Abstract
The role of gender and sex hormones is unclear in host response to lung injury, inflammation, and fibrosis. To examine gender influence on pulmonary fibrosis, male and female rats were given endotracheal injections of either saline or bleomycin. Female rats showed higher mortality rates and more severe fibrosis than did male rats, as indicated by higher levels of lung collagen deposition and fibrogenic cytokine expression. To clarify the potential role of female sex hormones in lung fibrosis, female rats were ovariectomized and treated with either estradiol or vehicle plus endotracheal injections of either saline or bleomycin. The results showed diminished fibrosis in the ovariectomized, bleomycin-treated rats without hormone replacement. Estradiol replacement restored the fibrotic response to that of the intact female mice in terms of lung collagen deposition and cytokine expression, which was accompanied by higher plasma estradiol levels. Furthermore, fibroblasts from bleomycin-treated rats exhibited increased responsiveness to estradiol treatment, causing dose-dependent increases in procollagen 1 and transforming growth factor-β1 mRNA expression relative to untreated controls. Taken together these findings suggest that female mice may have an exaggerated response to lung injury relative to male mice because of female sex hormones, which have direct fibrogenic activity on lung fibroblasts. This may provide a mechanism for a hormonally mediated intensification of pulmonary fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive form of interstitial lung disease. The median survival rates are reported to be ∼5 years, and most physicians consider it to be an ultimately fatal disease.1 In the United States, the mortality rate due to pulmonary fibrosis (PF) has increased from 40 to 56% between 1979 and 1991.2 PF is commonly characterized by some degree of lung inflammation and abnormal tissue repair, namely scarring, or the replacement of normal functional tissue with connective tissue.3,4 This process is now known to involve an intricate cytokine network that activates and mediates interactions between multiple cell types, resulting in the elevation of collagen gene expression and abnormal deposition of collagen in the lung.3–11
To date, a variety of animal models have been used to investigate mechanisms of PF and other types of fibrosis, including bleomycin-induced pulmonary fibrosis (BLM-IPF) in rodents and other animal species.5–12 However, only a few studies have dealt with the role of gender in PF. In vivo studies have found that female hamsters immunized with trinitrochloro-1-benzene were more susceptible to the development of PF than males.13 In humans, studies of several diseases involving connective tissue diseases may indicate the importance of gender in fibrosis. The incidence of familial IPF is higher among females than males, according to an early study, and it has been reported that women are diagnosed with both diffuse PF and Hermansky-Pudlak syndrome more often than men.14,15 It has also been documented that female cystic fibrosis patients have a worse prognosis than do their male counterparts.16 Current evidence suggests that women develop alcoholic liver disease at lower levels of alcohol intake and throughout a shorter period of time as compared to men, thus making them more susceptible to alcohol-induced liver injury than are males.17
With regard to the role of sex hormones on disease severity, much of the evidence suggests that the estrogen hormone, which is gender-specific, has a significant influence on disease progression. A significant increase in estradiol levels have been reported in the blood of female patients with alveolitis, histocytosis X, and lung sarcoidosis, with the magnitude of changes in hormone levels correlating with the severity of respiratory failure.18 Recently, topical estrogen has been found to be effective in wound healing in the elderly by alteration of inflammatory response and stimulation of matrix production, as shown by increased collagen deposition.19 In addition, a recent study has reported that administration of estradiol induced inflammatory response in the lateral prostate of castrated rats.20 Moreover immune reactivity is greater in females than males in both experimental animals and in humans. Thus sex hormones influence the onset and severity of immune-mediated pathological conditions by modulating lymphocytes, with immunoregulatory T cells appearing to be the most sensitive and responsive of the lymphoid cells to sex hormone exposure.21 In addition, it has been shown that estrogen can increase transforming growth factor (TGF)-β production by osteoblasts and other cells, and regulate apoptosis.22,23 However, despite this evidence, direct evidence linking gender, sex hormones, and lung disease remains lacking.
Objectives for this study were to explore the effects of gender and estradiol on lung fibrosis, and more specifically, to determine the mechanisms that enable estradiol to alter or intensify the fibrotic process. Using a well-characterized model of BLM-IPF in rats, the influence of both gender and estradiol on the progression of lung injury, inflammation, and fibrosis was analyzed using lung histopathology, cytokine expression, and collagen deposition in vivo, as well as by examination of morbidity and mortality rates. Whereas the gender study compared the response of male and female rats to BLM, the estradiol study compared the response of ovariectomized rats, with or without estradiol replacement therapy. The findings confirmed that female gender enhances lung fibrosis with female rats showing more extensive fibrosis, higher mortality rates, and significantly larger and more consolidated fibrotic lesions in response to BLM treatment. However ovariectomy diminished lung fibrosis, while estradiol replacement reversed this diminution. Furthermore, isolated lung fibroblasts from BLM-treated rats showed an altered phenotype as manifested by increased responsiveness to estradiol treatment, causing greater increases in procollagen 1 and TGF-β1 mRNA expression relative to untreated controls.
Materials and Methods
Induction of PF and Tissue Sampling
Specific pathogen-free, 3-month-old male and female Fisher 344 rats, weighing 180 to 200 g were obtained from Charles River Breeding Laboratories, Inc. (Wilmington, MA). These animals arrived in filtered cages, and were maintained in clean animal quarters separate from other laboratory animals. The rats were handled and maintained using microisolator techniques with daily veterinarian monitoring. The University of Michigan Committee on the Use and Care of Animals approved these experiments. Male and female rats were randomly chosen for the control or experimental groups. Seventy male rats were divided equally (14 per group) into five groups. An additional 70 female rats were also divided in the same manner. On day 0, half of the rats in each group were treated with 0.75 U/100 g body weight BLM (Blenoxane; Bristol-Myers Co., Evansville, IN) by endotracheal injection, as previously described.6–12 The remaining half received only sterile saline (SAL) endotracheally. Animals were examined daily, and body weights and survival rates recorded. One group each of the male and female rats were sacrificed under ketamine anesthesia on days 3 (group 1), 7 (group 2), 14 (group 3), 21 (group 4), and 28 (group 5) after BLM or SAL injection.6–12 At each time point, the lungs from three control and three experimental animals of each gender were used for extraction of RNA for Northern hybridization or polymerase chain reaction (PCR) analysis. From the remaining animals in each group, the lungs were rapidly dissected out and immediately inflated under 10 cm of pressure with phosphate-buffered saline (PBS) or 10% neutral buffered formalin, pH 7.2. After overnight fixation, lungs were embedded in paraffin.6–12 PBS-inflated lung tissues were used for frozen sections and used for immunohistochemistry. Serial sections (3 to 4 μm) of formalin-fixed tissue were used for routine histology and histochemistry. In addition, on day 21 after BLM challenge, lung tissue was harvested for analysis of fibrosis by hydroxyproline assay.
Ovariectomy in Rats
To clarify the relationship between female sex hormone and lung fibrosis, 78 3-month-old female Fisher 344 rats were used. Animals were divided equally into two groups, an ovariectomized group and a control group. The ovariectomy (OV) group underwent bilateral OV, and the sham or normal (N) operation group underwent a sham operation. When the hormonal status of the OV rats had stabilized at 3 weeks after OV, plasma estradiol levels were evaluated in all rats, and estradiol treatments commenced. The two groups were thus further divided equally into rats receiving estradiol (designated as +E) and those receiving vehicle (designated as +V) only.
Exogenous Estradiol Treatment in Ovariectomized Rats
Estradiol (17β-estradiol) was dissolved in propylene glycol and administered at 8 μg/kg/hour, a dose that will produce serum levels approximating those seen in vivo in rat pregnancy (258 pg/ml).24 After filling the drug into a pump (model 2004; Alza Corp., Palo Alto, CA), the pump was preincubated in PBS at 37°C for 24 hours. For control purposes, propylene glycol was administered at the same concentration as estradiol. Miniosmotic pumps were implanted into 1-cm subcutaneous pockets made in the nuchal area 3 weeks after OV for continuous delivery of estradiol or vehicle, with a group of OV and sham rats not receiving any estradiol or vehicle. Each group was then further divided into two equal groups receiving either endotracheal injections of SAL (indicated by +SAL) or BLM (indicated by BLM) 24 hours after implantation. The eight groups of rats were then referred to as OV + BLM, N + BLM, OV + V + SAL, OV + V + BLM, OV + E + BLM, N + V + SAL, N + V + BLM, or N + E + BLM. On day 14 after BLM challenge, lung tissue and blood were harvested for analysis of fibrosis, histopathology, cytokine, collagen expression, and plasma estradiol levels, using immunohistochemistry, reverse transcriptase (RT)-PCR, and enzyme-linked immunosorbent assays. In addition, on day 21 after BLM challenge, similarly treated groups of rats were sacrificed for harvesting of lung tissue for analysis of fibrosis by hydroxyproline assay. Plasma estradiol levels were also measured from these rats. The entire experiment was repeated twice with similar results.
Rat Lung Fibroblast Isolation and Culture
Rat lung fibroblasts were isolated from days 7 and 14 BLM-treated (BRLF) or SAL-treated (NRLF) female lung tissue as previously described.7,25 Fibroblasts used in this experiment were between cell passages 2 and 4 after isolation. Semiconfluent fibroblasts were washed three times and treated for 24 hours with various concentrations of either estradiol or vehicle in fresh serum-free Dulbecco’s modified Eagle’s medium containing 0.5% bovine plasma-derived serum. After 24 hours, the cells were harvested for analysis of cytokine and collagen gene expression. Fibroblast proliferation was estimated by [3H] thymidine incorporation in 96-well plates.7 In addition, fibroblasts were grown on Lab-Tek 4 chamber slides (Nalge Nunc International, Rochester, NY) and treated as above and the cells were used for immunostaining.
Lung Histopathology and Immunohistochemistry
General histological appearance of lung tissue was assessed after routine hematoxylin and eosin (H&E) staining, whereas Masson trichrome staining was used for assessment of collagen deposition. Lung cell identification was performed by histochemical and immunohistochemical techniques as previously described.10 Eosinophils were identified by either staining with chromotrope 2R (stains eosinophil granules) as previously described10 or by immunostaining of frozen lung sections with BMK-13 antibody. Monocytes and macrophages were identified by their mononuclear morphology and by immunostaining with ED1 antibody.10 T lymphocytes were identified by staining with CD3 antibody.10 Immunostaining also was performed to determine the effects of estradiol on TGF-β1 and collagen expression by fibroblasts. Semiconfluent NRLFs and BRLFs stimulated with estradiol in the manner described above were stained with rabbit anti-TGF-β antibody at a concentration of 5 μg/ml (R&D Systems, Minneapolis, MN), and anti-collagen l antibody at 1:200 dilution (Cymbus Biotech, Flanders, NJ) by immunoperoxidase and immunofluorescence techniques.25,26
Hydroxyproline Assay
To evaluate fibrosis, lungs were harvested from day 21 BLM-treated rats, homogenized, and assayed for hydroxyproline content as previously described.6
mRNA Analysis
At the indicated time points, animals were sacrificed and the lungs rapidly perfused with sterile PBS via the right ventricle until blanched. The lungs were rapidly dissected out and promptly suspended in guanidine isothiocyanate solution.6–12 Total RNA was isolated from lung tissues or cultured fibroblasts, and the poly(A)-rich fraction purified by oligo (dT) cellulose chromatography as previously described.4,9–12,26–28 Northern hybridization analysis was used to determine and quantify lung fibrogenic cytokine (TGF-β1, MCP-1, TNF-α), procollagens α1 (I), and α1 (III) mRNA, as previously described.8–12 The sequences for the oligonucleotide anti-sense probes are shown in Table 1.4,9,10,12,27–29 The blots were reprobed with a GAPDH probe for use as internal control for normalizing the data. RT-PCR was undertaken as before,29 using primers for TGF-β1, MCP-1, procollagen α1 (I), interleukin (IL)-4, and interferon (IFN)-γ, plus GAPDH as a control (primer sequences shown in Table 2).
Table 1.
Sequences of Rat Anti-Sense Oligonucleotide Probes
| Gene | Sequence | Reference |
|---|---|---|
| TGF-β1 | 5′-GAAGTTGGCATGGTAGCCCTTGGGCTCGTG-3′ | 12, 27, 28 |
| MCP-1 | 5′-AGTGAATGAGTAGCAGGAGGTGTGTGGGG-3′ | 10 |
| TNF-α | 5′-CCTTGAAGAGAACCTGGGAGTAGATA-3′ | 4 |
| Procollagen α1(I) | 5′-AGGGCCAGTCTCAGCACGGTCACCCTTGGC-3′ | 9 |
| Procollagen α1(III) | 5′-GTTCTTGCAGTGGTAGGTGATGTTCTGAGA-3′ | 9 |
| GAPDH | 5′-CACCCTGTTTTTGCTGTAGCCATATTCATTGTC-3′ | 29 |
Table 2.
Sequences of the Amplification Primers
| Gene | Primers | Product size (bp) | Corresponding sequence in mRNA |
|---|---|---|---|
| TGF-β1 | F: 5′-TACAGGGCTTTCGCTTCAGT-3′ | 394 | 1053–1446 |
| R: 5′-TGGTTGTAGAGGGCAAGGAC-3′ | |||
| MCP-1 | F: 5′-TATGCAGGTCTCTGTCACGC-3′ | 244 | 39–282 |
| R: 5′-TTCCTTATTGGGGTCAGCAC-3′ | |||
| IL-4 | F: 5′-CCAGGTCACAGAAAAAGGGA-3′ | 259 | 144–402 |
| R: 5′-CAGTGTTGTGAGCGTGGACT-3′ | |||
| IFN-γ | F: 5′-ACTGCCAAGGCACACTCATT-3′ | 350 | 71–420 |
| R: 5′-TCTGATGAGTTCATTGACCG-3′ | |||
| Procollagen α1(I) | F: 5′-TGCTGCCTTTTCTGTTCCTT-3′ | 179 | 4723–4901 |
| R: 5′-AAGGTGCTGGGTAGGGAAGT-3′ | |||
| GAPDH | F: 5′-ATGGGAAGCTGGTCATCAAC-3′ | 375 | 255–629 |
| R: 5′-CCACACTCTTCTGAGTGGCA-3′ |
F, Forward or sense primer; R, reverse or anti-sense primer.
Plasma Estradiol Assay
Circulating estradiol levels were measured using a commercially available estradiol enzyme-linked immunoassay kit (catalog no. 58225; Cayman Chemical Co., Inc., Ann Arbor, MI) according to the manufacturer’s instructions.
Morphometric Analysis
The number of lung monocytes/macrophages, T lymphocytes, and eosinophils were counted in tissue sections stained with specific antibodies as previously described.12 Positively stained cells were counted by light microscopy using the ×40 objective and an ocular grid. At least 30 randomly chosen, noncontiguous, and nonoverlapping high-power fields were counted in each lung segment. Five segments from each rat lung and a total of three to four rats per group were analyzed. Cell counts were expressed as the number of cells per high-power field. For determination of the number of TGF-β1- and collagen-expressing cells in normal or fibrotic lung fibroblasts treated with estradiol or vehicle, the numbers of positively stained cells as well as the total number of cells were counted from 10 randomly chosen noncontiguous high-power fields. Three slides per group were analyzed and the results were expressed as the percentage of positively stained cells.25
Statistical Analysis
All data were expressed as means ± SE, with n being the number of animals for each group. Differences between mean values from the various treatments and control groups were assessed for statistical significance by analysis of variance, and if significant were followed by analysis using Scheffé’s test for comparison between any two groups.9–12 A P value <0.05 was considered statistically significant.
Results
Effects of Gender on BLM-Induced Morbidity and Mortality
To determine the importance of gender in PF, male and female rats were treated with endotracheal injections of BLM or SAL on day 0. Animals were monitored daily for body weight and mortality rates were compared between male and female rats after BLM administration. Results showed that female rats had greater weight loss when compared to males (30 ± 1.0% versus 20 ± 1.1%) 2 to 3 weeks after BLM injection. Some of the female rats became very sick with severe respiratory distress as the disease progressed, resulting in respiratory failure and death. Females had a mortality rate of 80 ± 5.3%, whereas all male rats survived at 3 weeks after BLM treatment. Thus, female gender appeared to enhance the effects of BLM treatment.
Effect of Gender on Lung Fibrosis
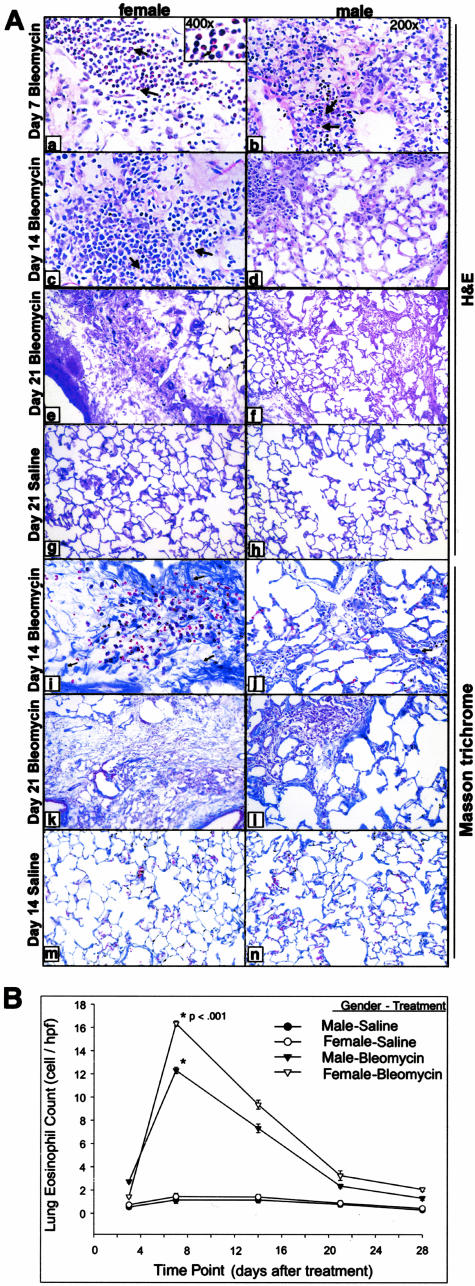
The amplitude of the lung fibrosis induced by BLM was determined by lung histopathology and lung hydroxyproline content. Examination of fixed lung tissue from day 7 treatment after BLM showed inflammation and early fibrotic lesions (Figure 1A). These became more extensive by day 14. Lungs from female rats had significantly larger and more consolidated fibrotic lesions with dramatic increase in overall lung cellularity at days 7 and 14 as compared to those from males. Chromotrope 2R staining revealed larger numbers of eosinophils and greater overall cellularity at day 7 female rats after BLM as compared to that in males (Figure 1A, a and b), with fibrotic lesions becoming more extensive and consolidated in females at days 14 and 21 than were observed in the males (Figure 1A; c to f and i to l). In contrast, none of the control lungs demonstrated significant morphological changes, showing no evidence of inflammation, eosinophil, or other inflammatory cell recruitment in either gender (Figure 1A; g, h, m, and n). The eosinophils were clustered in cellular areas where early fibrosis was evident (Figure 1A, arrows). Morphometric analysis of the lung tissue sections showed that significantly higher numbers of eosinophils in BLM-treated female lung tissue compared to that in the male (Figure 1B). ED-1 monocyte- and macrophage-positive cells were found within the alveolar septa and space and in larger numbers in female rat lung tissues compared to those in male tissues at day 7 after BLM treatment (data not shown). Masson trichrome staining of formalin-fixed tissue revealed greater and denser amounts of collagen deposition in female lung tissue at day 7 after BLM treatment compared to male lungs (data not shown), which became more extensive and apparent at days 14 and 21 in the female lungs, but still significantly less severe in male lungs (Figure 1A, i to l).
Figure 1.
Effects of gender on lung histopathology eosinophil recruitment. Representative chromotrope 2R-stained lung section from day 7, 14, and 21 female (a, c, e) and male (b, d, f) after BLM treatment and corresponding control at day 21 is shown (g, h). At day 7 after BLM, treated female lung sections show greater inflammatory cell infiltration and eosinophil (arrows and inset showing higher magnification) recruitment (a) compared to those in male lungs (b). Fibrotic lesions became more extensive at days 14 and 21 after BLM in female (c, e) than that observed in male rats (d, f). Saline-treated controls showed no sign of inflammation in both genders at the day 21 time point (g, h). A higher magnification view of the cellular areas shows dense clusters of polymorphonuclear cells that stained red with the chromotrope 2R (inset in a). Eosinophils in lung section were counted as described in Materials and Methods and the results are shown in B as the number of cells per high-power field. The values were presented as means ± SE (n = 4 per group). The increases in the number of eosinophils in female relative to respective male in BLM-treated animals were statistically significant (P < 0.001) for day 7. Masson trichrome-stained lung sections revealed greater amounts of collagen deposition in female lungs at days 14 and 21 after BLM treatment compared to males (i–l). In contrast, SAL controls corresponding to female and male rats did not show any abnormal collagen staining (m, n). Original magnification, ×400 (inset in a).
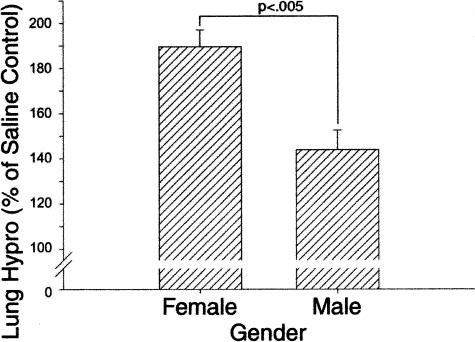
To confirm the morphological evidence of significant gender difference in lung fibrosis, female and male rat lung tissues from the day 21 (after BLM) time point were homogenized and assayed for hydroxyproline content. Consistent with the morphological findings, the results showed that female lungs had a significantly greater BLM-induced increase in total hydroxyproline content relative to that in male lungs (Figure 2). The lung hydroxyproline contents of control female and male rats treated with SAL only were not significantly different. These quantitative results confirmed the increased susceptibility of female rats to develop lung fibrosis in response to BLM-induced lung injury.
Figure 2.
Effects of gender on lung hydroxyproline content. PF was biochemically assessed by measurement of total lung hydroxyproline content on day 21 after BLM treatment. Results in BLM-treated lungs were expressed as a percentage of the corresponding SAL-treated controls, and shown as means ± SE (n = 10) for both female and male experimental groups. Lung hydroxyproline content was significantly higher in BLM-treated female versus male rats (P < 0.005). The lung hydroxyproline contents of male and female SAL-treated control rats were not significantly different.
Effects of Gender on BLM-Induced Lung Collagen Expression
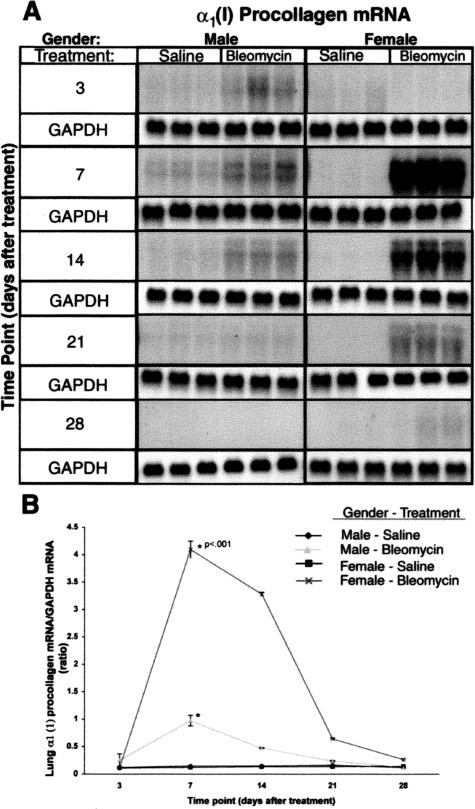
Previous studies have shown that endotracheal BLM administration in male rats causes rapid development of lung fibrosis, characterized by a transiently increased number of contractile, filament-laden parenchymal cells, increased lung collagen synthesis, and deposition.9 Because lung histopathology, collagen staining, and hydroxyproline data indicated greater BLM-induced lung collagen deposition in female relative to male rats, the effect of gender on lung collagen gene expression was examined. The amounts of lung procollagen α1 (I) and α1 (III) mRNA were analyzed by Northern blotting.9 Consistent with the histopathology and hydroxyproline analysis, the results showed higher mRNA levels for procollagens α1 (I) (Figure 3) in lungs of treated females after BLM relative to those in male rats. Furthermore the kinetics of increase were different in female rats versus those in male rats. Procollagen α1 (I) mRNA in lungs of treated female rats after BLM was elevated between days 7 and 14 with subsequent gradual decline toward control levels, whereas the lesser increases in male rats appear to start earlier on day 3 and up to day 14 (Figure 3A). Quantitative analysis of the blots using a radioactivity imaging system and after normalization to the GAPDH signal, indicated peak increases of procollagen α1 (I) in BLM-treated female rats at days 7 and 14, which were significantly higher than those in male rats at the same time points, with a subsequent gradual decline toward control levels in lungs of either gender (Figure 3B). The control SAL-treated groups showed no significant differences between female and male lungs at any of the time points. Control hybridization with a GAPDH probe showed no significant variation in GAPDH mRNA (Figure 3A). A similar result was seen for up-regulation of lung collagen α1 (III) mRNA (data not shown).
Figure 3.
Effect of gender on collagen gene expression. Total lung RNA from control and BLM-treated female and male animals at indicated time points were subjected to Northern blot analysis for determination of procollagen α1 (I) mRNA as described in Materials and Methods. A: Autoradiographs for collagen mRNA and GAPDH are shown. GAPDH mRNA was used to confirm uniform RNA loading and for use as a normalization factor. Each lane represents a RNA sample from a single animal. B: Bands on the autoradiographs were quantitated by an Ambis radioactive-imaging system. The data represent means ± SE of results from three animals at each time point. The increases in procollagen mRNA in female relative to male lungs were statistically significant (P < 0.01) for days 7 and 14 after BLM treatment.
Effects of Gender on BLM-Induced Lung Cytokine Expression
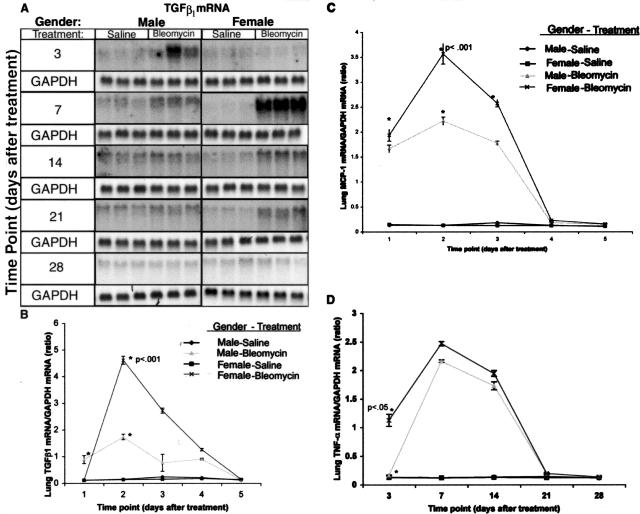
Previous studies have shown that several fibrogenic cytokines such as TGF-β1, MCP-1, and tumor necrosis factor (TNF)-α have potential roles in fibrosis in the lung and other organs.10–12 To quantitate expression of these fibrogenic cytokines, their mRNA levels in both female and male lung tissues were analyzed by Northern blotting. Consistent with the increased fibrosis in female versus male rats, the results showed significantly higher BLM-induced increases in TGF-β1, MCP-1, and TNF-α mRNAs in female versus male lungs (Figure 4). Quantitation of lung cytokine mRNAs showed significant up-regulation of TGF-β1 mRNA in lungs of treated female rats after BLM between days 7 and 14 with peak increase at day 7, whereas up-regulation occurred in male rats between days 3 and 7 (Figure 4, A and B). MCP-1 mRNA was up-regulated between days 3 and 21 with peak increases on day 7 for both sexes, but the elevated levels were significantly higher in female rats versus male rats (Figure 4C). Up-regulation of TNF-α occurred between days 3 and 14 in females, and between days 7 and 14 for males with peak increases on day 7 for both sexes, however the increase in female lungs was only statistically significant at the day 3 time point (Figure 4D). Control animals showed no significant changes in mRNA levels at any of the time points examined. As with the procollagen data, lung GAPDH mRNA levels were not significantly different at all time points in both SAL controls and BLM-treated animals (Figure 4A), and they were used for normalization of the results for the cytokine mRNA determinations.
Figure 4.
Lung cytokine mRNA expression. Total lung RNA from control and BLM-treated female and male rats at the indicated time points was subjected to Northern blot for determination of TGF-β1, MCP-1, and TNF-α mRNAs as described in Materials and Methods. The autoradiographs for TGF-β1 and GAPDH mRNAs are shown in A, with the GAPDH mRNA level being used to document uniform RNA loading and to normalize the cytokine mRNA results. The results of direct quantitation using an Ambis radioactivity imaging system for TGF-β1, MCP-1, and TNF-α mRNA levels are shown in B, C, and D, respectively. Each lane represented an RNA sample from a single animal and the results are shown as means ± SE of three animals at each time point. B: The levels of TGF-β1 mRNA in day 7 BLM-treated female rats were significantly (P < 0.001) higher than those in male rats. C: The increases in MCP-1 mRNA in females relative to respective male were statistically significant (P < 0.001) for days 3, 7, and 14 after BLM treatment. D: TNF-α mRNA was also significantly higher in female versus male lungs (P < 0.01) on day 3 after BLM treatment.
Effects of Ovarian Dysfunction with Exogenous Estradiol Supplementation on BLM-Induced Morbidity and Mortality
Gender studies indicated that in response to BLM-induced lung injury, female rats had increased severity of disease and decreased survival rates compared to male rats. This may be related to the modulating effect of female sex hormones on processes related to the fibrotic process. To clarify such a potential relationship between female sex hormones and lung fibrosis, the effects of OV with or without rescue with exogenous estradiol treatment were examined in female rats. Animals were monitored daily for body weight and mortality rates compared among all groups of rats after BLM administration. The results showed that whereas the ovariectomized rats receiving vehicle (OV + V + BLM) only had zero mortality at day 21 after BLM injection, the estradiol treated ovariectomized (OV + E + BLM) rats showed more severe disease and a 20 ± 1.2% mortality rate. Estradiol supplementation in sham-operated normal (N + E + BLM) rats also showed severe disease and comparable mortality rate. All saline (+SAL)-treated control groups exhibited zero mortality. Thus, estrogen appeared to enhance the effects of BLM treatment.
The Effects of Ovarian Dysfunction with Estradiol Supplementation on Lung Fibrosis
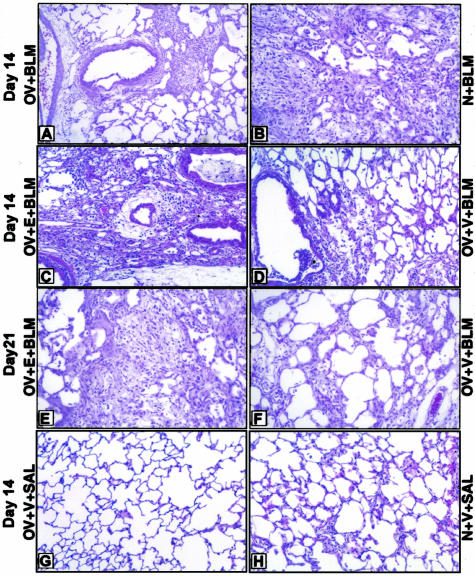
To assess the effects of OV and estradiol on lung fibrosis, histopathology was first assessed (Figure 5). Morphological evaluation on day 14 post-BLM treated lung sections showed a diminished fibrosis and lower numbers of monocytes, macrophages, and eosinophils in ovariectomized (OV + BLM) relative to sham-operated (N + BLM) rats (Figure 5, A and B). This diminution in ovariectomized rats was overcome by estradiol supplementation (OV + E + BLM rats) but not by vehicle (OV + V + BLM rats) as shown in Figure 5, C and D. Control lungs from all saline (+SAL)-treated groups did not demonstrate any significant morphological changes at the same time point (Figure 5, E and F). To appreciate the amplitude of lung injury and inflammation induced by BLM in all groups, the number of inflammatory cells in lung tissue at day 14 after BLM administration was counted morphometrically. ED-1-positive cells (monocytes and macrophages) were found within the alveolar septa and space and were counted as described in Materials and Methods. The results showed that the percentage of macrophages, monocytes, and eosinophils in lungs of ovariectomized rats treated with vehicle only (OV + V + BLM) was significantly lower than in the other BLM-treated groups (Table 3). In contrast, the number of lung CD3-positive T lymphocytes in the OV + V + BLM group was greater than in lungs of rats in the OV + E + BLM and other groups (Table 3). Masson trichrome staining showed reduced collagen deposition in lungs of rats in the OV + V + BLM compared to the OV + E + BLM group (data not shown).
Figure 5.
The effects of ovarian dysfunction on lung histology. H&E-stained lung sections from each treatment group are shown. A: OV + BLM; B: N + BLM; C: OV + E + BLM; D: OV + V + BLM; E: OV + V + SAL; F: N + V + SAL. Lung sections from ovariectomized rats on day 14 after BLM (A) showed a lower numbers of monocytes, macrophages, and eosinophils and less fibrosis compared with corresponding sham-operated group (B). This diminution was overcome by restoration of estradiol levels in ovariectomized BLM-treated group (C, E) relative to corresponding control (D, F). In contrast, control SAL-treated lungs from ovariectomized or normal received vehicle rats did not demonstrate any evidence of inflammation (G and H, respectively). N, normal sham operation; E, 17β-estradiol; V, vehicle.
Table 3.
Pulmonary Cell Populations in Rats at Day 14 after Saline or Bleomycin Treatment
| Group | Monocytes/macrophages (cells/hpf) | Eosinophils (cells/hpf) | T lymphocytes (cells/hpf) |
|---|---|---|---|
| OV + bleomycin | 25 ± 0.47 | 6 ± 0.22 | 11 ± 0.15 |
| N + bleomycin | 40 ± 1.27* | 8 ± 0.32* | 7 ± 0.38* |
| OV + V + bleomycin | 25 ± 0.74 | 6 ± 0.18 | 12 ± 0.29 |
| OV + E + bleomycin | 38 ± 1.10* | 8 ± 0.30* | 8 ± 0.23* |
| OV + V + saline | 3 ± 0.23 | 1 ± 0.15 | 2 ± 0.17 |
| N + V + bleomycin | 39 ± 0.69* | 8 ± 0.20* | 8 ± 0.23* |
| N + E + bleomycin | 41 ± 1.09* | 8 ± 0.15* | 8 ± 0.22* |
| N + V + saline | 4 ± 0.24 | 1 ± 0.09 | 2 ± 0.12 |
OV, ovariectomy; N, normal sham-operated group; V, vehicle; E, estadiol.
Significant differences P < 0.01 in values measured in bleomycin-treated group compared to the OV + V + bleomycin.
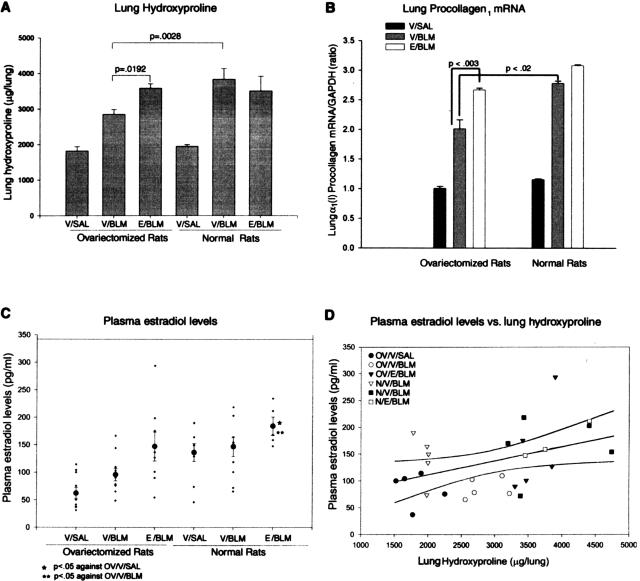
To obtain quantitative biochemical confirmation, hydroxyproline analysis of lung homogenates from all groups at day 21 after BLM challenge was undertaken. The results showed that the total lung hydroxyproline content of rats in the OV + V + BLM group was significantly lower than that in the OV + E + BLM and N + V + BLM groups (Figure 6A). This reduction in fibrosis in OV + V + BLM rats was accompanied by significantly reduced lung α1(1) procollagen mRNA levels at the day 14 time point when compared with the OV + E + BLM and N + V + BLM groups (Figure 6B). These results were consistent with the histological findings, thus confirming the role of the ovary and estradiol in the increased susceptibility of female rats to develop lung fibrosis in response to BLM.
Figure 6.
Effects on lung collagen and plasma estradiol. The effects of OV with or without estradiol replacement therapy are shown for lung hydroxyproline content on day 21 after BLM (A), lung α1(1) procollagen mRNA on day 14 after BLM (B), plasma estradiol level on day 21 after BLM (C), and comparison of plasma estradiol levels with lung hydroxyproline content in all groups (D). Lung hydroxyproline content, procollagen I mRNA, and plasma estradiol levels from normal and ovariectomized rats that received estradiol (E) or vehicle (V) and treated with BLM or SAL are shown as means ± SE (n = 5). A: Lung hydroxyproline content was significantly higher in ovariectomized and sham-operated rats that received estradiol and BLM (E/BLM) than in ovariectomized rats that received vehicle and BLM (V/BLM). Whole-lung RNA was isolated from all groups of rats at day 14 and analyzed for α1(1) procollagen and GAPDH (for normalization) mRNA by RT-PCR as described in Materials and Methods. The results were normalized to the GAPDH mRNA signal and expressed as the ratio of α1(1) procollagen to GAPDH mRNA signals. Means ± SE from three animals in each group are shown. B: Significantly higher α1(1) procollagen mRNA levels were seen as a result of BLM and estradiol treatment (E/BLM) in ovariectomized and normal rats compared to that in ovariectomized rats that received vehicle (V/BLM). Three weeks after BLM challenge, blood was collected and plasma estradiol levels measured by enzyme-linked immunosorbent assay. C: Results showed a significant decrease in plasma estradiol levels at 3 weeks after OV (V/BLM) with return to normal levels after estradiol (E/BLM) replacement therapy. D: Linear regression analysis was attempted to see if plasma estradiol levels would correlate with lung hydroxyproline content. The regression line (r = 0.405, P = 0.029) is shown with boundary lines indicating the 95% confidence intervals. Although the correlation was relatively weak, it was statistically significant.
Effect of OV with Estradiol Supplementation on Plasma Estradiol Level
On day 21 after OV, 3 weeks after BLM challenge, blood was collected and plasma estradiol levels were measured by enzyme-linked immunosorbent assay. Results showed a decrease in plasma estradiol levels after OV with return toward normal levels after implantation of mini osmotic pumps containing estradiol but not vehicle only (Figure 6C). Higher plasma estradiol levels in BLM-treated rats also showed higher lung hydroxyproline content (Figure 6D) and associated with higher mortality and morbidity rate.
The Effects of Ovarian Dysfunction with Estradiol Supplementation on BLM-Induced Lung Cytokine Expression
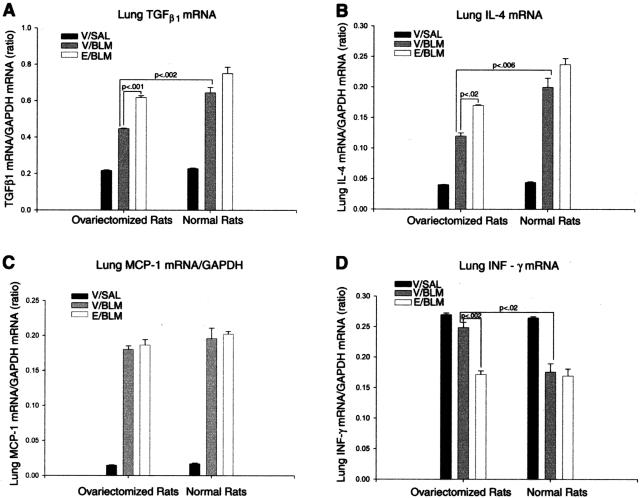
To identify relationships between estradiol and the ability to enhance expression of inflammatory and fibrogenic cytokines, lung TGF-β1, IL-4, MCP-1, and IFN-γ mRNA levels were examined by RT-PCR. The results indicated that there was a significantly reduced expression of TGF-β1 and IL-4 mRNA in ovariectomized (OV + V + BLM) rats relative to sham-operated controls (N + V + BLM) at day 14 after BLM treatment (Figure 7, A and B). This reduction in TGF-β1 and IL-4 mRNA expression in ovariectomized rat lungs was negated by estradiol supplementation (OV + E + BLM group), which was significantly higher than the levels in lungs of ovariectomized rats receiving vehicle only (OV + V + BLM group). However the expression of MCP-1 was not significantly affected by OV with or without estradiol treatment (Figure 7C). In contrast to the effects on TGF-β1 and IL-4 expression, IFN-γ mRNA levels were increased by OV, which was reversed by estradiol supplementation (Figure 7D). Thus these results suggest that the female gender via estradiol can enhance the response to BLM-induced lung injury by enhancing the production of type 2 cytokines and collagen synthesis, but reducing the expression of IFN-γ.
Figure 7.
The effects of ovarian dysfunction on BLM lung cytokine expression. Lung TGF-β1 (A), IL-4 (B), MCP-1 (C), and IFN-γ (D) mRNAs were examined by RT-PCR analysis as described in the legend to Figure 6. A, B: At day 14 after BLM treatment the levels of TGF-β1 (P < 0.001) and IL-4 (P < 0.02) mRNAs were significantly higher as a result of estradiol treatment (E/BLM) in both ovariectomized and normal rats compared to those in ovariectomized rats receiving vehicle (V/BLM) only. C: However the expression of MCP-1 was not significantly affected by estradiol treatment. D: Whereas the expression of IFN-γ mRNA was significantly lower (P < 0.002) in ovariectomized rats receiving estradiol (E/BLM) and in normal rats receiving vehicle (V/BLM) relative to that in ovariectomized rats treated with vehicle (V/BLM). Estradiol had no significant effect in normal rats (V/BLM versus E/BLM).
Effects of Estradiol on Fibrotic Lung Fibroblasts in Vitro
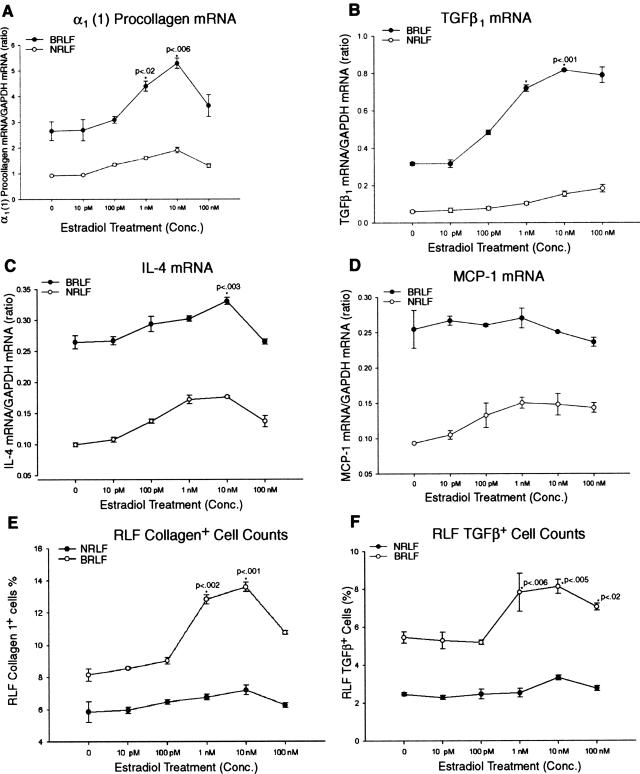
In view of the in vivo importance of estradiol in affecting lung fibrosis, its ability to directly affect fibroblast function in vitro was examined. Rat lung fibroblasts isolated from BLM-treated (BRLF) and control (NRLF) rats were exposed to various concentrations of either estradiol or vehicle. The cells were then analyzed for collagen and cytokine mRNAs by RT-PCR. Estradiol treatment caused a dose-dependent increase in procollagen α1 (I) mRNA levels in BRLFs that peaked at 10 nmol/L (Figure 8A). Only slight increases in procollagen mRNA were noted in NRLFs, and these were significantly lower than those seen in BRLFs. This difference in responsiveness was also observed with respect to TGF-β1 and IL-4 mRNA levels. Thus relative to untreated control cells, estradiol treatment on BRLF caused a dose-dependent increase in these mRNA species that were significantly higher than the levels seen in similarly treated NRLFs (Figure 8, B and C). However, the expression of MCP-1 was not significantly affected by estradiol treatment in both BRLFs and NRLFs (Figure 8D). To further evaluate the in vitro expression of TGF-β and collagen type 1, the effects of estradiol on lung fibroblasts were evaluated by immunocytochemistry. The results showed that estradiol-treated BRLFs expressed a significantly higher number of cells expressing TGF-β, and collagen type 1 compared with untreated control BRLFs (Figure 8, E and F), consistent with the higher expression of procollagen α1(1) and TGF-β1 mRNA in estradiol-treated BRLFs. Thus estradiol appears to have a selective profibrogenic effect on isolated lung fibroblasts. These results demonstrated that estradiol could directly activate selective lung fibroblast functions and this may provide a mechanistic basis as to how female sex hormones may represent a risk factor for the development of more severe PF.
Figure 8.
Effects of estradiol on fibrotic lung fibroblasts in vitro. Rat lung fibroblasts from BLM -treated (BRLF) and normal (NRLF) rats were isolated and treated with various concentration of estradiol. The cells were harvested for analysis of procollagen α1 (I) (A), TGF-β1 (B), IL-4 (C), and MCP-1 (D) mRNAs by RT-PCR. The procollagen, TGF-β1, and IL-4 mRNAs gradually increased after treatment with increasing doses of estradiol, with significant increases at concentrations of 1 and 10 nmol/L (only at 10 nmol/L for IL-4) in BRLF compared untreated control BRLF. D: The expression of MCP-1 was not significantly affected by estradiol treatment. Collagen type 1 and TGF-β1 expression was also assessed by immunohistochemistry and expressed as a percentage of total cells positively stained by the respective anti-collagen (E) or TGF-β1 (F) antibodies.
Discussion
There is evidence to suggest that gender affects the severity of lung disease, such as IPF and asthma.14–17 Several reports support this speculation that the female gender may represent a risk factor for development of more severe respiratory or other fibrotic diseases.30 For example there is a clear-cut gender-based difference in the prevalence and natural history of asthma, a fibrotic disease of the airways. Previous studies show that girls with asthma have poorer lung function,31 relapse more frequently after emergency room visits,32 and have a poorer prognosis than boys.31,32 Recent studies also indicate that the hospital admission rate is consistently higher among women 25 to 55 years of age and that cough sensitivity is also heightened in females.33–35 A study of 13,651 asthmatic patients showed that the disease pattern was related to sex and age.36 Males dominate the incidence of asthma until age of 14, but from 14 to 17 years of age, the pattern changes such that by age 17 females outnumber the male incidence. At age 50, the gap begins to narrow, such that at age 60 gender-based differences are few, probably because of the reduced sex hormone production after menopause. This observation suggests that hormones may influence asthma, and thus may affect disease outcome.36 The proposal that asthmatic airway wall thickening may be a fibrotic response also supports the notion that gender may play a role in lung fibrosis.37 In agreement with these results, recent studies have further documented that the incidence of focal sclerosing glomerulonephritis is higher in women than men.30 In contrast another study shows that men with IPF have a decreased survival rate than women.38 However, this study was based on survival analysis of patients with this disease, without controlling for factors such as the stage or severity of disease, occupation, age (with respect to menopause), or average life expectancy differences among men and women. Only 38% of the study population is female, and the average age was 64 years (postmenopausal age). Because the study population contains postmenopausal patients, the potential role of sex hormones, especially estrogen, cannot be evaluated. There could potentially be confounding factors related to the use of hormone replacement therapy, but that is not addressed in these studies as well. Similarly while a study using a different model of PF in hamsters found enhanced susceptibility to fibrosis in females,13 another revealed no major gender-related differences in the bronchiolar epithelia metaplasia in rats in response to chronic inhalation of ozone.39
With regard to the influence of ovarian hormones on disease progression, several reports support the claim that female reproductive hormones are involved in altered immune response leading to diseases such as systemic lupus erythematosus (SLE) and primary pulmonary hypertension. SLE, which primarily affects young women, involves the respiratory system more commonly than does any other collagen vascular disease and it may affect virtually all components of the respiratory system, including the upper airway, lung parenchyma, pulmonary vasculature, pleura, and respiratory muscle.40 A recent study found that lupus patients with a longer duration of prednisone therapy were more likely to develop carotid arteriosclerosis.41 Another disease typically affecting young women, primary idiopathic pulmonary hypertension may also have an autoimmune basis that is influenced by host immunogenetics.42 Moreover, there is evidence to suggest that tamoxifen, a synthetic nonsteroidal antiestrogen, may be effective in the treatment of idiopathic retroperitoneal fibrosis.43 One may speculate from this that estrogen, which is gender-specific, may have a significant influence on fibrosis and/or the inflammation that often accompanies the fibrotic response. However ovarian dysfunction that enhanced the formation of granuloma formation in rats has been reported.44 Thus reports on the role of gender on PF are few and controversial, and there are no reports on the influence of gender or sex hormone on BLM-IPF.
This study attempts to meet that need by examining for possible gender-based differences in a model of BLM-induced lung injury and fibrosis. Using adult female and male rats, the results indicated that relative to male rats, female rats had greater morbidity and mortality in their response to BLM. Additionally lungs of female rats exhibited more severe and extensive interstitial fibrosis than those of male rats according to histopathological examination. Furthermore, female lung tissue showed greater degrees of lung inflammation and fibrosis that were accompanied by a higher degree of lung collagen deposition as assessed by lung hydroxyproline content. This enhanced fibrotic response in female rats was also reflected in the higher mRNA levels for procollagens α1 (I), α1 (III), as well as by greater increases in fibrogenic cytokine expression. Taken together, these findings provide support for the conclusion that there are significant gender-dependent differences in the response to lung injury, and that the female gender may represent a risk factor for the development of more severe PF. The mechanism for this observed difference might involve genetic, immune system, or hormonal factors, or some combination of these factors. Based on the gender differences, the female sex hormone is a likely factor to influence the severity of disease and decreased survival rates. The mechanism may be related to the modulating effect of estrogen hormone on the inflammatory or immune response (eg, via activation of mononuclear phagocytes, eosinophils, and/or T lymphocytes), or more directly, on fibroblast function. Activated inflammatory cells and/or fibroblasts could then release fibrogenic cytokines (TGF-β1, TNF-α, IL-4), resulting in recruitment and activation of fibroblasts/myofibroblasts with consequent collagen deposition and development of severe and progressive fibrosis.
To evaluate this potential role of estrogen, the effects of OV with or without estradiol replacement therapy were examined using the BLM model. The results revealed that OV caused a significant reduction in the response to BLM, in terms of morbidity, mortality, lung cytokine expression, and fibrosis. This reduction in responsiveness to BLM could be reversed by estradiol replacement therapy, thus suggesting that female rats were more responsive to BLM than males, primarily because of the higher level of estrogen production by the former. Moreover there was some correlation between the plasma estradiol levels and the degree of fibrosis. Interestingly, the effect of estradiol is to promote monocyte/macrophage and eosinophil influx, while suppressing T-cell influx. These effects on the inflammatory/immune response were reflected as well in the expression of certain cytokines. Thus OV blunted the BLM-induced lung IL-4 and TGF-β expression while suppressing IFN-γ expression. These were all reversed by estradiol replacement therapy. The suppressive effect of estradiol on IFN-γ expression is consistent with the anti-fibrotic activity of this cytokine.45–49 Thus decreased IFN-γ expression by estradiol is expected to enhance fibrosis, which was observed in these series of experiments. Taken together these findings suggest that the female gender via estrogen was able to enhance PF, at least in part by altering the inflammatory/immune responses, perhaps by modulating cytokine expression.
Finally, possible effects of estrogen on fibroblast function were evaluated to see if an additional mechanism may be mediated directly in this manner. Fibroblasts from BLM-treated rats exhibited an altered phenotype as manifested by increased responsiveness to estradiol treatment, causing dose-dependent increases in procollagen 1, IL-4, and TGF-β1 mRNA expression relative to untreated controls. Interestingly and consistent with the in vivo data estradiol did not significantly affect MCP-1 expression in these cells, indicating that the effect of estradiol is selective. These in vitro findings suggest an additional mechanism as to how female sex hormones may represent a risk factor for development of more severe lung fibrosis. Further studies may enable the development of new therapeutic modalities for female patients.
Acknowledgments
We thank Dr. Stephen J. Weiss for critical review of the manuscript; and Bridget McGarry, Matthew Ullenbruch, Lisa K. Riggs, and Elizabeth A. Horn for excellent technical assistance.
Footnotes
Address reprint requests to Dr. Mehrnaz Gharaee-Kermani, 5259 LSI, Department of Internal Medicine, University of Michigan Medical School, 210 Washtenaw, Box 2216, Ann Arbor, MI 48109-2216. E-mail: nazy@umich.edu.
Supported by the American Lung Association of Michigan (grant to M.G.-K.) and the National Institutes of Health (grants HL28737, HL31963, and HL52285 to S.H.P.).
References
- Hiwatari N, Shimura S, Sasaki T, Aikawa T, Ando Y, Ishihara H, Seskizawa K, Sasakima H, Takishima T. Prognosis of idiopathic pulmonary fibrosis in patients with mucous hypersecretion. Am Rev Respir Dis. 1991;143:182–185. doi: 10.1164/ajrccm/143.1.182. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Etzel RA, Parrish RG. Pulmonary fibrosis deaths in the United States, 1979–1991; an analysis of multiple-cause mortality data. Am J Respir Crit Care Med. 1996;153:1548–1552. doi: 10.1164/ajrccm.153.5.8630600. [DOI] [PubMed] [Google Scholar]
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Collart MA, Grau GE, Sappino AP, Vassalli P. Requirement of tumor necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245–247. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Phan SH. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Dis. 2001;7:1083–1103. doi: 10.2174/1381612013397573. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Nozaki Y, Hatano K, Phan SH. Lung interlukin-4 gene expression in a murine model of bleomycin-induced pulmonary fibrosis. Cytokine. 2001;15:138–147. doi: 10.1006/cyto.2001.0903. [DOI] [PubMed] [Google Scholar]
- Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of interleukin-4 (IL-4) in lung injury and fibrosis. J Immunol. 2003;70:2083–2092. doi: 10.4049/jimmunol.170.4.2083. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Phan SH. Lung interleukin-5 expression in murine bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1997;16:438–447. doi: 10.1165/ajrcmb.16.4.9115755. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gharaee-Kermani M, McGarry B, Phan SH. In situ hybridization analysis of rat lung al (I), and a2 (I) collagen gene expression in pulmonary fibrosis induced by endotracheal bleomycin injection. Lab Invest. 1994;70:192–202. [PubMed] [Google Scholar]
- Zhang K, Gharaee-Kermani M, Jones ML, Warren JS, Phan SH. Lung monocyte chemoattractant protein-1 gene expression in bleomycin-induced pulmonary fibrosis. J Immunol. 1994;153:4733–4741. [PubMed] [Google Scholar]
- Zhang K, Gharaee-Kermani M, McGarry B, Remick D, Phan SH. TNF-α mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J Immunol. 1997;158:954–959. [PubMed] [Google Scholar]
- Zhang K, Flanders KC, Phan SH. Cellular localization of transforming growth factor-β expression in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1995;147:352–361. [PMC free article] [PubMed] [Google Scholar]
- Kimura R, Hu H, Stein-Streilein J. Delayed-type hypersensitivity responses regulate collagen deposition in the lung. Immunology. 1992;77:550–555. [PMC free article] [PubMed] [Google Scholar]
- Barzo P. Familial idiopathic fibrosing alveolitis. Eur J Respir Dis. 1985;66:350–352. [PubMed] [Google Scholar]
- Wockel W, Sultz J. Diffuse pulmonary fibrosis and Hermansky-Pudlak syndrome. Thorax. 1995;50:591–591. doi: 10.1136/thx.50.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey M, Farewell J. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol. 1996;143:1007–1017. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- Jarvelainen HA, Lukkari TA, Heinaro S, Sippel H, Lindros KO. The antiestrogen toremifene protects against alcoholic liver injury in female rats. J Hepatol. 2001;35:46–52. doi: 10.1016/s0168-8278(01)00050-2. [DOI] [PubMed] [Google Scholar]
- Tadzhiev FS, Kokosov AN, Il’kovich MM, Syromiatnikova NV. Steroid hormone and trace elements content of the blood in patients with disseminated processes of the lungs. Probl Tuberk. 1989;11:6–9. [PubMed] [Google Scholar]
- Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MWH. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155:1137–1146. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette CL. Sex hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate. 1988;12:271–286. doi: 10.1002/pros.2990120310. [DOI] [PubMed] [Google Scholar]
- Ahmed SA, Penhale WJ, Tatal N. Sex hormones, immune responses, and autoimmune diseases. Am J Pathol. 1985;121:531–551. [PMC free article] [PubMed] [Google Scholar]
- Oursler MJ, Cortese C, Keeting P, Anderson MA, Bonde SK, Riggs BL, Spelsberg TC. Modulation of transforming growth factor-β production in normal human osteoblast-like cells by 17β-estradiol and parathyroid hormone. Endocrinology. 1991;129:3313–3320. doi: 10.1210/endo-129-6-3313. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Witchell CM, Metcalfe JC. Tamoxifen elevates transforming growth factor-β and suppresses diet-induced formation of lipid lesions in mouse aorta. Nat Med. 1995;1:1067–1073. doi: 10.1038/nm1095-1067. [DOI] [PubMed] [Google Scholar]
- Douglas JG. Estrogen effects on angiotensin receptors are modulated by pituitary in female rats. Am J Physiol. 1987;252:57–62. doi: 10.1152/ajpendo.1987.252.1.E57. [DOI] [PubMed] [Google Scholar]
- Zhang H-Y, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast α-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148:352–361. [PMC free article] [PubMed] [Google Scholar]
- Gharaee-Kermani M, McGarry B, Lukacs N, Huffnagle G, Egan RW, Phan SH. The role of IL-5 in bleomycin-induced pulmonary fibrosis. J Leukocyte Biol. 1998;64:657–666. doi: 10.1002/jlb.64.5.657. [DOI] [PubMed] [Google Scholar]
- Phan SH, Gharaee-Kermani M, Wolber F, Ryan US. Stimulation of rat endothelial cell transforming growth factor-β production by bleomycin. J Clin Invest. 1991;87:148–154. doi: 10.1172/JCI114964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor β1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779–17784. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- Tso JY, Sun HO, Kao YH, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. J Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrmann M, Bohle A. The long-term prognosis of benign nephrosclerosis accompanied by focal glomerosclerosis and renal cortical interstitial fibrosis designated so-called decompensated benign nephrosclerosis. Pathol Res Practice. 1998;194:571–576. doi: 10.1016/S0344-0338(98)80047-2. [DOI] [PubMed] [Google Scholar]
- Weiss ST, Segal MR, Tager IB, Tosteson TD, Redline S, Speizer FE. Effects of asthma on pulmonary function in children. A longitudinal population-based study. Am Rev Respir Dis. 1992;145:58–64. doi: 10.1164/ajrccm/145.1.58. [DOI] [PubMed] [Google Scholar]
- Butz AM, Eggleston P, Alexander C, Rosenstein BJ. Outcomes of emergency room treatment of children with asthma. J Asthma. 1991;28:255–264. doi: 10.3109/02770909109073382. [DOI] [PubMed] [Google Scholar]
- Harju T, Keistinen T, Tuuponen T, Kivela SL. Hospital admissions of asthmatics by age and sex. Allergy. 1996;51:693–696. [PubMed] [Google Scholar]
- Gold DR, Wypij D, Wang X, Speizer EF, Pugh M, Ware JH, Ferris BG, Jr, Dockery DW. Gender-and-race-specific effects of asthma and wheeze on level and growth of lung function in children in six U.S. cities. Am J Respir Crit Care Med. 1994;149:1198–1208. doi: 10.1164/ajrccm.149.5.8173760. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Kasahara K, Kamio Y, Naruse M, Hashimoto T, Matsuda T. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J. 1996;9:1624–1626. doi: 10.1183/09031936.96.09081624. [DOI] [PubMed] [Google Scholar]
- Crawford WA, Beedham CG. The changing demographic pattern in asthma related to sex and age. Med J Aust. 1976;1:430–434. [PubMed] [Google Scholar]
- Minshall EM, Leung DYM, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGFβ1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Helmers RA, Galvin JR, Van Fossen DS, Frees KL, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:450–454. doi: 10.1164/ajrccm.149.2.8306044. [DOI] [PubMed] [Google Scholar]
- Stockstill BL, Chang L-Y, Menache MG, Mellick PW, Mercer RR, Crapo JD. Bronchiolarized metaplasia and interstitial fibrosis in rat lungs chronically exposed to high ambient levels of ozone. Toxicol Appl Pharmacol. 1995;134:251–263. doi: 10.1006/taap.1995.1191. [DOI] [PubMed] [Google Scholar]
- Murin S, Weidemann HP, Matthay RA. Pulmonary manifestations of systemic lupus erythematosus. Clin Chest Med. 1998;19:641–665. doi: 10.1016/s0272-5231(05)70108-8. [DOI] [PubMed] [Google Scholar]
- Manzi S, Selzer F, Sutton-Tyrrell K, Fitzgerald SG, Rairie JE, Tracy RP, Kuller LH. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Asherson RA, Harris EN, Bernstein RM, Mackworth-Young CG, Hughes GR. Immunological studies in primary idiopathic pulmonary hypertension. Eur J Rheumatol Inflamm. 1984;7:75–79. [PubMed] [Google Scholar]
- Allendorff J, Riegel W, Kohler H. Regression of retroperitoneal fibrosis by combination therapy with tamoxifen and steroids. Med Klin. 1997;92:439–443. doi: 10.1007/BF03042577. [DOI] [PubMed] [Google Scholar]
- Shirai M, Sato A, Chida K. The influence of ovarian hormones on the granulomatous inflammatory process in the rat lung. Eur Respir J. 1995;8:272–277. doi: 10.1183/09031936.95.08020272. [DOI] [PubMed] [Google Scholar]
- Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res. 1995;21:791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signaling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341:1264–1269. doi: 10.1056/NEJM199910213411703. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, Gerard C, Noble PW. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB, Rose NR. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]