Abstract
Lack of understanding of the molecular mechanisms and pathogenesis of impaired healing in chronic ulcers is a serious health issue that contributes to excessive limb amputations and mortality. Here we show that β-catenin and its downstream targets in keratinocytes, c-myc, and keratins K6 and K16, play important roles in the development of chronic wounds. In contrast to normal epidermis, we observed a significant nuclear presence of β-catenin and elevated c-myc expression at the nonhealing wound edge of chronic ulcers from 10 patients. In vitro studies indicated that stabilization of nuclear β-catenin inhibited wound healing and keratinocyte migration by blocking epidermal growth factor response, inducing c-myc and repressing the K6/K16 keratins (cytoskeletal components important for migration). The molecular mechanism of K6/K16 repression involved β-catenin and arginine methyltransferase (CARM-1) acting as co-repressors of glucocorticoid receptor monomers. We conclude that activation of the β-catenin/c-myc pathway(s) contributes to impaired healing by inhibiting keratinocyte migration and altering their differentiation. The presence of activated β-cate-nin and c-myc in the epidermis of chronic wounds may serve as a molecular marker of impaired healing and may provide future targets for therapeutic intervention.
The integrity of the skin depends on the specific attachments of its keratinocytes to the extracellular matrix and to each other. Keratinocytes are programmed to maintain this integrity and are the first cells to respond to injury. Wounding promotes activation of keratinocytes that trigger keratinocyte migration and proliferation, which is paralleled by changes in keratinocyte adhesion and the cytoskeletal content.1,2 However, sometimes this reliable program fails, resulting in a chronic wound (ulcers).3,4 Consequential to the extended life span of the modern human population and increased prevalence of diabetes, we are faced with epidemic proportions of chronic ulcers.5,6 The total prevalence of diabetes in the United States is estimated as high as 18.2 million or 6.3% of the population in the year 2002 (National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States found online at: http://www.cdc.gov/diabetes/pubs/estimates.htm). Diabetic foot ulcers are estimated to occur in 15% of all patients with diabetes, and precede 84% of all lower-leg amputations, which are at epidemic proportions in the elderly, as well as in the diabetic populations.7–9 The current lack of understanding of pathogenesis of impaired healing in chronic ulcers contributes to excessive amputations and is an extremely serious health issue. Nonetheless, there are only three FDA-approved thera-peutic modalities for such ulcers.6,10–13 The efforts to develop new therapies are hampered by the lack of knowledge of the mechanisms responsible for the pathologies, and the corresponding molecular targets for intervention.
Differentiating keratinocytes are characterized by two major types of cell adhesions: desmosomes and adherens junctions. Desmosomes are multimolecular complexes containing, as major components, two glycoproteins, desmocollin and desmoglein, two armadillo proteins, plakoglobin and plakophilin, and the plakin family protein desmoplakin.14–16 Adherens junctions are characterized by the presence of E-cadherin, α- and β-catenins, and γ-catenin (plakoglobin) at the membrane.17,18 β-Catenin is a multifunctional protein that plays an important role during embryonic development and neoplasia as a mediator of the Wnt signaling pathway.19,20 When the Wnt pathway is quiescent, β-catenin participates in adherens junctions.17 When β-catenin becomes cytoplasmic it gets phosphorylated and targeted for ubiquitination and degradation. Activation of the Wnt pathway inhibits this phosphorylation, leading to cytosolic stabilization of β-catenin, which consequently translocates to the nucleus where it binds Tcf-Lef transcription factors and regulates transcription.21,22 Among downstream targets of β-catenin transcriptional pathway is the oncogene c-myc.23 Activation of c-myc affects epidermal biology directly relevant to wound healing. Although c-myc is required for transition from the G1 to the S phase of the cell cycle and it promotes proliferation of transit-amplifying cells, deregulation of c-myc depletes epidermal stem cells, causing the inability of the tissue to react to injury.24–27 Furthermore, targeted overexpression of c-myc in basal keratinocytes leads to impairment of keratinocyte migration and consequently inhibition of wound healing in a transgenic mouse model.24
Experimental results of the roles of β-catenin and c-myc mostly originate from various mouse models whereas, to the best of our knowledge, the roles of β-catenin and c-myc in impairment of wound healing in human skin have never been investigated in patients.24–29 Interestingly, we found that c-myc has a particular transcriptional pattern during normal wound healing in human skin and that inhibitors of wound healing, glucocorticoids (GC), induce c-myc expression. In addition, we found that stabilization of β-catenin inhibits keratinocyte migration and wound healing of human skin in organ culture and that β-catenin participates in GC signaling and repression of the keratin genes that participate in cytoskeletal network and keratinocyte migration. Lastly, we found activation of both β-catenin and c-myc in the nonhealing edge of patients with chronic wounds. Therefore, we propose that nuclearization of β-catenin and induction of c-myc participate in inhibition of wound healing and contribute to impairment of healing in chronic wounds.
Materials and Methods
Plasmids
Plasmids pK14CAT, pK6CAT, pK16CAT, β-catenin, and CARM-1 have been described previously.30,31 The plasmid containing GRE-CAT was a gift from Dr. P. Chambon (France). All plasmids were grown using Promega kit (Promega, Madison, WI) and commercial protocols.32
Northern Blot
RNA isolation and purification was performed using Triazol (Invitrogen, Carlsbad, CA) extraction and subsequently Qiagen RNeasy kit column purification (Qiagen, Valencia, CA) followed by Northern blot as described.31 c-myc and GAPDH probes were generated as described.33 Densitometry tracing of the films was performed using GS-800 calibrating densitometer (Bio-Rad, Hercules, CA) and the image was quantified using Quantity One 4.1.1 program (Bio-Rad). The values were normalized to the loading control (GAPDH) for each condition.
Cell Growth and Transient Transfections
Normal human epidermal keratinocytes were grown as described.31,34 Cells were transfected at 80% confluency using polybrene with the dimethyl sulfoxide shock method as previously described.35 Cells were washed and incubated in the basal medium without epidermal growth factor (EGF) and bovine pituitary extract the day before transfection until the time of harvesting. Each transfection contained 5 μg/dish of keratin-CAT construct. The cells were then incubated with or without 0.1 μmol/L glucocorticoid dexamethasone (Sigma Chemical Co., St. Louis, MO) dissolved in ethanol and harvested 48 hours later. CAT assays were performed using FastCat (Molecular Probes, Eugene, OR) following a commercial protocol. Cell extracts used for CAT assay were normalized by total protein determined by protein assay (Bio-Rad). Thirty μg of protein were used for each reaction. CAT assay values were quantified by Fluor Imager 575 (Molecular Dynamics, Piscataway, NJ). All experiments were performed in duplicates, at least three times.
Keratinocyte Migration Assay
Primary human keratinocytes were grown to 80% confluency. Twenty-four hours before the experiment cells were transferred to basal KBM medium (Life Technologies, Inc., Grand Island, NY). Before the scratch, cells were treated with 8 μg/ml mitomycin C (ICN, Irvine, CA) for 1 hour and washed with basal media. Scratches were performed as previously described.36 Cells were incubated with 20 μmol/L LiCl or 25 ng/ml of EGF for 24 and 48 hours, rephotographed, and cell migration was quantified as previously described.36,37 Thirty measurements were taken for each experimental condition and distance coverage by cells moving into the scratch wound area was quantified. Three images were analyzed per condition, per time point, and averages and standard deviations were calculated.
Skin Specimens
Specimens of normal human skin were obtained as discarded tissue after reduction mammoplasty (approved protocol H #9796-03) and maintained as described.36 Topical GC treatment was performed by daily application of Cormax (Clobetasol Propionate Cream 0.05%; Oclassen Pharmaceuticals, Inc.) using a sterile Q-tip applicator.
Wounding Experiments
Wounds were created using 4-mm punch biopsies through the reticular dermis and a rim of cells participating in wound healing was collected by repunching around the initial wounded area. Each time point was collected in parallel with an unwounded skin specimen of the same donor. All specimens were collected and either stored in RNAlater (Ambion, Austin, TX) or frozen in OCT compound (Tissue Tek, Reading, CA) for immunocytochemistry. To activate β-catenin wounded skin was maintained on the air-liquid interface in the presence or absence of 20 mmol/L LiCl.38 Wounds were quantified by planimetry as described previously.36
Histology and Immunocytochemistry
Chronic ulcer skin specimens were obtained as discarded tissue after debridement procedures on consented patients (approved protocol 01-0960(001)03sux). Samples were fixed in formalin and routinely processed for paraffin embedding. Samples from 10 different patients were analyzed independently. Paraffin-embedded tissue was sectioned and 5-μm-thick sections were stained with hematoxylin and eosin. The sections were analyzed using a Nikon microscope and digital images were obtained using a Spot RTcolor camera.
For staining human tissues and cultured cells a c-myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:100 dilution at 4°C using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) as previously published.39 Keratinocytes were grown on chamber slides to 70% confluency (Lab-Tek, Naperville, IL) and treated with 0.1 μmol/L dexamethasone (Sigma) or 20 mmol/L LiCl. Cells were fixed in 70% methanol for 10 minutes and permeabilized with 0.1% Triton X-100 for 10 minutes.
Human tissues were stained with β-catenin antibody (BD Transduction Laboratories, Lexington, KY) as previously described40 or E-cadherin antibody (Santa Cruz Biotechnology) using 1:200 or K6 antibody (a gift from Dr. Pierre Coulombe, Johns Hopkins, Baltimore, MD41) at 1:1500 dilution in 5% bovine serum albumin and visualized using a secondary fluorescein isothiocyanate anti-mouse IgG antibody 1:150 (Sigma). All sections were mounted with mounting media containing propidium iodide or 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) to help visualization of the nuclear staining whereby the nuclear presence of β-catenin was visualized by the change of red color to orange or yellow. All negative controls were prepared by substitution of the primary antibody with an irrelevant antibody. The sections were analyzed using a Carl Zeiss microscope (Carl Zeiss, Thornwood, NY) and digital images were collected using Adobe TWAIN_32 program.
Quantification of the nuclei positive for either c-myc or β-catenin was performed by three blinded lab members and medians and SD were calculated. All experiments were performed in triplicates, in which three to five images per condition, per each time point were independently quantified.
Results
Histopathology of Chronic Ulcers Reveals Impaired Keratinocyte Activation and Differentiation
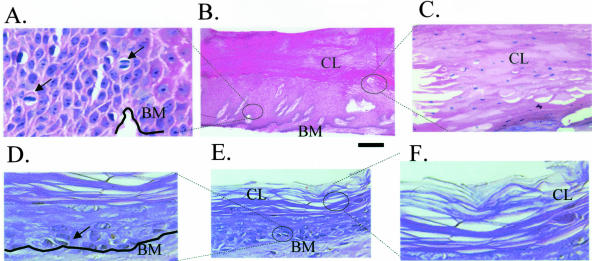
To characterize the epidermis at the nonhealing edge of chronic ulcers we analyzed the histopathology of chronic wounds from the actual patients (Figure 1; A to C) and compared it with the epidermis of normal skin (Figure 1; D to F). We found epidermis from chronic ulcers to be very thick, hyperproliferative, containing mitotically active cells in suprabasal layers (Figure 1, compare A with D). Lastly, no epithelial migration was detected (absence of the epithelial tongue), suggesting incomplete keratinocyte activation. We also found epidermis to be hyper- and parakeratotic (presence of nuclei in cornified layer), suggesting incomplete keratinocyte differentiation (Figure 1, compare B with E). Interestingly, the abnormalities found are consistent with previous findings of c-myc overexpression in transgenic mouse models24,25,42,43 as well as suppression of cytoskeletal components K6 and K16.44,45 Therefore, we hypothesized that induction of c-myc and suppression of K6/K16 may lead to inhibition of keratinocyte migration in human skin, perhaps contributing to the development of chronic wounds.
Figure 1.
Histology of chronic wounds is consistent with activation of c-myc. A to C: Histology of chronic ulcers. A: Higher magnification shows mitotically active cells found in suprabasal layers (arrows) indicating aberrant proliferation. B: Low magnification shows thickened, hyperproliferative, hyper- and parakeratotic epidermis. C: High magnification shows parakeratosis (nuclei present in cornified layer) indicating inappropriate differentiation. D to F: Histology of normal skin. D: Mitosis only in basal layer of epidermis; E: low magnification; F: cornified layer, high magnification. BM, basal membrane; CL, cornified layer.
c-myc Is Repressed during the Wound-Healing Process and Induced by a Wound-Healing Inhibitor
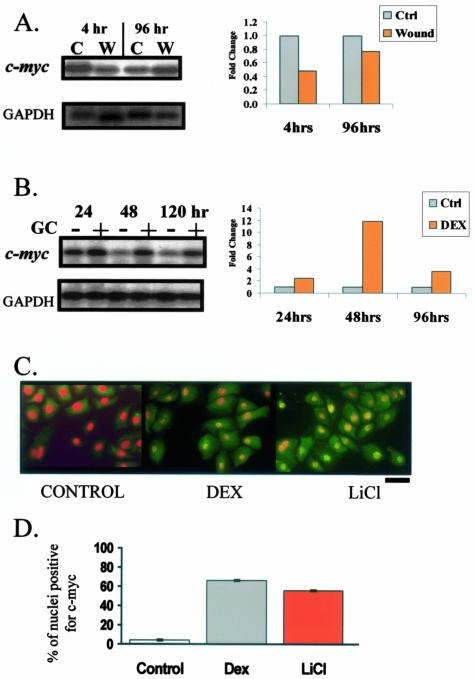
To test the role of c-myc in wound healing we used a human skin organ culture wound model. Normal skin was wounded by 4-mm punch biopsy and maintained at air-liquid interface. Cells participating in the wound-healing response were harvested at 4 hours (immediate-early response) and 96 hours (intermediate response—exponential phase in which keratinocytes are actively proliferating and migrating) after wounding. c-myc expression was measured by Northern blots. Interestingly, we observed a particular expression pattern of c-myc during wound healing: its mRNA was repressed at 4 hours but derepressed by 96 hours after wounding (Figure 2A). Conversely, when we used inhibitors of wound healing, topical GC,46 we found significant induction of c-myc mRNA (Figure 2B). Thus, we identified a differential expression pattern of c-myc: suppressed in early wound healing and induced by a wound-healing inhibitor.
Figure 2.
c-myc is differentially regulated by wound healing and its inhibitor, GC. Northern blots with mRNA isolated from acute wounds 4 and 96 hours after wounding (A) and topical GC treatment of human skin (B). c-myc was repressed in early wound healing, while induced by GC. Bar graphs show quantification of the Northern blots by densitometry. C: Immunofluorescence of primary human keratinocytes incubated with GC and LiCl stained with c-myc-specific antibody. To better visualize its nuclear presence we counterstained the nuclei. c-myc is visualized by green fluorescein isothiocyanate and its nuclear presence changes their color from red (see control) to orange/yellow (see treated cells). Both GC (middle) and LiCl, ie, stabilized β-catenin (right) induce c-myc as evident by positive nuclear staining. D: Graph represents average ± SD of percent of nuclei positive for c-myc for several independent experiments.
To determine whether GC indeed activate c-myc expression, we incubated primary human keratinocytes with either GC or lithium chloride (LiCl). LiCl is known to, by stabilizing nuclear localization of β-catenin, activate c-myc.47 To determine the nuclear presence of c-myc, cells were stained with c-myc-specific antibody and quantified. As predicted, we found that both GC and LiCl induce expression and nuclear localization of c-myc (Figure 2, C and D). c-myc was found to be nuclear in more than 70% of cells treated with GC and in 65% of LiCl-treated cells (positive control), whereas it was nuclear in only 1% of the untreated cells (negative control). Taken together, our findings that c-myc is repressed in human epidermis during early wound healing and induced by GC, coupled with the results from mouse models,24,25,42,43 suggest that overexpression of c-myc in the early stage of wound healing might inhibit keratinocyte migration, causing impairment in healing.
c-myc Is Induced in the Nonhealing Epidermal Edge of Chronic Ulcers
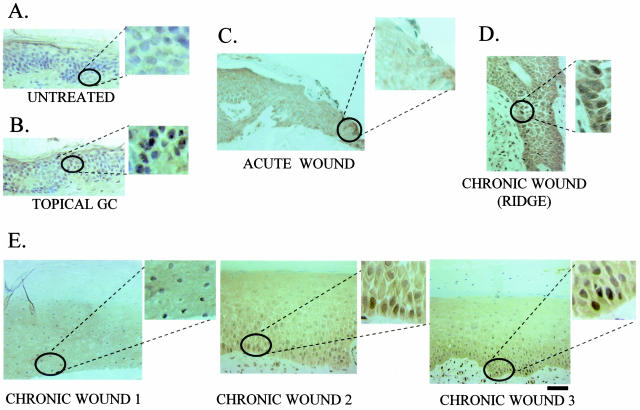
To test if the c-myc overexpression participates in chronic wounds we measured its activation in nonhealing wound biopsies from patients with chronic ulcers using a c-myc-specific antibody. We used normal human skin (negative control), skin treated with topical GC (positive control), and an acute wound edge (additional control). As expected, we found that epidermis of normal skin does not contain c-myc (Figure 3A) (in agreement with previous findings24). Topical GC treatment induced c-myc in human epidermis (Figure 3B), which was expected based on our results presented in Figure 2. Furthermore, wounding of normal skin did not induce c-myc in the epidermis of the acute wound edge (Figure 3C). Importantly, we found strong activation of c-myc in the epidermis at the nonhealing edge of chronic ulcers (Figure 3, D and E). c-myc was strongly expressed throughout the epidermis but was more prominent in layers closer to the basal membrane (Figure 3D). Furthermore, we found activation of c-myc at the nonhealing epithelial edge of chronic ulcers, irrespective of the type of the ulcer, ie, diabetic foot or pressure ulcers. This suggests that activation of c-myc may contribute to the overall epidermal pathology of chronic wounds.
Figure 3.
c-myc as a marker of inhibition of wound healing in chronic ulcers in vivo. Immunohistochemistry of skin samples stained with c-myc-specific antibody. A: Normal human skin shows absence of c-myc. B: Skin treated with topical GC shows induction of c-myc. C: Epidermis at the edge of an acute wound shows absence of c-myc, thus confirming that c-myc is not activated in acute wound healing. D and E: c-myc is induced in chronic ulcers in vivo. D: Most prominent induction of c-myc in the basal layer of epidermis; E: c-myc is activated throughout the epidermis at the nonhealing edge of a chronic ulcers. Insets show enlarged images of nuclear staining.
β-Catenin, the Activator of c-myc, Participates in the Inhibition of Wound Healing
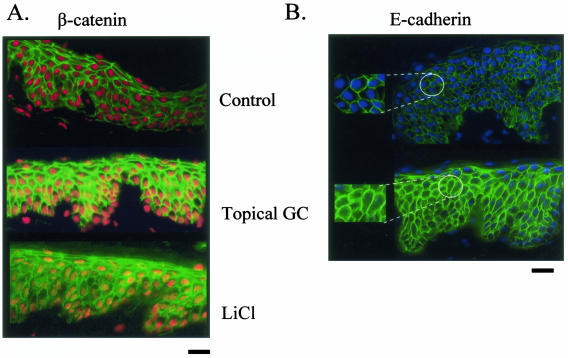
The activation of c-myc in chronic wounds raises the question of the role of its activator, β-catenin, in inhibition of wound healing. To test if wound-healing inhibitor, GC, activates the β-catenin pathway, we treated human skin with topical GC and determined β-catenin localization using a β-catenin-specific antibody. We found that GC causes nuclear accumulation of β-catenin in human epidermis. A robust nuclear localization of β-catenin in epidermis treated with topical GC was found, whereas in untreated skin, β-catenin was found on the membrane and not in the nuclei (Figure 4A). Furthermore, we found prominent cytoplasmic localization of E-cadherin in epidermis treated with GC (Figure 4B). We conclude that wound-healing inhibitors, GC, activate the β-catenin pathway, suggesting that β-catenin may play a role in the inhibition of wound healing.
Figure 4.
Topical GC activates β-catenin pathway in the epidermis of human skin. A: Immunofluorescence of normal human skin treated either with topical GC or LiCl (positive control) reveals nuclear β-catenin (visualized by orange/yellow nuclei) in treated skin whereas it is on the membrane in untreated skin (red nuclei indicate absence of signal). B: Immunofluorescence shows that E-cadherin remains membrane-associated in untreated skin whereas it becomes internalized (cytoplasmic) in GC-treated skin. Insets show enlarged images.
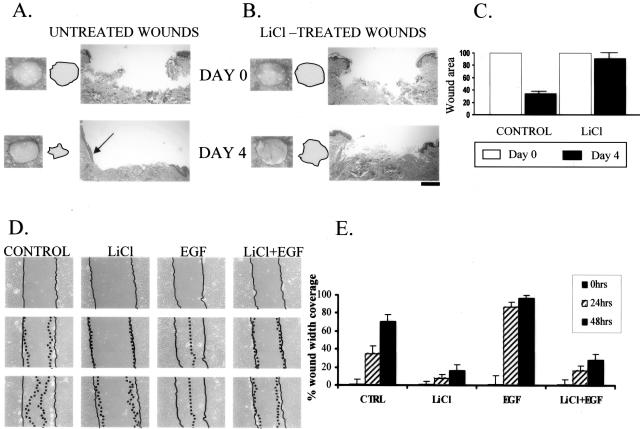
To test this we measured the healing rates of wounded skin in the presence or absence of LiCl, a β-catenin activator. Wound healing was measured 4 days after wounding when healing is in its exponential phase and keratinocyte migration is progressing. The healing rate was measured by planimetry and evaluated by histology (Figure 5; A to C). Interestingly, stabilization of nuclear β-catenin completely inhibited wound healing, thus converting an acute wound into a chronic wound phenotype (Figure 5; A to C).
Figure 5.
Activation of β-catenin inhibits wound healing and keratinocyte migration. LiCl (ie, nuclear β-catenin) causes delayed wound healing in human skin organ culture wounds. Both gross pictures of untreated (A) and LiCl-treated wounds (B) as well as their histology are shown. Arrow points at epithelial tongue indicating active healing in untreated wounds whereas it is absent in the LiCl-treated wounds. Filled circles indicate the wound surfaces. C: Quantification of the wound size by planimetry shows 70% healing rate of untreated wounds and only 12% healing rate for LiCl-treated wounds. D: LiCl inhibits migration of primary human keratinocytes in wound scratch assay when compared with untreated cells. Inhibition is prominent even in first 24 hours and was further sustained through 48 hours. EGF (positive control) stimulated migration and wound was completely closed after 48 hours. Importantly, this activation of endogenous β-catenin completely blocked EGF-stimulated migration. Full lines indicate initial wound area; dotted lines demarcate migrating front of cells. E: Histograms indicate the average coverage of scratch wounds widths in percent relative to baseline wound width at the day 0 and 24 and 48 hours after LiCl, EGF, and LiCl/EGF treatments.
β-Catenin Inhibits Keratinocyte Migration
If stabilization of β-catenin leads to inhibition of healing and activation of its downstream target, c-myc, inhibits keratinocyte migration, one may expect that stabilization of β-catenin should inhibit keratinocyte migration. To test if stabilized, nuclear β-catenin affects keratinocyte migration during wound healing we used an in vitro wound scratch assay. We incubated keratinocytes in the presence and absence of LiCl and/or with EGF (positive control), and observed and quantified keratinocyte migration during 48 hours. We found that LiCl, by stabilizing β-catenin, inhibited keratinocyte migration by 70%, whereas EGF promoted it by 50% (Figure 5, D and E). Interestingly, when introduced simultaneously, LiCl efficiently blocked the EGF-stimulated migration. Therefore, β-catenin contributes to the development of a chronic wound by inhibiting keratinocyte migration not only directly by activating c-myc, but also indirectly by blocking the effects of other growth factors and cytokines.
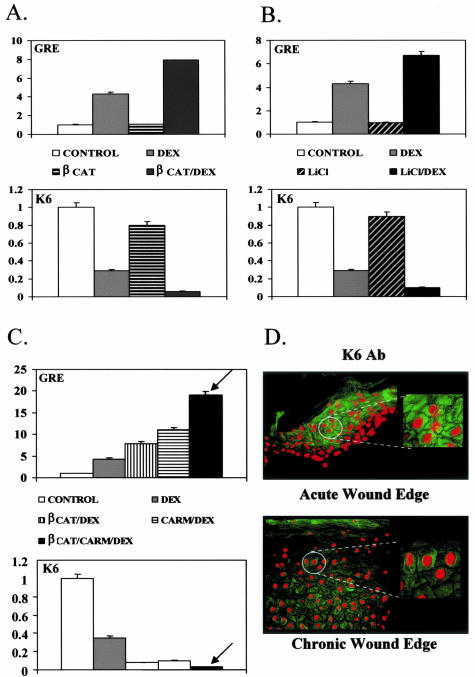
To examine further the molecular mechanism through which β-catenin participates in the inhibition of keratinocyte migration, we focused on the cytoskeletal components that participate in migration, keratins K6/K16. To test if β-catenin participates in K6/K16 suppression by GC we used co-transfection experiments with primary human keratinocytes. By itself, β-catenin did not affect K6/K16 expression. Surprisingly however, in the presence of GC, β-catenin acted as a co-repressor of the glucocorticoid receptor (GR) further suppressing K6 expression (Figure 6A). To test if endogenously activated β-catenin acts as a co-repressor of GR, we incubated keratinocytes with LiCl that stabilizes β-catenin instead of transfecting the expression plasmid. We found that endogenously activated β-catenin also acts as a co-repressor of GR (Figure 6B). Moreover, the protein arginine methyltransferase CARM-1 enhances this co-repression. The level of suppression of K6 by GC is significantly stronger in the presence of co-transfected CARM-1 and β-catenin (Figure 6C), suggesting that the complex that suppresses K6 promoter contains GR, β-catenin, and CARM-1. We found that K16, the partner of K6, was regulated in a similar manner (data not shown). We have shown that by participating in the GC-mediated repression of K6/K16 transcription as a co-repressor with CARM1, β-catenin contributes to the inhibition of keratinocyte migration through altering the cytoskeletal network.
Figure 6.
Molecular mechanism of inhibition of keratinocyte migration by β-catenin involves GR and CARM-1 and cytoskeletal component K6 keratin. A: Graph represents quantitative CAT assay after co-transfection of human keratinocytes with K6-CAT promoter showing that β-catenin enhances repression of K6 by the GC, dexamethasone (DEX), thus acting as a co-repressor of GR. B: Similarly, LiCl treatment (ie, endogenously activated β-catenin) also enhances repression of K6 by DEX. C: The β-catenin-mediated co-repression is further enhanced by arginine methyltransferase CARM-1 (arrow), indicating that GR, β-catenin, and CARM-1 act as a repressor complex that suppresses K6. D: Sections of chronic wounds stained with K6-specific antibody (bottom) revealed marked repression of K6 levels when compared with acute wound (top).
To further test the significance of K6 suppression in development of a chronic wound keratinocyte phenotype, we used K6-specific antibody to test for its presence in the nonhealing epidermal edge of chronic wounds and compared it to the edge of an acute wound. As expected, we found strong activation of K6 at the edge of the acute wound (Figure 6D, top). The levels of K6 were severely reduced in the epidermis of the nonhealing edge of a chronic wound (Figure 6D, bottom). Taken together, we conclude that suppression of K6 also contributes to the chronic wound keratinocyte phenotype, consistent with our histopathology findings that indicate incomplete keratinocyte activation (Figure 1).
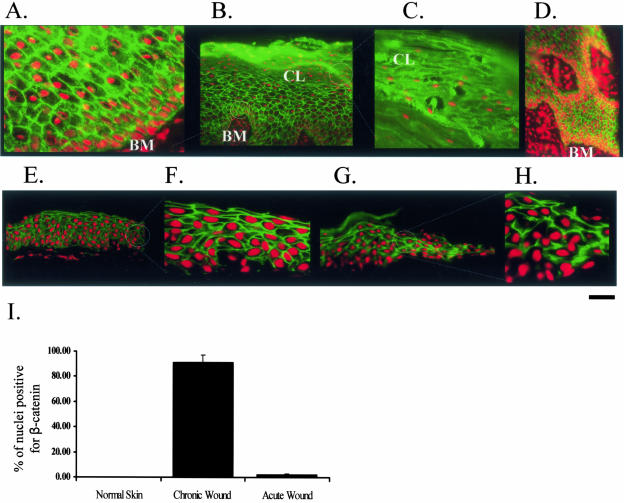
β-Catenin Is Induced in the Nonhealing Epidermal Edge of Chronic Ulcers
To test if β-catenin is indeed activated in chronic wounds we used biopsies of nonhealing edge from patients with chronic ulcers and stained sections with β-catenin-specific antibody (Figure 7). We used acute wound edge as well as normal (unwounded) skin as controls (Figure 7). We found robust nuclear localization of β-catenin in keratinocyte nuclei throughout epidermis of chronic ulcers, but neither in the epidermis of normal skin nor acute wound (Figure 7, compare A to D with E to H). β-Catenin was found in a majority (87%) of total nuclei in the epidermis of chronic wounds (Figure 7I). In contrast, less than 3% of the cells of the acute wounds and 1% of the cells of normal skin were found with nuclear β-catenin, suggesting that activation of β-catenin transcriptional pathway is specific for the chronic wounds. Interestingly, the nuclear localization of β-catenin persisted even in nuclei present in the corneal layer (Figure 7C), suggesting that β-catenin has a sustained transcriptional activity in epidermis of chronic ulcers. Lastly, we found much stronger nuclear signals in the layers closer to the basal and in keratinocytes surrounding granulation tissue islands present throughout epidermis, indicating possible signaling cross talk between the granulation tissue to the keratinocytes (Figure 7D). Therefore, nonhealing epidermal edge of a chronic wound contains cells with activated c-myc and β-catenin pathways, which contribute to its nonhealing phenotype along with K6/K16 repression.
Figure 7.
β-Catenin is activated in chronic wounds in vivo. Immunofluorescence with β-catenin-specific antibody. Prominent nuclearization of β-catenin is found in epidermis at the nonhealing edge of a chronic ulcer. Higher magnification shows nuclear presence of β-catenin in epidermis (A) and in parakeratotic cornified layer (C). B: Low magnification shows nuclear β-catenin signal throughout epidermis. D: The nuclearization signal is strongest toward basal layers, around granulation tissue of the actual edge. E and F: Control: normal skin. Normal epidermis of human skin shows β-catenin localized only to the membrane and not in the nucleus: low magnification (E) and enlarged (F). G and H: Control: acute wound. Epidermis at the edge of the acute wound shows β-catenin localized only at the membrane and not in the nucleus. G: Low magnification and H: enlarged. I: Histogram represents average ± SD of percent of nuclei positive for β-catenin from two different ulcers and several different sections.
Discussion
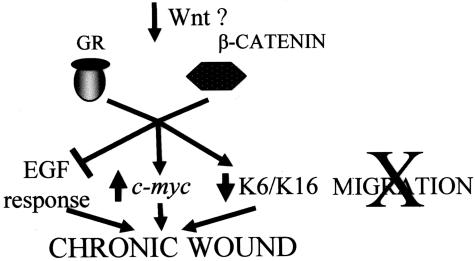
Our findings shed new light on the molecular mechanisms underlying the development of chronic wounds. Sustained activation of β-catenin and c-myc throughout the epidermis of the actual chronic ulcers, combined with β-catenin-mediated inhibition of keratinocyte migration, delayed wound healing and inhibition of EGF response, identifies β-catenin and c-myc as molecular inhibitors of wound healing (Figure 8).
Figure 8.
Cartoon summarizes our findings suggesting the molecular mechanism through which β-catenin and c-myc may participate in development of chronic wounds.
Possible Mechanisms of the Inhibition of Wound Healing by β-Catenin/c-myc
There are several possible mechanisms through which activation of β-catenin and c-myc may inhibit wound healing. For example, overexpression of c-myc was described to lead epidermal stem cells into frequent cycling.24,25,43 Therefore, inappropriate activation of β-catenin/c-myc pathway at the initiation of wound healing may cause the activation of the cell cycle of the epidermal stem cells, thus leading to their depletion at the wound site. As a consequence, keratinocytes appear to be trapped between the differentiation and activation pathways. They are hyperproliferative, which is consistent with activation, but they cannot migrate, thus their activation pathway is incomplete. This is also consistent with the findings of marked K6 repression in chronic wounds. Furthermore, a thick cornified layer is consistent with differentiation, but it is nucleated suggesting incomplete differentiation. Indeed, hyperproliferative epidermis with parakeratotic cornified layers was found at the nonhealing edge of a chronic ulcer, which is also consistent with findings in transgenic mouse models.24,25,42,43
The next logical question would be if β-catenin activates c-myc in the chronic wound, what activates β-catenin in that environment? It is very tempting to implicate the canonical Wnt pathway, as an activator of β-catenin. The complexity of the wound-healing process requires simultaneous input of signals from multiple cell types at the site of a wound, changing the differentiation into a wound-healing pathway. Wnt signals are potent regulators of development and maintenance of skin and its appendages48 and conceivably, their inappropriate activation may trigger the development of a chronic wound keratinocyte phenotype. Alternatively, an inhibitor of Wnt pathway, such as Dkk, may be suppressed, thus allowing activation of Wnt/β-catenin pathway. We are currently investigating these possibilities.
Chronic Wound Environment and β-Catenin/c-myc Activation
Several hallmarks of the chronic wound environment such as persistent inflammation, diminished activity of growth factors, and decreased angiogenesis may be affected by β-catenin/c-myc. For example, both c-myc and β-catenin pathways were found induced in the chronic inflammatory environment, such as rheumatoid arthritis.49–51 c-myc was found to inhibit the expression of PDGF-BB and its receptor,52 whereas basic fibroblast growth factor and EGF were shown to induce c-myc expression.53–55 This means that in addition to the wound-healing stimulatory effects these growth factors may also stimulate the negative feed back loop in a chronic wound environment by sustaining c-myc expression. Lastly, activation of Wnt/β-catenin pathway inhibits proliferation of human endothelial cells and increases their adhesion in vitro, suggesting inhibition of angiogenesis, one of the key elements in development of chronic wounds.56 In contrast, activation of Wnt/β-catenin pathway is important contributor of angiogenic response during tumor development and progression.57,58 This would further suggest a particular tissue context and cell specificity of angiogenic response to Wnt/β-catenin stimulation.
The Role of GC in β-Catenin/c-myc Pathways
In addition to activating c-myc, our results showed that β-catenin acts as a co-repressor of GR contributing to K6/K16 repression, thus altering the cytoskeleton necessary for keratinocyte migration. β-Catenin has been found to participate in active transcriptional repression and to interact with many transcription factors and their co-factors1,59 including the members of the hormone receptor family, such as retinoic acid receptors and androgen receptor.30,60,61 It has been shown that β-catenin and CARM-1 bind each other in a co-activator complex with AR, but it was not known if this complex has a co-repressing capacity.30 We have shown previously that GC suppress K6/K16 expression through a unique molecular mechanism that involves four monomers of GR.31,36 Here we define a novel role of β-catenin in this mechanism. Although GC are well known inhibitors of wound healing our data indicate their possible role in chronic wound environment.
Conclusion
Overall, our findings suggest a model of chronic wound phenotype initiation in which keratinocytes at the wound edge become targeted by canonical Wnt pathway(s), thus activating β-catenin. Activation of β-catenin inhibits keratinocyte migration through different mechanisms: by causing activation of c-myc, by blocking the EGF effects, and by synergizing with GC to suppress K6/K16 thus causing cytoskeletal changes. All these effects lead to inhibition of keratinocyte migration, and deregulation of their growth and differentiation (Figure 8). Although β-catenin signaling has been implicated in epithelial development and oncogenesis its role in wound healing has never been postulated. This further illustrates the importance of tissue context specificity, because β-catenin in the context of malignant tissue promotes invasion whereas in the context of a wound environment does the opposite.
Acknowledgments
We thank Dr. Miki Blumenberg for his generous support and helpful criticisms, Dr. Jiri Zavadil for assistance in quantification of our migration assays, Dr. Pierre Coulombe for generous gift of K6 antibody, and Dr. Cindy Loomis and Dr. Irwin Freedberg for critically reviewing the manuscript.
Footnotes
Address reprint requests to M. Tomic-Canic, New York University School of Medicine, The Ronald O. Perelman Department of Dermatology, 550 First Ave., TH100, New York, NY, 10016. E-mail: tomicm01@med.nyu.edu.
Supported by the National Institutes of Health (grants AR45974, NR08029, DK59424, DK43093, T3207190) and the American Diabetes Association.
References
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirfel J, Magin TM, Reichelt J. Keratins: a structural scaffold with emerging functions. Cell Mol Life Sci. 2003;60:56–71. doi: 10.1007/s000180300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic-Canic M, Magnus SA, Oscar MA. Epidermal repair and the chronic wound. Rovee DT, Maibach HI, editors. Boca Raton: CRC Press LLC,; The epidermis in wound healing. 2004:pp 25–57. [Google Scholar]
- Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2005;97:173–183. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361:1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- Brem H, Tomic-Canic M, Taranovskaya A, Ehlich HP, Baskin-Bay E, Gill K, Carasa G, Weinberger S, Entero H, Vladeck B. Healing of elderly patients with diabetic foot ulcers, venous stasis ulcers, and pressure ulcers. Surg Technol Int. 2003;11:161–167. [PubMed] [Google Scholar]
- Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. Harris MI, Cowie C, Stern MP, editors. Washington DC: U.S. Government Printing Office,; Diabetes in America. 1995:pp 409–428. [Google Scholar]
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513–521. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- Yoshikawa TT. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J Am Geriatr Soc. 2002;50:S226–S229. doi: 10.1046/j.1532-5415.50.7s.2.x. [DOI] [PubMed] [Google Scholar]
- Brem H, Young J, Tomic-Canic M, Isaacs C, Ehrlich HP. Clinical efficacy and mechanism of bilayered living human skin equivalent (HSE) in treatment of diabetic foot ulcers. Surg Technol Int. 2003;11:23–31. [PubMed] [Google Scholar]
- Brem H, Balledux J, Bloom T, Kerstein MD, Hollier L. Healing of diabetic foot ulcers and pressure ulcers with human skin equivalent: a new paradigm in wound healing. Arch Surg. 2000;135:627–634. doi: 10.1001/archsurg.135.6.627. [DOI] [PubMed] [Google Scholar]
- Nagai MK, Embil JM. Becaplermin: recombinant platelet derived growth factor, a new treatment for healing diabetic foot ulcers. Exp Opin Biol Ther. 2002;2:211–218. doi: 10.1517/14712598.2.2.211. [DOI] [PubMed] [Google Scholar]
- Sibbald RG. Apligraf living skin equivalent for healing venous and chronic wounds. J Cutan Med Surg. 1998;3:S24–S28. [PubMed] [Google Scholar]
- Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1:208–216. doi: 10.1038/35043032. [DOI] [PubMed] [Google Scholar]
- Green KJ, Jones JC. Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB J. 1996;10:871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- McGrath JA, McMillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, Garrod DR, Eady RA. Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nat Genet. 1997;17:240–244. doi: 10.1038/ng1097-240. [DOI] [PubMed] [Google Scholar]
- Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Millar SE. WNTs: multiple genes, multiple functions. J Invest Dermatol. 2003;120:7–8. doi: 10.1046/j.1523-1747.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- Rowlands TM, Pechenkina IV, Hatsell SJ, Pestell RG, Cowin P. Dissecting the roles of beta-catenin and cyclin D1 during mammary development and neoplasia. Proc Natl Acad Sci USA. 2003;100:11400–11405. doi: 10.1073/pnas.1534601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Kemler R. Curbing the nuclear activities of beta-catenin. Control over Wnt target gene expression. EMBO Rep. 2000;1:24–28. doi: 10.1093/embo-reports/kvd012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. The stem cell compartment in human interfollicular epidermis. J Dermatol Sci. 2002;28:173–180. doi: 10.1016/s0923-1811(02)00003-8. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Biro S, Fu YM, Yu ZX, Epstein SE. Inhibitory effects of antisense oligodeoxynucleotides targeting c-myc mRNA on smooth muscle cell proliferation and migration. Proc Natl Acad Sci USA. 1993;90:654–658. doi: 10.1073/pnas.90.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandarillas A, Watt FM. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 1997;11:2869–2882. doi: 10.1101/gad.11.21.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon SS, Nadesan P, Poon R, Alman BA. Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp Cell Res. 2004;293:267–274. doi: 10.1016/j.yexcr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, Alman BA. Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci USA. 2002;99:6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SS, Li H, Lee YH, Widelitz RB, Chuong CM, Stallcup MR. Synergistic coactivator function by coactivator-associated arginine methyltransferase (CARM) 1 and beta-catenin with two different classes of DNA-binding transcriptional activators. J Biol Chem. 2002;277:26031–26035. doi: 10.1074/jbc.M110865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoja N, Komine M, Jho SH, Blumenberg M, Tomic-Canic M. Novel mechanism of steroid action in skin through glucocorticoid receptor monomers. Mol Cell Biol. 2000;20:4328–4339. doi: 10.1128/mcb.20.12.4328-4339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho SH, Radoja N, Im MJ, Tomic-Canic M. Negative response elements in keratin genes mediate transcriptional repression and the cross-talk among nuclear receptors. J Biol Chem. 2001;276:45914–45920. doi: 10.1074/jbc.M103144200. [DOI] [PubMed] [Google Scholar]
- Li D, Turi TG, Schuck A, Freedberg IM, Khitrov G, Blumenberg M. Rays and arrays: the transcriptional program in the response of human epidermal keratinocytes to UVB illumination. FASEB J. 2001;15:2533–2535. doi: 10.1096/fj.01-0172fje. [DOI] [PubMed] [Google Scholar]
- Randolph RK, Simon M. Characterization of retinol metabolism in cultured human epidermal keratinocytes. J Biol Chem. 1993;268:9198–9205. [PubMed] [Google Scholar]
- Jiang CK, Connolly D, Blumenberg M. Comparison of methods for transfection of human epidermal keratinocytes. J Invest Dermatol. 1991;97:969–973. doi: 10.1111/1523-1747.ep12491889. [DOI] [PubMed] [Google Scholar]
- Lee B, Vouthounis C, Stojadinovic O, Brem H, Im M, Tomic-Canic M. From an enhanceosome to a repressosome: molecular antagonism between glucocorticoids and EGF leads to inhibition of wound healing. J Mol Biol. 2005;345:1083–1097. doi: 10.1016/j.jmb.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim M, Smart RC. Lithium stabilizes the CCAAT/enhancer-binding protein alpha (C/EBPalpha) through a glycogen synthase kinase 3 (GSK3)-independent pathway involving direct inhibition of proteasomal activity. J Biol Chem. 2003;278:19674–19681. doi: 10.1074/jbc.M301356200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J Biol Chem. 2002;277:26573–26580. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- Munne A, Fabre M, Marinoso ML, Gallen M, Real FX. Nuclear beta-catenin in colorectal tumors: to freeze or not to freeze? Colon Cancer Team at IMAS. J Histochem Cytochem. 1999;47:1089–1094. doi: 10.1177/002215549904700813. [DOI] [PubMed] [Google Scholar]
- McGowan KM, Coulombe PA. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J Cell Biol. 1998;143:469–486. doi: 10.1083/jcb.143.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M, Gardner C, Li ER, Arnold I, Watt FM. Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development. 2003;130:2793–2808. doi: 10.1242/dev.00462. [DOI] [PubMed] [Google Scholar]
- Waikel RL, Wang XJ, Roop DR. Targeted expression of c-Myc in the epidermis alters normal proliferation, differentiation and UV-B induced apoptosis. Oncogene. 1999;18:4870–4878. doi: 10.1038/sj.onc.1203040. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Bundman DS, Roop DR. Delayed wound healing in keratin 6a knockout mice. Mol Cell Biol. 2000;20:5248–5255. doi: 10.1128/mcb.20.14.5248-5255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Coulombe PA. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J Cell Biol. 2003;163:327–337. doi: 10.1083/jcb.200305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer HD, Fassler R, Werner S. Glucocorticoid-regulated gene expression during cutaneous wound repair. Vitam Horm. 2000;59:217–239. doi: 10.1016/s0083-6729(00)59008-6. [DOI] [PubMed] [Google Scholar]
- Roeser T, Stein S, Kessel M. Nuclear beta-catenin and the development of bilateral symmetry in normal and LiCl-exposed chick embryos. Development. 1999;126:2955–2965. doi: 10.1242/dev.126.13.2955. [DOI] [PubMed] [Google Scholar]
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- Roivainen A, Soderstrom KO, Pirila L, Aro H, Kortekangas P, Merilahti-Palo R, Yli-Jama T, Toivanen A, Toivanen P. Oncoprotein expression in human synovial tissue: an immunohistochemical study of different types of arthritis. Br J Rheumatol. 1996;35:933–942. doi: 10.1093/rheumatology/35.10.933. [DOI] [PubMed] [Google Scholar]
- Michael VV, Alisa KE. Cell cycle implications in the pathogenesis of rheumatoid arthritis. Front Biosci. 2000;5:D594–D601. doi: 10.2741/volin. [DOI] [PubMed] [Google Scholar]
- Sen M, Reifert J, Lauterbach K, Wolf V, Rubin JS, Corr M, Carson DA. Regulation of fibronectin and metalloproteinase expression by Wnt signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum. 2002;46:2867–2877. doi: 10.1002/art.10593. [DOI] [PubMed] [Google Scholar]
- Oster SK, Marhin WW, Asker C, Facchini LM, Dion PA, Funa K, Post M, Sedivy JM, Penn LZ. Myc is an essential negative regulator of platelet-derived growth factor beta receptor expression. Mol Cell Biol. 2000;20:6768–6778. doi: 10.1128/mcb.20.18.6768-6778.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, He H, Zigler JS, Jr, Iwata T, Ibaraki N, Reddy VN, Carper D. bFGF suppresses serum-deprivation-induced apoptosis in a human lens epithelial cell line. Exp Cell Res. 1999;249:123–130. doi: 10.1006/excr.1999.4450. [DOI] [PubMed] [Google Scholar]
- Izadnegahdar MF, Rathanaswami P, Shah RM. Effects of EGF and TGFbeta1 on c-myc gene expression and DNA synthesis in embryonic hamster palate mesenchymal cells. Anat Rec. 1999;254:453–464. doi: 10.1002/(SICI)1097-0185(19990401)254:4<453::AID-AR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chrysogelos SA, Dickson RB. EGF receptor expression, regulation, and function in breast cancer. Breast Cancer Res Treat. 1994;29:29–40. doi: 10.1007/BF00666179. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Smith SK, Charnock-Jones DS. Wnt-1 signaling inhibits human umbilical vein endothelial cell proliferation and alters cell morphology. Exp Cell Res. 2003;291:415–425. doi: 10.1016/j.yexcr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–6054. [PubMed] [Google Scholar]
- Hanai J, Gloy J, Karumanchi SA, Kale S, Tang J, Hu G, Chan B, Ramchandran R, Jha V, Sukhatme VP, Sokol S. Endostatin is a potential inhibitor of Wnt signaling. J Cell Biol. 2002;158:529–539. doi: 10.1083/jcb.200203064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantjes H, Barker N, van Es J, Clevers H. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol Chem. 2002;383:255–261. doi: 10.1515/BC.2002.027. [DOI] [PubMed] [Google Scholar]
- Song LN, Herrell R, Byers S, Shah S, Wilson EM, Gelmann EP. Beta-catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol Cell Biol. 2003;23:1674–1687. doi: 10.1128/MCB.23.5.1674-1687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran V, Pishvaian M, Salimuddin, Byers S. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9:1415–1418. doi: 10.1016/s0960-9822(00)80088-3. [DOI] [PubMed] [Google Scholar]