Abstract
X-linked anhidrotic/hypohidrotic ectodermal dysplasia (EDA) is caused by mutations in the (EDA) gene, which is required for the morphogenesis of ectoderm-derived tissues. Although EDA function in skin appendage development has been studied in Eda mutant “Tabby” mice, we have recently identified characteristic abnormalities in the ocular surface, an ectoderm-derived tissue. Histology of eyes of Tabby males revealed that 1) as previously reported, mice lacked meibomian glands; 2) >80% developed corneal lesions such as neovascularization, keratitis, ulceration, and keratinization identifiable from 9 weeks of age; and 3) > 80% showed ocular surface inflammation (blepharitis and conjunctivitis) when housed in a standard environment. Strikingly, both corneal defects and inflammation were prevented in Tabby mice bearing a transgene for the Eda-A1 isoform, but meibomian glands were restored little if at all. These findings suggest that intact ocular surface health is EDA dependent and that Tabby corneal abnormalities are not solely dependent on meibomian gland lipid secretion. Alternatively, susceptibility to inflammation and other phenotypes could result from failure of the usual EDA receptor to activate nuclear factor-κB transcription factors. This can be further tested in Tabby and Tabby-EDA transgenic mice, which provide unique models of severe ocular surface disease.
Ectodermal dysplasia (ED) comprises a group of genetic syndromes characterized by abnormalities in at least two ectodermal-derived tissues: sweat glands, hair, teeth, and nails. There are greater than 175 different ectodermal dysplasia syndromes with varying inheritance patterns and phenotypes. X-linked anhidrotic ectodermal dysplasia (EDA) is the most frequent form of ED, characterized by abnormal formation of ectoderm-derived tissues.1,2 The responsible gene for X-linked EDA encodes a transmembrane protein, ectodysplasin A,3,4 a new member of the tumor necrosis factor ligand superfamily. Human patients and mouse models demonstrate that EDA interacts with its receptor EDAR and a receptor adaptor protein EDARADD5–8 to activate downstream nuclear factor-κB (NF-κB) transcription factors, but the range and mechanism of EDA actions are only partially known.9–11
The phenotype of Tabby mice, the mouse counterpart of human EDA, was initially reported in the 1970s.12,13 Documented abnormalities in skin appendages included the absence of sweat glands and some types of hair follicles, as well as abnormal teeth.12,14 Some glandular tissues, including submandibular, preputial, and meibomian glands13,15 were also absent in Tabby mice; however, apart from the absence of meibomian glands, the eye, which is partially derived from ectoderm, had not been examined and reported in detail.
During the husbandry of a colony of Tabby animals, we noted that with increasing age, mutant mice demonstrated abnormal visual behavior and appeared to have impaired vision. This suggested that there might be additional effects of EDA on the eye, and we have observed a range of ocular surface phenotypes in the mutant mice. They include corneal epithelial defects, keratitis, corneal ulceration, neovascularization, keratinization, blepharitis, and conjunctivitis. Furthermore, as we had previously observed for guard hair and sweat glands,16,17 a transgene encoding mouse ectodysplasin A1 protein isoform also prevented most ocular pathology. These mice also permit the demonstration that EDA can affect ocular health independent of its effect on meibomian glands. Thus, ocular surface homeostasis in mice is EDA dependent, and the Tabby and Tabby-EDA transgenic mice provide a unique model for ocular surface disease.
Materials and Methods
The eyes from EDA mutant Tabby mice (C57BL/6J-Aw-J-Ta6J) (27 males), mEDA-A1 transgenic Tabby (4 males), transgenic mice in wild-type background (4 males), and wild-type controls (12 males) ranging from 4 to 45 weeks of age were examined. Mice were housed in two different facilities. One is a pathogen-free barrier facility; the other is the conventional facility with free access. Twenty mice studied below were from the barrier, and 27 were from the conventional facility (see Table 1). Treatment of animals was in compliance with Institutional Guidelines, and all animal study protocols were approved by an Institutional Review Board.
Table 1.
Number of Male Mice Analyzed
| Strain | Mice from barrier | Mice from conventional facility | Total |
|---|---|---|---|
| TA | 15 (5–45W) | 12 (4–40W) | 27 |
| TATG | 0 | 4 (9–12W) | 4 |
| WT | 5 (5–45W) | 7 (9–21W) | 12 |
| WTTG | 0 | 4 (13–25W) | 4 |
Numbers in parentheses indicate age of mice. w, weeks
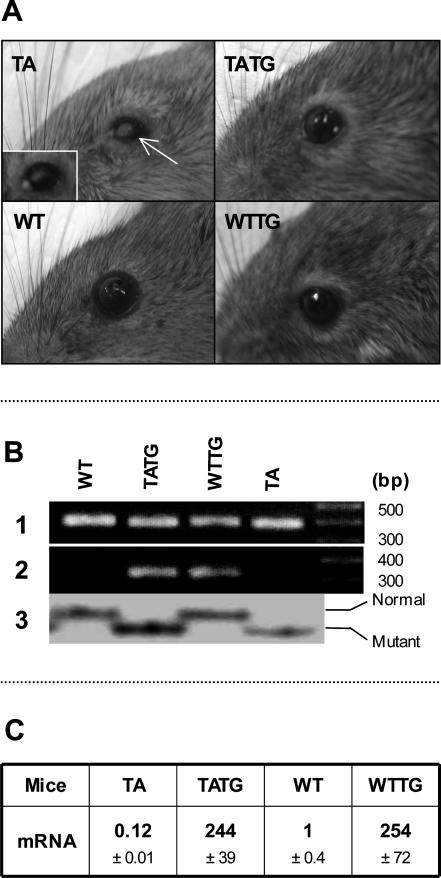
Generation of mEDA-A1 transgenic mice was reported previously.17 Briefly, linearized mouse EDA-A1 cDNA under a tetracycline-controllable promoter was injected into C57BL eggs, and potential founders were mated to heterozygous Tabby females. Tabby females carrying the transgene were then mated to Tet-off male mice [C57BL/6J-TgN (MMTVtTA); Jackson Laboratory, Bar Harbor, ME] to activate the transgene. Sample photographs of mice are in Figure 1A. The genetic background of transgenic mice was detected by single-strand confirmational polymorphism (Figure 1B).16 Transgene expression levels were determined by Taqman one-step real-time polymerase chain reaction (PCR) with RNA samples from age-matched adult back skin (Applied Biosystems, Foster City, CA) of transgenic, Tabby, and wild-type mice (Figure 1C).18 Real-time PCR demonstrated significantly lower expression of EDA in Tabby skin (10% wild type) and, in agreement with our previous studies,17 significantly higher expression of the transgene in transgenic mice (250-fold wild type).
Figure 1.
Phenotype, genotype, and mRNA expression of transgenic mice. A: Gross eye phenotype. Narrow palpebral fissure (blepharophimosis), thickened eyelid margins, and missing hair around eyelashes, but normal eyelashes in Tabby male mice (TA). Note white (arrow) and red corneal opacities (insert, also mucus) in Tabby (TA). mEDA-A1 transgene (TATG) almost fully rescued Tabby phenotype. Note “yellow hair ring” around eyelids in TATG and wild-type transgenic (WTTG) mice. B: Genotyping of transgenic mice. Lane 1, 390-bp PCR product from endogenous genomic EDA; lane 2, 330 bp of PCR product only from transgene; lane 3, SSCP for genetic background detection. PCR product from Tabby is 1 bp shorter than wild type, so that it moves faster on gel electrophoresis. C: Expression level of EDA in mice. Note lower EDA expression in Tabby (TA) and very high expression in transgenic mice (TATG and WTTG).
Direct determinations of relative expression levels of EDA and its receptor EDAR were extended to microdissected cornea, conjunctiva, and back skin of 9-week-old male mice from the pathogen-free facility. Real-time PCR was carried out on total RNAs that were isolated with Trizol (Invitrogen, Carlsbad, CA), reprecipitated with phenol:chloroform:isoamyl alcohol (25:24:1; Invitrogen) to eliminate contaminating protein that can inhibit real-time PCR, and again reprecipitated with lithium chloride (final concentration, 2.5 mol/L; Ambion) to remove DNA contamination. RNAs obtained by this procedure gave consistent results with high efficiency in real-time PCR. The Taqman probe/primer sets for EDA and EDAR have been described previously.17,18 The expression levels of EDA and EDAR were normalized to GAPDH with a rodent-specific probe/primer set (Applied Biosystems).
Gross eye phenotypes were examined before histological studies for all 47 mice. For histological analyses, one eye with intact eyelids and conjunctiva was removed from sacrificed mice under visualization with a dissecting microscope and fixed in 10% buffered formalin. Eye samples were then embedded in methacrylate, sectioned through the vertical pupillary optic nerve axis, and stained with hematoxylin and eosin (H&E); the other eye from each animal was embedded in OCT, snap frozen, and sectioned as described above.
Results
Corneal Lesions and Resultant Blindness in Tabby Mutant Mice
Tabby male mice housed in conventional or barrier facilities (see Materials and Methods) demonstrated narrowed palpebral fissures, thickened eyelid margins, and loss of hair along the eyelid margins. Most also had dramatic corneal defects.
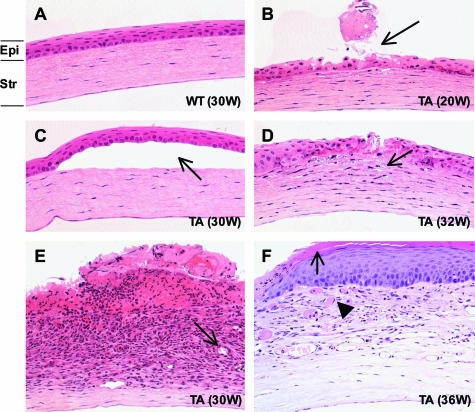
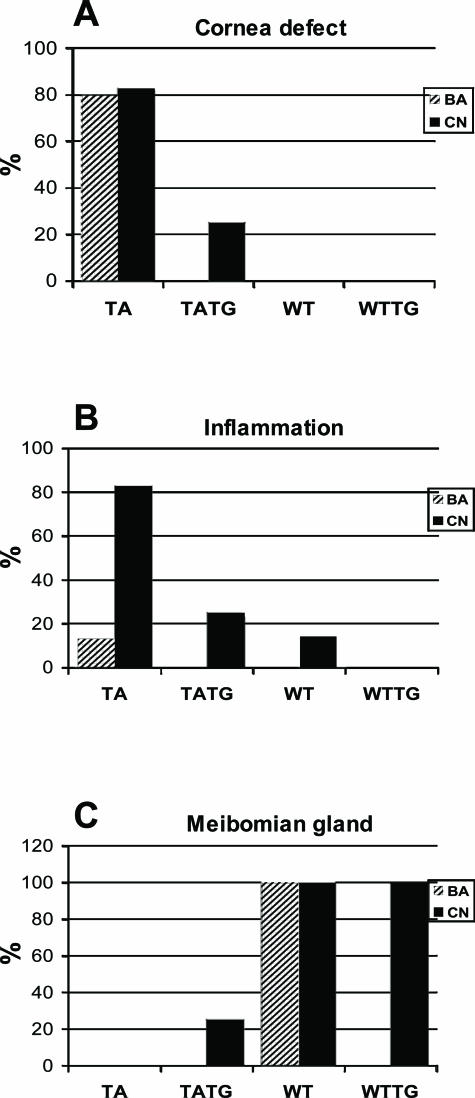
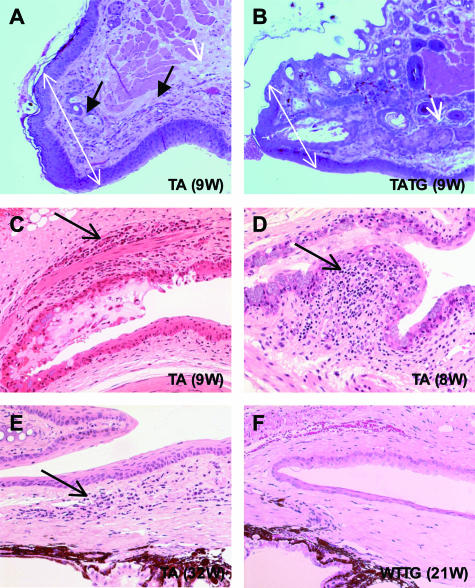
Nine of 15 Tabby male mice housed in a barrier facility demonstrated grossly visible white or white and red central, elevated corneal opacities at around 23 weeks of age (Figure 1A, TA and insert); several had abundant mucus in the eye; and one had an even smaller palpebral fissure than the others. Only three Tabby mice showed no grossly visible corneal defects. Histological analysis revealed corneal abnormalities including central epithelial defect, bullae formation, keratitis, ulceration, keratinization, and neovascularization (Figure 2, arrows in B to F show various corneal lesions of Tabby males). Corneal abnormalities were observed histologically as early as 4 weeks, and severe blinding lesions such as corneal neovascularization were demonstrated at 9 weeks, but in general, the severity of pathological changes increased with age: progressing from mild corneal epithelial defect to neovascularization, keratitis to ulceration and keratinization (Table 2). Limbitis, conjunctivitis to blepharitis were also observed (see below). Wild-type male mice housed in the same facility did not show any ocular surface abnormalities (Figures 1A and 2A). Similar corneal abnormalities were found in Tabby mice housed in the conventional facility (Figure 3, A compared with wild types in C). In summary, histological analysis revealed that various corneal abnormalities were found in 80% of Tabby males housed in the barrier and 83% of Tabby males from conventional facility (Figure 4A). However, inflammatory lesions were significantly less in Tabby males housed in the barrier compared with the Tabby mice from conventional facility (see below).
Figure 2.
Photomicrographs showing corneal pathology in Tabby males from the pathogen-free facility. A: Normal histology of wild-type cornea at 30 weeks. B to F: Tabby corneas. B: Superficial corneal epithelial defect (arrow). C: Bullous keratopathy; separation between epithelium and stroma (arrow). D: Keratitis characterized by inflammatory cells (arrow) infiltrating the cornea. E: Ulceration, keratitis, hemorrhage, and neovascularization (arrow). F: Keratinization (arrow) and neovascularization (arrowhead). Epi, epithelium, Str, stroma. Numbers in parentheses represent age of mice (H&E staining at an original magnification of ×200).
Table 2.
Time Course of Pathogenesis in Tabby Cornea
| Age (wk) | No. of mice* | Pathology |
|---|---|---|
| 4–5 | 1/2 | Descemet’s detachment |
| 8–9 | 4/4 | Epithelial defect, epithelial edema, neovascularization |
| 12–13 | 2/2 | Neovascularization, epithelial acanthosis |
| 16–19 | 1/3 | Mild keratinization |
| 20–25 | 4/5 | Neovascularization, keratitis, ulcer |
| 30–36 | 6/7 | Neovascularization, keratitis, ulcer, bullae formation, keratinization |
| 39–45 | 4/4 | Neovascularization, keratitis, ulcer, scar |
Number of abnormal mice/number of mice examined.
Figure 3.
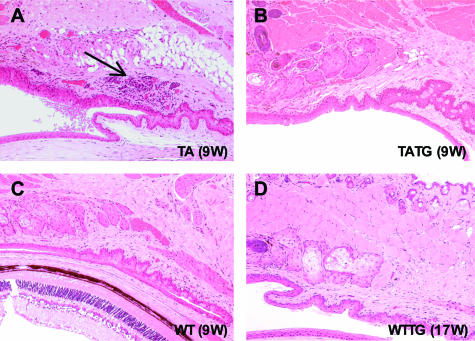
Photomicrographs showing rescue of corneal lesions by EDA-A1 transgene in Tabby mice. A: Age-matched Tabby control shows acanthosis (arrow) and neovascularization (arrowhead) in cornea. B: Cornea lesions were recovered to wild type in EDA-A1 transgenic Tabby mice. Wild-type mouse with normal cornea (C) and EDA-A1 transgenic wild-type mice (D) show normal cornea morphology. H&E staining at an original magnification of ×200.
Figure 4.
Summary scores of ocular phenotypes of Tabby mice. A: Corneal lesions in 80% of Tabby males housed in the pathogen free and 83% in the conventional facility (TA). One of four TATG mice showed minimal corneal keratinization (TATG), and no lesions were found in wild-type (WT) or transgenic mice in wild-type background (WTTG). B: Majority of the Tabby males housed in the conventional facility developed ocular surface inflammation (TA). Inflammation was decreased in transgenic Tabby (TATG) mice housed in the same facility. C: Tabby males lack meibomian glands (TA). EDA-A1 transgene failed to rescue meibomian glands in Tabby mice (TATG). BA, pathogen-free facility; CN, conventional facility.
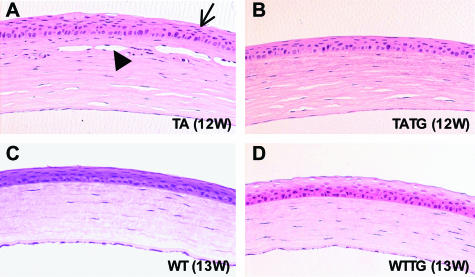
A transgene coding for the EDA-A1 isoform in Tabby mice essentially completely prevented the ocular phenotypes. Among the grossly visible wild-type features restored by EDA-A1 transgene were nearly normal sized palpebral fissures and hair along the eyelid margins (Figure 1A, TATG). No clinically visible corneal lesions were observed in the transgenic Tabby mice. Histological analysis revealed no evidence of corneal abnormalities, including neovascularization and overall normal ocular surface morphology [except for minimal corneal keratinization in one mouse (Figures 3B and 4A)], demonstrating significant rescue of corneal lesions by EDA-A1 transgene. Also, in contrast to the blunt, thicker eyelid margins in Tabby, transgenic Tabby mice demonstrated tapered eyelid margins and thinner skin around eyelids characteristic of wild-type animals (Figure 5, A and B, double-headed arrows).
Figure 5.
Photomicrographs showing ocular surface inflammation in Tabby mice. A: Thickened eyelids (double-headed white arrows), absence of meibomian glands (single-headed white arrow), blepharitis (single-headed black arrows), and sparse hair follicles in Tabby eyelids (upper skin). B: Nearly normal eyelids, with partially rescued meibomian gland and fully restored hair follicles (upper skin) in EDA-A1 transgenic Tabby mice. C: Conjunctivitis (arrow) in Tabby. D: Follicular conjunctivitis in the fornix (arrow). E: Limbitis showing inflammatory cells (arrow) infiltrating the junction of the cornea and sclera. F: Normal conjunctiva, limbus, and cornea in transgenic wild-type mice. H&E staining; original magnifications in A and B, ×100; in C to F, ×200.
The transgenic Tabby animals showed a single distinctive trait that was also seen in transgenic wild-type mice: transgenic eyelids showed a ring of “yellow hair” with slightly thicker than wild-type skin around the eyelashes (Figure 1A, TATG and WTTG). This may be a result of hypertrophic sebaceous glands caused by the EDA-A1 transgene (see below).17 The EDA-A1 transgenic mice on wild-type background showed no other abnormal ocular features (Figure 1A, WTTG). Also, darker guard hair was restored around transgenic Tabby eyes (Figure 1A, compare TATG hair coat with TA).
Susceptibility to Inflammation in Tabby Mice and Its Prevention by an EDA-A1 Transgene
In addition to the lack of meibomian glands, Tabby males housed in the conventional facility demonstrated dramatic ocular surface abnormalities. Ten of 12 Tabby males showed various manifestations of ocular surface inflammation. Five had blepharitis, four with conjunctivitis, and one had both conditions (Figures 4B and 5, C to E, arrows indicate various inflammatory lesions), demonstrating a propensity for ocular inflammation in these mice. Similar to corneal lesions, inflammation was clearly seen from 8 weeks of age (Figure 5, C and D). In contrast, only one of seven wild-type mice in the same facility showed minimal blepharitis (Figure 4B, black column for WT). The increased susceptibility to inflammation was apparently dependent on environmental challenges in conventional housing, because Tabby mice kept in the barrier (pathogen-free) facility showed no infectious-related features. Only 2 of 15 mice demonstrated mild conjunctivitis, and none had blepharitis, limbitis, or other inflammatory processes (Figure 4B, hatched bar for TA). However, central corneal epithelial defects persisted and even progressed to corneal ulceration and neovascularization in these older Tabby mice. Because a diseased ocular surface represents the loss of a physical barrier to infection, for the possible presence of bacteria eyes from two Tabby mice from each of the facilities, conventional and pathogen-free, were examined with gram stain. There was no evidence of gram-positive or -negative bacteria, indicating a lack of active infection (data not shown); however, these results do not exclude the involvement of pathogens.
As observed for the cornea, an EDA-A1 transgene sharply restored eyelid abnormalities, especially increasing the resistance to inflammation. Among four transgenic Tabby mice housed in the conventional facility, only one showed mild conjunctivitis, and none developed blepharitis (Figures 4B and 6B, TATG).
Figure 6.
Photomicrographs showing prevention of inflammation by EDA-A1 transgene in Tabby mice hosted in the conventional facility. Tabby (A), transgenic Tabby (B), and wild-type (C) mice are littermates housed in the same cage. A: Moderate conjunctivitis (arrow) in Tabby. B to D: No inflammation in transgenic Tabby (B), wild-type (C) or transgenic wild-type mice (D). H&E staining at an original magnification of ×100.
Meibomian glands produce the lipid layer of the tear film, which is critical to maintenance of tear film stability. Meibomian gland dysfunction can result in evaporative tear deficiency and secondary mechanical damage to the ocular surface. A healthy ocular surface requires the appropriate complex of the lipid, mucin, and aqueous components of the tears. The meibomian gland aplasia seen in Tabby mice results in absence of meibum, leading to enhanced tear evaporation, and may indirectly increase the risk of ocular surface inflammation. The status of meibomian glands in transgenic mice was therefore analyzed in some detail. Histological sections showed partial restoration of meibomian glands in one transgenic Tabby animal, but in agreement with our previous studies,17 no restoration at all was seen in the other three animals studied (Figure 4C, TATG). Meibomian gland loss is therefore not the primary factor in increasing eyelid inflammation or blepharitis in Tabby mice.
EDA-A1 transgenic Tabby mice also showed significant hypertrophy of sebaceous glands associated with hair follicles of eyelids (Figure 5B, upper skin; data not shown). This was similar to findings in back skin17 and was also seen in EDA-A1 transgenic wild-type mice, which showed no eyelid defects.
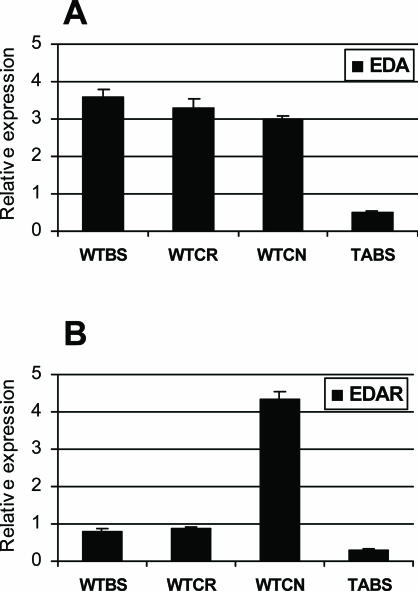
The expression levels of EDA and its receptor EDAR were directly determined in tissues by quantitative real-time PCR. They were comparable in back skin and cornea (Figure 7, A and B). Interestingly, EDAR expression, but not EDA, was fourfold higher in conjunctiva than in back skin (Figure 7B). These data indicate that the EDA pathway is active in both cornea and conjunctiva and are also consistent with in situ studies in which EDA and EDAR expression was detected in the cornea and conjunctiva of embryonic eyelids, respectively.19
Figure 7.
Quantitative real-time PCR showing expression of EDA and EDAR. Relative expression level of EDA and its receptor, EDAR, in wild-type cornea (WTCR) and conjunctiva (WTCN) compared with wild-type (WTBS) and Tabby (TABS) back skin. Real-time PCR expression levels of EDA (A) and EDAR (B). Data normalized to GAPDH.
Discussion
The X-linked anhidrotic ectodermal dysplasia (EDA) gene encodes ectodysplasin, a protein that regulates development of ectoderm-derived tissues.3,4 Whereas meibomian gland abnormalities have been described in EDA, ocular surface abnormalities had not been documented. Here, we discuss the possible mechanism of action of EDA in relation to the eye phenotypes caused by inactivation of the EDA gene.
Corneal Neovascularization and Keratinization in Tabby Mutant Mice
Absence of meibomian glands in Tabby mice was reported 30 years ago, and a report published during preparation of this manuscript highlighted alterations of the meibomian glands in EDA patients detected by “meibomianoscopy” as a reliable ocular feature of ectodermal dysplasia syndromes.13,20 However, there has been little standardized phenotypic study of the ocular surface in animals or EDA patients. During a reexamination of Tabby mice and their ectodermal structures, we noticed abnormalities of the cornea and other ocular surface structures that appeared to affect visual behavior (Figure 1A). Severe central corneal neovascularization and keratinization were the predominant lesions that were associated with corneal opacification consistent with loss of vision. Confirming the role of EDA, these ocular defects were almost completely prevented by the provision of the EDA-A1 isoform from a transgene (Figures 3 and 6).
The corneal defects in Tabby mice could have resulted from mechanical- or desiccation-related damage in the absence of the protective lipid layer of the tear film produced by meibomian glands. We verified that all of the Tabby mice lacked meibomian glands; but in contrast to the significant prevention of cornea defects in transgenic Tabby mice, meibomian glands were generally not restored (Figure 4C). Thus, the lack of meibomian glands could not be the sole cause of the cornea defects. Baum and Bull21 have proposed that corneal changes are a primary sign of ectodermal dysplasia syndromes, starting with mild epithelial defect and ending with stromal scarring and vascularization. Our data support this notion, and EDA function appears to be required for intact corneal physiology regardless of whether meibomian glands were present.
Corneal abnormalities in Tabby male mice progress with aging (see Results), indicating that EDA is required more for maintenance than for morphogenesis of ocular surface structures. This is consistent with our previous finding of disparate effects of EDA on hair follicles and sebaceous glands.17 Expression levels of EDA and its receptor EDAR in adult cornea are comparable with levels in back skin, the classical target organ of EDA, consistent with the notion that an active EDA pathway in cornea is involved in ocular surface physiology (Figure 7). Corneal lesions were seen in about 80% of Tabby male mice, much more than the observed 19.4% of EDA human patients.20 The lower prevalence in patients may result from protective treatment such as instating lubricant ocular solutions.
Tabby Mouse Susceptibility to Inflammation as a New Aspect of EDA Function
In contrast to constitutive corneal defects, which showed relatively high penetrance in both facilities, strikingly increased susceptibility to inflammation was only observed in the context of conventional mouse housing. Tabby mice from the pathogen-free facility showed no significant increase, but blepharitis and conjunctivitis were observed in animals raised in conventional facilities (Figure 4B). No specific pathogens have been identified in the conventional facility; but the EDA-A1 transgene prevented the inflammation, indicating that it too is contingent on EDA loss.
EDA may prevent inflammation by supporting ocular surface homeostasis. As seen in the cornea, the action of EDA is apparently involved in maintenance rather than morphogenesis; we similarly propose that the inflammation susceptibility may not be solely attributable to the relative absence or presence of meibomian glands.
Regarding possible mechanistic features, EDA operates through the NF-κB signal pathway, and mice disrupted in NF-κB were recently reported to display ocular phenotypes nearly identical to those described here in Tabby mice: skin changes and absence of meibomian glands were observed along with conjunctivitis, corneal neovascularization, and keratinization consistent with loss of vision.22 One might then suppose that EDA and NF-κB mutations both directly affect the immune system and thereby influence inflammatory responses. However, unlike mutations in NEMO or other downstream NF-κB pathway genes, which invariably show immune system abnormalities,22–24 true EDA mutations (in EDA, its receptor EDAR, or the auxiliary protein EDARADD) show only skin appendage dysplasias, with no evidence of any effect on the immune system (see Online Mendelian Inheritance in Man entries 305100 and 300291 for details). Thus, high expression of EDAR in conjunctiva25 (Figure 7B) suggests a higher activity of EDA pathway; but its use of NF-κB apparently supports the ocular surface rather than intervening directly in the inflammation process. This is a new and different feature of EDA action.
One can ask whether the ocular defects in the absence of EDA occur in other species. It could be relevant that among a number of reported animal models,26–28 ocular surface inflammation has thus far been noted only for EDA in the dog, with conjunctivitis and mucoid discharge,27 but this is the first report of such effects in Tabby mice, a strain that has been studied since the 1930s. Thus, the ocular defects may be widespread but not yet characterized in other species.
There are reports of one or two patients with ill-defined “corneal abnormalities” or scars and decreased corneal sensation, which have often been attributed to the recurrent inflammation or tear deficiency common in these patients.20,29 However, human studies have not described detailed examination of the cornea and have focused on the status of the meibomian glands. In addition, longitudinal patient studies are lacking. Further study of the Tabby model should provide a dissection of the pathogenic mechanisms of the corneal lesions, which appear to be distinct from the meibomian gland abnormalities, along with the identification of possible downstream targets for intervention.
Footnotes
Address reprint requests to Chi-Chao Chan, M.D., Immunopathology Section, Laboratory of Immunology, National Eye Institute, National Institute of Health, Building 10, Room 10N103, Bethesda, MD 20892-1857. E-mail: chanc@nei.nih.gov.
C.-Y.C. and J.A.S. contributed equally to this work.
References
- Pinheiro M, Freire-Maia N. Ectodermal dysplasias: a clinical classification and a causal review. Am J Med Genet. 1994;53:153–162. doi: 10.1002/ajmg.1320530207. [DOI] [PubMed] [Google Scholar]
- Priolo M, Silengo M, Lerone M, Ravazzolo R. Ectodermal dysplasias: not only ‘skin’ deep. Clin Genet. 2000;58:415–430. doi: 10.1034/j.1399-0004.2000.580601.x. [DOI] [PubMed] [Google Scholar]
- Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, Munoz F, Morgan D, Clarke A, Baybayan P, Chen EY, Ezer S, Saarialho-Kere U, de la Chapelle A, Schlessinger D. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet. 1996;13:409–416. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Pispa J, Hartung AJ, Du Y, Ezer S, Jenks T, Shimada T, Pekkanen M, Mikkola ML, Ko MS, Thesleff I, Kere J, Schlessinger D. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc Natl Acad Sci USA. 1997;94:13069–13074. doi: 10.1073/pnas.94.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet. 1999;22:370–374. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- Monreal AW, Ferguson BM, Headon DJ, Street SL, Overbeek PA, Zonana J. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet. 1999;22:366–369. doi: 10.1038/11937. [DOI] [PubMed] [Google Scholar]
- Headon DJ, Emmal SA, Ferguson BM, Tucker AS, Justice MJ, Sharpe PT, Zonana J, Overbeek PA. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414:913–916. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- Yan M, Zhang Z, Brady JR, Schilbach S, Fairbrother WJ, Dixit VM. Identification of a novel death domain-containing adaptor molecule for ectodysplasin-A receptor that is mutated in crinkled mice. Curr Biol. 2002;12:409–413. doi: 10.1016/s0960-9822(02)00687-5. [DOI] [PubMed] [Google Scholar]
- Yan M, Wang LC, Hymowitz SG, Schilbach S, Lee J, Goddard A, de Vos AM, Gao WQ, Dixit VM. Two-amino acid molecular switch in an epithelial morphogen that regulates binding to two distinct receptors. Science. 2000;290:523–527. doi: 10.1126/science.290.5491.523. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Pispa J, Jung HS, Nieminen P, Mikkola M, Wang X, Saarialho-Kere U, Galceran J, Grosschedl R, Thesleff I. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development. 2002;129:2541–2553. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- Kumar A, Eby MT, Sinha S, Jasmin A, Chaudhary PM. The ectodermal dysplasia receptor activates the nuclear factor-kappaB, JNK, and cell death pathways and binds to ectodysplasin A. J Biol Chem. 2001;276:2668–2677. doi: 10.1074/jbc.M008356200. [DOI] [PubMed] [Google Scholar]
- Gruneberg H. The tabby syndrome in the mouse. Proc R Soc Lond B Biol Sci. 1971;179:139–156. doi: 10.1098/rspb.1971.0086. [DOI] [PubMed] [Google Scholar]
- Gruneberg H. The glandular aspects of the tabby syndrome in the mouse. J Embryol Exp Morphol. 1971;25:1–19. [PubMed] [Google Scholar]
- Vielkind U, Hardy MH. Changing patterns of cell adhesion molecules during mouse pelage hair follicle development. 2. Follicle morphogenesis in the hair mutants, Tabby and downy. Acta Anat (Basel) 1996;157:183–194. doi: 10.1159/000147880. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Zhou YM, Trump G, Melnick M. Ectodysplasin receptor-mediated signaling is essential for embryonic submandibular salivary gland development. Anat Rec. 2003;271A:322–331. doi: 10.1002/ar.a.10045. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Durmowicz MC, Hartung AJ, Hudson J, Ouzts LV, Donovan DM, Cui CY, Schlessinger D. Ectodysplasin-A1 is sufficient to rescue both hair growth and sweat glands in Tabby mice. Hum Mol Genet. 2001;10:2973–2981. doi: 10.1093/hmg/10.26.2973. [DOI] [PubMed] [Google Scholar]
- Cui CY, Durmowicz M, Ottolenghi C, Hashimoto T, Griggs B, Srivastava AK, Schlessinger D. Inducible mEDA-A1 transgene mediates sebaceous gland hyperplasia and differential formation of two types of mouse hair follicles. Hum Mol Genet. 2003;12:2931–2940. doi: 10.1093/hmg/ddg325. [DOI] [PubMed] [Google Scholar]
- Cui CY, Durmowicz M, Tanaka TS, Hartung AJ, Tezuka T, Hashimoto K, Ko MS, Srivastava AK, Schlessinger D. EDA targets revealed by skin gene expression profiles of wild-type, Tabby and Tabby EDA-A1 transgenic mice. Hum Mol Genet. 2002;11:1763–1773. doi: 10.1093/hmg/11.15.1763. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Aebischer T, Hulsken J, Birchmeier W, Klemm U, Scheidereit C. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development. 2001;128:3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- Kaercher T. Ocular symptoms and signs in patients with ectodermal dysplasia syndromes. Graefes Arch Clin Exp Ophthalmol. 2004;242:495–500. doi: 10.1007/s00417-004-0868-0. [DOI] [PubMed] [Google Scholar]
- Baum JL, Bull MJ. Ocular manifestation of ectrodactyly ectodermal dysplasia, cleft lip-palate syndrome. Am J Ophthalmol. 1974;78:211–216. doi: 10.1016/0002-9394(74)90078-6. [DOI] [PubMed] [Google Scholar]
- Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, Wood P, Rabia SH, Headon DJ, Overbeek PA, Le Deist F, Holland SM, Belani K, Kumararatne DS, Fischer A, Shapiro R, Conley ME, Reimund E, Kalhoff H, Abinun M, Munnich A, Israel A, Courtois G, Casanova JL. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- Courtois G, Smahi A, Reichenbach J, Doffinger R, Cancrini C, Bonnet M, Puel A, Chable-Bessia C, Yamaoka S, Feinberg J, Dupuis-Girod S, Bodemer C, Livadiotti S, Novelli F, Rossi P, Fischer A, Israel A, Munnich A, Le Deist F, Casanova JL. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J Clin Invest. 2003;112:1108–1115. doi: 10.1172/JCI18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Levy O, Brodeur SR, Krzewski K, Roy RM, Niemela JE, Fleisher TA, Bonilla FA, Geha RS. Human nuclear factor κB essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Pispa J, Mikkola ML, Mustonen T, Thesleff I. Ectodysplasin, Edar and TNFRSF19 are expressed in complementary and overlapping patterns during mouse embryogenesis. Gene Expr Patterns. 2003;3:675–679. doi: 10.1016/s1567-133x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kuwahara Y, Kondo M, Naruse K, Mitani H, Wakamatsu Y, Ozato K, Asakawa S, Shimizu N, Shima A. The medaka rs-3 locus required for scale development encodes ectodysplasin-A receptor. Curr Biol. 2001;11:1202–1206. doi: 10.1016/s0960-9822(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Casal ML, Jezyk PF, Greek JM, Goldschmidt MH, Patterson DF. X-linked ectodermal dysplasia in the dog. J Hered. 1997;88:513–517. doi: 10.1093/oxfordjournals.jhered.a023146. [DOI] [PubMed] [Google Scholar]
- Drogemuller C, Distl O, Leeb T. Partial deletion of the bovine ED1 gene causes anhidrotic ectodermal dysplasia in cattle. Genome Res. 2001;11:1699–1705. doi: 10.1101/gr.182501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins M, Waring F. Absent meibomian glands and reduced corneal sensation in hypohidrtoic ectodermal dysplasis. J Ped Ophthalmol Strabismus. 1981;18:44–47. doi: 10.3928/0191-3913-19810701-09. [DOI] [PubMed] [Google Scholar]