Abstract
Chronic allograft nephropathy is characterized by chronic inflammation and fibrosis. Because retinoids exhibit anti-proliferative, anti-inflammatory, and anti-fibrotic functions, the effects of low and high doses of 13-cis-retinoic acid (13cRA) were studied in a chronic Fisher344→Lewis transplantation model. In 13cRA animals, independent of dose (2 or 20 mg/kg body weight/day) and start (0 or 14 days after transplantation) of 13cRA administration, serum creatinine was significantly lower and chronic rejection damage was dramatically reduced, including subendothelial fibrosis of preglomerular vessels and chronic tubulointerstitial damage. The number of infiltrating mononuclear cells and their proliferative activity were significantly diminished. The mRNA expression of chemokines (MCP-1/CCL2, MIP-1α/CCL3, IP-10/CXCL10, RANTES/CCL5) and proteins associated with fibrosis (plasminogen activator inhibitor-1, transforming growth factor-β1, and collagens I and III) were strikingly lower in treated allografts. In vitro, activated peritoneal macrophages of 13cRA-treated rats showed a pronounced decrease in protein secretion of inflammatory cytokines (eg, tumor necrosis factor-α, interleukin-6). The suppression of the proinflammatory chemokine RANTES/CCL5 × 13cRA in fibroblasts could be mapped to a promoter module comprising IRF-1 and nuclear factor-κB binding elements, but direct binding of retinoid receptors to promoter elements could be excluded. In summary, 13cRA acted as a potent immunosuppressive and anti-fibrotic agent able to prevent and inhibit progression of chronic allograft nephropathy.
Chronic allograft nephropathy (CAN) is an important cause of graft loss in renal transplantation.1,2 It is a complex disease resulting from interactions between humoral and cellular immune responses and nonimmunological factors.1–3 Clinically, CAN is manifested by a gradual deterioration of renal function often accompanied by hypertension and proteinuria. Histopathologically, it is characterized by hyalinosis and fibrosis of preglomerular vessels, transplant glomerulopathy, glomerulosclerosis, interstitial fibrosis with a variable degree of mononuclear cell infiltrate, and tubular atrophy.2,3
The cellular and molecular mechanisms involved in development of CAN have not been elucidated in detail. A number of proinflammatory and fibrogenic mediators such as interleukin (IL)-1, interferon (IFN)-γ, transforming growth factor (TGF)-β, platelet-derived growth factor, endothelin, and angiotensin II are thought to be involved in various stages of the chronic inflammatory and reparative process.1 There are indications that chemokines such as RANTES/CCL5 and IP-10/CXCL10 also contribute to chronic rejection.4 These proteins are synthesized and secreted by both tissue-infiltrating inflammatory cells as well as by activated graft parenchymal cells.3 It may be postulated that master switches or transcription factors regulate this inflammatory network.
The anti-inflammatory and anti-proliferative actions of retinoids (derivatives of vitamin A) have long been known.5,6 Retinoids have predominantly been used for treatment of hyperplastic skin disease or hematopoietic malignancies (eg, leukemias).6 Retinoids act via specific nuclear retinoic acid (RAR) and retinoid X (RXR) receptors, with α, β, and γ subtypes. These receptors are broadly expressed in the rat and human kidney7 as well as by immunocompetent cells such as B and T cells and monocytes/macrophages.8,9 The retinoid receptors regulate expression of target genes either by direct binding to retinoic acid response elements or indirectly by influencing other transcription factors such as AP-1 (activator protein-1),10 NF-κB (nuclear factor-κB),11 and CREB (cAMP-responsive element binding protein).12
In experimental models of glomerulonephritis, retinoids have recently been shown to inhibit the proliferation of mesangial cells, lower the number of infiltrating monocytes, and reduce extracellular matrix deposition without signs of vitamin A toxicity.13,14 In addition, retinoids have been shown to positively influence arterial remodeling after balloon catheter injury by reducing neointimal formation.15 In an animal model of vein grafting, intimal hyperplasia was inhibited by a retinoid derivative.16
We have previously observed that 13-cis-retinoic acid (13cRA) ameliorated rejection phenomena and preserved graft function in acute models of renal transplantation.17 Based on these results we postulated that retinoids could prevent or moderate CAN. In addition, the potent anti-proliferative actions of retinoids led us to propose that early stages of CAN might be reversed through treatment. We examined the effects of two doses of 13cRA, a retinoic acid derivative currently in clinical use,6 in chronic models of rat renal transplantation. Using doses of 13cRA that correspond to those used in humans significant preservation of renal function, morphology, inhibition of immune cell infiltration and proliferation, and reduction in intragraft, inflammatory cytokine/chemokine expression was observed.
Materials and Methods
Animals
Male inbred Lewis (LEW, RT11) and Fisher (F344, RT11v1) rats were purchased from Charles River GmbH, Sulzfeld, Germany. Lewis rats were used as recipients of Fisher kidney allografts. The donors as well as the recipients weighed ∼200 to 220 g at time of renal transplantation. Animal experimentation was performed according to German laws on animal protection.
Kidney Transplantation
Transplantation was performed under ether drop anesthesia. The left kidney of the donor rat was isolated, perfused with ice-cold isotonic sodium chloride solution, excised, and transplanted orthotopically into a weight-matched Lewis recipient. In the recipient, the left renal vein and artery were mobilized and clamped, the ureter was cut and the left kidney was excised. End-to-end anastomosis of renal vessels and of ureter, without ureteral stenting, were performed with 10-0 nonabsorbable nylon sutures. Total ischemic time of the donor kidney varied between 30 and 45 minutes. All transplant kidneys with hydronephrosis, which was evaluated both macroscopically and by light microscopy, were excluded from the experimental groups.
Experimental Protocol
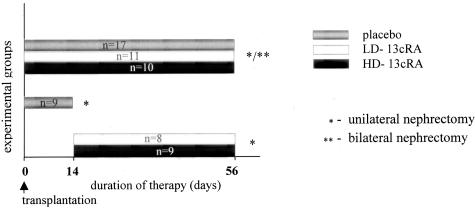
In a first set of experiments, animals were randomly allocated to five experimental groups. Two placebo groups—placebo-14 days (n = 9) and placebo-56 days (n = 17), Fisher to Lewis allografts fed with standard rat cow for 2 and 8 weeks, respectively; two prevention groups: LD-8 weeks group (n = 11) and HD-8 weeks group (n = 10), in which animals were treated with low-dose (LD) and high-dose (HD), respectively, 13cRA for 8 weeks starting at the day of renal transplantation and two therapy groups: LD-6 weeks group (n = 8) and HD-6 weeks group (n = 9), in which rats were treated with low, respectively, HD-13cRA for 6 weeks starting at day 14 after transplantation, when chronic damage could already be seen. The LD of isotretinoin was 2 mg/kg body weight/day and the HD 20 mg/kg body weight/day, administered orally. None of the recipients was treated with any other immunosuppressant.
In the first set of experiments the native contralateral (right) kidney was kept in situ as an internal control for the renal effects of 13cRA. The experimental set-up is schematically shown in Figure 1. In the second set of experiments, performed for determination of graft function the native contralateral (right) kidney was excised at day 49, and renal function tests were performed at day 56 after renal transplantation; three groups were compared placebo group (n = 5), LD-8 weeks group (n = 5), and HD-8 weeks group (n = 5).
Figure 1.
Experimental design: placebo, rats without any treatment; LD-13cRA, low-dose 13cRA; HD-13cRA, high-dose 13cRA.
13cRA (Isotretinoin)
To improve homogeneity and oxidative stability, 13cRA (F. Hoffmann-La Roche, Basel, Switzerland) was first incorporated into a lactose-gelatin granular carrier substance including 5% ascorbic acid (Sigma-Aldrich, Deisenhofen, Germany) using a wet granulation method.13 Rats were fed after 6 p.m. when lights were turned off. Animals were pair-fed to ascertain comparable calorie intake in placebo- and 13cRA-treated animals. The rats had free access to tap water.
Systolic Blood Pressure Measurement
Systolic blood pressure was determined at weeks 2, 4, and 6 after renal transplantation by tail cuff plethysmography under light ether anesthesia.18
Biochemical Analysis
Blood samples were obtained at the time of sacrifice and serum creatinine (enzymatic method), urea nitrogen, calcium, glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, and alkaline phosphatase were determined with a Hitachi 911 autoanalyzer (Roche, Mannheim, Germany).
Solid-Phase Retinoid Extraction and High Performance Liquid Chromatographic Analysis
Under dimmed yellow light, isopropanol (1.5 ml) was added to plasma samples (0.5 ml, 3:1 v:v).16 After vortexing for 5 minutes at room temperature and centrifugation (5000 rpm, 10 minutes), the supernatant was diluted 1:3 (v:v) with 2% ammonium acetate. Samples were loaded on C2 cartridges (ICT, Bad Homburg, Germany) that were preconditioned with 3.4 ml of methanol followed by 0.6 ml of 2% ammonium acetate solution. After loading, the cartridges were washed with 3 ml of 15% acetonitril/85% in 0.5% ammonium acetate. Samples were extracted in 250 μl of methanol and stored in amber light glass tubes at −20°C until used for high performance liquid chromatography analysis.19,20
Histology and Morphometry
At sacrifice organs (kidneys, heart, lung, thymus, liver, spleen, pancreas, femur) were quickly blotted free of blood, weighed (with the exception of lung, thymus, pancreas), and processed as needed for histology, immunohistology, and molecular analysis. For histology the organs were cut into 1-mm slices and either immersion-fixed in 4% formaldehyde in phosphate-buffered saline (PBS) pH 7.35 (PBS: 99 mmol/L NaH2PO4, 108 mmol/L NaH2PO4, and 248 mmol/L NaCl) for 24 hours at 4°C, or fixed in Methacarn (60% methanol, 30% chloroform, 10% acetic acid) for 8 hours and then embedded in paraffin. In addition tissue slices were frozen in liquid nitrogen and stored at −80°C, until used for immunohistology or RNA isolation.
Light microscopy was performed on 3-μm sections stained by periodic acid-Schiff, hematoxylin and eosin, and Goldner trichrome. Morphometric evaluation of acute rejection and chronic damage was done according to the Banff classification.21 In addition results were corroborated by a different evaluation scheme as has been described.17,22,23
Glomerulosclerosis was defined as 0, no sclerosis; 0.5, sclerosis of less than 25% of capillary loops; 1, sclerosis of 26 to 50% of the capillary loops; 2, sclerosis of 51 to 75% of the capillary loops; 3, sclerosis of more than 75% of the capillary loops. Glomerulosclerosis score was calculated as the sum of all specific injury indices, whereby the index of glomeruli with degree 0.5 was multiplied by 0.5, that of degree 1 × 1, that of degree 2 × 2, that of degree 3 × 3 (see Supplemental Table A and Supplemental Figures A-E at http://ajp.amjpathol.org).
Chronic tubulointerstitial damage was defined as broadening of the basement membrane of the tubuli with flattened epithelium, tubular atrophy, and interstitial matrix increase. It was evaluated as 0.5, focal chronic damage and 1, diffuse chronic damage. Tubulointerstitial damage was judged in 20 high-power fields of cortex (objective, ×40), and the tubulointerstitial damage score was calculated as described for the glomerulosclerosis score.
In addition, morphometric analysis was performed using a semiautomatic image analyzing system (Leica Q600; Qwin, Cambridge, UK), to evaluate the chronic changes in preglomerular vessels, glomeruli, and tubulointerstitium. All cross-section of arteries from each kidney were examined (×40 objective). To measure fibrointimal changes a ratio was calculated between the surface having as outline the lamina elastica interna and the free luminal area. Mesangial matrix increases were determined by point-counting method. The results were expressed as a fraction of glomerular surface area in 50 glomeruli. Chronic tubulointerstitial changes were expressed as a percentage of the total tubulointerstitial area, obtained after exclusion of glomeruli in 20 fields (objective, ×20) of cortex and outer stripe of outer medulla.
Immunohistochemistry
Immunohistochemical staining was performed on 3-μm sections of paraffin-embedded tissue, using mouse anti-rat monoclonal antibodies against ED1 (Serotec, Oxford, UK) to demonstrate monocytes/macrophages in Methacarn-fixed tissues, CD8 (Serotec) to define cytotoxic T cells, Ki67-clone MIP 5 (Dianova, Hamburg, Germany) for the detection of proliferating cells. The last two antibodies were applied to formaldehyde-fixed and microwave-treated tissue sections. An alkaline phosphatase anti-alkaline phosphatase detection system was applied for visualization (DAKO, Hamburg, Germany).
Glomerular-positive cells were counted in at least 50 glomerular cross sections and given as the mean per glomerular section; interstitial-positive cells were counted in 20 high-power fields (×40) of cortex and outer medulla and recorded as mean per high-power field. To localize collagens, streptavidin-biotin-enhanced horseradish peroxidase immunostaining was performed, using rabbit anti-rat polyclonal antibodies against collagen I (Biogenesis, Poole, UK) and collagen III (Chemicon, Temecula, CA).
The extent of the staining was evaluated as 0, no staining detectable; 1, faint; 2, moderate; and 3, intense staining. A degree-specific staining index was defined as the percentage of the fields with the respective degree of staining in 20 high-power fields of cortex and outer medulla. The staining score was calculated as the sum of the specific staining indices, whereby the index of the fields with degree 1 was multiplied by 1, that of degree 2 × 2, that of degree 3 × 3. Controls, omitting the first antibody or replacing the first antibody by a respective mouse or rabbit nonimmune IgG, for each paraffin block tested were negative.
Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Tissue mRNA
Total RNA was extracted by the method of Chomczynski and Sacchi.24 RNA quality was monitored by agarose electrophoresis. Ten μg of the total RNA were used for the first-strand cDNA synthesis with Superscript II reverse transcriptase and oligo d(T)12–18 primer (LifTechnologies, Karlsruhe, Germany). RT-PCR products from three animals per group were obtained: 1, control group, nontransplanted Fisher344 rat kidneys; 2, placebo; and 3, LD-8 weeks groups from the first set of experiments. Real-time PCR was performed by LightCycler using the LightCycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostics, Mannheim, Germany). The primer sequences for target genes used in this study are shown in Table 1. Gene expression of 12 genes was investigated by LightCycler as described.25
Table 1.
Sequences of Primers Used in This Study
| Gene: | Gene bank accession number | Sense (5′ → 3′) | Anti-sense (5′ → 3′) | Amplicon (bp) |
|---|---|---|---|---|
| IP-10/CXCL10 | (U22520) | AAAGCGGTGAGCCAAAGAAG | AGGAGAAACAGGGACAGTTAGG | 150 |
| MCP-1/CCL2 | (M57441) | GCTGACCCCAATAAGGAATG | GTTGTGGAAAAGAGAGTGGATG | 176 |
| RANTES/CCL5 | (U06436) | TGCTGCTTTGCCTACCTCTC | CTTGAACCCACTTCTTCTCTGG | 151 |
| MIP-1α/CCL3 | (U06435) | CTATGGACGGCAAATTCCAC | AGGCATTCAGTTCCAGCTC | 167 |
| TGF-β1 | (X52498) | GCAACACGTAGAACTCTACC | CCCTGTATTCCGTCTCCTTG | 153 |
| HO-1 | (J02722) | AACACAAAGACCAGAGTCCC | ACTGAGTGTGAGGACCCATC | 158 |
| PAI-1 | (M24067) | TTTGTGTTCCAGTCACACTC | ATCTGTCTATCTGCTGCCC | 153 |
| GAPDH | (M17701) | AACATCATCCCTGCATCCAC | CTGCTTCACCACCTTCTTG | 180 |
| Collagen I | (Z78279) | GCAACATGGAGACAGGTCAG | CCTTCGCTTCCATACTCGAAC | 154 |
| Collagen III | (X70369) | GCCTCCCAGAACATTACATACC | TGCAGCCATCCTCTAGAAC | 162 |
| Interferon-γ | (AF010466) | GCGTCTTGGTTTTGCAGCTC | ACCGTCCTTTTGCCAGTTCC | 170 |
| IL-10 | (X60675) | AAAGCAAGGCAGTGGAGCAG | CGCCGGGTGGTTCAATTTTTC | 145 |
Macrophages and Cytokine Profile
Thioglycolate (3%, w/v; 10 ml) was injected intraperitoneally in two groups of Lewis rats (n = 6 rats/group), pretreated for 14 days with 13cRA (2 mg/kg body weight/day) and placebo, respectively. After 72 hours peritoneal macrophages were harvested with 40 ml of RPMI containing 10% fetal calf serum. Cells were seeded at 1 × 106 per well and incubated for 6 hours in RPMI medium containing 1% streptomycin/penicillin, 1% l-glutamine, and 25 mmol/L Hepes without stimuli. Cells and supernatants were then harvested for protein analyses. Protein expressions of IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, GM-CSF (granulocyte-macrophage colony-stimulating factor), tumor necrosis factor-α (TNF-α), and IFN-γ were determined by Bio-Plex cytokine assay (Bio-Rad Laboratories, Munich, Germany) according to the manufacturer’s instructions. Supernatant values were referred to the total cellular protein as determined by Lowry and colleagues.26
Fibroblasts and Molecular Action of 13cRA on RANTES/CCL5 and IP10/CXCL10
Normal adult human dermal fibroblasts (NHDF6447; BioWhittaker, Europe) and the cell line K4 (cell line of fibroblasts immortalized with SV40 virus) were incubated in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) with 10% heat-inactivated fetal calf serum. Cells were used between passages 3 to 9.
RANTES/CCL5 and IP-10/CXCL10 Measurement
For induction of RANTES/CCL5 production the cells were trypsinized and seeded in a 96-well plate (enzyme-linked immunosorbent assay) or a 6-well plate (TaqMan RT-PCR) for 24 hours in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. Then the medium was replaced with fetal calf serum-free medium, followed by further incubation of the cells for 48 hours. After this time the stimulation of both fibroblasts and K4 cells was performed. In dose-response experiments, maximal stimulation was found at 25 or 50 ng/ml TNF-α and 50 ng/ml IFN-γ (Peprotech, Inc.) (data not shown).
Real-time RT-PCR was performed on a TaqMan ABI 7700 sequence detection system (PE Biosystems, Weiterstadt, Germany) using the procedure as described.27 Sequences with following gene bank accession numbers served for the design of a predeveloped TaqMan assay: AF043341 (human RANTES/CCL5), F439522 (human IP-10/CXCL10), and M33197 (human GAPDH) as housekeeping gene. All of these assay reagents did not amplify genomic DNA samples. Controls consisting of ddH2O were negative in all runs. Measurement of RANTES/CCL5 protein was performed using the Duo Set ELISA development system (R&D). This assay uses the quantitative sandwich immunoassay technique. The minimum detectable concentration of RANTES/CCL5 was 32.5 pg/ml.
Promoter-Reporter Plasmids, Transient Transfection, and Luciferase Reporter Gene Assays
Deletions and site-specific mutants of the human RANTES/CCL5 promoter sequence were based on previously described pGL3-based plasmid constructs (pGL3; Promega, Madison, WI).27–29 The tk-Renilla plasmid (Promega) was used to normalize transfection data. Per data point, 5 × 105 of proliferating K4 cells were transfected using Superfect Reagent (Qiagen, Hilden, Germany). Three hours after transfection, the cells were stimulated with TNF-α (Peprotech, Inc.) for 24 hours, with or without 10−5 mol/L 13cRA. The Dual-Luciferase reporter assay (Promega) was performed as described. The results are presented as a ratio of Photinus/Renilla-Luciferase and are representative of more than three experiments.
Electrophoretic Mobility Shift Assay (EMSA)
Dermal fibroblasts were stimulated with TNF-α (25 ng/ml) and/or IFN-γ (50 ng/ml) for 2 and 12 hours, and nuclear protein was isolated by using the high-salt extraction method with small modifications.28,29 For EMSA, 2.5 μg of nuclear protein were incubated with 40 kcpm of 32P-labeled oligonucleotide coding for the region of interest. Binding conditions for NF-κB and GAS-ISRE were as described.28,29
Antibodies and Gel Shift Oligonucleotides
Transcription factor-specific antibody reagents were purchased from Santa Cruz Biotechnology, Heidelberg, Germany, p50 (sc-114); p52; p65 (sc-109); c-Rel (sc-71X); rel-B (sc-226). Oligonucleotide sequences for consensus or mutant DNA-binding domains were purchased from Santa Cruz [NF-κB (sc-2505/sc-2511) GAS/ISRE (sc-2537/sc-2538)].
Statistical Analyses
Data are given as mean values ± SEM. Data were analyzed by using the nonparametric Mann-Whitney test or analysis of variance and Bonferroni’s multiple comparison test as appropriate. A P value <0.05 was considered to show a significant difference.
Results
As shown in Table 2, body weights between placebo- and 13cRA-treated animals were similar at day 56. No significant differences in the absolute and relative weight of organs (eg, kidney, heart, liver, femur) were observed between the placebo and treated groups. In all experimental groups systolic blood pressure remained in the normal range and did not change throughout the experimental period (Tables 3 and 4). Mean serum creatinine levels were significantly lower in the LD-13cRA-treated groups than in the untreated transplanted rats (second set of experiments) (2.4 ± 1.3 in LD-13cRA and 3.8 ± 1.0 in HD-13cRA versus 8.1 ± 2.4 mg/dl in placebo group, P < 0.05). Although a slight increase in triglyceride concentration and alkaline phosphatase activity was noted in rats treated with 13cRA, the levels did not reach significance between groups. Cholesterol levels were not influenced by treatment with 13cRA. No clinical signs of retinoid toxicity, ie, hair loss, cheilitis, conjunctivitis, and no histological signs of liver damage and bone loss were found at both doses in the treated animals.
Table 2.
Body Weight and Relative Organ Weights in the Experimental Groups 56 Days after Transplantation
| Groups | Body weight (BW) (g) | Relative organ weights (g/100 g BW)
|
||||
|---|---|---|---|---|---|---|
| Allograft kidney | Endogenous kidney | Heart | Liver | Femur | ||
| Placebo 14 days | 267.3 ± 22.5 | 0.56 ± 0.07 | 0.42 ± 0.02 | n.d. | n.d. | n.d. |
| Placebo 56 days | 318.3 ± 89.0 | 0.29 ± 0.02 | 0.43 ± 0.01 | 0.32 ± 0.02 | 2.48 ± 0.11 | 0.53 ± 0.02 |
| LD-6 weeks | 320.8 ± 5.8 | 0.25 ± 0.02 | 0.40 ± 0.01 | 0.30 ± 0.01 | 2.44 ± 0.09 | 0.59 ± 0.02 |
| LD-8 weeks | 309.7 ± 4.5 | 0.24 ± 0.02 | 0.32 ± 0.01 | 0.31 ± 0.01 | 2.47 ± 0.04 | 0.53 ± 0.03 |
| HD-6 weeks | 316.1 ± 6.7 | 0.19 ± 0.01 | 0.44 ± 0.01 | 0.29 ± 0.02 | 2.38 ± 0.02 | 0.52 ± 0.02 |
| HD-8 weeks | 300.1 ± 4.5 | 0.20 ± 0.01 | 0.47 ± 0.02 | 0.30 ± 0.02 | 2.53 ± 0.05 | 0.52 ± 0.01 |
| P value | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
n.s., Not significant placebo versus 13cRA-treated groups; n.d., not determined.
Table 3.
Systolic Blood Pressure at Different Time Points throughout the Experimental Period
| Groups | Systolic blood pressure (mm Hg) after transplantation
|
||
|---|---|---|---|
| At 2 weeks | At 4 weeks | At 6 weeks | |
| Placebo 56 days | 110.0 ± 2.8 | 120.6 ± 5.2 | 119.4 ± 5.1 |
| LD-6 weeks | 115.0 ± 4.3 | 120.8 ± 7.1 | 117.5 ± 6.3 |
| LD-8 weeks | 109.4 ± 3.5 | 120.6 ± 4.05 | 119.4 ± 5.9 |
| HD-6 weeks | 115.0 ± 4.6 | 126.4 ± 2.6 | 122.1 ± 6.0 |
| HD-8 weeks | 117.5 ± 4 | 111.3 ± 4.3 | 124.4 ± 2.7 |
| P value | n.s. | n.s. | n.s. |
n.s., Not significant placebo versus 13cRA-treated groups; n.d., not determined.
Table 4.
Effects of 13cRA on Graft Morphology
| Score | Placebo 14 days, n = 9, no. (%) | Placebo 56 days, n = 17, no. (%) | LD-6 weeks, n = 8, no. (%) | LD-8 weeks, n = 11, no. (%) | HD-6 weeks, n = 9, no. (%) | HD-8 weeks, n = 10, no. (%) | |
|---|---|---|---|---|---|---|---|
| Mesangial matrix increase (mm) | mm0 | 1 (11.1) | 0 (0.0) | 3 (37.5) | 2 (18.2) | 1 (11.1) | 2 (20.0) |
| mm1 | 7 (77.7) | 2 (11.7) | 2 (25.0) | 8 (72.7) | 3 (33.3) | 8 (80.0) | |
| mm2 | 1 (11.1) | 6 (35.3) | 3 (37.5) | 1 (9.1) | 4 (44.4) | 0 (0.0) | |
| mm3 | 0 (0.0) | 9 (52.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ††† | ** | *** | * | *** | |||
| Allograft glomerulopathy (cg) | cg0 | 6 (66.6) | 0 (0.0) | 0 (0.0) | 3 (27.3) | 1 (11.1) | 3 (30.0) |
| cg1 | 3 (33.3) | 2 (11.7) | 7 (87.5) | 6 (54.5) | 7 (77.7) | 7 (70.0) | |
| cg2 | 0 (0.0) | 11 (64.7) | 1 (12.5) | 2 (18.2) | 1 (11.1) | 0 (0.0) | |
| cg3 | 0 (0.0) | 4 (23.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ††† | **† | *** | ***† | *** | |||
| Glomerulosclerosis (gs) | gs0 | 1 (11.1) | 0 (0.0) | 4 (50.0) | 7 (63.4) | 5 (55.5) | 7 (70.0) |
| gs1 | 4 (44.4) | 3 (17.6) | 3 (37.5) | 4 (36.6) | 4 (44.4) | 3 (30.0) | |
| gs2 | 4 (44.4) | 4 (23.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| gs3 | 0 (0.0) | 10 (58.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (..0.0) | |
| †† | ***† | ***†† | ***† | ***†† | |||
| Fibrous intimal thickening (cv) | cv0 | 4 (44.4) | 1 (5.9) | 3 (37.5) | 7 (63.4) | 4 (44.4) | 7 (70.0) |
| cv1 | 2 (22.2) | 2 (17.6) | 4 (50.0) | 4 (36.6) | 4 (44.4) | 2 (20.0) | |
| cv2 | 2 (22.2) | 6 (35.3) | 1 (12.5) | 0 (0.0) | 1 (11.1) | 1 (10.0) | |
| cv3 | 1 (11.1) | 8 (47.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| † | ** | *** | *** | *** | |||
| Interstitial fibrosis (ci) | ci0 | 1 (11.1) | 0 (0.0) | 3 (37.5) | 5 (45.4) | 2 (22.2) | 4 (40.0) |
| ci1 | 2 (22.2) | 2 (17.6) | 4 (50.0) | 5 (45.4) | 6 (66.6) | 5 (50.0) | |
| ci2 | 5 (55.5) | 2 (17.6) | 0 (0.0) | 1 (9.1) | 1 (11.1) | 1 (10.0) | |
| ci3 | 1 (11.1) | 13 (76.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| †† | ** | ***†† | ***† | ***† | |||
| Tubular atrophy (ct) | ct0 | 1 (11.1) | 1 (5.9) | 3 (37.5) | 6 (54.5) | 2 (22.2) | 4 (40.0) |
| ct1 | 4 (44.4) | 2 (11.7) | 3 (37.5) | 4 (36.4) | 5 (55.5) | 5 (50.0) | |
| ct2 | 3 (33.3) | 3 (17.6) | 1 (12.5) | 1 (9.1) | 2 (22.2) | 1 (10.0) | |
| ct3 | 1 (11.1) | 11 (64.7) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| † | ** | ***† | ** | *** |
Histological grading was done according to Banff classification 1997.21 The grading scale is from 0 to 3 (0, none present; 1, mild; 2, moderate; 3, severe alterations); c-denotes chronic; glomerulosclerosis was judged accordingly being gs0, no sclerosis; gs1, sclerosis in up to 25%; gs2, to 50%; gs3, more than 50% of all glomeruli.
n.s., Not significant.
< 0.05,
< 0.01,
< 0.001 versus placebo 56 days;
< 0.05,
< 0.01,
< 0.001 versus placebo 14 days.
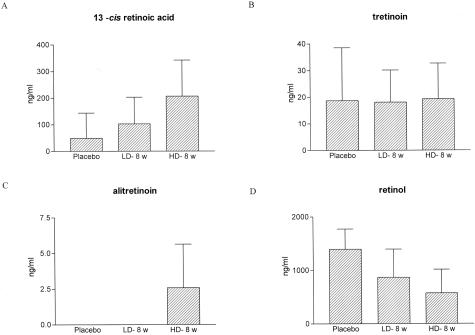
13cRA levels in plasma increased from <10 ng/ml in control animals to 54 ± 36 ng/ml in animals treated with LD-13cRA and 274 ± 14 ng/ml in animals treated with HD-13cRA. In contrast, the levels of tretinoin were not changed. Alitretinoin levels were detectable in the HD-13cRA group. Retinol levels in untreated animals were 1394 ± 379 ng/ml (n = 4) and significantly lower in treated animals [868 ± 526 ng/ml in LD-treated (n = 4) and 758 ± 296 ng/ml (n = 3) in HD-treated animals] (Figure 2).
Figure 2.
Retinoid plasma levels measured by reverse-phase high performance liquid chromatography. A: Significant dose-dependent increase of 13cRA in plasma (approximately fourfold increase in plasma at a 10-fold dose increase). B and C: Tretinoin levels remained unchanged whereas alitretinoin concentrations tended to increase during HD-13cRA. D: Retinol levels were reduced in association with 13cRA treatment indicating a retinol esterification.
Reduction of Damage by 13cRA
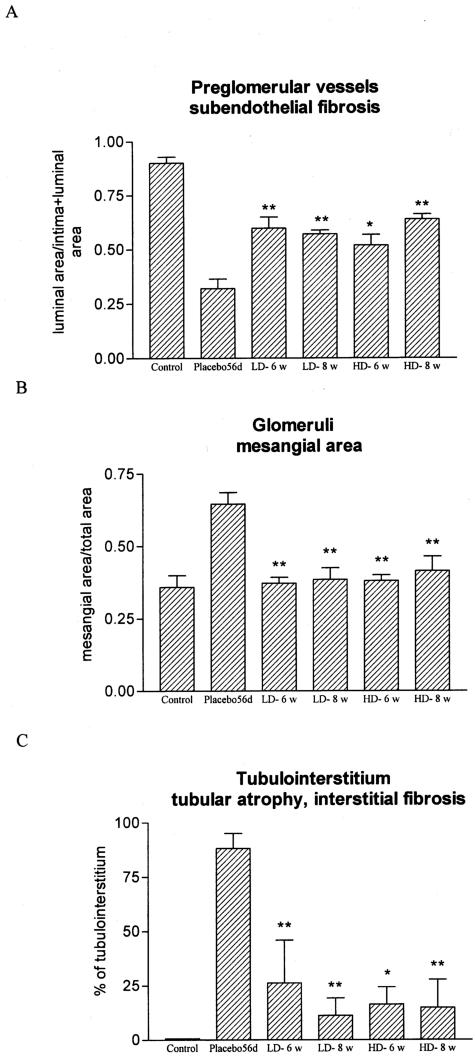
At day 56 after transplantation a severe obliteration with fibrosis and a transmural infiltration of mononuclear cells was observed in the preglomerular arteries of untreated allografts. 13cRA very markedly reduced the vascular rejection score at both the LD and HD treatment (8 week therapy: placebo 83.6 ± 10.4 versus LD-13cRA 22.8 ± 8.3; P < 0.01) (Supplemental Tables A and B, and Supplemental Figure A at http://ajp.amjpathol.org). By morphometric analysis the subendothelial fibrosis was significantly inhibited in 13cRA allografts compared to the placebo group (P < 0.01) as shown in Figure 3A and Figure 4, A and B. In all groups studied glomerulitis was not pronounced 56 days after transplantation (Supplemental Figure B at http://ajp.amjpathol.org). Chronic glomerular injury with mesangial matrix increase and thickening and splitting of the basement membranes corresponding to transplant glomerulopathy were found in the placebo group at day 56. By contrast, the 13cRA-treated grafts showed well preserved glomerular structure (Table 4; Figure 3B and Figure 4, C to H). Glomerulosclerosis was strikingly lower in rats treated with either LD or HD of 13cRA independent of whether the 13cRA was given immediately at transplantation or started 14 days after transplantation (8 weeks therapy: placebo 85.1 ± 13.1 versus LD-13cRA 4.1 ± 2.4; P < 0001) Tubulointerstitial rejection score and chronic tubulointerstitial damage were markedly lowered by 13cRA treatment in all groups as compared with the placebo group (Table 4; Supplemental Tables A and B and Supplemental Figures C and E at http://ajp.amjpathol.org). Morphometric measurements of the chronic tubulointerstitial changes in the grafts detected 60 to 70% less injury by 13cRA for all groups compared with the placebo group (P < 0.01) as presented in Figure 3C and Figure 4, G and H).
Figure 3.
Effects of isotretinoin on chronic graft injury (morphometric analyses). A: The free luminal area of the preglomerular vessels were 50% higher in 13cRA-treated groups than in placebo grafts. B: 13cRA significantly reduced mesangial matrix expansion in the grafts because controls of the right native kidneys of the placebo animals were used. C: The chronic tubulointerstitial changes were lowered by 60 to 70% by treatment with 13cRA. *P < 0.05 and **P < 0.01 versus placebo.
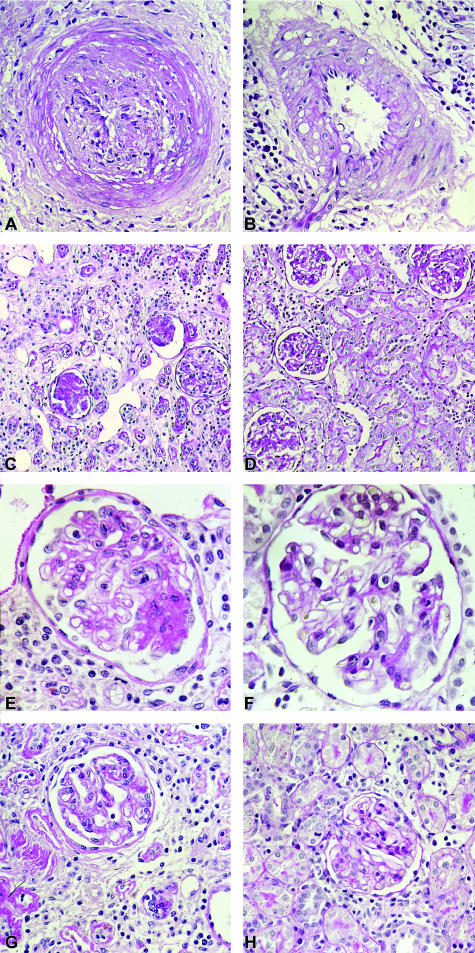
Figure 4.
Light microscopy of renal allografts 56 days after transplantation without (A, C, E, G) and with (B, D, F, H) LD-13cRA administration started 14 days after transplantation. A: Preglomerular artery with significant obliteration by subendothelial matrix increase and with sticking of mononuclear cells to the endothelium and infiltration of subendothelial area and media by mononuclear cells in untreated renal allograft (placebo). B: In renal allograft with LD-13cRA therapy preglomerular artery with only a few mononuclear cells sticking to endothelium without significant narrowing of lumen. C, E, G: In untreated allograft, glomeruli with mesangial matrix increase, broadening and splitting of peripheral basement membrane, and increased number of intracapillary mononuclear cells; surrounding tubulointerstitium characterized by an increase in interstitial mononuclear cells, a rise in matrix surrounding collapsed atrophic tubules, which are focally infiltrated by mononuclear cells. E and G: Glomerulus and tubulointerstitium at higher magnification. D: Glomeruli in 13cRA-treated allograft with good preservation of structure; surrounding tubulointerstitium with focal sparse mononuclear infiltrate; majority of tubules regular. F and H: Glomerulus and tubulointerstitium at higher magnification. A–F: Periodic acid-Schiff stain. Original magnifications: ×400 (A, B, E, F); ×100 (C, D); ×200 (G, H).
At day 14 after transplantation in vehicle-treated rats we observed morphological signs of early CAN (Table 4; Supplemental Table A and Supplemental Figures D and E at http://ajp.amjpathol.org). 13cRA given from day 14 after transplantation to day 56 resulted in significantly lower values for mesangial matrix increase, allograft glomerulopathy, fibrous intimal thickening, tubular atrophy, and interstitial fibrosis than in allografts without treatment on day 56. Interstitial fibrosis even was lower in HD-13cRA kidneys than in renal grafts 2 weeks after transplantation (Tables 4 and 5). Glomerulosclerosis was reduced by factors 2 to 3 compared to placebo and was significantly lower than in allografts 14 days after transplantation. Tubulointerstitial mononuclear cell infiltration was significantly inhibited in all 13cRA groups (P < 0.01) (Table 5 and Figure 5). Nevertheless, more than 50% of monocytes/macrophages and CD8-positive T cells remained in the interstitium (Table 5). Cell proliferation of interstitial cells, as determined by Ki-67 immunohistochemistry, was reduced by more than 60% after 13cRA treatment (Table 5).
Table 5.
Effects of 13cRA on Intragraft Cell Infiltration and Proliferation
| Groups | ED1(+)
|
CD8(+)
|
Ki67(+)
|
|---|---|---|---|
| Cells/tubulointerstitial HPF | |||
| Placebo 14 days | 149.6 ± 47.617 | 258.3 ± 10.4117 | 60.7 ± 4.617 |
| Placebo 56 days | 73.9 ± 6.8††† | 124.9 ± 14.9†† | 18.6 ± 2.7†† |
| LD-6 weeks | 48.2 ± 12.7*††† | 77.6 ± 10.1†† | 6.9 ± 0.8*†† |
| LD-8 weeks | 34.4 ± 6.5**††† | 68.1 ± 3.8**†† | 3.9 ± 0.8 ***†† |
| HD-6 weeks | 38.5 ± 5.0**††† | 69.2 ± 8.3**†† | 5.1 ± 0.3**†† |
| HD-8 weeks | 41.9 ± 4.4**††† | 65.4 ± 8.7**†† | 5.3 ± 0.4**†† |
n.s., Not significant.
< 0.05,
< 0.01,
<0.001 versus placebo 56 days;
< 0.05,
< 0.01,
< 0.001 versus placebo 14 days.
The values for the placebo 14-days group, which already have been depicted in reference 17 have been added to facilitate interpretation of the data set.
Figure 5.
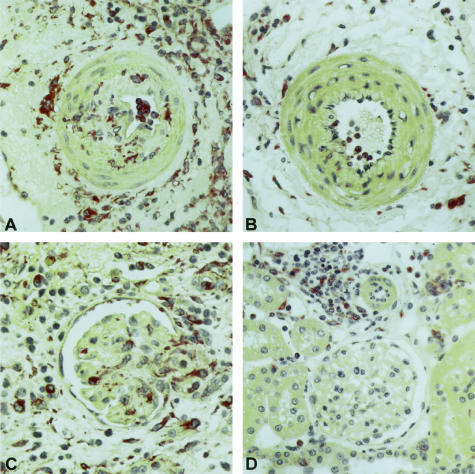
A–F: Immunohistology for monocytes/macrophages (ED1) in renal allografts without (A, C) and with (B, D) LD-13cRA administration immediately after transplantation. A: Preglomerular artery with pronounced infiltration of endothelium, subendothelial area, and media with ED1-positive monocytes/macrophages. B: Preglomerular artery in renal allograft after 13cRA therapy with only few monocytes/macrophages sticking to endothelium. The periadventitial area contains still a significant amount of ED1-positive monocytes/macrophages. C: Glomerulus with surrounding tubulointerstitium with increased number of ED1-positive monocytes/macrophages within the glomerular capillaries, within the mesangium and interstitium with focal infiltration of tubules by ED1-positive cells. D: In renal allografts with 13cRA administration only a few monocytes/macrophages in glomerulus and tubulointerstitium with regularly differentiated tubules. A–D: APAAP stain.
mRNA Expression of Proinflammatory Cytokines/Chemokines
The proinflammatory chemokines MCP-1/CCL-2, MIP-1α/CCL-3, IP-10/CXCL10, and RANTES/CCL5 were up-regulated in placebo allografts as compared to normal rat kidneys. Treatment with LD-13cRA led to a significant down-regulation in the mRNA expression of these chemokines, almost to levels observed in normal kidney (P < 0.001) (Table 6). 13cRA therapy was also associated with a significant decrease in the intragraft mRNA expression of the Th1 cytokine IFN-γ and the Th2 cytokine IL-10 (Table 6).
Table 6.
Effect of 13cRA on mRNA Expression of Different Cytokines/Chemokines in the Allografts by Real-Time RT-PCR (First Set of Experiments); as Controls Normal Fisher344 Rat Kidneys Were Used
| Cytokine/GAPDH | Groups
|
||
|---|---|---|---|
| Control | Placebo 56 days | LD-8 weeks | |
| IFN-γ | 102.1 ± 24.9 | 24370.0 ± 2511 | 2838.0 ± 659.5*** |
| IL-10 | 70.1 ± 6.7 | 6304.0 ± 1270 | 944.4 ± 18.0** |
| RANTES/CCL5 | 2.8 ± 0.3 × 10−3 | 407.5 ± 65.7 × 10−3 | 16.2 ± 1.7 × 10−3*** |
| IP-10/CXCL10 | 7.2 ± 1.1 × 10−3 | 252.8 ± 2.4 × 10−3 | 41.8 ± 25.6 × 10−3*** |
| MCP-1/CCL2 | 1.5 ± 0.5 × 10−3 | 102.8 ± 9.2 × 10−3 | 10.4 ± 1.1 × 10−3*** |
| MIP-1 α/CCL3 | 0.2 ± 0.03 × 10−3 | 22.2 ± 4.6 × 10−3 | 1.1 ± 0.2 × 10−3*** |
| TGF-β1 | 5.9 ± 1.8 × 10−3 | 132.5 ± 25.9 × 10−3 | 20.5 ± 6.9 × 10−3*** |
| PAI-1 | 0.6 ± 0.2 × 10−3 | 54.1 ± 6.4 × 10−3 | 1.8 ± 0.5 × 10−3*** |
| Collagen I | 1.1 ± 0.5 × 10−3 | 30.4 ± 6.6 × 10−3 | 4.0 ± 0.7 × 10−3*** |
| Collagen III | 2.8 ± 0.3 × 10−3 | 50.0 ± 9.6 × 10−3 | 8.3 ± 2.0 × 10−3*** |
, P < 0.01 and
, P < 0.001 placebo versus 13cRA-treated groups.
Fibrosis-Associated Cytokines and Extracellular Matrix Deposition
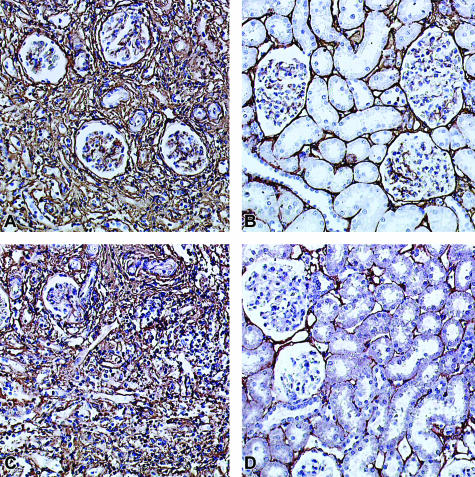
Renal cortical expression of PAI-1 (plasminogen activator inhibitor-1) mRNA, and TGF-β1 mRNA, were consistently up-regulated in the placebo group as compared with normal control kidneys. This expression was markedly reduced in the 13cRA-treated allografts (P < 0001) (Table 6). The mRNA expression of collagens I and III as assessed by real-time RT-PCR was also decreased approximately sevenfold by LD-13cRA as compared with the placebo group (P < 0001) (Table 6). Immunostaining score for collagen I and III showed a significant reduction of these proteins in 13cRA-treated allografts by more than twofold (P < 0001) (Table 7 and Figure 6).
Table 7.
Effect of 13cRA on Collagen Deposition in the Tubulointerstitium of the Grafts
| Groups | Collagen I (staining score) | Collagen III (staining score) |
|---|---|---|
| Placebo 56 days | 257.0 ± 21.9 | 224.1 ± 19.0 |
| LD-6 weeks | 113.0 ± 10.3*** | 106.0 ± 10.5*** |
| LD-8 weeks | 105.0 ± 6.8*** | 100.6 ± 7.8*** |
| HD-6 weeks | 155.0 ± 18.9** | 114.2 ± 6.0** |
| HD-8 weeks | 108.0 ± 10.4*** | 111.0 ± 13.3** |
, P < 0.01 and
, P < 0.001 versus placebo.
Figure 6.
A–D: Immunohistology for collagen I and collagen III in renal allografts without (A, C) and with (B, D) LD-13cRA therapy started immediately after transplantation. A and C: Collagen I (A) and collagen III (C) with strong expression in glomeruli and tubulointerstitium in renal allografts 56 days after transplantation. B and D: In renal allografts treated with LD-13cRA a significant reduction of collagen I (B) and collagen III (D) in interstitium.
Effects of 13cRA on Macrophages and Fibroblasts
Cytokine Secretion of Peritoneal Macrophages
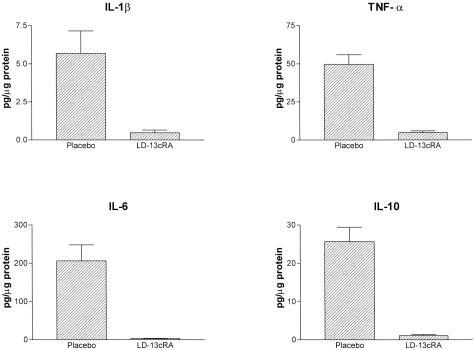
Although the 13cRA-treated animals demonstrated a marked reduction in ED1 infiltration, the overall level of monocyte/macrophage infiltration was still significantly increased as compared to normal kidney samples. To determine the effect of 13cRA on monocyte/macrophage activity, thioglycolate elicited peritoneal macrophages were isolated from rats with and without 13cRA treatment for 14 days. Secreted protein expression of nine representative inflammatory cytokines (IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, GM-CSF, TNF-α, and IFN-γ) was assessed in the supernatant of activated peritoneal macrophages 6 hours after isolation. 13cRA significantly lowered secretion of all of the cytokines tested (Figure 7).
Figure 7.
Protein expression of selected cytokines in the supernatant of thioglycolate elicited peritoneal macrophages without and with 13cRA treatment.
Effects of 13cRA on Fibroblasts
To assess the effect of 13cRA on the resident cells linked to fibrosis and chronic damage, the regulation of genes linked to inflammation and fibrosis in fibroblasts was studied.4 13cRA mediated suppression of TNF-α/IFN-γ-induced chemokine expression (RANTES/CCL5 and IP-10/CXCL10) at the level of mRNA expression, protein production and secretion, and at the level of promoter activity. In primary human dermal fibroblasts 13cRA (10−5 mol/L) treatment reduced the immediate (at 6 hours) activation of IP-10/CXCL10 mRNA by twofold and the late (24 hours) activation of RANTES/CCL5 mRNA by approximately fourfold.
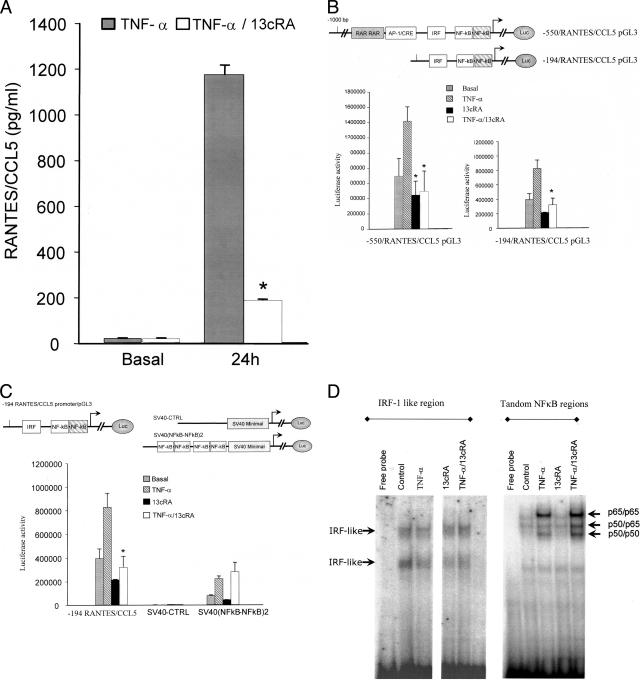
The effect of 13cRA on the TNF-α induced secretion of RANTES/CCL5 protein by fibroblasts was determined using enzyme-linked immunosorbent assay (Figure 8A). 13cRA treatment reduced RANTES/CCL5 secretion in a dose-dependent manner with statistically significant reduction seen at 10−8 mol/L (data not shown). To characterize the molecular mechanism of action of 13cRA the effects of treatment on transcriptional control of RANTES/CCL5 was studied using RANTES-promoter reporter constructs and EMSAs.30
Figure 8.
The effect of 13cRA on regulation of RANTES/CCL5 in fibroblast cells. A: RANTES/CCL5 protein expression in primary fibroblasts induced by stimulation with 25 ng/ml TNF-α at 24 hours; inhibition by 13cRA (10−5) mol/L by >80%. B: Inhibition of RANTES/CCL5 × 13cRA at the level of promoter activity. Transient transfection of a RANTES promoter-reporter gene construct into human K4 fibroblast cell line with induction by TNF-α stimulation and suppression by 13cRA. Depletion of the potential RAR elements identified in the promoter without elimination of the suppressive effect of 13cRA. C: Cloning of the dual NF-κB elements as a dimer in pGL-3 SV40 provector with TNF-α induction of reporter gene activity (to the left comparison to promoter of Figure 7C), no effect of 13cRA on SV40 (NF-κB-NF-κB)2. D: EMSA using the IRF and NF-κB regions of the RANTES/CCL5 promoter. 13cRA without influence on the induction or composition of EMSA complex formation. *P < 0.05 to stimulation value.
A full-length RANTES/CCL5 promoter-reporter construct transfected into K4 cells (SV40-immortalized human fibroblast cell line) showed induction of reporter gene activity after stimulation with TNF-α. This induction was suppressed by 13cRA (Figure 8B). A series of functional promoter elements have been previously described in the CCL5 promoter. An Ap1/cre-like region (−187 to −196) mediates some transcriptional effects. Two retinoic acid-binding consensus sites for RARF/RAR.01 (− strand) agtgaGCTCatcagtttcc (core 0.857 and matrix 0.903 score) and (+ strand) gatgaGCTCactctagatg (core 0.857 and matrix 0.903 score) (http://www.genomatix.de) are found just upstream of this region. In addition, an IRF-like element (−144 to −116) has been shown to be functional in fibroblasts.27–29,31 Additional functional elements include dual NF-κB elements at (−69 to −59) and (−55 to −34).30,31
To assess the functional role of the RAR elements, a deletion of the complete region containing both the RAR and AP1/CRE elements was tested. Although the construct showed the reduced activity associated with elimination of the AP1 element, the resultant TNF-α-induced reporter gene activity was still inhibited by treatment with 13cRA. Although this cannot absolutely rule out a role for these elements, it clearly demonstrates that other mechanisms control sensitivity to 13cRA (Figure 8B).
The IRF-like element and tandem NF-κB sites play central roles in mediating the TNF-α induced transcription of CCL5.31 The role of 13cRA on the tandem NF-κB sites was assessed using an enhancer trap vector in which a SV40 minimal promoter drives luciferase expression. The CCL5 NF-κB elements were cloned as a dimer (four elements in total) in front of the minimal SV40 promoter. Although the reporter construct responded to TNF-α stimulation, it was insensitive to treatment with 13cRA (Figure 8C). The sites have previously been shown to bind members of the Rel family of transcription factors in response to stimulation with TNF-α. EMSA assays using the tandem NF-κB elements showed no effect on complex formation. Two NF-κB complexes were found induced in response to TNF-α stimulation.30 Consistent with the enhancer trap experiment, no changes in the composition of the EMSA shift was seen after treatment with 13cRA (Figure 8D). The second element, IRF-1, did not work in the enhancer trap assay (data not shown), but potential changes induced by 13cRA were studied on EMSA using the 25-bp IRF-like region as probe. The results showed the two major complexes previously described.28,30 The complexes were not altered by TNF-α stimulation. Treatment with 13cRA did not alter the complexes and did not generate new bands.
Discussion
In a rat model of CAN 13cRA primarily preserved renal function and structural integrity of the grafts on a long-term basis. Importantly, 13cRA also considerably slowed progression of CAN. The Fisher 344 to Lewis rat model of chronic rejection is a model of CAN between rat strains differing only in minor histocompatibility antigens.32 Because the T cell-mediated effects are relatively minor, the model allows focus on the role of innate immune responses within a limited T cell-moderated inflammatory background. Characteristic full-blown histological changes of CAN develop in the allografts as early as 4 weeks.33 The key glomerular lesions of mesangial expansion, widening and splitting of basement membranes, segmental glomerular sclerosis and the subendothelial thickening of preglomerular vessels, the interstitial fibrosis, and tubular atrophy, are similar to those seen in human kidney recipients with CAN.34,35 In human kidney grafts macrophages represent a seminal leukocyte population that infiltrate grafts with CAN. A similar infiltrate is seen in this animal model.36,37 A number of cytokines/chemokines (such as TNF-α, IL-1β, IFN-γ, MCP-1/CCL2) and growth factors (TGF-β), known to induce proliferation and increase in matrix production and fibrosis were shown to be up-regulated in the allografts.36 These factors were all found to be significantly reduced by treatment with 13cRA (Figures 3 to 5 and Table 6).
The central hypothesis driving these experiments is that master regulators may coordinate or modulate the complex interactions of cellular and humoral rejection factors. Nuclear receptors such as glucocorticoid and vitamin D receptors have been shown to possess complex immunosuppressive effects. In former experiments we have demonstrated that retinoids can act as potent agents to modulate inflammatory and fibrosing processes.13,14,17 Retinoids can act through retinoic acid (RAR) and retinoid X (RXR) receptors, members of the nuclear receptor family. Mesangial and tubular epithelial cells express retinoid receptors as do endothelial and inflammatory cells, ie, monocytes/macrophages.7,9,38 Nuclear retinoid receptors influence gene transcription either directly through retinoic acid-responsive elements binding to control region in genes or more indirectly through binding or modulation of other transcription factors, among them activator-protein 1 (AP-1) or NF-κB.10,11
In the studies outlined here, 13cRA was applied at two different doses. The dose of 20 mg/kg body weight exceeded dosages generally applied in clinical settings by 10- to 20-fold.39 This dose was chosen to avoid missing potential immunosuppressive effects detectable only at HD as suggested in earlier reports.6 We show that a dose of 13cRA of 2 mg/kg body weight corresponding to the high dosage limit in humans39 was as efficient as the high dose. As expected, 13cRA levels in plasma increased in a dose-dependent manner being approximately fourfold higher in HD- than in LD-treated animals. The retinol and tretinoin levels in untreated animals were similar to the ones previously reported.40 As is seen in humans, 13cRA led to a dose-dependent decrease in retinol concentrations.6 13cRA effects are thought to be mediated by tretinoin and alitretinoin after isomerization. Alitretinoin could be detected in plasma in the HD group indicating an enzymatic transformation of 13cRA to alitretinoin in tissues.41,42
13cRA in both doses significantly reduced the morphological signs of acute rejection. In 13cRA-treated groups serum creatinine, determined in bilaterally nephrectomized allografts, was significantly lower as compared to placebo groups demonstrating that the morphological improvement was translated into an improved renal function. As all animals had systolic arterial blood pressure values in the normal range, the renoprotective effect of 13-cRA could not be attributed to systemic hemodynamic changes. In rats with experimental glomerulonephritis, 13cRA and other retinoids even appeared to inhibit the increases in arterial pressure associated with glomerulonephritis.13,14
Kirkman and colleagues43 reported prolonged survival in a bilaterally nephrectomized renal transplant model in cynomolgus monkeys treated intravenously with 2-trifluororetinin, an aromatic retinoid, at a dose of 2 mg/kg/day; histological data were not provided. The cellular basis of this survival benefit was not investigated. Cytotoxic T-cell activity was suppressed by the same retinoid in sponge matrix allografts.8 Severe gastrointestinal and skeletal toxicity of this compound were observed in these experiments8,42 that were not seen in the experiment reported here.
Beneficial effects of 13cRA and other receptor-specific retinoids on glomerular and tubulointerstitial integrity have been reported by our group in experimental models of inflammatory renal disease.13,14 The marked decrease in intimal hyperplasia of the graft vessels by 13cRA administration in this study correlated with reports for different chronic vascular damage models; tretinoin at high doses has been reported to decrease intimal thickening after balloon catheter injury in the rat carotid artery and to lower vein graft intimal hyperplasia in New Zealand White rabbits.15,16 In our previous report, allografts treated with 13cRA for 14 days showed also a marked reduction in acute morphological damage and acute rejection.
One characteristic feature of evolving CAN is the proliferative activity of infiltrated mononuclear cells and resident interstitial cells. The number of proliferating cells, as documented with Ki67-positive cells, was markedly reduced in the 13cRA-treated allografts in glomeruli as well as tubulointerstitium. Apart from malignant cells, retinoids have been shown to inhibit the proliferation of mesangial, vascular smooth muscle cells, endothelia, as well as tubular epithelia.9,13,44 There seem to be several pathways by which retinoids slow cellular growth; one is inhibition of AP-1, but it may also involve the inhibition of cyclin D1 and the stimulation of p21 and p27, inhibitors of the cell cycle.10,44 Activated macrophages and T lymphocytes produce a number of cytokines/chemokines, which perpetuate and amplify inflammation. These agents can also partly act as fibrogenic signal molecules.36,45 IFN-γ, the signature cytokine of Th1 cells, as well as the chemokines RANTES/CCL5, IP-10/CXCL10, MCP-1/CCL2, and MIP-1α/CCL3 are implicated in the acute and chronic inflammatory rejection processes associated with CAN.3,4,36 These cytokines were strongly down-regulated in the allografts treated with 13cRA.
The dominant pathological process of CAN is an excessive accumulation of extracellular matrix proteins leading to fibrosis. Monocyte/macrophage cell infiltrates are the major subpopulation of mononuclear cells in fibrotic allografts and are thought to be key mediators in initiating fibrosis by providing profibrotic factors.36,37 A 50% reduction in the number of infiltrating monocytes/macrophages (ED1+) as well as CD8+ T cells in tubulointerstitium was seen after 13cRA treatment. Thus, although reduced mononuclear cell infiltrates may explain part of the effects of 13cRA the treated grafts still showed relatively high numbers of monocytes as compared to controls; these monocytes were apparently inactivated with regard to inflammatory stimuli.
CAN is characterized by an elevated expression of TGF-β1 and PAI-1 proteins.33 In chronic renal allograft dysfunction a relationship between TGF-β mRNA expression and the intensity of cellular infiltration has been described.45 TGF-β1 promotes extracellular matrix accumulation by increasing matrix production and potentially by inhibition of matrix degradation (by induction of PAI-1).46 Retinoids may have moderated fibrosis at least in part by reducing TGF-β1 expression (Table 6). In experimental mesangioproliferative glomerulonephritis 13cRA also reduced the level of TGF-β1 gene expression significantly more than tretinoin.47
To further dissect the cellular and molecular mechanisms of inhibition of inflammation and fibrosis by 13cRA two cells centrally involved in these processes: pure monocytes/macrophages and fibroblasts were analyzed with regards to a direct influence of 13cRA on their cytokine profile. In addition, the effect of 13cRA on transcriptional regulation of an inflammatory and fibrogenic chemokine RANTES/CCL5 was studied.
In activated peritoneal macrophages the treatment of rats with 13cRA led to a marked inhibition of cytokine secretion, corroborating the results obtained at the mRNA level in the whole allograft. These data suggest that the suppression of macrophage effector activity may also contribute to the immunosuppressive effect of 13cRA. Although monocytes/macrophages have been reported to be the dominant-acting immunocompetent cell in the model studied, a significant contribution of T cells cannot be negated.
We next tried to elucidate whether 13cRA could also influence mesenchymal cells and if so, how these events may be moderated. To this end transcriptional effects in fibroblasts on the exemplary chemokine gene RANTES/CCL5 expressed in response to proinflammatory (TNF-α) stimulation were studied. The results showed that although induction of the gene was inhibited at the transcriptional level, this was not necessarily mediated through consensus RAR elements found in the immediate upstream region of the CCL5 promoter. Elimination of the RAR elements did not influence 13cRA suppression of promoter activity. In addition, reporter gene studies and EMSA results suggest that 13cRA does not act directly through the modulation of levels of NF-κB or IRF-1. EMSA experiments failed to demonstrate a change in the constitutive or TNF-α-induced complexes for IRF-1 or NF-κB.48 Recently it has been shown that a higher order promoter structure referred to as a promoter module comprising two or more factors working in concert can impart selective transcriptional effects.48 A promoter module comprised of NF-κB and IRF-1 factors has been defined.48 Although indirect, the overall results of the CCL5 promoter analysis suggest that 13cRA may act at the level of the promoter initiation complex, potentially through a promoter module composed of IRF-1 and NF-κB. Thus, it appears difficult at this time to predict how individual genes may be influenced by 13cRA because the retinoids can apparently act at different molecular levels, eg, interaction with transcription factors, binding to response elements of promoter regions through retinoid receptors, and modulating promoter initiation complexes.5
These suppressive effects of 13cRA on the activity of a dominant subgroup of intragraft mononuclear cells, monocytes/macrophages, and on the activity of resident cells involved in fibrotic changes, the fibroblasts are inferential to explain the anti-inflammatory and anti-fibrotic effects of 13cRA in CAN because macrophages and fibroblasts were not studied in situ. Although the experimental in vivo system used here de-emphasized contributions from alloreactive T cells, 13cRA may clearly also act on T cells or other cells (eg, tubular epithelia).
In summary, our data show, that in experimental models of CAN, 13cRA monotherapy led to a significant preservation of renal function and to a decrease of rejection phenomena. CAN was not only inhibited in its development but early chronic changes did not progress when therapy was started 14 days after transplantation. 13cRA in a dose corresponding to dosages used in humans is a potent immunosuppressive and anti-fibrotic agent in experimental renal allografts. Its actions appear directly immunosuppressive and anti-fibrotic as evidenced by effects on chronic inflammatory processes and reduction of fibroblast activity. 13cRA may represent a novel therapeutic option in transplantation, specifically in the prevention of CAN, which up-to-date cannot be successfully treated.
Supplementary Material
Acknowledgments
C. Schmidt provided excellent technical help.
Footnotes
Address reprint requests to Hermann-Josef Gröne M.D., Dept. of Cellular and Molecular Pathology, German Cancer Research Center, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany. E-mail: h.-j.groene@dkfz.de.
Supported by the German Research Foundation (grants FOR 406-1/2 and SFB 405 B5 to H.-J.G.).
J.A. and E.K. contributed equally to the work.
Supplemental material for this article appears on http://ajp.amjpathol.org.
References
- Häyry P, Aavik E, Savolainen H. Mechanisms of chronic rejection. Transplant Proc. 1999;31:5S–8S. [PubMed] [Google Scholar]
- Kouwenhoven EA, Ijzermans JNM, de Bruin RWE. Etiology and pathophysiology of chronic transplant dysfunction. Transpl Int. 2000;13:385–401. doi: 10.1007/s001470050721. [DOI] [PubMed] [Google Scholar]
- Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- Haskell CA, Ribeiro S, Horuk R. Chemokines in transplant rejection. Curr Opin Investig Drugs. 2002;3:399–405. [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- Zouboulis CC, Orfanos CE. Retinoids. Millikan LE, editor. New York: Marcel Dekker,; Drug Therapy in Dermatology. 2000:pp 171–233. [Google Scholar]
- Yang T, Michele DE, Park J, Smart AM, Lin Z, Brosius FC, III, Schnermann JB, Briggs JP. Expression of peroxisomal proliferator-activated receptors and retinoid X receptors in the kidney. Am J Physiol. 1999;277:F966–F973. doi: 10.1152/ajprenal.1999.277.6.F966. [DOI] [PubMed] [Google Scholar]
- Orosz CG, Zinn NE, Bishop DK, Leppink DL, Faherty D, Ferguson RM. Analysis of retinoid-mediated immunosuppression in vivo. Effects of Ro-23-6457 on cellular alloimmune responses. Immunopharmacology. 1991;22:49–58. doi: 10.1016/0162-3109(91)90055-4. [DOI] [PubMed] [Google Scholar]
- Kreutz M, Fritsche J, Ackermann U, Krause S, Andreesen R. Retinoic acid inhibits monocyte to macrophage survival and differentiation. Blood. 1998;91:4796–4802. [PubMed] [Google Scholar]
- Simonson MS. Anti-AP-1 activity of all-trans retinoic acid in glomerular mesangial cells. Am J Physiol. 1994;267:F805–F815. doi: 10.1152/ajprenal.1994.267.5.F805. [DOI] [PubMed] [Google Scholar]
- Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G, Im SY, Lee JW, Kim TS. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. J Biol Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- Chakravarti D, LaMorte VJ, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM. Role of CBP/P-300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- Schaier M, Lehrke I, Schade K, Morath CH, Shimizu F, Kawachi H, Gröne HJ, Ritz E, Wagner J. Isotretinoin alleviates renal damage in rat chronic glomerulonephritis. Kidney Int. 2001;60:2222–2234. doi: 10.1046/j.1523-1755.2001.00056.x. [DOI] [PubMed] [Google Scholar]
- Lehrke I, Schaier M, Schade K, Morath C, Waldherr R, Ritz E, Wagner J. Retinoid receptor-specific agonists alleviate experimental glomerulonephritis. Am J Physiol. 2002;282:F741–F751. doi: 10.1152/ajprenal.00026.2001. [DOI] [PubMed] [Google Scholar]
- Miano JM, Kelly LA, Artacho CA, Nuckolls TA, Piantedosi R, Blaner WS. All-trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation. 1998;98:1219–1227. doi: 10.1161/01.cir.98.12.1219. [DOI] [PubMed] [Google Scholar]
- Leville CD, Dassow MS, Seabrook GR, Jean-Claude JM, Towne JB, Cambria RA. All-trans retinoic acid decreases vein graft intimal hyperplasia and matrix metalloproteinase activity in vivo. J Surg Res. 2000;90:183–190. doi: 10.1006/jsre.2000.5887. [DOI] [PubMed] [Google Scholar]
- Kiss E, Adams J, Grone HJ, Wagner J. Isotretinoin ameliorates renal damage in experimental acute renal allograft rejection. Transplantation. 2003;76:480–489. doi: 10.1097/01.TP.0000066354.31050.5A. [DOI] [PubMed] [Google Scholar]
- Gröne HJ, Helmchen U. Impairment and recovery of the clipped kidney in two kidney, one clip hypertensive rats during and after antihypertensive therapy. Lab Invest. 1986;54:645–655. [PubMed] [Google Scholar]
- Collins MD, Eckhoff C, Chahoud I, Bochert G, Nau H. 4-Methylpyrazole partially ameliorated the teratogenicity of retinol and reduced the metabolic formation of all-trans-retinoic acid in the mouse. Arch Toxicol. 1992;66:652–659. doi: 10.1007/BF01981505. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Schröder M, Roos TC, Chandraratna RAS, Reichert U, Merk HF, Orfanos CE, Zouboulis CHC. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J Invest Dermatol. 2000;115:321–327. doi: 10.1046/j.1523-1747.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- Hermann M, Shaw S, Kiss E, Camici G, Buhler N, Chenevard R, Luscher TF, Grone HJ, Ruschitzka F. Selective COX-2 inhibitors and renal injury in salt-sensitive hypertension. Hypertension. 2005;45:193–197. doi: 10.1161/01.HYP.0000153053.82032.bf. [DOI] [PubMed] [Google Scholar]
- Scheuer H, Gwinner W, Hohbach J, Grone EF, Brandes RP, Malle E, Olbricht CJ, Walli AK, Grone HJ. Oxidant stress in hyperlipidemia-induced renal damage. Am J Physiol. 2000;278:F63–F74. doi: 10.1152/ajprenal.2000.278.1.F63. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Porubsky S, Schmid H, Bonrouhi M, Kretzler M, Malle E, Nelson PJ, Grone HJ. Influence of native and hypochlorite-modified low-density lipoprotein on gene expression in human proximal tubular epithelium. Am J Pathol. 2004;164:2175–2187. doi: 10.1016/S0002-9440(10)63775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Cohen CD, Grone HJ, Gröne EF, Nelson PJ, Schlöndorff D, Kretzler M. Laser microdissection and gene expression analysis on formaldehyde-fixed archival tissue. Kidney Int. 2002;61:125–132. doi: 10.1046/j.1523-1755.2002.00112.x. [DOI] [PubMed] [Google Scholar]
- Fessele S, Boehlk S, Mojaat A, Miyamoto NG, Werner T, Nelson EL, Schlondorff D, Nelson PJ. Molecular and in silico characterization of a promoter module and C/EBP element that mediate LPS-induced RANTES/CCL5 expression in monocytic cells. FASEB J. 2001;15:577–579. doi: 10.1096/fj.00-0459fje. [DOI] [PubMed] [Google Scholar]
- Boehlk S, Fessele S, Mojaat A, Miyamoto NG, Werner T, Nelson EL, Schlöndorff D, Nelson PJ. ATF and Jun transcription factors, acting through an Ets/CRE promoter module mediate lipopolysaccharide inducibility of the chemokine RANTES in monocytic Mono Mac 6 cells. Eur J Immunol. 2000;30:1102–1112. doi: 10.1002/(SICI)1521-4141(200004)30:4<1102::AID-IMMU1102>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Andoh A, Fujino S, Bamba S, Araki Y, Okuno T, Bamba T, Fujiyama Y. IL-17 selectively down-regulates TNF-alpha-induced RANTES gene expression in human colonic subepithelial myofibroblasts. J Immunol. 2002;169:1683–1687. doi: 10.4049/jimmunol.169.4.1683. [DOI] [PubMed] [Google Scholar]
- Nelson PJ, Ortiz BD, Pattison JM, Krensky AM. Identification of a novel regulatory region critical for expression of the RANTES chemokine in activated T lymphocytes. J Immunol. 1996;157:1139–1148. [PubMed] [Google Scholar]
- White E, Hildemann WH, Mullen Y. Chronic kidney allograft reactions in rats. Transplantation. 1969;8:602–617. doi: 10.1097/00007890-196911000-00007. [DOI] [PubMed] [Google Scholar]
- Shihab FS, Tanner AM, Shao Y, Weffer MI. Expression of TGF-β1 and matrix proteins is elevated in rats with chronic rejection. Kidney Int. 1996;50:1904–1913. doi: 10.1038/ki.1996.512. [DOI] [PubMed] [Google Scholar]
- Diamond JR, Tilney NL, Frye J, Ding G, McElroy J, Pesek-Diamond I, Yang H. Progressive albuminuria and glomerulosclerosis in a rat model of chronic renal allograft rejection. Transplantation. 1992;54:710–716. doi: 10.1097/00007890-199210000-00028. [DOI] [PubMed] [Google Scholar]
- Olsen TS. Pathology of allograft rejection. Burdick JF, Racusen LC, Solez K, William GM, editors. New York: Marcel Dekker,; Kidney Transplant RejectionDiagnosis and Treatment. 1991:pp 333–339. [Google Scholar]
- Nadeau KC, Azuma H, Tilney NL. Sequential cytokine dynamics in chronic rejection of rat renal allografts: roles for cytokines RANTES and MCP-1. Proc Natl Acad Sci USA. 1995;92:8729–8733. doi: 10.1073/pnas.92.19.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock WH, Whitley D, Tullius SG, Heemann UW, Wasowska B, Baldwin WM, Tilney NL. Cytokines, adhesion molecules, and the pathogenesis of chronic rejection of rat renal allografts. Transplantation. 1993;56:643–650. doi: 10.1097/00007890-199309000-00028. [DOI] [PubMed] [Google Scholar]
- Sugarawa A, Sanno N, Takahashi N, Osamura RY, Abe K. Retinoid X receptors in the kidney: their protein expression and functional significance. Endocrinology. 1997;138:3175–3180. doi: 10.1210/endo.138.8.5351. [DOI] [PubMed] [Google Scholar]
- Orfanos CE, Zouboulis CC. Oral retinoids in the treatment of seborrhea and acne. Dermatology. 1998;196:140–147. doi: 10.1159/000017848. [DOI] [PubMed] [Google Scholar]
- Kelleher SL, Lonnerdal B. Long-term marginal intakes of zinc and retinol affect retinol homeostasis without compromising circulating levels during lactation in rats. J Nutr. 2001;131:3237–3242. doi: 10.1093/jn/131.12.3237. [DOI] [PubMed] [Google Scholar]
- Rollmann O, Vahlquist A. Oral isotretinoin (13-cis-retinoic acid) therapy in severe acne: drug and vitamin A concentrations in serum and skin. J Invest Dermatol. 1986;86:384–389. doi: 10.1111/1523-1747.ep12285651. [DOI] [PubMed] [Google Scholar]
- IARC Working Group Lyon: IARC,; Retinoids/ IARC Handbooks of Cancer Prevention. 1999:pp 145–176. [Google Scholar]
- Kirkman RL, Barrett LV, Carter P, Reed MH, Shapiro ME. RO 23–6457 prolongs survival of vascularized allografts in rodents and primates. J Surg Res. 1990;48:304–307. doi: 10.1016/0022-4804(90)90063-8. [DOI] [PubMed] [Google Scholar]
- Wakino S, Kintscher U, Kim S, Jackson S, Yin F, Nagpal S, Chandraratna RA, Hsueh WA, Law RE. Retinoids inhibit proliferation of human coronary smooth muscle cells by modulating cell cycle regulators. Arterioscler Thromb Vasc Biol. 2001;21:746–751. doi: 10.1161/01.atv.21.5.746. [DOI] [PubMed] [Google Scholar]
- Hamar P, Szabo A, Muller V, Heemann U. The involvement of activated T cells and growth-factor production in the early and late phase of chronic kidney allograft nephropathy in rats. Transpl Int. 2002;15:446–454. doi: 10.1007/s00147-002-0446-5. [DOI] [PubMed] [Google Scholar]
- Fogo AB. Progression and potential regression of glomerulosclerosis. Kidney Int. 2001;59:804–827. doi: 10.1046/j.1523-1755.2001.059002804.x. [DOI] [PubMed] [Google Scholar]
- Morath C, Dechow C, Lehrke I, Haxsen V, Waldherr R, Floege J, Ritz E, Wagner J. Effects of retinoids on the TGF-beta system and extracellular matrix in experimental glomerulonephritis. J Am Soc Nephrol. 2001;12:2300–2309. doi: 10.1681/ASN.V12112300. [DOI] [PubMed] [Google Scholar]
- Naschberger E, Werner T, Vicente AB, Guenzi E, Topolt K, Leubert R, Lubeseder-Martellato C, Nelson PJ, Sturzl M. Nuclear factor-kappaB motif and interferon-alpha-stimulated response element co-operate in the activation of guanylate-binding protein-1 expression by inflammatory cytokines in endothelial cells. Biochem J. 2004;379:409–420. doi: 10.1042/BJ20031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.