Abstract
Thymic abnormalities are present in ∼80% of myasthenia gravis (MG) patients, and the thymus seems to be the main site of autosensitization to the acetylcholine receptor. In view of findings that the innate immune system can generate an autoimmune response, we studied the expression of Toll-like receptors (TLRs) 2 to 5, key components of innate immunity signaling pathways, in 37 thymuses from patients with autoimmune MG. TLR4 mRNA levels were significantly greater in thymitis (hyperplasia with diffuse B-cell infiltration) and involuted thymus than in germinal center hyperplasia and thymoma. By immunohistochemistry and confocal microscopy, cells positive for TLR4 protein were rarely detected in thymoma. However, in thymitis TLR4 protein was mostly found on epitheliomorphic (cytokeratin-positive) cells located in close association with clusters of acetylcholine receptor-positive myoid cells in thymic medulla and also at the borders between cortical and medullary areas. B cells were never TLR4-positive. TLR4 protein was also present in remnant tissue of involuted thymus. This is the first finding of a possible link between innate immunity and MG. We speculate that in a subgroup of MG patients, an exogenous or endogenous danger signal may activate the innate immune system and give rise to TLR4-mediated mechanisms contributing to autoimmunity.
Myasthenia gravis (MG) is a well-characterized autoimmune disease of the neuromuscular junction.1 In most MG cases, the effectors are autoantibodies against the nicotinic acetylcholine receptor (AChR) in the postsynaptic muscle endplate. These antibodies block AChR function or provoke its degradation, resulting in impaired neuromuscular transmission and the characteristic symptoms of fatigue and muscle weakness.1 AChR-specific T cells have been isolated from peripheral blood and thymuses of MG patients and propagated in vitro,2 suggesting that T cells play a key role in antibody production. However, healthy individuals also have blood CD4+ T cells that can be activated in the presence of various autoantigens, including AChR.3 These findings suggest that autoreactive T cells are not deleted during normal T-cell maturation and that the crucial step in initiating an autoimmune disease could be the activation or penetration into the target organ of pre-existing autoreactive T cells, rather than the generation of new ones.3
The thymus is almost certainly implicated in MG pathogenesis.1 Thymic alterations (hyperplasia with germinal centers, hyperplasia with diffuse B-cell proliferation, or thymoma) are found in ∼80% of MG patients4 and thymectomy improves the clinical course of the disease.5,6 In fact the thymus is suspected to be the main site of autosensitization to AChR, because thymic epithelial cells (TECs) and muscle-like (myoid) cells express AChR on their surfaces. In addition, AChR-specific T-cell lines can be cloned from thymus, cultured thymic lymphocytes produce AChR-specific autoantibodies, and numerous germinal centers are present within the thymic medulla.1 However, what triggers autosensitization (loss of self-tolerance) remains a mystery.
The innate immune system is the first line of defense against pathogen invasion. Its effectors are dendritic cells, macrophages, natural killer (NK) cells, and NKT cells, as well as receptors (including Toll-like receptors, TLRs) and soluble components. There is evidence that elements of the innate immune system can generate signals that may potentiate the development of an autoimmune reaction.7,8 In experimental autoimmune MG, depletion of NK1.1-positive cells during the antigen-priming period drastically reduces clinical symptoms and AChR-specific T-cell proliferation.9 In MG patients, Vα24 NKT cell levels are significantly increased and produce high quantities of interferon-γ and interleukin (IL)-4.10 Because Vα24 NKT cells appear to be produced intrathymically,11,12 Reinhardt and Melms10 suggested that an altered microenvironment in the MG thymus might promote enhanced survival or even positive selection of these cells.
TLRs play a critical role in pathogen recognition and activation of the innate immune response. They are expressed differentially on immune and other cell types and appear to have the ability to respond to diverse foreign stimuli.8 As far as we are aware, no information on TLR expression in MG patients is available. We therefore performed a study to investigate TLR expression in thymus specimens from MG patients, grouped according to thymic pathology (hyperplasia with diffuse B-cell infiltration, referred to as thymitis, hyperplasia with germinal centers, thymoma, and involuted thymus).13–15
Materials and Methods
Patients
MG patients were diagnosed on the basis of fluctuating muscle weakness, fatigability, and neurophysiological and immunological criteria.16 Table 1 summarizes their clinical features. Written informed consent was obtained from all patients or their parents/guardians for thymectomy. Normal thymuses (n = 4) were obtained during heart surgery in cardiopathic patients in whom autoimmune diseases had been ruled out.
Table 1.
Clinical Features of MG Patients
| Thymus histology | Involuted (n = 12) | Hyperplasia (n = 11) | Thymitis (n = 6) | Thymoma (n = 9) |
|---|---|---|---|---|
| Sex (F/M) | 9/3 | 9/2 | 3/3 | 5/4 |
| Age at disease onset | 30.3 ± 9.3 | 26.1 ± 9.5 | 30 ± 7.1 | 48.1 ± 20.2 |
| Disease onset (less than/more than 40 years) | 10/2 | 10/1 | 6/- | 4/5 |
| Age at surgery | 36.5 ± 11.9 | 27.9 ± 9.8 | 31.5 ± 7.8 | 49.7 ± 19.8 |
| Ab AChR-positive | 9 | 10 | 3 | 9 |
| Ab AChR (pmol/ml) mean ± SD | 21.5 ± 24 | 21.5 ± 33.8 | 8 ± 10.7 | 11.2 ± 6.7 |
| Maximal disease severity* (NADIR) | IIIa = 2 | IIIa = 10 | IIIa = 3 | IIIa = 1 |
| IIIb = 5 | IVb = 1 | IIIb = 3 | IIIb = 5 | |
| IVb = 5 | IVb = 3 | |||
| Immunosuppressive therapy | 12 | 5 | 4 | 7 |
Classification according to the recommendations for clinical research standards by the Task Force of the Medical Scientific Advisory Board of the MGFA.
Thymus Processing
Thymic specimens were fixed in 10% formalin for histopathological evaluations or immediately frozen in isopentane, precooled in liquid nitrogen, and stored in liquid nitrogen pending polymerase chain reaction (PCR) and immunolocalization. Thymic hyperplasia (n = 12), thymitis (n = 6), and involution (n = 11) were defined according to standard histopathological criteria;13–15 thymoma (n = 9) according to the World Health Organization classification.17
Total RNA Extraction and cDNA Synthesis
Total RNA was extracted from 10 to 20 mg of thymic tissue using RNAwiz reagent (Ambion, Woodward Austin, TX) and treated with DNase I (Ambion). Random-primed cDNA was prepared using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions and stored at −20°C pending PCR amplification.
Semiquantitative PCR Analysis
Transcripts of TLRs 2 to 5 were assayed by the following semiquantitative method: a constant amount of cDNA (corresponding to 100 ng of total RNA) was amplified in a PCR reaction containing 1× PCR buffer (Finnzymes, Espoo, Finland), 0.1 mmol/L of each dNTP (Applied Biosystems, Foster City, CA), 1 U of DynaZyme DNA polymerase (Finnzymes), and 1 μmol/L each of the specific primers for each sequence. Primers sequences were: TLR2: forward 5′-GCCAAAGTCTTGATTGATTGG-3′, reverse 5′-TTGAAGTTCTCCAGCTCCTG-3′ (amplified fragment length, 346 bp); TLR3: forward 5′-AAATTGGGCAAGAACTCACAGG-3′, reverse 5′-GTGTTTCCAGAGCCGTGCTAA-3′, (amplified fragment length, 320 bp); TLR4: forward 5′-TGGATACGTTTCCTTATAAG-3′, reverse 5′-GAAATGGAGGCACCCCTTC-3′, (amplified fragment length, 507 bp); TLR5: forward 5′-CTCTGTTCCTCATGACCATCC-3′, reverse 5′-AGGTCAGATAAGCACCTGCC-3′ (amplified fragment length, 396 bp). Amplification conditions were 32 cycles of 94°C for 1 minute, annealing temperature according to primer sequence for 1 minute, and 72°C for 1 minute. The PCR products were separated by 2% agarose gel electrophoresis and the fluorescence intensity of the products quantitated with Quantity One software (Gel Doc EQ Gel Documentation System; Bio-Rad Laboratories, Hercules, CA). TLR expression was normalized to that of β-actin.18 The results were expressed as ratios of band intensities (±SEM) and grouped according to thymic histology.
Real-Time Quantitative PCR
Primers and probe for TLR4 were identified using the Primer Express software package (Applied Biosystems). Primer and probe sequences were: TLR4 forward 5′-CAGAGTTTCCTGCAATGGATCA-3′; TLR4 reverse 5′-TGCTTATCTGAAGGTGTTGCACAT-3′; TLR4 probe: 5′-AGGCAGCTCTTGGTGGAAGTTGAACGA-3′ (Applied Biosystems). Optimal concentrations for TLR4 primers and probe were determined according to the manufacturer’s instructions. For CD14 and MD-2 determinations, predesigned functionally tested assays (Applied Biosystems) were used. Each cDNA sample (corresponding to 100 ng of total RNA) was amplified in triplicate using a GeneAmp 5700 sequence detection system (Applied Biosystems) in a PCR volume of 25 μl containing the TaqMan Universal PCR Master Mix (containing AmpliTaq Gold DNA polymerase), 900 nmol/L of each TLR4-specific primer, and 250 nmol/L of TLR4-specific probe, alternatively 1 μl of predesigned CD14 and MD-2 primers and probes. GAPDH mRNA (Applied Biosystems) was used as endogenous control. The appearance of the specific fluorescence signal from the probe was analyzed using Sequence Detector Software (version 1.6, Applied Biosystems). The relative expression of TLR4, CD14, and MD-2 in thymic tissue was normalized to GAPDH and calculated from the formula 2−ΔΔCT as described in the manufacturer’s instructions. Normalized TLR4, CD14, and MD-2 CT values for healthy thymuses were used as calibrators.
The efficiency of TLR4 and GAPDH amplification was evaluated by measuring CT values after serial dilutions of the cDNA from template blood lymphocytes and production of a linear regression curve (log to base 2 of dilution against measured increase in cycle threshold, CT). Assuming that 100% efficiency corresponded to a slope of 3.32, the actual efficiency of TLR4 and GAPDH amplification was calculated: 3.185 and 3.43, respectively. To determine whether the efficiencies of the target and GAPDH were comparable, a validation experiment was performed. Log serial cDNA dilutions were plotted against average CT for TLR4 minus the average CT for GAPDH (ΔCT); if the amplification efficiencies for the two transcripts are similar, the absolute value of the slope is <0.1; in our conditions it was 0.0356.
Antibodies
The following primary antibodies were used for immunohistochemistry: rabbit polyclonal anti-TLR4 (working dilution, 1:20) (Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal pan anti-cytokeratin antibody, marker for epithelial cells (1:50, clone AE1/AE3; Zymed Laboratories, South San Francisco, CA); mouse monoclonal antibody anti-CD1a, a specific marker for thymic cortex (1:50, clone 010; DAKO, Glostrup, Denmark); rabbit polyclonal antibody anti-CD3, a specific marker for T cells (1:200, DAKO); mouse monoclonal antibody anti-CD20, a specific marker for B cells (1:100, clone L26; DAKO); mouse monoclonal antibody anti-CD21, specific marker for follicular dendritic cells (1:50, clone 1F8; DAKO); mouse monoclonal antibody anti-human dendritic reticulum cells (1:10, clone R4/23; DAKO); mouse monoclonal antibody anti-desmin to reveal myoid cells (1:100, D33; DAKO).
Immunohistochemistry
Routine histological analyses were performed on 6-μm-thick cryostat cut sections of thymus from all 37 MG patients and all 4 controls. Immunohistochemistry was performed on all six thymitis samples, three involuted thymuses, two thymomas, and all four healthy thymuses. Frozen sections were fixed in 96% ethanol for 15 minutes. After air-drying for 40 minutes, sections were incubated in 3% hydrogen peroxide for 5 minutes. After two rapid washes in water and Tris-buffered saline (TBS), sections were incubated with TBS plus 0.5% bovine serum albumin for 30 minutes at room temperature. Incubation with primary antibodies was performed for 1 hour at room temperature. After a 5-minute wash in TBS, sections were incubated with peroxidase-labeled polymer conjugated to secondary antibodies (Envision, DAKO); staining was completed by 5 minutes of incubation with 3,3′-diaminobenzidine according to the manufacturer’s instructions (Envision, DAKO). As negative control on serial sections primary antibody was omitted.
Double Immunohistochemistry
Confocal microscopy was performed on all six thymitis samples, three involuted thymuses, two thymomas, and all four healthy thymuses. Four-μm-thick cryostat cut sections were prepared and stained with mouse monoclonal antibody against TLR4 (Santa Cruz Biotechnology), in combination with rabbit polyclonal antibody against cytokeratin (Novocastra, Newcastle, UK) as described above, followed by incubation with Cy2-conjugated goat anti-mouse IgG and Cy3-conjugated goat anti-rabbit IgG (Jackson Immunoresearch Laboratories, West Grove, PA). Fluorescence images were captured from a confocal laser-scanning microscope (EclipseE600; Nikon, Tokyo, Japan and LaserSharp 2000 software; Bio-Rad, Hercules, CA).
Histometric Study of TLR4- and Cytokeratin-Positive Cells
On serial sections from all thymitis specimens and three involuted thymus specimens, the numbers of antibody-positive cells in 10 adjacent field areas (for a total area of 3.12 mm2 as viewed in a wide-angle Leitz Diaplan microscope with a ×10 eyepiece and a ×40 objective) were counted.19,20 For involuted thymuses field areas with thymic tissue residue were chosen.
Statistical Analysis
StatView software, release 5.01, was used for the statistical analyses (SAS Institute Inc., Cary, NC). All group comparisons were performed using analysis of variance. P values <0.05 were considered significant. Significant differences were further analyzed using post hoc Bonferroni/Dunn test for multiple comparisons. P values <0.005 were considered significant.
Results
Transcript Expression of TLR2 to TLR5
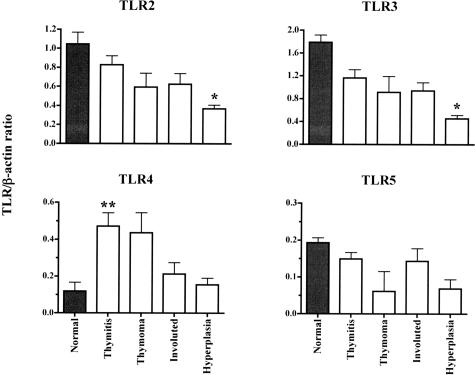
We used semiquantitative PCR to determine the expression of TLRs 2 to 5 in 37 MG thymuses, whose clinical characteristics are summarized in Table 1, and four nonpathological thymuses from young patients. All these TLRs were expressed in thymic tissues (Figure 1). Analysis of variance revealed significant differences between groups for all TLRs, except for TLR5. Post hoc tests (with P value corrected for multiple comparisons) showed that TLR2 and TLR3 mRNA levels were significantly higher in healthy thymus than MG-associated thymuses. By contrast TLR4 mRNA levels were higher in pathological than healthy thymuses, and levels differed significantly between thymitis and hyperplastic thymuses (P = 0.001).
Figure 1.
Steady-state TLR mRNA levels in MG thymitis, thymoma, involuted thymuses, and hyperplasia, and in healthy thymuses. The results are expressed as band intensities relative to β-actin and are plotted as means ± SEM. In general mRNA levels of TLR2, TLR3, and TLR5 are higher in healthy than pathological thymuses, with significant differences for hyperplasia for TLR2 and TLR3 (*P < 0.005, Bonferroni/Dunn test). By contrast, TLR4 mRNA expression was much higher in thymitis and thymoma than other specimens, with a statistically significance difference between thymitis and hyperplasia (**P < 0.005, Bonferroni/Dunn test).
High Expression of TLR4 Transcript in Thymitis and Involuted Thymus
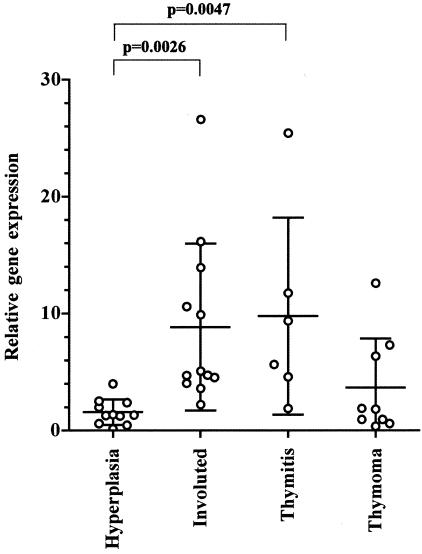
Subsequently, we accurately quantified TLR4 transcript levels in thymus specimens by real-time PCR using TLR4 expression in nonpathological thymuses as calibrator. Mean TLR4 (±SD) mRNA levels were significantly higher in thymitis (9.79 ± 8.43) and involuted thymus (8.85 ± 7.13) than in hyperplastic thymus (1.58 ± 1.10) (P = 0.0047 and 0.0026, respectively). Differences in TLR4 mRNA levels between thymoma (3.66 ± 4.21) and thymitis and involuted thymus were not significant, although TLR4 mRNA levels in thymoma are numerically lower (Figure 2).
Figure 2.
Overexpression of TLR4 mRNA in thymitis and involuted thymus. The transcript was analyzed by real-time PCR starting from total RNA extracted from MG thymuses. TLR4 levels were higher in thymitis and involuted thymus compared with hyperplastic thymus (P = 0.0047 and P = 0.0026, respectively). Horizontal bars are means with SDs. Values obtained from healthy thymuses were used as calibrator as required by the 2−ΔΔCT method.
Normal Expression of TLR4 Accessory Molecules in All Thymus Specimens, Irrespective of Pathology
We investigated by real-time PCR the expression of CD14 and MD-2 [accessory molecules required for TLR4-mediated responses to lipopolysaccharide (LPS)]21,22 in thymus specimens of MG patients and controls. We found that both CD14 and MD-2 transcripts were expressed in all specimens at similar levels with no difference between the thymic pathology groups. Table 2 shows the mean CD14 and MD-2 (±SD) mRNA levels in MG thymuses. Statistical analysis of the transcript levels did not show any significant differences between thymic alterations.
Table 2.
CD14 and MD-2 Transcript Levels in All Pathological and Normal Thymuses
| Thymus histology | CD14 (mean ± SD) | MD-2 (mean ± SD) |
|---|---|---|
| Hyperplasia | 3.68 ± 2.16 | 1.42 ± 1.03 |
| Involuted thymuses | 3.00 ± 1.78 | 1.06 ± 0.64 |
| Thymitis | 5.06 ± 3.53 | 2.10 ± 0.38 |
| Thymoma | 3.58 ± 2.68 | 1.27 ± 0.88 |
For each group of MG thymuses, the mean and SD are reported. The relative expression of TLR4 accessory molecules, CD14 and MD-2, were obtained by real-time PCR technique starting from total RNA extracted from thymus specimens. Values obtained from healthy thymuses were used as calibrator as required by the 2-ΔΔCT method (see Materials and Methods section).
Immunolocalization of TLR4 Protein in Thymus
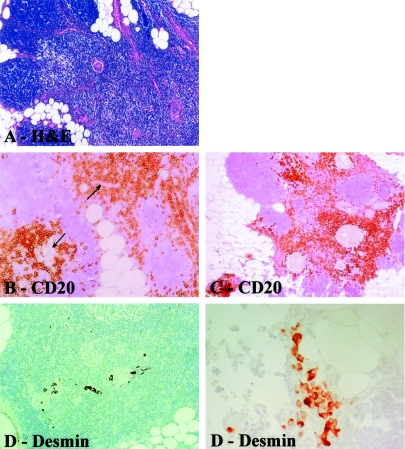
All thymitis cases were histologically similar, corresponding to thymitis with B-cell proliferation as described by Müller-Hermelink and colleagues14 and Kirchner and colleagues.15 Briefly, division of glands by fibro-fatty fibrous septa was maintained and each lobule consisted of a relatively dark cortex and paler medulla (Figure 3A). Rare lymphatic follicles were seen in one case. Perivascular spaces were filled by B lymphocytes (Figure 3B). The basal membrane between perivascular spaces and the medulla was only occasionally disrupted (periodic acid-Schiff reaction and silver methenamine, not shown). Rounded or spindle-shaped myoid cells were numerous and generally clustered in the medulla around Hassall’s corpuscles (Figure 3, D and E). The corpuscles were surrounded by T and B cells (Figure 3C). Polyclonal plasma cells were present both in cortex and medulla. Dendritic cells were always present in medulla.
Figure 3.
Photomicrographs showing histopathological characteristics of thymitis. A: H&E staining shows thymus gland with darker cortex (left) and paler medullary region (center and right). Note preserved lobular structure of cortex. B and C: Immunostainings with anti-CD20 antibody reveal B cells (CD20+), which are infiltrating perivascular spaces and surround Hassall’s corpuscles. D: Note Hassall’s corpuscles (center) surrounded by rounded and spindle-shaped myoid cells (desmin+). E: Clusters of desmin-positive myoid cells associated with indentated border within perithymic adipose tissue. Original magnifications: ×10 (A); × 20 (B–D); ×40 (E).
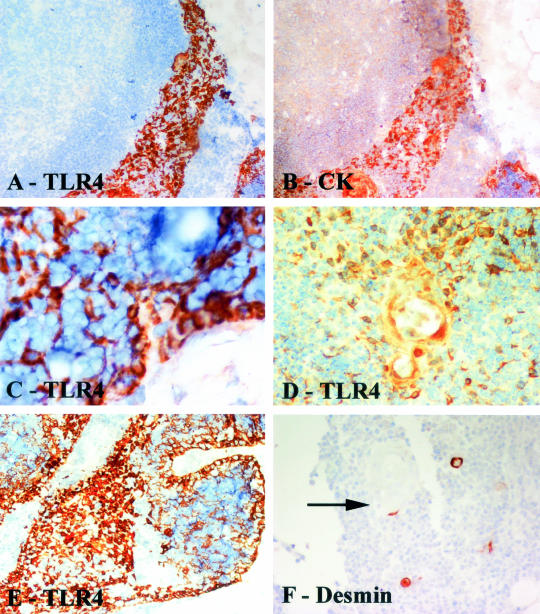
In thymitis specimens, TLR4 positivity was found in epitheliomorphic cells (Figure 4A), which in consecutive sections were intensely stained with anti-cytokeratin antibody (Figure 4B). These cells always surrounded lobulated borders, in close connection with the fibrous capsule, and were also present at the border between cortical and medullary regions (Figure 4; C to E). Myoid cells were frequently clustered in close proximity to clusters of TLR4-positive cells (Figure 4F). B cells were negative for TLR4 (data not shown).
Figure 4.
Immunostaining of thymitis specimens. A and B are serial sections: A, TLR4 immunostaining; B, cytokeratin immunostaining. Note that the TLR4-positive cytokeratin-positive TECs form a band adjacent to the cortex. C: TLR4 stains pericortical epithelial cells interspersed between cortical lymphoid cells. D: TLR4-positive cells clustered around a Hassall’s corpuscle. E: Pericortical and medullary staining of epithelial cells by TLR4. F: Immmunostaining to reveal myoid cells shows that these occasional cells are often associated with Hassall’s corpuscles (arrow). Tissue sections counterstained with hematoxylin. Original magnifications: ×20 (A, B, F); ×40 (C–E).
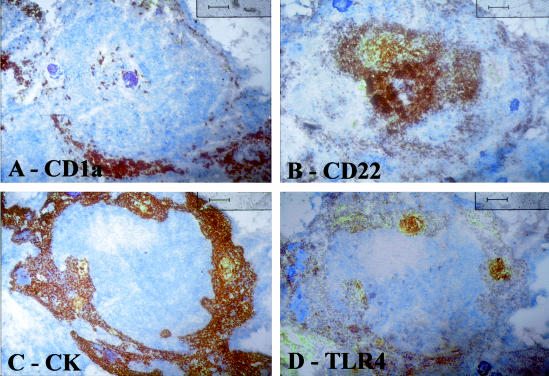
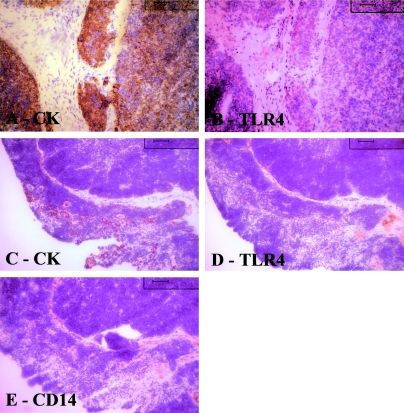
In involuted thymus specimens, the remnant islands of thymic tissue with Hassall’s bodies were positive for TLR4; in serial sections the same cells were cytokeratin-positive; when present, TLR4 positivity had a similar distribution to that observed in thymitis (Figure 5). In thymoma (Figure 6, A and B) TLR4 positivity was rarely found: the few TLR4-positive cells (Figure 6B) were usually shown to be macrophages on adjacent sections. In thymus from healthy children, positivity was sporadic apparently in macrophages and occasionally in epithelial cells (Figure 6).
Figure 5.
Serial sections of an involuted thymus. A: CD1a (thymocyte) staining; B: CD22 staining; C: cytokeratin staining; D: TLR4 staining. Note that TLR4 positivity is closely associated with epithelial (cytokeratin-positive) cells and that B cells CD22+ are negative for TLR4. All counterstained with hematoxylin. Original magnifications, ×10.
Figure 6.
A, B: Serial sections of thymoma; C–E: healthy young thymus. In A, cytokeratin staining reveals abundant epithelial cells, whereas B show few cells positive for TLR4. In normal thymus cytokeratin immunostaining (C) and macrophage immunostaining (E) reveal that these cells are fairly abundant. D: By contrast TLR4-positive cells are uncommon. Tissue sections counterstained with hematoxylin. Original magnifications: ×20 (A, B); ×10 (C–E).
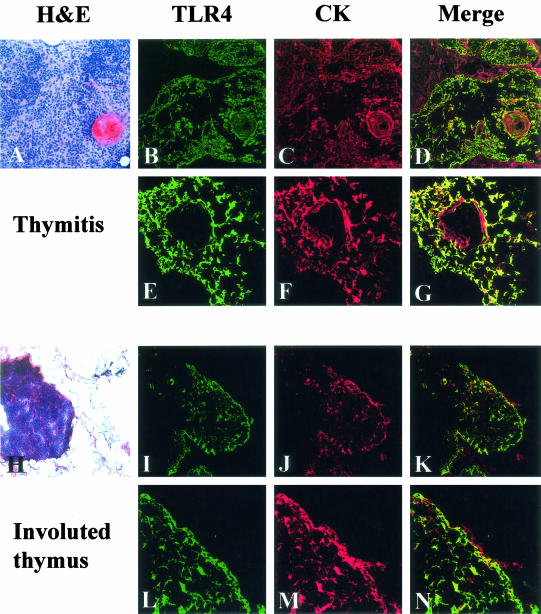
To further characterize TLR4 expression in the thymuses we performed double-immunofluorescence stainings for TLR4 and cytokeratin; representative results for thymitis (Figure 7; A to G) and involuted thymus (Figure 7; H to N) are shown. TLR4 signal (Figure 7; B, E, I, and L) was co-expressed mainly by cytokeratin-positive cells (Figure 7; C, F, J, and M, merged images Figure 7; D, G, K, and N). Numbers of cytokeratin-positive and TLR4-positive cells were determined on equivalent areas of serial sections. In thymitis, the mean numbers of cyto-keratin-positive and TLR4-positive cells per unit area were 125.83 ± 24.79 and 120.83 ± 35.92, respectively. In involuted thymuses the mean numbers of cytokeratin-positive and TLR4-positive cells were 70.33 ± 26.76 and 19.67 ± 23.03, respectively.
Figure 7.
Expression of TLR4 (B, E, I, L) on cytokeratin-positive cells (C, F, J, M) in thymitis (A–G) and involuted thymus (H–N), viewed under confocal microscope. Merged images (D, G, K, N) of TLR4 (green) and cytokeratin (red) show co-localization (yellow) of TLR4 and cytokeratin positivity. In thymitis samples, the double labeling is restricted to around Hassall’s bodies as shown by single immunostaining (Figure 4D). In involuted thymus, double immunofluorescence is positive on residual TECs. Original magnifications: ×10 (A, H); ×20 (B–D, I–K); ×40 (E–G); ×60 (L–N).
Discussion
TLRs seem to be key factors in innate immune system activation;8,23 in turn innate immune system activation (T-helper type-1 cell response) can be involved in the induction of acquired immunity.8,23 Recognition of pathogens or pathogen products by TLRs is followed by synthesis of proinflammatory cytokines that help sustain an inflammatory process. In rheumatoid arthritis, innate immune system activation has been shown to be part of the pathogenetic process,24–26 whereas enhanced expression of TLR3 and TLR4 has been observed in inflamed CNS tissue from multiple sclerosis patients.27 To our knowledge no data on TLR expression in MG patients have been reported previously.
Ten human TLR genes have so far been identified.28 We analyzed the expression of TLRs 2 to 5, the best characterized TLRs, that recognize ligands of both exogenous (gram-positive and gram-negative bacteria and other pathogens) and endogenous (heat-shock proteins, polypeptides, fibrinogen) origin.8,28 TLRs are expressed differentially in immune and other cell types, with highest transcript levels found in peripheral blood mononuclear cells, spleen, and tissues exposed to the external environment.29–31 Normal human thymus expresses all TLR transcripts but at lower levels than spleen.30
Using semiquantitative reverse transcriptase-PCR we found that transcripts of TLRs 2 to 5 were expressed in all thymus samples analyzed (Figure 1). TLR2 and TLR3 transcripts were mainly expressed in healthy thymuses, confirming previously reported data on tissue expression of TLRs;30 conversely, TLR4 transcripts were up-regulated in thymitis and thymoma (Figure 1) relative to healthy thymus.
We therefore quantified TLR4 levels in pathological and normal thymuses using the more sensitive and accurate real-time PCR technique.32 We found that highest levels of TLR4 expression were present in involuted thymus and thymitis, whereas in hyperplastic thymus and thymoma expression was low with little variation between patients (Figure 2). The real-time PCR results were supported by the protein immunostaining that showed positivity for TLR4 protein and expression confined to specific areas and cell types in thymitis and involuted thymuses (Figures 4 and 5), whereas thymoma thymuses showed only weak or sporadic positivity for TLR4 (Figure 6). Healthy tissue showed sporadic TLR4 positivity (Figure 6).
Confocal microscopy was consistent with these results showing that TLR4 was mainly expressed on TECs in thymitis and involuted thymus (Figure 7). Counting of cytokeratin- and TLR4-positive cells on adjacent sections in thymitis and involuted thymuses showed that the number of cytokeratin-positive cells (TECs) increased with the number of TLR4-positive cells and showed that most TECs were TLR4-positive. These findings indicate in MG patients with thymitis or involuted thymus, the thymus is activated, consistent with the idea it is the site of immunological reactions (usually AChR-specific activation of T cells and B-cell expansion)1 crucial for MG pathogenesis.
Thymic involution is found in 20 to 30% of MG patients (28.5% in our series),33 ∼40% of whom achieve true remission after thymectomy.6 This latter finding is difficult to reconcile with the prevalent opinion that thymic atrophy is nonpathological in MG, as it is in healthy people,4 and hence uninvolved in the disease process. Our finding that TLR4 expression is increased in involuted thymus adds further support to the idea that involuted thymus is involved in MG pathogenesis, and that a search for immunopathological alterations in involuted MG thymuses would be worthwhile.
Thymitis with diffuse B-cell proliferation (all six thymitis cases in the present study) is characterized by absence of the germinal centers that are present in lymphofollicular hyperplasia4 and is thought by some to be an early-stage hyperplasia that eventually progresses to the lymphofollicular stage with germinal centers. Lymphofollicular hyperplasia is typically found after azathioprine treatment.15,34 However, in our thymitis patients, time between disease onset and surgery was similar to that of the other groups and none of them had received azathioprine.
It is noteworthy that in patients with up-regulated thymic TLR4 transcripts (those with thymitis or involution), the TLR4 protein was found selectively on the TECs present in the medulla, and also occasionally at the border between cortex and medulla. These areas were rich in perivascular infiltrating B cells and also contained myoid cell clusters, both of which were always TLR4-negative (Figures 4, 5, and 7). There is evidence that medullary TECs are involved in MG pathogenesis via several mechanisms, including induction of pathogenic rather than regulatory behavior in nearby developing T cells, aberrant encounters with autoreactive T or B cells, and intimate contact between medullary epithelial bands and germinal centers.35 Medullary TECs, which are usually positive for AChR in MG, are in fact plausible candidates for initial autosensitization of T cells against AChR epitopes, giving rise to early autoantibody production with subsequent germinal center formation and expansion of antibody specificities.36 In this regard we note that Cohen-Kaminsky and colleagues37 demonstrated that, after stimulation of TECs with tumor necrosis factor-α plus LPS, both the number of cells expressing IL-6 mRNA and the quantities of cytoplasmic IL-6 mRNA per cell were increased, indicating that cytokines and LPS can modulate IL-6 production. LPS activation of TLR4 and subsequent activation of the TLR4-MyD88-NF-κB pathway may be responsible for expression of IL-6 and other proinflammatory cytokines.23 This proposed scenario is reinforced by our findings that CD14 and MD-2—essential for the innate response to LPS via TLR421,22—are efficiently transcribed in MG thymus. Our recent unpublished real-time PCR data on molecules downstream to TLR4 activation (eg, IL-6, NF-κB, and IκB) indicate differentially increased levels of these transcripts in different MG-associated thymic pathologies. Thus, in MG patients without thymoma, TLR4 expression on TECs, as demonstrated by confocal microscopy (Figure 7) could be a key step in the process leading to tolerance escape, reinforcing other data suggesting that TECs are involved in the immunopathogenesis of MG without thymoma.1
Levinson and colleagues38 recently described a model in which inflammation induced in the thymic medulla promotes the entry of peripheral CD4+ T cells into the thymus. This finding provides further evidence that an inflammatory reaction to an AChR-unrelated antigen within the medulla can facilitate entry of peripheral AChR-specific CD4+ T cells to the medulla where they would encounter a microenvironment favoring the immunopathogenetic process leading to MG. Intrathymic TLR4 up-regulation in thymitis and involuted thymus may represent a biological manifestation of such an inflammatory event.
Turning to consider to the MG cases with thymoma, we note that TLR4 mRNA expression in thymoma was low and the protein was detected only rarely. This finding is consistent with the view that mechanisms of MG pathogenesis in patients with thymoma differ from those in patients with a nonneoplastic thymus. In MG with thymoma, aberrant expression of neurofilaments, AChR, or titin epitopes is thought to give rise to the abnormal positive selection of autoreactive T cells;4 these may be activated locally or peripherally by mechanisms different from those observed in nonthymoma patients, and in particular endogenous or exogenous trigger factors may not play a significant role, consistent with the finding of no TLR4 up-regulation in thymoma tissue.
To conclude, ours is the first study to demonstrate up-regulation of an innate immunity-related molecule in the thymus of MG patients, suggesting a possible link between the effector arm of innate immunity and autoimmunity. We speculate that in a subgroup of MG patients an exogenous or endogenous danger signal (possibly proinflammatory cytokines) might activate the innate immune system giving rise to TLR4-mediated mechanisms contributing to autoimmunity. This hypothesis needs to be validated by further studies in vitro and on the experimental model of MG.
Acknowledgments
We thank Don Ward for critical discussion and help with the English; Mrs. C. Kluc, Gruppo Multimedica, for technical assistance; Dr. G. Finocchiaro and his group at our institute for advice on the real-time PCR technique; Dr. R. Spreafico, Dr. G. Battaglia, and their collaborators at our institute for advice on confocal microscopy; Dr. R. Pedotti for critical reading of the manuscript; and all physicians of the Neurology IV Department at Besta Institute for referring the patients.
Footnotes
Address reprint requests to Dr. Pia Bernasconi, Department of Neurology IV, Istituto Nazionale Neurologico “Carlo Besta”, Via Celoria 11, 20133 Milan, Italy. E-mail: pbernasconi@istituto-besta.it.
Supported by the Italian Ministry of Health (grants RF2001-94 to F.C. and RF2002-159 to R.M.).
References
- Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2:797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- Sommer N, Harcourt GC, Willcox N, Beeson D, Newsom-Davis J. Acetylcholine receptor-reactive T lymphocytes from healthy subjects and myasthenia gravis patients. Neurology. 1991;41:1270–1276. doi: 10.1212/wnl.41.8.1270. [DOI] [PubMed] [Google Scholar]
- Marx A, Wilisch A, Schultz A, Gattenlohner S, Nenninger R, Müller-Hermelink HK. Pathogenesis of myasthenia gravis. Virchows Arch. 1997;430:355–364. doi: 10.1007/s004280050044. [DOI] [PubMed] [Google Scholar]
- Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review). Neurology. 2000;55:7–15. doi: 10.1212/wnl.55.1.7. [DOI] [PubMed] [Google Scholar]
- Mantegazza R, Baggi F, Bernasconi P, Antozzi C, Confalonieri P, Novellino L, Spinelli L, Ferrò MT, Beghi E, Cornelio F. Video-assisted thoracoscopic extended thymectomy and extended transsternal thymectomy (T-3b) in non-thymomatous myasthenia gravis patients: remission after 6 years of follow-up. J Neurol Sci. 2003;212:31–36. doi: 10.1016/s0022-510x(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Shi F-D, Ljunggren HG, Sarvetnick N. Innate immunity and autoimmunity: from self-protection to self-destruction. Trends Immunol. 2001;22:97–101. doi: 10.1016/s1471-4906(00)01821-4. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Shi F-D, Wang HB, Li H, Hong S, Taniguchi M, Link H, Van Kaer L, Ljunggren HG. Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nat Immunol. 2000;1:245–251. doi: 10.1038/79792. [DOI] [PubMed] [Google Scholar]
- Reinhardt C, Melms A. Elevated frequencies of natural killer T lymphocytes in myasthenia gravis. Neurology. 1999;52:1485–1487. doi: 10.1212/wnl.52.7.1485. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Rivera MN, Park S-H, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Levitsky HJ, Golumbek PT, Pardoll DM. The fate of CD4−8− T cell receptor-alpha beta+ thymocytes. J Immunol. 1991;146:1113–1117. [PubMed] [Google Scholar]
- Eimoto T, Kusano T, Ando K, Kikuchi M, Shirakusa T, Kawanami S. Nonneoplastic and nonhyperplastic thymus in myasthenia gravis. An immunohistochemical study with double immunoenzymatic labeling of basement membrane and cellular components. Am J Clin Pathol. 1990;94:36–43. doi: 10.1093/ajcp/94.1.36. [DOI] [PubMed] [Google Scholar]
- Müller-Hermelink HK, Marx A, Kirchner T. Thymus. Damianov I, Lindner J, editors. St Louis: Mosby Year-Book,; Anderson’s Pathology. 1996:pp 1218–1243. [Google Scholar]
- Kirchner T, Schalke B, Melms A, von Kugelgen TV, Müller-Hermelink HK. Immunohistological patterns of non-neoplastic changes in the thymus in myasthenia gravis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52:237–257. doi: 10.1007/BF02889966. [DOI] [PubMed] [Google Scholar]
- Jaretzki A, III, Barhon RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America Myasthenia gravis. Recommendations for clinical research standards. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- Marx A, Müller-Hermelink HK. From basic immunobiology to the upcoming WHO-classification of tumors of the thymus. The Second Conference on Biological and Clinical Aspects of Thymic Epithelial Tumors and Related Recent Developments. Pathol Res Pract. 1999;195:515–533. doi: 10.1016/s0344-0338(99)80001-6. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-β1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis PSJ, Whitehead R. Mitosis counting—a need for reappraisal. Hum Pathol. 1981;12:3–4. doi: 10.1016/s0046-8177(81)80235-3. [DOI] [PubMed] [Google Scholar]
- Elston CW, Ellis IO. Pathological factors in breast cancer. 1. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva CJ, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex: transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptors signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Klinman D. Does activation of the innate immune system contribute to the development of rheumatoid arthritis? Arthritis Rheum. 2003;48:590–593. doi: 10.1002/art.10852. [DOI] [PubMed] [Google Scholar]
- Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, Michel BA, Seger RA, Gay S, Lauener RP. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Muzio M, Polentarutti N, Bosisio D, Manoj Kumar PP, Mantovani A. Toll-like receptor family and signalling pathway. Biochem Soc Trans. 2000;28:563–566. doi: 10.1042/bst0280563. [DOI] [PubMed] [Google Scholar]
- Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Rossi CR, Pilati P, Nitti D, Marincola FM. Quantitative real-time PCR: a powerful ally in cancer research. Trends Mol Med. 2003;9:189–195. doi: 10.1016/s1471-4914(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Mantegazza R, Baggi F, Antozzi C, Confalonieri P, Morandi L, Bernasconi P, Andreetta F, Simoncini O, Campanella A, Beghi E, Cornelio F. Myasthenia gravis (MG): epidemiological data and prognostic factors. Ann NY Acad Sci. 2003;998:413–423. doi: 10.1196/annals.1254.054. [DOI] [PubMed] [Google Scholar]
- Schalke BC, Mertens HG, Kirchner T, Wegener S, Müller-Hermelink HK. Long-term treatment with azathioprine abolishes thymic lymphoid follicular hyperplasia in myasthenia gravis. Lancet. 1987;2:682. doi: 10.1016/s0140-6736(87)92460-3. [DOI] [PubMed] [Google Scholar]
- Roxanis I, Micklem K, Willcox N. True epithelial hyperplasia in the thymus of early-onset myasthenia gravis patients: implications for immunopathogenesis. J Neuroimmunol. 2001;112:163–173. doi: 10.1016/s0165-5728(00)00415-x. [DOI] [PubMed] [Google Scholar]
- Shiono H, Roxanis I, Zhang W, Sims GP, Meager A, Jacobson LW, Liu JL, Matthews I, Wong YL, Bonifati M, Micklem K, Stott DI, Todd JA, Beeson D, Vincent A, Willcox N. Scenarios for autoimmunization of T and B cells in myasthenia gravis. Ann NY Acad Sci. 2003;998:237–256. doi: 10.1196/annals.1254.026. [DOI] [PubMed] [Google Scholar]
- Cohen-Kaminsky S, Delattre RM, Devergne O, Rouet P, Gimond D, Berrih-Aknin S, Galanaud P. Synergistic induction of interleukin-6 production and gene expression in human thymic epithelial cells by LPS and cytokines. Cell Immunol. 1991;138:79–93. doi: 10.1016/0008-8749(91)90134-w. [DOI] [PubMed] [Google Scholar]
- Levinson AI, Zheng Y, Gaulton G, Moore J, Pletcher CH, Song D, Wheatley LM. A new model linking intrathymic acetylcholine receptor expression and the pathogenesis of myasthenia gravis. Ann NY Acad Sci. 2003;998:257–265. doi: 10.1196/annals.1254.027. [DOI] [PubMed] [Google Scholar]