Abstract
Oxidative stress is involved in the pathogenesis of diabetes and its cardiovascular complications. Metallothionein (MT), a stress-response protein, is significantly increased in the liver and kidney of diabetic animals. We examined whether diabetes also induces cardiac MT synthesis through oxidative damage and whether MT overexpression protects the heart from injury. Diabetes was induced in mice by single injection of streptozotocin (STZ), and cardiac MT mRNA and protein levels were measured 2 weeks and 2 months after STZ treatment. Diabetes significantly increased cardiac MT synthesis 2 weeks and 2 months after STZ treatment, with no change in cardiac metals including zinc, copper, and iron. Serum and cardiac vasopeptide endothelin and inflammatory cytokine tumor necrosis factor-α were also significantly increased in diabetic hearts, as were the ratio of oxidized to reduced glutathione and the immunohistochemical staining of 3-nitrotyrosine and 4-hydroxynonenal. To explore the biological importance of increased MT synthesis in the heart, MT-overexpressing transgenic mice were treated with STZ and then examined 2 months later. A loss of inotropic reserve, uncovered during β-adrenergic stimulation, and the presence of cardiac fibrosis, shown by increased Sirius red staining of collagen, were evident in the wild-type diabetic mice but not in the MT-overexpressing transgenic diabetic mice. These results suggest that diabetes-induced cardiac MT expression likely associates with systemic increases in endothelin-1 and tumor necrosis factor-α and the resulting cardiac oxidative stress. Overexpressing cardiac MT significantly protects the heart from diabetes-induced injury.
Diabetes and its cardiovascular complications are related to multiple pathogenic factors, including hyperglycemia, hyperlipidemia, and inflammatory response.1–3 However, the pivotal mediator for the pathogenesis of diabetes and its cardiovascular complications is oxidative stress, directly or indirectly derived from the multiple factors mentioned above.2,3 Oxidative stress is defined as the imbalance between the production of reactive oxygen and nitrogen species (ROS and RNS) and antioxidant capacity.3,4 Diabetes impairs cardiac antioxidant capacity, showing decreases in enzymatic (superoxide dismutase, catalase, glutathione peroxidase) and nonenzymatic (vitamin C, E, or A) antioxidant defenses, as well as total radical-trapping antioxidant capacity in the heart.4–6 In addition to impaired defenses, diabetes also causes ROS and RNS overproduction in the hearts of diabetic animals and patients.2–4 In response to the diabetes-derived oxidative stress, however, cardiac compensatory mechanisms to protect the heart from these deleterious effects, such as induction of heat shock protein and metallothionein (MT), may take place7–9 because MT is up-regulated in the heart challenged by other oxidative stresses.10,11
MT is a cysteine-rich, metal-binding protein. Four major isoforms of MT have been identified in mammalian tissues, but MT-I and MT-II are the major isoforms found in most tissues.10,12 Several physiological roles have been proposed for MT, including detoxification of potentially toxic heavy metals, such as cadmium (Cd), and homeostasis of essential metals, such as zinc (Zn) and copper (Cu). MT is also a potent antioxidant, interacting with various ROS and RNS more efficiently than do other antioxidants in cell-free systems.10,12–14 MT exists in most organs, including in animal and human hearts, and is inducible to a high level by various oxidative or pathogenic stresses.9–12 Zn and Cu, major metals that bind to MT under physiological conditions, significantly induce MT synthesis in various organs.10,12,14
We have demonstrated that MT levels increase in response to chronic stress, such as allogenic and isogenic organ transplantation,15 which may be induced by inflammation factors including interleukin (IL)-6 and tumor necrosis factor (TNF)-α.15–17 Enhanced expression of hepatic and renal MT in diabetic rats was also indicated in our previous study.18 A literature search showed organ-specific patterns for diabetes-induced MT expression.19,20 Diabetes-induced renal MT was metal-dependent, whereas hepatic MT was metal-independent.18–20 MT plays a critical role in Zn and Cu homeostasis, which is required to maintain normal cellular functions in different tissues, and diabetes-derived abnormal homeostasis of Zn or Cu may be related to the development of diabetic cardiovascular complications.21,22 To date, however, it remains unclear whether diabetes affects cardiac MT expression.
The antioxidant function of MT in the heart has been extensively implicated.10,23 Previous induction of cardiac MT by various agents significantly prevented hearts from oxidative damage.23–26 Using a cardiac-specific MT-overexpressing transgenic mouse, significant protection of the heart from diabetes-induced injury was also observed in our previous studies and others.27–31 However, in these transgenic mice, MT was overexpressed only in cardiac myocytes and was ∼20- or 100-fold higher than that of control mice. Such high level of cardiac-specific MT expression would be impossible to be manipulated by pharmaceutical approach such as administration of MT inducers.
This study, therefore, was aimed: 1) to investigate the effect of diabetes on cardiac MT expression by measuring MT mRNA and protein contents; 2) to explore the mechanisms for cardiac MT induction by measuring cardiac metal levels (Zn, Cu, and iron, Fe), systemic and cardiac vasopeptide (endothelin-1, ET-1), and inflammation factor (TNF-α, IL-6) levels, and cardiac oxidative damage; and 3) to explore the biological importance of MT expression at an inducible level in preventing diabetes-induced cardiac injury by comparing MT-overexpressing transgenic (MT-TG) with their wild-type (WT) diabetic mice.
Materials and Methods
Diabetic Mouse Model
FVB mice, obtained from Harlan Bioproducts for Science, Inc. (Indianapolis, IN), MT-TG mice, and WT control mice (C57BL/6), purchased from The Jackson Laboratory (Bar Harbor, ME), were housed in the University of Louisville Research Resources Center at 22°C with a 12-hour light/dark cycle and free access to rodent chow and tap water. All animal procedures were approved by the Institutional Animals Care and Use Committee, which is certified by the American Association for Accreditation of Laboratory Animals Care. Eight-week-old male mice were intraperitoneally given a single dose of streptozotocin (STZ; Sigma Chemical Co., St. Louis, MO) at 150 mg/kg body weight, dissolved in sodium citrate buffer (pH 4.5). On day 3 after STZ treatment, whole blood glucose obtained from mouse tail-vein was detected using a SureStep complete blood glucose monitor (LifeScan, Milpitas, CA). STZ-treated mice with whole blood glucose higher than 250 mg/dl were considered as diabetic. Mice serving as vehicle controls were given the same volume of sodium citrate.32 Experimental measurements were performed in the control and diabetic mice 2 weeks and 2 months after STZ treatment because these will reflect both early and late response of the heart to diabetes. To avoid the direct effect of STZ on cardiac MT expression a group of insulin-treated diabetic mice was included for 2-week experiments, as described previously.32 For 2-month experiments, the insulin-treated diabetes group was not included because MT protein turnover is ∼3 or 4 days in most tissues and its expression in such late period after STZ treatment will be unlikely affected.10,23–26
Measurements of Blood Glycated HB-A1, and Serum Triglyceride, ET-1, IL-6, and TNF-α
Whole blood was collected from dorsal vena cava of the anesthetized animals. Noncoagulated whole blood was used to measure glycated Hb-A1. Serum was prepared using a serum separator apparatus (Becton Dickinson, Rutherford, NJ) to measure triglyceride level using a kit from Sigma Chemical Co. and ET-1, IL-6, and TNF-α levels using their corresponding enzyme-linked immunosorbent assay kits from Cayman Chemical Co. (Ann Arbor, MI).33,34 Selection of IL-6 and TNF-α was because these inflammatory factors were found to be increased in diabetic mice in our previous studies34 and are also a potent MT inducer.15–17 Selection of ET-1 is because we have demonstrated that ET-1 plays important roles in mediating the enhanced MT expression in the liver and kidney of diabetic rats,18 and also the development of diabetic cardiomyopathy.35,36
MT mRNA Measurement by Northern Blot Assay
Total cellular RNA was extracted from frozen tissues using the Trizol reagent. Random-primed first-strand cDNA was prepared from total cellular RNA using Superscript II and amplified by polymerase chain reaction (PCR) using mouse MT II primers (sense primer, 5′tctcgtcgatcttcaacc-3′; anti-sense, 5′-ggcttctacatggtctatttac-3′). The purified PCR fragment was subcloned into a PCR II vector as a template for synthesis of the cRNA probe. The plasmid was linearized with NCO I, and the anti-sense cRNA probe was produced using the T7 RNA polymerase Maxiscript kit (Ambion, Austin, TX) in the presence of α32-UTP (Amersham, Piscataway, NJ). Total cellular RNA extracted from heart samples (15 μg/lane) was fractionated by electrophoresis through denaturing (0.66 mol/L formaldehyde in 1% agarose) gel, downward transferred onto nylon membranes, and vacuum-dried at 80°C for 2 hours. Membranes were prehybridized for 1 hour and then hybridized with the anti-sense cRNA probes, as described above, in the hybridization solution (Ambion) at 68°C for 16 hours. Membranes were then washed under high-stringency conditions (final wash 0.1× standard saline citrate at 68°C) while monitored with a Geiger counter. Radioactivity was recorded on X-ray film.
Immunohistochemical Examinations for Cardiac MT, 3-Nitrotyrosine, and 4-Hydroxynonenal
Expression of cardiac MT, 3-nitrotyrosine (3-NT), and 4-hydroxynonenal (4-HNE) was examined by immunohistochemical staining with monoclonal mouse anti-MT antibody (Zymed Laboratories, Inc., San Francisco, CA), rabbit anti-NT antibody (Upstate Biotechnology, Lake Plaid, NY), and rabbit anti-HNE antibody (Alpha Diagnostic Int., San Antonio, TX). Cardiac tissues were collected from diabetic FVB mice 2 weeks and 2 months after STZ treatment. After deparaffinization and redehydration, the slides were subjected to quenching endogenous peroxidase activity using 3% H2O2 for 10 minutes at room temperature. Nonspecific binding sites were blocked by 5% normal rabbit serum for 30 minutes. Sections were incubated with primary antibodies overnight at 4°C. The sections were then incubated with biotinylated rabbit anti-mouse IgG for 20 minutes, followed by incubation with streptavidin-horseradish peroxidase for 20 minutes. The antibody binding sites were visualized by incubation with a diaminobenzidine-H2O2 solution. Methyl green counterstaining for MT staining and no counterstaining was used for 3-NT and 4-HNE staining. Semiquantitative analysis for 3-NT and 4-HNE was performed by computerization of the percentage of the positive staining from five samples in each group with two sections for each sample and five images for each section.
MT Protein Measurement by Western Blot and Cadmium-Hemoglobin Assays
Cardiac tissues were homogenized with a lysis buffer containing 25 mmol/L Tris-HCl (pH 7.4), 0.5 mol/L EDTA, 0.5 mol/L EGTA, 1 mmol/L phenylmethyl sulfonyl fluoride, 25 μg/ml leupeptin, and 0.1% Chaps and centrifuged at 12,000 rpm at 4°C in a Beckman GS-6R centrifuge for 10 minutes. The suspension was collected and protein concentration was determined. The sample was diluted in loading buffer (40 mmol/L Tris-HCl, pH 6.8, 1% sodium dodecyl sulfate, 50 mmol/L dithiothreitol, 7.5% glycerol, 0.003% bromophenol blue), heated at 95°C for 5 minutes, and then subjected to electrophoresis on 16% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel at 120 V. Proteins were transferred from gels to polyvinylidene difluoride membrane. Membranes were incubated in 2.5% glutaraldehyde solution for 1 hour, and then were rinsed briefly with Tris-buffered saline buffer and blocked in blocking buffer (5% milk and 0.5% bovine serum albumin) for 1 hour, followed by three washes with Tris-buffered saline containing 0.1% Tween 20. The rabbit anti-MT antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a dilution of 1:300 was used and antigen-antibody complexes were then visualized by ECL kit (Amersham).
MT levels in the heart were determined by a cadmium-hemoglobin (Cd-hem) assay.15,18 Briefly, an aliquot of the cardiac protein suspension was diluted with 10 mmol/L Tris-HCl buffer (pH 7.4) and incubated with 10 ppm 109Cd solution with known specific activity to saturate the metal-binding sites of MT. Excess Cd was removed by adding hemoglobin and centrifugation. The Cd concentrations in the final supernatant were calculated from the radioactivity of the 109Cd, which were measured by a γ counter and converted to MT concentration on the basis of 7 g-atoms of Cd/MT. Total hepatic MT concentration was expressed as μg/g wet tissue.
Measurements of Cardiac Metals
Cardiac metal concentrations were determined by using inductively coupled argon plasma emission spectroscopy (model 35608; Thermo ARL-VG Elemental, Franklin, MA) after lyophilization and digestion of the tissues with nitric acid and hydrogen peroxide.37 Metal levels were expressed as μg/g dry tissue.
Measurements of Cardiac ET-1, IL-6, and TNF-α
Heart samples for measurements of ET-1 and TNF-α were prepared according to our previous publication.34 Briefly, heart samples were homogenized in 3 vol of ice-cold Ripa buffer. After incubation on ice for 30 minutes, samples were centrifuged twice at 20,000 × g for 10 minutes at 4°C. The resulting supernatants were used for assay. The TNF-α levels were detected by ET-1, IL-6, and TNF-α enzyme-linked immunosorbent assay kits, and expressed as pg/g heart tissue.33,34
Cardiac Reduced and Oxidized Glutathione
Cardiac GSH and oxidized GSH (GSSG) concentrations were determined by the glutathione-disulfide reductase and 5′5-dithiobis (2-nitrobenzoic acid) recycling assay, as described previously.38 Briefly, cardiac tissues were homogenized with 5% sulfosalicylic acid and centrifuged at 10,000 × g for 5 minutes. The supernatant of each sample was divided into two aliquots. One aliquot was directly used to measure total glutathione and another aliquot was first treated with 2-vinylpyridine to block the GSH, followed by measuring the GSSG. The reduced GSH value was determined by subtracting GSSG from total glutathione, and the ratio of GSSG/GSH in mol was used as the index of the existence of oxidative stress in the present study.
Assessment of Left Ventricular Hemodynamic Performance
General measurements of cardiac performance were performed using in situ left ventricle (LV) hemodynamic analysis as described previously.39 Mice were anesthetized using tribromoethanol (0.5 mg/g body weight, by 0.25 ml/g i.p. injection). After the catheter was slowly put in the LV the animal was allowed to stabilize for 20 to 30 minutes before recording of the waveform for up to 2 hours. Isoproterenol was used to generate β-adrenergic stimulation of the LV function. The right femoral vein was cannulated with PE 10 tubing and connected to a Harvard Apparatus/22 microinjecion pump for the infusion of isoproterenol. After 30- to 45-minute stabilization period, isoproterenol was infused at a rate of 0.02 ml/min (1.6 ng isoproterenol/minute/g body weight) for 1 minute at each time. Cardiac functional changes in response to isoproterenol were recorded immediately after the infusion for 20 to 30 minutes at which time cardiac function was recovered to baseline.
Histopathological Examination for Cardiac Fibrosis
Cardiac fibrosis was examined by Sirius red staining of collagen as described in our previous study.40 For Sirius red staining, sections of 5 μm thickness, prepared as described for immunohistochemical staining, were stained with 0.1% Sirius red F3BA and 0.25% Fast green FCF, and the Sirius red-stained sections were assessed for the proportion of fibrosis (collagen) in the heart tissues using computer-assisted image analysis system as described above for 3-NT and 4-HNE.
Statistic Analysis
All measurements are expressed as mean ± SD. The data were analyzed by analysis of variance and the Newman-Keuls multiple comparison test. Differences between groups were considered significant at P < 0.05.
Results
STZ-Induced Diabetic Model
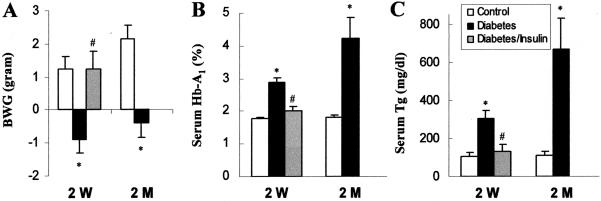
Mice treated with STZ were considered diabetic if their whole blood glucose levels are equal to or more than 250 mg/dl. Some of the diabetic mice were treated with insulin, called insulin-treated diabetic mice; this group is to control for any possible direct effect of STZ on cardiac MT expression independent of diabetes. Body weight measurement showed that diabetic mice had significantly lower body weight gain than control mice (Figure 1A). Glycated Hb-A1 and serum triglyceride were significantly increased in the diabetic mice (Figure 1, B and C). All these changes were prevented by insulin treatment, which is consistent with our previous studies.32
Figure 1.
STZ-induced diabetic model. Body weight gain (BWG, A), glycated hemoglobin (Hb-A1, B), and serum triglyceride (Tg, C) were measured in the control (white columns), diabetic mice (black columns), and insulin-treated diabetic mice (gray columns) 2 weeks (2 W) and 2 months (2 M) after STZ treatment. *P < 0.05 versus control; #P < 0.05 versus diabetes.
Cardiac MT Induction in Response to Diabetes
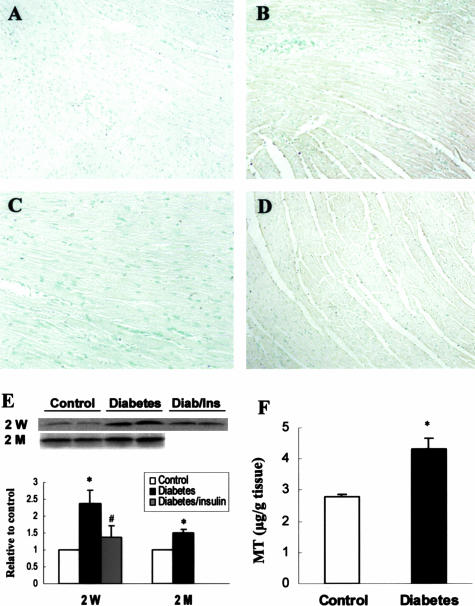
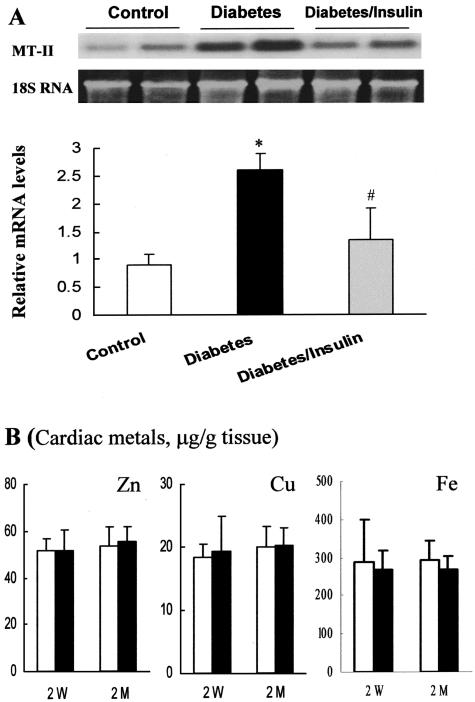
Diabetes-induced cardiac MT synthesis in the hearts of diabetic mice 2 weeks and 2 months after STZ treatment was first evidenced by immunohistochemical staining (Figure 2; A to D), and was quantitatively analyzed by Western blot (Figure 2E). In addition, quantitative analysis was also performed by Cd-hem assay in control and diabetic mice 2 months after STZ treatment (Figure 2F). These results showed that MT content was significantly increased in the hearts of diabetic mice 2 weeks (2.5-fold) and 2 months (1.5-fold) after STZ treatment, but not changed in the hearts of insulin-treated diabetic mice, suggesting that the induction of cardiac MT is diabetes related. To investigate whether the increased MT protein was due to up-regulation of its transcriptional level, MT-II mRNA was analyzed by Northern blot in the hearts of diabetic mice 2 weeks after STZ treatment (Figure 3A). Results showed that diabetes induced a significant increase in MT-II mRNA expression, and insulin treatment significantly prevented the diabetes-increased expression of cardiac MT mRNA.
Figure 2.
Diabetes-induced cardiac MT protein expression. Cardiac MT protein was measured by immunohistochemical staining (A–D), Western blot (E), and Cd-hem (F) assays in the diabetic mice 2 weeks (2 W) and 2 months (2 M) after STZ treatment. A: Control; B: diabetes at 2 weeks; C: insulin-treated diabetic mice at 2 weeks; D: diabetes at 2 months. In F, white column and black column represent control and diabetic mice 2 months after STZ treatment. *P < 0.05 versus control; #P < 0.05 versus diabetes.
Figure 3.
Diabetes-induced cardiac MT mRNA expression and metal changes. Cardiac MT mRNA was measured by Northern blot in the diabetic mice 2 weeks after STZ treatment. Cardiac metals were measured in the control (white columns) and diabetic mice (black columns) 2 weeks (2 W) and 2 months (2 M) after STZ treatment. *P < 0.05 versus control; #P < 0.05 versus diabetes.
No Change of Cardiac Metals in Response to Diabetes
Although metals such as Zn and Cu are the most common inducer for MT synthesis, no change of cardiac Zn, Cu, and Fe was found in the diabetic mice either 2 weeks or 2 months after STZ treatment (Figure 3B). To confirm the experimental model, hepatic MT and Zn contents were also measured. Hepatic MT induction (MT μg/g tissue: 16.5 ± 3.2 in diabetic liver versus 5.8 ± 0.4 in control liver, P < 0.05) was accompanied by a significant decrease in hepatic Zn level (μg/g tissue: 101.3 ± 0.6 in diabetic liver versus 90.2 ± 0.8 in control liver, P < 0.05, for 2 weeks after STZ treatment, and 104.6 ± 1.3 in diabetic liver versus 88.5 ± 2.3 in control liver, P < 0.05, for 2 months after treatment), as observed by our and other previous studies using diabetic rats.18,20
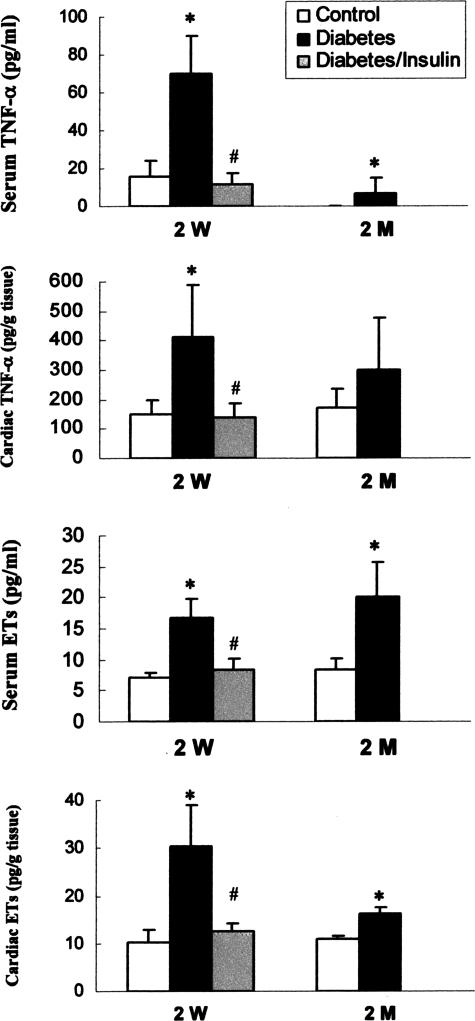
Increases in Serum and Cardiac TNF-α and ET-1 Levels in Response to Diabetes
Because diabetes is a systemic problem, whether the cardiac MT induction is associated with systemic response was explored. Serum levels of total ET-1 and TNF-α were measured by enzyme-linked immunosorbent assay method and significantly increased in the diabetic mice 2 weeks and 2 months after STZ treatment (Figure 4). Cardiac levels of TNF-α (2 weeks after STZ treatment) and ET-1 (both 2 weeks and 2 months after STZ treatment) were also elevated in the diabetic mice. These changes observed in diabetic group were completely prevented by insulin treatment (Figure 4). Undetectable levels of serum and cardiac IL-6 were noted in both control and diabetic mice under current experimental condition (data not shown).
Figure 4.
Diabetes-induced increases in serum and cardiac TNF-α and ETs. Serum and cardiac TNF-α and ET-1 were measured using specific enzyme-linked immunosorbent assay kits as described in Material and Methods, 2 weeks (2 W) and 2 months (2 M) after STZ treatment. *P < 0.05 versus control; #P < 0.05 versus diabetes.
Induction of Cardiac Oxidative Stress in Response to Diabetes
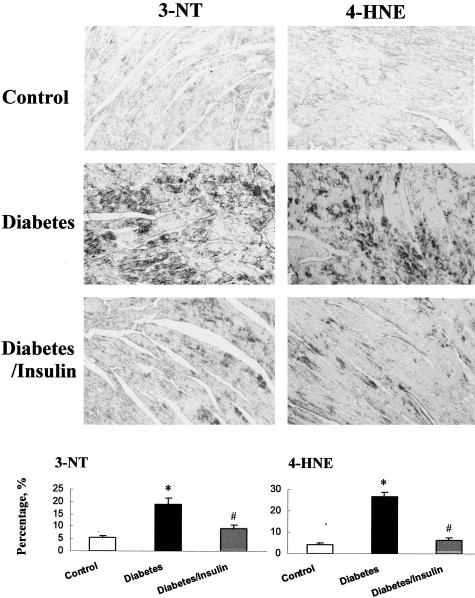
Increases of ET-1 and TNF-α in serum and in the heart may contribute to cardiac oxidative stress and damage.4,33,34,41 Therefore, the ratio of GSSG/GSH was measured in the hearts of control and diabetic mice 2 weeks after STZ treatment, and significantly increased (approximately threefold) in the diabetic hearts compared to control (0.014 ± 0.002 in the hearts of control mice versus 0.037 ± 0.001 in the hearts of diabetic mice 2 weeks after STZ treatment, P < 0.05), suggesting the existence of cardiac oxidative stress. To further explore the existence of oxidative stress-caused damage in this experimental model, 3-NT and 4-HNE were examined by immunohistochemical staining (Figure 5). Significant increases in 3-NT and 4-HNE staining were observed in diabetic hearts but not in the hearts of control and insulin-treated mice (Figure 5).
Figure 5.
Diabetes-induced cardiac oxidative damage. Immunohistochemical stainings for 3-nitrotyrosine (3-NT) and 4-hydroxynonenal (4-HNE) were performed in control, diabetic mice, and insulin-treated diabetic mice 2 weeks after STZ treatment. Semiquantitative analysis was performed by using computer-imaging system for the percentage of positive staining, as described in Material and Methods. *P < 0.05 versus control; #P < 0.05 versus diabetes.
Diabetes-Induced Cardiac Injury was Prevented in MT-TG Diabetic Mice
Our previous studies27–29 have shown the significant prevention of diabetic cardiomyopathy in the cardiac-specific MT-overexpressing transgenic mice, which is consistent with other studies.30,31 It should be mentioned that in those mice MT is ∼20- or 100-fold higher than control mice and also only in the cardiac myocytes. However, up-regulated MT synthesis in response to diabetes is unable to reach to 20-fold higher relative to control level and also not only in the heart. Therefore, whether systemically overexpressing MT with a cardiac MT content at the level similar to diabetes-induced cardiac MT expression can prevent diabetes-induced cardiac injury was examined using the MT-TG mice in the following experiments.
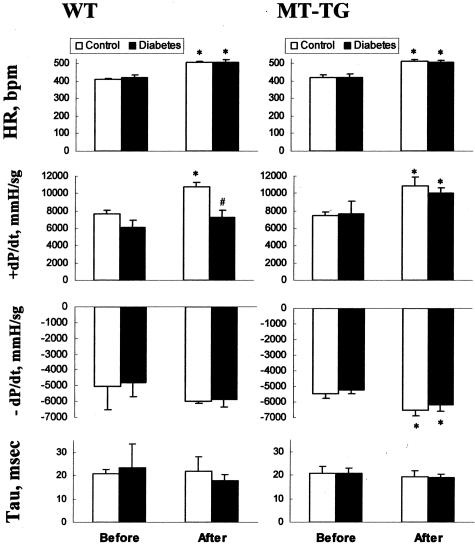
In these MT-TG mice MT is overexpressed in all tissues including the heart.42 Our measurement of cardiac MT in these mice was approximately threefold higher (12.3 ± 1.0 μg/g tissue) in the MT-TG mice than that in the WT mice (3.8 ± 0.3 μg/g tissue, P < 0.05). These mice were given STZ to induce diabetes, as described above in experiments using FVB mice. Two months after STZ treatment, cardiac function and fibrotic effect were evaluated by LV hemodynamic analysis and Sirius red staining of collagen. As shown in Figure 6, between control and diabetic groups either in the WT or in the MT-TG mice there were no significant differences for the basal heart rate (HR), the basal maximum rate of intraventricular pressure rise (+dP/dt) and basal minimum rate of intraventricular pressure fall (−dP/dt) during ventricular contraction, and tau, the constant of pressure decay, although there was a trend of decreased +dP/dt in the WT diabetic mice.
Figure 6.
Comparison of cardiac function changes between MT-TG and WT mice. LV hemodynamic performance was examined in the control (white columns, n = 4) and diabetic (black columns, n = 5 or 6) groups from WT and MT-TG mice. The representative endpoints including HR, +dP/dt, −dP/dt, and tau were examined by basal condition (before) and after isoproterenol stimulating condition (after). *P < 0.05 versus basal line (before); #P < 0.05 versus WT.
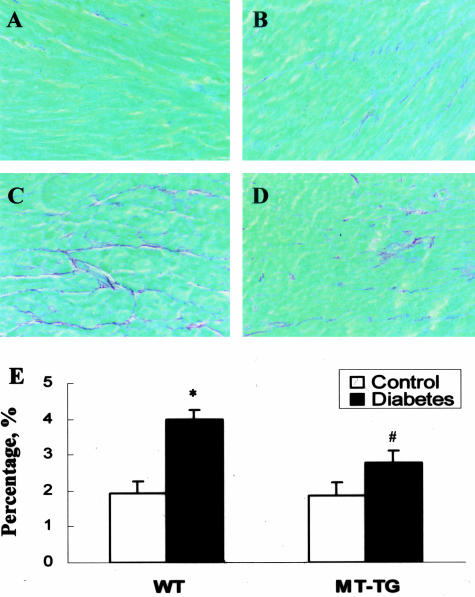
After isoproterenol stimulation, HR was significantly increased in both the control and diabetic groups either from the WT and MT-TG mice. The values of +dP/dt in both WT and MT-TG control mice were significantly increased (Figure 6, white columns). More important, this value in the MT-TG diabetic mice was also significantly increased in response to isoproterenol stimulation, similar to that in the MT-TG control group; however, it was almost not changed in the WT diabetic mice (Figure 6). The remarkable loss of inotropic reserve uncovered during β-adrenergic stimulation suggests the cardiac dysfunction in WT diabetic mice, whereas MT-TG diabetic mice still preserve this important cardiac function. Histopathological examination by Sirius red staining of collagen showed the existence of cardiac fibrosis in the WT diabetic mice (Figure 7; A, C, and E), but not in the MT-TG diabetic mice, (Figure 7; B, D, and E).
Figure 7.
Diabetes-induced cardiac fibrosis. Cardiac fibrosis was evaluated by sirius red staining of collagen in the heart of control (A, B, or white columns in E) and diabetic (C, D, or black columns in E) groups between WT (A, C) and MT-TG (B, D) mice 2 months after STZ treatment. *P < 0.05 versus control; #P < 0.05 versus WT diabetes.
Discussion
Diabetes-Induced MT Synthesis in the Heart
In this study, we demonstrated for the first time that as an antioxidant and stress-responsive protein, MT was also up-regulated at mRNA and protein levels in the hearts of diabetic mice 2 weeks and 2 months after STZ treatment. There were several pieces of indirect evidence in support of diabetes-induced up-regulation of cardiac MT. Besides hyperglycemia, hyperlipidemia is also a key feature in diabetic patients even for type 1 diabetics,1–4 as indicated in the present study (Figure 1C). Puskas and colleagues43 fed rats with high-fat food for 8 weeks to induce a hyperlipidemia model in which MT-II mRNA, discovered by microarray and defined by real-time PCR analysis, was increased approximately twofold to threefold in the heart as compared to normal rats. In another study, using skeletal muscle from diabetic rats and humans, increased expression of MT-II mRNA, discovered by microarray analysis, was also documented.44 The increased expression degree (twofold to threefold) and isoform of the MT (MT-II) in the heart in response to these diabetes-related stresses are same as those observed in the present study (Figures 2 and 3).
Possible Mechanisms Underlying the Cardiac MT Induction in Response to Diabetes
Up-regulation of MT expression was observed in the liver and kidney of STZ-induced diabetic rats, but seems not to be directly due to metal alterations.18–20 The present study, showing no change in cardiac Zn and Cu contents, and decrease in hepatic Zn content with a significant induction of cardiac and hepatic MT in response to diabetes, suggests that cardiac or hepatic MT induction in response to diabetes was not related to metal contents. The possibility of the direct effect of STZ on cardiac MT induction in the present study was also excluded because of the complete prevention of cardiac MT induction by insulin treatment in diabetic mice 2 weeks after STZ treatment. This is consistent with a previous study in which renal and hepatic MT induction in STZ diabetic rats were completely prevented by insulin treatment.45 Although we did not include an insulin-treated diabetic group in the diabetic mice 2 months after STZ treatment, the increased expression of cardiac MT in diabetic mice is most unlikely due to STZ direct effect because MT protein turnover is ∼3 or 4 days in most organs.10,23–26 In support of this notion, renal and hepatic MT contents were found to be significantly increased in spontaneously ob/ob diabetic mice and BB diabetic rats.46,47 Therefore, the cardiac MT expression in response to diabetic mice should not be related to STZ direct effect.
A significant increase in serum and cardiac ET-1 levels were observed in diabetic mice 2 weeks or 2 months after STZ treatment (Figure 4). Vasopeptide ET-1 plays an important role in the induction of hepatic and renal MT synthesis in response to diabetes.20 We demonstrated that in STZ diabetic rats, hepatic and renal MT contents at both mRNA (MT-II) and protein levels were significantly increased 6 months after diabetes onset. Application of ET-1-specific agonist Bosentan significantly prevented, in part, increases in MT levels in the liver and kidney. The important role of ET-1 in diabetes-induced MT synthesis was further supported by an in vitro study,48 in which MT-II mRNA expression and protein production were elevated in endothelial cells exposed to high levels of glucose, and further enhanced by the addition of exogenous ET-1 into the cultures. Addition of ET receptor blockers to these cultures partially prevented MT production.48 The partial prevention of diabetes- or high-level glucose-induced MT expression by blocking ET system suggests the involvement of other mechanisms in MT induction in response to diabetes.
The induction of MT synthesis by cytokines has been extensively documented under both in vivo and in vitro conditions.10,12 For example, radiation-induced MT protein synthesis was demonstrated mainly in the in vivo condition, whereas a very weak effect was observed in the in vitro conditions due to lack of a systemic inflammatory response.12 One of the major cytokines effective in inducing MT synthesis is TNF-α.10,12 TNF-α is expressed at a very low level in the normal heart49 but is significantly increased in response to various stresses including diabetes.34,50 In the present study, both serum and cardiac TNF-α levels were significantly elevated in the diabetic mice 2 weeks or 2 months (only serum level) after STZ treatment (Figure 4, A and B). Therefore the increased systemic and cardiac TNF-α may be one of the mechanisms responsible for cardiac MT induction in response to diabetes.
Induction of MT protein expression by oxidative stress has been observed in the rats and mice exposed to chemicals or radiation.9,12 Cardiac oxidative stress is found to be a major cause for diabetic cardiomyopathy, which may relate to multiple factors including what were observed in the present studies: hyperglycemia, hyperlipidemia, and inflammatory response such as induction of ET-1 and TNF-α in response to diabetes (Figures 1 and 4). As mentioned earlier, oxidative stress includes overproduction of ROS or RNS and suppression of antioxidant capacity. GSH, a free radical scavenger, plays an important role in the maintenance of protein sulfhydryl groups.4,36 During inactivation of ROS such as hydrogen peroxides, GSH is converted to oxidized form (GSSG); therefore, an increased GSSG:GSH ratio is used as the index of oxidative stress.36 Consistent with the results of previous work,30 an increase in the GSSG:GSH ratio was found in the hearts of diabetic mice 2 weeks after STZ, suggesting the existence of cardiac oxidative stress in the current diabetic model. Increasing evidence indicates that superoxide overproduction derived from diabetes plays a critical role in the development of diabetic cardiovascular complications2–7 because it interacts with nitric oxide to form peroxynitrite or derives hydrogen peroxide in the presence of superoxide dismutase, and then causes protein nitration or lipid peroxidation. In support of this notion, significant increases in 3-NT and 4-HNE protein adducts, detected by immunohistochemical staining, were observed in the diabetic hearts (Figure 5). Therefore, we conclude that cardiac MT induction is most likely due to the occurrence of cardiac oxidative stress associated with diabetic multiple factors, including increased systemic increases ET-1 and TNF-α.
Possible Biological Importance of Diabetes-Induced MT Synthesis
MT can suppress production of proinflammatory factors including TNF-α51 and protect the heart from various oxidative damage.10,19,25,41 Therefore, cardiac MT synthesis in response to diabetes may be an adaptive mechanism to compensate for cardiac TNF-α increase and associated oxidative damage. We have shown that cardiac cell death significantly increased after exposed to TNF-α in vitro and MT can significantly inhibit this effect.41 We also noted that cell death takes place in the heart of diabetic mice and rats, with a high incidence at early stage (7 to 14 days after STZ treatment) than that at later stage.32,52 Therefore, the lower incidence of cardiac cell death in the later stage may be related to cardiac MT induction as an adaptive mechanism in the heart. In fact, MT induction has been indicated in the preconditioned heart11 and cardiac-specific overexpressing MT significantly protected from ischemia/reperfusion-induced cardiac damage.53
However, if MT is up-regulated in response to diabetes as an adaptive response to protect the heart from damage, why does it not prevent the occurrence of diabetic cardiomyopathy? We hypothesize that the up-regulated MT expression observed under these conditions in the WT mice is secondary to diabetes-caused systemic injury and organ damage. This damage induces MT induction but also simultaneously initiates the pathogenesis of diabetic cardiomyopathy. Therefore, the diabetes-induced MT expression in the heart does not prevent the initiation of diabetic cardiomyopathy, but may prevent diabetes-induced further cardiac damage and consequently slow the progression of the diabetic cardiomyopathy. To prevent the occurrence of diabetic cardiomyopathy, pre-enhanced cardiac MT content may be requested. In line with this notion, the present study provides the evidence that MT-TG mice were significantly resistant to diabetes-induced cardiac injury. Although the present study only presents a cardiac examination between MT-TG and WT diabetic mice at a relatively short period (≤2 months) after diabetes induction, at which time point typical features of cardiomyopathy such as diastolic dysfunction may be still not developed, a moderate cardiac dysfunction was still evident, shown by a significant loss of cardiac inotropic reserve under stimulating condition and an increase in cardiac fibrosis. These moderate abnormalities were completely prevented in the MT-TG mice, which is consistent with our previous studies and others.27–31
These results suggest that to prevent the development of diabetic cardiomyopathy requires a pre-up-regulated MT in the heart. This is a very important and clinically relevant issue because MT is inducible by physical stress such as exercise and MT inducer such as Zn.10,12 However, the overexpressed MT in the transgenic mice used in these previous studies27–31 is only in the cardiac myocytes and also 20- or 100-fold higher than control level, both which are impossible to be manipulated by pharmaceutical approach such as administration of MT inducer. Our earlier study has indicated that in a cardiac-specific catalase-overexpressing transgenic mouse model, which was also proved to prevent diabetic cardiomyopathy by others,54 catalase protection against adriamycin-induced cardiac damage is gene dose-dependent.55 Neither a line with 15-fold increase nor a line with 200-fold or greater increase in cardiac catalase over normal level provided apparent protection, and a line with 100-fold increase in the cardiac catalase provided a maximal protection.55 Whether MT cardiac protection from diabetes is also dose-dependent and whether MT expression in the heart at an inducible level provides cardiac protection from diabetes will be critical issues before exploring its clinical application. Therefore, an innovative and important finding of the present study is to provide the direct evidence using a nonorgan-specific MT-TG mouse model that cardiac MT content at an inducible level such as induced by Zn25,26 provides a significant protection from diabetes-induced cardiac injury.
Acknowledgments
We thank Mr. Don Mosley for his technological assistance.
Footnotes
Address reprint requests to Dr. Lu Cai, Department of Medicine, University of Louisville School of Medicine, 511 South Floyd St., MDR 533, Louisville, KY 40202. E-mail: l0cai001@louisville.edu.
Supported in part by the Jewish Hospital Foundation, Louisville, KY (grant JHF 010808); Philip Morris USA, Inc. (grant PM020187); the American Diabetes Association (grant ADA020667 to L.C.); and the National Institutes of Health (grants HL63760 and HL59225 to Y.J.K.).
Y.S. and J.W. contributed equally to this study.
Y.J.K. is a Distinguished University Scholar of the University of Louisville.
References
- Chatham JC, Forder JR, McNeill JH. Norwell: Kluwer Academic Publishers,; The Heart in Diabetes, (ed 1) 1996 [Google Scholar]
- Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26:1589–1596. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- Da Ros R, Assaloni R, Ceriello A. Antioxidant therapy in diabetic complications: what is new? Curr Vasc Pharmacol. 2004;2:335–341. doi: 10.2174/1570161043385538. [DOI] [PubMed] [Google Scholar]
- Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001;1:181–193. doi: 10.1385/ct:1:3:181. [DOI] [PubMed] [Google Scholar]
- Joyeux M, Faure P, Godin-Ribuot D, Halimi S, Patel A, Yellon DM, Demenge P, Ribuot C. Heat stress fails to protect myocardium of streptozotocin-induced diabetic rats against infarction. Cardiovasc Res. 1999;43:939–946. doi: 10.1016/s0008-6363(99)00185-6. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Barrett CJ, Sun SY, Schultz HD. Oxidative stress impairs cardiac chemoreflexes in diabetic rats. Am J Physiol. 2000;279:H2176–H2187. doi: 10.1152/ajpheart.2000.279.5.H2176. [DOI] [PubMed] [Google Scholar]
- Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hanninen O, Sen CK. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol. 2004;97:605–611. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P. Expression of acute-phase response proteins in retinal Muller cells in diabetes. Invest Ophthalmol Vis Sci. 2005;46:349–357. doi: 10.1167/iovs.04-0860. [DOI] [PubMed] [Google Scholar]
- Yin X, Wu H, Chen Y, Kang YJ. Induction of antioxidants by adriamycin in mouse heart. Biochem Pharmacol. 1988;56:87–93. doi: 10.1016/s0006-2952(98)00099-9. [DOI] [PubMed] [Google Scholar]
- Kang YJ. The antioxidant function of metallothionein in the heart. Proc Soc Exp Biol Med. 1999;222:263–273. doi: 10.1046/j.1525-1373.1999.d01-143.x. [DOI] [PubMed] [Google Scholar]
- Onody A, Zvara A, Hackler L, JR, Vigh L, Ferdinandy P, Puskas LG. Effect of classic preconditioning on the gene expression pattern of rat hearts: a DNA microarray study. FEBS Lett. 2003;536:35–40. doi: 10.1016/s0014-5793(03)00006-1. [DOI] [PubMed] [Google Scholar]
- Cai L, Satoh M, Tohyama C, Cherian MG. Metallothionein in radiation exposure: its induction and protective role. Toxicology. 1999;132:85–98. doi: 10.1016/s0300-483x(98)00150-4. [DOI] [PubMed] [Google Scholar]
- Cai L, Klein JB, Kang YJ. Metallothionein inhibits peroxynitriteinduced DNA and lipoprotein damage. J Biol Chem. 2000;275:38957–38960. doi: 10.1074/jbc.C000593200. [DOI] [PubMed] [Google Scholar]
- Cai L, Cherian MG. Zinc-metallothionein protects from DNA damage induced by radiation better than glutathione and copper- or cadmium-metallothioneins. Toxicol Lett. 2003;136:193–198. doi: 10.1016/s0378-4274(02)00359-4. [DOI] [PubMed] [Google Scholar]
- Cai L, Deng DX, Jiang J, Chen S, Zhong R, Cherian MG, Chakrabarti S. Induction of metallothionein synthesis with preservation of testicular function in rats following long term renal transplantation. Urol Res. 2000;28:97–103. doi: 10.1007/s002400050145. [DOI] [PubMed] [Google Scholar]
- Courtade M, Carrera G, Paternain JL, Martel S, Carre PC, Folch J, Pipy B. Metallothionein expression in human lung and its varying levels after lung transplantation. Toulouse Lung Transplantation Group. Chest. 1998;113:371–378. doi: 10.1378/chest.113.2.371. [DOI] [PubMed] [Google Scholar]
- Baba HA, Schmid KW, Takeda A, Wichter T, Gradaus R, Erren M, Plenz G, Grabellus F, Tjan TD, Deng MC. Metallothionein: localization in human transplant endomyocardium, relation to cytokines and allograft function. J Heart Lung Transplant. 1999;18:963–971. doi: 10.1016/s1053-2498(99)00061-3. [DOI] [PubMed] [Google Scholar]
- Cai L, Chen S, Evans T, Cherian MG, Chakrabarti S. Endothelin-1-mediated alteration of metallothionein and trace metals in the liver and kidneys of chronically diabetic rats. Int J Exp Diabetes Res. 2002;3:193–198. doi: 10.1080/15604280214281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L. Metallothionein as an adaptive protein prevents diabetes and its toxicity. Nonlinearity Biol Toxicol Med. 2004;2:89–103. doi: 10.1080/15401420490464367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Failla ML. Metallothionein metabolism in the liver and kidney of the streptozotocin-diabetic rat. Comp Biochem Physiol B. 1998;90:439–445. doi: 10.1016/0305-0491(88)90101-0. [DOI] [PubMed] [Google Scholar]
- Aguilar MV, Laborda JM, Martinez-Para MC, Gonzalez MJ, Meseguer I, Bernao A, Mateos CJ. Effect of diabetes on the tissular Zn/Cu ratio. J Trace Elem Med Biol. 1998;12:155–158. doi: 10.1016/S0946-672X(98)80004-7. [DOI] [PubMed] [Google Scholar]
- Karahan SC, Deger O, Orem A, Ucar F, Erem C, Alver A, Onder E. The effects of impaired trace element status on polymorphonuclear leukocyte activation in the development of vascular complications in type 2 diabetes mellitus. Clin Chem Lab Med. 2001;39:109–115. doi: 10.1515/CCLM.2001.019. [DOI] [PubMed] [Google Scholar]
- Nath R, Kumar D, Li T, Singal PK. Metallothioneins, oxidative stress and the cardiovascular system. Toxicology. 2000;155:17–26. doi: 10.1016/s0300-483x(00)00273-0. [DOI] [PubMed] [Google Scholar]
- Naganuma A, Satoh M, Imura N. Specific reduction of toxic side effects of adriamycin by induction of metallothionein in mice. Jpn J Cancer Res. 1988;79:406–411. doi: 10.1111/j.1349-7006.1988.tb01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Naganuma A, Imura N. Involvement of cardiac metallothionein in prevention of adriamycin induced lipid peroxidation in the heart. Toxicology. 1988;53:231–237. doi: 10.1016/0300-483x(88)90216-8. [DOI] [PubMed] [Google Scholar]
- Ali MM, Frei E, Straub J, Breuer A, Wiessler M. Induction of metallothionein by zinc protects from daunorubicin toxicity in rats. Toxicology. 2002;179:85–93. doi: 10.1016/s0300-483x(02)00322-0. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Cai L. Metallothionein suppression of diabetic cardiomyopathy by inhibition of hyperglycemia-induced oxidative stress. Free Radic Biol Med. 2001;31(Suppl 1):33. [Google Scholar]
- Cai L, Kang YJ. Metallothionein prevents diabetic cardiomyopathy. Toxicol Sci. 2001;60:13. [Google Scholar]
- Cai L, Li W, Sun X, Li Y, Kang YJ. Prevention of diabetic cardiomyopathy by metallothionein through suppression of hyperglycemia-induced oxidative stress and cell death. Toxicol Sci. 2002;66(Suppl 1):288. [Google Scholar]
- Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes. 2002;51:174–181. doi: 10.2337/diabetes.51.1.174. [DOI] [PubMed] [Google Scholar]
- Ye G, Metreveli NS, Ren J, Epstein PN. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes. 2003;52:777–783. doi: 10.2337/diabetes.52.3.777. [DOI] [PubMed] [Google Scholar]
- Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Li Y, Zhou Z, Roberts AM, Cai L, Myers SR, Wang L, Schuchke DA. Elevation of serum endothelins and cardiotoxicity induced by particulate matter (PM2.5) in rats with acute myocardial infarction. Cardiovasc Toxicol. 2002;2:253–261. doi: 10.1385/ct:2:4:253. [DOI] [PubMed] [Google Scholar]
- Song Y, Song Z, Zhang L, McClain CJ, Kang YJ, Cai L. Diabetes enhances lipopolysaccharide-induced cardiac toxicity in the mouse model. Cardiovasc Toxicol. 2003;3:363–372. doi: 10.1385/ct:3:4:363. [DOI] [PubMed] [Google Scholar]
- Chen S, Evans T, Mukherjee K, Karmazyn M, Chakrabarti S. Diabetes-induced myocardial structural changes: role of endothelin-1 and its receptors. J Mol Cell Cardiol. 2000;32:1621–1629. doi: 10.1006/jmcc.2000.1197. [DOI] [PubMed] [Google Scholar]
- Hileeto D, Cukiernik M, Mukherjee S, Evans T, Barbin Y, Downey D, Karmazyn M, Chakrabarti S. Contributions of endothelin-1 and sodium hydrogen exchanger-1 in the diabetic myocardium. Diabetes Metab Res Rev. 2002;18:386–394. doi: 10.1002/dmrr.322. [DOI] [PubMed] [Google Scholar]
- Elsherif L, Ortines RV, Saari JT, Kang YJ. Congestive heart failure in copper-deficient mice. Exp Biol Med (Maywood) 2003;228:811–817. doi: 10.1177/15353702-0322807-06. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, Kang Y James. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med (Maywood) 2002;227:214–222. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]
- Li Y, Gu Y, Song Y, Zhang L, Kang YJ, Prabhu SD, Cai L. Cardiac functional analysis by electrocardiography, echocardiography and in situ hemodynamics in streptozotocin-induced diabetic mice. J Health Sci. 2004;50:356–365. [Google Scholar]
- Jiang Y, Kang YJ. Metallothionein gene therapy for chemical-induced liver fibrosis in mice. Mol Ther. 2004;10:1130–1139. doi: 10.1016/j.ymthe.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Klein JB, Wang GW, Zhou Z, Buridi A, Kang YJ. Inhibition of tumor necrosis factor-alpha-dependent cardiomyocyte apoptosis by metallothionein. Cardiovasc Toxicol. 2002;2:209–218. doi: 10.1007/s12012-002-0005-4. [DOI] [PubMed] [Google Scholar]
- Iszard MB, Liu J, Liu Y, Dalton T, Andrews GK, Palmiter RD, Klaassen CD. Characterization of metallothionein-I-transgenic mice. Toxicol Appl Pharmacol. 1995;133:305–312. doi: 10.1006/taap.1995.1155. [DOI] [PubMed] [Google Scholar]
- Puskas LG, Nagy ZB, Giricz Z, Onody A, Csonka C, Kitajka K, Hackler L, Jr, Zvara A, Ferdinandy P. Cholesterol diet-induced hyperlipidemia influences gene expression pattern of rat hearts: a DNA microarray study. FEBS Lett. 2004;562:99–104. doi: 10.1016/S0014-5793(04)00189-9. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involves a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- Failla ML, Kiser RA. Altered tissue content and cytosol distribution of trace metals in experimental diabetes. J Nutr. 1981;111:1900–1909. doi: 10.1093/jn/111.11.1900. [DOI] [PubMed] [Google Scholar]
- Failla ML, Gardell CY. Influence of spontaneous diabetes on tissue status of zinc, copper, and manganese in the BB Wistar rat. Proc Soc Exp Biol Med. 1985;180:317–322. doi: 10.3181/00379727-180-42182. [DOI] [PubMed] [Google Scholar]
- Kennedy ML, Failla ML. Zinc metabolism in genetically obese (ob/ob) mice. J Nutr. 1987;117:886–893. doi: 10.1093/jn/117.5.886. [DOI] [PubMed] [Google Scholar]
- Apostolova MD, Chen S, Chakrabarti S, Cherian MG. High-glucose-induced metallothionein expression in endothelial cells: an endothelin-mediated mechanism. Am J Physiol. 2001;281:C899–C907. doi: 10.1152/ajpcell.2001.281.3.C899. [DOI] [PubMed] [Google Scholar]
- Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- Depre C, Young ME, Ying J, Ahuja HS, Han Q, Garza N, Davies PJ, Taegtmeyer H. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol. 2000;32:985–996. doi: 10.1006/jmcc.2000.1139. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Hidalgo J. Metallothionein treatment reduces proinflammatory cytokines IL-6 and TNF-alpha and apoptotic cell death during experimental autoimmune encephalomyelitis (EAE). Exp Neurol. 2001;170:1–14. doi: 10.1006/exnr.2001.7675. [DOI] [PubMed] [Google Scholar]
- Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol. 2003;3:219–228. doi: 10.1385/ct:3:3:219. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Li Y, Sun X, Sun X. Antiapoptotic effect and inhibition of ischemia/reperfusion-induced myocardial injury in metallothionein-overexpressing transgenic mice. Am J Pathol. 2003;163:1579–1586. doi: 10.1016/S0002-9440(10)63514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996;271:12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]