Abstract
Spatial memory of animals is usually tested in navigation tasks that do not allow recognition and recall processes to be separated from the mechanisms of goal-directed locomotion. In the present study, place recognition was examined in rats (n = 7) confined in an operant chamber mounted on the periphery of a slowly rotating disk (diameter 1 m, angular velocity 9°/s). The animals were passively transported over a circular trajectory and were rewarded for bar pressing when they passed across a 60°-wide segment of the path. This segment was recognizable with reference to room landmarks visible from the operant box. Responding defined in the coordinate system of the room increased when the chamber entered the 60°-wide approach zone, culminated at the entrance into the reward sector, was decreased inside it by eating the available reward, and rapidly declined to zero at the exit from this zone. When reward was discontinued, the skewed response distribution changed into a symmetric one with a maximum in the center of the reward sector. With advancing extinction, the response peak in the reward sector decreased in most rats proportionally to the overall decline of bar pressing. The rewarded and nonrewarded response patterns indicate that passively transported rats can recognize their position in the environment with an accuracy comparable to that of actively navigating animals and that location-driven operant responding can serve as a useful tool in the analysis of the underlying neural mechanisms.
Although recall and recognition tasks are the main tools used in human memory research (1, 2), they are less clearly defined in animal psychology. This is particularly true for recall because unassisted retrieval of information stored in the memory of the subject can be easily assessed in verbal reports of humans, but is almost impossible to isolate from the often complex behavior of animals. Thus, in experiments conducted in the Morris water maze (3), navigation to the hidden escape platform can be considered as demonstrating recall of its allothetic coordinates, but, at the same time, as a series of recognition steps corresponding to places passed on the way to the goal. In fact, a direct swim from the start to the goal suggests that the animal first recognizes its own position in the pool, then recalls the perceptually unavailable location of the goal, and finally computes the azimuth of the shortest direction to the goal. As the animal approaches the goal, the recall component of the task is gradually replaced by the recognition component, which becomes dominant in the vicinity of the goal. In this particular case, existence of recall is indicated by the initial direction of the swim, which cannot be produced without retrieval of information contained in the memory.
In the navigation setting, place recognition implies activation of the corresponding cognitive map containing both the location of the goal and the present position of the subject relative to salient landmarks. The correctness of the corresponding recognition and recall processes is confirmed by the success of the goal-directed locomotion. The progress of navigation is monitored by a series of recognition steps, confirming or disproving the expectations based on the cognitive map employed. The discrepancy between expectation and reality leads to corrections ranging from minor changes of swim direction to complete abandonment of the pursued trajectory and to onset of a new search or an attempt to use a different map.
The overlap of the processes implementing recognition of the current location of the subject and locomotion to the recalled position of the goal makes it difficult to dissociate the underlying physiological mechanisms and examine them separately. This is particularly important in research into the role of hippocampal place cells (4), which are believed to form the cellular substrate of cognitive maps (5) and which may take part in the processes of place recall, place recognition, and place navigation. It is not clear, however, whether the firing of place cells represents response to a specific sensory input (visual, vestibular, kinesthetic) corresponding to the current position of the animal in the charted environment or whether it also reflects the anticipation of the position to which the performed or intended movement will lead (6). Foster et al. (7) have addressed this problem in rats in which firing fields of hippocampal place cells were first established during pellet chasing and then retested when the same animal wrapped in a towel was passively transported into and out of the firing field. The results of this study were inconclusive, however, because the spatially selective activity of hippocampal place cells was suppressed by restraint. This may either indicate that restraint precludes retrieval of movement-space associations or that the passively transported animal does not pay attention to its position in the environment. The purpose of the present study is to eliminate the latter possibility by developing a place-recognition task in which a rat confined in an operant chamber is passively transported over a circular path and rewarded by food for bar pressing emitted during passage of this chamber through a definite region of the environment defined in the reference frame of the room. Because place recognition triggers, in this case, a nonlocomotor go—no go reaction comparable to the “true-false” decision used in recognition-memory tasks in human subjects (8)—the task does not require active locomotion of the animal, and this mechanism cannot, therefore, be responsible for any place-cell activation. Preliminary descriptions of the place-recognition task have been reported (9, 10).
Methods
Animals.
Seven adult male Long–Evans rats (350–450 g) obtained from the breeding colony of the Institute of Physiology, Czech Academy of Sciences (Prague) were used for the experiment. They were housed in groups of two in a temperature-controlled room (20°C) with a natural light/dark cycle. Water was freely available, but access to food was restricted to maintain the rats at 85% of their free feeding weight.
Apparatus.
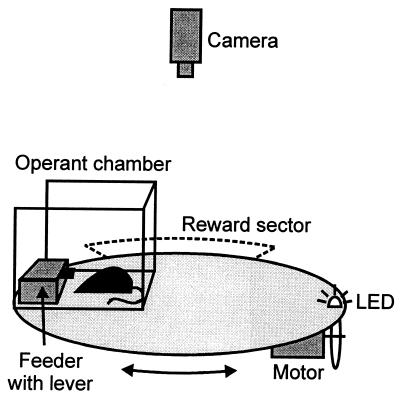
The apparatus (Fig. 1) consisted of an elevated circular arena (70 cm above the floor, 104 cm in diameter) that could be rotated with an angular velocity of 9°/s. On the periphery of the arena, an operant chamber (30 × 30 × 40 cm) with three transparent perspex walls and open centrifugal side was mounted. It contained a feeder (18 × 6 × 9 cm) equipped with a lever and a supply of 20-mg pasta pellets, accessible through a window (1 × 1.5 cm) in the feeder wall occluded by a sliding plate. This plate was moved aside by a solenoid when the animal was rewarded. The position of the feeder in the room was monitored by a television camera connected to a computer. The camera was located above the center of the arena and was set up for detecting an infrared light-emitting diode on the perimeter of the arena. The computer controlled the movement of the arena and access to pellets and recorded bar presses. The apparatus was located near the southern wall (80 cm) in a room (4 × 4 m) with many cues along the walls (tables, windows, door, shelves).
Figure 1.
The apparatus used in the place recognition experiments.
Pretraining.
Rats used in this experiment underwent extensive training in earlier versions of the task and were pretrained to reach a stable performance level in its current modification.
Place-Recognition Task.
A rat was placed in the operant chamber with the feeder. The transparent walls of the box allowed the rat to survey not only the nearly 90°-wide segment facing the feeder but the entire room. Bar presses were rewarded only when the feeder was passing through a 60°-wide reward sector of the circular trajectory defined in the coordinate system of the room. This induced an increased density of bar pressing in or close to the reward sector.
The rotation continued for 1 s after each “effective” bar press and then stopped for 5 s. Bar presses emitted during these 6 s (ineffective bar presses) were not rewarded and had no effect on the movement of the arena. Presses emitted outside this time interval within the target sector were rewarded by opening the feeder during the next 5-s stop. The above procedure was adopted to prevent delivery of food while the arena was rotating, when its consumption would distract the animal's attention. The 1-s rotation after each bar press guaranteed that the arena could not be held at one place but moved in at least 9° steps. Stopping the arena for 5 s after an incorrect bar press increased the accuracy of the place-recognition response because, in this way, each error was punished by delaying the entrance into the reward sector. All bar presses emitted when the arena was moving (i.e., the effective bar presses and the ineffective ones emitted during the first second after the effective bar press) were used in the evaluation of bar pressing.
Changing the direction of rotation at pseudorandom intervals prevented the rat from solving the task as a fixed interval schedule. The nine points where the arena changed the direction of rotation were ±90°, ±135°, 180°, ±225°, and ±270° far from the center of the reward sector. The length of the rotation in the same direction ranged from 225° to 540°. The rotation was programmed in such a way that in the absence of bar pressing the feeder spent an equal amount of time in each sector after passing through the reward location 10 times. This interval, a “hypercycle,” was used as a unit to measure the duration of a session. The hypercycle lasted 400 s and increased in duration by 5 s for each effective bar press emitted at a longer then 6-s interval, i.e., when the arena was moving.
Data Evaluation.
The data were analyzed by ANOVA with repeated measures followed by post hoc comparisons where appropriate.
Results
Rewarded Place Recognition.
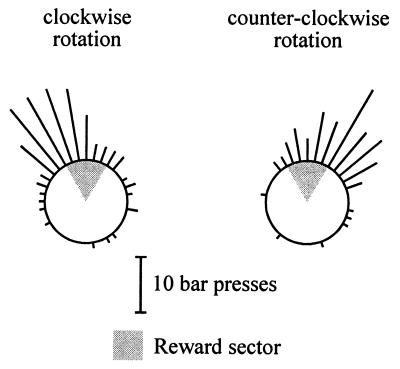
Rats pretrained during 14 days to asymptotic performance in recognizing reward sectors oriented to the north, east, south, and west were trained on four subsequent days to recognize the above sectors again. Each session consisted of eight hypercycles formed by 40 clockwise (CW) and 40 counterclockwise (CCW) passes through the reward sector. Fig. 2 illustrates the results obtained in one session in the form of circular histograms (10° bins) corresponding to the circular trajectory of the operant chamber.
Figure 2.
Circular histogram of bar pressing emitted by a typical rat during one session of rewarded bar pressing. The CW and CCW histograms correspond each to 40 passes through the reward sector.
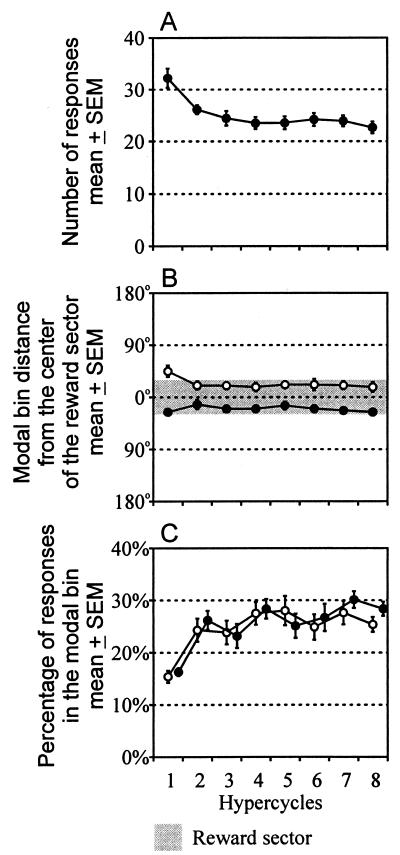
At the beginning of the experiment, bar pressing tended to be uniformly distributed all over the circular path, but, as soon as several rewarded bar presses made it possible to identify the target sector, bar pressing almost disappeared in the opposite sections of the trajectory and concentrated in the approach path to the expected reward. The transformation of the response pattern was completed in the second hypercycle and remained unchanged in the final four hypercycles. This intrasession development is illustrated in Fig. 3, showing the mean number of all emitted responses per hypercycle (Fig. 3A), the average distance of the modal bin from the center of the reward sector (Fig. 3B), and the percentage of responses in the modal bin (Fig. 3C). ANOVA with repeated measures showed a significant main effect of the hypercycle factor [F(7, 42) = 6.629, P < .001]. Newman–Keuls test indicated that only the first hypercycle was significantly different from all the remaining ones. Two-way ANOVA with repeated measures performed on the data illustrated by Fig. 3B showed a significant main effect of the sense direction of rotation [F(1, 6) = 43.838, P < .001], but no significant main effect of hypercycles [F(7, 42) = 1.262, not significant (n.s.)] and no significant interaction [F(7, 42) = 1.569, n.s.]. Similar ANOVA for Fig. 3C demonstrated a significant main effect of the hypercycles [F(7, 42) = 10.744, P < .001], but no significant main effect of the direction of rotation [F(1, 6) = .702, n.s.] and no significant interaction [F(7, 42) = .690, n.s.]. Newman–Keuls tests performed separately for the CW and CCW rotations indicated that the first hypercycle was significantly different from all remaining ones in the amplitude of the modal bin.
Figure 3.
Development of bar pressing in the eight successive hypercycles forming a session. Means of results obtained in seven rats each trained in four different goal locations. Abscissa: Ordinal number of the hypercycle. (A) Mean (±SEM) number of responses per hypercycle. (B) Mean (±SEM) angular distance of the 20° modal bin of the response histogram from the center of the reward sector. ● and ○ denote CW and CCW rotation, respectively. (C) Mean (±SEM) percentage of responses in the 20° modal bin. ● and ○ denote CW and CCW rotation, respectively.
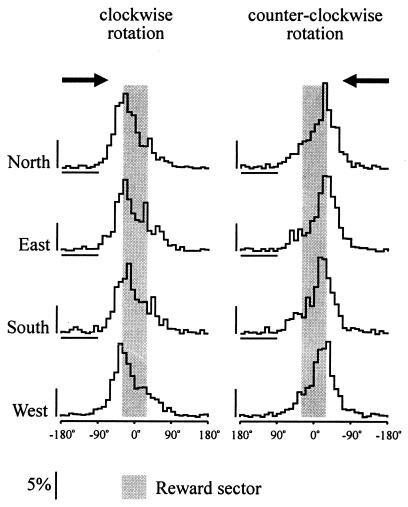
The normalized histograms of bar pressing recorded in the seven rats during the last six hypercycles at the four locations of the reward sector were essentially similar (Fig. 4). Evaluation of the modal angle with two-way ANOVA with repeated measures showed a significant main effect of the direction of rotation [F(1, 6) = 29.199, P < .002], but no significant main effect of target location [F(3, 18) = .567, n.s.] and no significant interaction [F(3, 18) = .895, n.s.].
Figure 4.
Histograms of the bar pressing emitted during CW and CCW passes (indicated by arrows) of the operant chamber through the reward sector in four different locations. Bin width, 10°. Ordinate: Mean percentage of responses in individual bins. Abscissa: Angular distance from the center of the reward sector.
The histograms in Fig. 4 show that the bar-pressing rate started to increase about 60° before the reward sector boundary and reached maximum in the last bin before or first bin after this boundary. Response rate then dropped rather abruptly in the first half of the reward sector to about 50% of the maximum and then slowly declined until the operant level was reached 60° after the exit boundary. A mirror response pattern was produced when the operant chamber traversed the reward sector in the opposite direction. With the reward sector in the north, peak response rates were observed at the approximate azimuths 330° or 30° when the arena rotated CW or CCW, respectively. The 60° angular distance between these response peaks corresponded to the reward sector width, but did not depend on the location of the reward sector nor on the salience, size, and distance of the remote landmarks. Thus, the landmarks visible in the north and west were more distant from the arena (about 2–3 m) than those present in the east (1 m) and south (0.2 m), but this difference was reflected neither in the accuracy nor in the amplitude of the response peaks.
The steep increase of the response rate and its abrupt drop after the first rewarded bar press is obviously because of a major change of behavior taking place at this time: the nonrewarded bar pressing is replaced by consummatory activity triggered by the first opening of the feeder. The hungry animal spends all 5 s of the interrupted rotation at the open feeder and needs a further 2–5 s to finish eating and return to the bar. Although theoretically the rat can emit up to five rewarded responses when passing through the reward sector, the delayed return to the bar decreases the number of actually produced responses to one or two. This is illustrated by Fig. 5, showing the position of the Skinner box during the first three and the last passes through the reward sector in the fourth hypercycle and the locations where effective (●) or ineffective (○) responses were emitted by the seven rats. The density of effective responses in the last 30° section before the reward sector boundary indicates that the rat pressed the bar as soon as the arena started to move again, i.e., that sometimes the rats advanced through this region at steps not much exceeding the 9° displacement produced by the 1-s rotation triggered by an effective bar press.
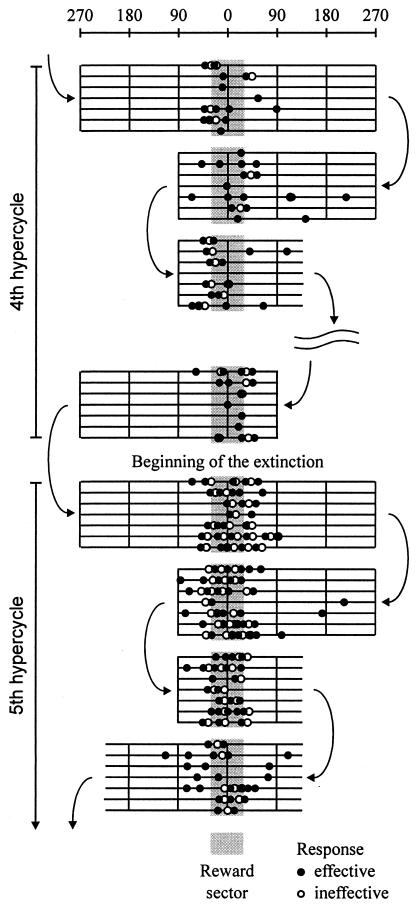
Figure 5.
Transition of rewarded to nonrewarded bar pressing at the onset of extinction. The seven lines in each block indicate the passage of individual rats over the trajectory extent (the upper scale) with the location of each bar press denoted by ● or ○ for the effective and noneffective responses, respectively. The first three blocks in the fourth (rewarded) and the fifth (nonrewarded) hypercycle share the same trajectory. The extinction-induced response change is illustrated by the difference between the last rewarded passage and the first nonrewarded passage through the reward sector. The curved arrows indicate the direction of the passive transport.
Absence of reward after effective bar pressing increases responding in the anticipation zone but decreases it in the exit zone. This differential reaction to the same event indicates that the animal's response depends not only on the recognition of the actual position of the Skinner box but also on the direction of rotation and on the anticipation of the immediate consequences of the actual movement of the arena. The importance of the latter factors is best documented by the observation that bar pressing often stops after the last rewarded bar press, obviously because the animal recognizes that it has already left the reward sector and is moving away from it.
Place Recognition During Extinction.
The interfering effect of consummatory activity on the recognition-triggered bar pressing can be removed by elimination of reward when the Skinner box passes across the reward sector. In a series of experiments, the rats were trained to a new reward sector, and, after they reached asymptotic performance in the fourth hypercycle, rewarding was discontinued in subsequent hypercycles. The absence of reward caused a dramatic change of the response pattern in the first three nonrewarded passes through the reward sector. This is shown in Fig. 5, indicating that the incidence of effective bar pressing was higher inside than outside the reward sector and that it was actually higher in the central 30° of the reward sector than in the 30° arc corresponding to the approach maximum seen when the bar pressing was rewarded. This pattern was found during the fifth hypercycle in all rats, but, with continued extinction, responding gradually declined in all parts of the trajectory and almost ceased in the last hypercycles.
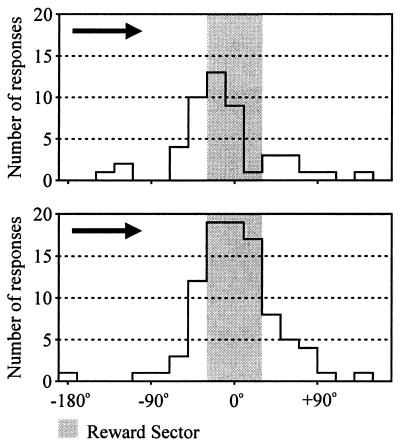
Fig. 6 shows the cumulative histogram of bar pressing emitted in the first three passes through the reward sector at the onset of extinction by all seven rats. The symmetric distribution reaches about 66% of the peak response rate at the boundary of the reward sector. Comparison of the distribution of anticipatory bar pressing in the matched rewarded passes with the corresponding segment of the extinction distribution showed that the two distributions are not statistically different and that in the absence of reward the projected maximum of the anticipatory bar pressing distribution would reach an amplitude exceeding the anticipatory peak by 50% in the center of the reward sector. This comparison suggests that the recognition process is based on a holistic assessment of the reward sector rather than on mere recognition of its boundaries.
Figure 6.
Histographic comparison of operant responding in the corresponding three rewarded (Upper) and the first three nonrewarded passes (Lower) shown in Fig. 5. Ordinate: Total number of responses emitted by all seven rats. Abscissa: Negative values indicate angular distance from the approaching center of the reward sector, positive values the distance from the already passed center of the reward sector.
Discussion
Although the results of the above experiments show the rats' proficiency in place recognition, it is less clear whether they demonstrate implicit (procedural) or explicit (declarative) memory (11, 12), i.e., whether they are accomplished by the operation of an automatic learning mechanism beyond the reach of awareness or by conscious reflection and judgement (13). The neural mechanisms involved can range from temporal anticipation, delayed conditioning, and priming to conscious awareness indispensable for human recall, i.e., to processes differentially affected in various types of human amnesia (14). Although procedural and declarative memory cannot be easily differentiated in animal models of amnesia, it is generally believed that spatial learning and navigation can serve as a counterpart of declarative memory in animals (5). Place navigation is seriously impaired by hippocampectomy (15), which does not, however, disrupt other types of learning. Klement et al. (16) demonstrated that the place-recognition task used in the present study is blocked during tetrodotoxin-induced (5 ng TTX in 1 μl saline) inactivation of both dorsal hippocampi. The functional ablation procedure does not interfere with bar pressing, the frequency of which increases more than twice after TTX injection, but which is uniformly distributed all over the circular trajectory of the operant box and shows no peak corresponding to the location of the goal sector. This result indicates that place recognition is similar to place navigation in that they are both hippocampus-dependent tasks. On the other hand, it is necessary to stress that the procedural components of such tasks are not impaired by blockade of the hippocampus. Hippocampal inactivation prevents a rat overtrained in the Morris water maze from swimming directly to the goal (presumably because it cannot recall the exact coordinates of the goal), but does not interfere with the procedural components of navigation, e.g., with the search strategy, a motor skill allowing the animal to find the goal by visiting all possible locations in the pool (17). In the place-recognition task, elimination of the hippocampus can be similarly compensated by increased bar pressing, which may provide delivery of the same reinforcement albeit at the expense of a higher effort.
The bar-pressing distribution in the reinforced place-recognition task might suggest that the rat recognizes only the entrance into the reward sector marked by the modal peak of responding in the narrow 20° bin at its border and that after the first rewarded bar press it continues to respond until the first nonrewarded response indicates that it is outside the reward sector. Such an interpretation is obviously incorrect, however, because responding at the exit from the reward sector does not always stop after a nonrewarded bar press, but is often terminated by the last rewarded bar press inside the reward sector. Discontinuation of bar pressing under such circumstances indicates the animal's recognition that it has left the reward sector and that the probability of reward is zero.
The anticipatory responding on the 60° approach to the reward sector cannot be explained by associative mechanisms alone, because these responses are never reinforced. Simple conditioning with a noncorrection procedure would produce maximum responding inside the reward sector. It is conceivable that the response pattern observed in the present study is a result of a deliberate trade-off between the nonrewarded responses needed for the earliest detection of the entrance into the reward sector and the possibility to access the open feeder repeatedly when the first rewarded bar press occurs at the entrance into the goal sector. Proximity to the reward sector alone cannot account for the observed changes of responding, which express anticipation of reinforcement availability on approach to the goal and absence of reward on exit from it.
Place Recognition During Extinction.
After the delivery of reward suddenly stops, rats' responding to the position of the operant chamber is no longer disturbed by the interfering effect of consummatory activity. The bar-pressing rate increases almost to the theoretical maximum of five effective responses during a single passage through the previously rewarded sector. Because the maximum effective response rate is flattened by this procedurally imposed ceiling, the motivation of the animal is better reflected by the increased rate of ineffective bar presses (emitted when the operant box is not moving), the incidence of which culminated in the center of the reward sector and started to decline at its exit border. The symmetry of the bar press distribution during extinction shows that the animal appreciates the rewarded sector as a single region in which the probability of reward increases smoothly from the sector boundaries to its center. The availability of reward does not influence the anticipatory response distribution, which overlaps with the distribution obtained in the same animal in the first extinction passes. The asymmetry of the distribution of rewarded bar presses is due to the blockade of the extinction peak by eating. Slightly faster decline of the exit portion of the distribution may reflect the appearance of the first nonrewarded bar press after a series of rewarded ones. This can signal to the animal that it has left the reward zone and that the reward probability has decreased to zero.
The extinction process leads, after the first three or four nonrewarded cycles, to changes of responding that are different in individual animals. The response peak in the reward sector decreased in most rats proportionally to the overall decline of bar pressing. Some animals reduced responding in the reward sector and increased bar pressing in other parts of the trajectory in an obvious attempt to check the possibility that the reward sector moved to a different location. This behavior resembling the search strategy typical for finding the location of the goal position at the beginning of a new session was, however, soon abandoned in the absence of reward. In the third and fourth extinction hypercycles, bar pressing almost disappeared in six of seven animals.
Place Recognition and Place Navigation.
The specific feature of the place-recognition task described in the present study is passive transport of the animal, not its movement, over a circular path, which can be also accomplished by active navigation. Moser and colleagues (18, 19) have recently published a series of reports describing place navigation in a ring-shaped pool containing, at a fixed position, an escape platform made available after the animal had swum a full circle. After the animal has learned the task, its performance was examined in a probe trial in which the rat usually spent up to 40% of the search in the target area instead of the 12.5% corresponding to chance level. Some of these experiments used a computer-controlled on-demand platform (20, 21), which is raised from the bottom of the pool only when the rat has spent a criterion dwell time in the target area. Successful solution of such a task requires that the rat slows down swimming or starts treading water in the target area, i.e., emits a sort of operant activity comparable to the bar pressing used in the present experiment. Similarity with the present study could be further increased by replacing active locomotion of the animal at least partly by rotation of water (22), the movement of which must be counteracted by the animal if it has to raise the platform by spending the criterion time in the trigger area.
Circular paths are also generated in the standard water maze when an overtrained rat searches a new position of the platform at the 50% distance between the center of the pool and the wall (23). Efficient target finding can only be achieved when the rat succeeds in following a trajectory with sufficient accuracy comparable to uniform distribution of bar pressing in rats searching for a new location of the reward sector in the present experiment.
At a more general level, the analogy can be extended to the comparison of place recognition extinction with the water maze probe trial. In neither case does the absence of reinforcement initially interfere with responding. Only after the animal has convinced itself that the spatially defined operant response (bar pressing during passage through a certain region or visit of a definite part of the pool) does not provide the expected reward (food or escape platform), will it stop the ineffective responding and start a new search (distributed bar pressing or visiting all parts of the pool).
The comparison of the above observations suggests that the spatial decisions made during active locomotion and passive transport are essentially similar and are probably supported by the same neural mechanisms. It is concluded that examination of place cells during performance of the place-recognition task by passively transported rats may allow better control of the conditions influencing their activity (position, rate, and direction of movement) and contribute to better understanding of their role in spatial behavior.
Acknowledgments
We thank R. G. M. Morris for helpful suggestions and L. Nadel for critical reading of the manuscript. We appreciate the practical help and advice of E. Pastalkova and A. A. Fenton for the conception and realization of the experiments. We are particularly thankful to Yu Kaminsky for the development of the computerized system that made these experiments possible and to A. Zahalka for the construction of the apparatus. The research was supported by Grant 309/97/0555 from the Granting Agency of the Czech Republic.
Abbreviations
- CW
clockwise
- CCW
counterclockwise
- n.s.
not significant
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040576197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040576197
References
- 1.Hintzman D L. The Psychology of Learning and Memory. San Francisco: Freeman; 1978. p. 456. [Google Scholar]
- 2.Tulving E. Elements of Episodic Memory. New York: Oxford Univ. Press; 1983. [Google Scholar]
- 3.Morris R G M. Learn Motiv. 1981;12:239–260. [Google Scholar]
- 4.O'Keefe J, Dostrofsky J. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. p. 570. [Google Scholar]
- 6.Muller R U, Kubie J L. J Neurosci. 1989;9:4101–4110. doi: 10.1523/JNEUROSCI.09-12-04101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster T C, Castro C A, McNaughton B L. Science. 1989;244:1580–1582. doi: 10.1126/science.2740902. [DOI] [PubMed] [Google Scholar]
- 8.Shepard R N. J Verbal Learn Verbal Behav. 1967;6:156–163. [Google Scholar]
- 9.Bures J. In: Perception, Memory and Emotion: Frontiers in Neuroscience. Ono T, McNaughton B L, Rolls E T, Nishijo H, editors. Oxford: Elsevier; 1996. pp. 291–303. [Google Scholar]
- 10.Bures J, Fenton A A, Kaminsky Yu, Wesierska M, Zahalka A. Neuropharmacology. 1998;37:689–699. doi: 10.1016/s0028-3908(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 11.Schacter D L. J Exp Psychol Learn Mem Cognit. 1987;13:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- 12.Squire L R. Memory and Brain. New York: Oxford Univ. Press; 1987. [Google Scholar]
- 13.Mandler J M. Ann NY Acad Sci. 1990;608:485–516. doi: 10.1111/j.1749-6632.1990.tb48907.x. [DOI] [PubMed] [Google Scholar]
- 14.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 15.Jarrard L M. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 16.Klement D, Pastalkova E, Kelemen E, Fenton A A. Physiol Res. 1999;48:S84. [Google Scholar]
- 17.Whishaw I Q. Brain Res Bull. 1989;23:347–358. doi: 10.1016/0361-9230(89)90221-9. [DOI] [PubMed] [Google Scholar]
- 18.Hollup S A, Donnett J G, Jeffery K J, Moser M-B, Moser E J. Soc Neurosci Abstr. 1999;25:1623. [Google Scholar]
- 19.Steffmach H-A, Moser E J, Moser M-B. Soc Neurosci Abstr. 1999;25:1622. [Google Scholar]
- 20.Buresova O, Krekule I, Zahalka A, Bures J. J Neurosci Methods. 1985;15:63–72. doi: 10.1016/0165-0270(85)90062-7. [DOI] [PubMed] [Google Scholar]
- 21.Spooner R I W, Thompson A, Hall J, Morris R G M, Salter S H. Learn Mem. 1994;1:203–211. [PubMed] [Google Scholar]
- 22.Moghaddam M, Bures J. Neurobiol Learn Mem. 1997;68:239–251. doi: 10.1006/nlme.1997.3800. [DOI] [PubMed] [Google Scholar]
- 23.Bures J, Buresova O. In: Machinery of Mind. John E R, editor. Boston: Birkhauser; 1990. pp. 291–310. [Google Scholar]