Abstract
Apolipoprotein E (apoE) alleles determine the age-adjusted relative risk (ɛ4 > ɛ3) for Alzheimer's disease (AD). ApoE may affect AD pathogenesis by promoting deposition of the amyloid-β (Aβ) peptide and its conversion to a fibrillar form. To determine the effect of apoE on Aβ deposition and AD pathology, we compared APPV717F transgenic (TG) mice expressing mouse, human, or no apoE (apoE−/−). A severe, plaque-associated neuritic dystrophy developed in APPV717F TG mice expressing mouse or human apoE. Though significant levels of Aβ deposition also occurred in APPV717F TG, apoE−/− mice, neuritic degeneration was virtually absent. Expression of apoE3 and apoE4 in APPV717F TG, apoE−/− mice resulted in fibrillar Aβ deposits and neuritic plaques by 15 months of age and substantially (>10-fold) more fibrillar deposits were observed in apoE4-expressing APPV717F TG mice. Our data demonstrate a critical and isoform-specific role for apoE in neuritic plaque formation, a pathological hallmark of AD.
Multiple lines of evidence suggest that the deposition of amyloid-β (Aβ) peptides is an early pathogenic event in Alzheimer's disease (AD) that initiates a cascade of changes ultimately resulting in neuronal dysfunction, neurodegeneration, and eventual death (1). Conversion of Aβ from a soluble to an aggregated, insoluble form(s) with a β-sheet conformation may be central to its accumulation and possibly for its detrimental effects (2). The formation of a prominent neuritic dystrophy (e.g., neuritic plaques) is likely to account for a significant amount of neuronal and accompanying cognitive dysfunction in AD (3). Whether, how, and what form of Aβ causes this prominent neuritic dystrophy is unclear. Understanding the pathogenesis of neuritic degeneration and its relationship to Aβ deposition and aggregation may allow for development of preventive treatments.
Transgenic (TG) mice that develop age- and region-dependent Aβ deposition have provided a major advance in AD research (4, 5). These mice allow for the study of both disease pathogenesis and potential treatment strategies targeted at Aβ deposition and fibrillogenesis as well as their consequences such as neuritic degeneration (4–7). One protein that may play a role in Aβ deposition and neuritic degeneration is apolipoprotein E (apoE). We observed a severe, plaque-associated neuritic dystrophy in APPV717F TG mice with most fibrillar Aβ deposits surrounded by both large and fine dystrophic neurites. Importantly, we found that apoE is required for the extensive, plaque-associated neuritic degeneration. In APPV717F TG, apoE−/− mice, extensive, nonfibrillar Aβ deposits developed; however, Aβ-associated neuritic degeneration almost never was observed. Astrocyte-specific expression of human apoE3 and E4 in APPV717F TG, apoE−/− mice ultimately restored fibrillar Aβ deposition by 15 months of age, with expression of apoE4 having a markedly greater effect on neuritic plaque formation than apoE3. Our data strongly suggest a critical and isoform-specific role of apoE in influencing Aβ deposition and structure in vivo. Moreover, apoE appears to be required for the progressive development of Aβ-associated neuritic degeneration.
Materials and Methods
Animals and Tissue Preparation.
APPV717F+/+, apoE−/−, and APPV717F +/+, apoE+/+ mice (8) on an outbred background [(Swiss Webster × C57BL/6 × DBA/2) × C57BL/6] were utilized for breeding in these experiments. APPV717F +/+, apoE−/− mice were bred with apoE−/− animals on a C57BL/6 background (The Jackson Laboratory), and APPV717F +/+, apoE+/+ were bred with C57BL/6 mice (The Jackson Laboratory). F1 progeny from each of these breedings were compared with each other. APPV717F +/+, apoE−/− were also crossed with both glial fibrillary acidic protein (GFAP)-apoE3+/− (line 37) and GFAP-apoE4+/− (lines 22 and 11) TG mice (9, 10). F1 progeny of this cross were compared with each other. The GFAP-apoE mice had been backcrossed four generations to apoE−/− mice on a C57BL/6 background (The Jackson Laboratory). Animals were screened for the presence of the APPV717F and GFAP-apoE transgenes by PCR as described (8, 9). Tissue analysis was performed exactly as described (10).
Histological Analysis.
Tissue sections were cut in the coronal plane at 40 μm on a freezing sliding microtome from the genu of the corpus callosum through the caudal extent of the hippocampus. For qualitative analysis of Aβ immunoreactivity (IR), sections were immunostained as described (10). Thioflavine-S staining was performed as described (8). The de Olmos silver stain was performed as described (11). In experiments in which sections were stained with the de Olmos silver method as well as with thioflavine-S, the silver staining was performed first. Quantitative analysis of Aβ deposits was assessed with unbiased stereology as described (10). To determine the density of neuritic plaques, three equally spaced coronal sections from the rostral to the caudal extent of the hippocampus were stained with the de Olmos silver method. The number of neuritic plaques was counted in these sections under ×100 magnification. The area of the hippocampus in these sections was determined by utilizing a charge-coupled device camera linked to a computer screen with nih image 1.57. To determine the density of thioflavine-S-positive plaques in APPV717F +/−, apoE3, or apoE4+/− mice, the number of thioflavine-S-positive-stained deposits was counted in a 0.015-mm2 area in the superior blade of the molecular layer of the dentate gyrus in each of three equally spaced sections from the rostral to caudal extent of the hippocampus in each animal.
Biochemical Analysis.
ApoE, amyloid precursor protein (APP), and lipoprotein receptor-related protein (LRP) levels in brain tissue were determined by semiquantitative Western blotting as described (10). Antibody to LRP was a gift of J. Herz (Univ. of Texas, Southwestern). Aβ ELISA was performed as described (10).
Statistical Analysis.
Quantitative data are presented as mean ± SEM and analyzed by t test with significance set at P < 0.05.
Results
Severe Neuritic Pathology Is Present in APPV717F Mice.
Immunohistochemistry with antibodies to certain neuronal markers as well as electron microscopy have revealed evidence of swollen, distorted neurites associated with Aβ deposits in the APPV717F TG mouse brain (6). We analyzed APPV717F TG mice hemizygous (+/−) for the transgene stained with the de Olmos silver degeneration method (11) as well as several other silver stains routinely used in the diagnosis of AD. We found that by 12 months of age, numerous neuritic plaques were detected in the hippocampus and neocortex (Figs. 1 and 2). The de Olmos method was much more sensitive for detecting the marked neuritic dystrophy present in APPV717F TG mice. It was particularly useful at identifying large, distended, plaque-associated dystrophic neurites but also detected dystrophic neurites of smaller caliber (Figs. 1 and 2). Neuritic plaque density increased with age in APPV717F +/− TG mice. In the hippocampus, there were, on average, 7.6 neuritic plaques/mm2 at 12 months of age, whereas at 15 months, there were 23 neuritic plaques/mm2 (Fig. 4A). This markedly abnormal neuritic dystrophy was not observed in age-matched control, non-TG mice of the same or of other genetic backgrounds (data not shown).
Figure 1.
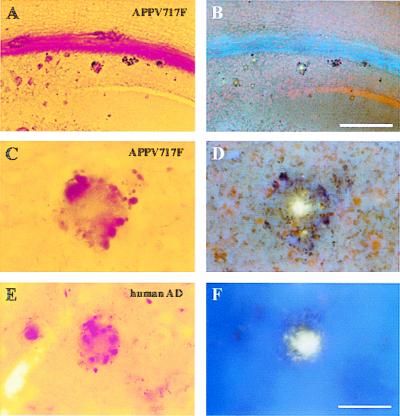
Neuritic plaques in APPV717F TG mice are thioflavine-S-positive. A section containing the hippocampus from a 12-month-old APPV717F +/− TG mouse [low power (A and B); high power (C and D)] and the temporal neocortex of an AD brain [high power (E and F)] were stained with the de Olmos silver degeneration method (A, C, and E), and the same sections were counterstained with thioflavine-S and visualized via epifluorescence microscopy with a UV filter (B, D, and F). Most of the neuritic plaques stained with this method had a thioflavine-S-positive core. [Bar in B = 100 μm (A and B); bar in F = 17 μm (C–F).]
Figure 2.
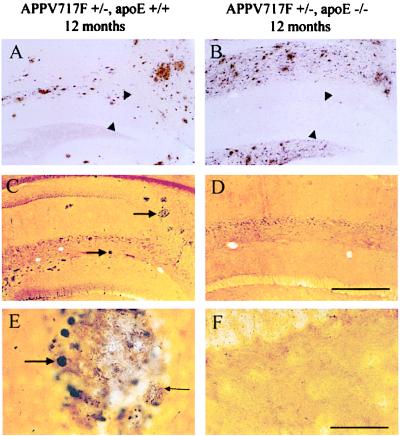
Aβ deposition and neuritic plaques in APPV717F TG mice in the presence and absence of apoE. Sections of the hippocampus from APPV717F +/−, apoE+/+ (A, C, and E) and APPV717F +/−, apoE−/− mice (B, D, and E) at 12 months of age were examined for Aβ IR (A and B) and the de Olmos silver degeneration method (C–F). Aβ-IR deposits were observed in the hippocampus of APPV717F TG mice in the presence (A) and absence (B) of apoE; however, the distribution of Aβ deposits within the hippocampus was different. The area between arrowheads in A and B is the molecular layer of the dentate gyrus. Both low- (C and D) and higher-power (E and F) photomicrographs within the hippocampus reveal the presence of numerous neuritic plaques only in the presence (C and E) as opposed to in the absence (D and F) of apoE. Arrows in C point to neuritic plaques. Large arrow in E points to a large, distended, dystrophic neurite in a plaque, and small arrow in E points to fine dystrophic neurites. [Bar in D = 180 μm (A–D); bar in F = 30 μm (E and F).]
Figure 4.
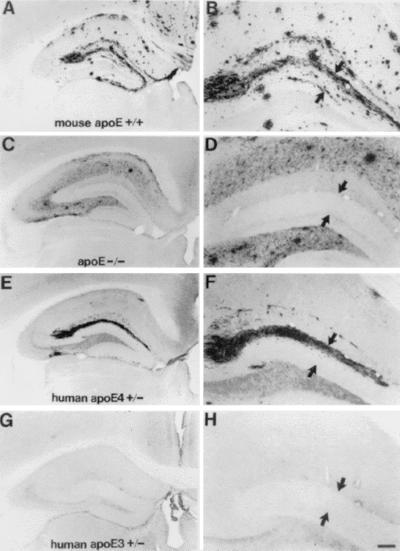
Human apoE restores the pattern of Aβ-IR deposits in the hippocampus of APPV717F +/− TG mice to that seen in animals expressing mouse apoE. Photomicrographs are shown of Aβ-IR deposits representative of the median values of their respective groups from APPV717F +/− TG mice either apoE+/+ (A and B), apoE−/− (C and D), apoE4+/− (E and F), or apoE3+/− (G and H) at 15 months of age. Although all mice expressing mouse apoE and some expressing human apoE at this age had Aβ-IR deposits in the molecular layer of the dentate gyrus (between arrowheads in B, D, F, and H), in the absence of apoE, Aβ-IR deposits were never observed in this layer. Significantly more E4-expressing (eight of nine, 89%) as compared with E3-expressing (two of six, 33%) mice developed Aβ deposition in the molecular layer. [Bar in H = 150 μm (A, C, E, and G) and 60 μm (B, D, F, and H).]
Neuritic Plaque Formation Requires apoE Expression.
To explore further the relationship of the neuritic plaque formation to Aβ deposition, we performed double labeling of both APPV717F TG and AD brain with the de Olmos silver stain and thioflavine-S. In the APPV717F TG brain, thioflavine-S stains only fibrillar amyloid. We found that silver-positive, dystrophic neurites surrounded and appeared to be limited in location to those Aβ deposits that were thioflavine-S-positive (Fig. 1). The appearance of these dystrophic neurites was very similar to those seen surrounding thioflavine-S-positive deposits in AD brain by using the same method (Fig. 1). These findings support the possibility that the process of structural changes in which Aβ is converted from a “soluble/diffuse” form to a “fibrillar” form results in the development of neuritic degeneration. One protein that may be involved in these structural changes in Aβ is apoE. ApoE is associated with Aβ in plaques within the AD brain (12, 13). It is also colocalized with fibrillar Aβ in APPV717F TG mice (14) and has been shown to have profibrillogenic activity in vitro (15, 16). Our previous studies have shown that APPV717F TG mice on an apoE−/− background have reduced Aβ deposition and levels in the hippocampus as compared with APPV717F TG mice expressing wild-type murine apoE (apoE+/+) (8, 10, 14). Interestingly, at later ages, Aβ deposition does occur in APPV717F TG, apoE−/− mice; however, virtually all deposits were thioflavine-S-negative (8, 10, 14). However, these studies did not address whether the Aβ deposits that do accumulate in the absence of apoE are associated with damage to surrounding neurons or their processes.
To determine the role of apoE in the development of AD-like neuritic dystrophy, we looked for evidence of neuritic pathology in APPV717F +/−, apoE+/+ and APPV717F +/−, apoE−/− mice at 12–15 months of age. Though APPV717F +/−, apoE−/− mice did not manifest neocortical Aβ deposits, they did develop significant Aβ deposition in the hippocampus. Although the average amount of Aβ deposition is somewhat less in the absence of apoE, in some animals, Aβ deposition is as extensive as that seen in APPV717F TG mice expressing mouse apoE (Figs. 2 A and B and 4 A–D). However, the anatomical distribution pattern of Aβ staining in the hippocampus is different in apoE−/− mice. Aβ deposits in APPV717F +/−, apoE+/+ mice were always prominent and abundant in the outer molecular layer of the dentate gyrus (terminal zone of the perforant pathway), as well as in the stratum oriens and radiatum of the hippocampus (Figs. 2A and 4 A and B). In contrast, Aβ deposits in APPV717F +/−, apoE−/− mice were more diffuse and prominent in the hilus of the dentate gyrus as well as the stratum oriens and radiatum (Figs. 2B and 4 C and D). Aβ deposition was not seen in the outer molecular layer of the dentate gyrus in the absence of apoE through 15 months of age. Interestingly, despite the presence of large Aβ burdens in some APPV717F +/−, apoE−/− mice, we found that very few to no neuritic plaques develop in these animals through 15 months of age (Figs. 2 D and F and 3A). Antibodies to phosphorylated neurofilament and synaptophysin revealed the same results (data not shown). Whereas the volume of hippocampal Aβ deposition in APPV717F +/−, apoE−/− mice was substantial (Fig. 3B), the level of total Aβ and Aβ42 as measured by ELISA was significantly less than that found in APPV717F +/−, apoE+/+ mice (Fig. 3C). The ratio of Aβ42/Aβ total, however, was not modified by the absence of apoE in this model (data not shown). Thus, the effect of apoE on fibrillogenesis in vivo does not appear to be through alteration of Aβ42-to-total Aβ ratio. This finding and the fact that APP levels do not differ between animals with and without apoE (8, 10, 14) suggest that apoE is influencing Aβ metabolism, structure, and/or clearance after being processed and released from APP.
Figure 3.
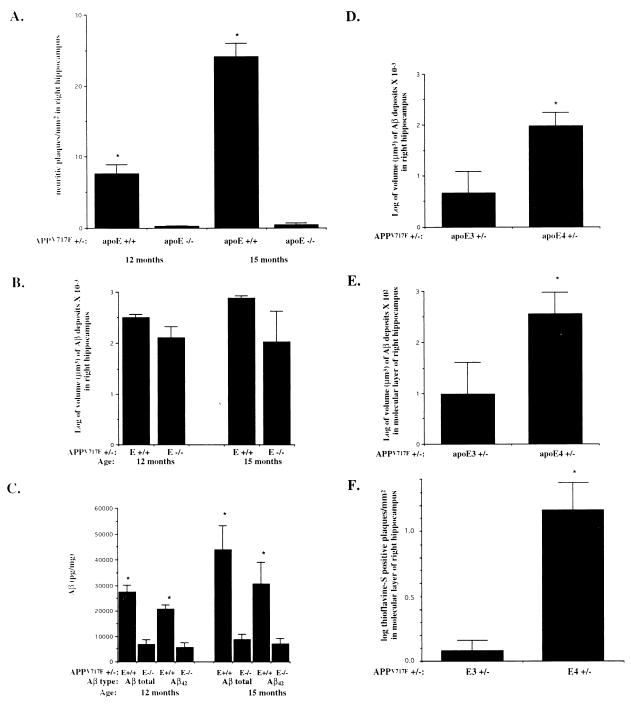
Quantitation of neuritic plaques, hippocampal Aβ, and thioflavine-S-positive deposits in APPV717F +/− TG mice expressing mouse, human, or no apoE. (A) Hippocampal neuritic plaques were prominent in APPV717F +/−, apoE+/+ mice at 12 months and increased by more than 3-fold by 15 months of age (n = 4, both time points). In APPV717F +/−, apoE−/− mice (n = 6, both time points), neuritic plaques were virtually absent and did not increase in number between 12 and 15 months of age (*, P < 0.0001 compared with apoE−/−). Neuritic plaques were identified with the de Olmos method. (B) The total volume of Aβ-IR deposits in the right hippocampus was determined in APPV717F +/− TG mice that were apoE+/+ (n = 4) or apoE−/− (n = 6) at 12 months of age and apoE+/+ (n = 4) or apoE−/− (n = 6) at 15 months of age. Volume of Aβ deposits was determined by using unbiased stereological methods. There was no statistical difference in the size of the right hippocampus between any of the groups of mice (data not shown). (C) Aβ ELISA for total Aβ and Aβ42 was assessed in the left hippocampus from the same mice used in A. *, P < 0.05, comparing apoE+/+ with apoE−/−. The total volume of Aβ-IR deposits in the right hippocampus (D) and in the molecular layer of dentate gyrus (E) was determined in APPV717F +/− TG mice that were either apoE3+/− (line 37, n = 6) or apoE4+/− (line 22, n = 9) at 15 months of age. Volume of Aβ deposits was determined by using unbiased stereological methods. There was no statistical difference in the size of the right hippocampus or the molecular layer of the dentate gyrus between the groups (data not shown). (F) The mean density of thioflavine-S-positive plaques was assessed in the molecular layer of the dentate gyrus of the same mice. *, P < 0.05 (D and E); *, P < 0.001, comparing apoE3+/− with apoE4+/− (F). Data in B, D, E, and F were log-transformed and tested for normality before statistical analysis.
Expression of Human apoE Results in Fibrillar Aβ Deposition and Neuritic Plaques.
To determine whether human apoE is also required for the development of fibrillar Aβ deposition and neuritic plaques in APPV717F TG mice, we bred APPV717F +/+, apoE−/− mice to apoE3 and apoE4 TG mice expressing human apoE isoforms under the control of the astrocyte-specific GFAP promoter (GFAP-apoE). These mice are on a mouse apoE−/− background and express human apoE at levels similar to that seen in wild-type, mouse apoE+/+ animals (9, 10). Furthermore, we utilized apoE TG mice in which apoE3 and apoE4 protein levels are indistinguishable from each other (10). We have shown recently that in contrast to mouse apoE and no apoE, human apoE isoforms actually suppress early Aβ deposition and Aβ levels in 9-month-old APPV717F +/− mice (10). To determine whether Aβ deposition would occur at later time points in the presence of human apoE, we examined APPV717F +/− TG mice that were apoE3+/− and apoE4+/− TG at 12 and 15 months of age. At 15 months of age there was a clear change in Aβ deposition observed in APPV717F +/−, apoE3 and apoE4+/− mice. Most E4-positive and some E3-positive animals had begun to develop Aβ deposits in the hippocampus in the same pattern as that seen in the presence of mouse apoE (Figs. 4 and 5). Aβ deposition was prominent in the outer molecular layer of the dentate gyrus. Compact Aβ deposits also were seen in the stratum oriens and radiatum. In addition, although not as abundant as in the presence of mouse apoE at this age, thioflavine-S-positive, neuritic plaques were now observed in those APPV717F +/−, apoE3 and E4+/− TG mice with Aβ deposits (Fig. 5). Although Aβ deposition occurred in the presence of both apoE3 and apoE4, there was a significant isoform-specific difference in the amount of deposition, with greater Aβ deposition in apoE4 as compared with E3-expressing mice (Figs. 3 D and E and 4 E–H). In fact, 89% of APPV717F +/−, E4 line 22+/− mice had hippocampal Aβ deposits whereas only 33% of APPV717F +/−, E3 line 37+/− mice had Aβ deposits. APPV717F +/−, E4 line 11+/− mice (n = 6) that express similar levels of apoE4 as E4 line 22 mice also had similar levels of hippocampal Aβ deposition when compared with E4 line 22 animals at 15 months of age (data not shown). Interestingly, several APPV717F +/−, apoE4-expressing mice at 15 months also had marked neocortical Aβ deposition, a result not observed in the apoE3-expressing mice at this age.
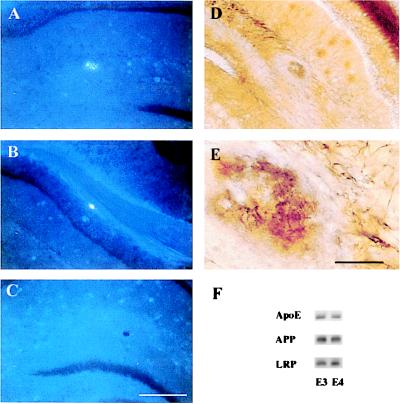
Figure 5.
Fibrillar Aβ deposits and neuritic plaques develop in the presence of human apoE. Coronal sections through the hippocampus of an APPV717F +/−, apoE4+/− (A, B, D, and E) and an APPV717F +/−, apoE−/− mouse at 15 months of age were stained with thioflavine-S (A, B, and C) or the de Olmos silver degeneration method. Thioflavine-S-positive plaques were observed in the molecular layer of the dentate gyrus (A) and corpus callosum (B) of this apoE4-expressing mouse whereas no thioflavine-S-positive deposits were found in apoE−/− mice (C). In addition to fibrillar Aβ, neuritic plaques also were found in the hippocampus of apoE4-expressing mice (D and E). [Bar in C = 180 μm (A–D); bar in E = 30 μm (E).] (F) Representative Western blot analysis of apoE, APP, and LRP in hippocampal lysates of APPV717F +/− TG mice expressing apoE3 (line 37) or apoE4 (line 22) at 15 months of age. There were no significant differences in the level of apoE, APP, or LRP between the apoE3- and apoE4-expressing mice, as shown here qualitatively or quantitatively assessed by densitometric analysis (data not shown).
In addition to total Aβ deposition, we also compared the density of thioflavine-S-positive plaques in apoE3- vs. apoE4-expressing mice. Strikingly, there was an even greater difference between apoE3- and apoE4-expressing mice in this parameter. There was a greater than 10-fold higher density of thioflavine-S-positive deposits in the outer molecular layer of the dentate gyrus of the apoE4 vs. the apoE3 animals (P < 0.001) (Fig. 3F). Importantly, and as with mouse apoE, all fibrillar Aβ deposits in these animals were associated with a neuritic dystrophy. As found previously, there were no significant differences in the level of apoE protein when comparing APPV717F +/− TG mice expressing apoE3 (line 37) and apoE4 (line 22) at 15 months of age (Fig. 5F). Levels of human APP and the low-density LRP also did not differ between E3- and E4-expressing mice.
Discussion
The ɛ4 allele of apoE was the first genetic risk factor identified for sporadic and late-onset familial AD (17). Many hypotheses have been put forward as to how apoE4 serves as an AD risk factor. However, direct evidence supporting these different hypotheses predominantly are based on in vitro systems. Several studies have suggested that the binding of apoE and Aβ is somehow linked to Aβ deposition and amyloid formation. For example, neuropathological studies in AD have shown that there were more Aβ deposits in E4-positive than E4-negative subjects (18, 19). Our in vivo report details a direct apoE isoform-specific effect on the mass, structure, and consequences of amyloid deposition in the brain. That apoE4 had greater effects than apoE3 on Aβ burden as well as an even greater effect on fibrillar Aβ deposition and neuritic plaques in this in vivo system strongly supports the hypothesis that apoE's effects on Aβ deposition, fibrillogenesis, and neuritic plaque formation underlie the role apoE plays as a genetic risk modifier for AD. Most importantly, our data also suggest that mouse or human apoE is a critical cofactor for the pathogenesis of AD-like neuritic degeneration.
Neuritic plaques are one of the classical hallmarks of AD pathology. These are composed of extracellular accumulations of the amyloidogenic peptide, Aβ, a variety of other plaque-associated proteins (e.g., apoE), activated glial cells, and degenerating nerve processes. Plaque-associated dystrophic neurites are likely to markedly disrupt neuronal function. For example, morphological changes such as those in dendritic curvature found in AD have been suggested by mathematical modeling to underlie functional deterioration in memory retrieval and higher-order functioning (3). Prior data strongly suggest that apoE is critical in vivo for the conversion of Aβ into mature amyloid fibrils, and our current data suggest it is also critical for neuritic degeneration. We have reported previously (8) and confirmed recently in an older cohort of APPV717F +/− mice (14) that apoE expression is important for fibrillar (thioflavine-S-positive) Aβ (amyloid) deposition. Significantly, despite essentially equivalent amounts of hippocampal Aβ-IR deposits in 21-month-old APPV717F +/−, apoE+/+ and apoE−/− mice, virtually no thioflavine-S-positive deposits were observed in mice lacking apoE. Our current data therefore demonstrate the critical importance of converting diffuse, thioflavine-S-negative Aβ-IR deposits to those that are thioflavine-S-positive. Thus, our findings argue that the apoE protein is important for AD pathology because it facilitates fibrillar amyloid to form in vivo and that the latter results in toxicity to neurites. The fibrillar Aβ may be directly neurotoxic or it could be indirectly toxic because of an inflammatory response. Ultimately, it will be important to discern how human and mouse apoE influence not only AD pathology but also cognitive changes associated with AD. This may be possible in transgenic models; however, it will be difficult to determine by using APPV717F mice. These animals demonstrate marked spatial learning impairments at young ages before Aβ deposition (20).
We have reported previously that in young APPV717F TG mice, human apoE expression appears to enhance Aβ clearance relative to APPV717F TG mice lacking apoE (10). Differences in apoE-mediated Aβ clearance is consistent with the fact that both apoE3 and apoE4 delay Aβ deposition relative to murine apoE and no apoE (10). This mechanism also may be consistent with our current in vivo data that show that apoE4 has a greater effect on Aβ deposition than apoE3. Interestingly, even at 15 months of age, expression of endogenous mouse apoE at levels similar to human apoE results in significantly more Aβ deposition. Our recent studies suggest that species differences in the primary sequence of apoE influence the form and structure of apoE/lipoproteins produced by astrocytes (21). It is possible that these alterations influence interactions with Aβ and subsequent amyloid deposition and neuritic pathology. ApoE has been shown to bind to Aβ both in vitro (17, 22) and in vivo (12, 13, 23). When apoE is associated with lipid, in vitro experiments reveal more Aβ associated in an SDS-stable complex with apoE3 than with apoE4 (22). That apoE forms a complex not only with fibrillar Aβ but also with “soluble” Aβ in human brain (23, 24) further supports the notion that apoE-Aβ interactions before Aβ deposition are likely to regulate Aβ clearance. Like other Aβ-associated molecules such as apoJ (25), α2-M (26–28), and transthyretin (29), apoE-containing lipoproteins in the brain may “sequester” Aβ and facilitate its cellular uptake and degradation locally by cells or its removal from the brain into the systemic circulation. Transport of apoE/Aβ complexes from the brain extracellular space back into the systemic circulation either through bulk cerebrospinal fluid flow or through specific apoE receptors at the blood–brain barrier also may be an important avenue of apoE/Aβ clearance from the brain.
In sum, our in vivo results suggest that at a critical species- or isoform-specific concentration of apoE and Aβ, a threshold is reached that is required for mature, fibrillar Aβ to deposit in the brain. That this process is associated with a gross neuritic dystrophy and is isoform-dependent (apoE4 ≫ apoE3) offers significant insight, we believe, into how apoE and apoE isoforms are likely to contribute to the genetic risk for developing AD.
Acknowledgments
We thank the Neuropathology Core of the WU ADRC for providing AD brain tissue and technical assistance as well as Eugene Johnson, Jr., and Ron DeMattos for their advice and comments. This work was supported by grants from the Alzheimer's Association (RG3-96-26) and the Ruth K. Broad Foundation, a Paul Beeson Faculty Scholar Award from the American Federation for Aging Research, and National Institutes of Health Grant AG13956 to D.M.H.
Abbreviations
- Aβ
amyloid-β
- AD
Alzheimer's disease
- TG
transgenic
- apoE
apolipoprotein E
- GFAP
glial fibrillary acidic protein
- IR
immunoreactivity
- LRP
lipoprotein receptor-related protein
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050004797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050004797
References
- 1.Selkoe D J. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 2.Lansbury P T J. Neuron. 1997;19:1151–1154. doi: 10.1016/s0896-6273(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 3.Knowles R B, Wyart C, Buldyrev S V, Cruz L, Urbanc B, Hasselmo M E, Stanley H E, Hyman B T. Proc Natl Acad Sci USA. 1999;96:5274–5279. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Nature (London) 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 5.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Youkin S, Yang F, Cole G. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 6.Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D. J Neurosci. 1996;16:5795–5811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irizarry M C, McNamara M, Fedorchak K, Hsiao K, Hyman B T. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bales K R, Verina T, Dodel R C, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone E M, Little S P, Cummins D J, et al. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Wu S, Bu G, Onifade M K, Patel S N, LaDu M J, Fagan A M, Holtzman D M. J Neurosci. 1998;18:3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtzman D M, Bales K R, Wu S, Bhat P, Parsadanian M, Fagan A M, Chang L K, Sun Y, Paul S M. J Clin Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wozniak D F, Brosnan-Watters G, Nardi A, McEwen M, Corso T D, Olney J W, Fix A S. Brain Res. 1996;707:165–179. doi: 10.1016/0006-8993(95)01230-3. [DOI] [PubMed] [Google Scholar]
- 12.Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- 13.Wisniewski T, Frangione B. Neurosci Lett. 1992;135:235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- 14.Bales K R, Verina T, Cummins D J, Du Y, Fishman C E, DeLong C A, Piccardo P, Petegnief V, Ghetti B, Paul S M. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Yee A, Brewer H B, Das S, Potter H. Nature (London) 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 16.Castano E M, Prelli F, Wisniewski T, Golabek A, Kumar R A, Soto C, Frangione B. Biochem J. 1995;306:599–604. doi: 10.1042/bj3060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strittmatter W J, Saunders A M, Schmechel D, Pericak-Vance M, Enghild J, Salvesen G S, Roses A D. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmechel D E, Saunders A M, Strittmattter W J, Crain B J, Hulette C M, Joo S H, Pericak-Vance M A, Goldgaber D, Roses A D. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebeck G W, Reiter J S, Strickland D K, Hyman B T. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 20.Dodart J-C, Meziane H, Mathis C, Bales K R, Paul S M, Ungerer A. Behav Neurosci. 1999;113:982–990. doi: 10.1037//0735-7044.113.5.982. [DOI] [PubMed] [Google Scholar]
- 21.Fagan A M, Holtzman D M, Munson G, Mathur T, Schneider D, Chang L K, Getz G S, Reardon C A, Lukens J, Shah J A, et al. J Biol Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- 22.LaDu M J, Falduto M T, Manelli A M, Reardon C A, Getz G S, Frail D E. J Biol Chem. 1994;269:23404–23406. [PubMed] [Google Scholar]
- 23.Russo C, Angelini G, Dapino D, Piccini A, Piombo G, Schettini G, Chen S, Teller J K, Zaccheo D, Gambetti P, et al. Proc Natl Acad Sci USA. 1998;95:15598–15602. doi: 10.1073/pnas.95.26.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Permanne B, Perez C, Soto C, Frangione B, Wisniewski T. Biochem Biophys Res Commun. 1997;240:715–720. doi: 10.1006/bbrc.1997.7727. [DOI] [PubMed] [Google Scholar]
- 25.Choi-Miura N H, Ihara Y, Fukuchi K, Takeda M, Nakano Y, Tobe T, Tomita M. Acta Neuropathol. 1992;83:260–264. doi: 10.1007/BF00296787. [DOI] [PubMed] [Google Scholar]
- 26.Qiu W Q, Borth W, Ye Z, Haass C, Teplow D B, Selkoe D J. J Biol Chem. 1996;271:8443–8451. doi: 10.1074/jbc.271.14.8443. [DOI] [PubMed] [Google Scholar]
- 27.Du Y, Ni B, Glinn M, Dodel R C, Bales K R, Zhang Z, Hyslop P, Paul S M. J Neurochem. 1997;69:299–305. [PubMed] [Google Scholar]
- 28.Narita N, Holtzman D M, Schwartz A L, Bu G. J Neurochem. 1997;69:1904–1911. doi: 10.1046/j.1471-4159.1997.69051904.x. [DOI] [PubMed] [Google Scholar]
- 29.Goldgaber D, Schwarzman A I, Bhasin R, Gregori L, Schmechel D, Saunders A M, Roses A D, Strittmatter W J. Ann NY Acad Sci. 1993;695:139–143. doi: 10.1111/j.1749-6632.1993.tb23042.x. [DOI] [PubMed] [Google Scholar]