Abstract
The pathology associated with tuberous sclerosis complex (TSC) shows diverse phenotypes that suggest abnormal signaling of multiple pathways. Besides the negative regulatory role of the TSC1/TSC2 proteins on mTOR, we have reported an effect on β-catenin signaling at the level of the degradation complex in vitro. The TSC1/TSC2 complex associates with GSK3 and Axin and promotes β-catenin degradation to inhibit Wnt-stimulated TCF/LEF-dependent transcription. Here, we show that β-catenin and its effectors, cyclin D1 and connexin 43, were up-regulated in TSC-related angiomyolipomas and lymphangioleiomyomatosis. This was supported by the failure of three disease-causing TSC2 missense mutants to inhibit Wnt signaling. Further, the interaction between TSC1/TSC2 and components of the β-catenin degradation complex was dependent on Wnt stimulation such that binding of tuberin to GSK3 and Axin was reduced in the presence of Wnt whereas the tuberin-Dishevelled interaction was increased. GSK3 activity played a role in regulating the assembly/stability of the degradation complex. Inhibition of GSK3 by lithium chloride reduced its association with TSC1 whereas disruption of GSK3-phosphorylation sites in TSC1 reduced interaction between TSC2 and TSC1. Collectively, our data provide further evidence that β-catenin signaling plays a role in TSC pathogenesis in vivo and suggest a novel role of GSK3 in modulating the TSC1/TSC2 complex through TSC1 phosphorylation.
Tuberous sclerosis complex (TSC) is a multiorgan disorder of benign hamartomas with rare potential for malignant transformation.1 It is classified as a hereditary tumor syndrome along with other members of the phakomatoses including Cowden’s disease and Peutz-Jeghers syndrome. Significant overlap in the function of the respective genes associated with these three diseases has recently been uncovered. Specifically, PTEN, the gene responsible for Cowden’s disease, inhibits growth factor-dependent PI3K/Akt signaling by acting as a phosphatase for PIP3. LKB1 is a serine/threonine kinase involved in Peutz-Jeghers syndrome whose function is to phosphorylate AMPK, a key enzyme in the cellular energy-sensing mechanism.2,3 Both Akt and AMPK directly phosphorylate TSC2, one of two proteins affected in TSC, to inhibit or activate its activity, respectively.4,5 In turn, TSC2 complexes with TSC1 to exert a negative regulatory role on Rheb via TSC2 GAP activity, which leads to down-regulation of mTOR signaling and inhibition of protein synthesis mediated by p70S6K and 4E-BP1.6 Thus, TSC1/TSC2 plays a pivotal role in integrating signals stemming from growth factor and energy availability in the regulation of cell growth. The biochemical relationship of the four tumor suppressor proteins (PTEN, LKB1, TSC1, TSC2) is highlighted by the dysregulation of the mTOR pathway in all three syndromes.
Despite the common molecular mechanism, the clinical spectrum and diagnostic criteria of these diseases are quite distinct. For example, patients with Cowden’s disease manifest with malignancies of the breast and thyroid, those with Peutz-Jeghers syndrome are predisposed to gastrointestinal polyps and carcinoma, whereas TSC typically affects the pediatric population with seizures, mental retardation and autism, and benign proliferation of the kidneys and lungs in older patients.1,7,8 Among the explanations that have been proposed to account for these differences, we postulate that the four tumor suppressor gene products may share a common pathway (ie, mTOR) and also possess other unique functions. For example, we have previously reported that on Wnt stimulation, co-expression of TSC1 and TSC2 represses β-catenin-dependent transcriptional activity.9 This raises the possibility that some of the phenotypic features of TSC may be the consequences of abnormal β-catenin signaling.
The biology of TSC overlaps with the known function of the β-catenin pathway. Collective observations from cortical tubers, angiomyolipomas (AML), lymphangioleiomyomatosis (LAM), and other TSC pathologies point to underlying defects in cell differentiation and migration, in addition to proliferation and growth. These important cellular processes are known to be regulated by the Wnt/β-catenin pathway through the combined effects of β-catenin on transcriptional activation and on cell adhesion.10 In response to secreted growth factors such as those in the Wnt family, signaling is initiated at the membrane receptors, Frizzled and LRP5/6, to activate dishevelled (Dsh). Dishevelled in concert with Frat1, blocks the activity of the β-catenin degradation complex to increase the cytoplasmic pool of β-catenin available for nuclear translocation and subsequent co-activation of the TCF/LEF family of transcription factors. A number of target genes including c-myc, cyclin D1, vascular endothelial growth factor, and connexin 43, are thought to mediate the effects of β-catenin during tumorigenesis.11–14 In addition to its signaling role, β-catenin binds type 1 cadherins and α-catenin to link cell adhesion proteins to the actin cytoskeleton and regulate cell morphology, adhesion, and migration. The integrity of the cadherin/catenin complex is modulated by multiple phosphorylation events including tyrosine phosphorylation of β-catenin by receptor tyrosine kinases leading to complex disassembly and promotion of nuclear β-catenin signaling. Although much remains unclear, recent studies are beginning to shed light on the interconnection between the membrane activity of β-catenin with that of its nuclear function.10 We hypothesize that modulation of β-catenin by TSC1/TSC2 may provide an alternative pathway that may account for some of the unique abnormalities seen in TSC compared to other phakomatoses.
In this study, we provide further evidence linking TSC1/TSC2 to the β-catenin signaling pathway by examination of the expression of β-catenin and its effectors in TSC-related pathology, the effects of disease-causing TSC2 missense mutations on β-catenin-dependent luciferase activity, complex formation between TSC1/TSC2 with Dsh and the GSK3 degradation complex, and the influence of GSK3 activity on the complex. Our results strongly support a biological role of the TSC proteins in the β-catenin pathway, and reveal a novel mechanism of GSK3-dependent TSC1-TSC2 interaction.
Materials and Methods
Chemicals and Plasmids
The antibodies for connexin-43 and hamartin were purchased from Zymed (San Francisco, CA), cyclin D1 from Rockland (Gilbertsville, PA), actin and Flag (M2) from Sigma (St. Louis, MO), GSK3 and β-catenin from BD Biosciences Pharmingen (San Diego, CA), Axin and TSC1 from Upstate (Charlottesville, VA), Dsh (H75) and TSC2(C20) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), HA (12CA5) from Roche (Indianapolis, IN), and smooth muscle actin from DAKO (Carpinteria, CA). Polyclonal anti-hamartin was prepared as described previously.15 All other antibodies were purchased from Cell Signaling (Beverly, MA). Secondary antibodies and ECL reagents were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). The Elite ABC and M.O.M. kits, diaminobenzidine, and hematoxylin QS were purchased from Vector Laboratories (Burlingame, CA). Eosin was obtained from Richard Allen Scientific (Kalamazoo, MI). LY 294002 was obtained from Calbiochem (La Jolla, CA) and LiCl from Sigma. The TNT Quick Coupled Transcription/Translation system was purchased from Promega (Madison, WI). [γ-32P] ATP was obtained from Perkin-Elmer (Boston, MA). Purified GSK3 was purchased from Upstate. The SilverQuest silver staining kit, LipofectAMINE, and Plus reagent were purchased from Invitrogen (Carlsbad, CA). Tsc2 mutant constructs were created by polymerase chain reaction mutagenesis and sequenced as described previously.15 Expression constructs included Flag-Dsh (gift of Randall Moon, University of Washington, Seattle, WA), Xenopus GSK-3 Flag(XG73),16 GSK-3 S9A, and Xenopus Axin-myc (gift of David Kimmelman, University of Washington, Seattle, WA), myc-tagged WT and 357A/390A TSC1 (gift of Kun-Liang Guan, University of Michigan, Ann Arbor, MI), mWnt-1 (gift from Marian Waterman, University of California, Irvine, CA), ΔN-Tcf-4,17 c-β-galactosidase (β-gal),18 and the TOPFLASH reporter construct.17
Animals
The Eker rat strain harboring a germ-line Tsc2 mutation is as previously described.19 The Tsc2+/− knockout mouse was obtained from David Kwiatkowski (Harvard University, Boston, MA). All work related to animals was in accordance with the protocol approved by the Animal Care Committee, University of Washington, Seattle, WA.
Immunohistochemistry
Slides mounted with human AML and LAM sections were obtained from Elizabeth Henske (Fox Chase Cancer Center, Philadelphia, PA) and Vera Krymyskya (University of Pennsylvania, Philadelphia, PA). Rodent kidney samples were fixed in formalin, paraffin-embedded, and 5-μm sections were cut. Slides were deparaffinized, rehydrated, and washed with phosphate-buffered saline (PBS). After antigen retrieval in 0.1 mmol/L sodium citrate (pH 6.0) and quenching of endogenous peroxidase activity with 1% H2O2, samples were blocked with 5% normal goat serum before incubation with primary antibodies overnight at 4°C. Negative controls were treated with 5% normal goat serum without the primary antibodies. Signals were processed according to supplied protocol (Elite ABC or M.O.M. kit). Slides were counterstained with hematoxylin QS, dehydrated, and mounted using Permount (Fischer Scientific, Santa Clara, CA).
Western Blotting
Both normal and tumor kidney tissue were homogenized in ice-cold RIPA buffer [1% Nonidet P-40 (NP-40), 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 0.15 mol/L NaCl, 10 mmol/L Tris (pH 7.2), 0.025 mol/L β-glycerophosphate (pH 7.2), 2 mmol/L ethylenediamine tetraacetic acid, and 50 mmol/L sodium fluoride] with protease and phosphatase inhibitors (0.05 mmol/L 4-(2-aminoethyl)benzenesulfonyl fluoride, HCI (AEBSF), 10 μg/ml aprotinin, 10 μg/ml pepstatin, 1 mmol/L orthovanadate, 10 μg/ml leupeptin, 1 mmol/L microcystin LR). The protein concentration was measured using the BCA protein assay (Pierce, Rockford, IL). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to Immobilon-P membranes (Millipore, Bedford, MA), and blotted with antibodies according to manufacturer recommendations.
Luciferase Reporter Assay
Assessment of β-catenin activity was performed using the TOPFLASH assay as previously described.9 Briefly, cells (HEK293T) were transfected with the Tcf/LEF reporter construct (TOPFLASH)17 using LipofectAMINE Plus reagents. Vectors encoding TSC1, TSC2, or TSC2 (R905Q, S1498N, S1704T) mutants were co-transfected with or without Wnt-1. A vector encoding β-gal was co-transfected as a transfection control while a dominant-negative Tcf-4 (ΔN-Tcf-4) was used as a negative control for activation. A reporter vector containing mutated Tcf/LEF-binding sites (FOPFLASH)17 served as a control for background activity. Luciferase activity was analyzed using an EG&G Berthold Autolumet LB953 luminometer (Perkin-Elmer Instruments, Bad Wildbad, Germany), and normalized to corresponding β-gal values.
Immunoprecipitation
For immunoprecipitation of exogenous proteins, HEK293T cells were transfected with the constructs as shown. Empty vector (pcDNA3) was co-transfected as needed to equalize the total amount of transfected DNA. For drug treatments, cells were treated for 25 hours with 20 mmol/L LiCl or 20 μmol/L LY294002. Cells were harvested 48 or 28 hours after transfection in PBS and then lysed in 0.5% NP-40 lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.5% NP-40, 2.5 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L NaVO4, 1 mmol/L phenylmethyl sulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). Protein concentrations were measured using the BCA protein assay (Pierce). One hundred μg of whole cell lysates were incubated with the appropriate antibodies overnight at 4°C. Two hours after the addition of Protein A or G Sepharose (Sigma), samples were washed with 0.5% NP-40 lysis buffer. Samples were then analyzed by 7% SDS-PAGE, transferred to Immobilon-P membranes (Millipore), and immunoblotted as shown.
For immunoprecipitation of endogenous proteins, HEK293T cells were transfected with the Wnt-1 construct or empty vector. Cells were harvested the following day in lysis buffer (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.5% NP-40, 0.5 μg/ml leupeptin, 1.0 μg/ml pepstatin, 0.2 mmol/L phenylmethyl sulfonyl fluoride). Equal amounts of protein were immunoprecipitated using the anti-tuberin polyclonal antibody and blotted with anti-bodies against GSK3, Axin, and Dsh as previously described.9 For immunoprecipitation of drug-treated cells, HEK293 cells were treated for 19 hours with 20 mmol/L LiCl or 20 μmol/L LY294002. Cells were harvested in 0.5% NP-40 lysis buffer (see above). Five hundred μg of lysates were immunoprecipitated using the anti-GSK3 monoclonal antibody and blotted as shown.
In Vitro Kinase Assay
The kinase assay was performed as described by Eldar-Finkelman and colleagues.20 Briefly, TNT reactions were performed using the myc-TSC1 WT, myc-TSC1 357A/390A (T7), or myc-Axin (SP6) constructs per the manufacturer’s instructions. Axin, TSC1 WT, or 357A/390A were immunoprecipitated from either 30 μl TNT reaction (TSC1) or 90 μl (Axin) by incubating with antibodies for Myc (9B11, Cell Signaling) in harvest buffer (0.5% NP-40, 150 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.4, 0.5 μg/ml leupeptin, 2.8 μg/ml aprotinin, 2 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L dithiothreitol, 1 mmol/L sodium orthovanadate, 0.5 mmol/L AEBSF, 1 μmol/L microcystin), for 2 hours followed by protein A/G Sepharose overnight. The beads were washed twice with harvest buffer, twice with high-salt wash buffer (same as harvest buffer with the addition of 350 mmol/L NaCl), split to two tubes, then washed twice with kinase buffer (20 mmol/L Hepes, pH 7.5, 10 mmol/L magnesium acetate, 2 mmol/L dithiothreitol) and resuspended in an equal liquid-to-bead volume of kinase buffer. Purified GSK3 was added to half of the tubes immediately before adding a 5× reaction mixture, bringing the samples to a final concentration of 20 mmol/L Tris-HCl, pH 7.4, 10 mmol/L magnesium acetate, 0.0002% Triton X-100, 100 μmol/L cold ATP, and 10 μCi [γ-32P] ATP. The beads were incubated for 20 minutes at 30°C with shaking (800 rpm). The reactions were stopped by adding prewarmed 4× SDS-PAGE sample buffer and heating at 95°C. Samples were resolved by SDS-PAGE and the gels were exposed to film for detection of bands. Bands from the gel were excised and counted for radioactivity using a Packard Tricarb 2100TR liquid scintillation counter (United Technologies Packard, Downers Grove, IL). To determine the amount of TSC1 or Axin in the GSK3 kinase reaction, IPs were resolved by SDS-PAGE and the gel was silver stained. The amount of protein was determined by using a standard curve of purified Tau within the same gel. Purified Tau protein was also used as a control substrate for GSK-3 activity.
Results
β-Catenin and Its Effectors Are Up-Regulated in TSC Pathology
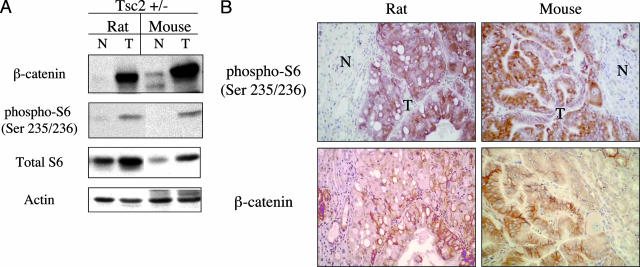
We have previously shown that spontaneous renal tumors from the Tsc2+/− rats expressed high levels of β-catenin.9 One of its downstream targets, cyclin D1, was also abundantly expressed in these lesions and did not respond to a short exposure to rapamycin in vivo.21 This suggests that the elevated β-catenin/cyclin D1 was not mediated by mTOR activity. To further demonstrate the in vivo relevance of the β-catenin pathway in TSC pathology, we examined the expression of β-catenin and its effectors in TSC human and mouse lesions. Consistent with our previous observations, immunoblot analysis showed significantly higher levels of β-catenin expression in renal tumors derived from Tsc2+/− rats and mice compared with adjacent normal kidneys (Figure 1A). Concomitantly, these lesions also expressed high levels of phospho-S6(Ser235/236) indicative of mTOR activation secondary to the loss of Tsc2 function in the tumors. Immunohistochemical analysis on serial sections of renal tumors from these two rodent models confirmed tumor-specific expression of β-catenin associated with overlapping phospho-S6 immunoreactivity (Figure 1B). Thus, these Tsc2-related renal tumors exhibited dual activation of the β-catenin and mTOR pathways. Of note, the distribution of β-catenin staining within the tumors was more variable than that of phospho-S6 suggesting that β-catenin activation may be limited to a certain subset of tumor cells (Figure 1B).
Figure 1.
β-catenin expression in renal tumors of rodent models of TSC. A: Western blot analysis of β-catenin in Tsc2-related renal tumors derived from the Eker rat and Tsc2+/− mouse. N, normal kidney; T, tumor. B: Immunohistochemistry of Tsc2+/− rodent kidneys. Serial sections of each kidney were stained with anti-phospho-S6 and anti-β-catenin antibodies. Original magnifications, ×200.
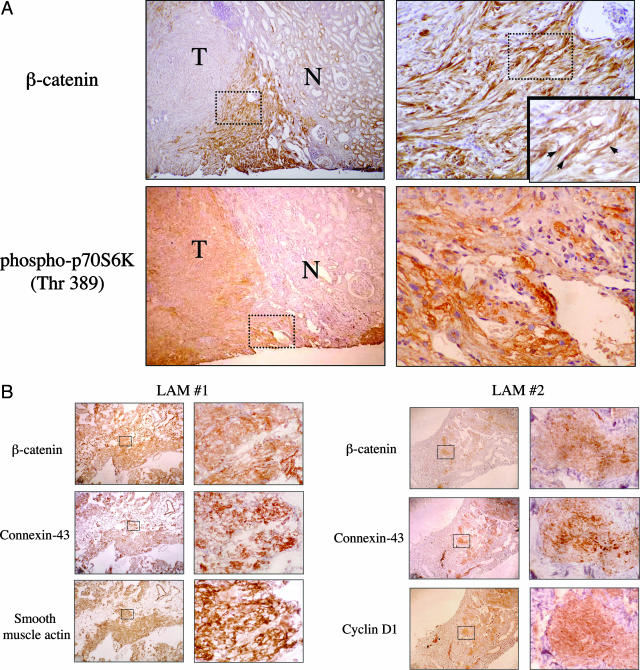
To extend these findings to human TSC lesions, immunohistochemistry was performed on paraffin-fixed sections of AML and LAM tissues obtained from patients with TSC. In all five AMLs examined, expression of β-catenin was high compared to adjacent normal kidneys (Figure 2A). Similarly, three of three LAM tissue samples stained strongly with anti-β-catenin antibodies (Figure 2B). As in the rodent pathology, the distribution of β-catenin expression in these tumors was heterogeneous. Most apparent in the larger AML lesions, β-catenin staining was concentrated near the tumor boundaries next to the normal tissue, whereas elevated phospho-p70S6K expression, another indicator of increased mTOR activity, was more evenly distributed throughout the lesions (Figure 2A). As seen in the rodent tumors, cells staining for β-catenin co-expressed phospho-p70S6K in the human TSC lesions. The peripheral tumor localization of β-catenin staining may indicate a role of this pathway functioning at the interface between the tumor and adjacent normal tissue. Of note, nuclear staining for β-catenin can be seen within these areas (Figure 2A, inset) consistent with up-regulation of β-catenin signaling.
Figure 2.
β-catenin and its effectors in human TSC pathology. A: Representative immunohistochemical staining of AMLs for β-catenin and phospho-p70S6K in serial sections. N, normal kidney; T, tumor. Arrows indicate nuclear β-catenin staining. B: Representative immunohistochemical staining of LAM for β-catenin, connexin 43, cyclin D1, and smooth muscle actin in serial sections. Original magnifications: ×40 (left column); ×200 (right column); ×400 (insets).
Cyclin D1 and connexin 43 have been shown to be downstream targets of β-catenin.13 Here, we examined their expression in LAM tissues by immunohistochemistry and used smooth muscle actin as a marker of the ab-normal tissues. Figure 2B illustrates the expression of β-catenin, connexin 43, and cyclin D1 in representative serial sections of LAM nodules. In contrast, adjacent normal lung parenchyma showed negative staining for all three. Collectively, the observations from two independent rodent models of TSC and multiple human tissues strongly suggest that up-regulation of β-catenin signaling occurs in TSC pathology consistent with a role of TSC1/TSC2 in negatively mediating Wnt signaling.
Wild-Type but Not Mutant TSC2 Negatively Regulates β-Catenin Signaling
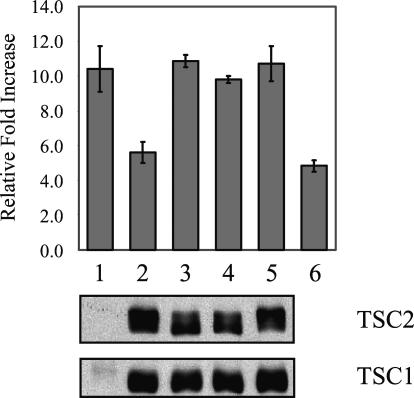
Tuberous sclerosis arises from mutations of either the TSC1 or TSC2 gene. The majority of the disease-causing TSC1 mutations are associated with loss of protein expression, whereas some TSC2 mutations encode abnormal protein secondary to missense mutations. Here, we examined the ability of these disease-causing missense TSC2 mutants to suppress Wnt-stimulated β-catenin-dependent transcription. Among 11 TSC2 mutants known to be associated with the human TSC phenotype tested,22 three (R905Q, S1498N, S1704T) expressed stable proteins at levels that were comparable to that of the wild-type gene (Figure 3); the remainder were expressed at significantly lower levels (data not shown), consistent with previous reports.15 Each of the stable TSC2 mutants was tested in a TOPFLASH reporter assay for β-catenin-dependent activity. Plasmids encoding TSC1 and TSC2 were transiently expressed in HEK293T cells along with the TOPFLASH reporter and β-galactosidase construct to normalize for transfection efficiency. Luciferase activities with or without Wnt stimulation were measured relative to β-galactosidase expression and normalized to the expression level of wild-type TSC2 (except for the positive and negative controls).
Figure 3.
Effects of disease-causing TSC2 mutants on Wnt-induced β-catenin transcriptional activities. HEK293T cells were transfected with the TCF/LEF reporter construct (TOPFLASH), Wnt-1, TSC1, and various TSC2 expression constructs. Luciferase activities in arbitrary light units were normalized to β-galactosidase values for transfection efficiency and to TSC2 expression levels, and plotted as the relative increase in activity in Wnt-stimulated samples compared with unstimulated samples. Results are expressed as the average fold-increase in luciferase activities from three experiments. Western blots of TSC1 and TSC2 are shown on the bottom. Lane 1, vector control; lane 2, wild-type TSC2; lane 3, R905Q; lane 4, S1498N; lane 5, S1704T; lane 6, ΔN-Tcf4 as negative control.
Wnt stimulation alone (vector alone) resulted in a 10-fold increase in luciferase activity (Figure 3, lane1). This was reduced to under sixfold in the presence of wild-type TSC1/TSC2 (Figure 3, lane 2) at a level that was similar to that seen with the ΔN-Tcf4-negative control (Figure 3, lane 6). Expression of each of the three missense TSC2 mutants showed no inhibitory effect on Wnt-induced luciferase activities (Figure 3, lanes 3 to 5). Similar results were obtained using a reporter for cyclin D1 transcription (data not shown). These data show a strong correlation between disease-causing TSC2 mutations and the loss of the ability to suppress Wnt-stimulated β-catenin activity.
TSC1/TSC2 Interact with the β-Catenin Degradation Complex in a Wnt-Dependent Manner
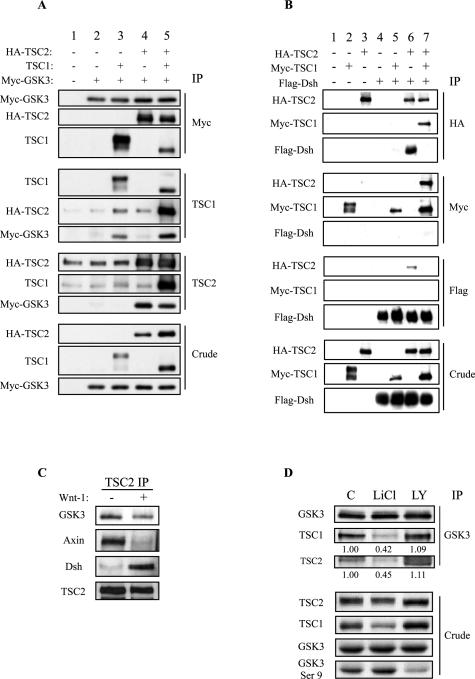
To explore the mechanism of the TSC genes on β-catenin signaling, we investigated the relationship between TSC1/TSC2 and the β-catenin degradation complex. It has been shown that the TSC1/TSC2 complex can associate with GSK3 and Axin in co-immunoprecipitation analysis of exogenously expressed proteins.9 Here, we show that GSK3 can separately bind to tuberin and hamartin when these proteins were individually expressed in HEK293T cells (Figure 4A). This was further confirmed in co-immunoprecipitation experiments of endogenous proteins in Tsc1−/− and Tsc2−/− cells (data not shown). To determine whether other components of this signaling cascade also associate with TSC1/TSC2, we examined the interaction between TSC1/TSC2 and Dsh. Dsh mediates the signaling stemming from Frizzled, a seven-transmembrane receptor that is activated on engagement with the Wnt ligand. Flag-tagged Dsh was overexpressed in HEK293T cells together with Myc-tagged TSC1 and/or HA-tagged TSC2. Cell lysates were immunoprecipitated with anti-HA, anti-Myc, or anti-Flag antibodies. Figure 4B illustrates the ability of Dsh to associate with TSC2 but not TSC1. Further, the interaction between TSC2 and TSC1 or Dsh appeared to be mutually exclusive. In the presence of all three proteins, TSC2 preferentially associated with TSC1.
Figure 4.
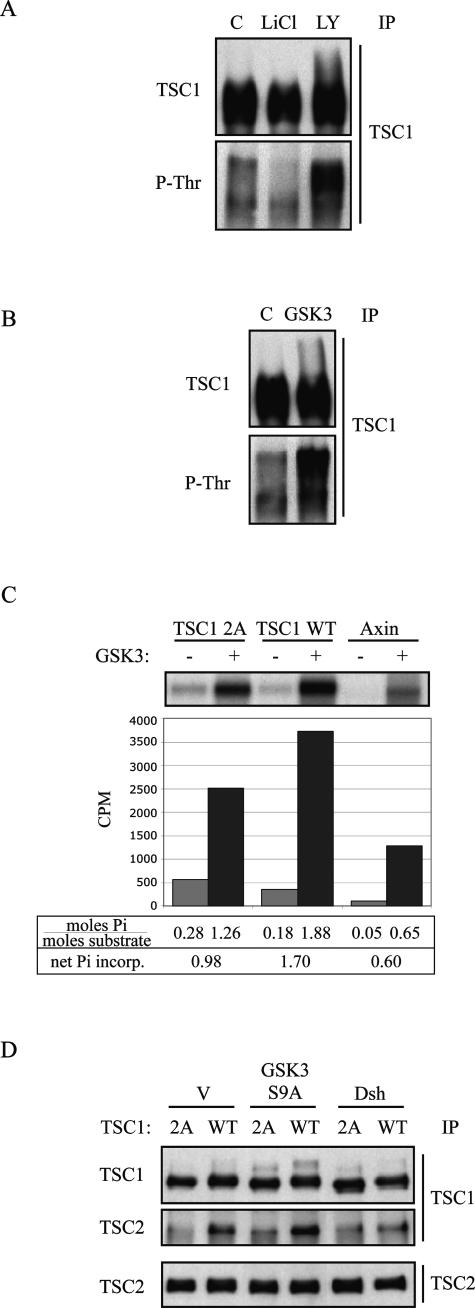
Interaction between TSC1/TSC2 and the β-catenin degradation complex. A: Association between exogenous GSK3 and TSC1/TSC2 in HEK293T cells. Samples were transfected/co-transfected with the indicated vectors. Forty-eight hours after transfection cell lysates were immunoprecipitated with the indicated antibodies (top: Myc IP; middle: TSC1 IP; bottom: TSC2 IP). Immunoprecipitated samples were analyzed by SDS-PAGE and Western blot (IB) for the indicated proteins. Crude extracts show the expression levels of individual proteins in cell lysates (bottom). B: Association between exogenous Dsh and TSC1/TSC2 in HEK293T cells. Samples were transfected/co-transfected with the following constructs: lane 1, empty vector (pcDNA3); lane 2, Myc-tagged hamartin; lane 3, HA-tagged tuberin; lane 4, Flag-tagged Dsh; lane 5, Myc-tagged hamartin and Flag-tagged Dsh; lane 6, HA-tagged tuberin and Flag-tagged Dsh; lane 7, Myc-tagged hamartin, HA-tagged tuberin, and Flag-tagged Dsh. In addition, samples in lanes 2 to 4 were co-transfected with empty vector to obtain a total of 1.6 μg of DNA. Twenty-eight hours after transfection cell lysates were immunoprecipitated with the indicated antibodies (top: HA IP; middle: Myc IP; bottom: Flag IP). Immunoprecipitated samples were analyzed by SDS-PAGE and Western blot as in A. C: Association between endogenous GSK3, Axin, Dsh, and tuberin in HEK293T cells in the presence or absence of Wnt-1. Twenty-four hours after Wnt-1 transfection, cell lysates were immunoprecipitated with anti-tuberin antibodies, separated by SDS-PAGE, and immunoblotted for the indicated proteins. D: Effects of LiCl and LY294002 on interaction between endogenous hamartin, tuberin, and GSK3. HEK293T cells were treated with 20 mmol/L LiCl, 20 μmol/L LY294002, or control for 19 hours, lysed, and subjected to immunoprecipitation with anti-GSK3 antibodies. The numbers below the blots are the relative intensities of the bands indicated by the ratio of treated versus control IP based on values normalized to the amount of immunoprecipitated proteins to which the antibody was directed. Crude extracts show effects of the drugs on GSK3 (Ser9) phosphorylation and steady-state levels of hamartin and tuberin.
To determine the physiological relevance of these interactions, we tested if the endogenous proteins can interact in a manner that is dependent on Wnt stimulation. In the absence of Wnt, tuberin co-immunoprecipitated GSK3, Axin, and to a minimal extent, Dsh (Figure 4C). On Wnt stimulation, significantly more tuberin was associated with Dsh but the amount of GSK3 or Axin associated with TSC2 decreased significantly. The inverse relationship of tuberin interaction between Dsh and GSK3/Axin is consistent with the putative role of Dsh in promoting the disassembly of the β-catenin degradation complex and in doing so, associating with TSC2.
One of the consequences of Wnt stimulation is to down-regulate GSK3 activity resulting in stabilization of β-catenin. We postulate that the Wnt-dependent interaction of TSC1/2 with the GSK3 complex may be dependent on GSK3 activity. To examine this relationship, a pharmacological approach was used to modulate GSK3 activity. Lithium chloride (LiCl) is a known inhibitor of GSK3 that has been used to mimic the effects of Wnt. LiCl treatment leads to an increase in the N-terminal phosphorylation of GSK3 (ie, Ser9/21),23 which results in inactivation of this protein kinase. Conversely, LY294002 inhibits PI3K and Akt activities and thereby reduces GSK3 phosphorylation and increases its activity [Figure 4D, see GSK3(Ser9) panel in crude extracts]. After exposure of LiCl in HEK293T cells, immunoprecipitation using anti-GSK3 antibodies showed that the interaction of endogenous GSK3 with TSC1 and TSC2 was significantly reduced compared to control (Figure 4D). On the other hand, treatment with LY294002 slightly increased the relative amounts of co-immunoprecipitated TSC1 and TSC2. Thus, GSK3 activity has a significant effect on its ability to interact with TSC1/TSC2 similar to the observations made with Wnt stimulation.
GSK3 Phosphorylates TSC1 and Mediates TSC1-TSC2 Interaction
It has been shown that GSK3 can phosphorylate Axin and APC in addition to β-catenin, and that the phosphorylation status of these proteins affects their interaction and the stability of the degradation complex.24 We hypothesized that the TSC proteins, which contain consensus sequences for the GSK3 recognition site, S/TXXXS/TP, may also be physiological substrates of GSK3. Here, we will focus on TSC1 because LiCl significantly reduced the steady state level of hamartin without significant change in tuberin (Figure 4D). To determine whether GSK3 phosphorylates TSC1 in vivo, HEK293T cells were treated with LiCl or LY294002, followed by immunoprecipitation of hamartin and blotting with an anti-hamartin or an anti-phospho-threonine antibody. Figure 5A shows reduced immunoreactivity to anti-phospho-threonine antibody after LiCl treatment and increased immunoreactivity after LY294002 treatment. Further, cells overexpressing wild-type GSK3 showed higher levels of anti-phospho-threonine reactivity (Figure 5B). These observations suggest that modulation of GSK3 activity affects the state of hamartin phosphorylation. To confirm this, in vitro kinase assays were performed using in vitro translated products of TSC1 and Axin, a known physiological substrate of GSK3. Figure 5C shows that purified GSK3 catalyzed the incorporation of 32Pi into both TSC1 and Axin. Under these conditions, there were a net 1.7 mol of Pi incorporation per mol of TSC1, compared to 0.6 mol of Pi per mol of Axin, suggesting that TSC1 is indeed a GSK3 substrate. A Scansite search for potential GSK3 recognition motifs in TSC1 identified two sites with high homology scores: T390 and T357. Disruption of these sites by threonine → alanine substitution (TSC1–2A mutant) resulted in significant reduction (∼40%) in Pi incorporation (ie, 0.98 mol of Pi per mol of TSC1). Other GSK3 phosphorylation sites must exist in TSC1 because the TSC1–2A mutant still incorporated ∼1 mol of Pi per mol of TSC1. Furthermore, using a kinase-deficient mutant, GSK3 (K85A), we found a substantial decrease (∼45%) in 32P-incorporation of TSC1 confirming the effects of GSK3 activity on hamartin phosphorylation (data not shown).
Figure 5.
Phosphorylation of TSC1 by GSK3 affects TSC1-TSC2 interaction. A: HEK293T cells were treated with LiCl, LY294002, or control. Lysates were immunoprecipitated by anti-hamartin antibodies, and Western blots were probed with anti-hamartin and anti-phospho-threonine antibodies. B: HEK293T cells were transiently transfected with GSK3 or vector control, and analyzed as in A. C: In vitro kinase assays using in vitro translated TSC1 [wild-type and 2A(T357A/T390A)] and Axin as substrates. Samples were incubated with or without purified GSK3 kinase. Values indicating the absolute amount of Pi incorporation per mole of substrate are shown below. The differences between the GSK3(+) and GSK3(−) samples representing the net Pi incorporation attributable to GSK3 activity are also shown. D: The effects of GSK3 activity on TSC1-TSC2 interaction. HEK293T cells were transfected with the indicated vectors. Twenty-four hours after transfection, cell lysates were immunoprecipitated using anti-TSC1 antibodies, analyzed by SDS-PAGE, and immunoblotted for TSC1 and TSC2 proteins. The bottom panel shows the expression level of TSC2 protein in cell lysates. V, empty vector.
Based on the current model, GSK3 activity promotes the stability and function of the degradation complex. We hypothesized that phosphorylation of TSC1 by GSK3 may also affect its interaction with the complex. Here, we show that alanine substitutions at 390 and 357 of TSC1 significantly reduced its interaction with TSC2 (Figure 5D). Further, expression of an active GSK3 mutant (S9A) promoted the TSC1-TSC2 complex whereas expression of Dsh, which inhibits GSK3 activity, significantly reduced TSC1-TSC2 association (Figure 5D). Collectively, these results suggest that the interaction between the TSC proteins and the β-catenin degradation complex is regulated by Wnt and more specifically by the activity of the protein kinase, GSK3. Further, we show for the first time that TSC1 is a physiological substrate of GSK3 and that TSC1 phosphorylation by GSK3 plays a role in its interaction with TSC2.
Discussion
The diverse phenotypes found in TSC suggest possible involvement of multiple signaling pathways. Recently, much focus has been placed on the role of TSC1/TSC2 in modulating mTOR activity through Rheb, but the impact of this and other pathways on the observed pathological effects of proliferation, differentiation, and migration remains poorly understood. Evidence to suggest additional or alternative mechanisms besides the mTOR pathway include: 1) the relative lack of effect of rapamycin on vascular endothelial growth factor secretion in Tsc2−/− cells compared to that of Trichostatin A, a histone acetylase inhibitor;25 2) the unresponsiveness of cyclin D1 and β-catenin expression to rapamycin in the Eker rat renal tumors;21 and 3) the recent findings that rapamycin failed to reduce the number of precursor lesions during the initiation phase of renal tumorigenesis in the Eker rat.26 This led us to further examine the role of TSC1/TSC2 in the β-catenin pathway. The latter has been implicated in multiple cellular processes such as cell proliferation, survival, differentiation, polarity, and migration,27 which are all relevant to TSC pathology.
Biochemical analyses have demonstrated that TSC1/TSC2 regulates β-catenin stability and protein expression, inhibits Wnt-induced β-catenin-dependent transcriptional activity, and interacts with the GSK3-degradation complex.9 In this study, we provide further functional evidence that TSC2 plays a role in the β-catenin pathway by highlighting 1) the expression of β-catenin and its effectors, cyclin D1 and connexin 43, in TSC-related AML and LAM tissues as well as tumors derived from rodent models of TSC; 2) the effects of disease-causing missense mutations of TSC2 on β-catenin activity; 3) the inverse relationship in the Wnt-dependent interaction between endogenous Dsh and GSK3/Axin and TSC1/TSC2; and 4) the identification of TSC1 as a physiological substrate of GSK3 and the ability of GSK3 to modulate TSC1-TSC2 interaction. Together, the data strongly support a biological link between the TSC and β-catenin pathways.
The consistent elevated expression of β-catenin and its effectors in multiple, independently derived TSC-related lesions is highly suggestive of a direct effect of the loss of TSC1/2 function in these tumors rather than a secondary event such as acquired mutations of the APC or β-catenin genes. The presence of nuclear β-catenin signals in AML further supports β-catenin activity in TSC pathology. Whether β-catenin expression occurs as an initiating or subsequent event of tumorigenesis has yet to be determined; studies are currently in progress to determine the role of β-catenin in tumor initiation and/or progression.
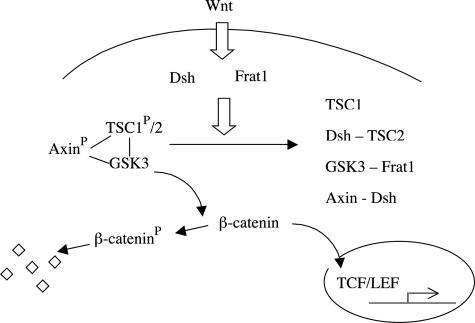
Our findings that TSC1-TSC2-GSK3-Dsh interaction is mediated by Wnt signaling are consistent with the known action of GSK3 on Axin and APC. In our model, the TSC1/2 complex plays an analogous role as the tumor suppressor APC in regulating the β-catenin degradation complex (Figure 6). On activation of GSK3, TSC1 and TSC2 proteins associate with an active GSK3/Axin complex (Figure 4, C and D).9 GSK3 phosphorylates Axin to promote Axin stability. Our data demonstrate that GSK3 also phosphorylates TSC1, which in turn promotes the formation of the TSC1/TSC2 complex (Figure 5, A to D). The formation of the TSC1/TSC2 complex is required to inhibit β-catenin transcriptional activity, and is sufficient to promote the degradation of β-catenin.9 Consistent with this, two Tsc2+/− rodent models were found to have increased levels of β-catenin in renal tumors (Figure 1, A and B). An increase in β-catenin levels was also demonstrated in human AML and LAM tissues (Figure 2, A and B). Furthermore, TSC2 mutants identified from TSC patients examined for relative TOPFLASH reporter activity demonstrated a lack of inhibition of β-catenin activity (Figure 3). Altogether, our results support a model in which GSK3 regulates the formation of the TSC1/TSC2 complex, which in turn regulates β-catenin activity (Figure 6).
Figure 6.
Model of TSC1/TSC2 in the canonical β-catenin signaling pathway. See text for details.
In this study, we have identified two putative GSK3-phosphorylation sites in TSC1 that account for ∼40% of the phosphate incorporation on stimulation by GSK3. These sites (T357, T390) were found to be critical for the interaction between TSC1 and TSC2 and are situated within the known tuberin-binding domain of TSC1. Given that the function of the TSC genes is highly dependent on their association, the finding that GSK3 activity can modulate TSC1 phosphorylation and its interaction with TSC2 may have broader implications in the regulation of the TSC signaling pathway beyond that of β-catenin. Astrinidis and colleagues28 previously found TSC1(T417) as a CDK1 phosphorylation site, which when phosphorylated inhibits TSC1-TSC2 function without interfering with their association. Thus, we propose that differential phosphorylation of TSC1 may act in an opposing manner to modulate its function, similar to the effects of phosphorylation by AKT and AMPK on TSC2. Future studies will identify other relevant GSK3-dependent phosphorylation sites, as well as other protein kinases that may play a role in hamartin prephosphorylation.
GSK3 has been implicated in multiple pathways including the Akt/mTOR and β-catenin pathways. Thus, there is a potential for cross talk between PI3K and Wnt signaling. Fukumoto and colleagues29 found that Wnt induces Akt activity and promotes binding of Akt to the β-catenin degradation complex in the presence of Dsh to activate downstream transcriptional activity. Other studies have shown that stimulation of PI3K by insulin/IGF-1 can also modulate β-catenin activity.30 Recently, the gene involved in the Peutz-Jeghers syndrome, LKB1, was found to modulate both the mTOR and β-catenin pathways similar to that of TSC1/TSC2. LKB1 phosphorylates AMPK in vivo to modulate TSC2 and mTOR activities under conditions of energy stress.5 Its orthologue in Xenopus, XEEK1, was found to modulate Wnt signaling in early vertebrate development by cooperating with Wnt to enhance β-catenin activity.31 In cell culture studies, LKB1 inhibited Dsh-induced β-catenin transcriptional activity.32 The latter relationship would seem consistent with the notion that LKB1 acts as a tumor suppressor gene. It remains unclear if the increased cancer susceptibility associated with Peutz-Jeghers syndrome is caused by the inappropriate activation of the β-catenin and/or the mTOR pathways, and if the influence of LKB1 on β-catenin is mediated by TSC2. Nonetheless, these independent observations linking TSC1/2 and LKB1 to both the mTOR and β-catenin signaling cascades provide novel molecular insights in the interdependence of key cellular processes such as proliferation, differentiation, cell-cell interaction, and migration.
Downstream targets of β-catenin may help to explain some of the observed phenotypic heterogeneity in TSC. For example, the β-catenin target, connexin 43, has been associated with increased invasiveness of malignant glioma cells.33 This may be relevant to LAM pathogenesis in light of our finding that these lesions expressed high levels of connexin 43. Recent genetic studies in LAM suggested their derivation from pre-existing AML based on their similarities in genetic alterations at the TSC2 locus.34 This led to the metastatic model that certain smooth muscle-like cells in AMLs can invade and spread to the lungs where LAM develops. The effects of the TSC genes on β-catenin-dependent connexin 43 signaling deserve further consideration. Vascular endothelial growth factor is another β-catenin effector that is found to be up-regulated in TSC pathology.35,36 In vitro data suggest that the mTOR pathway may only account for a portion of the vascular endothelial growth factor activity,25 leaving open the possibility of a contribution from the β-catenin pathway. Finally, the abnormal phenotype of the cortical tubers in TSC displaying unusual patterns of neuronal and glial differentiation remains to be explained by known TSC gene functions. There is much evidence to suggest a role of β-catenin in brain development. For example, forced overexpression of β-catenin in the central nervous system results in encephalomegaly in the mice.37,38 Further, neurogenin 1 is found to be a target of β-catenin and affects cortical neuronal differentiation.39 Additionally, immunocytochemical analysis of sporadic cases of dysplastic neurons has reported the abnormal expression of components of the Wnt/β-catenin pathway.40
In summary, the influence of β-catenin on cell proliferation, differentiation, polarity, and migration can potentially account for some of the observed abnormalities associated with TSC pathology. The concurrent expression of β-catenin and mTOR effectors in AML and LAM suggests that the TSC phenotype may culminate from the additive effects of abnormal activation of both the β-catenin and mTOR pathways, and that the regulatory role of the TSC genes on these pathways is not mutually independent. Moreover, if β-catenin plays a role in TSC, this provides an alternative approach in the treatment of TSC-related disorders based on the recent discovery of small molecule β-catenin inhibitors.41 Future in vivo studies using transgenic animals mutated for specific components of these pathways will help to dissect the relative functional contribution of these two pathways in TSC-related tumorigenesis.
Acknowledgments
We thank Elizabeth Henske and Vera Krymyskya for providing unstained sections of AML and LAM tissues, Kun-Liang Guan for TSC1(2A) construct, David Kimmelman for Axin and GSK3 expression vectors, Randall Moon for Flag-Dsh and critical review of the manuscript, and Jean Campbell for discussion.
Footnotes
Address reprint requests to Raymond S. Yeung, Department of Surgery, University of Washington, 1959 NE Pacific St., Box 356410, Seattle, WA 98195. E-mail: ryeung@u.washington.edu.
Supported in part by grants from the National Institutes of Health (CA61889, CA102662) and the LAM Foundation.
Current address of B.C.M.: University of Toronto, Dept. of Dentistry, Fitzgerald Bldg., 150 College St., Rm. 243, Toronto, Ontario, M5S 3E2 Canada.
References
- Gomez MR. Definition and criteria for diagnosis. Gomez MR, Sampson JR, Whittemore VH, editors. New York: Oxford University Press,; Tuberous Sclerosis Complex. 1999:pp 10–23. [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:1–16. doi: 10.1186/1475-4924-2-28. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci USA. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- Lim W, Olschwang S, Keller JJ, Westerman AM, Menko FH, Boardman LA, Scott RJ, Trimbath J, Giardiello FM, Gruber SB, Gille JJ, Offerhaus GJ, de Rooij FW, Wilson JH, Spigelman AD, Phillips RK, Houlston RS. Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology. 2004;126:1788–1794. doi: 10.1053/j.gastro.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Mak BC, Takemaru K, Kenerson HL, Moon RT, Yeung RS. The tuberin-hamartin complex negatively regulates beta-catenin signaling activity. J Biol Chem. 2003;278:5947–5951. doi: 10.1074/jbc.C200473200. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci. 1998;111:1741–1749. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Wu R, Schwartz DR, Darrah D, Reed H, Kolligs FT, Nieman MT, Fearon ER, Cho KR. Role of beta-catenin/T-cell factor-regulated genes in ovarian endometrioid adenocarcinomas. Am J Pathol. 2002;160:1229–1238. doi: 10.1016/s0002-9440(10)62550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, Randazzo F, Gundel R, Warren RS, Escobedo J, Aukerman SL, Taylor RN, Fantl WJ. Beta-catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–3153. [PubMed] [Google Scholar]
- Aicher LD, Campbell JS, Yeung RS. Tuberin phosphorylation regulates its interaction with hamartin. Two proteins involved in tuberous sclerosis. J Biol Chem. 2001;276:21017–21021. doi: 10.1074/jbc.C100136200. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Kimelman D. Overexpression of Xgsk-3 disrupts anterior ectodermal patterning in Xenopus. Dev Biol. 1996;175:256–264. doi: 10.1006/dbio.1996.0113. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci USA. 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Argast GM, Foord O, Fischer EH, Krebs EG. Expression and characterization of glycogen synthase kinase-3 mutants and their effect on glycogen synthase activity in intact cells. Proc Natl Acad Sci USA. 1996;93:10228–10233. doi: 10.1073/pnas.93.19.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62:5645–5650. [PubMed] [Google Scholar]
- Jones AC, Shyamsundar MM, Thomas MW, Maynard J, Idziaszczyk S, Tomkins S, Sampson JR, Cheadle JP. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet. 1999;64:1305–1315. doi: 10.1086/302381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAulay K, Hajduch E, Blair AS, Coghlan MP, Smith SA, Hundal HS. Use of lithium and SB-415286 to explore the role of glycogen synthase kinase-3 in the regulation of glucose transport and glycogen synthase. Eur J Biochem. 2003;270:3829–3838. doi: 10.1046/j.1432-1033.2003.03777.x. [DOI] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG., Jr TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- Kenerson HL, Dundon TA, Yeung RS. Effects of rapamycin in the Eker rat model of tuberous sclerosis. Pediatr Res. 2005;57:67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Astrinidis A, Senapedis W, Coleman TR, Henske EP. Cell cycle-regulated phosphorylation of hamartin, the product of the tuberous sclerosis complex 1 gene, by cyclin-dependent kinase 1/cyclin B. J Biol Chem. 2003;278:51372–51379. doi: 10.1074/jbc.M303956200. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Vaidya B, Patel D, Herlyn M. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and beta-catenin pathways. Cancer Res. 2001;61:7318–7324. [PubMed] [Google Scholar]
- Ossipova O, Bardeesy N, DePinho RA, Green JB. LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nat Cell Biol. 2003;5:889–894. doi: 10.1038/ncb1048. [DOI] [PubMed] [Google Scholar]
- Spicer J, Rayter S, Young N, Elliott R, Ashworth A, Smith D. Regulation of the Wnt signalling component PAR1A by the Peutz-Jeghers syndrome kinase LKB1. Oncogene. 2003;22:4752–4756. doi: 10.1038/sj.onc.1206669. [DOI] [PubMed] [Google Scholar]
- Zhang W, Nwagwu C, Le DM, Yong VW, Song H, Couldwell WT. Increased invasive capacity of connexin43-overexpressing malignant glioma cells. J Neurosurg. 2003;99:1039–1046. doi: 10.3171/jns.2003.99.6.1039. [DOI] [PubMed] [Google Scholar]
- Henske EP. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosom Cancer. 2003;38:376–381. doi: 10.1002/gcc.10252. [DOI] [PubMed] [Google Scholar]
- Liu MY, Poellinger L, Walker CL. Up-regulation of hypoxia-inducible factor 2alpha in renal cell carcinoma associated with loss of Tsc-2 tumor suppressor gene. Cancer Res. 2003;63:2675–2680. [PubMed] [Google Scholar]
- El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 2003;63:5173–5177. [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Cotter D, Honavar M, Lovestone S, Raymond L, Kerwin R, Anderton B, Everall I. Disturbance of Notch-1 and Wnt signalling proteins in neuroglial balloon cells and abnormal large neurons in focal cortical dysplasia in human cortex. Acta Neuropathol (Berl) 1999;98:465–472. doi: 10.1007/s004010051111. [DOI] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]