Abstract
Thrombospondin (TSP)-2-null dermal fibroblasts display an attachment defect that results from increased matrix metalloproteinase (MMP)-2 levels in their conditioned media. To investigate the molecular mechanisms responsible for this defect, we analyzed the activity of tissue transglutaminase (tTG) in TSP-2-null dermal fibroblasts and in tissues of TSP-2-null mice. tTG functions as a co-receptor for β1 and β3 integrins and stabilizes extracellular matrix proteins by introduction of isopeptide cross-links. Cell-surface tTG activity was reduced in TSP-2-null cells (0.50 ± 0.05 arbitrary units versus 0.84 ± 0.07 for wild type; P ≤ 0.05), and addition of MMP-2 to the culture medium of wild-type cells caused a 35% reduction in cell-surface tTG activity. tTG was susceptible to proteolysis by MMP-2 in vitro, and addition of the MMP inhibitor TIMP-2 to TSP-2-null cells restored tTG activity (0.3 ± 0.08 for untreated cells; 0.71 ± 0.09 with TIMP-2). TSP-2-null mice had reduced tTG activity in skin, as measured by incorporation of fluorescein isothiocyanate-labeled cadaverine, and a threefold increase in acetic acid-extracted dermal collagen. Furthermore, isopeptide cross-links were reduced in both uninjured skin and in excisional wounds of TSP-2-null mice, as determined by morphometric immunohistochemical analysis, indicating that isopeptide cross-links are important for the stabilization of the collagenous matrix in dermis. These findings provide a mechanism for the reduced adhesion of TSP-2-null fibroblasts and an explanation for the increased collagen solubility and fragility of TSP-2-null skin.
The phenotype of the thrombospondin (TSP)-2-null mouse has provided investigators with a number of important clues to the regulation of the assembly and stabilization of the extracellular matrix (ECM). TSP-2-null fibroblasts show a marked adhesive defect, and mice that lack TSP-2 have fragile skin of low tensile strength,1 but heal excisional skin wounds more rapidly and with less scarring than controls.2 Reduced tensile strength and tissue integrity have also been documented in the pregnant cervix3 and in the injured myocardium of the TSP-2-null mouse.4 At the level of the light microscope, collagen fibers are disorganized in dermis1 and in granulation tissue of healing skin wounds,2,5 and collagen fibrils show an abnormal size distribution with irregular contours,1 and abnormalities in fibroblast-matrix interactions in the electron microscope.6
A pathogenesis for these matrix abnormalities was suggested by the findings that matrix metalloproteinase (MMP)-2 levels are increased in cultured fibroblasts of TSP-2-null mice, and that the adhesive defect in these cells could be reversed by inhibitors of MMP-2.7 Elevated levels of MMP-2 were also documented in tissues of TSP-2-null mice after a variety of injuries, eg, in the foreign body reaction to subcutaneously implanted biomaterials,8 in healing skin wounds,5 in the circumferentially stressed pregnant cervix,3 and in injured myocardium.4 In some of these tissues, MMP-9 was also increased. An explanation for accumulation of MMP-2 in tissues of TSP-2-null mice was provided by the demonstration that MMP-2 is normally cleared from the pericellular environment by the formation of a TSP-2-MMP-2 complex that is endocytosed by the LRP1 scavenger receptor.9
These findings, although implicating proteolysis by MMP-2 in the generation of matrix abnormalities in TSP-2-null mice, raise the question of the target of that proteolysis. MMP-2 is capable of degrading many matrix proteins, including type I collagen and fibronectin,10,11 but there is evidence that much of the proteolytic activity of MMP-2 in the extracellular milieu is neutralized by the tight-binding inhibitor, TIMP-2.12 However at the cell surface, pro-MMP-2 is activated by MT1-MMP in a complex with TIMP-2 and free, active MMP-2 is released.13 There is ample evidence for a role of MMP-2 and other MMPs in regulating cell adhesion and other cellular functions by proteolysis of cell-surface proteins.14,15
Our attention was drawn to the possibility that tissue transglutaminase (transglutaminase 2; tTG) might serve as a target of the elevated levels of MMP-2 in TSP-2-null mice for several reasons. As shown by Akimov and Belkin,16 cell-surface tTG interacts with β1 and β3 integrins and mediates their association with the gelatin-binding domain of fibronectin, which lacks intrinsic integrin-binding sites. tTG also increases the size of focal adhesions and increases adhesion-dependent phosphorylation of focal adhesion kinase. These properties are not shared by a functional homologue of tTG, factor XIIIA, and do not depend on the catalytic activity of tTG.16 Reduced levels of tTG could account for both reduced cell adhesion and abnormal collagen fibrillogenesis in TSP-2-null mice, since it is now recognized that assembly of collagen monomers into fibers occurs in close association with the cell surface,17,18 and this association could involve collagen-binding β1 integrins. Finally, tTG is known to stabilize the ECM by the introduction of γ-glutamyl-ε-lysyl isopeptide bond cross-links into many ECM proteins, including collagens and fibronectin.19
Indeed, we show in this study that tTG is susceptible to proteolysis by MMP-2 in vitro, and that tTG activity is reduced in both fibroblasts in culture and in tissues from TSP-2-null mice. A reduction in tTG activity could therefore account for the inferior structural properties of connective tissues, as summarized above, and for the increased extractability of collagen from the dermis of TSP-2-null mice, as demonstrated in this study. Furthermore, the content of isopeptide cross-links is reduced in TSP-2-null skin and excisional skin wounds. We therefore propose that a reduction in intact tTG protein and activity is a major cause of the connective tissue abnormalities in TSP-2-null mice.
Materials and Methods
Mice
All mice used in this study were on a mixed C57BL6/129SvEMS+Ter background.
Cell Culture
After euthanasia, mice (four of each genotype) were shaved and skinned. Subcutaneous fat was removed by scraping, and the skin was treated with 0.25% w/v trypsin in Dulbecco’s modified Eagle’s medium (DMEM) at 4°C overnight to detach the epidermis from the dermis. The dermis was then cut into small pieces with a scalpel and digested with 0.25% w/v collagenase in DMEM at 37°C for 3 to 5 hours. The resulting cells were collected by centrifugation, washed twice with DMEM, plated, and maintained in DMEM supplemented with 10% fetal calf serum.
Cell Attachment Assays
Cell attachment assays were performed in 96-well plates coated with fibronectin or vitronectin as previously described.7 Briefly, cultured fibroblasts were trypsinized, washed, and 100 μl of a cell suspension (104 cells) was added to each well. Cells were allowed to attach for 1 hour at 37°C, unattached cells were removed by washing the plates with DMEM, and attachment was quantified by addition of 20 μl of Cell Titer 96 solution (Promega, Madison, WI). After incubation at 37°C for 1 hour, the absorbance at 650 nm was measured in a microplate reader (Molecular Devices, Sunnyvale CA). MMP-2 (25 to 100 ng/ml) (a generous gift from Dr. C.M. Overall, University of British Columbia, Vancouver, BC, Canada) was added to the culture medium for 9 hours to determine its effects on tTG activity and cell attachment.
Analysis of Cellular tTG Activity
Fibroblasts were suspended in 0.1 mmol/L biotinylated cadaverine (Molecular Probes, Eugene, OR) and plated in fibronectin-coated 96-well plates at 2 × 103 cells/well. After incubation at 37°C for 2 hours, the cells were washed and lysed with deoxycholate. Incorporation of biotinylated cadaverine into soluble protein was detected by addition of avidin-horseradish peroxidase and tetra-methyl benzidine, followed by measurement of absorbance at 450 nm. DNA concentrations of the cell lysates were measured in a fluorimeter using SYBR green dye, and were used to normalize the cell content of the wells. Antibodies to laminin 5 (DAKO, Glostrup, Denmark) were used to test for contamination of fibroblasts with epidermal keratinocytes. Recombinant mouse TIMP-2 (Chemicon, Temicula, CA) was used to inhibit MMP-2 activity.
Proteolysis of tTG in Vitro
Pro-MMP-2 was activated for 30 minutes at 4°C with 1 mmol/L p-aminophenylmercuric acetate (Sigma Chemical Co., Saint Louis, MO). Purified tTG (5 μg; Diarect Ag, Freiburg, Germany) was incubated with activated MMP-2 (0.3 μg) for 1 hour at 37°C in a buffer containing 0.05 mol/L Tris-HCl, pH 7.5, 50 mmol/L NaCl, 1 mmol/L CaCl2, and 10 μmol/L ZnCl2. The digestion products were analyzed by 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Western analysis for tTG was performed with a polyclonal antibody (Neo-markers, Fremont, CA).
Extraction of Collagen from Mouse Skin
Three mice of each genotype were euthanized, shaved, and the skins were removed. After removal of subcutaneous tissue by scraping, the skins were weighed, minced, and extracted in phosphate-buffered saline at 4°C overnight with gentle stirring. The mince was then centrifuged, the supernate discarded, and the sediment extracted with 0.5 N of acetic acid (30:1, v/w) for 2 days at 4°C with gentle stirring. The mixture was centrifuged, the supernate was filtered, and NaCl was added to a final concentration of 10% to precipitate the collagen. After centrifugation, the supernatant was discarded, 0.1 N acetic acid was added to the collagen pellet, and the slurry dialyzed against 0.1 N of acetic acid to dissolve the salt-free collagen. The mince was then subjected to a second extraction and purification. The resulting soluble collagens were combined and lyophilized.
Activity of tTG in Skin Homogenates
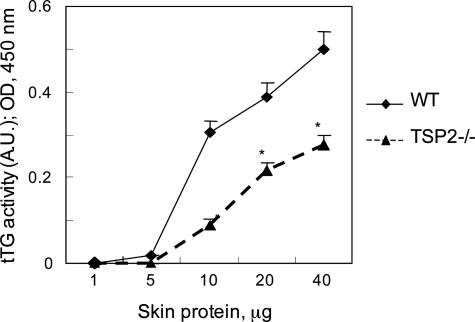
Four mice of each genotype were euthanized and shaved, and skin was removed from the back. Skins were minced and homogenized with a Polytron homogenizer in 2 ml of phosphate-buffered saline containing 2 mmol/L phenylmethyl sulfonyl fluoride and 1 μg/ml each of aprotinin, leupeptin, and pepsin A (Sigma Chemical Co.). Protein content was determined by the bicinchoninic acid method according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA). tTG activity in the skin homogenate was measured as described above.
Analysis of tTG Activity in Excisional Wounds
Four mice of each genotype, ∼3 months of age and sex-matched, were anesthetized with avertin and 6-mm excisional wounds were made as described previously.2 Wounded animals were housed individually. Uninjured skin and excised wounds were fixed in 10% zinc-formalin buffer (Z-fix; Anatech, Battle Creek, MI), paraffin-embedded, and sectioned at 5 μm thickness, as described previously.2 The measurement of tTG activity in situ was performed as previously described.20 Briefly, cryostat sections were washed with a solution containing a cocktail of protease inhibitors and then incubated with fluorescein isothiocyanate cadaverine (Molecular Probes) in 2.5 mmol/L CaCl2 for 1 hour at room temperature. For negative controls, CaCl2 was replaced with 2 mmol/L ethylenediamine tetraacetic acid. Visualization and quantification were performed using fluorescence microscopy.
Immunohistochemical Analysis of Isopeptide-Bond Cross-Links in Tissues
Analysis of cross-link formation was quantified by immunodetection of isopeptide bonds with specific antibodies to ε-lysyl γ-glutaminyl cross-links (Abcam, Cambridge, MA) in paraffin-embedded sections, as described previously.21,22 All examinations were performed with a Nikon Eclipse 800 microscope (Tokyo, Japan). For morphometric analyses, images were captured with a digital camera and analysis was performed with Metamorph software (Universal Corp., West Chester, PA), as described previously.8 Four sections per sample were analyzed.
Statistical Analysis
All results are expressed as means ± SEM. Statistical significance was evaluated by the two-tailed unpaired Student’s t-test for comparisons between the means of two groups. A value of P < 0.05 was considered to be statistically significant.
Results
Cell-Surface Activity of tTG Is Reduced in Primary Fibroblast Cultures from TSP-2-Null Mice
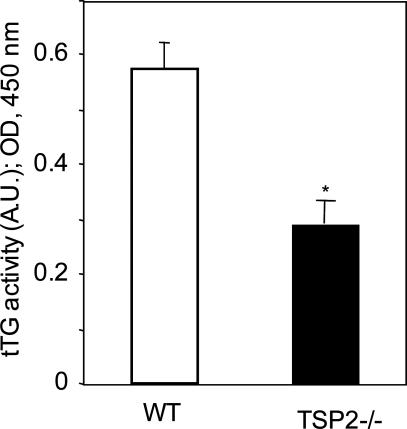
To evaluate the basis for the adhesive defect observed in TSP-2-null cells, dermal fibroblasts were isolated from both wild-type (WT) and TSP-2-null mice. Cell-surface-mediated tTG activity was measured by incorporation of biotinylated cadaverine (a substrate for tTG) into soluble proteins. Cell-surface tTG activity was significantly reduced in mutant in comparison with WT cells (0.50 ± 0.05 arbitrary units versus 0.84 ± 0.07 for WT, P ≤ 0.05; Figure 1). To assess possible contamination with keratinocytes, which are rich in tTG, fibroblasts were plated on chamber slides at a density of 105 cells/well. Cells were allowed to attach and were then stained with an antibody to laminin 5, a keratinocyte marker. Less than 1% of the cell population stained positively (data not shown).
Figure 1.
Cell-surface tTG activity in dermal fibroblasts from WT and TSP-2-null mice. tTG activity was quantified by incorporation of biotinylated cadaverine into soluble proteins. The bar graphs represent the averages of four determinations; mean ± SEM; *P ≤ 0.05. A.U., arbitrary units. This experiment was repeated four times with similar results.
Purified tTG Is a Substrate for MMP-2
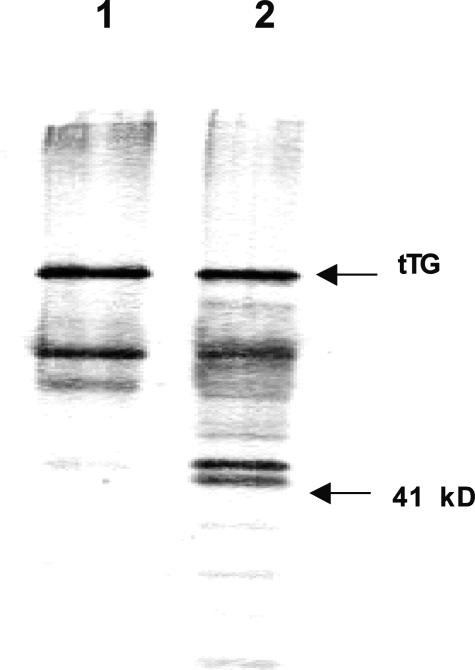
To determine whether the reduced adhesion of TSP-2-null fibroblasts could be attributed directly to increased MMP-2 levels, we subjected purified tTG to proteolysis by MMP-2. Pro-MMP-2 was incubated with p-aminophenylmercuric acetate and the conversion of zymogen to the active form of the enzyme was confirmed by gelatin zymography (data not shown). The proteolytic digest of tTG was subjected to SDS-PAGE and Western blot analysis. As shown in Figure 2, additional low molecular weight fragments of tTG were generated by MMP-2.
Figure 2.
Proteolysis of tTG in vitro. tTG was incubated with MMP-2 for 1 hour at room temperature. The digest was examined by Western analysis of SDS gels with an anti-tTG antibody. Lane 1, tTG; lane 2, fragments generated by cleavage of tTG with MMP-2. The positions of migration of tTG (68 kd) and a molecular weight standard (41 kd) are indicated.
Addition of Exogenous MMP-2 to WT Fibroblasts Reduces tTG Activity and Attachment, and an Inhibitor of MMP-2 Increases tTG Activity
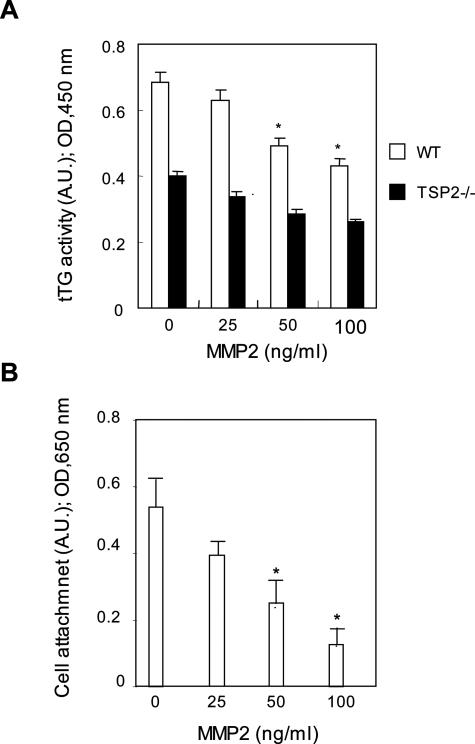
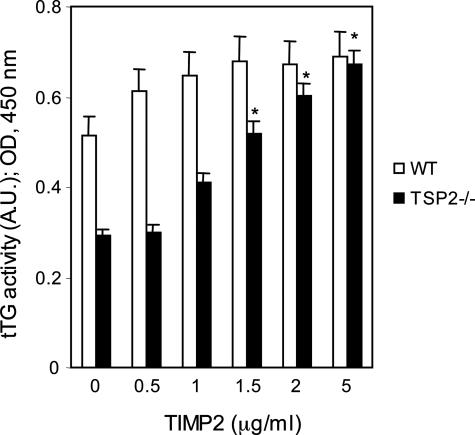
To provide additional support for the possibility that increased MMP-2 activity is responsible for the reduced activity of cell-surface tTG in TSP-2-null fibroblasts, exogenous, purified active MMP-2 was added to WT fibroblasts. Addition of 100 ng/ml of MMP-2 to cultured WT dermal fibroblasts caused a 35% reduction in tTG activity (Figure 3A). The addition of MMP-2 also resulted in a dose-dependent reduction in the adhesive properties of WT cells (Figure 3B). In support of these findings, the MMP-2 inhibitor, TIMP-2, increased tTG activity in TSP-2-null fibroblasts to levels similar to those found in WT cells (0.68 ± 0.07 arbitrary units, TIMP-2; 0.70 ± 0.1; Figure 4). We conclude, on the basis of the results shown in Figures 1 to 4, that the adhesive defects observed in TSP-2-null fibroblasts are a consequence of partial proteolysis of tTG by the elevated levels of MMP-2 present in these cells.
Figure 3.
Addition of MMP-2 to WT fibroblasts reduces cell-surface tTG activity and cell adhesion. A: Quantification of tTG activity after addition of purified MMP-2 to WT fibroblasts. B: Quantification of attachment after addition of MMP-2 to WT fibroblasts. The bar graphs represent the averages of four determinations; mean ± SEM; *P ≤ 0.05. A.U., arbitrary units. This experiment was repeated three times with similar results.
Figure 4.
Addition of the MMP-2 inhibitor, TIMP-2, to TSP-2-null fibroblasts increases cell-surface tTG activity. The bar graphs represent the averages of four determinations; mean ± SEM; *P ≤ 0.05. A.U., arbitrary units. This experiment was repeated twice with similar results.
Increased Collagen Extractability and Reduced tTG Activity in TSP-2-Null Skin
To determine whether the reduced tTG activity documented in cultured TSP-2-null fibroblasts could also account for some of the phenotypic features of TSP-2-null mice, we first assessed the ability of 0.5 N acetic acid to extract dermal collagen. The extractability of collagen from TSP-2-null skin was found to be increased threefold in comparison with that from WT skin (Table 1). Examination of the collagens by SDS-PAGE failed to reveal lower molecular weight bands in the extracts from TSP-2-null skin, as might be expected if the increased solubility of TSP-2-null collagen resulted from partial pro-teolysis by MMP-2 (data not shown). Furthermore, measurement of tTG activity, based on incorporation of biotinylated cadaverine into proteins solubilized from homogenates of dermis, indicated that the enzymatic activity was significantly reduced in skin of mutant mice, in comparison with WT skin (Figure 5). These results support a role for tTG in stabilizing the ECM in dermis.
Table 1.
Extractability of Collagen from Mouse Skin
| Genotype* | Skin (g)† | Extracted collagen (mg) | Collagen yield (mg/g skin) |
|---|---|---|---|
| TSP2+/+ | 2.7 ± 1.6 | 4.2 ± 3.1 | 1.3 ± 0.7 |
| TSP2−/− | 3.2 ± 1.8 | 16 ± 9.8 | 4.2 ± 2.1‡ |
Collagen was extracted with 0.5 N of acetic acid at 4°C. Mice averaged 25 to 30 g in weight and varied between 3 and 4 months of age. Values are means ± SD. The extractability of TSP2-null skin exceeded that of WT by threefold.
n = 3 each group.
Wet weight.
P ≤ 0.05.
Figure 5.
tTG activity in skin homogenates. The epidermal layer was removed and tTG activity in dermal homogenates was quantified by incorporation of biotinylated cadaverine into soluble proteins. The graphs represent the averages of three determinations; mean ± SEM; *P ≤ 0.05. This experiment was repeated twice with similar results.
Reduced tTG Activity and Isopeptide Bond Cross-Links in TSP-2-Null Skin and Excisional Skin Wounds
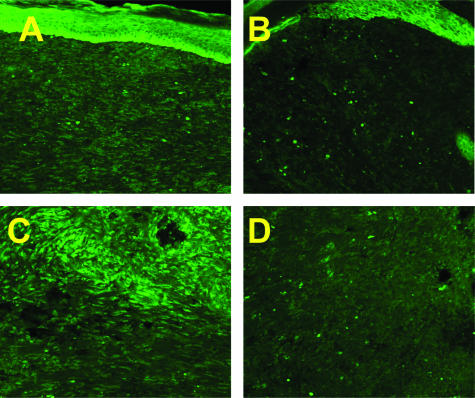
tTG activity in intact, uninjured skin was found to be below the level for reliable detection by fluorescence microscopy. However, the enzyme has been reported to be induced in response to injury.23 We therefore chose a well-characterized injury model, full-thickness excisional skin wounds, to compare the activity of this enzyme in WT and TSP-2-null-mice. To detect endogenous tTG activity, cryosections of day 10 wounds, a time at which levels of MMP-2 are elevated, were incubated with fluorescein isothiocyanate-labeled cadaverine, and in situ tTG activity was tested. Reduced tTG activity was evident by fluorescence microscopy in sections of granulation tissue of TSP-2-null wounds, in comparison with sections of WT wounds (Figure 6).
Figure 6.
Incorporation of fluorescein isothiocyanate-labeled cadaverine in day 10 wounds. Cryosections of day 10 wounds were incubated with fluorescein isothiocyanate-labeled cadaverine, and incorporation into soluble proteins was visualized by fluorescence microscopy. Reduced tTG activity is evident in TSP-2-null (B, D) in comparison with WT wounds (A, C). Original magnifications: ×100 (A, B); ×200 (C, D).
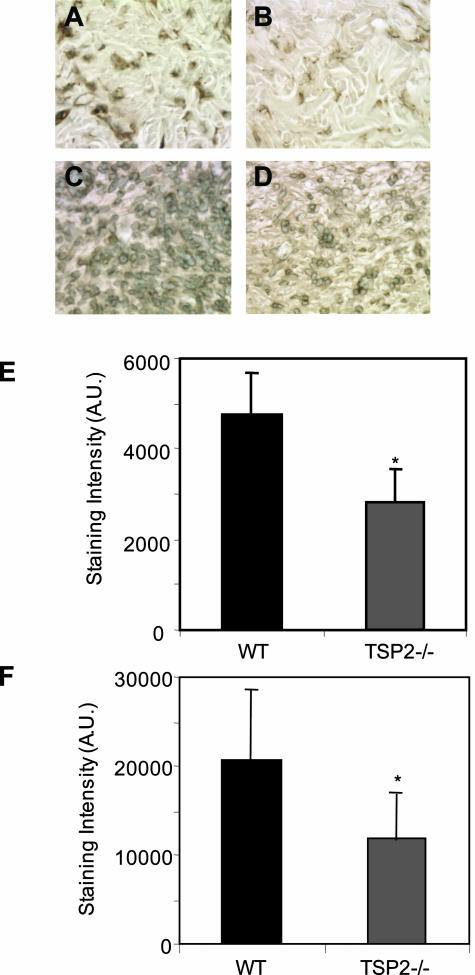
Levels of isopeptide bond cross-links were also evaluated in skin and excisional wounds of TSP-2-null and WT mice. Uninjured skin and day 10 wound sections were stained with antibodies to cross-links and peroxidase-conjugated secondary antibodies, visualized by light microscopy, and quantified with Metamorph software. In comparison with WT tissues, isopeptide cross-links in TSP-2-null mice were reduced in both uninjured skin (Figure 7; A, B, E) and in day 10 wounds (Figure 7; C, D, F).
Figure 7.
Detection of isopeptide bonds in dermis and wounds. Isopeptide bond formation is decreased in both uninjured skin (B) and day 10 wounds (D) of TSP-2-null animals in comparison with WT tissue (A, C). Sections were stained with antibodies to isopeptide bonds, and visualized by the peroxidase reaction. Metamorph software was used to quantify the staining intensity in skin (E) and in wounds (F). A.U., arbitrary units. Original magnifications, ×400.
Discussion
The TSP-2-null mouse has proved to be a useful experimental animal in which to study the role of angiogenesis in reactions to injury, tumor growth and metastasis, and the mechanisms involved in the assembly and stabilization of the ECM. In previous publications, both from our own laboratory and in collaboration with other scientists, we had identified elevated levels of MMP-2 in cultured fibroblasts and in tissues of TSP-2-null mice,3–5,7–9 and considered the relation between the increase in protease activity and the reduced cell adhesion and tissue integrity observed in these mice. In this study we have shown that a target of MMP-2 is cell-surface tTG on fibroblasts, and possibly on other cells. MMP-2 is capable of digesting tTG in vitro, and the addition of MMP-2 to cultured WT fibroblasts results in a significant reduction in tTG activity, with a concomitant reduction in cell attachment. Furthermore, the concentration of isopeptide bond cross-links, the product of catalysis by tTG, was reduced in uninjured dermis and in dermal wound tissue of TSP-2-null mice. While this article was in preparation, Belkin and colleagues24 also reported that tTG is a substrate for MMP-2, acting in concert with MT1-MMP in vitro. The size of proteolytic fragments of tTG generated by MMP-2/MT1-MMP in experiments described by Belkin and colleagues24 are very similar to those shown in Figure 2.
tTG, also known as TG2, is a member of a family of eight homologous proteins, and is widely expressed in mammalian tissues, not only on cell surfaces but also in the cytosol and the nucleus. The enzyme catalyzes many posttranslational modifications of ECM proteins, such as amine incorporation, acylation, esterification, and deamidation, in addition to cross-linking.19 Its cross-linking function is also thought to be important in the lens, in the stabilization of apoptotic cell bodies and insoluble protein aggregates that cause neuronal death, and in mineralization of bone.19 In addition, tTG is involved in a number of physiological processes that are independent of its cross-linking function, including its role as a GTP-binding protein in the regulation of cell-cycle progression,25 in receptor-mediated signal transduction,26 and in cell-matrix interactions.27 The latter findings are consistent with the observations that overexpression of tTG in fibroblasts increased cell attachment,28 and that reduced expression in endothelial cells, by use of anti-sense technology, resulted in decreased attachment and spreading.29
Given the widespread distribution of tTG and its multiple functions, it is surprising that the phenotype of tTG-null mice is relatively mild.30,31 tTG-null mice are viable and fertile, and are born with the expected Mendelian frequency. In particular, the induction of apoptosis appears to be primarily normal. We have found that the extractability of collagen from the skins of tTG-null mice (kindly provided by Dr. R.M. Graham, Victor Chang Cardiac Research Institute, Darlinghurst, NSW Australia) did not differ from that of control mice (N.Y. and P.B., unpublished observations). However, there was a small but statistically significant decrease in the viability of tTG-null thymocytes after dexamethasone treatment, and in attachment of tTG-null fibroblasts.30 The lack of more serious defects in tTG-null mice can be attributed, in part, to compensation by other TGs, specifically to the small amounts of TG1 (keratinocyte TG) and factor XIIIA that have been detected in tissues of both control and tTG-null mice.30,31
To what extent can we ascribe the diverse matrix abnormalities in the TSP-2-null mouse to a reduction in functional tTG? It should first be emphasized that our measurements of tTG activity in fibroblasts in culture and in tissues of TSP-2-null mice were meant to provide us with an indication of the levels of protein that were active in catalysis. These determinations would therefore not detect partial proteolysis that did not interfere with the catalytic function of tTG. However, as shown in Figure 3 and by Belkin and colleagues,24 proteolysis of tTG with MMP-2 results in the loss of both the catalytic and adhesive activities of the protein. We believe that the sum of evidence presented here, together with the experiments cited above, argue strongly for proteolysis of tTG by MMP-2 as a major cause of the cellular adhesive defect in TSP-2-null fibroblasts in vitro. However, the increased levels of MMP-2 in TSP-2-null fibroblasts are also capable of increased partial proteolysis of the collagen substratum in culture (T.R.K. and P.B., in preparation).
The pathogenesis of the matrix abnormalities in the TSP-2-null mouse, in vivo, is somewhat more difficult to assess, but we believe that the evidence also points to a major role for a reduction of tTG activity in generating these abnormalities. The defects in the mouse include electron microscopic evidence for abnormal collagen fibrillogenesis, and reduced tissue integrity in dermis, myocardium, and cervix (see Introduction for references). It is evident that tTG activity is reduced in excisional skin wounds of TSP-2-null mice (Figure 6; we were unable to assess tTG activity accurately in intact uninjured skin), and isopeptide bond cross-links are reduced in uninjured skin and wounds from these animals (Figure 7).
The increased extractability of collagen from TSP-2-null skin with cold acetic acid, coupled with the reduction in tTG activity and isopeptide bond cross-links, strongly suggests that reduced cross-linking rather than increased limited proteolysis is responsible for the increase in solubility of the protein. This interpretation is supported by the absence of evidence for proteolysis of the extracted collagen from TSP-2-null mice, although it is possible that partially proteolyzed collagen may have been lost during purification. We initially considered the possibility that decreased lysyl- and hydroxylysyl-derived cross-links were responsible for the increase in extractability of collagen from TSP-null dermis. However, quantification by high performance liquid chromatography of hydroxylysinonorleucine and dihydroxylysinonorleucine, two major reducible intermolecular cross-links in dermal type I collagen, revealed no differences between WT and TSP-2-null dermis (L.A. and P.B., unpublished observations). Although it is known that tTG is capable of cross-linking a variety of extracellular proteins,19,22,32 to our knowledge this is the first demonstration of a role for tTG-catalyzed isopeptide bond cross-links in the stabilization of fibers composed of type I collagen in vivo.
We present the following hypothesis for a mechanism by which cell-surface-associated tTG could participate in collagen fibrillogenesis. As first demonstrated by Birk and Trelstad,17 fibroblasts compartmentalize their adjacent pericellular space by extension of long cellular extensions. Nascent collagen fibrils are subsequently assembled in these extracellular compartments, in close association with the cell surface. In an electron microscopic study, we have shown distinct abnormalities in fibroblast-collagen fibril interactions in TSP-2-null neonatal tendon.6 Additional evidence for the participation of the cell surface in collagen fibrillogenesis has recently been presented by Canty and colleagues.18 Building on the work of Birk and Trelstad,17 Canty and colleagues18 have shown that conversion of procollagen to collagen and collagen fibrillogenesis are initiated in Golgi-derived exocytic vesicles, which they term Golgi to plasma membrane carriers (GPCs). GPCs, together with their cargo of nascent collagen fibrils, are then targeted to protrusions in the plasma membrane, termed fibripositors, which project into deep channels between adjacent cells, or possibly in channels formed by a single cell. Secretion of the nascent or initial collagen fibrils then occurs from the tips of fibripositors, and subsequent packing of fibrils into hexagonal bundles takes place in this relatively specialized extracellular environment in close apposition with the plasma membrane. We suggest that increased levels of active MMP-2 could proteolyze cell-surface tTG in this environment, and thus interfere with normal collagen fibrillogenesis.
In conclusion, we have documented an important role for tTG, and its ability to introduce covalent intermolecular cross-links in collagen matrices, in the stabilization of the ECM. Thus, a deficiency of tTG activity can account for many of the matrix abnormalities that have been encountered in the TSP-2-null mouse.33 The importance of isopeptide bonds in the cross-linking of collagen fibers has not been fully appreciated. These findings raise the possibility that local gene therapy that achieves an increase in tTG activity, eg, by introduction of tTG mRNA,34 or a decrease, eg, by use of siRNA, could be used to manipulate the physical properties of connective tissues.
Acknowledgments
We thank Tristan Hartzell for assistance with the preparation of extractable collagen.
Footnotes
Address reprint requests to Paul Bornstein M.D., Department of Biochemistry, Box 357350, University of Washington, Seattle, WA 98195. E-mail: bornsten@u.washington.edu.
Supported by the National Institutes of Health (grant AR 45418 to P.B.), National Science Foundation Grant EEC 9529161, and the American Heart Association (beginning grant-in-aid to A.A.).
Current address of T.R.K.: Departments of Pathology and Biomedical Engineering, Yale University School of Medicine, New Haven, CT.
References
- Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Tam JW, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- Kokenyesi R, Armstrong LC, Agah A, Artal R, Bornstein P. Thrombospondin 2 deficiency in pregnant mice results in premature softening of the uterine cervix. Biol Reprod. 2004;70:385–390. doi: 10.1095/biolreprod.102.014704. [DOI] [PubMed] [Google Scholar]
- Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, Janssen BJ, Debets JJ, Smits JF, Daemen MJ, Crijns HJ, Bornstein P, Pinto YM. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- Agah A, Kyriakides TR, Letrondo N, Bjorkblom B, Bornstein P. Thrombospondin 2 levels are increased in aged mice: consequences for cutaneous wound healing and angiogenesis. Matrix Biol. 2004;22:539–547. doi: 10.1016/j.matbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Kyriakides TR, Yang Z, Armstrong LC, Birk DE. Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J Invest Dermatol Symp Proc. 2000;5:61–66. doi: 10.1046/j.1087-0024.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu YH, Yang Z, Huynh G, Bornstein P. Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am J Pathol. 2001;159:1255–1262. doi: 10.1016/S0002-9440(10)62512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase: inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Lambert E, Dassé E, Haye B, Petitfrère E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Werb Z, Vu TH, Rinkenberger JL, Coussens LM. Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS. 1999;107:11–18. doi: 10.1111/j.1699-0463.1999.tb01521.x. [DOI] [PubMed] [Google Scholar]
- Ray JM, Stetler-Stevenson WG. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J. 1995;14:908–917. doi: 10.1002/j.1460-2075.1995.tb07072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimov SS, Belkin AM. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: a role in TGFbeta-dependent matrix deposition. J Cell Sci. 2001;114:2989–3000. doi: 10.1242/jcs.114.16.2989. [DOI] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol. 2004;165:553–563. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Johnson TS, Abo-Zenah H, Skill JN, Bex S, Wild G, Brown CB, Griffin M, El Nahas AM. Tissue transglutaminase: a mediator and predictor of chronic allograft nephropathy? Transplantation. 2004;77:1667–1675. doi: 10.1097/01.tp.0000131171.67671.3c. [DOI] [PubMed] [Google Scholar]
- Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenard P, Bresson-Hadni S, El Alaoui M, Chevallier DA, Vuitton DA, Ricard-Blum S. Transglutaminase-mediated cross-linking is involved in the stabilization of extracellular matrix in human liver fibrosis. J Hepatol. 2001;35:367–375. doi: 10.1016/s0168-8278(01)00135-0. [DOI] [PubMed] [Google Scholar]
- Haroon ZA, Hettasch JM, Lai TS, Dewhirst MW, Greenberg CS. Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. FASEB J. 1999;13:1787–1795. doi: 10.1096/fasebj.13.13.1787. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Zemskov EA, Hang J, Akimov SS, Sikora S, Strongin AY. Cell-surface-associated tissue transglutaminase is a target of MMP-2 proteolysis. Biochemistry. 2004;43:11760–11769. doi: 10.1021/bi049266z. [DOI] [PubMed] [Google Scholar]
- Mian S, el Alaoui S, Lawry J, Gentile V, Davies PJ, Griffin M. The importance of the GTP-binding protein tissue transglutaminase in the regulation of cell cycle progression. FEBS Lett. 1995;370:27–31. doi: 10.1016/0014-5793(95)00782-5. [DOI] [PubMed] [Google Scholar]
- Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, Im MJ, Graham RM. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994;264:1593–1596. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem. 2001;276:18415–18422. doi: 10.1074/jbc.M010135200. [DOI] [PubMed] [Google Scholar]
- Balklava Z, Verderio E, Collighan R, Gross S, Adams J, Griffin M. Analysis of tissue transglutaminase function in the migration of Swiss 3T3 fibroblasts: the active-state conformation of the enzyme does not affect cell motility but is important for its secretion. J Biol Chem. 2002;277:16567–16575. doi: 10.1074/jbc.M109836200. [DOI] [PubMed] [Google Scholar]
- Jones RA, Nicholas B, Mian S, Davies PJ, Griffin M. Reduced expression of tissue transglutaminase in a human endothelial cell line leads to changes in cell spreading, cell adhesion and reduced polymerisation of fibronectin. J Cell Sci. 1997;110:2461–2472. doi: 10.1242/jcs.110.19.2461. [DOI] [PubMed] [Google Scholar]
- Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001;276:20673–20678. doi: 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- De Laurenzi V, Melino G. Gene disruption of tissue transglutaminase. Mol Cell Biol. 2001;21:148–155. doi: 10.1128/MCB.21.1.148-155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodeling of extracellular matrices: the role of transglutaminases. Connect Tissue Res. 2000;41:1–27. doi: 10.3109/03008200009005638. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Hartzel T, Huynh G, Bornstein P. Regulation of angiogenesis and matrix remodeling by localized, matrix-mediated antisense gene delivery. Mol Ther. 2001;3:842–849. doi: 10.1006/mthe.2001.0336. [DOI] [PubMed] [Google Scholar]