Abstract
Acne is the most common skin disease, causing significant psychosocial problems for those afflicted. Currently available agents for acne treatment, such as oral antibiotics and isotretinoin (Accutane), have limited use. Thus, development of novel agents to treat this disease is needed. However, the pathophysiology of acne inflammation is poorly understood. Before new therapeutic strategies can be devised, knowledge regarding molecular mechanisms of acne inflammation is required. We report here that transcription factors nuclear factor-κB and activator protein-1 are activated in acne lesions with consequent elevated expression of their target gene products, inflammatory cytokines and matrix-degrading metalloproteinases, respectively. These elevated gene products are molecular mediators of inflammation and collagen degradation in acne lesions in vivo. This new knowledge enables a rational strategy for development of pharmacological agents that can target the inflammation and matrix remodeling that occurs in severe acne.
Acne vulgaris is a disorder of sebaceous follicles.1 Found primarily on the face and upper trunk (chest and back), these specialized follicles have prominent sebaceous glands associated with them. Acne is the most common skin disease, estimated to affect ∼80% of individuals at some point between the ages of 11 and 30 years.2 Although not life-threatening, acne can adversely impact psychosocial development, and may cause significant emotional problems, depression, and even suicide.3
Despite its common occurrence, the pathogenesis of acne is not fully understood. Excessive shedding of epithelial cells from the walls of follicles combined with increased amounts of sebum produced by associated sebaceous glands are two important factors that contribute to follicular obstruction.4,5 This obstruction leads to the formation of microcomedos, which are believed to be the precursor lesions of acne.5 These microcomedos may evolve into clinically visible comedos (blackheads and whiteheads) or inflammatory lesions (papules, pustules, or cysts). Propionibacterium acnes (P. acnes) are gram-positive, anaerobic diphtheroids that are part of the normal skin flora.6 In the generation of inflammatory lesions, proliferation of P. acnes in the obstructed follicles is believed to be critical.6,7 This is based on in vitro data that P. acnes stimulate monocytes to produce proinflammatory mediators and chemotactic factors,7,8 and time-tested clinical observations that antibiotics that inhibit P. acnes improve inflammatory acne.
It is a well established clinical fact that inadequate control of acne inflammation can later lead to scarring. Scarring is the most unfortunate and undesirable sequela of acne, because once formed, it is not amenable to pharmacological treatment and requires invasive procedures such as chemical peels, dermabrasion, or laser resurfacing to improve its appearance.9,10 These procedures are not only costly, but they themselves are associated with further risks of scarring and pigmentary abnormalities. Thus, preventing scar formation is a major goal in the treatment of inflammatory acne.
Currently, inflammatory acne is treated with antibiotics (both oral and topical) or oral isotretinoin (Accutane). Although widely used and often effective, antibiotics have a significant limitation, which is development of resistant strains of P. acnes.11–13 Oral isotretinoin is by far the most effective treatment for severe inflammatory acne, routinely delivering dramatic improvements.14,15 However, because of its established teratogenicity, isotretinoin use has been restricted. Furthermore, the recently publicized, controversial risk of depression/suicidal ideation has made isotretinoin use even more problematic.16,17 Therefore, current treatment options for inflammatory acne are suboptimum. Rational development of new treatments for acne necessitates a molecular description of mechanisms involved in acne inflammation in vivo because no animal model exists. Here we describe the key inflammatory signaling pathways that are activated in facial acne lesions from human patients.
Materials and Methods
Tissue Samples
We studied skin samples from 16 patients (4 men and 12 women; mean age, 25 years; range, 13 to 39 years) with inflammatory acne. Before skin biopsy, patients must not have used any systemic (4 weeks) or topical (2 weeks) acne medications. Skin of one to two inflammatory lesions (red raised papules, n = 16, or pustules, n = 2, with a ring of erythema) and an adjacent, uninvolved normal area was obtained from the face of each patient using a 3-mm punch biopsy instrument. Routinely used in dermatology, 3-mm punch cleanly cuts a cylindrical core of epidermis, dermis, and subcutis (fat). After the plug of tissue is removed, 5-0 prolene suture is placed to close the circular opening. Seven days after the procedure, the suture is removed. A 3-mm linear scar is formed, but it blends well with the surrounding skin and becomes difficult to appreciate, especially on the face. The skin specimens were OCT-embedded for immunohistological analysis and in situ zymography, and snap-frozen for all other assays. The study was approved by the institutional review board at the University of Michigan, and all patients provided written informed consent before entering the study.
Measurement of Matrix Metalloproteinase (MMP) and Procollagen mRNA Levels
Total RNA was extracted using a commercial kit (RNeasy Mini kit; Qiagen, Chatsworth, CA). One 3-mm punch biopsy of acne lesion or normal face typically yielded ∼1 μg of total RNA. cDNA synthesis was performed with 0.1 μg of total RNA, in a volume of 50 μl. Reverse transcription was performed using a TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA). Real-time polymerase chain reaction (PCR) was performed in duplicate with 2 μl of cDNA for all genes of interest and an internal control gene (36B4) using TaqMan Universal PCR Master Mix kit (Applied Biosystems) and a 7700 sequence detector system (Applied Biosystems). Thus, one 3-mm punch biopsy was sufficient to quantify expression of at least 100 genes. All liquid handling procedures were performed with a calibrated robotic workstation (Biomek 2000; Beckman Coulter, Inc., Hialeah, FL) to ensure accuracy and reproducibility. Target gene mRNA levels (number of molecules/10 ng total RNA) were quantified based on a standard curve and normalized to 36B4 mRNA levels. PCR primers and probes for MMP-1, MMP-3, MMP-9, procollagen I, procollagen III, and 36B4 (control gene) were produced by a custom oligonucleotide synthesis service (Applied Biosystems). Cycle threshold (CT) values for genes of interest ranged from ∼22 to 28. CT value for 36B4 was ∼20. Duplicate CT values did not vary by more than 3%, and were well within the linear range of quantitation, based on standard curves.
Measurement of Cytokine mRNA Levels
mRNA levels of 12 different cytokines [interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12 p35, IL12 p40, IL-15, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α] were measured using the TaqMan Cytokine Gene Expression Plate I kit (Applied Biosystems). Reverse transcriptase (RT)-PCR was performed with 18s-rRNA as endogenous control, and the data were analyzed using a 7700 sequence detector system (Applied Biosystems).
Western Analysis of MMP-8 Protein
In three patients, MMP-8 protein levels in supernatants from skin homogenates were determined by Western blot analysis, as previously described.18 Rabbit polyclonal MMP-8 antibody (Chemicon, Temecula, CA) was used. Equal amounts of protein (100 μg/lane) were analyzed for each sample from an acne lesion and uninvolved adjacent skin.
Immunohistology
For immunohistological analysis, 6-μm skin cryosections were air-dried and fixed in paraformaldehyde and processed as previously described.18 Slides were incubated (1 hour) with mouse monoclonal antibodies for c-Jun (1:200; Transduction Laboratories, Lexington, KY), MMP-1 (1:250, Chemicon), MMP-3 (1:50, Chemicon), MMP-9 (1:50, Chemicon), or procollagen I [SP1.D8 developed by Dr. Heniz Furthmayer and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA; and PIC, a mouse monoclonal antibody (clone PC8-7, IgG1) obtained from PanVera Corp. (Madison, WI), which detects procollagen type I C-terminal peptide antibodies]. Sections were then incubated with a biotinylated horse anti-mouse antibody (30 minutes) followed by avidin biotin peroxidase complex (1/500 for 30 minutes). 3-Amino-9-ethyl carbazole (Sigma Chemical Co., St. Louis, MO) was used as chromogen. All sections were counterstained with hematoxylin. Substitutes of isotype control immunoglobulin for each primary antibody yielded no detectable staining.
Immunofluorescence
Seven-μm frozen sections were fixed in 2% paraformaldehyde and endogenous peroxidase activity quenched with 0.3% H2O2. For nuclear factor (NF)-κB p50, sections were preincubated with normal horse serum then stained with a rabbit polyclonal p50 antibody (H-119, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA). For NF-κB p65, sections were preincubated with normal goat serum then stained with a mouse monoclonal p65 antibody (F-6, 1:200; Santa Cruz Biotechnology). Binding of the antibodies was visualized by biotinylated goat anti-rabbit (Vector Laboratories, Burlingame, CA) for p50, and by biotinylated horse anti-mouse (Vector Laboratories) in combination with Texas Red (Calbiochem, San Diego, CA) for p65. Isotype control immunoglobulin that was substituted for each primary antibody yielded no detectable staining.
In Situ Zymography
In situ zymography using fluorescein isothiocyanate gelatin and resorufin-casein substrates has been described previously.19 Similar procedures were followed with the use of fluorescein-labeled collagen (Elastin Products, Owensville, MO) that was coated (4 mg/ml) directly onto slides without the addition of agarose.
Degraded/Fragmented Collagen
Quantitative assay for collagen fragmentation in skin was performed as previously described.20,21 Briefly, skin biopsies were homogenized in Tris buffer (20 mm, pH 7.3) and centrifuged. The pellet, containing the collagenous extracellular matrix, was resuspended in either Tris buffer alone or Tris buffer with α-chymotrypsin (75 μg/150 μl) and incubated for 8 hours at 37°C. At the end of the incubation period, the reaction tubes were centrifuged at 10,000 × g for 10 minutes. Supernatants were collected and assayed for hydroxyproline using automated amino acid analysis. The pellet containing nonfragmented collagen was resuspended in Tris buffer, subjected to acid hydrolysis (6 N HCl), and then assayed for hydroxyproline. The amount of hydroxyproline in the supernatant (from fragmented collagen) was expressed as a percentage of the total (supernatant plus pellet).
Statistical Analysis
Paired comparison of involved acne lesions and uninvolved skin was the basic structural design of these studies. Comparisons were analyzed with the paired t-test for normally distributed data, or the Wilcoxon signed-rank test for nonnormal data. Summary statistics are presented as means ± 1 SE. All P values are two-tailed. The data were analyzed with SAS statistical software (SAS Institute, Inc., Cary, NC).
Results
NF-κB Is Activated in Inflammatory Acne Lesions in Vivo
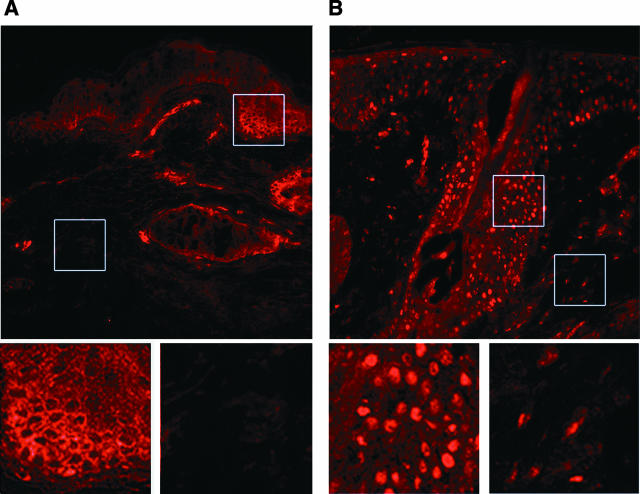
NF-κB is a transcription factor critical for up-regulation of many proinflammatory cytokine genes.22,23 Human skin expresses p65 (rel-A) and p50 (NF-κB1) that form a functional NF-κB complex.18 In the nonactivated state, p65 and p50 are tethered to the inhibitor IκBα in the cytoplasm, which primarily prevents the NF-κB complex from entering the nucleus. However, in response to extracellular proinflammatory signals, IκBα is degraded, thus allowing the NF-κB complex to translocate to the nucleus where it activates target gene transcription. Therefore, immunohistology with antibodies that recognize p50 or p65 show predominantly cytoplasmic staining (exclusion from nucleus) in the nonactivated state, whereas in the activated state p65 and p50 are mostly localized in the nucleus (solid dot pattern). In clinically normal uninvolved skin of acne patients, p65 staining is limited to the cytoplasm, in follicular and perifollicular epidermis, displaying a chicken wire-type of staining pattern, due to exclusion from the nucleus (Figure 1A). In the skin of inflammatory acne lesions however, p65 is localized in nuclei, with a solid dot-staining pattern (Figure 1B). Similar staining patterns were seen with anti-p50 antibody (data not shown). These data clearly indicate that NF-κB is activated in inflammatory acne lesions in vivo.
Figure 1.
Activation of NF-κB in skin specimens from inflammatory acne lesions. An inflammatory papule (B) and adjacent clinically normal skin (A) were obtained from acne patients. A component of NF-κB, p65 protein, was detected with biotinylated antibody in combination with Texas Red. p65 protein is stained red by this technique. A, inset, reveals cytoplasmic staining in a chicken-wire pattern in the epidermis, whereas B, inset, demonstrates a nuclear solid dot pattern. The specimens are from one subject and are representative of the findings in involved and uninvolved tissue from four patients.
Proinflammatory Cytokine Genes Are Activated in Inflammatory Acne Lesions in Vivo
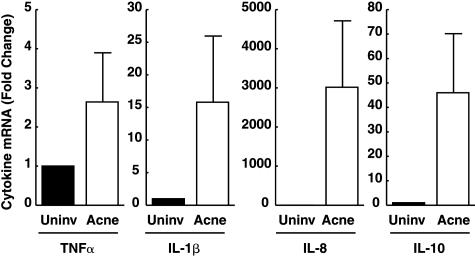
The finding that NF-κB is activated in inflammatory acne lesions suggests that transcripts for NF-κB-regulated cytokine genes may be elevated in lesions. Using real-time RT-PCR technology, we quantified mRNA levels of several cytokine genes in inflammatory acne lesions. Acne facial lesions had significantly greater mRNA levels of TNF-α (2.6-fold, P < 0.04), IL-1β (16-fold, P < 0.007), IL-8 (3015-fold, P < 0.001), and IL-10 (46-fold, P < 0.001) compared to uninvolved normal adjacent skin (Figure 2). Other cytokine gene transcript levels were not elevated in acne lesions. mRNA levels of IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-12 (p35 and p40), and IL-15 were similar in lesional and nonlesional skin. Elevated expression of IL-8, a well-known chemokine, can serve to attract circulating cells, into the tissue. Indeed, in lesional skin of acne, there is a marked increase in the presence of polymorphonuclear leukocytes (PMNs) (as revealed by neutrophil elastase staining), as compared to the uninvolved skin (data not shown). Similarly lymphocytes were prominently visible in inflammatory acne lesions as compared to normal controls (fourfold increase, P < 0.02; n = 10).
Figure 2.
Enhanced expression of inflammatory cytokines in inflammatory acne lesions. Skin was obtained from inflammatory papules and uninvolved areas of facial acne. Tissue specimens were assayed for cytokine mRNA levels using real-time quantitative RT-PCR. The values shown are means, with standard errors indicated by the bars. For each cytokine, mRNA levels were significantly higher in inflammatory acne than in uninvolved control skin (TNF-α, P < 0.05; IL-1β, P < 0.007; IL-8, P < 0.001; IL-10, P < 0.001; n = 8). ▪, uninvolved skin; □, acne lesion.
Activator Protein (AP)-1 Is Activated in Inflammatory Acne Lesions in Vivo
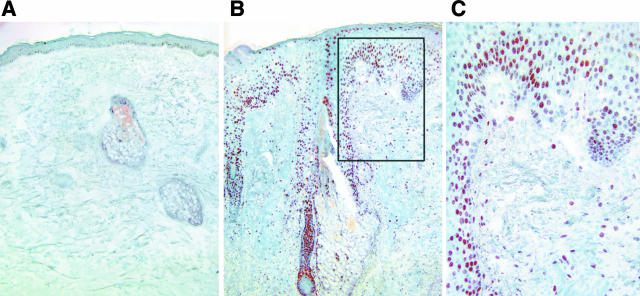
AP-1 is another important transcription factor involved in inflammation. Proinflammatory stimuli induce AP-1 gene and protein expression. AP-1 is composed of jun and fos family members. Unlike NF-κB, whose component levels are relatively constant, AP-1 components may be rapidly induced in response to proinflammatory stimuli. In normal human skin, cJun is limiting, while cFos is continually expressed at relatively high levels.24 In uninvolved normal skin, cJun expression is minimal and limited to nuclei of basal keratinocytes (Figure 3A). In inflammatory acne lesions, cJun expression is markedly elevated in the nucleus both in follicular and perifollicular epidermis, as well as in dermal cells, indicating that AP-1 activation has occurred in these regions (Figure 3, B and C).
Figure 3.
cJun, a component of AP-1 transcription factor, is induced in inflammatory acne lesions. An inflammatory papule (B) and adjacent uninvolved skin (A) were obtained from acne patients. A component of AP-1, cJun protein, was detected by peroxidase immunohistology. A: In uninvolved skin cJun expression is minimal. In inflammatory acne lesions, however, cJun staining is markedly intranuclear and prominent in follicular and perifollicular epidermis and in dermis (C is inset of B). The specimens are from one subject and are representative of the findings in involved and uninvolved tissue from eight patients.
AP-1-Regulated Matrix-Degrading Metalloproteinases Are Elevated in Acne Lesions in Vivo
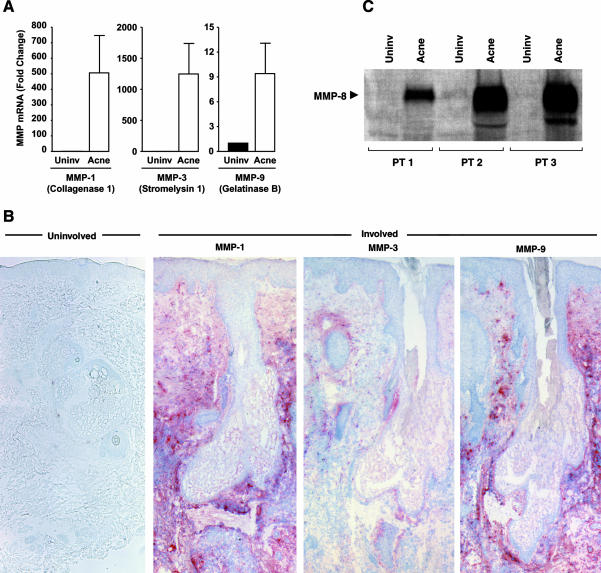
A consequence of AP-1 activation is enhanced transcription of AP-1-regulated genes. Among the many genes that AP-1 regulates are several MMPs. Of the many MMPs identified, MMP-1 (collagenase-1), MMP-3 (stromelysin I), MMP-8 (collagenase 2), MMP-9 (92-kd gelatinase or collagenase-4), and MMP-13 (collagenase 3) possess AP-1 response elements in their gene promoters.25–27 MMP-1 is critical in the degradation of mature collagen because it initiates site-specific cleavage within the triple-helical domain of type I and other fibrillar collagens.27 MMP-8 and MMP-13 are also collagenases that can initiate collagen degradation. After initial cleavage by collagenase, other MMPs, such as gelatinase and stromelysin, can further degrade collagen fragments.27 In inflammatory acne lesions, MMP-1 (506-fold), MMP-3 (829-fold), and MMP-9 (12-fold) mRNA levels were significantly elevated (P < 0.05, n = 8) compared to uninvolved facial controls (Figure 4A). Consistent with the mRNA data, MMP protein expression, revealed through immunohistology, was markedly increased in the perifollicular dermis of acne lesions as compared to the uninvolved skin (Figure 4B). Neutrophil collagenase, or MMP-8, as implied in its name, is produced by PMNs, and is stored within the cell. A consequence of PMN infiltration into tissue is delivery of MMP-8 to the site, where on secretion and activation, it can contribute to matrix degradation. MMP-8 is highly active against type I collagen.28 Consistent with our findings mentioned above that numerous PMNs are found in inflammatory lesions of acne, MMP-8 protein levels, as measured by Western blot, were markedly elevated (dark thick bands) in lesional skin compared to nonlesional skin (Figure 4C).
Figure 4.
Enhanced expression of AP-1 regulated MMPs in inflammatory acne lesions. Skin was obtained from inflammatory papules and uninvolved areas of facial acne. For MMP mRNA levels, real-time RT-PCR was used. The values shown are means, with standard errors indicated by the bars. A: For each MMP, mRNA levels were significantly higher in inflammatory acne than in uninvolved skin (MMP-1, P < 0.008; MMP-3, P < 0.006; MMP-9, P < 0.003; n = 6; ▪, uninvolved skin; □, acne lesion). MMP-1, -3, and -9 protein expression levels were assessed by immunohistology. B: Consistent with their mRNA data, the expression of three MMP proteins (revealed as brown staining) was increased in acne lesions as compared to uninvolved skin. Neutrophil collagenase (MMP-8) expression in inflammatory acne lesions was assessed with Western blot analyses. An inflammatory papule and adjacent uninvolved skin were obtained from three acne patients. C: Bands from the Western blot analyses are shown.
Collagen-Degrading Enzyme Activities Are Present, and Degraded/Fragmented Collagen Is Increased in Skin from Inflammatory Acne, but Not from Uninvolved Normal Skin
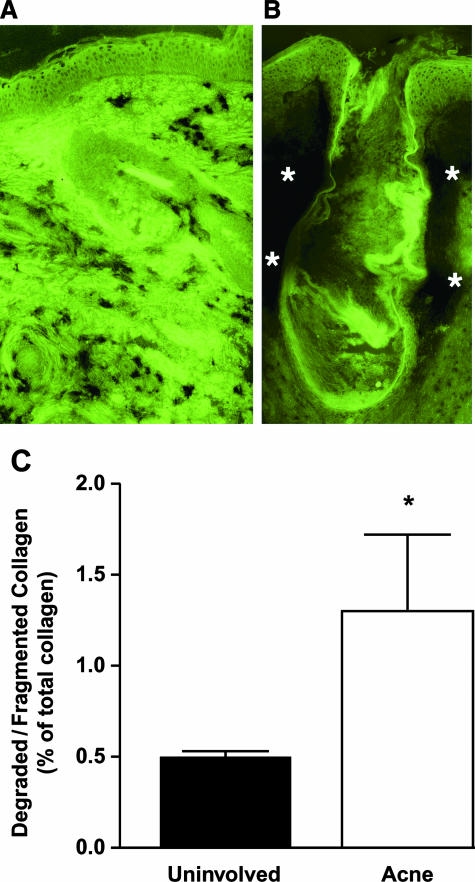
In inflammatory acne, there was a marked loss of fluorescence due to collagen-degrading activities around and within the follicle (Figure 5B). An increase in MMP activity in human skin in vivo, is expected to produce greater amounts of degraded/fragmented collagen in the tissue. A quantitative method of detecting fragmented collagen was used as a second and independent approach to address matrix destruction in acne. In the lesional skin of inflammatory acne, there was a significant 2.6-fold increase (P < 0.04, n = 5) in degraded/fragmented collagen greater than the level present in nonlesional facial skin (Figure 5C).
Figure 5.
Induction of collagenolytic activity and increased degraded collagen levels in skin specimens from acne patients. Collagenase activity in inflammatory acne (B) and uninvolved skin (A) was measured with the use of fluorescein-labeled collagen as substrate. The specimens are from one subject and are representative of the findings in tissue from three patients. The green color is fluorescein-labeled collagen that was coated onto a glass slide. The skin section was laid on top of the slide and incubated for 24 hours to allow collagenase in the tissue to degrade the fluorescein-labeled collagen on the slide. Darkened areas, especially noticeable in B (asterisks) are due to the degradation of colored substrate by collagenase. For C skin was obtained from inflammatory papules and uninvolved areas of facial acne. α-Chymotrypsin-sensitive degraded/fragmented collagen was expressed as a percentage of total skin collagen (ratio of hydroxyproline levels). *P < 0.04; n = 5; ▪, uninvolved skin; □, acne lesion.
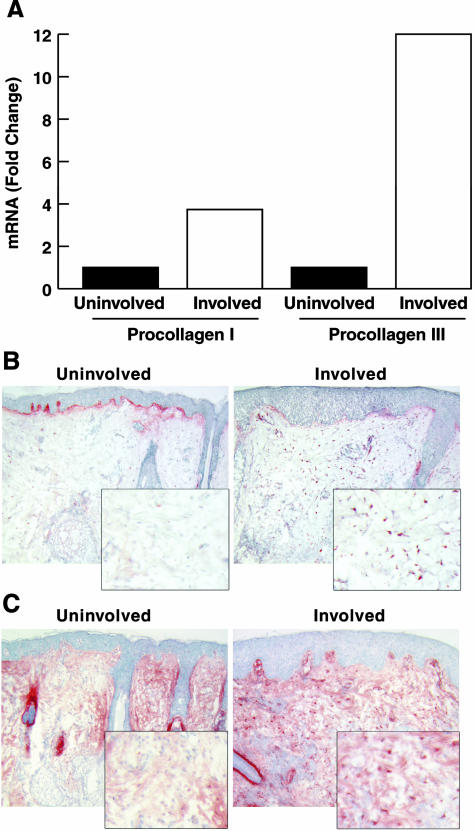
Procollagen I and III mRNA Levels Are Increased and Procollagen I Protein Levels Are Increased within Dermal Fibroblasts in Lesional Skin of Acne
Coincident with increased matrix degradation mediated by MMPs, derived from resident skin cells as well as brought into skin via PMNs, we found an increase in procollagen I and III mRNA levels in the lesional skin of inflammatory acne (Figure 6A). Immunohistological studies with two different procollagen I antibodies (SP1.D8 and PIC) reveal that dermal fibroblasts show greater intracellular immunostaining, indicative of stimulated protein synthesis (Figure 6, B and C).
Figure 6.
Type I and type III procollagen synthesis is induced in inflammatory acne lesions. An inflammatory papule and adjacent uninvolved skin were obtained from an acne patient. A: Tissue specimens were assayed for type I and type III procollagen mRNA levels using real-time quantitative RT-PCR. Data are expressed as fold greater than the values of uninvolved skin. 36B4 mRNA was used to normalize the expression level of other genes. Immunostaining of procollagen I in uninvolved and involved skin was performed with SP.1D (B) and PIC antibodies (C). Positively stained dermal fibroblasts are much more numerous in the inflammatory acne lesion, as compared to the uninvolved skin.
Discussion
The molecular description of inflammatory acne in human skin in vivo has not been previously made. We have obtained directly from human facial skin, evidence for activation of intracellular signaling cascades associated with two important transcription factors involved in inflammation and matrix destruction, NF-κB and AP-1. The NF-κB pathway was activated as evidenced by nuclear localization of p50 and p65 in follicular and perifollicular epidermis in inflammatory acne. Consistent with NF-κB activation, we found a marked increase in inflammatory cytokine gene transcripts in active acne lesions. Among those that were increased were TNF-α and IL-1β (primary cytokines), which are critically important in inflammation. These proinflammatory cytokines will not only amplify the NF-κB signaling pathways that originally led to their production through cell surface receptor activation (an autocrine loop), but will also stimulate nearby cells in a paracrine manner. For example, TNF-α and IL-1β are known to up-regulate adhesion molecules, such as ICAM-1 and VCAM-1 on endothelial cells.29,30 Thus, the recent observation that ICAM-1, E-selectin, and VCAM-1 expression levels on the luminal surface of endothelial cells are increased in inflammatory acne papules may be a consequence of TNF-α and IL-1β induction in the milieu.31 The elaboration of adhesion molecules is necessary to slow the flow of circulating inflammatory cells for their eventual diapedsis into the inflamed tissue.29,30 Elevated levels of IL-8, a well-known chemokine, found in inflammatory acne lesions would aid in the recruitment of these inflammatory cells. Because the IL-8 gene is regulated by NF-κB elevated IL-8 expression in acne likely arises by NF-κB activation.32 In addition, as a secondary cytokine, IL-8 is induced by the primary cytokines TNF-α and IL-1β. Besides TNF-α, IL-1β, and IL-8, we found significant elevation of IL-10 in acne lesions. As a well known anti-inflammatory/immunosuppressive cytokine produced by lymphocytes, macrophages, and keratinocytes,33–35 IL-10 induction may serve as a dampening mechanism for acne inflammation.
TNF-α and IL-1β acting through their specific cell surface receptors stimulate mitogen-activated protein (MAP) kinases, which results in induction and activation of AP-1.36,37 We have previously demonstrated that cJun expression is limiting for AP-1 activation in human skin in vivo.24 Therefore, increased levels of TNF-α and IL-1β observed in inflammatory acne would be expected to induce cJun and AP-1 activity. Indeed, we saw a clear induction of cJun in follicular and perifollicular epidermis, as well as in dermal cells of acne lesions. AP-1 is a critical regulator of induced expression of MMP-1, -3, and -9. These three AP-1-regulated MMPs were markedly elevated in inflammatory acne lesions. Immunolocalization of MMP-1, -3, and -9 was predominantly in the dermis, indicating that the MMPs synthesized in the epidermis were transported to the dermis. This was not an unexpected finding because the MMPs are secreted proteins, and the basement-membrane zone, which separates the epidermis from the dermis, readily allows the passage of proteins between the two compartments.38,39 Together, these MMPs were very efficient in degrading collagen matrix as evidenced by in situ zymography. More than a 2.5-fold increase in degraded (fragmented) collagen in the lesional skin of acne provided an independent, quantitative confirmation of matrix breakdown in inflammatory acne. The degradation of dermal matrix will be followed by its synthesis and repair, which cannot be perfect. Indeed, we found that expression levels of procollagens I and III are elevated in inflammatory acne lesions, and this was accompanied with an increase in transforming growth factor-β1 (data not shown). Most of the imperfect wound healing response would leave clinically undetectable deficits in the organization or composition, or both, of the extracellular matrix. However, if such imperfections occur to a significant extent throughout time, along with sustained and marked increase in procollagen synthesis, acne scarring may become clinically noticeable.
Although clinical manifestation of inflammatory acne is unique, our findings indicate that molecular pathways that drive the inflammatory state are not. Thus, the uniqueness of acne inflammation is not in the nature of the signaling cascade but in the localization of the process (ie, specialized sebaceous follicles). Future studies are needed to determine the importance of local milieu in initiating the inflammatory cascade in acne. Studies such as ours with tissue directly acquired from human patients cannot typically achieve the same level of mechanistic depth and/or proof of causality, relative to those using more tractable animal and in vitro systems. Nonetheless our work on human acne is the first of its kind as de novo research, and provides valuable information on the disease processes in man. An acne model derived from our study identifies several novel pharmacological targets to interrupt the inflammatory acne process (Figure 7). Indeed, some anti-inflammatory activity observed with the use of oral antibiotics may relate to their action in the inflammatory cascade. With growing difficulty in using oral antibiotics and isotretinoin, understanding molecular mechanisms of acne inflammation and scarring will enable newer approaches to combat this common and often disfiguring skin disease.
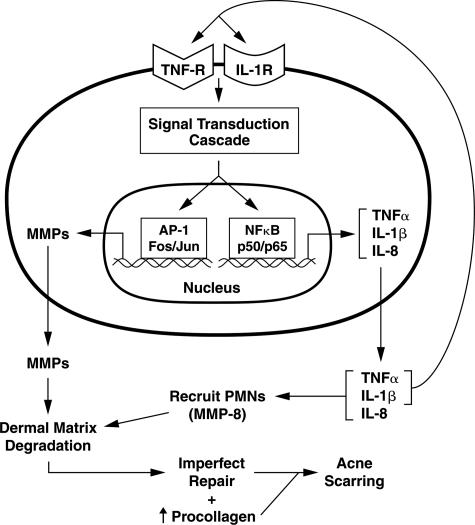
Figure 7.
Hypothetical model of the pathophysiology of inflammatory acne and dermal damage. In inflammatory acne lesions, NF-κB signaling is activated. As a consequence, NF-κB-driven inflammatory cytokine genes (eg, TNF-α and IL-1β) are induced. These primary cytokines will propagate the inflammatory response by acting on endothelial cells to elaborate adhesion molecules (eg, ICAM-1) to facilitate recruitment of inflammatory cells into the skin. TNF-α and IL-1β will also stimulate the production of secondary cytokines, such as IL-8, which can aid in chemotaxis of inflammatory cells. By working through their cell surface receptors, TNF-α and IL-1β not only amplify the NF-κB signaling cascade, but also activate MAP kinases to stimulate AP-1-mediated gene transcription. As a consequence of AP-1 activation (cJun induction), AP-1-driven MMPs are elaborated by resident skin cells. Along with MMP-8 and neutrophil elastase brought in by PMNs, they degrade the matrix. This is followed by matrix synthesis and repair, which is imperfect. Most of the imperfections would leave clinically undetectable deficits in the organization or composition, or both, of the extracellular matrix. However, when they occur to a significant extent throughout time, accompanied by sustained procollagen synthesis, acne scarring becomes clinically visible.
Acknowledgments
We thank Jennifer Burger, MD, Margaret Brown, MD, and Joyce Lee, MD, for assisting in patient recruitment and tissue procurement; Laura VanGoor for graphics presentation; and Ted Hamilton for statistical analysis.
Footnotes
Address reprint requests to Sewon Kang, M.D., Department of Dermatology, University of Michigan Medical Center, 1910 Taubman Center, Ann Arbor, MI 48109. E-mail: swkang@umich.edu.
Supported in part by the Babcock Endowment for Dermatological Research and the National Institutes of Health (K24 to S.K.).
Presented in part at the annual meeting of the Society for Investigative Dermatology, Los Angeles, CA, May 15 to 18, 2002.
Current address of S.C. and J.H.C.: Department of Dermatology, Seoul National University College of Medicine, Seoul, Korea.
References
- Brown S, Shalita A. Acne vulgaris. Lancet. 1998;351:1871–1876. doi: 10.1016/S0140-6736(98)01046-0. [DOI] [PubMed] [Google Scholar]
- Kraning K, Odland G. Prevalence, morbidity, and cost of dermatological diseases. J Invest Dermatol. 1979;73:395–401. doi: 10.1111/1523-1747.ep12541101. [DOI] [PubMed] [Google Scholar]
- Koo J, Smith L. Psychologic aspects of acne. Pediatr Dermatol. 1991;8:185–188. doi: 10.1111/j.1525-1470.1991.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Knutson D. Ultrastructural observations in acne vulgaris: the normal sebaceous follicle and acne lesions. J Invest Dermatol. 1974;62:288–307. doi: 10.1111/1523-1747.ep12676804. [DOI] [PubMed] [Google Scholar]
- Lavker R, Leyden J. Lamellar inclusions in follicular horny cells: a new aspect of abnormal keratinization. J Ultrastruct Res. 1979;69:362–370. doi: 10.1016/s0022-5320(79)80053-2. [DOI] [PubMed] [Google Scholar]
- Marples R, Leyden J, Stewart R, Mills O, Kligman A. The skin microflora in acne vulgaris. J Invest Dermatol. 1974;62:37–41. doi: 10.1111/1523-1747.ep12676718. [DOI] [PubMed] [Google Scholar]
- Webster G, Leyden J. Characterization of serum-independent polymorphonuclear leukocyte chemotactic factors produced by Propionibacterium acnes. Inflammation. 1980;4:261–269. doi: 10.1007/BF00915027. [DOI] [PubMed] [Google Scholar]
- Vowels B, Yang S, Leyden J. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158–3165. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao S, Dover J, Arndt K, Kaminer M. Scar management: keloid, hypertrophic, atrophic, and acne scars. Semin Cutan Med Surg. 2002;21:46–75. doi: 10.1016/s1085-5629(02)80719-2. [DOI] [PubMed] [Google Scholar]
- Hirsch R, Lewis A. Treatment of acne scarring. Semin Cutan Med Surg. 2001;20:190–198. doi: 10.1053/sder.2001.27557. [DOI] [PubMed] [Google Scholar]
- Leyden J, McGinley L, Cavalieri S, Webster G, Mills O, Kligman A. Propionibacterium acnes resistance to antibiotics in acne patients. J Am Acad Dermatol. 1983;8:41–45. doi: 10.1016/s0190-9622(83)70005-8. [DOI] [PubMed] [Google Scholar]
- Eady E, Jones C, Tipper J, Cove J, Cunliffe W, Layton A. Antibiotic resistant propionibacteria in acne: need for policies to modify antibiotic usage. BMJ. 1993;306:555–556. doi: 10.1136/bmj.306.6877.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady E, Cove J, Blake J, Holland K, Cunliffe W. Recalcitrant acne vulgaris: clinical, biochemical, and microbiological investigation of patients not responding to antibiotic treatment. Br J Dermatol. 1988;118:415–423. doi: 10.1111/j.1365-2133.1988.tb02437.x. [DOI] [PubMed] [Google Scholar]
- Peck G, Olsen T, Yoder F, Strauss J, Downing D, Pandya M, Butkus D, Arnaud-Battndier J. Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N Engl J Med. 1979;300:329–333. doi: 10.1056/NEJM197902153000701. [DOI] [PubMed] [Google Scholar]
- Strauss J, Rapini R, Shalita A, Konecky E, Pochi P, Comite H, Exner J. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490–496. doi: 10.1016/s0190-9622(84)80100-0. [DOI] [PubMed] [Google Scholar]
- Wysowski D, Pitts M, Beitz J. Depression and suicide in patients treated with isotretinoin. N Engl J Med. 2001;344:460. doi: 10.1056/NEJM200102083440616. [DOI] [PubMed] [Google Scholar]
- Jick S, Kremers H, Vasilakis-Scaramozza C. Isotretinoin use and risk of depression, psychotic symptoms, suicide, and attempted suicide. Arch Dermatol. 2000;136:1231–1236. doi: 10.1001/archderm.136.10.1231. [DOI] [PubMed] [Google Scholar]
- Fisher G, Datta S, Talwar H, Wang Z, Varani J, Kang S, Voorhees J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Galis Z, Sukhova G, Libby P. Microscopic localization of active proteases by in situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J. 1995;9:974–980. doi: 10.1096/fasebj.9.10.7615167. [DOI] [PubMed] [Google Scholar]
- Fligiel S, Varani J, Datta S, Kang S, Fisher G, Voorhees J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- Bank R, Krikken M, Beekman B, Stoop R, Maroudas A, Lafeber F, Koppele J. A simplified measurement of degraded collagen in tissues: application in healthy, fibrillated and osteoarthritic cartilage. Matrix Biol. 1997;16:233–243. doi: 10.1016/s0945-053x(97)90012-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor R. Role of the NFκB pathway in the pathogenesis of human disease states. Curr Mol Med. 2001;1:287–296. doi: 10.2174/1566524013363816. [DOI] [PubMed] [Google Scholar]
- Boone D, Lee E, Libby S, Gibson P, Chien M, Chan F, Madonia M, Burkett P, Ma A. Recent advances in understanding NFκB regulation. Inflamm Bowel Dis. 2002;8:201–212. doi: 10.1097/00054725-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Fisher G, Talwar H, Lin J, Lin P, McPhillips F, Wang Z, Li X, Wan Y, Kang S, Voorhees J. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J Clin Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P, Karin M. Specific members of the Jun protein family regulate collagenase expression in response to various extracellular stimuli. Matrix Suppl. 1992;1:156–164. [PubMed] [Google Scholar]
- Crowe D, Tsang K, Shemirani B. Jun N-terminal kinase 1 mediates transcriptional induction of matrix metalloproteinase 9 expression. Neoplasia. 2001;3:27–32. doi: 10.1038/sj.neo.7900135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore W, Bodden M, Windsor L, Birkedal-Hansen B, DeCarlo A, Engler J. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Hirose T, Patterson C, Pourmotabbed T, Mainardi C, Hasty K. Structure-function relationship of human neutrophil collagenase: identification of regions responsible for substrate specificity and general proteinase activity. Proc Natl Acad Sci. 1993;90:2569–2573. doi: 10.1073/pnas.90.7.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Read M, Neish A, Whitley M, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- Ledebur H, Parks T. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NFκB site and p65 homodimers. J Biol Chem. 1995;270:933–943. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- Jeremy A, Holland D, Roberts S, Thomson K, Cunliffe W. Inflammatory events are involved in acne lesions initiation. J Invest Dermatol. 2003;121:20–27. doi: 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Santana M, Rosenstein Y. What it takes to become an effector T cell. J Cell Physiol. 2003;195:392–401. doi: 10.1002/jcp.10258. [DOI] [PubMed] [Google Scholar]
- Okura Y, Jee Y, Matsumoto Y. Acquired thymic tolerance to autoimmune encephalomyelitis is associated with activation of peripheral IL-10-producing macrophages/dendritic cells. Int Immunol. 2003;15:437–446. doi: 10.1093/intimm/dxg044. [DOI] [PubMed] [Google Scholar]
- Kondo S. The roles of keratinocyte-derived cytokines in the epidermis and their possible responses to UVA-irradiation. J Invest Dermatol Symp Proc. 1999;4:177–183. doi: 10.1038/sj.jidsp.5640205. [DOI] [PubMed] [Google Scholar]
- Whitmarsh A, Davis R. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Claret F, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383:453–457. doi: 10.1038/383453a0. [DOI] [PubMed] [Google Scholar]
- Kazama T, Yaoita E, Ito M, Sato Y. Charge-selective permeability of dermo-epidermal junction: tracer studies with cationic and anionic ferratins. J Invest Dermatol. 1988;91:560–565. doi: 10.1111/1523-1747.ep12476939. [DOI] [PubMed] [Google Scholar]
- Okubo T, Sano S. Functional aspects of dermo-epidermal junction. Acta Derm Venereol Suppl (Stockh) 1973;73:121–128. [PubMed] [Google Scholar]