Abstract
Wegener’s granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome, and idiopathic pauci-immune necrotizing crescentic glomerulonephritis are associated with myeloperoxidase (MPO)-specific anti-neutrophil cytoplasmic autoantibodies (ANCAs). Clinical and experimental evidence indicates that ANCA and proinflammatory stimuli of infectious origin act synergistically to cause vasculitis. We tested this hypothesis in a recently developed mouse model of anti-MPO IgG-induced glomerulonephritis by using bacterial lipopolysaccharide (LPS) as the proinflammatory stimulus. Systemic administration of LPS dose dependently increased renal injury induced by anti-MPO IgG as demonstrated by increased glomerular crescent formation and glomerular necrosis. In the early phase, LPS enhanced anti-MPO IgG-induced glomerular neutrophil accumulation. Furthermore, a transient induction of circulating tumor necrosis factor (TNF)-α levels, followed by a marked increase in circulating MPO levels, was observed on administration of LPS. In vitro, anti-MPO IgG induced a respiratory burst in murine neutrophils only after priming with TNF-α. Finally, anti-TNF-α treatment attenuated, but did not prevent, the LPS-mediated aggravation of anti-MPO IgG-induced glomerulonephritis. In conclusion, our study demonstrates that ANCA and proinflammatory stimuli act synergistically to induce vasculitic disease and suggests potential benefits of inhibiting TNF-α bioactivity in treating human ANCA-associated necrotizing crescentic glomerulonephritis.
Wegener’s granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome, and idiopathic pauci-immune necrotizing crescentic glomerulonephritis (NCGN) are forms of small-vessel vasculitis of unknown etiology that are strongly associated with anti-neutrophil cytoplasmic autoantibodies (ANCAs).1 ANCAs comprise a group of autoantibodies directed against proteins contained in the lysosomal compartments of neutrophils and monocytes. The primary target antigens have been identified as proteinase 3 (Pr3), a 29-kd neutral serine proteinase, and myeloperoxidase (MPO), a 140-kd protein involved in the generation of reactive oxygen species.2 To date, detection of ANCAs has proven to be a helpful diagnostic tool and many clinical studies have confirmed that Pr3-ANCA and MPO-ANCA are highly specific for Wegener’s granulomatosis and microscopic polyangiitis, respectively.2
Since the discovery of ANCAs, numerous clinical and laboratory studies have been performed to determine whether ANCAs are directly involved in the pathogenesis of vasculitis and glomerulonephritis. The overall concept derived from these studies is that ANCA-induced vasculitis is a two-hit process in which ANCAs together with proinflammatory stimuli, most likely of infectious origin, are required for the induction of full-blown disease. In support of this model are clinical observations dem-onstrating that relapses in disease activity are often preceded by rising ANCA levels.3–5 Furthermore, the frequent observation of infectious episodes before diagnosis and/or relapse suggests that these may play a role in the pathogenesis of ANCA-associated disease.6–9 Consistent with these clinical observations are in vitro studies that have shown that ANCAs are capable of activating neutrophils and monocytes primed with proinflammatory cytokines, resulting in an oxidative burst, degranulation, production of cytokines, and endothelial cell damage.10–15
Recently, an experimental animal model of anti-MPO-induced NCGN was developed that involves the adoptive transfer of mouse MPO-reactive splenocytes into immune-deficient mice.16 These mice developed severe NCGN with pathological features that were remarkably similar to human anti-MPO-associated glomerulonephritis. In addition, it was demonstrated that passive transfer of murine anti-mouse MPO antibodies alone into either immune-deficient or wild-type (WT) mice also induces NCGN, although of a substantially milder form. Thus, the association between ANCAs, small vessel vasculitis, and infections suggests that, besides ANCAs, a second (nonspecific) proinflammatory signal is necessary to induce full-blown disease. To test this hypothesis, we used the experimental mouse model of anti-MPO antibody-induced NCGN and investigated the effects of bacterial lipopolysaccharide (LPS), as a model (pro-) inflammatory stimulus, on disease severity.
Materials and Methods
Mice
Mpo−/− mice, backcrossed to a C57BL/6 background for six times, were genotyped using polymerase chain reaction-amplified DNA from tail clippings.17 WT female C57BL/6 (8 to 12 weeks of age) were obtained from Harlan (Horst, The Netherlands). Mice were kept according to University of Maastricht animal facility regulations, and all experiments were approved by the local Animal Care and Experimentation Committee.
Purification of Murine MPO
Murine MPO (muMPO) was purified from WEHI-3, a myeloid cell line, that was grown in HEPES-buffered McCoy5A medium containing penicillin/streptomycin and 10% fetal calf serum. When a density of 1.5 × 106 cells/ml was reached, cells were harvested, resuspended in buffer A containing 6.7 mmol/L sodium acetate, 3.0 mmol/L MgCl2, 3.0 mmol/L NaCl, 0.5 mmol/L phenyl-methyl sulfonyl fluoride, and 1% CTAB, and lysed by Dounce homogenization on ice. After stirring (2 hours, 4°C), insoluble particles were removed by centrifugation (14,000 × g, 30 minutes, 4°C), and the supernatant was dialyzed overnight at 4°C against buffer B containing 100 mmol/L sodium acetate (pH 6.3) and 100 mmol/L NaCl. Next, CaCl2, MgCl2, and MnCl2 were added to a final concentration of 1 mmol/L each, and the solution was mixed with concanavalin A-Sepharose (Amersham Biosciences, Roosendaal, The Netherlands). After centrifugation and removal of supernatant, the concanavalin A was resuspended in several washes of buffer B with 750 mmol/L methyl α-d-mannopyranoside to elute the MPO (overnight, 4°C). The samples of buffer B containing MPO (as judged by OD at 428 nm) were then dialyzed against buffer C, containing 50 mmol/L sodium acetate (pH 8.5 to 9) and 100 mmol/L NaCl, loaded onto a Mono S cation exchange column (Bio-Rad, Veenendaal, The Netherlands), eluted with 1 mol/L NaCl, and dialyzed against phosphate-buffered saline (PBS). Purity was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Preparation of Pathogenic Mouse Anti-Murine MPO IgG and Control Mouse Anti-Bovine Serum Albumin (BSA) IgG
Mpo−/− mice were immunized as reported previously16 with minor modifications. Briefly, mice received an intraperitoneal injection of 10 μg of muMPO in complete Freund’s adjuvant (Difco, Detroit, MI) on day 0, followed by intraperitoneal booster injections in incomplete Freund’s adjuvant on days 21 and 36. On day 42, blood was obtained and mice were sacrificed. Antibody titers were monitored by enzyme-linked immunosorbent assay (ELISA) as described previously.16 In short, microtiter plates were coated overnight with muMPO (0.5 μg/ml) and blocked with BSA. Then plates were incubated with mouse sera (1:100 starting dilution), followed by incubation with alkaline phosphatase-conjugated goat anti-mouse IgG antibodies. 4-Nitrophenyl phosphate (pNPP) was used as substrate, and wells were analyzed spectrophotometrically at 405 nm.
To obtain anti-BSA IgG, a separate group of Mpo−/− mice was immunized with BSA following the protocol described above. Normal serum from C57BL/6 mice was obtained from Harlan. IgG was isolated from pooled sera by 50% ammonium sulfate precipitation followed by protein G column affinity chromatography. IgG-containing fractions were concentrated by ultrafiltration (Centriplus; Millipore, Amsterdam, The Netherlands) and dialyzed against PBS. Protein concentrations were determined using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL). The anti-MPO titer of each batch was checked by ELISA as described above, using a sample of anti-MPO IgG with previously established pathogenicity as a reference. Endotoxin concentrations were determined by the limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD) and depending on the isolation ranged from 0.04 ng/ml to 1.2 ng/ml.
Induction of Glomerulonephritis by Passive Transfer of Anti-MPO IgG
C57BL/6 mice received a dose of 100 μg/g body weight of sterile-filtered (0.2 μm filter; Schleicher & Schuell, Dassel, Germany) anti-MPO IgG by intraperitoneal injection. Where stated, groups of mice additionally received a single intraperitoneal injection of 5.0, 0.5, or 0.05 μg/g of LPS (Escherichia coli, serotype 026-B6, n = 4 to 5 in each group; Sigma, St. Louis, MO) dissolved in sterile PBS 1 hour after the administration of IgG. Control mice were injected with anti-BSA IgG (100 μg/g, n = 5) followed by 5.0 μg/g of LPS 1 hour later, or with LPS (5.0 μg/g, n = 4) alone. Circulating anti-MPO IgG was monitored by ELISA as described above, using a serum pool from MPO-immunized Mpo−/− mice as reference. Results are expressed as a percentage of the absorbance units (OD 405 nm) of a positive control pool serum.
Measurement of Serum MPO and Tumor Necrosis Factor (TNF)-α Levels
In a separate experiment, the effect of intraperitoneal injection of LPS on serum TNF-α and MPO levels was determined. Blood was taken from mice 1 week before, 1 hour after, and 1 and 6 days after the administration of 0.05, 0.5, or 5.0 μg/g LPS (n = 4 in each group) in endotoxin-free 0.9% saline. Samples were centrifuged and serum was taken and stored at −20°C. To detect circulating MPO, we generated a mouse anti-mouse MPO monoclonal antibody (mAb) from splenocytes obtained from muMPO-immunized Mpo−/− mice using standard procedures. The resulting mAb (IgG1, designated 8F4) recognized murine MPO and cross-reacted with rat but not human MPO as determined by direct ELISA and immunohistochemistry on normal spleen sections (data not shown). Using this antibody, a catching ELISA was developed for the detection of murine MPO in sera as follows. Microtiter plates were coated with Fcγ fragment-specific goat anti-mouse IgG (3.2 μg/ml, 100 μl/well; Jackson ImmunoResearch), incubated for 48 hours at 4°C, and blocked with 1% BSA in PBS for 30 minutes at room temperature. Plates were then incubated with 8F4 (1.0 μg/ml, 100 μl/well) for 1 hour at room temperature. Next, plates were incubated with appropriately diluted serum samples for 1 hour at room temperature, followed by incubation with polyclonal rabbit anti-human MPO (14 μg/ml; DakoCytomation, Glostrup, Denmark) for 1 hour at room temperature and incubation with alkaline phosphatase-labeled polyclonal goat anti-rabbit IgG for 1 hour at room temperature. Finally, plates were incubated with pNPP for 30 minutes, and results were analyzed spectrophotometrically at 405 nm. Concentrations were calculated from a standard curve of purified murine MPO (range, 2.5 to 100 ng/ml).
TNF-α levels were determined by capture ELISA as described previously.18 Briefly, plates were coated overnight with TN3 (5 μg/ml). After blocking with BSA, samples were added to the plate for 1 hour at room temperature. Plates were then incubated for 1 hour with rabbit anti-mouse TNF polyclonal antibody followed by incubation with peroxidase-labeled goat anti-rabbit IgG for 1 hour. The assay was developed with O-phenylene diamine, the reaction was stopped with 4 N H2SO4, and the OD was measured at 490 nm. Concentrations were calculated from a standard curve of recombinant murine TNF-α (range, 0.02 to 40 ng/ml).
Anti-TNF-α Treatment
To investigate the effects of TNF-α depletion on disease induction, groups of mice received a single intraperitoneal dose of the anti-murine TNF-α antibody TN3 (500 μg/mouse, endotoxin concentration <10 pg/ml), a complementarity-determining regions-grafted murine IgG2a (n = 8; kindly provided by Celltech, Slough, UK) or isotype control (mAb L2-3D9, endotoxin concentration <10 pg/ml, n = 7),19,20 in sterile PBS, 24 hours before anti-MPO IgG and LPS (0.5 μg/g) administration. When given 24 hours in advance, this dose of TN3 completely inhibited TNF-α activity in sera of mice taken 1 hour after intraperitoneal injection of 0.5 μg/g LPS, as determined by cytotoxicity assay using the murine fibrosarcoma cell line WEHI 164 as described previously.21 All mice were sacrificed 6 days after disease induction.
Laboratory and Pathological Evaluation of Disease Induction
At the times indicated, mice were bled and sacrificed. Urine samples were tested by dipstick (Bayer, Mijdrecht, The Netherlands) for hematuria, proteinuria, and leukocyturia and scored on a 0 to 4+ scale. Blood urea nitrogen and creatinine levels were determined in sera collected at the time of sacrifice by an enzymatic degradation assay on a Synchron LX20 PRO (Beckman Coulter Inc., Fullerton, CA). Tissue samples were taken from both kidneys and processed for light microscopy, immunofluorescence, and immunohistochemistry. For light microscopy, renal tissue samples were fixed in 4% formaldehyde and embedded in paraffin. Sections (1.5 μm) were cut and hematoxylin and eosin (H&E) and periodic acid-Schiff staining were performed. For each animal, a crescent score was determined by evaluating crescent formation in 50 consecutive glomerular cross sections. Only glomeruli that had two or more layers of cells in Bowman’s space were considered crescentic. Similarly, a glomerular necrosis score was determined for each animal by evaluating segmental or global glomerular capillary necrosis in 50 consecutive glomerular cross sections. Analysis was performed in a blinded manner using coded slides.
Phenotypic analysis of the inflammatory cell infiltrate was performed on 4-μm cryostat sections fixed in 100% acetone at room temperature. The following primary antibodies were used: rat anti-mouse CD45, rat anti-mouse neutrophils (clone NIMP-R14),22 rat anti-mouse CD68 (macrophages, clone FA11),23 and rat anti-mouse CD3 (clone KT3). Endogenous peroxidase activity was blocked with 0.05% H2O2 in PBS. Rabbit anti-rat IgG-PO and goat anti-rabbit IgG-PO (both DakoCytomation) were used as secondary and tertiary antibodies, respectively. Antibody binding was visualized using 3-amino-9-ethylcarbazole and H2O2 as substrates. Sections were counterstained with hematoxylin. Glomerular cell infiltrates were determined by counting the number of positive cells in 30 glomerular cross sections per kidney section. In experiments in which effects of LPS on early (day 1) neutrophil recruitment were studied the number of glomeruli containing neutrophil aggregates was also scored. An aggregate was defined as a homotypic aggregate of three or more neutrophils and was evaluated in 30 glomerular cross sections.
Mouse IgG and fibrin deposits were detected by immunofluorescence using rabbit anti-mouse IgG-Alexa Fluor 488 (Molecular Probes, Leiden, The Netherlands) and rabbit anti-human fibrinogen-fluorescein isothiocyanate (DakoCytomation). MPO deposits were detected using biotinylated mouse anti-mouse MPO (8F4) followed by streptavidin-Alexa 488 (Molecular Probes). Endogenous avidin and biotin were blocked using a streptavidin/biotin blocking kit (Vector Laboratories, Burlingame, CA).
Superoxide Anion Assay
To determine the in vitro capacity of polyclonal mouse anti-murine MPO antibodies to induce an oxidative burst in murine neutrophils, we used the superoxide dismutase inhibitable (SOD) ferricytochrome C assay.24 Peritoneal exudate cells (PECs) were obtained by flushing of the peritoneal cavities of three to four WT or Mpo−/− mice with sterile Hanks’ balanced salt solution (HBSS) 4 hours after intraperitoneal administration of 1 ml of 3% thioglycollate. As determined on May Grünwald Giemsa-stained cytospots, the percentage of neutrophils in these cell preparations was ≥55%. Cells were centrifuged and resuspended at a concentration of 2 × 106 cells/ml in HBSS. The remainder of the procedure was performed at 37°C. Cells were incubated with cytochalasin B (5 μg/ml; Serva, Heidelberg, Germany) for 5 minutes, followed by incubation with recombinant murine TNF-α (10 ng/ml; R&D, Abingdon, UK) or an equal volume of HBSS for 10 minutes. Next, 96-well microtiter plates were incubated with 200 μl of cell suspension (4 × 105 cells/well), ferricytochrome C (70 μmol/L; Sigma, Zwijndrecht, The Netherlands), stimulus, and either SOD (42 U/ml, Sigma) or an equal volume of HBSS. As stimulus, 250 μg/ml of anti-muMPO IgG or normal mouse IgG was used. Phorbol ester myristate acetate (200 ng/ml, Sigma) served as a positive control. Optical density at 550 nm was measured every 15 minutes for 60 minutes and SOD-inhibitable production of superoxide was determined as ΔOD at 550 nm. Each test was performed in triplicate and experiments were repeated three (Mpo−/− cells) to four (WT cells) times.
Statistical Analysis
Data are expressed as means ± SD and were analyzed using the unpaired two-tailed Student’s t-test using Graphpad Prism 4.01 for Windows (Graphpad Software, San Diego CA). For semiquantitative data from dipstick analysis, a ranked analysis of variance test was used to evaluate differences across all groups. When significant differences were detected with analysis of variance, the Duncan test was used to evaluate statistical differences between specific groups. A P value ≤0.05 was considered statistically significant.
Results
Development of Circulating MPO-ANCA and Urinary Abnormalities after Passive Transfer of Anti-MPO IgG
On passive transfer of anti-MPO IgG, circulating anti-MPO antibodies were detected in all subjected mice. The level of circulating anti-MPO was highest on day 1 and had declined at the time of sacrifice (day 6). On day 1, no differences were detected in levels of circulating anti-MPO antibodies between the experimental groups (Table 1). However, on day 6, mice that received the highest dose of LPS (5 μg/g) had a significantly lower anti-MPO titer compared to mice receiving anti-MPO alone (Table 1). No anti-MPO reactivity was detected in sera from mice treated with anti-BSA IgG and LPS (0.5 μg/g) or LPS alone.
Table 1.
Anti-MPO Antibody Titers after Passive Transfer of Anti-MPO IgG
| Groups | n | Anti-MPO antibody titer (%AU (OD 405 nm) of positive control)
|
|
|---|---|---|---|
| Day 1 | Day 6 | ||
| Anti-MPO | 5 | 85.2 ± 10.0 | 54.4 ± 12.2 |
| Anti-MPO + 5 μg/g LPS | 5 | 77.4 ± 13.5 | 38.2 ± 8.1* |
| Anti-MPO + 0.5 μg/g LPS | 5 | 83.6 ± 2.7 | 45.0 ± 16.2 |
| Anti-MPO + 0.05 μg/g LPS | 4 | 82.5 ± 3.1 | 59.0 ± 6.8 |
| Anti-BSA + LPS 5 μg/g | 5 | 0 | 0 |
P < 0.04 compared to anti-MPO alone.
By day 1, hematuria had developed in all mice that received anti-MPO antibodies with or without LPS, which persisted until the time of sacrifice at day 6 (Table 2). Most of these mice also had proteinuria and leukocyturia at day 6. Hematuria and leukocyturia measured at day 6 tended to be more severe in mice treated with anti-MPO IgG and 5.0 μg/g or 0.5 μg/g LPS, than in mice treated with anti-MPO IgG alone (Table 2). At day 6, serum creatinine and blood urea nitrogen levels were within normal range in all mice. No urinary abnormalities above background could be detected in mice treated with anti-BSA and 5.0 μg/g LPS, or with LPS alone (Table 2).
Table 2.
Urinalysis and Renal Function at 6 Days after Disease Induction
| Groups | n | Hematuria | Proteinuria | Leukocyturia | Creatinine (μmol/L) | BUN (mmol/L) |
|---|---|---|---|---|---|---|
| Anti-MPO | 5 | 2.7 | 2.8 | 0.8 | 15.6 ± 7.0 | 8.1 ± 2.9 |
| (1–3) | (2–4) | (0–2) | ||||
| Anti-MPO + 5 μg/g LPS | 5 | 3.4* | 2.6 | 1.4 | 19.2 ± 2.6 | 8.0 ± 0.9 |
| (3–4) | (2–3) | (0–2) | ||||
| Anti-MPO + 0.5 μg/g LPS | 5 | 3.8* | 3.2 | 2.6† | 18.2 ± 2.4 | 7.5 ± 1.8 |
| (3–4) | (2–4) | (2–3) | ||||
| Anti-MPO + 0.05 μg/g LPS | 4 | 2.3 | 2.3 | 1.0 | 21.3 ± 2.6 | 7.0 ± 1.6 |
| (1–3) | (1–3) | (1–2) | ||||
| Anti-BSA + LPS 5 μg/g | 5 | 0 | 1.4 | 0 | 18.5 ± 2.4 | 8.6 ± 1.1 |
| (1–2) |
Hematuria, proteinuria, and leukocyturia were tested by dipstick and scored on 0 to 4+ scale. Values represent mean scores per group. Samples taken before antibody injection showed mean proteinuria 1.0+, hematuria 0+, and leukocyturia 0+.
P < 0.005.
P < 0.008.
LPS Aggravates Anti-MPO IgG-Induced NCGN
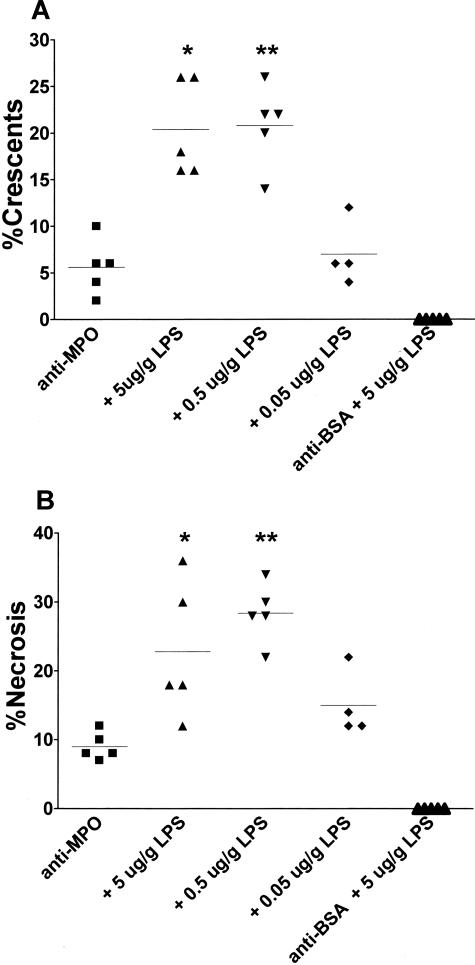
Intraperitoneal administration of anti-MPO IgG into WT mice induced a focal and segmental NCGN in all subjected mice at day 6, with a mean of 5.6% (range, 2 to 10%) crescentic and 9.0% (range, 7 to 12%) necrotic glomeruli (Figures 1 and 2). Administration of 5.0 or 0.5 μg/g LPS 1 hour after administration of anti-MPO IgG resulted in a marked increase in the severity of glomerulonephritis, as judged by the percentage of glomeruli containing crescents or fibrinoid necrosis. Addition of 5.0 μg/g LPS resulted in 20.4% (range, 16 to 26%) crescentic and 22.8% (range, 12 to 36%) necrotic glomeruli, whereas addition of 0.5 μg/g LPS resulted in 20.8% (range, 14 to 26%) crescentic and 28.4% (range, 22 to 34%) necrotic glomeruli (Figures 1 and 2). Addition of 0.05 μg/g LPS did not lead to more severe disease compared to anti-MPO IgG alone, and administration of 100 μg/g anti-BSA IgG and 5.0 μg/g LPS, or LPS alone, did not result in any histological abnormalities (Figures 1 and 2).
Figure 1.
Effect of LPS on pathological findings in C57BL/6 mice 6 days after passive transfer of anti-MPO IgG. A: Glomerular crescent formation expressed as a percentage of glomerular crescents in each animal. *P = 0.0005, **P = 0.0002 compared to anti-MPO IgG alone. B: Glomerular necrosis expressed as a percentage of glomeruli with necrosis in each animal. Horizontal lines represent mean percentages in each group. *P = 0.02, **P = 0.001 compared to anti-MPO IgG alone.
Figure 2.
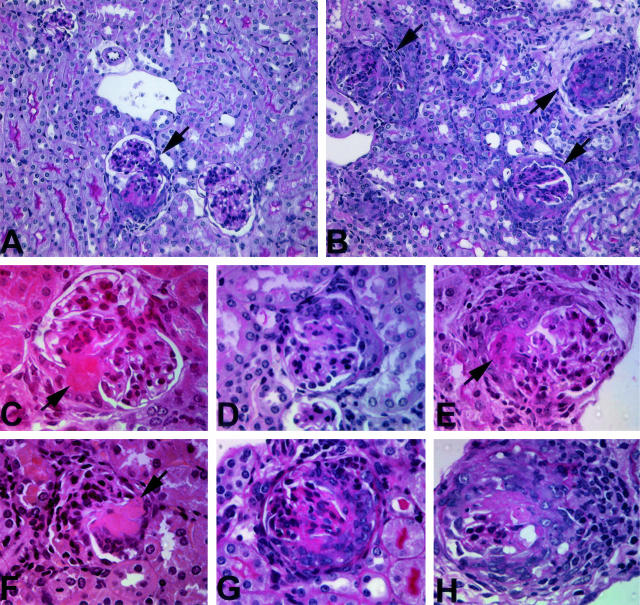
Effect of LPS on renal tissue injury in C57BL/6 mice 6 days after passive transfer of anti-MPO IgG. A: Overview of renal tissue of a mouse that received anti-MPO IgG. One small crescentic glomerulus is shown (arrow). B: Overview of renal tissue in a mouse that received anti-MPO IgG and 0.5 μg/g LPS. Increased numbers of crescentic glomeruli are observed (arrows). C: Segmental fibrinoid necrosis in a glomerulus from a mouse that received anti-MPO IgG. D: Small cellular crescent in a mouse that received anti-MPO IgG. E: Glomerulus with segmental fibrinoid necrosis and crescent formation in a mouse that received anti-MPO IgG. F: Segmental fibrinoid necrosis in a glomerulus from a mouse that received anti-MPO IgG and 0.5 μg/g LPS. G: Large cellular crescent in mouse that received anti-MPO IgG and 0.5 μg/g LPS. H: Large cellular crescent with breaks in Bowman’s capsule and periglomerular accumulation of inflammatory cells in a mouse that received anti-MPO IgG and 0.5 μg/g LPS. A, B, D, E, G, H: PAS stain; C, F: H&E stain. Original magnifications: ×320 (A, B); ×400 (C–H).
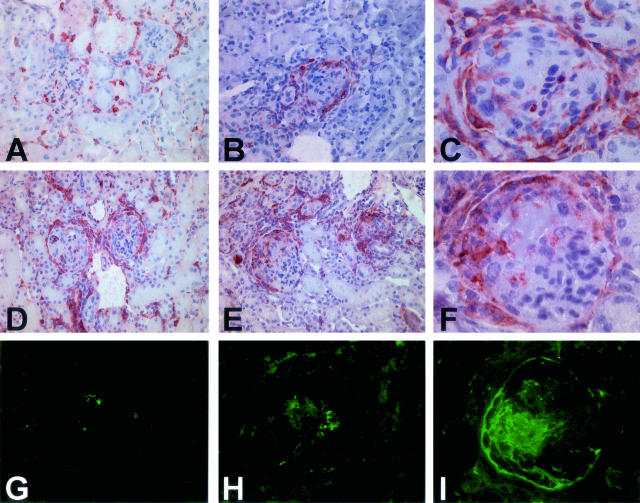
Phenotypic analysis of the inflammatory cell infiltrate 6 days after administration of anti-MPO IgG demonstrated intraglomerular influx of CD45+ve leukocytes, the majority of which were found to be FA11+ macrophages (Figure 3, A and B; Table 3). A further increase in the number of glomerular infiltrating CD45+ leukocytes and FA11+ macrophages was observed on addition of 5.0 or 0.5 μg/g LPS (Figure 3, C to F; Table 3). At this time point, numbers of intraglomerular CD3+ T cells and neutrophils were only slightly elevated in anti-MPO-treated mice with or without LPS as compared to nontreated C57BL/6 mice or mice that had received LPS (5.0 μg/g) alone. Immunofluorescence revealed marked focal and segmental glomerular deposition of fibrin in anti-MPO-treated mice with or without LPS at day 6 that corresponded with sites of segmental necrosis or crescent formation (Figure 3I). In contrast, on day 6 glomerular staining for MPO and IgG was absent or scanty (Figure 3, G and H).
Figure 3.
Effect of LPS on inflammatory cell recruitment in C57BL/6 mice 6 days after passive transfer of anti-MPO IgG. A: Moderate intraglomerular and interstitial infiltration of CD45+ve leukocytes in a mouse that received anti-MPO IgG. B: Intra- and periglomerular influx of FA11+ve macrophages in a mouse that received anti-MPO IgG. C and D: Marked intra- and periglomerular and interstitial influx of CD45+ve leukocytes in a mouse that received anti-MPO IgG and 0.5 μg/g LPS. E and F: Marked intra- and periglomerular and interstitial influx of FA11+ve macrophages in a mouse that received anti-MPO IgG and 0.5 μg/g LPS. G: Immunofluorescent staining for MPO in a mouse that received anti-MPO IgG and 0.5 μg/g LPS. H: Immunofluorescent staining for mouse IgG in a mouse that received anti-MPO IgG and 0.5 μg/g LPS. I: Immunofluorescent staining for fibrin in a mouse that received anti-MPO IgG and 0.5 μg/g LPS showing prominent staining corresponding to crescent formation. Original magnifications: ×200 (A, B, D, E); ×630 (C, F); ×400 (G–I).
Table 3.
Immunophenotyping of Intraglomerular Inflammatory Cell Infiltrate at 6 Days after Disease Induction
| Groups | n | CD45+ leukocytes | FA11+ macrophages | CD3+ T cells | PMNs |
|---|---|---|---|---|---|
| Anti-MPO | 5 | 1.82 ± 0.19 | 1.34 ± 0.13 | 0.44 ± 0.20 | 0.39 ± 0.10 |
| Anti-MPO + 5 μg/g LPS | 5 | 3.72 ± 0.51* | 2.24 ± 0.07* | 0.55 ± 0.14 | 0.48 ± 0.19 |
| Anti-MPO + 0.5 μg/g LPS | 5 | 3.56 ± 0.41* | 2.45 ± 0.19* | 0.43 ± 0.14 | 0.39 ± 0.10 |
| Anti-MPO + 0.05 μg/g LPS | 4 | 1.95 ± 0.14 | 1.48 ± 0.22 | 0.23 ± 0.03 | 0.21 ± 0.07 |
| LPS 5 μg/g | 4 | 1.08 ± 0.10 | 0.44 ± 0.15 | 0.31 ± 0.13 | 0.22 ± 0.08 |
| WT | 4 | 0.77 ± 0.26 | 0.36 ± 0.20 | 0.17 ± 0.06 | 0.09 ± 0.07 |
P < 0.0001 compared to anti-MPO alone. Numbers represent mean numbers ± SD of positive cells per glomerular cross section.
LPS Enhances Anti-MPO IgG-Induced Early Neutrophil Recruitment
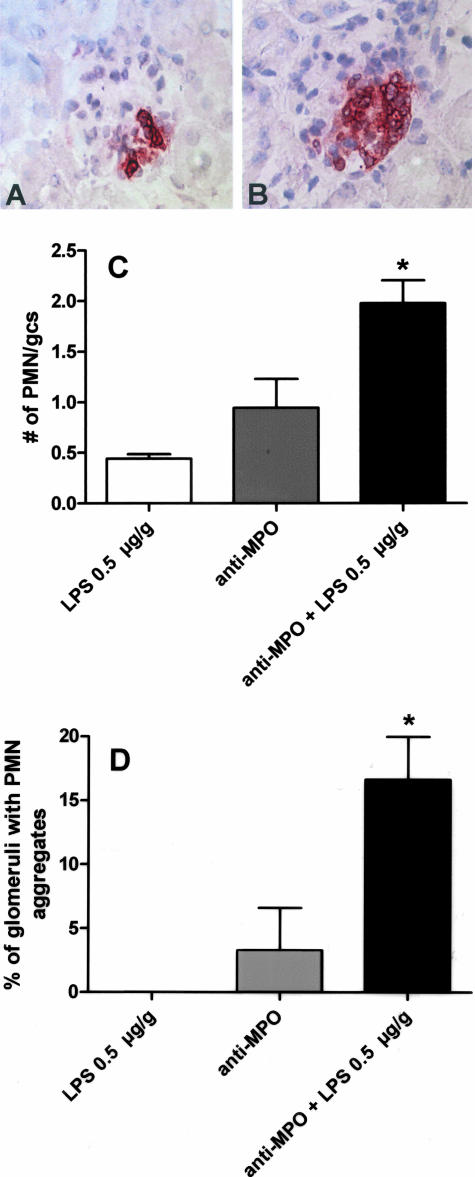
At day 1, anti-MPO IgG-treated mice showed increased glomerular influx of neutrophils that occasionally appeared as small neutrophilic aggregates (Figure 4B). This early glomerular influx of neutrophils was markedly enhanced on addition of 0.5 μg/g LPS resulting in large neutrophilic aggregates (Figure 4C). In contrast, injection of 0.5 μg/g LPS alone induced only a slight increase in the number of intraglomerular neutrophils, whereas glomerular neutrophil aggregation was not observed (Figure 4).
Figure 4.
Effect of LPS on neutrophil influx and aggregation in C57BL/6 mice 1 day after passive transfer of anti-MPO IgG. A: Glomerulus from a mouse that received anti-MPO IgG showing segmental neutrophil influx and formation of a small neutrophilic aggregate. B: Glomerulus from a mouse that received anti-MPO and 0.5 μg/g LPS showing massive neutrophil influx and homotypic aggregation. C and D: Quantification of glomerular neutrophil influx (C) and the percentage of glomeruli that contained homotypic neutrophil aggregates (D) in mice (n = 3 in each group) that received LPS (0.5 μg/g) alone, anti-MPO IgG alone, or anti-MPO and LPS (0.5 μg/g). gcs, glomerular cross-section. *P = 0.008. Original magnifications, ×400 (A and B).
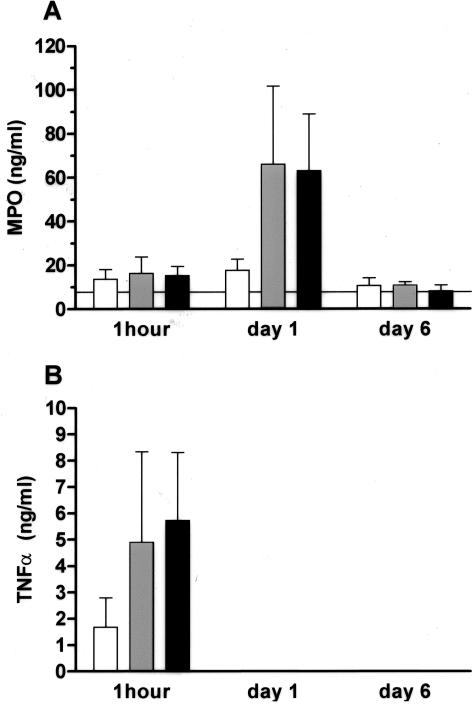
LPS Increases Serum MPO and TNF-α Levels
In a separate experiment, the effect of a single dose of 5.0, 0.5, or 0.05 μg/g LPS on serum MPO and TNF-α levels was determined. As shown in Figure 5, intraperitoneal administration of 5.0, 0.5, and 0.05 μg/g LPS led to a rise in serum TNF-α levels 1 hour after injection. In case of the high (5.0 μg/g) and medium (0.5 μg/g) dose of LPS, this was followed by a rise in serum MPO levels at day 1. TNF-α levels were undetectable at day 1 and day 6 after administration, whereas at the latter time point MPO levels had returned to baseline. Administration of saline alone did not lead to alterations in TNF-α and MPO levels (data not shown).
Figure 5.
Serum MPO and TNF-α levels in C57BL/6 mice that received 0.05 (white bars), 0.5 (gray bars), or 5 μg/g (black bars) of LPS by intraperitoneal injection. The horizontal line indicates the mean serum MPO levels in serum samples taken before treatment. In these pretreatment samples, no TNF-α could be detected.
Pretreatment with Anti-TNF-α Antibody Attenuates Glomerulonephritis Induced by Anti-MPO IgG and LPS
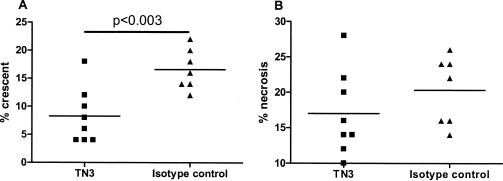
To investigate the involvement of TNF-α in the LPS-mediated aggravation of anti-MPO IgG-induced glomerulonephritis, a neutralizing anti-murine TNF-α antibody (TN3) was used. In these experiments, mice received an intraperitoneal injection of 500 μg TN3 or isotype control on day −1, followed by administration of anti-MPO IgG and 0.5 μg/g LPS on day 0. In both groups, mice developed hematuria and leukocyturia to a similar degree as measured by dipstick analysis. However, pathological analysis at day 6 revealed that pretreatment with TN3 significantly attenuated glomerular crescent formation, whereas no difference was found in the extent of glomerular necrosis (Figure 6). In both groups, renal function on day 6 was unimpaired (blood urea nitrogen 7.8 ± 1.4 versus 7.4 ± 2.1 and creatinine 19.± 4.1 versus 19.3 ± 1.9 in TN3 and isotype control antibody-treated mice, respectively). Immunohistochemical analysis showed that pretreatment with TN3 significantly attenuated glomerular influx of CD45+ total leukocytes and FA11+ macrophages (Table 4). No differences were observed in intraglomerular numbers of neutrophils and T cells (Table 4). By immunofluorescence, glomerular IgG staining was absent or scanty in both groups.
Figure 6.
Effect of anti-TNF-α treatment on NCGN induced by anti-MPO IgG and LPS (0.5 μg/g). A: Quantification of glomerular crescent formation on anti-TNF-α or isotype control treatment. Glomerular crescent formation is expressed as a percentage of glomerular crescents in each animal. B: Quantification of glomerular necrosis on anti-TNF-α or isotype control treatment. Glomerular necrosis is expressed as a percentage of glomeruli with necrosis in each animal.
Table 4.
Immunophenotyping of Intraglomerular Inflammatory Cell Infiltrate at 6 Days after Anti-TNF-α Antibody or Isotype Control Antibody (L2-3D9) Treatment
| Groups | n | CD45+ Leukocytes | FA11+ macrophages | CD3+ T cells | PMNs |
|---|---|---|---|---|---|
| Anti-MPO + 0.5 μg/g LPS + L2-3D9 | 8 | 3.03 ± 0.52* | 2.26 ± 0.62* | 0.33 ± 0.14 | 0.37 ± 0.10 |
| Anti-MPO + 0.5 μg/g LPS + TN3 | 7 | 2.23 ± 0.41 | 1.53 ± 0.29 | 0.37 ± 0.08 | 0.35 ± 0.14 |
P < 0.005 compared to anti-MPO + 0.5 μg/g LPS + TN3. Numbers represent mean numbers ± SD of positive cells per glomerular cross section.
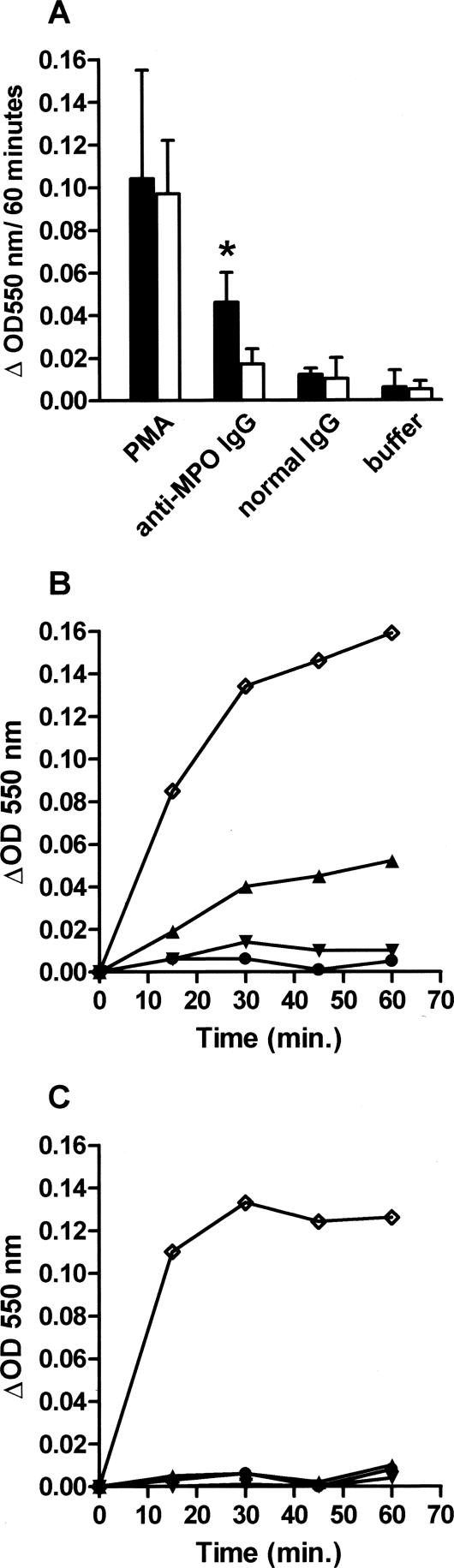
Anti-MPO IgG Induces Superoxide Anion Production in TNF-α-Primed Peritoneal Exudate Cells in Vitro
The capacity of anti-MPO antibodies to induce neutrophil activation in vitro was determined using the SOD inhibitable ferricytochrome C assay. Incubation of unprimed peritoneal exudate cells with normal mouse IgG or anti-MPO IgG did not induce superoxide production above background (data not shown). However, when peritoneal exudate cells were pretreated with 10 ng/ml of murine TNF-α for 10 minutes, incubation with anti-MPO IgG resulted in a modest but significant increase in superoxide production as compared to normal mouse IgG or buffer alone (Figure 7, A and B). Importantly, anti-MPO IgG did not induce superoxide anion production in TNF-α-primed, MPO-deficient peritoneal exudate cells (Figure 7, A and C). Phorbol ester myristate acetate-induced superoxide anion production in these cells was, however, similar to that observed in WT peritoneal exudate cells (Figure 7A).
Figure 7.
A: Superoxide anion production in TNF-α (10 ng/ml)-primed peritoneal exudate cells (4 × 105 cells/well) derived from WT C57BL/6 or Mpo−/− mice as measured by the SOD inhibitable ferricytochrome C assay. Anti-MPO IgG (250 μg/ml) induces significant superoxide anion production in TNF-α-primed peritoneal exudate cells from WT C57BL/6 mice (black bars) as compared to normal mouse IgG (250 μg/ml) or buffer alone. In contrast, no superoxide anion production was detected in TNF-α-primed peritoneal exudate cells from MPO−/− mice (white bars) on stimulation with anti-MPO IgG. Results represent mean ΔOD 550 nm ± SD of three to four experiments performed in triplicate. *P = 0.02 compared to anti-MPO IgG on MPO-deficient cells. B and C: Time course of superoxide anion production in response to phorbol ester myristate acetate (⋄, 200 ng/ml), anti-MPO IgG (▴, 250 μg/ml), normal mouse IgG (▾, 250 μg/ml), or buffer alone (•) using TNF-α-primed peritoneal exudate cells from WT C57BL6 (B) or Mpo−/− mice (C). Results represent mean ΔOD 550 nm from single experiments performed in triplicate.
Discussion
In the present study, three main findings are reported that are relevant to the pathogenesis of MPO-ANCA-induced glomerulonephritis. First, it was demonstrated that systemic administration of bacterial LPS markedly aggravates anti-MPO-induced NCGN. This effect was dependent on the dose of LPS administered and appeared to be related to LPS-induced circulating levels of TNF-α and MPO. Second, LPS-mediated aggravation of anti-MPO IgG-induced glomerulonephritis was attenuated, but not prevented, by pretreatment with neutralizing anti-TNF-α antibodies. Finally, in vitro experiments showed that murine anti-MPO IgG induces an oxidative burst in TNF-α-primed peritoneal exudate cells, an observation that is consistent with human data showing ANCA mediated neutrophil activation.
In our studies, glomerulonephritis induction by anti-MPO IgG alone is less severe compared to similar groups of mice reported in the companion paper by Xiao and colleagues. The most plausible explanation for this difference in disease severity is the route of injection of the anti-MPO antibodies. In our studies, anti-MPO antibodies were injected intraperitoneally whereas in the companion article the antibodies were administered intravenously. This could potentially lead to different levels of circulating anti-MPO antibodies but we have not formally tested this. Another explanation may be that the antibody batches used in these two studies differed in the amount of pathogenic anti-MPO antibodies. Importantly, however, in both studies, transfer of anti-MPO antibodies induced crescentic glomerulonephritis in all of the tested mice, which emphasizes the pathogenic potential of these autoantibodies. Our observation that LPS dose dependently aggravates anti-MPO-induced glomerulonephritis provides support for the hypothesis that ANCAs and proinflammatory stimuli act synergistically in inducing full-blown disease in humans. With respect to the mechanisms underlying this effect several possibilities may be considered and will be discussed below.
First, as shown here and by many others, systemic administration of LPS results in an immediate, transient increase in serum TNF-α levels. From in vitro experiments on human polymorphonuclear leukocytes, it is well known that priming with TNF-α is necessary for the induction of a respiratory burst by sera or purified IgG from ANCA-positive patients.10–15 We confirmed this in our mouse model by showing that anti-MPO IgG induced an oxidative burst in peritoneal exudate cells only after pretreatment with TNF-α, and only when those cells were obtained from MPO-competent mice. Second, we observed that administration of LPS synergistically increased anti-MPO IgG-induced early glomerular neutrophil recruitment. The number of infiltrating polymorphonuclear leukocytes was significantly increased in anti-MPO-treated mice with or without LPS that were sacrificed 1 day after disease induction. These results suggest an important role for polymorphonuclear leukocytes in the initiation of anti-MPO IgG-induced disease. The number of lesional polymorphonuclear leukocytes had decreased on day 6, which most likely is a reflection of the progression of the inflammatory response that is dominated by macrophages. This is in line with in vitro data, demonstrating an important role for priming of endothelial cells with TNF-α25 or LPS26 in anti-MPO IgG-induced neutrophil adhesion. Subsequently, these adherent neutrophils may become activated by MPO-ANCA causing endothelial cell injury.27 Despite aggravation of renal injury by LPS as observed by histopathological analysis, renal function in these mice was not impaired. Apparently, the number of affected glomeruli was not sufficient to cause elevations in serum creatinine and blood urea nitrogen levels.
The requirement for TNF-α priming in ANCA-mediated neutrophil activation is in part explained by the observation that primed neutrophils translocate the ANCA antigens to their cell surface making them accessible for interaction with the autoantibodies. In the case of MPO-ANCA, studies have clearly demonstrated that expression of MPO is essential for MPO-ANCA-mediated neutrophil activation because neutrophils obtained from MPO-deficient individuals are nonresponsive.28 However, inconsistent data exist on whether MPO-ANCA-mediated neutrophil activation involves MPO expressed on the cell surface of primed neutrophils. Whereas some authors describe an enhanced surface expression of MPO on primed neutrophils, others have been unable to demonstrate this effect.10,12,13,15 In preliminary experiments using flow cytometry, we have not been able to convincingly demonstrate surface MPO expression on either peripheral neutrophils from LPS-treated mice or peritoneal exudate cells treated with LPS in vitro. One possibility is that priming induces cell surface MPO expression in minute amounts that are below the detection limits of our assay system but this clearly needs further investigation. Alternatively, other mechanisms may be involved in MPO-ANCA-mediated neutrophil activation. Studies by Hess and colleagues10 have shown that resting human neutrophils exposed to supernatants of degranulated autologous neutrophils expressed MPO, but not Pr3, on their cell surface and became responsive to anti-MPO autoantibodies. Interestingly, high levels of circulating MPO correlate with disease severity in human MPO-ANCA-associated glomerulonephritis.8 In the present study, we found that doses of LPS that aggravated anti-MPO IgG-induced glomerulonephritis also gave rise to increased levels of circulating MPO. Based on these observations we speculate that circulating MPO, in the presence of high levels of anti-MPO antibodies, may disperse MPO-ANCA-mediated activation to resting neutrophils resulting in amplification of the inflammatory response.
As described above, ANCA-induced neutrophil activation is enhanced on priming with proinflammatory stimuli, in particular TNF-α. This is a potent proinflammatory cytokine produced by many cell types, and there is mounting evidence that TNF-α plays a major role in glomerular inflammation and scarring.29 The actions of TNF-α are numerous and include the stimulation of release of other cytokines and chemokines, and the induction of adhesion molecule expression on the endothelium.30 To directly investigate the contribution of TNF-α on the LPS-mediated aggravation of anti-MPO IgG-induced glomerulonephritis, we treated mice with a neutralizing anti-TNF-α antibody before disease induction. It was found that TNF-α -inhibition reduced glomerular crescent formation whereas development of glomerular necrosis was unaffected. These results are in agreement with studies in TNF-α-deficient mice, in which the development of accelerated anti-glomerular basement antibody (GBM) glomerulonephritis is also attenuated but not completely prevented.31 On the other hand, complete prevention of glomerular crescent formation has been observed in a rat model of anti-GBM glomerulonephritis on treatment with the soluble TNF-α receptor P55.32 Our observations suggest that, as yet unknown, TNF-α-independent effects also play a role in the LPS-mediated aggravation of anti-MPO IgG-induced glomerulonephritis but this clearly needs to be investigated in more detail.
In conclusion, the results of the present study are in agreement with the hypothesis that in ANCA-associated diseases, autoantibody-mediated effects and proinflammatory signals synergize in causing vascular inflammation by promoting neutrophil adhesion to the endothelium and enhancing neutrophil activation. Furthermore, our findings indicate that therapeutic strategies aimed at neutralizing TNF-α bioactivity could be beneficial in the treatment of ANCA-associated diseases. In fact, such clinical studies have already been initiated and in a first preliminary publication, promising results have been reported.33
Footnotes
Address reprint requests to P. Heeringa, Department of Clinical and Experimental Immunology, Cardiovascular Research Institute Maastricht, University Maastricht, PO Box 616, 6200 MD Maastricht, The Netherlands. E-mail: p.heeringa@immuno.unimaas.nl.
Supported by the Dutch Kidney Foundation (grant C01.1927 to D.H., A.V.E., J.W.C.T., P.H.) and the National Institutes of Health (grant NIDDK PO1 DK58335 to H.X. and J.C.J.).
References
- Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- Kallenberg CG, Brouwer E, Weening JJ, Tervaert JW. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- Han WK, Choi HK, Roth RM, McCluskey RT, Niles JL. Serial ANCA titers: useful tool for prevention of relapses in ANCA-associated vasculitis. Kidney Int. 2003;63:1079–1085. doi: 10.1046/j.1523-1755.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- Boomsma MM, Stegeman CA, van der Leij MJ, Oost W, Hermans J, Kallenberg CG, Limburg PC, Tervaert JW. Prediction of relapses in Wegener’s granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43:2025–2033. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tervaert JW, van der Woude FJ, Fauci AS, Ambrus JL, Velosa J, Keane WF, Meijer S, van der Giessen M, van der Hem GK, The TH, Kallenberg GCM. Association between active Wegener’s granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–2465. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Hogan S, Carey TS, Jennette JC. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The Glomerular Disease Collaborative Network. Ann Intern Med. 1990;113:656–663. doi: 10.7326/0003-4819-113-9-656. [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Rees AJ, Pussell BA, Lockwood CM, Mitchison RS, Peters DK. Relapses in Wegener’s granulomatosis: the role of infection. Br Med J. 1980;281:836–838. doi: 10.1136/bmj.281.6244.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura Y, Minoshima S, Kamiya Y, Tanaka U, Nakabayashi K, Kitamoto K, Nagasawa T, Sasaki T, Suzuki K. Serum myeloperoxidase and serum cytokines in anti-myeloperoxidase antibody-associated glomerulonephritis. Clin Nephrol. 1993;40:256–264. [PubMed] [Google Scholar]
- Capizzi SA, Specks U. Does infection play a role in the pathogenesis of pulmonary vasculitis? Semin Respir Infect. 2003;18:17–22. doi: 10.1053/srin.2003.50002. [DOI] [PubMed] [Google Scholar]
- Hess C, Sadallah S, Schifferli JA. Induction of neutrophil responsiveness to myeloperoxidase antibodies by their exposure to supernatant of degranulated autologous neutrophils. Blood. 2000;96:2822–2827. [PubMed] [Google Scholar]
- Franssen CF, Huitema MG, Muller Kobold AC, Oost-Kort WW, Limburg PC, Tiebosch A, Stegeman CA, Kallenberg CG, Cohen Tervaert JW. In vitro neutrophil activation by antibodies to proteinase 3 and myeloperoxidase from patients with crescentic glomerulonephritis. J Am Soc Nephrol. 1999;10:1506–1515. doi: 10.1681/ASN.V1071506. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettritz R, Schreiber A, Luft FC, Haller H. Role of mitogen-activated protein kinases in activation of human neutrophils by antineutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2001;12:37–46. doi: 10.1681/ASN.V12137. [DOI] [PubMed] [Google Scholar]
- Rarok AA, Limburg PC, Kallenberg CG. Neutrophil-activating potential of antineutrophil cytoplasm autoantibodies. J Leukoc Biol. 2003;74:3–15. doi: 10.1189/jlb.1202611. [DOI] [PubMed] [Google Scholar]
- Reumaux D, Vossebeld PJ, Roos D, Verhoeven AJ. Effect of tumor necrosis factor-induced integrin activation on Fc gamma receptor II-mediated signal transduction: relevance for activation of neutrophils by anti-proteinase 3 or anti-myeloperoxidase antibodies. Blood. 1995;86:3189–3195. [PubMed] [Google Scholar]
- Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentener MA, Greve JW, Maessen JG, Buurman WA. Role of tumour necrosis factor in the enhanced sensitivity of mice to endotoxin after exposure to lead. Immunopharmacol Immunotoxicol. 1989;11:321–334. doi: 10.3109/08923978909005373. [DOI] [PubMed] [Google Scholar]
- Sheehan KC, Ruddle NH, Schreiber RD. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989;142:3884–3893. [PubMed] [Google Scholar]
- Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Intratracheal instillation of lipopolysaccharide in mice induces apoptosis in bronchial epithelial cells: no role for tumor necrosis factor-alpha and infiltrating neutrophils. Am J Respir Cell Mol Biol. 2001;24:569–576. doi: 10.1165/ajrcmb.24.5.4156. [DOI] [PubMed] [Google Scholar]
- Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- Lopez AF, Strath M, Sanderson CJ. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br J Haematol. 1984;57:489–494. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- Masaki T, Chow F, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Heterogeneity of antigen expression explains controversy over glomerular macrophage accumulation in mouse glomerulonephritis. Nephrol Dial Transplant. 2003;18:178–181. doi: 10.1093/ndt/18.1.178. [DOI] [PubMed] [Google Scholar]
- Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46:211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Radford DJ, Luu NT, Hewins P, Nash GB, Savage CO. Antineutrophil cytoplasmic antibodies stabilize adhesion and promote migration of flowing neutrophils on endothelial cells. Arthritis Rheum. 2001;44:2851–2861. doi: 10.1002/1529-0131(200112)44:12<2851::aid-art473>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ewert BH, Becker ME, Jennette JC, Falk RJ. Antimyeloperoxidase antibodies induce neutrophil adherence to cultured human endothelial cells. Renal Fail. 1995;17:125–133. doi: 10.3109/08860229509026249. [DOI] [PubMed] [Google Scholar]
- Ewert BH, Jennette JC, Falk RJ. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992;41:375–383. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- Reumaux D, De Boer M, Meijer AB, Duthilleul P, Roos D. Expression of myeloperoxidase (MPO) by neutrophils is necessary for their activation by anti-neutrophil cytoplasm autoantibodies (ANCA) against MPO. J Leukoc Biol. 2003;73:841–849. doi: 10.1189/jlb.1102567. [DOI] [PubMed] [Google Scholar]
- Lan HY. Therapeutic effects of cytokine blockade in glomerulonephritis. Nephrol Dial Transplant. 1998;13:7–9. doi: 10.1093/ndt/13.1.7. [DOI] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Le Hir M, Haas C, Marino M, Ryffel B. Prevention of crescentic glomerulonephritis induced by anti-glomerular membrane antibody in tumor necrosis factor-deficient mice. Lab Invest. 1998;78:1625–1631. [PubMed] [Google Scholar]
- Karkar AM, Smith J, Pusey CD. Prevention and treatment of experimental crescentic glomerulonephritis by blocking tumour necrosis factor-alpha. Nephrol Dial Transplant. 2001;16:518–524. doi: 10.1093/ndt/16.3.518. [DOI] [PubMed] [Google Scholar]
- Booth A, Harper L, Hammad T, Bacon P, Griffith M, Levy J, Savage C, Pusey C, Jayne D. Prospective study of TNFalpha blockade with infliximab in anti-neutrophil cytoplasmic antibody-associated systemic vasculitis. J Am Soc Nephrol. 2004;15:717–721. doi: 10.1097/01.asn.0000114554.67106.28. [DOI] [PubMed] [Google Scholar]