesangial cells,24 and moreover TGF-β1-induced glomerulopathy is prevented by 17β-estradiol in vivo.25 These findings demonstrate that estrogen may inhibit the expression of extracellular matrix by interfering with TGF-β action. However, inhibition of the TGF-β pathway may not be sufficient for preventing all renal abnormalities in diabetic nephropathy. Although the treatment with anti-TGF-β antibody ameliorated renal insufficiency and glomerulosclerosis, it failed to reverse albuminuria in db/db mice.26 In contrast, in this study, we provide evidence that 17β-estradiol does ameliorate albuminuria, in addition to its beneficial effects on histological alteration, in db/db mice. The discrepancy suggests potential mechanisms involving estrogen other than anti-TGF-β action. Consistent with our findings, 17β-estradiol has been shown to cause significant reductions in albuminuria as well as glomerulosclerosis and tubulointerstitial damage in uninephrectomized SHRsp rats. Kang et al27 recently examined macro- and microvascular changes in five of six remnant kidney models in both male and female rats and found that female rats showed less proteinuria and prominent vascular remodeling associated with increased expression of vascular endothelial growth factor. Furthermore, mice lacking estrogen receptor-α exhibit proteinuria and progression of glomerulonephritis, suggesting that estrogen exerts its essential role in maintaining a healthy renal vasculature through estrogen receptor-α.28
However, the effect of estrogen on diabetic nephropathy remains controversial. Rosenmann et al8 showed that the incidence of glomerulosclerosis was increased in ovariectomized Cohen diabetic rats treated with estrogen. Furthermore, estrogen has been shown to contribute to the development of glomerulosclerosis in Otsuka Long-Evans Tokushima Fatty rats.10 The discrepancy between these results and ours may be explained by variable susceptibilities to estrogen in the different experimental animal strains. In fact, it has been shown that the responsiveness to estrogen could be controlled by genetic traits related to those that determine the susceptibility to glomerular scarring.29,30
17β-Estradiol suppressed hyperglycemia, obesity, and food consumption in db/db mice. Estrogen has been shown to suppress or delay the development of diabetes31 and to affect insulin sensitivity.32 We cannot exclude the possibility that normalization of metabolic conditions by 17β-estradiol contributed to the amelioration of renal damage. We therefore examined the effect of RLX, one of the selective estrogen receptor modulators, to further clarify the effect of estrogen on renal damage, because it is known that RLX does not affect insulin sensitivity or glycemic control in postmenopausal women with type 2 diabetes.33 RLX prevented the progression of mesangial expansion and fibronectin accumulation in db/db mice without affecting hyperglycemia and obesity, but it failed to reduce urinary albumin excretion. Therefore, we conclude that RLX has a beneficial effect on diabetic kidney disease independent of glycemic control and that 17β-estradiol may have additional actions through improving metabolic conditions.
Our in vitro results demonstrated that RLX suppressed TGFβ1-induced fibronectin promoter activity as well as AP-1 activation in cultured mesangial cells. Because, as we previously reported, AP-1 is responsible for TGF-β1-induced fibronectin expression in mesangial cells,34 these results suggest that RLX suppresses TGF-β1-stimulated fibronectin expression possibly in part by inhibiting AP-1 activity. In contrast, it has been shown that RLX may stimulate AP-1 activity with estrogen receptors.35 Thus, further studies are necessary to elucidate the mechanism by which RLX inhibits TGF-β1-induced fibronectin expression.
To our knowledge, this is the first study to demonstrate that RLX has a beneficial effect on the kidney in vivo. Because RLX does not have the harmful effects of estrogen, such as carcinogenesis in the breasts and uterus,36 but has presumably preserved beneficial effects on the vascular system, RLX may be more useful than estrogen as a clinical therapy for diabetic nephropathy.
Footnotes
Address reprint requests to Daisuke Koya, MD, Department of Medicine, Shiga University of Medical Science, Otsu, Shiga, 520-2192, Japan. E-mail: koya@belle.shiga-med.ac.jp.
References
- Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- U. S. Renal Data System The National Institutes of Health, Bethesda, MD: NIDDK,; USRDS 2000 Annual Data Report. 2000 [Google Scholar]
- Ballard DJ, Humphrey LL, Melton LJ, Frohnert PP, Chu PC, O’Fallon WM, Palumbo PJ. Epidemiology of persistent proteinuria in type II diabetes mellitus: population-based study in Rochester, Minnesota. Diabetes. 1998;37:405–412. doi: 10.2337/diab.37.4.405. [DOI] [PubMed] [Google Scholar]
- Gross ML, Adamczak M, Rabe T, Harbi NA, Krtil J, Koch A, Hamar P, Amann K, Ritz E. Beneficial effects of estrogens on indices of renal damage in uninephrectomized SHRsp rats. J Am Soc Nephrol. 2004;15:348–358. doi: 10.1097/01.asn.0000105993.63023.d8. [DOI] [PubMed] [Google Scholar]
- Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol. 2004;15:1546–1556. doi: 10.1097/01.asn.0000128219.65330.ea. [DOI] [PubMed] [Google Scholar]
- Kwan G, Neugarten J, Sharman M, Ding Q, Fotadar U, Lei J, Silbiger S. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50:1173–1179. doi: 10.1038/ki.1996.425. [DOI] [PubMed] [Google Scholar]
- Silbiger S, Lei J, Ziyadeh FN, Neugarten J. Estradiol reverses TGF-β1-stimulated type 4 collagen gene transcription in murine mesangial cells. Am J Physiol. 1998;274:F1113–F1118. doi: 10.1152/ajprenal.1998.274.6.F1113. [DOI] [PubMed] [Google Scholar]
- Rosenmann E, Yanko L, Cohen AM. Female sex hormone and nephropathy in Cohen diabetic rat (genetically selected sucrose-fed). Horm Metab Res. 1984;16:11–16. doi: 10.1055/s-2007-1014683. [DOI] [PubMed] [Google Scholar]
- Gades MD, Stern JS, Goor HV, Nguyen D, Johnson PR, Kaysen GA. Estrogen accelerates the development of renal disease in female obese Zucker rats. Kidney Int. 1998;53:130–135. doi: 10.1046/j.1523-1755.1998.00746.x. [DOI] [PubMed] [Google Scholar]
- Tomiyoshi Y, Sakemi T, Aoki S, Miyazono M. Different effects of castration and estrogen administration on glomerular injury in spontaneously hyperglycemic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Nephron. 2002;92:860–867. doi: 10.1159/000065442. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. J Am Med Assoc. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Krueger K, Cohen FJ, Eckert S, Ensrud K, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. J Am Med Assoc. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K, Rautaharju P, Harpar KD, MORE Investigators Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene) randomized trial. J Am Med Assoc. 2002;287:847–857. doi: 10.1001/jama.287.7.847. [DOI] [PubMed] [Google Scholar]
- Walsh BW, Paul S, Wild RA, Dean RA, Tracy RP, Cox DA, Anderson PW. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2000;85:214–218. doi: 10.1210/jcem.85.1.6326. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Acharya A, Lei J, Silbiger S. Selective estrogen receptor modulators suppress mesangial cell collagen synthesis. Am J Physiol Renal Physiol. 2000;279:F309–F318. doi: 10.1152/ajprenal.2000.279.2.F309. [DOI] [PubMed] [Google Scholar]
- Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKCβ inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- Hugo C, Pichler R, Gordon K, Schmidt R, Amieva M, Couser WG, Furthmayr H, Johnson RJ. The cytoskeletal linking proteins, moesin and radixin, are upregulated by platelet-derived growth factor, but not basic fibroblast growth factor in experimental mesangial proliferative glomerulonephritis. J Clin Invest. 1996;97:2499–2508. doi: 10.1172/JCI118697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono M, Chen S, Hong SW, Cruz MCI, Ziyadeh FN. Smad pathway is activated in the diabetic mouse kidney and Smad3 mediates TGF-β-induced fibronectin in mesangial cells. Biochem Biophys Res Commun. 2002;296:1356–1365. doi: 10.1016/s0006-291x(02)02084-3. [DOI] [PubMed] [Google Scholar]
- Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications in IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Medve I, Lei J, Silbiger SR. Estradiol suppresses mesangial cell type IV collagen synthesis via activation of the MAP kinase cascade. Am J Physiol. 1999;277:F875–F881. doi: 10.1152/ajprenal.1999.277.6.F875. [DOI] [PubMed] [Google Scholar]
- Guccione M, Silbiger S, Lei J, Neugarten J. Estradiol upregulates mesangial cell MMP-2 activity via the transcription factor AP-2. Am J Physiol Renal Physiol. 2002;282:F164–F169. doi: 10.1152/ajprenal.0318.2000. [DOI] [PubMed] [Google Scholar]
- Potier M, Elliot SJ, Tack I, Lenz O, Striker GE, Striker LJ, Karl M. Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol. 2001;12:241–251. doi: 10.1681/ASN.V122241. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor-β in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Zdunek M, Silbiger S, Lei J, Neugarten J. Protein kinase CK2 mediates TGF-beta1-stimulated type IV collagen gene transcription and its reversal by estradiol. Kidney Int. 2001;60:2097–2108. doi: 10.1046/j.1523-1755.2001.00041.x. [DOI] [PubMed] [Google Scholar]
- Nielsen CB, Krag S, Osterby R, Flyvbjerg A, Nyengaard J, Forman A, Wogensen L. Transforming growth factor beta1-induced glomerulopathy is prevented by 17beta-estradiol supplementation. Virchows Arch. 2004;444:561–566. doi: 10.1007/s00428-004-1006-4. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN, Hoffman BB, Han DC, Iglesias-de la Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression and glomerular mesangial matrix expression by treatment with monoclonal anti-TGF-β antibody in db/db diabetic mice. Proc Natl Acad Sci USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-H, Yu ES, Yoon K-I, Johnson R. The impact of gender on progression of renal disease: potential role of estrogen-mediated vascular endothelial growth factor regulation and vascular protection. Am J Pathol. 2004;164:679–688. doi: 10.1016/S0002-9440(10)63155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim GL, Kis LL, Warner M, Gustafsson JA. Autoimmune glomerulonephritis with spontaneous formation of splenic germinal centers in mice lacking the estrogen receptor alpha gene. Proc Natl Acad Sci USA. 2004;101:1720–1724. doi: 10.1073/pnas.0307915100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, Karl M, Zheng F, Elliot SJ, Striker GE, Striker LJ. Estrogen-related abnormalities in glomerulosclerosis-prone mice: reduced mesangial cell estrogen receptor expression and prosclerotic response to estrogens. Am J Pathol. 2002;160:1877–1885. doi: 10.1016/S0002-9440(10)61134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot SJ, Karl M, Berho M, Poiter M, Zheng F, Leclercq B, Striker GE, Striker LJ. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am J Pathol. 2003;162:1441–1448. doi: 10.1016/S0002-9440(10)64277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JT, Foglia VG, Rodrigues RR. The effects of steroids on the incidence of diabetes in rats after subtotal pancreatectomy. Endocrinology. 1950;46:111–121. doi: 10.1210/endo-46-1-111. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Alonso A, Grueso NA, Diaz F, Esteban MM, Fernandez S, Patterson AM. Role of 17β-estradiol administration on insulin sensitivity in the rat: implications for the insulin receptor. Steroid. 2002;67:993–1005. doi: 10.1016/s0039-128x(02)00073-9. [DOI] [PubMed] [Google Scholar]
- Andersson B, Johannsson G, Holm G, Bengtsson BA, Sashegyi A, Pavo I, Mason T, Anderson PW. Raloxifene does not affect insulin sensitivity or glycemic control in postmenopausal women with type 2 diabetes mellitus: a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:122–128. doi: 10.1210/jcem.87.1.8168. [DOI] [PubMed] [Google Scholar]
- Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-γ ligands inhibit TGF-β1-induced fibronectin expression in glomerular mesangial cells. Diabetes. 2004;53:200–208. doi: 10.2337/diabetes.53.1.200. [DOI] [PubMed] [Google Scholar]
- Peach K, Webb P, Kuiper G, Nilsson S, Gustafsson JA, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Goldfrank D, Haytoglu T, Frishman WH. Raloxifene, a new selective estrogen receptor modulator. J Clin Pharmacol. 1999;39:767–774. doi: 10.1177/00912709922008416. [DOI] [PubMed] [Google Scholar]