Abstract
Type II NaPi cotransporters mediate epithelial phosphate (Pi) reabsorption. In mammals the type IIb protein is expressed in the small intestinal apical membrane and other epithelia; it is not expressed in the renal proximal tubule where we find the type IIa isoform. To look for molecular determinant(s) involved in apical expression of type IIb cotransporters, we have made deletion mutations within the C-terminal tails of mouse IIb (mIIb) and human IIb (hIIb) transporter proteins. The constructs were fused to the enhanced green fluorescent protein and transiently transfected into intestinal CaCo2-cells. Both mIIb and hIIb were located exclusively in the apical membrane of the cells. For mIIb, the removal of a cysteine cluster or the last three amino acids (TVF) had no effect on the location of the protein. However, truncation at the level of the conserved L691/689 prevented the apical membrane expression of both mIIb and hIIb, respectively, and the mutated proteins were located in endosomal and lysosomal structures. A similar expression pattern of the mIIb and hIIb constructs was found in renal proximal tubular opossum kidney cells. Our data suggest that L691/689 is involved in mechanisms leading to an apical expression of type IIb NaPi cotransporters.
Keywords: epithelial polarity, enhanced green fluorescent protein
The asymmetrical distribution of transporter proteins in the plasma membrane of epithelial cells is essential for the vectorial transport of solutes. Type II NaPi cotransporters are located in the apical (brush border) membrane and mediate Pi transport in different epithelia (1, 2). The type IIb NaPi cotransporter is located in small intestine, type II alveolar pneumocytes, and other tissues, whereas the type IIa isoform is expressed mainly in the renal proximal tubule (3, 4).
The type IIa and type IIb transporters are homologous proteins (≈70% similarity) with most of the differences in the cytoplasmic-oriented termini (3). In a recent study on transfections with type IIa and type IIb proteins we found that the type IIb protein is exclusively apically expressed in several cell lines of different epithelial origin whereas the type IIa transporter is expressed only in renal epithelial cell lines, and proper apical location is observed only in opossum kidney (OK) cells (proximal tubular origin) (5). This finding indicates that besides molecular motifs at the level of the protein the cellular context may crucially contribute to proper polarized expression.
The mechanism(s) by which newly synthesized proteins are expressed at either of the two plasma membrane domains are not fully understood; “targeting” and/or “scaffolding” mechanisms can be involved. Newly synthesized apical and basolateral proteins seem to be transported together from the endoplasmic reticulum (ER) to the trans-Golgi network and then to the subapical compartment (SAC). SAC is an important intermediate in the delivery of proteins to specific early endosomes destined either to the apical or the basolateral membrane (6). The mechanisms by which SAC is able to discriminate between apical and basolateral endosomes are not clear. Two basolateral sorting motifs located in the cytoplasmic tail of transmembrane proteins have been identified: tyrosine and di-leucine-based motifs (7, 8). The presence of a basolateral determinant within transmembrane domains also has been suggested (9). For apical sorting, several mechanisms have been proposed. One involves the interaction of proteins with rafts, sphingolipids, and cholesterol-rich and detergent-insoluble membranes (10). Another depends on the presence of apical sorting signals in the cytoplasmic domain of membrane proteins (11, 12). Furthermore, the involvement of ecto- and transmembrane domains also has been suggested (13–15).
The aim of the present study was to obtain information about molecular determinants involved in the apical expression of type IIb NaPi cotransporters. The present experiments show that a conserved L691 (mouse IIb, mIIb)/689 (human IIb, hIIb) is essential for the (apical) membrane localization of type IIb NaPi cotransporters. This leucine residue therefore could be part of a targeting motif and/or a motif required for membrane stabilization of the transporter protein.
Methods
NaPi-pEGFP Construction and Mutagenesis.
The cDNAs encoding the wild-type (WT) mIIb and hIIb cotransporters were subcloned into the red-shifted enhanced green fluorescent protein (EGFP) variant vector pEGFP-C1 (CLONTECH). Briefly, single restriction sites were introduced at the 5′ and 3′ ends of the WT cDNAs through PCR: SacI–SalI for mIIb and SalI–BamHI for hIIb. The cDNAs then were ligated into the pEGFP-C1, which was digested by the same restriction enzymes. All cDNAs were fused at the carboxyl terminal end of the EGFP (NaPi-pEGFP).
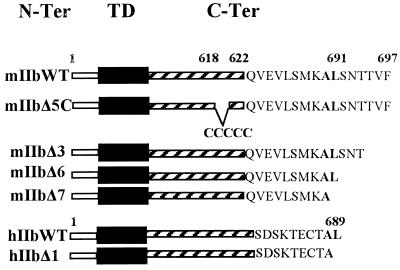
The truncated mutants were constructed by introducing the stop codon TAG at different positions within the C-terminal tail of the WT cDNAs (Fig. 1). For these constructs, site-directed mutagenesis was performed by using NaPi-pEGFP as templates and complementary sense and antisense primers, both of them containing the stop codon in the middle of their sequence. After PCRs, the templates were digested by Dnp1.
Figure 1.
Schematic representation of WT and mutants of type IIb NaPi cotransporters. The N-terminus (N-Ter), the transmembrane (TD), and the C-terminus (C-Ter) domains are indicated in open, filled, and striped bars, respectively. The numbers given at the beginning or the end of the bars indicate the position of the amino acids. The spaces indicate the removal of the amino acids. Also shown are the different sequences of the C-terminal tails of mIIb and hIIb cotransporters.
To generate mIIbΔ5C, which lacks the five-cysteine residues located in the C-terminal tail of mIIb, a ThaI site was introduced at position 618 of the mIIb cDNA, which originally contains the same site at position 622 (Fig. 1). The nucleotides encoding the five cysteines then were removed by digesting the mutated cDNA with ThaI. After purification, mIIbΔ5C was inserted into pEGFP-C1 as described for mIIbWT.
All mutations were confirmed by sequencing.
Cell Culture and Transient Transfections.
Colon adenocarcinoma cells (CaCo2 cells) were grown in DMEM complemented with 20% FCS, 44 mM NaHCO3, 20 mM Hepes, 4 mM l-glutamine, 1% nonessential amino acids, 100 units/ml of penicillin, and 100 mg/ml streptomycin, at 37°C in 10% CO2-90% air. OK cells were grown in DMEM/Ham's F-12 medium (1:1) complemented with 10% FCS, 22 mM NaHCO3, 20 mM Hepes, 2 mM l-glutamine, 50 units/ml of penicillin, and 50 mg/ml streptomycin, at 37°C in 5% CO2-95% air. For transfections, CaCo2 and OK cells were grown on coverslips in 35-mm dishes. Subconfluent cultures were transfected with 3 μl of Fugene (Boehringer Mannhein) and 1 μg plasmids. Cells were processed for analysis by microscopy once they reached confluency, 2 or 3 days after transfection. At least five independent experiments were performed on transiently transfected cells. Attempts to obtain stably transfected cell lines expressing the EGFP-fused cotransporter have failed, though it was previously possible to establish cell lines containing the WT NaPi-2 cotransporter (16, 17).
Analysis by Confocal Microscopy.
Confluent cells were fixed, and the actin and/or expressed proteins were stained as described (5). The immunodetection of hIIb was performed with a rabbit polyclonal antibody raised against an N-terminal peptide (3). For the immunodetection of ER, trans-Golgi network, endosomes, and lysosomes, we used mAbs against human p63, giantin, transferrin receptor, and lamp1, respectively (18–22). The coverslips were mounted by using Dako-glycerol containing 2.5% 1.4-diazabicyclo-(2.2.2) octane (Sigma) as a fading retardant. Confocal images were taken by using a Leica TCSSP (Wetzlar, Germany) laser scan microscope equipped with a ×63 oil immersion objective.
Results and Discussion
Similar to the type IIa transporter (23, 24), the type IIb NaPi cotransporter most likely has eight transmembrane domains, with cytoplasmic N and C termini (3). Most of the differences between both transporters lay within the cytoplasmic tails (3).
The type IIb proteins showed proper apical expression after transfection in different epithelial cell lines (CaCo2, OK, LLC-PK1, and MDCK cells) whereas for the type IIa transporter a “correct” apical expression was obtained only in renal proximal tubular OK cells (5). To identify potential subdomains (motifs) involved in the apical expression of the type IIb transporter protein, we performed transient transfection experiments with truncations in the C-terminal tail of mIIb and hIIb cotransporters (Fig. 1). As indicated in Methods, we were unable to establish stably transfected cell lines.
Cellular Localization of hIIb Cotransporter in CaCo2 Cells.
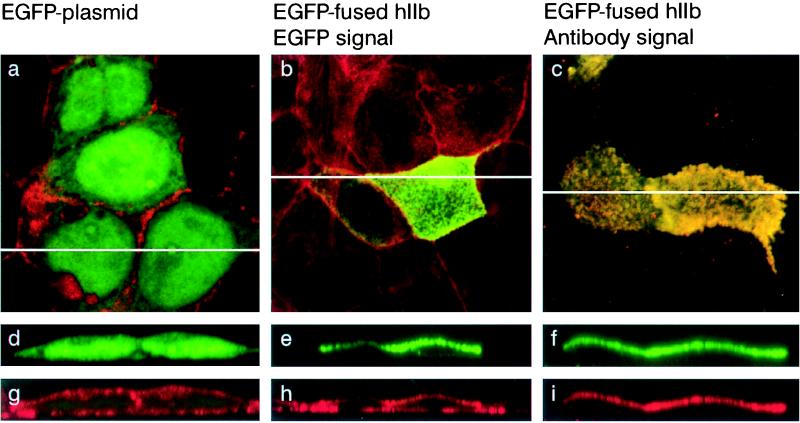
We studied the location of WT as well as the different truncated NaPi cotransporters by detecting the intrinsic fluorescence of the EGFP-fused cotransporter proteins. As shown in Fig. 2, the fluorescence of the empty vector was found only intracellularly. In contrast, the signal of the EGFP-fused hIIb was detected only in the apical membrane (Fig. 2). The immunostaining of the hIIb protein superimposed with the intrinsic fluorescence of the EGFP-fused hIIb (Fig. 2). No immunostaining was detected in the nontransfected cells (data not shown). Thus, the EGFP-related fluorescence of hIIb reflects the cellular location of the cotransporter. Similar data were obtained previously with mIIb (5).
Figure 2.
Expression of hIIb cotransporter in CaCo2 cells. Cells were transfected with EGFP-fused hIIb cotransporter or EGFP plasmid and processed for confocal microscopy. (a–c) Focal planes. (d–i) xy cross-sections. (a, d, and g) Cells transfected with empty plasmid. (b, e, and h) Cells transfected with the EGFP-fused hIIb. (c, f, and i) Cells transfected with EGFP-fused hIIb and stained with anti-hIIb antibody. The endogenous EGFP fluorescence is shown in green and actin and anti-hIIb antibody staining in red. In a–c the fluorescence signals were merged.
Noninvolvement of a C-Terminal Tail Cysteine Cluster.
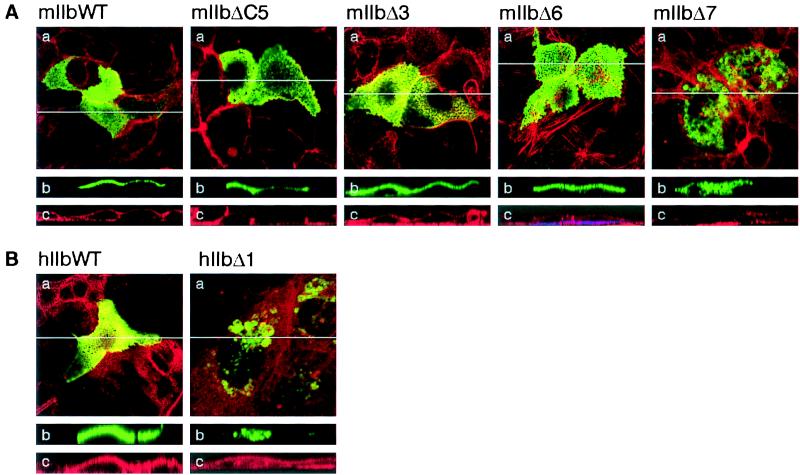
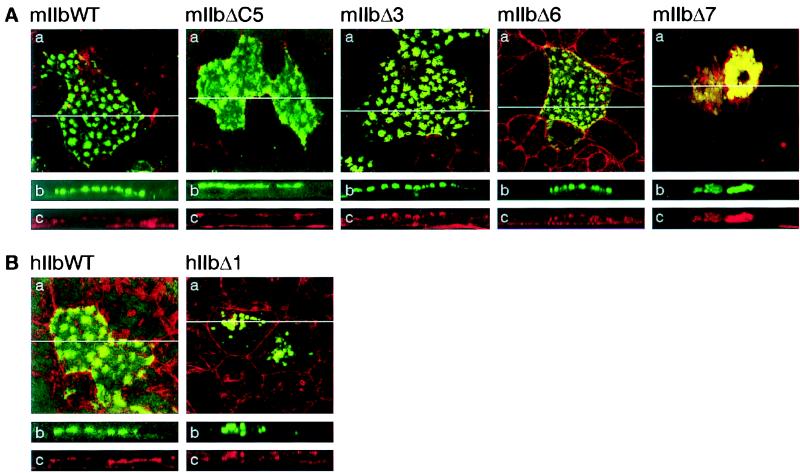
Cysteine clusters can be involved in plasma membrane delivery and/or stabilization of proteins via palmitoylation, e.g., for NHE1 antiporter (26), for interaction of different proteins with rafts and with caveolae-coated membrane invaginations (27, 28), and for G0 protein α subunits (28–30). The C-terminal tail of the type IIb cotransporter contains a specific cluster of cysteine residues that is not present in type IIa (3, 25). To investigate the role of the cysteine cluster, we analyzed the distribution of the mIIbΔ5C mutant (Fig. 1) in CaCo2 cells. The mIIbΔ5C protein was located in the apical membrane of the cells, and no difference was detected between the expressions of mIIbΔ5C and the WT (Fig. 3A). Thus, the cysteine cluster is not involved in apical expression, i.e., targeting and/or membrane stabilization of the mIIb transporter protein.
Figure 3.
Cellular distribution of mIIb and hIIb deletion mutants in CaCo2 cells. Cells were transfected with WT mIIb (mIIbWT, A) or hIIb (hIIbWT, B) cotransporters or with the mutants mIIbΔC5, mIIbΔ3, mIIbΔ6, mIIbΔ7, and hIIbΔ1 and processed for confocal microscopy. (a) Focal planes. (b and c) The xy cross-sections. The endogenous EGFP fluorescence is shown in green (b) and the actin staining in red (c); a merge of the fluorescence signals is shown in a.
Involvement of a C-Terminal Tail Leucine Residue.
The last three amino acids of the mIIb (TVF) strongly resemble the PDZ binding motif TXL/F, where X is any amino acids (31). Binding to proteins containing PDZ domains is involved in the membrane targeting and/or stabilization of several proteins (11, 32–34), e.g., of the Cl− channel CFTR (cystic fibrosis transmembrane conductance regulator) via interaction with EBP50 (35). To investigate a potential role of the three terminal amino acids of the mIIb transporter protein, we analyzed the expression of the mIIbΔ3 construct (Fig. 1). Confocal microscopy analysis showed that also the mIIbΔ3 protein still had an apical expression (Fig. 3A). Therefore, an interaction of these three amino acids of NaPi IIb with proteins carrying PDZ domains is not required for its apical expression. Nevertheless, we cannot exclude the necessity of PDZ domain interactions within other parts of the transporter protein (36, 37). Removal of the last six amino acids (SNTTVF) also had no effect on the apical expression of mIIb transporter protein (mIIbΔ6, Fig. 3A). However, additional removal of L691 abolished the apical expression, and the mutated cotransporter was intracellularly located (mIIbΔ7, Fig. 3A).
Comparison of mIIb and hIIb sequences revealed that the two proteins are highly homologous (more than 90%). The C-terminal tail of mIIb has six additional amino acids (SNTTVF), and upstream of this sequence only two of 10 amino acids are conserved (shown in bold in Fig. 1): A690 and L691 in mIIb corresponding to A688 and L689 in hIIb. We studied whether L689 also has a role in the apical expression of hIIb, as shown above for mIIb. As expected, removal of this leucine impaired the membrane expression, and the mutant was detected in intracellular compartments (hIIbΔ1, Fig. 3B).
Above data indicate that L691 (mIIb)/689 (hIIb) is involved in the routing to or in the stabilization of the IIb transporters at the apical membrane. Leucine residues within dileucine motifs are important for endocytosis and basolateral targeting (38). In addition, it has been shown that leucine residues are involved in protein–protein interaction, e.g., in the interaction of the β2-adrenergic receptor with the regulatory factor NHERF (39).
Intracellular Fate of Misslocated Transporters (mIIbΔ7/hIIbΔ1).
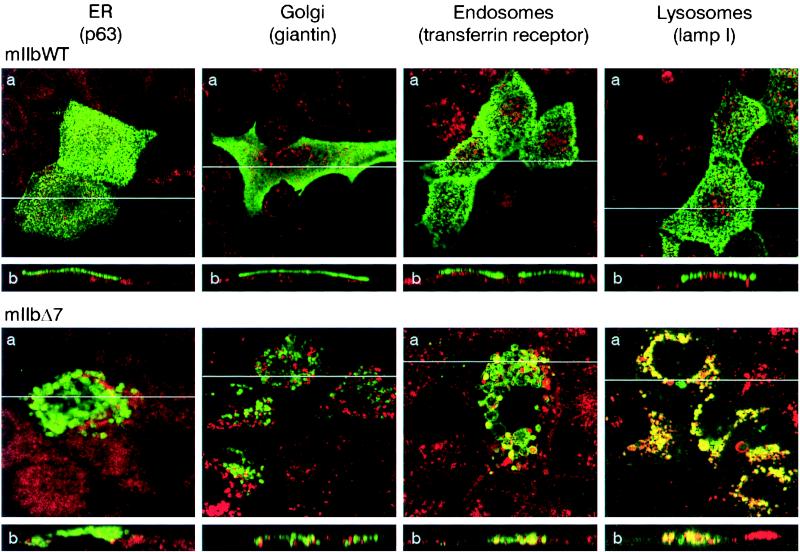
We attempted to identify the intracellular compartment where the IIb transporters are accumulated after removal of the L691/689. For that purpose, we studied the colocalization of the WT and mutant proteins with proteins residing in the ER (p63), the Golgi apparatus (giantin), the endosomes (transferrin receptor), and the lysosomes (lamp1) (18–22). As shown in Fig. 4, neither the WT nor the mIIbΔ7 mutant colocalized with p63 or giantin. Unlike the WT, mIIbΔ7 strongly colocalized with the lysosomal marker lamp1 (Fig. 4). We also observed partial colocalization of mIIbΔ7 with the transferrin receptor, an endosomal marker (Fig. 4). Similar colocalizations were detected with the hIIbΔ1 mutant (data not shown).
Figure 4.
Colocalization of mIIb and mIIbΔ7 with different intracellular compartment markers in CaCo2 cells. Cells were transfected with WT mIIb cotransporter (mIIbWT) or the mutant mIIbΔ7. They were stained with antibodies againts p63 (ER), giantin (Golgi), transferrin receptor (Endosomes), and lamp1 (Lysosomes) and processed for confocal microscopy. (a) The focal planes. (b) The xy cross-sections. The endogenous EGFP fluorescence is shown in green, the antibodies stainings in red, and the overlapping of both in yellow.
The ER and Golgi compartments are involved in “quality control,” and missfolded proteins are unable to leave these compartments (40–42). The above results suggest that removal of the L691/689 yields a cotransporter that is conformationaly competent to leave the ER and Golgi. The detection of both mIIbΔ7 and hIIbΔ1 in lysosomal compartments indicated that the truncated cotransporters are targeted to degradation. Based on the partial colocalization of the truncated cotransporters with the transferrin receptor, two possibilities for lysosomal location can be envisioned: (i) Transferrin receptors are present in early/recycling endosomes but not in the late endosomal/lysosomal pathway (43). Thus, localization in early/recycling endosomes could suggest that the mutants mIIbΔ7/hIIbΔ1 were expressed at the plasma membrane but were quickly internalized because of their instability in the plasma membrane. Such a mechanism has been reported for the epithelial γ-aminobutyric acid transporter (BGT-1) (44). (ii) On the other hand, the colocalization of the transferrin receptor and the mIIbΔ7/hIIbΔ1 proteins also could be explained by their presence in the SAC (45). From the SAC, transferrin receptors could be routed to the membrane whereas the mutated cotransporters could be targeted to the lysosomes. Therefore, the L691/689 truncations could affect either the targeting and/or the stability of the type IIb NaPi cotransporter in the membrane. Because we did not observe an apical location of mIIbΔ7/hIIbΔ1 proteins in several experiments, we favor the interpretation of inappropriate apical delivery as contributing to a major extent to endosomal and finally lysosomal location.
L689/691 Also Is Involved in Apical Expression of Type IIb Proteins in OK Cells.
To test whether the removal of L691/689 affects the apical expression of the type IIb cotransporter independent of the cellular environment (cellular context), we studied the expression of the WT and several mutants in a renal cell line (OK cells). We previously have shown that the mIIb cotransporter was mostly apically expressed in OK cells, although a weak basolateral staining was observed in about 30% of transfected cells (5, 17). We found that mIIb, mIIbΔ3, and mIIbΔ6 had a similar apical expression pattern, whereas the mutant mIIbΔ7 was intracellularly detected (Fig. 5A), as indicated by the lack of the mosaic pattern characteristic for the apical surface of OK cells (5). The same results were observed for hIIb cotransporter (Fig. 5B), which indicates that L689/691 also determines the apical expression in OK cells. Because of the lack of antibodies crossreacting with OK cell proteins residing in intracellular compartments we could not characterize the intracellular fate of the truncated transporters in OK cells, but assume similar behavior as in CaCo2 cells.
Figure 5.
Cellular distribution of mIIb and hIIb deletion mutants in OK cells. Cells were transfected with WT mIIb (mIIbWT, A) or hIIb (hIIbWT, B) cotransporters or with the mutants mIIbΔC5, mIIbΔ3, mIIbΔ6, mIIbΔ7, and hIIbΔ1 and processed for confocal microscopy. (a) Focal planes. (b and c) The xy cross-sections. The endogenous EGFP fluorescence is shown in green (b) and the actin staining in red (c); a merger of the fluorescence signals is shown in a.
In summary, the L691/689 seems to be crucial for the apical membrane expression of IIb NaPi cotransporters. Its role is beyond the maturation of the protein in ER and Golgi and primarily might involve an altered membrane delivery although a reduced apical membrane stability also can contribute. Finally, the cysteine cluster has no apparent function in the apical expression of IIb NaPi cotransporters.
Acknowledgments
We thank Hans-Peter Hauri and Paul Cannon (Roche Bioscience) for providing us with the antibodies for the different intracellular compartments and with hNaPi IIb, U. Ziegler for helpful technical advice in the analysis by the confocal microscope, and C. Gasser for assistance in preparing the figures. This work was supported by Swiss National Science Foundation Grant (to H.M.), the Hartmann Müller-Stiftung (Zürich), the Olga Mayenflsch-Stiftung, the Schwerzerische Bank-Gesellschaft (Zürich; Bu70417–1), and Novartis Foundation.
Abbreviations
- EGFP
enhanced green fluorescent protein
- mIIb
mouse IIb
- hIIb
human IIb
- ER
endoplasmic reticulum
- SAC
subapical compartment
- WT
wild type
- OK
opossum kidney
References
- 1.Murer H, Biber J. Curr Opin Nephrol Hypertens. 1994;3:504–510. doi: 10.1097/00041552-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Murer H, Biber J. Pflügers Arch. 1997;433:379–389. doi: 10.1007/s004240050292. [DOI] [PubMed] [Google Scholar]
- 3.Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J. Proc Natl Acad Sci USA. 1998;95:14564–14569. doi: 10.1073/pnas.95.24.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traebert M, Hattenhauer O, Murer H, Kaissling B, Biber J. Am J Physiol. 1999;277:L868–L873. doi: 10.1152/ajplung.1999.277.5.L868. [DOI] [PubMed] [Google Scholar]
- 5.Hernando, N., Sheikh, S., Karim-Jimenez, Z., Galliker, H., Forgo, J., Biber, J. & Murer, H. (2000) Am. J. Physiol., in press. [DOI] [PubMed]
- 6.van Ijzendoorn S C D, Hoekstra D. Trends Cell Biol. 1999;9:144–149. doi: 10.1016/s0962-8924(99)01512-3. [DOI] [PubMed] [Google Scholar]
- 7.Mellman I. Cold Spring Harb Symp Quant Biol. 1995;60:745–752. doi: 10.1101/sqb.1995.060.01.080. [DOI] [PubMed] [Google Scholar]
- 8.Roush D L, Gottardi C J, Naim H Y, Roth M G, Caplan M J. J Biol Chem. 1998;273:26862–26869. doi: 10.1074/jbc.273.41.26862. [DOI] [PubMed] [Google Scholar]
- 9.Alonso M A, Fan L, Alarcòn B. J Biol Chem. 1997;272:30748–30752. doi: 10.1074/jbc.272.49.30748. [DOI] [PubMed] [Google Scholar]
- 10.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 11.Muth T R, Ahn J, Caplan M J. J Biol Chem. 1998;273:25616–25627. doi: 10.1074/jbc.273.40.25616. [DOI] [PubMed] [Google Scholar]
- 12.Chuang J Z, Sung C H. J Cell Biol. 1998;142:1245–1256. doi: 10.1083/jcb.142.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbeil D, Boileau G, Lemay G, Crine P. J Biol Chem. 1992;267:2798–2801. [PubMed] [Google Scholar]
- 14.Dunbar L A, Courtois-Coutry N, Roush D L, Muth T R, Gottardi C J, Rajendran V, Geibel J, Kashgarian M, Caplan M J. Acta Physiol. Scand. 1998. Suppl., 643, 289–295. [PubMed] [Google Scholar]
- 15.Jacob R, Preuss U, Panzer P, Alfalah M, Quack S, Roth M G, Naim H, Naim H Y. J Biol Chem. 1999;274:8061–8067. doi: 10.1074/jbc.274.12.8061. [DOI] [PubMed] [Google Scholar]
- 16.Quabius E S, Murer H, Biber J. Am J Physiol. 1996;270:F220–F228. doi: 10.1152/ajprenal.1996.270.1.F220. [DOI] [PubMed] [Google Scholar]
- 17.Pfister M F, Lederer E, Forgo J, Ziegler U, Lotscher M, Quabius E S, Biber J, Murer H. J Biol Chem. 1997;272:20125–20130. doi: 10.1074/jbc.272.32.20125. [DOI] [PubMed] [Google Scholar]
- 18.Schweizer A, Fransen J A, Bachi T, Ginsel L, Hauri H P. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linstedt A D, Hauri H P. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schweizer A, Ericsson M, Bachi T, Griffiths G, Hauri H P. J Cell Sci. 1993;104:671–683. doi: 10.1242/jcs.104.3.671. [DOI] [PubMed] [Google Scholar]
- 21.Vollenweider F, Kappeler F, Itin C, Hauri H P. J Cell Biol. 1998;142:377–389. doi: 10.1083/jcb.142.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson H, Kappeler F, Hauri H P. J Biol Chem. 1999;274:15080–15084. doi: 10.1074/jbc.274.21.15080. [DOI] [PubMed] [Google Scholar]
- 23.Magagnin S, Werner A, Markovich D, Sorribas V, Stange G, Biber J, Murer H. Proc Natl Acad Sci USA. 1993;90:5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert G, Traebert M, Hernando N, Biber J, Murer H. Pflügers Arch. 1999;437:972–978. doi: 10.1007/s004240050869. [DOI] [PubMed] [Google Scholar]
- 25.Feild J A, Zhang L, Brun K A, Brooks D P, Edwards R M. Biochem Biophys Res Commun. 1999;258:578–582. doi: 10.1006/bbrc.1999.0666. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Singh D, Fliegel L. Arch Biochem Biophys. 1998;358:116–124. doi: 10.1006/abbi.1998.0833. [DOI] [PubMed] [Google Scholar]
- 27.Shenoy-Scaria A M, Dietzen D J, Kwong J, Link D C, Lublin D M. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milligan G, Parenti M, Magee A I. Trends Biochem Sci. 1995;20:181–187. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- 29.Mumby S M, Kleuss C, Gilman A G. Proc Natl Acad Sci USA. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Trible R P, Samelson L E. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 31.Songyang Z, Fanning A S, Fu C, Xu J, Marfatia S M, Chishti A H, Crompton A, Chan A C, Anderson J M, Cantley L C. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 32.Kim E, Niethammer M, Rothschild A, Jan Y N, Sheng M. Nature (London) 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 33.Kornau H C, Schenker L T, Kennedy M B, Seeburg P H. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 34.Panzer P, Preuss U, Joberty G, Naim H Y. J Biol Chem. 1998;273:13861–13869. doi: 10.1074/jbc.273.22.13861. [DOI] [PubMed] [Google Scholar]
- 35.Short D B, Trotter K W, Reczek D, Kreda S M, Bretscher A, Boucher R C, Stutts M J, Milgram S L. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- 36.Cuppen E, Gerrits H, Pepers B, Wieringa B, Hendriks W. Mol Biol Cell. 1998;9:671–683. doi: 10.1091/mbc.9.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Huizen R, Miller K, Chen D M, Li Y, Lai Z C, Raab R W, Stark W S, Shortridge R D, Li M. EMBO J. 1998;17:2285–2297. doi: 10.1093/emboj/17.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mellman I. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 39.Hall R A, Premont R T, Chow C W, Blitzer J T, Pitcher J A, Claing A, Stoffel R H, Barak L S, Shenolikar S, Weinman E J, et al. Nature (London) 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 40.Kopito R R. Cell. 1997;88:427–430. doi: 10.1016/s0092-8674(00)81881-4. [DOI] [PubMed] [Google Scholar]
- 41.Saraste J, Kuismanen E. Semin Cell Biol. 1992;3:343–355. doi: 10.1016/1043-4682(92)90020-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammond C, Helenius A. J Cell Biol. 1994;126:41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukherjee S, Ghosh R N, Maxfield F R. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 44.Perego C, Vanoni C, Villa A, Longhi R, Kaech S M, Frohli E, Hajnal A, Kim S K, Pietrini G. EMBO J. 1999;18:2384–2393. doi: 10.1093/emboj/18.9.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odorizzi G, Pearse A, Domingo D, Trowbridge I S, Hopkins C R. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]