Abstract
Experimental autoimmune myocarditis (EAM) can be induced in the Lewis rat by cardiac myosin or its cryptic S2-16 peptide epitope (amino acids1052 to 1076). To investigate cellular mechanisms and the role of antigen-presenting cells in regulation of myocarditis, we induced protection against EAM in Lewis rats by administration of S2-16 peptide in incomplete Freund’s adjuvant (IFA). Protection to EAM was associated with activation of S2-16-reactive splenocytes secreting high levels of interleukin (IL)-10 and reduced levels of interferon-γ and IL-2. Adoptive transfer of S2-16:IFA-induced splenocytes producing IL-10 suppressed myocarditis induction in syngeneic recipients, suggesting their regulatory cell nature. However, exposure of S2-16:IFA-induced cells to inflammatory cytokine IL-12 converted them to Th1 effectors that transferred EAM. Differentiated function of S2-16-reactive T cells in protected rats resulted from increased IL-10 production by dendritic cells (DCs). Purified DCs from S2-16:IFA-treated rats promoted S2-16-reactive CD4+ T cells to produce increased IL-10 and reduced interferon-γ. In addition, adoptive transfer of IL-10-producing DCs from S2-16:IFA-treated rats also induced protection to EAM in recipient rats. These studies demonstrated DCs and key cytokines, such as IL-10 and IL-12, regulated the fate of T cells in myocarditis development in the Lewis rat.
Myocarditis is an inflammatory heart disease that can be initiated by infectious pathogens.1–3 Dilated cardiomyopathy, which may follow myocarditis and represent the chronic stage of disease, is a major cause of heart failure and heart transplantation.4–6 Evidence suggests that autoimmune responses to cardiac antigens exposed after heart damage may play an important role in prolonged damage of myocardium.3,7–9 Nevertheless, little progress has been made in treating myocarditis by immunosuppression, because a complete understanding of key factors that regulate the pathogenic immune responses in autoimmune myocarditis are not well established.
Experimental autoimmune myocarditis (EAM) generated in susceptible mouse and rat strains by immunization with purified cardiac myosin or a specific pathogenic cardiac myosin peptide in adjuvant has been used to investigate the pathogenesis of myocarditis induced by autoimmune mechanisms.10–20 Many studies have shown that cardiac antigen-induced myocarditis is a T-cell-mediated disease.18,21–24 However, the active induction of EAM relies on the use of bacterial adjuvants [complete Freund’s adjuvant (CFA)] during immunization, suggesting that activation of the innate immune system is important in disease induction.25–27 Inflammatory cytokines such as interleukin (IL)-1, tumor necrosis factor (TNF)-α, and IL-12 promote myocarditis development in animals,28–31 whereas mice that lack TNF-Rp55 or are deficient in IL-12 signaling were protected from EAM.32,33 In vivo inhibition of co-stimulatory molecule B7-1 and CD40 also markedly decreased myocardial inflammation.34,35 A recent study directly demonstrated that cardiac antigen-loaded dendritic cells (DCs) induced autoimmune myocarditis when they were activated and transferred.36 Taken together, these studies suggest that EAM induction is closely associated with not only the myocarditic epitopes of cardiac myosin and their reactive T cells, but also with the activation of antigen-presenting cells (APCs) such as DCs by inflammatory cytokines.
Various strategies have been used to down-regulate cardiac myosin-specific immune responses in EAM.37–42 Nasal administration of cardiac myosin suppressed EAM in A/J mice, and blockade of IL-10 at the time of nasal administration of antigen abolished the effect of nasal tolerization.40,42 Intravenous administration of syngeneic splenocytes coupled with cardiac myosin before myocarditis induction also reduced the incidence and severity of myocarditis. Both T- and B-cell responsiveness was affected after tolerization.41 In addition, administration of a streptococcal M protein peptide, which has similarity to cardiac myosin and could induce myocarditis in mice, induced partial protection against coxsackieviral myocarditis.39 Immune tolerance approaches and mechanisms have also been studied in other autoimmune disease models such as experimental autoimmune encephalomyelitis and experimental autoimmune uveitis.43,44
The definition of tolerance is an antigen-specific unresponsiveness.45 Classic tolerance mechanisms include T-cell anergy and clonal deletion, but accumulating evidence suggests the importance of active immune suppression associated with various subtypes of regulatory T cells.46–49 Regulatory T cells occur naturally and could be developed de novo in central and peripheral lymphoid organs.47,48 It has been shown that DCs or cytokines such as IL-10 were required for induction of regulatory T cells.50,51 Therefore, APCs not only activate antigen-specific T cells, but also suppress activated T cells by certain direct and indirect mechanisms.
It has been widely reported that animals pretreated with antigen in incomplete Freund’s adjuvant (IFA) were protected from inflammatory responses induced by the same antigen in CFA.52–57 Regulatory/suppressive cells seem to be involved in this process, because the protection is transferable. The antigen:IFA-induced immunity is an ideal model system to study the regulatory mechanisms that control the self-reactive T cells. It has been suggested that APCs are not effectively activated after immunization with antigen in IFA that lacks mycobacteria.58–60 However, whether or how APCs are involved in active suppression of antigen:IFA-induced unresponsiveness has not been studied. The aim of this study was to induce protection to myocarditis by cardiac myosin peptide and IFA administration, and to further examine the cellular mechanisms and the role of DCs in the regulation of T-cell responses in a Lewis rat EAM model.
We previously identified a cryptic pathogenic peptide sequence of cardiac myosin S2 region (S2-16, amino acids 1052 to 1076), which induced EAM in Lewis rats by both active immunization and passive transfer of S2-16 peptide-specific T cells.20 S2-16-induced EAM was accompanied by up-regulation of inflammatory cytokine expression in myocardium and production by antigen-specific T cells. In this study, the S2-16-induced EAM model was used to investigate protection to EAM by peptide S2-16 and IFA treatment. We found that protection induced by S2-16:IFA treatment was accompanied by expansion of autoreactive T cells that had impaired interferon (IFN)-γ production and enhanced IL-10 production. Antigen-specific T cells from S2-16:IFA-treated rats had both regulatory and pathogenic potential that was controlled by DCs and their cytokines IL-10 and IL-12. The study directly demonstrated that DCs played a regulatory role in antigen and IFA-induced immune protection in EAM. The data link protection against EAM to both innate and adaptive immunity.
Materials and Methods
Animals and Antigens
Female Lewis rats (6 to 8 weeks old) were purchased from Harlan-Sprague-Dawley (Indianapolis, IN) and maintained in groups of three at the Animal Resources Facility on the campus of the University of Oklahoma Health Sciences Center (OUHSC). All animal studies were approved by the OUHSC animal care and use committee. Peptide S2-16 and S2-1 from the S2 region of cardiac myosin was synthesized and purified as a 25-mer by Genmed Synthesis Inc. (San Francisco, CA). The amino acid sequence of S2-16 peptide is KRKLEGDLKLTQESIMDLENDKQQL, and sequence of S2-1 peptide is SAEREKEMASMKEEFTRLKEALEKS.20
Induction of Active EAM and Protection
For induction of EAM by active immunization, rats were anesthetized with 10 mg of ketamine/0.2 mg of xylazine, and were injected in one hind footpad with 0.5 mg of S2-16 peptide emulsified in CFA (Sigma, St. Louis, MO) at 1:1 ratio (v/v, 0.25 ml for each rat). After immunization, the rats were given 1 × 1010 heat-killed Bordetella pertussis (Michigan Department of Public Health, Lansing, MI) on day 1 and day 3 intraperitoneally. Seven days after primary immunization, the rats were boosted subcutaneously with 0.5 mg of antigen emulsified in IFA (Sigma) at 1:1 ratio. Control rats received phosphate-buffered saline (PBS) plus adjuvants. Rats were sacrificed at day 21 by cardiac puncture under anesthesia. Heart, liver, and kidneys were fixed in 10% buffered formalin and imbedded in paraffin. Five-μm sections were cut and stained with hematoxylin and eosin for microscopic histological examination. Myocardium was blindly scored for the presence of histopathological myocarditis according to the scale: 0, normal; 1, mild (<5% of heart cross-section involved); 2, moderate (5 to 10% of cross-section involved); 3, marked (10 to 25% of cross-section involved); and 4, severe (>25% of cross-section involved). Valve, liver, and kidneys were also evaluated for cellular infiltrates.
To induce protection to EAM, rats were injected intraperitoneally with 1.0 mg or 1.5 mg of S2-16 peptide emulsified in IFA at 1:1 ratio (v/v, 0.5 ml for each rat). Control rats received S2-1:IFA or PBS:IFA by intraperitoneal injection. After 14 days, all rats were challenged by S2-16:CFA immunization or PBS:CFA as a control, as described in active induction of EAM. The rats were terminated at day 21 after challenge and evaluated for histological myocarditis. To induce suppression of EAM by cell transfer, rats were injected in one footpad with S2-16:IFA followed by subcutaneous boosts with S2-16:IFA on day 7. Control rats were injected with S2-1:IFA, or S2-16:CFA, or PBS:CFA. Two weeks after the first injection, splenocytes from immunized rats were isolated and cultured with S2-16 peptide (5 μg/ml) for 24 hours. In some experiments, recombinant murine IL-12 (2 ng/ml; Peprotech, Rocky Hill, NJ) or mouse anti-rat IL-10 monoclonal antibody (A5-7, 10 μg/ml; Pharmingen, San Diego, CA) were added to the cell culture. Cells were then harvested, counted, and injected intravenously into naïve 6- to 8-week-old Lewis rats (108 cells in 0.5 ml of PBS per rat). Recipients were concomitantly challenged by active immunization with S2-16 in CFA after transfer and were sacrificed at day 21 after challenge for histopathological examination. Some of the recipients were sacrificed 14 days after transfer without being challenged with S2-16:CFA.
Proliferation and Cytokine Assay
Spleens were obtained from rats and pressed through fine mesh screens. The single cell suspension was washed, counted by trypan blue exclusion, and resuspended to 5 × 106/ml in culture medium, containing RPMI 1640 (Life Technologies, Inc., Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 1% sodium pyruvate, 1% nonessential amino acids, and antibiotics (all from Life Technologies, Inc.). The cells were plated in 96-well tissue culture plates (Corning Inc., Corning, NY) in 100 μl of culture medium. Splenocytes were incubated at 37°C in 5% CO2 for 5 days with antigens at various concentrations before addition of 0.5 μCi tritiated thymidine (ICN, Irvine, CA). After 18 to 24 hours, cells were harvested onto filters with a MACH II M Harvester 96 (Wallac Inc., Gaithersburg, MD) and the tritiated thymidine incorporation was measured in a Betaplate liquid scintillation counter (Wallac, Turku, Finland). Values represent the stimulation index (stimulation index: mean test counts per minute/mean of medium control counts per minute). To determine cytokine production, splenocytes were cultured in medium in the presence of 10 μg/ml of antigens for 24 to 72 hours. In some experiments, mouse anti-rat IL-4 monoclonal antibody (OX-81, 5 μg/ml; PharMingen), mouse anti-rat IL-10 monoclonal antibody (A5-7, 5 μg/ml; PharMingen), or recombinant murine IL-12 (1 ng/ml, Peprotech) were added into the culture. Supernatant was collected, and analyzed for cytokine content by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s protocol (PharMingen). For in vitro stimulation assay of CD4+ T cells, splenocytes were incubated with a saturating concentration of magnetic anti-rat CD4 (OX-38) microbeads (Miltenyi Biotec, Auburn, CA) at 4°C for 20 minutes. After one wash, cells were separated magnetically on MS columns in a MACS separator (Miltenyi Biotec). Purified CD4+ T cells were then stimulated by 10 μg/ml of antigen together with various concentrations of purified DCs. Proliferative T-cell responses were assessed after 72 to 96 hours in culture medium at 37°C/5% CO2 by measuring tritiated thymidine incorporation.
Magnetic Cell Sorting of Splenic DCs, Stimulation of DCs for Cytokine Production, and Adoptive Transfer of DCs
Spleens were minced and digested with 2 mg/ml of collagenase D (Roche Diagnosis, Meylan, France) in RPMI 1640/1% fetal calf serum/10 mmol/L ethylenediamine tetraacetic acid for 45 minutes at 37°C. Digested material was pressed through steel mesh using a plunger of a syringe. Cell suspensions were then pipetted to disperse cells, filtered, and washed once with PBS/0.5% bovine serum albumin/2 mmol/L ethylenediamine tetraacetic acid. Supernatants were removed after centrifugation, and cell pellets were resuspended and layered on an equal volume of 14.5% (w/v) Nycodenz (Nycomed As, Oslo, Norway)/PBS solution and centrifuged for 13 minutes at 1800 × g and 4°C. Low-density cells at the top of the low-density solution were collected and washed once. Cells were then incubated with a saturating concentration of magnetic anti-rat DC (OX-62) microbeads (Miltenyi Biotec) at 4°C for 20 minutes. After one wash, cells were separated magnetically on MS columns in a MACS separator (Miltenyi Biotec). The purity of OX-62+ DCs is greater than 90% after such positive selection as measured by flow cytometry analysis. For cytokine measurement, sorted splenic rat DCs (1.25 × 105 cells) were cultured in 1 ml of culture medium in 24-well plates and incubated for 24 hours with various stimuli including 10 μg/ml of anti-CD40 monoclonal antibody (HM40-3, PharMingen), 2 μg/ml of lipopolysaccharide (LPS) (Sigma, St. Louis, MO), or 10 μg/ml of CpG oligonucleotide (Qiagen, Alameda, CA). TNF-α, IFN-γ, and IL-10 were measured by sandwich ELISA according to the manufacturer’s protocol (PharMingen). For transfer of DCs, OX-62+ DCs were isolated from rat spleens, and incubated with 10 μg/ml of S2-16 peptide, 10 μg/ml of anti-CD40 antibody (PharMingen), and 2 μg/ml of LPS (Sigma) for 12 hours. Some groups of DCs were also treated with 5 μg/ml of mouse anti-rat IL-10 antibody (PharMingen). Cells were then washed, and 106 DCs were adoptively transferred into naïve Lewis rats. Recipients were concomitantly challenged by active immunization with S2-16 in CFA after transfer and were sacrificed at day 21 after challenge for histopathological examination.
Flow Cytometry
Isolated splenic DCs (1 × 106) were stained with 1 μg of antibodies against rat DC (OX62) and MHC class II-PE (OX-6, all from PharMingen), and analyzed using a FACScalibur (Becton Dickinson, San Jose, CA) to evaluate the separation of DCs from rat spleen cells. Data were processed with Cellquest software (Becton Dickinson). Intracellular IL-10 production by CD4+ T cells was measured by flow cytometric staining according to BD cytofix/cytoperm kit manual (PharMingen). Anti-rat CD4-Cy-chrome (OX-35) and anti-rat IL-10-PE (A5-4, 1 μg/106 cells; all from PharMingen) were used to label the cells.
Quantification of Gene Expression by Real-Time Polymerase Chain Reaction (PCR)
Total RNA was extracted from 106 splenic DC samples using RNeasy mini kit (Qiagen, Valencia, CA). Reverse transcription was performed with aliquots of each RNA sample, random hexamers, and Superscript reverse transcriptase according to the protocol of the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was performed using an ABI Prism 7700 sequence detector (PE Applied Biosystems, Foster City, CA). All primers were designed using the Primer Express software (PE Applied Biosystems). The sequences of primer pairs specific for rat IL-12 (p40) and glyceraldehyde-3-phosphate dehydrogenases (G3PDH) are as follows: IL-12, ATCTGAAACTCCCCATGATGCT and CAGAGCTCCGAGTTCATTTTCC; G3PDH, TGCACCACCAACTGCTTAGC and GGCATGGACTGTGGTCATGAG. Each reaction contained 1 μl of cDNA, 0.75 μl of 50 mmol/L magnesium chloride, 4 μl of 5 μmol/L forward and reverse primer, 2.75 μl of distilled water, and 12.5 ml SYBR Green PCR Master Mix (PE Applied Biosystems). The total reaction volume was 25 μl. The negative control reaction contained distilled water as a template. Rat G3PDH was used as an endogenous control to allow for relative mRNA quantification. Cytokine mRNA levels are presented as the mean ± SEM fold increase in gene expression observed in triplicate wells of LPS and anti-CD40 antibody-treated DCs relative to untreated DCs.
Statistical Analysis
Means, SD, SEMs, and unpaired Student’s t-test or Mann-Whitney test were used to analyze the data using GraphPad Prism (GraphPad Software, San Diego, CA). Groups were considered statistically different if P ≤ 0.05.
Results
Prevention of EAM by S2-16 Peptide and IFA Treatment
Our previous work demonstrated that S2-16 peptide, a cryptic myocarditic epitope found in both human and rat cardiac myosin induced myocarditis in the Lewis rat.20 In this study, we investigate regulation of autoreactive T cells in the S2-16-induced EAM. Administration of autoantigen in IFA is well known to effectively induce antigen-specific unresponsiveness in animal models.54,55 As expected, Lewis rats given 1.5 mg of S2-16 peptide in IFA intraperitoneally 2 weeks before active immunization with 0.5 mg of the same antigen in CFA were protected from developing EAM (Table 1). The disease-positive control group of rats, which received PBS and IFA pretreatment before challenge with S2-16 peptide in CFA, experienced mild to severe myocarditis at the rate of >80% as expected for rats not protected. Another group of rats pretreated with a control peptide S2-1 and IFA also developed myocarditis on challenge with S2-16 in CFA (Table 1), and suggested that protection induced by S2-16:IFA treatment was not just a adjuvant effect, but was related to S2-16-specific lymphocytes. Pretreatment of rats with S2-16:CFA before the challenge resulted in some protection from myocarditis, but not as well as S2-16:IFA pretreatment. As a disease-negative control, rats pretreated with PBS:IFA before PBS:CFA challenge failed to develop myocarditis (Table 1). Dose titration experiments showed that 1.0 mg of S2-16 peptide administered intraperitoneally in IFA also provided protection from EAM (data not shown).
Table 1.
Induction of Myocarditis or Protection by Cardiac Myosin Peptide S2-16
| Pretreat-ment | Immunization | Positive/total | Mean histological score (1+–4+)* ± SD |
|---|---|---|---|
| S2-16:IFA | S2-16:CFA | 0/6 | 0† |
| PBS:IFA | S2-16:CFA | 5/6 | 2.6 ± 1.6 |
| S2-1:IFA | S2-16:CFA | 2/3 | 2.5 ± 2 |
| S2-16:CFA | S2-16:CFA | 1/3 | 0.3 ± 0.3 |
| PBS:IFA | PBS:CFA | 0/3 | 0‡ |
Lewis rats were pretreated with S2-16 peptide emulsified in IFA. Control groups of rats were pretreated with PBS or S2-1 peptide in IFA, or S2-16 in CFA. Fourteen days later, rats were challenged by immunization with S2-16 peptide or PBS in CFA as described in Materials and Methods. Animals were killed 21 days after challenge. Myocarditis was identified in fixed heart tissue sections by histopathological examination. In vitro analysis of proliferation and cytokine production of splenocytes are shown in Figure 1.
Lesions were scored histologically based on the following scale: 0, normal; 1, mild (less than 5% of cross section involved); 2, moderate (5 to 10% of cross section involved); 3, marked (10 to 25% of cross section involved); 4, severe (greater than 25% of cross section involved).
P < 0.005 for S2-16:IFA/S2-16:CFA-treated group versus PBS:IFA/S2-16:CFA-treated control group (Mann-Whitney test was used for in vivo results).
P < 0.05 for PBS:IFA/PBS:CFA-treated group versus PBS:IFA/S2-16:CFA-treated group.
S2-16-Reactive T Cells Proliferated in Protected Rats, but Produced Different Cytokines Compared to T Cells from Myocarditic Rats
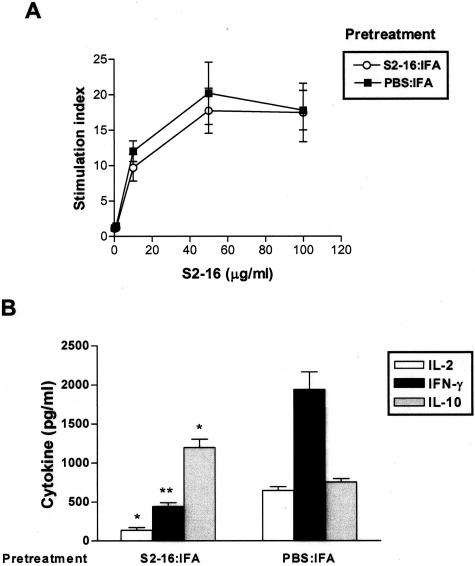
We tested the ability of T cells to recall proliferative responses against antigen in protected and diseased rats. Splenic lymphocytes from disease-positive control rats, which were treated with PBS:IFA before S2-16:CFA immunization, mounted recall proliferative responses to in vitro restimulation with S2-16 peptide in a dose-dependent pattern (Figure 1A). In comparison, a similar strength of recall proliferation was observed in S2-16:IFA-pretreated disease-protected rats (Figure 1A). When cytokine levels in cell culture supernatants were examined, we found that splenocytes from protected rats produced lower levels of Th1 cytokine IFN-γ and IL-2 as compared with disease-positive control rats (Figure 1B). However, IL-10, a regulatory cytokine, was significantly higher in protected rats than in diseased rats (Figure 1B). Th2 cytokine IL-4 production was undetectable for these groups of rats (data not shown). Therefore, S2-16-reactive T cells were not deleted or anergized in protected rats, but they produced low levels of Th1 cytokine IFN-γ and IL-2 and high levels of regulatory cytokine IL-10.
Figure 1.
S2-16:IFA-induced protection was associated with expansion of S2-16-reactive T cells producing low levels of IFN-γ and IL-2 but high levels of IL-10. Lewis rats were administered S2-16:IFA or control PBS:IFA 14 days before challenge by immunization with S2-16:CFA. Splenic lymphocytes were collected 21 days after challenge. A: Proliferative responses of splenocytes with different concentrations of S2-16 peptide. Proliferation was measured by 3H-thymidine incorporation. Results of proliferative assay were expressed as stimulation index (SI) (mean test counts per minute/mean of medium control counts per minute). B: IL-2, IL-10, and IFN-γ production by splenocytes cultured with S2-16. Supernatants were collected at 24 hours for IL-2, 48 hours for IFN-γ, and 72 hours for IL-10, and cytokine levels were measured by cytokine-specific ELISA. Error bars represent SEMs, and Student’s t-test was used to determine the significant differences between PBS:IFA-pretreated group and S2-16:IFA-pretreated group (*P ≤ 0.05, **P ≤ 0.005). Cytokine levels of cells cultured with medium alone were <20% of cytokine response to S2-16, and are not shown in figure.
S2-16:IFA-Induced Splenocytes Protected Naïve Rats from EAM
To further illustrate that S2-16:IFA-induced protection to EAM is a cellular process of active immune suppression, we performed adoptive transfer experiments. Lewis rats were treated with S2-16:IFA, and control rats were treated with S2-1:IFA, S2-16:CFA or PBS:CFA. Two weeks later, splenocytes from treated rats were collected, restimulated with S2-16 in vitro, and transferred intravenously into naïve syngeneic recipients. As shown in Table 2, S2-16:IFA-induced splenocytes did not transfer myocarditis to naïve recipient rats. Furthermore, they prevented myocarditis induction when recipients were challenged by active immunization with S2-16:CFA. Control peptide S2-1:IFA-induced splenocytes did not have such protective effects. By contrast, S2-16:CFA-induced splenocytes were pathogenic and adoptively transferred myocarditis into syngeneic recipients. Recipients of S2-16:CFA-induced splenocytes developed myocarditis after challenge (Table 2). As another control, cells from PBS:CFA-treated rats did not transfer EAM into recipients, and they also did not protect recipients from myocarditis (Table 2). Therefore, only S2-16:IFA-induced splenocytes played a regulatory role in controlling myocarditis.
Table 2.
Adoptive Transfer of S2-16:IFA-Induced Splenocytes Resulted in Protection or Myocarditis
| Donor treatment
|
Number of recipients with disease | Mean histological score of heart (0+–4+) | |
|---|---|---|---|
| In vivo | In vitroS2-16 plus | ||
| S2-16:IFA | — | 0/6 | 0 |
| S2-16:IFA | — | 0/6 (after challenge) | 0* |
| S2-1:IFA | — | 2/3 (after challenge) | 2.5 ± 1.5 |
| S2-16:CFA | — | 5/6 | 1.6 ± 0.6* |
| S2-16:CFA | — | 4/6 (after challenge) | 1.8 ± 1.1 |
| PBS:CFA | — | 0/6 | 0 |
| PBS:CFA | — | 3/3 (after challenge) | 2.5 ± 1.8 |
| S2-16:IFA | IL-12 | 5/6 | 1.7 ± 0.8* |
| PBS:CFA | IL-12 | 0/6 | 0 |
| S2-16:IFA | Anti-IL-10 | 3/3 (after challenge) | 3.6 ± 0.3 |
Lewis rats were treated with S2-16:IFA. Control rats were treated with S2-1:IFA, S2-16:CFA, or PBS:CFA. Spleen cells were obtained from each group of animals 2 weeks after treatment, and were cultured in vitro with peptide S2-16, or S2-16 plus recombinant murine IL-12, or S2-16 plus anti-rat IL-10 monoclonal antibody for 24 hours. Cell aliquot 108 was injected into each naïve Lewis rat intravenously. The recipients were sacrificed 2 weeks after adoptive transfer, and myocarditis was identified in the fixed heart sections by histopathological examination. Some recipients were concomitantly challenged by active immunization with S2-16:CFA after transfer.
P < 0.05 for S2-16:IFA cell recipients versus PBS:CFA cell recipients that were challenged by S2-16:CFA immunization. P < 0.05 for S2-16:CFA cell recipients versus PBS:CFA cell recipients, and for S2-16:IFA cell (IL-12 stimulated) recipients versus PBS:CFA cell (IL-12 stimulated) recipients (Mann-Whitney test).
IL-12 Stimulation or IL-10 Blockade Reversed Protection Induced by S2-16:IFA Splenocytes
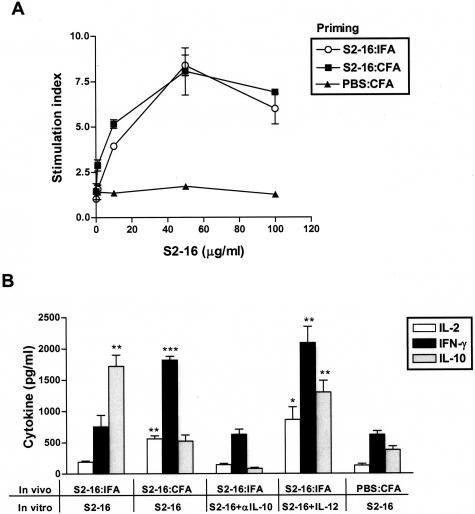
Study of splenocytes harvested from S2-16:IFA and S2-16:CFA-primed donor rats showed comparable proliferative responses to S2-16 peptide restimulation in vitro, whereas cells from control PBS:CFA-treated rats did not respond to S2-16 in the proliferation assay (Figure 2A). These data suggested that S2-16:IFA priming also activated autoreactive lymphocytes. When cytokines in the culture supernatants were measured, splenocytes from S2-16:IFA-treated donor rats did not produce high levels of Th1 cytokine IFN-γ and IL-2 as S2-16:CFA-induced cells did, but they produced an increased level of IL-10 regulatory cytokine (Figure 2B). Th2 cytokine IL-4 secretion for these groups of rats was still undetectable (data not shown). The low level of IFN-γ and IL-2 production in S2-16:IFA immunized rats was not reversed by adding anti-IL-10 antibody into the cell cultures, although IL-10 production was inhibited in this case (Figure 2B). In contrast, addition of IL-12, a key proinflammatory cytokine, significantly increased IFN-γ and IL-2 secretion of S2-16:IFA splenocytes (Figure 2B), which suggested that S2-16:IFA-primed lymphocytes still have the potential to polarize to Th1 effectors during an inflammatory stimulation.
Figure 2.
S2-16:IFA-primed splenocytes proliferated to S2-16 and produced low levels of IFN-γ and IL-2 but high levels of IL-10. Lewis rats were treated with S2-16:IFA, and S2-16:CFA or PBS:CFA as controls. Fourteen days later, splenic lymphocytes were collected and cultured with S2-16 peptide to determine their in vitro recall response. A: Proliferative response of splenocytes after culture with different concentrations of S2-16. Proliferation was measured by 3H-thymidine incorporation. B: Cytokine production of splenocytes immunized in vivo and treated in vitro as indicated in figure and in legend above. Cell culture supernatants were collected for measurement of IFN-γ, IL-2, and IL-10 by ELISA. In some experiments, recombinant murine IL-12 or anti-rat IL-10 monoclonal antibody were added together with S2-16 to the cell culture as described in Materials and Methods. Error bars represent SEMs, and Student’s t-test was used to determine the significant differences between PBS:CFA-treated group and S2-16:IFA- or S2-16:CFA-treated group (*P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.001).
Consistent with in vitro results, IL-12 stimulation converted the S2-16:IFA-induced splenocytes into pathogenic effectors that transferred EAM in five of six naïve syngeneic recipients (Table 2). In contrast, splenocytes from PBS:CFA-injected rats failed to transfer EAM into recipients even after culture with S2-16 in the presence of IL-12 (Table 2). More significantly, the protective effect of S2-16:IFA-induced splenocytes could be eliminated if IL-10 production from S2-16-reactive cells was blocked by an anti-rat IL-10 antibody before the transfer. Rats receiving anti-IL-10 antibody-treated S2-16:IFA splenocytes regained sensitivity to myocarditis induction (Table 2). Taken together, these data suggest in S2-16:IFA-treated donor rats, S2-16-reactive T cells were nonpathogenic due to reduced inflammatory cytokines IFN-γ and IL-2, and became protective by production of regulatory cytokine IL-10. S2-16:IFA-induced lymphocytes have both pathogenic and protective potential that depends on the cytokine production from S2-16-specific T cells.
Effect of S2-16:IFA Treatment on Cytokine Production of DCs
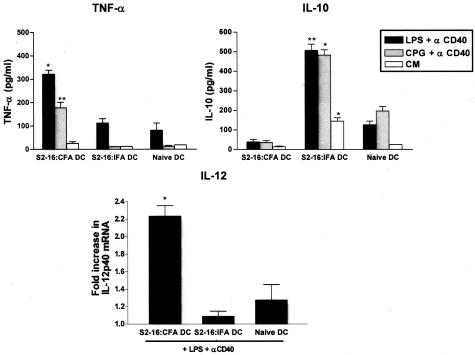
Because both cytokines IL-12 and IL-10 can be produced by APCs, especially DCs, we next tested whether the polarization of S2-16:IFA-induced lymphocytes is secondary to an alteration of DC function. When we measured the cytokine secretion of DCs, we found the production of proinflammatory cytokine TNF-α from DCs of S2-16:CFA-primed rats was much higher than S2-16:IFA-primed and untreated naive rats after LPS plus anti-CD40 antibody or CpG-containing oligonucleotide plus anti-CD40 antibody stimulation (Figure 3). The expression of inflammatory cytokine IL-12p40 mRNA was also up-regulated more than twofold in S2-16:CFA DCs after exposure to LPS and anti-CD40 antibody (Figure 3). In contrast, IL-10 production was highly induced for S2-16:IFA-induced DCs but not S2-16:CFA-induced DCs or naïve DCs after toll-like receptor (LPS or CpG) and CD40 stimulation (Figure 3). Therefore, these results show that S2-16:IFA-induced DCs produced less proinflammatory cytokines TNF-α and IL-12 but a higher amount of regulatory cytokine IL-10 than S2-16:CFA-induced DCs after activation.
Figure 3.
S2-16 and adjuvant treatment affected cytokine production of DCs. TNF-α production was increased in S2-16:CFA DCs as well as IL-12p40 mRNA, whereas increased IL-10 production was observed for S2-16:IFA DCs. Lewis rats were injected with S2-16:IFA or S2-16:CFA as a control, or left untreated. Fourteen days later, DCs were isolated from spleens by positive selection using OX62 monoclonal antibody and were stimulated by LPS or CpG-containing oligonucleotide with anti-rat CD40 antibody. TNF-α and IL-10 production from DCs in culture supernatants were measured by ELISA. IL-12p40 mRNA expression in rat DCs were measured by quantitative real-time PCR. Rat G3PDH was used as an endogenous control to allow for relative mRNA quantification. Cytokine mRNA levels are presented as fold increase in gene expression observed in triplicate wells of LPS and anti-CD40 antibody-treated DCs relative to untreated DCs. Error bars represent SEMs, and Student’s t-test was used to determine the significant differences between DCs from untreated rats and DCs from S2-16:IFA- or S2-16:CFA-treated rats (*P ≤ 0.05, **P ≤ 0.005).
Polarized CD4+ T-Cell Responses after Culture with DCs
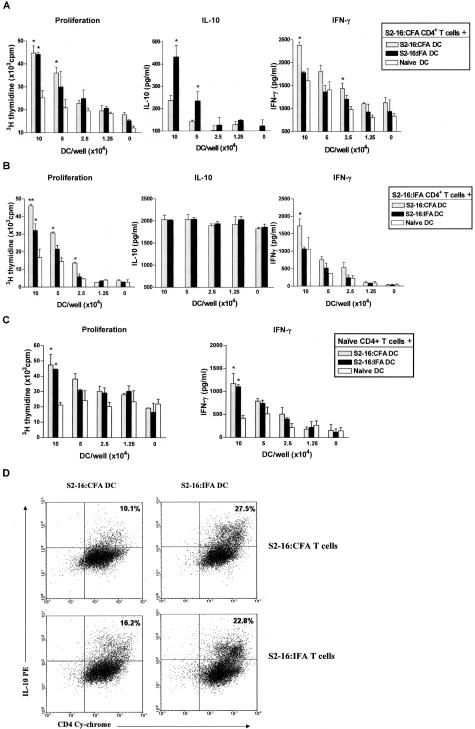
We then wanted to determine whether DCs derived from S2-16:IFA and S2-16:CFA-treated rats differed in stimulating T-cell reactivity to S2-16. To address this question, we co-cultured purified DCs from S2-16:IFA- or S2-16:CFA-treated and naïve rats at various concentrations with purified CD4+ T cells from these groups of rats, together with S2-16 peptide. After 96 hours, supernatants and cells were collected to measure the cytokine production and cell proliferation. As shown in Figure 4A, S2-16:CFA CD4+ T cells proliferated at comparable levels when cultured with S2-16:CFA DCs and S2-16:IFA DCs. However, S2-16:CFA CD4+ T cells were promoted by S2-16:IFA DCs to produce more IL-10 than when they were cultured with S2-16:CFA DCs (Figure 4A). In contrast, they produced the highest level of IFN-γ when cultured with S2-16:CFA DCs (Figure 4A). On the other hand, S2-16:IFA CD4+ T cells were found to be promoted by S2-16:CFA DCs to proliferate at a higher level and produce more IFN-γ; and in addition their IL-10 production was high when stimulated with either IFA DCs or CFA DCs (Figure 4B). Naïve CD4+ T cells showed minimal difference at proliferative responses and IFN-γ production after stimulation with either S2-16:CFA DCs or S2-16:IFA DCs, where their proliferation and IFN-γ production levels were higher than stimulation with naïve rat DCs (Figure 4C). This indicated that differentiated cytokine production and proliferation observed in Figure 4, A and B, did not result from DC proliferation, although both S2-16:CFA and S2-16:IFA DCs were activated and had T-cell-stimulating effects.
Figure 4.
Polarized CD4+ T-cell response after culture with DCs of S2-16 and adjuvant-treated rats: S2-16:IFA DCs promoted CD4+ T cells to produce more IL-10, whereas S2-16:CFA DCs promoted IFN-γ production from CD4+ T cells. Lewis rats were treated with S2-16:IFA or S2-16:CFA. Fourteen days later, CD4+ T cells and DCs were isolated from spleens of treated rats or untreated naïve rats, and co-cultured together with S2-16 peptide. Proliferation of cells was measured by 3H-thymidine incorporation after 96 hours. Cytokine production in cell culture supernatants was measured by ELISA. A: Proliferative response, IL-10, and IFN-γ production by CD4+ T cells from S2-16:CFA-treated rats after co-culture (5 × 105 CD4+ T cells) with DCs from three groups of rats at various concentrations. B: Proliferative response and IL-10 and IFN-γ production of CD4+ T cells from S2-16:IFA-treated rats after co-culture (5 × 105 CD4+ T cells) with DCs at various concentrations. C: Proliferative response and IFN-γ production of CD4+ T cells from naive rats after co-culture (5 × 105 CD4+ T cells) with DCs at various concentrations. Error bars represent SEMs. Mann-Whitney test was used to determine the difference between S2-16:IFA DC or S2-16:CFA DC versus naïve DC groups (proliferation and IFN-γ), and S2-16:IFA DC versus S2-16:CFA DC groups (IL-10). *P ≤ 0.05, **P ≤ 0.005. D: Intracellular staining suggested that S2-16:IFA DCs induced higher level IL-10 production from CD4+ T cells than S2-16:CFA DCs. DCs were purified from spleens of S2-16:CFA- or S2-16:IFA-treated rats, and cultured with purified CD4+ T cells from these groups of rats, together with S2-16 peptide. Cells were removed from co-culture on day 4 and stained for CD4+ cells and intracellular IL-10-positive cells. Numbers denote percentage of cells in each quadrant. The data are representative of two to three independent experiments with similar results.
We also performed intracellular IL-10 staining of DCs and CD4+ T cells in co-culture to more accurately examine the IL-10 production from T cells. Figure 4D showed that most of the IL-10 was produced by CD4+ T cells in the co-culture. In addition, S2-16:IFA DCs but not S2-16:CFA DCs, when co-cultured with CD4+ T cells from S2-16:CFA and S2-16:IFA-treated rats, promoted CD4+ T cells from both groups to produce more IL-10 (Figure 4D). Taken together, these results suggested that the intrinsic altered immune stimulatory capacity of DCs was responsible for functional polarization of S2-16-reactive T cells after S2-16:IFA administration.
Adoptive Transfer of IL-10-Producing DCs Prevented EAM Induction
To directly demonstrate that DCs contribute to the regulation of myocarditis, we performed a DC adoptive transfer. DCs were purified from S2-16:IFA-treated, control S2-1:IFA-treated, PBS:IFA-treated, S2-16:CFA-treated, or naive rats and stimulated in vitro with LPS and anti-CD40 together with S2-16 peptide for 12 hours before transfer. As shown in Table 3, transfer of DCs from S2-16:IFA-primed rats to recipient rats before S2-16:CFA challenge prevented myocarditis. Transfer of DCs from S2-1:IFA-primed or PBS:IFA-primed rats also resulted in protection against myocarditis, which suggested that the protection provided by DC transfer was not antigen-specific. In contrast, S2-16:CFA-induced DCs and naïve DCs did not have a protective effect. In addition, blocking of IL-10 production from S2-16:IFA DCs by anti-rat IL-10 antibody treatment in vitro inhibited their protective effects, as shown when recipients of these DCs developed myocarditis after challenge (Table 3). Therefore, the adoptive transfer directly demonstrated that DCs from S2-16:IFA-treated rats have a regulatory effect, and antibody against IL-10 reversed the effects of S2-16:IFA DCs on protection of EAM.
Table 3.
DCs from Tolerized Rats Transferred Tolerance to Naïve Rats
| Donor of DCs | Recipient with EAM after challenge | Mean score (0+–4+) ± SD |
|---|---|---|
| S2-16:IFA | 1/8 | 0 ± 0.3* |
| S2-16:IFA (+ anti-IL-10) | 4/6 | 1.9 ± 1.8 |
| S2-1:IFA | 0/3 | 0† |
| PBS:IFA | 0/3 | 0† |
| S2-16:CFA | 6/8 | 1.6 ± 1.1 |
| Naive | 4/5 | 1.7 ± 1.1 |
Lewis rats were treated with S2-16:IFA. Control rats were treated with S2-16:CFA, S2-1:IFA, PBS:IFA, or left untreated. Splenic DCs were obtained from each group of animals 2 weeks after treatment, and were cultured in vitro with peptide S2-16, together with LPS plus anti-rat CD40 antibody for 12 hours. One group of S2-16:IFA-treated rats were incubated with antibody against rat IL-10 in addition. DC aliquot (106) was injected into each naïve Lewis rat intravenously. The recipients were concomitantly challenged by active immunization with S2-16:CFA after transfer, and sacrificed 3 weeks after challenge. Myocarditis was identified in the fixed heart sections by histopathological examination.
P < 0.01 for groups: S2-16:IFA versus naïve, S2-16:IFA versus S2-16:IFA (+ anti-IL-10), and S2-16:IFA versus S2-16:CFA.
P < 0.05 for groups: S2-1:IFA versus naïve, and PBS:IFA versus naive.
Discussion
Our present study examined cellular mechanisms and the role of DCs in regulation of S2-16:IFA-induced protection to EAM. Cardiac myosin-derived cryptic pathogenic peptide S2-16:CFA-induced EAM was prevented in the Lewis rat by pretreatment with the same peptide in IFA. Adoptive transfer of S2-16:IFA-primed splenocytes suppressed the induction of myocarditis, however, those splenocytes could be rendered pathogenic after stimulation with IL-12. S2-16:IFA treatment induced altered cytokine secretion by autoreactive T cells and DCs. DCs were found to up-regulate their own production of IL-10 after S2-16:IFA treatment and to promote S2-16-specific T cells to produce higher levels of IL-10 and reduced IFN-γ. Adoptive transfer of anti-IL-10-treated S2-16:IFA splenocytes failed to protect recipients from myocarditis. Finally, S2-16:IFA-induced IL-10-producing DCs transferred tolerance, which directly demonstrated that DCs contributed to S2-16:IFA-induced protection.
In our Lewis rat EAM model, protection against myocarditis induced by S2-16:IFA treatment did not abrogate the activation of autoreactive T cells. The recall proliferation of S2-16:IFA-induced splenocytes was similar to that of S2-16:CFA-induced splenocytes. In addition, IgG1, IgG2a, and IgG2b antibodies against peptide S2-16 were detected in S2-16:IFA-treated rats and were comparable to those in CFA-primed rats (unpublished data). Therefore, both antibody production and T-cell proliferation responses were very similar in protected and diseased rats. Despite the strength of S2-16-reactive lymphocyte proliferation, no cellular infiltration was detectable in protected rat hearts, which was accompanied by a high level of regulatory cytokine IL-10 production by S2-16-specific T cells in those rats. These results do not support clonal deletion or anergy of autoreactive immune cells, although such possible mechanisms were indicated by some previous reports that studied antigen and IFA-induced immunity.54,55,57,61,62 The different results may be explained by administration of different antigen doses and routes that induce different levels of tolerance. Active regulation is an important mechanism of immune tolerance. Such tolerance does not necessarily mean the lack of immune responses, and can be associated with an activation of immune cells that have regulatory effects.63–65 We showed that peptide S2-16:IFA administration induced regulatory cells (T cells and DCs) that have protective effects. First, S2-16-reactive lymphocytes from protected rats produced a higher amount of IL-10. IL-10 has been implicated to be a suppressive cytokine that is produced by DCs and regulatory T cells and mediates the down-regulation of immune responses.66 Second, cells from S2-16:IFA-treated rats actively suppressed myocarditis induction on adoptive transfer into syngeneic rats. Third, IL-10 blockade of S2-16-reactive splenocytes by antibody treatment abrogated immune tolerance generation, which indicated the important role of IL-10 and regulatory cells in S2-16:IFA-induced protection. Although we only used in vitro anti-IL-10 antibody treatment in this study, we would think IL-10 blockade of S2-16-specific T cells in this way was effective enough to inhibit the protective nature of those T cells. A control nonmyocarditic peptide S2-1:IFA treatment did not have protective effects. As we expected, S2-16:IFA pretreatment or primed splenocytes also did not prevent cardiac myosin-induced myocarditis (data not shown), which indicated that S2-16 may not be the only myocarditic epitope in cardiac myosin. These results suggested that the protection was antigen-specific and required regulatory cytokine production by antigen-specific T cells. Further studies shall be done to determine whether IL-10-producing regulatory cells exert their effects by direct contact or indirect mechanisms.
On the other hand, T cells of S2-16:IFA-treated rats have myocarditic potential as well. Although splenocytes induced by S2-16:IFA produced lower amounts of Th1 cytokines IFN-γ and IL-2, these cytokines were primarily up-regulated when S2-16:IFA cells were stimulated with IL-12 or encountered DCs from S2-16:CFA-immunized rats. S2-16:IFA-induced cells regained their pathogenicity and transferred myocarditis into recipient rats after stimulation in vitro with IL-12. These results were consistent with previous studies that suggested that T cells induced by antigen:IFA may be in a transitional state and retain the ability to differentiate into pathogenic effectors under Th1-polarizing conditions.56,67,68 Our data also suggested that antigen:IFA-induced tolerance to myocarditis is a dynamic process and can be broken by strong inflammatory stimuli produced by APCs.68–70 After exposure to high doses of exogenous IL-12, S2-16:IFA-induced cells produced increased IFN-γ but not IL-10 in response to S2-16. The reversal of protection by IL-12 exposure, therefore, might be mediated by enhanced Th1 effector function that could overcome the protective effects of IL-10-producing regulatory cells. Although disease induction required Th1 effectors, the protection induced by S2-16:IFA was not simply a Th2 cytokine effect because it was antigen-specific and transferable. Because we did not investigate functional perspective of T-cell clones, we do not know if a single cell or clonal population can polarize under pressure from DCs, but our data strongly suggest that S2-16-reactive T cells can be controlled by DCs. S2-16 and IFA administration in our model may induce mixed T-cell populations that contain partially polarized T-helper cells and activated regulatory cells recognizing S2-16, and the result of immune responses depends on the balance of these cell functions. A mixed T-cell population may also be induced by S2-16:CFA treatment, in which activated Th1 effectors generally are more dominant than regulatory cells and may explain why S2-16:CFA pretreatment also induced some protection against myocarditis as shown in Table 1.
APCs play a key role in deciding the fate of a T-cell population.71,72 DCs are professional APCs specialized for the initiation of not only effector T cells but also regulatory T-cell immunity.51,73 The tolerogenic DCs have been implicated in some tolerance studies.45,74–76 For example, pulmonary DCs producing IL-10 have been shown to mediate tolerance induced by intranasal administration of antigen.74 DCs generated in the presence of agents that inhibit their maturation-induced T-cell unresponsiveness in vitro and in vivo.75,76 The role of DCs and their cytokines in antigen:IFA-induced protection have not been previously characterized. In our study, we showed that the altered function of S2-16-reactive T cells resulted from the effects of DCs. Activated DCs isolated from S2-16:IFA or CFA-primed rats secreted different cytokines, and also polarized S2-16-reactive CD4+ T cells to have differentiated function. A marked difference in IL-10 production of S2-16:IFA T cells after co-culturing with CFA DCs or IFA DCs was detected by flow cytometry analysis (Figure 4D) but not cytokine ELISA (Figure 4B). The difference might result from the sensitivity of different experimental techniques. Alternatively, the data may suggest that terminally differentiated IFA CD4+ T cells are more dominant than DCs and their IL-10 production is not easily changed by DCs. Despite the fact that S2-16:IFA CD4+ T cells produced high levels of IL-10, the T cells were still able to make IFN-γ under S2-16:CFA DC stimulation, which may suggest that different populations of T cells secrete different cytokines in response to the DCs. In our experiments, naïve T cells and activated CFA or IFA T cells gave similar proliferation profiles when cultured with DCs (Figure 4; A to C). The peak of proliferation of activated T cells may be earlier than for naïve T cells, and we therefore may not have observed it under our experimental conditions. In addition to our in vitro studies, we also showed that IL-12 and IL-10, key DC cytokines, were critical to control the fate of S2-16:IFA-induced S2-16-specific lymphocytes in vivo. Furthermore, S2-16:IFA-induced IL-10-producing DCs transferred tolerance or protection against myocarditis. Therefore, our results directly demonstrated that antigen-specific immunity or tolerance induced by S2-16:IFA was regulated by DCs secreting different cytokines.
It remains controversial how DCs are involved in the maintenance of tolerance to self-antigen especially at the periphery. It has been proposed that specialized regulatory DCs are involved in tolerance,77–80 however, the evidence for this is still fragmentary. Another more accepted concept is that the state of development or activation of DCs decides if those particular DCs will act as an immunogenic or tolerogenic mediator.50,81 The classical two-signal model proposes that immature DCs that deliver signal 1 in the absence of signal 2 induce the anergic or tolerance state in T cells.25 New evidence shows that DC maturation may be a process including several stages that could be distinguished not only by their activation marker expression but also by their cytokine production and migration capacity.73,82 Our study showed that DCs from S2-16:IFA- or CFA-treated rats had different cytokine production as well as different T-cell-polarizing function. Similar results have been shown in other models of immune tolerance.74,83 We were not able to observe a difference in their maturation state at the time point when we harvested cells, and DCs from both groups had mature DC features (data not shown). Other molecules on DCs that deliver signals, such as CD40, may also play an important role in determining cytokine production from T cells.67,83 DCs induced by S2-16:IFA immunization may exert their protective effect by promoting S2-16-specific T cells to reduce their Th1 polarization and increase IL-10 production. The regulation of T cells by DCs may not be antigen-specific, because DCs from PBS:IFA- and S2-1:IFA-treated rats also prevented myocarditis induction. Although DCs produced regulatory cytokines that influenced the T cells, it is the antigen-specific T cells that finally deliver protection against myocarditis. Therefore, the data clearly link protection to both innate and adaptive antigen-specific immune function. Because development of myocarditis may involve multiple myocarditic epitopes of cardiac myosin, such a DC-induced, antigen-specific T-cell-mediated protection may have potential application in the development of therapies for myocarditis. Defined mechanisms of how regulatory DCs control T-cell function in this model system is still under study. The ratio of IFN-γ-producing effector T cells and IL-10-producing regulatory T cells may be a dynamic process controlled by DCs and the cytokine environment that directs the outcome of S2-16-specific immune responses in our model system.
In summary, we demonstrated that antigen-specific protection induced by cardiac myosin peptide and IFA treatment in Lewis rat EAM was closely associated with antigen-specific T-cell function controlled by DCs and their secreted cytokines. In humans with myocarditis, it is likely that alteration in DC function and cytokine production may be important in development of disease. Modulation of cardiac-specific immune responses provides a powerful tool to investigate the pathogenic mechanisms involved in autoimmune myocarditis. Our study may also shed light on potential development of antigen-specific immune therapy to control chronic myocarditis.
Acknowledgments
We thank Dr. Sally Huber for critical review of the manuscript and Dr. Darise Farris and Dr. Juneann W. Murphy for helpful discussion.
Footnotes
Address reprint requests to Madeleine W. Cunningham, Ph.D., Department of Microbiology and Immunology, University of Oklahoma Health Sciences Center, Biomedical Research Center, Room 217, 975 NE 10th St., Oklahoma City, OK 73104. E-mail: madeleine-cunningham@ouhsc.edu.
Supported by the National Heart, Lung, and Blood Institute (grant HL 56267) and the American Heart Association (grant AHA-0215176Z).
References
- Leslie K, Blay R, Haisch C, Lodge A, Weller A, Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989;2:191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olinde KD, O’Connell JB. Inflammatory heart disease: pathogenesis, clinical manifestations, and treatment of myocarditis. Annu Rev Med. 1994;45:481–490. doi: 10.1146/annurev.med.45.1.481. [DOI] [PubMed] [Google Scholar]
- Feldman AM, McNamara D. Medical progress: myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- Fuster V, Gersh BJ, Giuliani ER, Tajik AJ, Brandenburg RO, Frye RL. The natural history of idiopathic dilated cardiomyopathy. Am J Cardiol. 1981;47:525–531. doi: 10.1016/0002-9149(81)90534-8. [DOI] [PubMed] [Google Scholar]
- Brown CA, O’Connell JB. Myocarditis and idiopathic dilated cardiomyopathy. Am J Med. 1995;99:309–314. doi: 10.1016/S0002-9343(99)80164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin JA, Bowles KR, Bowles NE. Etiologies of cardiomyopathy and heart failure. Nat Med. 1999;78:270–283. doi: 10.1038/6474. [DOI] [PubMed] [Google Scholar]
- Lange LG, Schreiner GF. Immune mechanisms of cardiac disease. N Engl J Med. 1994;330:1129–1135. doi: 10.1056/NEJM199404213301607. [DOI] [PubMed] [Google Scholar]
- Caforio AL, Goldman JH, Haven AJ, Baig KM, McKenna WJ. Evidence for autoimmunity to myosin and other heart-specific autoantigens in patients with dilated cardiomyopathy and their relatives. Int J Cardiol. 1996;54:157–163. doi: 10.1016/0167-5273(96)02593-4. [DOI] [PubMed] [Google Scholar]
- Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091–1100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990;57:250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- Liao L, Sindhwani R, Leinwand L, Diamond B, Factor S. Cardiac alpha-myosin heavy chains differ in their induction of myocarditis. Identification of pathogenic epitopes. J Clin Invest. 1993;92:2877–2882. doi: 10.1172/JCI116909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann KW, Zhao W, Griffin AC, Hickey WF. Identification of myocarditogenic peptides derived from cardiac myosin capable of inducing experimental allergic myocarditis in the Lewis rat. The utility of a class II binding motif in selecting self-reactive peptides. J Immunol. 1994;153:892–900. [PubMed] [Google Scholar]
- Inomata T, Hanawa H, Miyanishi T, Yajima E, Nakayama S, Maita T, Kodama M, Izumi T, Shibata A, Abo T. Localization of porcine cardiac myosin epitopes that induce experimental autoimmune myocarditis. Circ Res. 1995;76:726–733. [PubMed] [Google Scholar]
- Donermeyer DL, Beisel KW, Allen PM, Smith SC. Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart. J Exp Med. 1995;182:1291–1300. doi: 10.1084/jem.182.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pummerer CL, Luze K, Grassl G, Bachmaier K, Offner F, Burrell SK, Lenz DM, Zamborelli TJ, Penninger JM, Neu N. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97:2057–2062. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K, Takagaki Y, Aoyama N, Yokoyama H, Takehana H, Izumi T. A peptide fragment of beta cardiac myosin heavy chain (beta-CMHC) can provoke autoimmune myocarditis as well as the corresponding alpha cardiac myosin heavy chain (alpha-CMHC) fragment. Autoimmunity. 2001;34:177–185. doi: 10.3109/08916930109007382. [DOI] [PubMed] [Google Scholar]
- Cunningham MW. Cardiac myosin and the TH1/TH2 paradigm in autoimmune myocarditis. Am J Pathol. 2001;159:5–12. doi: 10.1016/S0002-9440(10)61665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Hemric ME, Kosanke SD, Factor SM, Quinn A, Cunningham MW. Induction of myocarditis and valvulitis in Lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am J Pathol. 2002;160:297–306. doi: 10.1016/S0002-9440(10)64373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Heuser JS, Kosanke SD, Hemric M, Cunningham MW. Cryptic epitope identified in rat and human cardiac myosin S2 region induces myocarditis in the Lewis rat. J Immunol. 2004;172:3225–3234. doi: 10.4049/jimmunol.172.5.3225. [DOI] [PubMed] [Google Scholar]
- Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147:2141–2147. [PubMed] [Google Scholar]
- Pummerer C, Berger P, Fruhwirth M, Ofner C, Neu N. Cellular infiltrate, major histocompatibility antigen expression and immunopathogenic mechanisms in cardiac myosin-induced myocarditis. Lab Invest. 1991;65:538–547. [PubMed] [Google Scholar]
- Kodama M, Matsumoto Y, Fujiwara M. In vivo lymphocyte-mediated myocardial injuries demonstrated by adoptive transfer of experimental autoimmune myocarditis. Circulation. 1992;85:1918–1926. doi: 10.1161/01.cir.85.5.1918. [DOI] [PubMed] [Google Scholar]
- Penninger JM, Neu N, Timms E, Wallace VA, Koh DR, Kishihara K, Pummerer C, Mak TW. The induction of experimental autoimmune myocarditis in mice lacking CD4 or CD8 molecules. [Corrected erratum appears in J Exp Med 1994 Jan 1;179(1):371]. J Exp Med. 1993;178:1837–1842. doi: 10.1084/jem.178.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- Ohashi PS, DeFranco AL. Making and breaking tolerance. Curr Opin Immunol. 2002;14:744–759. doi: 10.1016/s0952-7915(02)00406-5. [DOI] [PubMed] [Google Scholar]
- Lane JR, Neumann DA, Lafond-Walker A, Herskowitz A, Rose NR. Interleukin 1 or tumor necrosis factor can promote Coxsackie B3-induced myocarditis in resistant B10.A mice. J Exp Med. 1992;175:1123–1129. doi: 10.1084/jem.175.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura Y, Takeda K, Honda S, Hanawa H, Watanabe H, Kodama M, Izumi T, Aizawa Y, Seki S, Abo T. Recombinant murine interleukin-12 facilitates induction of cardiac myosin-specific type 1 helper T cells in rats. Circ Res. 1998;82:1035–1042. doi: 10.1161/01.res.82.10.1035. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Kurrer MO, Sonderegger I, Iezzi G, Tafuri A, Hunziker L, Suzuki S, Bachmaier K, Bingisser RM, Penninger JM, Kopf M. Activation of dendritic cells through the interleukin 1 receptor is critical for the induction of autoimmune myocarditis. J Exp Med. 2003;197:323–331. doi: 10.1084/jem.20021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabie N, Delfs MW, Westrich JR, Love VA, Stavrakis G, Ahmad F, Seidman CE, Seidman JG, Lichtman AH. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J Clin Invest. 2003;111:671–680. doi: 10.1172/JCI16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmaier K, Pummerer C, Kozieradzki I, Pfeffer K, Mak TW, Neu N, Penninger JM. Low-molecular-weight tumor necrosis factor receptor p55 controls induction of autoimmune heart disease. Circulation. 1996;95:655–661. doi: 10.1161/01.cir.95.3.655. [DOI] [PubMed] [Google Scholar]
- Afanasyeva M, Wang Y, Kaya Z, Stafford EA, Dohmen KM, Sadighi Akha AA, Rose NR. Interleukin-12 receptor/STAT4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-gamma-independent pathway. Circulation. 2001;104:3145–3151. doi: 10.1161/hc5001.100629. [DOI] [PubMed] [Google Scholar]
- Seko Y, Takahashi N, Azuma M, Yagita H, Okumura K, Yazaki Y. Effects of in vivo administration of anti-B7-1/B7-2 monoclonal antibodies on murine acute myocarditis caused by coxsackievirus B3. Circ Res. 1998;82:613–618. doi: 10.1161/01.res.82.5.613. [DOI] [PubMed] [Google Scholar]
- Seko Y, Takahashi N, Azuma M, Yagita H, Okumura K, Yazaki Y. Expression of costimulatory molecule CD40 in murine heart with acute myocarditis and reduction of inflammation by treatment with anti-CD40L/B7-1 monoclonal antibodies. Circ Res. 1998;83:463–469. doi: 10.1161/01.res.83.4.463. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- Estrin M, Smith C, Huber S. Antigen-specific suppressor T cells prevent cardiac injury in Balb/c mice infected with a nonmyocarditic variant of coxsackievirus group B, type 3. Am J Pathol. 1986;125:578–584. [PMC free article] [PubMed] [Google Scholar]
- Job LP, Lyden DC, Huber SA. Demonstration of suppressor cells in coxsackievirus group B, type 3 infected female Balb/c mice which prevent myocarditis. Cell Immunol. 1986;98:104–113. doi: 10.1016/0008-8749(86)90271-6. [DOI] [PubMed] [Google Scholar]
- Huber SA, Cunningham MW. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsackieviral myocarditis. J Immunol. 1996;156:3528–3534. [PubMed] [Google Scholar]
- Wang Y, Afanasyeva M, Hill SL, Kaya Z, Rose NR. Nasal administration of cardiac myosin suppresses autoimmune myocarditis in mice. J Am Coll Cardiol. 2000;36:1992–1999. doi: 10.1016/s0735-1097(00)00939-6. [DOI] [PubMed] [Google Scholar]
- Godsel LM, Wang K, Schodin BA, Leon JS, Miller SD, Engman DM. Prevention of autoimmune myocarditis through the induction of antigen-specific peripheral immune tolerance. Circulation. 2001;103:1709–1714. doi: 10.1161/01.cir.103.12.1709. [DOI] [PubMed] [Google Scholar]
- Kaya Z, Dohmen KM, Wang Y, Schlichting J, Afanasyeva M, Leuschner F, Rose NR. Cutting edge: a critical role for IL-10 in induction of nasal tolerance in experimental autoimmune myocarditis. J Immunol. 2002;168:1552–1556. doi: 10.4049/jimmunol.168.4.1552. [DOI] [PubMed] [Google Scholar]
- Harrison LC, Hafler DA. Antigen-specific therapy for autoimmune disease. Curr Opin Immunol. 2000;12:704–711. doi: 10.1016/s0952-7915(00)00166-7. [DOI] [PubMed] [Google Scholar]
- Link H, Xiao BG. Rat models as tool to develop new immunotherapies. Immunol Rev. 2001;184:117–128. doi: 10.1034/j.1600-065x.2001.1840111.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- Read S, Powrie F. CD4(+) regulatory T cells. Curr Opin Immunol. 2001;13:644–649. doi: 10.1016/s0952-7915(01)00273-4. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- Francois Bach J. Regulatory T cells under scrutiny. Nat Rev Immunol. 2003;3:189–198. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- Jonuleit H. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- Nussenzweig MC, Steinman RM. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch HC, Einstein ER, Csejtey J, Davis WJ. Protective action of the encephalitogen and other basic proteins in experimental allergic encephalomyelitis. Immunochemistry. 1968;5:567–575. doi: 10.1016/0019-2791(68)90092-x. [DOI] [PubMed] [Google Scholar]
- Swierkosz JE, Swanborg RH. Suppressor cell control of unresponsiveness to experimental allergic encephalomyelitis. J Immunol. 1975;115:631–633. [PubMed] [Google Scholar]
- Gaur A, Wiers B, Liu A, Rothbard J, Fathman CG. Amelioration of autoimmune encephalomyelitis by myelin basic protein synthetic peptide-induced anergy. Science. 1992;258:1491–1494. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- Marusic S, Tonegawa S. Tolerance induction and autoimmune encephalomyelitis amelioration after administration of myelin basic protein-derived peptide. J Exp Med. 1997;186:507–515. doi: 10.1084/jem.186.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BM, Chang JT, Shevach EM. CpG oligonucleotides are potent adjuvants for the activation of autoreactive encephalitogenic T cells in vivo. J Immunol. 2000;164:5683–5688. doi: 10.4049/jimmunol.164.11.5683. [DOI] [PubMed] [Google Scholar]
- Conant SB, Swanborg RH. Autoreactive T cells persist in rats protected against experimental autoimmune encephalomyelitis and can be activated through stimulation of innate immunity. J Immunol. 2004;172:5322–5328. doi: 10.4049/jimmunol.172.9.5322. [DOI] [PubMed] [Google Scholar]
- Warren HS, Vogel FR, Chedid LA. Current status of immunological adjuvants. Annu Rev Immunol. 1986;4:369–388. doi: 10.1146/annurev.iy.04.040186.002101. [DOI] [PubMed] [Google Scholar]
- Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Clayton JP, Gammon GM, Ando DG, Kono DH, Hood L, Sercarz EE. Peptide-specific prevention of experimental allergic encephalomyelitis. Neonatal tolerance induced to the dominant T cell determinant of myelin basic protein. J Exp Med. 1989;169:1681–1691. doi: 10.1084/jem.169.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidard L, Colarusso LJ, Benacerraf B. Specific T-cell tolerance may reflect selective activation of lymphokine synthesis. Proc Natl Acad Sci USA. 1995;92:2259–2262. doi: 10.1073/pnas.92.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–916. [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Ichikawa HT, Williams LP, Segal BM. Activation of APCs through CD40 or Toll-like receptor 9 overcomes tolerance and precipitates autoimmune disease. J Immunol. 2002;169:2781–2787. doi: 10.4049/jimmunol.169.5.2781. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. J Immunol. 2002;169:117–125. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J Immunol. 1999;163:2561–2567. [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere R, Malefyt W, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge KL, Gregg RK, Maldonado-Lopez R, Li L, Caprio JC, Moser M, Zaghouani H. On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity. J Exp Med. 2002;196:217–227. doi: 10.1084/jem.20011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Heath WR. Immunity or tolerance? That is the question for dendritic cells. Nat Immunol. 2001;2:988–989. doi: 10.1038/ni1101-988. [DOI] [PubMed] [Google Scholar]
- Martin E, O’Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity. 2003;18:155–167. doi: 10.1016/s1074-7613(02)00503-4. [DOI] [PubMed] [Google Scholar]