Abstract
Because oxidative stress has been strongly implicated in up-regulation of vascular endothelial growth factor (VEGF) expression in ischemic retinopathy, we evaluated the role of NAD(P)H oxidase in causing VEGF overexpression and retinal neovascularization. Dihydroethidium imaging analyses showed increased superoxide formation in areas of retinal neovascularization associated with relative retinal hypoxia in a mouse model for oxygen-induced retinopathy. The effect of hypoxia in stimulating superoxide formation in retinal vascular endothelial cells was confirmed by in vitro chemiluminescence assays. The superoxide formation was blocked by specific inhibitors of NAD(P)H oxidase activity (apocynin, gp91ds-tat) indicating that NAD(P)H oxidase is a major source of superoxide formation. Western blot and immunolocalization analyses showed that retinal ischemia increased expression of the NAD(P)H oxidase catalytic subunit gp91phox, which localized primarily within vascular endothelial cells. Treatment of mice with apocynin blocked ischemia-induced increases in oxidative stress, normalized VEGF expression, and prevented retinal neovascularization. Apocynin and gp91ds-tat also blocked the action of hypoxia in causing increased VEGF expression in vitro, confirming the specific role of NAD(P)H oxidase in hypoxia-induced increases in VEGF expression. In conclusion, NAD(P)H oxidase activity is required for hypoxia-stimulated increases in VEGF expression and retinal neovascularization. Inhibition of NAD(P)H oxidase offers a new therapeutic target for the treatment of retinopathy.
Retinopathy of prematurity and diabetic retinopathy are leading causes of blindness in infants and adults world wide.1 Studies of experimental animal models of retinopathy of prematurity and diabetes as well as patients with diabetic retinopathy indicate that the two diseases are characterized by retinal ischemia, excess production of the angiogenic growth factor vascular endothelial growth factor (VEGF), and pathological neovascularization within the retina and vitreous.2,3 Because no therapy is available to prevent retinal ischemia, ophthalmologists currently use laser photocoagulation to ablate the ischemic retinal tissue to suppress the pathological neovascularization. Preclinical studies using inhibitors of VEGF to prevent retinal neovascularization have shown promising results.4 Therefore, elucidation of the cellular and molecular mechanisms underlying ischemic retinopathy and control of VEGF expression is of major clinical importance.
Hypoxia is one of the most potent inducers of VEGF expression.5 Under in vivo conditions, ischemic hypoxia leads to increased generation of reactive oxygen species. NAD(P)H oxidase is a major source of superoxide generation during hypoxia and it has been suggested that NAD(P)H oxidase can serve as an oxygen sensor that is activated by hypoxia in various cell types.6–9 Vascular endothelial cell NAD(P)H oxidase consists of the same components found in phagocytic NAD(P)H oxidase—two membranous subunits, gp91phox and p22 phox; three cytosolic subunits p40phox, p47phox, and p67phox; and the small GTP binding protein, Rac.10–12
Oxidative stress has been strongly implicated in up-regulation of VEGF expression in patients and experimental animals with ischemic retinopathy and in tissue culture cells.13 Analyses in a rat model for type 2 diabetes have shown elevated levels of oxidative stress and H2O2 formation correlated with elevated retinal levels of VEGF and its receptors VEGF-R1 and VEGF-R2.14,15 Studies showing that anti-oxidant treatments attenuate diabetes-induced increases in retinal VEGF formation provide strong support for the potential role of reactive oxygen species in stimulating VEGF overexpression in vivo.16 Recent evidence shows that a gp91phox containing NAD(P)H oxidase is a major source of reactive oxygen species in endothelial cells.17 NAD(P)H oxidase has also been implicated in redox-mediated induction of VEGF expression in smooth muscle cells and keratinocytes.18–21 Thus, NAD(P)H oxidase may represent an important new target for control of retinal neovascularization in ischemic retinopathy. The goal of this research was to test and develop this idea and to determine the effects of inhibiting NAD(P)H oxidase on retinal neovascularization and VEGF expression in ischemic retinopathy.
Materials and Methods
Mouse Model of Ischemic Retinopathy
All experimental procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Committee for the Use of Animals in Research. Oxygen-induced retinopathy was induced in C57BL/6 mice using the protocol of Smith and colleagues22 as described previously.23,24 On postnatal day 7 (P7), mice were placed in 75% oxygen (hyperoxia) and after 5 days (P12) they were returned to room air. The hyperoxia exposure causes obliteration of the developing vessels in the posterior retina. This results in relative hypoxia and causes vitreoretinal neovascularization that begins at P14 to P15 and reaches a peak at P17.22 Age-matched mice raised in room air were used as normoxia controls. Groups of the hyperoxia-treated mice were injected with the NAD(P)H oxidase inhibitor apocynin (Sigma, St. Louis, MO) after return to room air. The apocynin was injected intraperitoneally (10 mg/kg in 50 μl of 0.9% saline) from P12 through P17. A group of mice injected with 0.9% saline (50 μl) served as vehicle controls. In another experiment groups of mice received apocynin or saline injections from P12 to P22.
Cultured Retinal Cells
Primary cultures of bovine retinal endothelial cells (passage 7 to 9) or rat Muller glial cells (passage 4 to 6)25 were used in these experiments. The effect of hypoxia on formation of reactive oxygen species was measured using chemiluminescence, dihydroethidium (DHE), and dichlorofluorescein (DCF) assays. For treatment, cells were placed in serum-free medium and then incubated in normoxia (21% O2, 160 mmHg) or hypoxia (1% O2, 7.6 mm Hg) for 6 hours. The hypoxia exposure was performed in a humidified incubator under the control of a PROOX model 110 oxygen regulator (Reming Bioinstruments, Redfield, NY). The oxygen level was continuously monitored using the PROOX oxygen sensor.
Analysis of Vaso-Obliteration and Neovascularization
Vascular distribution was analyzed in the neonatal retinas by using flat-mount preparations labeled with biotinylated Griffonia simplicifolia isolectin B4 (GSI) and Texas Red-conjugated avidin D as previously described.26 Retinas were viewed with LSM 510 confocal microscopy (Carl Zeiss, Thornwood, NY) and the images captured in digital format (Spot System; Diagnostic Instruments, Sterling Heights, MI). The areas of vaso-obliteration and of vitreoretinal neovascularization were quantified from the digital images in masked manner, using IP Lab Spectrum Scientific Image System (Signal Analytics, Vienna, VA).
Immunolocalization Studies
The retinal distribution of gp91phox was analyzed using an anti-gp91phox antibody (Transduction Laboratories, San Diego, CA) and Oregon Green-conjugated secondary antibody (Molecular Probes, Portland, OR). The co-localization of gp91phox protein with vascular endothelial cells was determined by double labeling with GSI and anti-CD 31 (BD Biosciences Pharmingen, San Diego, CA) and Texas Red-labeled secondary antibody. The co-localization with Muller glial cells was determined by double labeling with anti-glial fibrillary acidic protein (GFAP) (DAKO, Carpinteria, CA).
Western Blot
For analysis of gp91phox protein, pooled retinas were homogenized in a modified RIPA buffer [20 mmol/L Tris-HCl (pH 7.4), 2.5 mmol/L ethylenediamine tetraacetic acid, 50 mmol/L NaF, 10 mmol/L Na4P2O7, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate, 1 mmol/L phenylmethyl sulfonyl fluoride] and 75-μg protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and reacted with anti-gp91phox (1:1000) followed by horseradish peroxidase-linked secondary antibody and enhanced chemiluminescence (Amersham Pharmacia, San Francisco, CA). Membranes were stripped and reprobed for β-actin to demonstrate equal loading and results were analyzed using densitometry. For quantitation of VEGF levels, protein samples were processed according to the method described by Ferrara and Henzel.27 Briefly, protein samples from mouse retina or cultured endothelial cells (100 μg) were adjusted to a volume of 1 ml using 10 μmol/L Tris (pH 7.4) and 100 μmol/L NaCl and incubated overnight with 50 ml of equilibrated heparin-agarose beads (Sigma). Samples were boiled in sodium dodecyl sulfate-sample buffer (100°C, 10 minutes) to elute the proteins that were then electrophoresed in 4 to 20% Tris-HCl gradient gels (Bio-Rad Laboratories, Hercules, CA), transferred to nitrocellulose membranes, and probed with anti-VEGF antibody (Oncogene, San Diego, CA).
Reactive Oxygen Species Assays
DHE Imaging
Superoxide production in retinal tissue sections and cultured endothelial cells was assayed by using the oxidative fluorescent dye DHE. DHE is oxidized on reaction with superoxide to ethidium bromide, which binds to DNA in the nucleus and fluoresces red.28 For the in vivo studies serial cryosections from fresh-frozen retinas were first incubated in NAD(P)H (100 μmol/L) or NAD(P)H with PEG-SOD (400 U), the NAD(P)H blocking peptide gp91ds-tat (50 μmol/L) or apocynin (30 μmol/L) for 20 minutes followed by DHE with or without PEG-SOD (Sigma), gp91ds-tat, or apocynin (20 minutes, 37°C). Apocynin (4-hydroxy-3-methoxy-acetophenone) specifically blocks activity of NAD(P)H oxidase by interfering with the assembly of the cytosolic NAD(P)H oxidase components (p40phox, p47phox, p67phox) with the membranous components gp91phox and p22phox,29,30 whereas gp91ds-tat specifically inhibits NAD(P)H oxidase assembly by binding to p47phox and blocking its interaction with gp91phox.20,31,32 This peptide has been shown to specifically inhibit gp91phox-based oxidases in endothelial cells and neutrophils.33 The gp91ds-tat blocking peptide and the control peptide (scramb-tat) were prepared by the MCG Molecular Biology Core Laboratory and Invitrogen (Carlsbad, CA) as described by Rey and colleagues.31 DHE images from serial sections treated with or without inhibitors were obtained using a laser-scanning confocal microscope. Some sections from both groups were preincubated in cell permeable superoxide dismutase (PEG-SOD, 30 minutes). This treatment completely blocked the DHE staining, demonstrating specificity of the reaction for superoxide. For studies in vitro, equal numbers of endothelial cells in 24-well plates were incubated in serum-free medium with or without PEG-SOD, apocynin, gp91ds-tat, scramb-tat as gp91ds-tat control, or vehicle (0.01 N acetic acid in normal saline). The plate was then incubated for 6 hours in normoxia or hypoxia. The medium was then replaced by Earle’s balanced salt solution containing the same inhibitors and DHE (2 μmol) followed by incubation under the same conditions for 20 minutes. Images were collected by fluorescence microscopy and analyzed for reaction intensity in equal numbers of cells (10 cells from each field, five fields total) using the Metamorph Image System (Molecular Devices Corp., Downingtown, PA).
DCF
DCF the oxidation product of 2,7-dichlorodihydro-fluorescein diacetate (DHDCF), a marker of cellular oxidation by hydrogen peroxide, peroxynitrite, and hydroxy radicals.34 For in vivo assays retinas were lysed in buffer containing phosphate (50 mmol/L/L, pH 7.0), EGTA (1 mmol/L), protease inhibitors, and sucrose (150 mmol/L). Tissue was mechanically lysed using a glass/Teflon homogenizer in ice-cold buffer. Equal volumes of retinal lysates were incubated with DHDCF (10 μmol/L) for 60 minutes at 37°C. The specificity of the reaction was determined by incubating 100 μl of normal retinal lysate in buffer containing DHDCF with or without 4-phorbol 2-myristate 13-acetate, a known upstream stimulator of NAD(P)H oxidase and PEG-SOD, PEG-SOD/catalase, or gp91ds-tat. Fluorescence was measured in a CytoFluor 4000 spectrophotometer (Foster City, CA) with excitation of 450 nm and emission of 530 nm. For in vitro studies retinal endothelial cells were plated in 96-well plates and incubated in normoxia or hypoxia for 6 hours with and without PEG-SOD (400 U), gp91ds-tat (50 μmol/L), or scramb-tat (50 μmol/L). One hour before the end of the experiment the medium was replaced with Earle’s balanced salt solution containing the same inhibitors and DHDCF followed by incubation in normoxia or hypoxia for 60 minutes at 37°C. Fluorescence was measured as described above.
Chemiluminescence
Cells were plated in 96-well plates in serum-free medium with or without PEG-SOD (400 U) or NAD(P)H oxidase inhibitors, apocynin (30 μmol/L) or the gp91ds-tat blocking peptide (50 μmol/L),31 then incubated in normoxia or hypoxia. One hour before measuring superoxide the medium was replaced with 50 μl of Earle’s balanced salt solution with the same treatment and the plates were returned to the incubator. At the end of the hypoxia exposure 50 μl of Earle’s balanced salt solution containing 800 μmol/L of highly sensitive luminol derivative L-012 (Wako Pure Chemical Industries, Osaka, Japan)35 that was dissolved in dimethylsulfoxide was added to each well at final concentration of 400 μmol/L. Then chemiluminescence was measured using an automated microplate reader (Lumistar Galaxy; BMG Lab Technologies, Offenburg, Germany).
Measurement of Retinal Lipid Peroxidation
Lipid peroxide concentration was determined by a method that measures the amount of thiobarbituric acid reactivity by the amount of malondialdehyde (MDA) formed during acid hydrolysis of the lipid peroxide compound. Retinal tissue homogenates were prepared and reacted with sodium dodecyl sulfate, acetic acid, and thiobarbituric acid as described previously.16 Tetraethoxypropane was used to establish a standard curve. Lipid peroxide level was expressed in terms of ng MDA per mg protein.
Results
Induction of Superoxide Anion Formation by Hypoxia
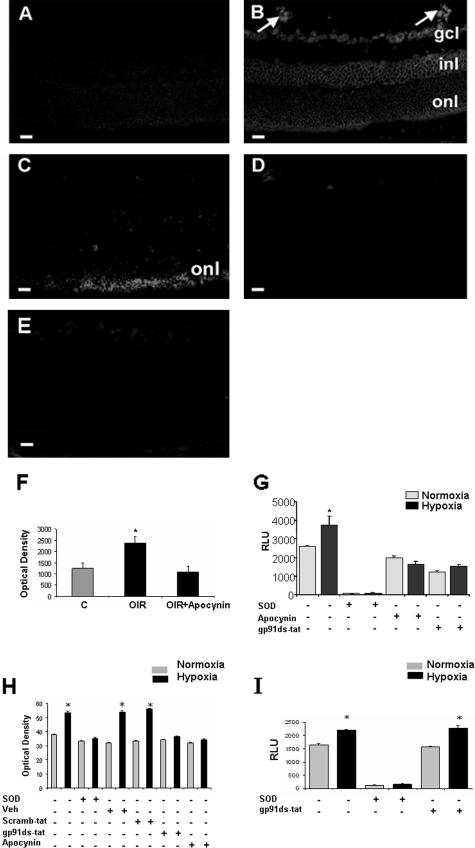
DHE imaging was used to localize superoxide formation in ischemic retinas at P17, which is the peak time of retinal neovascularization in this model. The normal control retinas were negative for the superoxide signal. Superoxide was detected within all cellular layers of the ischemic retina, but was prominently increased within areas of neovascularization (Figure 1). The DHE staining within the retinal vasculature was completely blocked in adjacent sections that were preincubated with the specific inhibitors of NAD(P)H oxidase activity apocynin or gp91ds-tat. The inhibitor treatment also greatly reduced the reaction within the cellular layers of the inner retina. Weak staining was sometimes evident within the nonvascular cells of the inhibitor-treated retinas and was most evident within the outer nuclear layer of the ischemic retinas. This residual staining is probably due to another source of superoxide formation such as mitochondrial oxidase. Specificity of the DHE reaction for superoxide anion was demonstrated by complete blockade of the staining with PEG-SOD. These DHE results were confirmed by using DCF assay, which showed a significant increase in oxidative stress in retinas of OIR mice. This increase was completely blocked in retinas of apocynin-treated mice (Figure 1F). The specificity of DCF assay was confirmed by positive control studies using 4-phorbol 2-myristate 13-acetate to activate NAD(P)H oxidase. This treatment caused an increase in DCF reaction that was inhibited by PEG-SOD/catalase and gp91ds-tat (data not shown).
Figure 1.
Superoxide generation in mouse retina was assessed in vitro by using the oxidative fluorescent dye DHE in retinal sections from mice maintained in room air (A) or exposed to hyperoxia for 5 days and returned to room air for 5 days (B–E). There was a marked increase in fluorescence intensity in the retinas and new vessels of the ischemic retina (arrows, B) as well as within the ganglion cell layer (gcl), inner nuclear layer (inl), and outer nuclear layer (onl). DHE reaction was reduced in retinal sections treated with gp91ds-tat (C), apocynin (D), or PEG-SOD (E). DCF assay of reactive oxygen species production in retina (F) showed a significant increase in retina during OIR that was inhibited in apocynin-treated mice (*P = < 0.05, n = 5). Chemiluminescence assay of superoxide production by retinal endothelial cells (G) or rat Muller cells (I) exposed to hypoxia (1% O2, 6 hours) shows a significant increase in the generation of superoxide anion. Treatment with the NAD(P)H oxidase inhibitors apocynin or the gp91ds-tat blocking peptide prevented the hypoxia-induced increase in endothelial cells but not in rat Muller cells. The signal was completely eliminated by treatment with PEG-SOD (*P = < 0.05, n = 4; RLU, relative light unit). DHE assay of superoxide production by endothelial cells (H) showed a significant increase in hypoxia-treated cells that was blocked by PEG-SOD, gp91ds-tat, and apocynin (*P = < 0.05, n = 5). Scale bars, 20 μm.
To study the role of vascular endothelial cell NAD(P)H oxidase in increasing superoxide anion formation in the ischemic retina, we exposed cultured retinal endothelial cells to hypoxia in the presence or absence of NAD(P)H oxidase inhibitors and assayed superoxide formation by chemiluminescence. These experiments showed significant increases in superoxide production in the hypoxia-treated endothelial cells (Figure 1G). Treatment with the NAD(P)H oxidase-blocking peptide gp91ds-tat or apo-cynin completely blocked the hypoxia-induced increase in superoxide formation. The generation of superoxide was eliminated by PEG-SOD, indicating the specificity of the assay for superoxide anion. These data were confirmed by DHE and DCF assays in retinal endothelial cells. DHE showed a significant increase in reactive oxygen species production in retinal endothelial cells treated with hypoxia, which was blocked by PEG-SOD, apocynin, and gp91ds-tat (Figure 1H). These results were confirmed by DCF reaction (data not shown). To test whether another type of retinal cell could be a potential source of NAD(P)H-derived superoxide we exposed Muller glial cells to hypoxia and measured superoxide generation using chemiluminescence. Hypoxia induced a significant increase in superoxide generation by Muller cells, this increase was not blocked by gp91ds-tat (Figure 1I).
Up-Regulation of gp91phox Protein by Retinal Ischemia
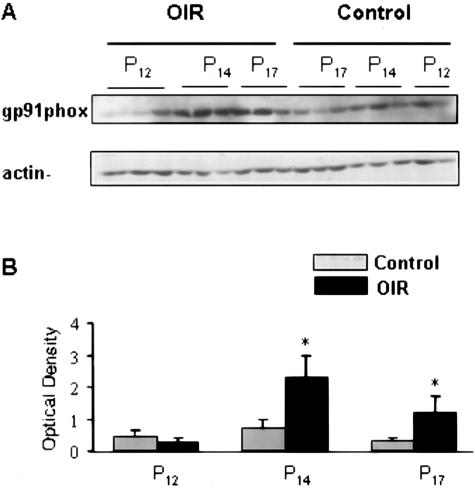
The electron transfer component of the phagocytic NAD(P)H oxidase resides in the catalytic subunit gp91phox, and its association with p22phox is essential for function of flavocytochrome b. Because gp91phox containing NAD(P)H oxidase has been shown to be a major source of superoxide generation by endothelial cells we investigated its expression during retinal ischemia. Western blot analysis showed a time-dependent increase in levels of gp91phox in the ischemic retina (Figure 2). The amount of gp91phox protein was significantly increased in the hypoxic retina and the maximal effect was evident at P14 when retinal neovascularization first begins. This protein expression pattern suggests that increased expression of gp91phox is involved in the induction of retinal neovascularization.
Figure 2.
Immunoblot analysis of gp91phox expression at P12, P14, and P17. A: Levels of gp91phox are markedly increased at P14 and P17 in retinas of mice raised in hyperoxia for 5 days and returned to room air for 5 days (OIR) as compared with age-matched controls (control) raised in normoxia. B: Results were quantified by densitometry (*P < 0.05 versus age-matched control, n = 6).
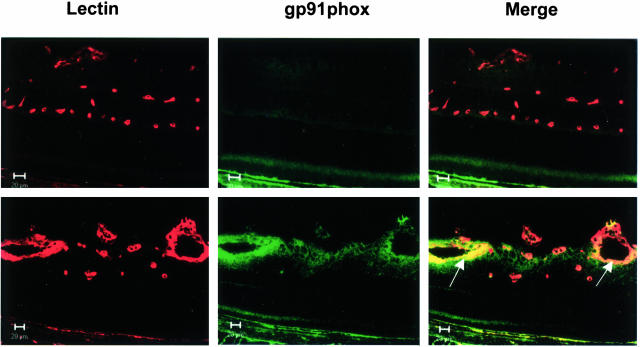
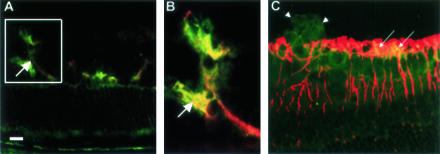
To examine the distribution of gp91phox protein expression in relation to retinal neovascularization, we used double-label immunofluorescence to co-localize gp91phox and the vascular endothelial cell marker GSI. This analysis showed very weak immunoreactivity for gp91phox within the inner retinal layers of the normoxia control mice (Figure 3). The GSI-labeled vascular endothelial cells were negative for gp91phox during normoxia. By contrast, the proliferating vessels in the ischemic retina were strongly immunoreactive for gp91phox. Further investigation using the vascular endothelial cell marker CD31 (PECAM-1) also showed co-localization with gp91phox, confirming that endothelial cells are a major source of gp91phox expression during ischemic retinopathy (Figure 4). Co-localization of gp91phox with glial cell marker GFAP showed occasional weak immunoreactivity in GFAP-positive processes in the nerve fiber layer suggesting limited gp91phox expression in retinal glia.
Figure 3.
Immunohistochemical localization of gp91phox and vascular endothelial cells. Retinal sections from normoxia control (top) and ischemic retinas (bottom) were reacted with GSI lectin to label the vessels (red) and gp91phox antibody (green). Merged images show low levels of gp91phox protein in the normal retina and marked increases in the ischemic retina, especially within the proliferating vessels (yellow, arrows). Scale bars, 20 μm.
Figure 4.
Immunohistochemical localization of gp91phox with vascular endothelial cells (A, B) and with retinal glia (C) at P17 in the ischemic retina. Double labeling with antibodies against CD31 (red) and gp91phox (green) shows that gp91phox co-localizes with vascular endothelial cells in the ischemic retina (yellow, arrows). Inset in A is shown at higher magnification in B. Double labeling with antibodies against GFAP (red) and gp91phox (green) shows a limited co-localization of gp91phox with retinal glia (yellow, arrows), whereas the neovascular tuft showed strong gp91phox expression (arrowheads). Scale bar, 20 μm.
Prevention of Retinal Neovascularization by Inhibition of NAD(P)H Oxidase
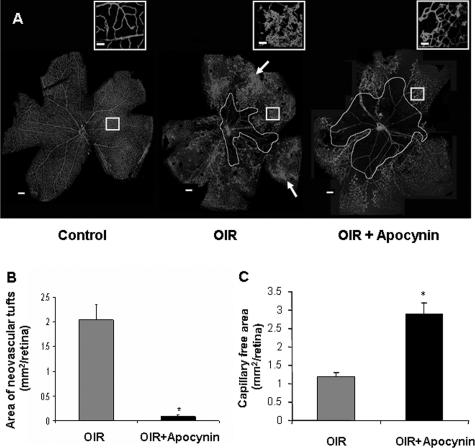
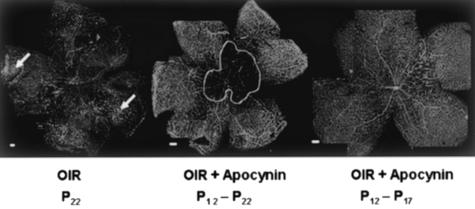
The above results showing that increases in NAD(P)H oxidase activity and gp91phox expression accompany retinal neovascularization suggest that NAD(P)H oxidase activity has a key role in ischemic retinopathy. To test this hypothesis, mice were treated with apocynin to inhibit NAD(P)H oxidase activity from P12 to P17, which is the period of retinal ischemia and neovascularization. This treatment almost completely blocked pathological neovascularization within the retina and vitreous as shown by retinal imaging and morphometric analysis (Figure 5, A and B). In addition to preventing the vitreoretinal neovascularization, the apocynin treatment also slowed the regrowth of new capillaries into the area of capillary dropout surrounding the optic disk. The area of the central capillary-free zone was substantially larger in retinas of the apocynin-treated mice (2.9 ± 0.3 mm2) than in the control group (1.2 ± 0.1 mm2) (Figure 5C). This inhibition of vessel regrowth was confirmed by additional studies showing continued inhibition of revascularization when the apocynin treatment was continued from P12 to P22 (Figure 6). This result indicates that NAD(P)H oxidase activity is required for both neovascularization and revascularization of the central retina. To test whether or not the apocynin effect on revascularization is reversible, we performed additional analyses in which mice were treated with apocynin from P12 to P17 and then allowed to recover until P22. This study showed that almost complete revascularization of the central retina occurred within 5 days after treatment was terminated, indicating that the apocynin effect in preventing revascularization is reversible (Figure 6).
Figure 5.
Flat-mounted retinas reacted with GSI lectin to localize neovascularization and capillary obliteration. A: Mice were maintained in hyperoxia from P7 to P12, returned to room air and treated with apocynin (10 mg/kg/day, ip) or vehicle from P12 to P17. Retina from P17 control mouse shows a normal vascular pattern. Retina from nontreated P17 hyperoxia-exposed mouse shows neovascular tufts in the mid-periphery (arrow) and a zone of capillary obliteration around the optic disk. Retina from apocynin-treated P17 mouse shows almost no neovascular tufts and a large capillary-free zone around the optic disk. B: Quantitative comparison of the area occupied by neovascular tufts in the ischemic retinas on P17 shows a significant decrease in neovascularization in the apocynin-treated mice as compared with the nontreated mice (*P < 0.05, n = 5). C: Measurement of the capillary-free zone in the ischemic retinas on P17 shows a significant decrease in revascularization of the central retina of apocynin-treated mice as compared with the nontreated mice (*P < 0.05, n = 5). Scale bars: 100 μm; 20 μm (inset).
Figure 6.
Flat-mounted retinas reacted with GSI lectin to localize neovascularization and capillary obliteration. Mice were maintained in hyperoxia from P7 to P12, returned to room air for 10 days and treated with apocynin (10 mg/kg/day, ip) or vehicle for various times. Retina of a nontreated ischemic mouse retina at P22 shows capillary tufts on the surface of retina (arrows) and a small capillary-free zone around the optic disk. Middle: Retina of a P22 mouse that had been treated with apocynin from P12 to P22, demonstrating the absence of capillary tufts, but persistence of the capillary-free zone around the optic disk. Right: Retina of a P22 mouse that had been treated with apocynin from P12 to P17 and allowed to recover for 5 days until P22, demonstrating the absence of capillary tufts and almost complete revascularization of the central retina. Scale bars, 100 μm.
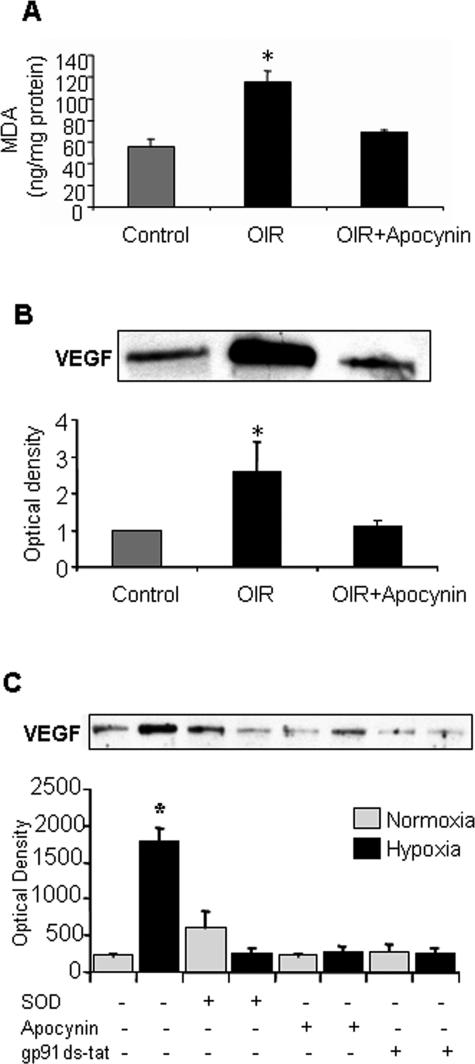
Biochemical analysis of lipid peroxidation levels in the ischemic mouse retina using the MDA assay confirmed the previously reported increases in lipid peroxidation during OIR.36 These experiments showed a twofold increase in lipid peroxidation at P14 during the period of relative retinal hypoxia (Figure 7A). This increase in lipid peroxidation was completely blocked by the apocynin treatment, providing further support for the role of NAD(P)H oxidase activity in oxidative stress during ischemic retinopathy.
Figure 7.
MDA analysis of lipid peroxidation (A) and Western blot analysis of VEGF expression (B) in mice maintained in hyperoxia from P7 to P12 and then returned to room air and treated with apocynin (10 mg/kg/day, ip) or vehicle for 2 days shows significant increases in lipid peroxidation and VEGF expression in the OIR retinas. Both effects were blocked by apocynin (*P < 0.05 versus control, n = 4 for MDA, n = 6 for Western blot). Western blot analysis of VEGF expression in retinal endothelial cells (C) exposed to normoxia (N) and hypoxia (H, 1% O2, 6 hours) shows a significant increase in VEGF expression that was completely inhibited by treatment with the NAD(P)H oxidase inhibitors apocynin (Apo), gp91ds-tat blocking peptide (BP), or PEG-SOD (*P < 0.05 versus normoxia control, n = 3).
Normalization of VEGF Expression by Inhibition of NAD(P)H Oxidase
An increase in VEGF expression is known to occur during ischemic retinopathy and is thought to play a key role in triggering retinal neovascularization.37 To see if apocynin’s action in blocking retinal neovascularization involves an effect in blocking the ischemia-induced increase in VEGF expression we determined the effects of apocynin treatment on VEGF protein levels. Western blots showed a significant increase in retinal VEGF protein during the period of pathological angiogenesis (2.6 ± 0.8-fold greater than control levels). This increase was completely blocked by apocynin treatment (Figure 7B). These results indicate that NAD(P)H oxidase activity is required for ischemia-induced increases in VEGF expression. To assess the role of NAD(P)H oxidase in hypoxia-induced VEGF up-regulation by endothelial cells, cells were maintained in hypoxia in the presence or absence of apocynin or gp91phox ds-tat. VEGF expression was then analyzed by Western blot. The expression of VEGF was significantly increased by the hypoxia treatment and this effect was completely blocked by inhibiting NAD(P)H oxidase (Figure 7C).
Discussion
Our results show that ischemia-induced retinal neovascularization is associated with an increase in activity of NAD(P)H oxidase as indicated by DHE and DCF assays of reactive oxygen species formation and the MDA assay of lipid peroxidation. Inhibition of NAD(P)H oxidase normalizes VEGF expression, suppresses retinal neovascularization, and prevents lipid peroxidation, indicating that NAD(P)H-derived oxidative stress has a fundamental role in the pathological process. To our knowledge this is the first study to demonstrate the role of NAD(P)H oxidase activity in hypoxia-induced increases in VEGF expression and retinal neovascularization. Data showing increases in expression of the NAD(P)H oxidase catalytic subunit gp91phox within the ischemic retina indicate that a gp91phox-based oxidase is a major source of the increase in oxidative stress. Co-localization of gp91phox with vascular endothelial cell markers in retinal sections suggests that vascular endothelial cells contribute to the ischemia-induced activation of NAD(P)H oxidase. The potential role of the vascular endothelium is further demonstrated by results of in vitro studies showing that hypoxia-induced activation of NAD(P)H oxidase in cultured retinal endothelial cells is correlated with an increase in VEGF expression that is blocked by inhibiting NAD(P)H oxidase activity. Results of the chemiluminescence, DHE, and DCF assays of reactive oxygen species production by retinal endothelial cells under hypoxic conditions confirmed NAD(P)H as a major source of oxidative stress in vascular endothelial cells during hypoxia.
The results of DHE imaging studies as well as DCF and MDA assays showed increased levels of reactive oxygen species formation on P17 in OIR model, which is the time when retinal neovascularization is at its peak.22 The DHE reaction was prominent within the proliferating new vessels as well as within the ganglion cell layer and the inner and outer nuclear layers. The suppressive effects of apocynin and gp91ds-tat on the DHE fluorescence signal within the areas of neovascularization suggest that vascular NAD(P)H oxidase is a major source of superoxide generation in the ischemic retina. Apocynin specifically blocks activity of NAD(P)H oxidase by interfering with the assembly of the cytosolic NAD(P)H oxidase components (p40phox, p47phox, p67phox) with the membranous components gp91phox and p22phox.29,30 The gp91ds-tat peptide specifically inhibits NAD(P)H oxidase assembly by binding to p47phox and blocking its interaction with gp91phox.20,31,32 This peptide has been shown to specifically inhibit gp91phox-based oxidases in endothelial cells and neutrophils.33 The efficacy of these inhibitors in blocking superoxide formation in both the proliferating new vessels in vivo and the hypoxia-treated retinal endothelial cells in vitro implies that a gp91phox-based oxidase is a primary source of the NAD(P)H oxidase activity during retinal ischemia. The role of vascular NAD(P)H in mediating hypoxia-induced oxidative stress in vascular endothelial cells was confirmed by the studies using three different approaches; DHE, DCF, and chemiluminescence. In all three assays, hypoxia caused a significant increase in reactive oxygen species formation. This effect was blocked by apocynin and gp91ds-tat indicating the role of NAD(P)H oxidase activity in the response.
Our Western blot studies confirmed significant increases in levels of gp91phox expression during the period of relative hypoxia in the ischemic retina. The expression of gp91phox protein remains elevated throughout the period of relative hypoxia, but is maximal at P14, which is the time when increases in VEGF expression become evident and when retinal neovascularization begins.22,38 Consistent with previous studies showing that the gp91phox-containing NAD(P)H oxidase is expressed in vascular endothelial cells.39 Our double-label immunolocalization analysis showed that the gp91phox co-localizes with cells that are also positive for the endothelial cell markers GSI lectin and CD-31. The immunolabeled sections from the retinas of control mice maintained in room air were weakly positive for gp91phox protein, suggesting that the protein is expressed at very low levels during normoxia conditions. The prominent increase in gp91phox in areas of neovascularization suggest that up-regulation of this protein has a key role in retinal neovascularization.
The ischemia-induced increase in lipid peroxidation was completely blocked by treatment of the animals with apocynin during the period of relative hypoxia, providing further evidence for the role of NAD(P)H oxidase activity in the pathological process. Lipid peroxidation is a recognized indicator of oxidative injury that occurs as fatty acids react with reactive oxygen species. Lipid peroxidation has been shown to be associated with retinal neovascularization during ischemic retinopathy in rats36 and in diabetic rat retinas16 and vitrectomy specimens from patients with proliferative diabetic retinopathy.40
Taken together our data show that oxidative injury in the ischemic retina is associated with increased expression of gp91phox and that inhibition of NAD(P)H oxidase prevents retinal neovascularization. Our finding that NAD(P)H oxidase inhibitors block hypoxia’s effects in increasing VEGF expression in cultured retinal endothelial cells underlines the potential importance of endothelial cell NAD(P)H oxidase as a critical source of reactive oxygen species in the ischemic retina. On the other hand our studies using the glial cell marker, GFAP showed only weak gp91phox expression by retinal glia. Moreover our study of retinal Muller glial cells in culture showed that hypoxia induces superoxide production, but that inhibition of NAD(P)H oxidase has no effect on the hypoxia-induced increase in superoxide. This suggests that hypoxia activates a different source of superoxide formation in this cell type. It has been shown that diabetes or hyperglycemia significantly increased superoxide production in Muller cells, primarily via activation of mitochondria oxidase.41
Our finding that both pathological vascular growth and revascularization of the central retina were prevented by apo-cynin suggests that NAD(P)H oxidase activity is required for both normal and pathological angiogenesis. Our in vivo and in vitro studies indicate that this could be in part due to inhibition of VEGF expression. Studies by other investigators have suggested additional mechanisms by which apocynin could inhibit neovascularization. For example, inhibition of gp91phox has been shown to interfere with the VEGF-mediated angiogenic signaling process.42,43 Inhibition of NAD(P)H oxidase with apocynin has been found to increase ocular blood flow to the iris, ciliary body, and choroid,44 and to improve microcirculation in peripheral nerves during diabetes.45 These results together suggest that inhibition of NAD(P)H oxidase could modulate neovascularization via different mechanisms: inhibiting VEGF expression, inhibiting VEGF signaling events, or improving retinal oxygenation. The fact that revascularization occurred normally when the treatment was terminated indicates that the apocynin effect is reversible. Thus, NAD(P)H oxidase inhibitors may be useful as acute therapy for blocking neovascularization during periods of elevated oxidative stress.
The mechanism by which hypoxia triggers activation of NAD(P)H oxidase is unknown. Observations associating the gp91phox catalytic subunit with oxygen-sensing processes in a variety of mammalian cells suggest that the enzyme functions as an oxygen sensor to produce reactive oxygen species in response to changes in oxygen tension.39 Although it has also been shown that oxygen sensing function is preserved in mutant and knockout mice that lack the gp91phox subunit, vascular cells express other gp91phox homologues that could serve a compensatory role.46 Further study is needed to determine the mechanism by which hypoxia triggers NAD(P)H oxidase activity in the ischemic retina. Nevertheless, the results of this study indicate that specific inhibitors of NAD(P)H oxidase could offer a new therapeutic strategy to block the development of retinal neovascularization during ischemic retinopathies.
Acknowledgments
We thank Dr. Adviye Ergul for providing the gp91ds-tat blocking peptide used in these experiments.
Footnotes
Address reprint requests to Ruth B. Caldwell, Ph.D., Vascular Biology Center, Medical College of Georgia, 1120 15th St., Augusta, GA 30912-2500. E-mail: rcaldwel@mail.mcg.edu.
Supported by the National Institutes of Health (EY04618, EY11766, and HL70215), the Fight For Sight, the American Heart Association, and a postdoctoral fellowship from the Juvenile Diabetes Research Foundation, International.
References
- Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, III, Klein R. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113:1538–1544. doi: 10.1001/archopht.1995.01100120068012. [DOI] [PubMed] [Google Scholar]
- Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19:442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Fu XW, Wang D, Nurse CA, Dinauer MC, Cutz E. NADPH oxidase is an O2 sensor in airway chemoreceptors: evidence from K+ current modulation in wild-type and oxidase-deficient mice. Proc Natl Acad Sci USA. 2000;97:4374–4379. doi: 10.1073/pnas.97.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Qiu H, Yoon HW, Huang S, Bunn HF. Identification of a cytochrome b-type NAD(P)H oxidoreductase ubiquitously expressed in human cells. Proc Natl Acad Sci USA. 1999;96:14742–14747. doi: 10.1073/pnas.96.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ER, Dratz EA, Gizachew D, Deleo FR, Yu L, Volpp BD, Vlases M, Jesaitis AJ, Quinn MT. Interaction of human neutrophil flavocytochrome b with cytosolic proteins: transferred-NOESY NMR studies of a gp91phox C-terminal peptide bound to p47phox. Biochem J. 1997;325:249–257. doi: 10.1042/bj3250249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger RH, Marti HH, Schuerer-Maly CC, Kvietikova I, Bauer C, Gassmann M, Maly FE. Hypoxic induction of gene expression in chronic granulomatous disease-derived B-cell lines: oxygen sensing is independent of the cytochrome b558-containing nicotinamide adenine dinucleotide phosphate oxidase. Blood. 1996;87:756–761. [PubMed] [Google Scholar]
- Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- Bayraktutan U, Draper N, Lang D, Shah AM. Expression of functional neutrophil-type NADPH oxidase in cultured rat coronary microvascular endothelial cells. Cardiovasc Res. 1998;38:256–262. doi: 10.1016/s0008-6363(98)00003-0. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Voest EE, Amano S, Beerepoot LV, Takashima S, Tolentino M, Kim RY, Rohan RM, Colby KA, Yeo KT, Adamis AP. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98:1667–1675. doi: 10.1172/JCI118962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EA, Guberski DL, Somogyi-Mann M, Grant MB. Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 2000;28:91–101. doi: 10.1016/s0891-5849(99)00216-6. [DOI] [PubMed] [Google Scholar]
- Ellis EA, Grant MB, Murray FT, Wachowski MB, Guberski DL, Kubilis PS, Lutty GA. Increased NADH oxidase activity in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 1998;24:111–120. doi: 10.1016/s0891-5849(97)00202-5. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol. 2003;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: role of the p22(phox)-containing NADPH oxidase. Circ Res. 2001;89:47–54. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med. 2002;32:1116–1122. doi: 10.1016/s0891-5849(02)00789-x. [DOI] [PubMed] [Google Scholar]
- Brandes RP. Role of NADPH oxidases in the control of vascular gene expression. Antioxid Redox Signal. 2003;5:803–811. doi: 10.1089/152308603770380115. [DOI] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem. 2002;277:33284–33290. doi: 10.1074/jbc.M203391200. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Brooks SE, Gu X, Samuel S, Marcus DM, Bartoli M, Huang PL, Caldwell RB. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:222–228. [PubMed] [Google Scholar]
- Gu X, Samuel S, El-Shabrawey M, Caldwell RB, Bartoli M, Marcus DM, Brooks SE. Effects of sustained hyperoxia on revascularization in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2002;43:496–502. [PubMed] [Google Scholar]
- Behzadian MA, Wang XL, Shabrawey M, Caldwell RB. Effects of hypoxia on glial cell expression of angiogenesis-regulating factors VEGF and TGF-beta. Glia. 1998;24:216–225. [PubMed] [Google Scholar]
- Al-Shabrawey M, El-Remessy A, Gu X, Brooks SS, Hamed MS, Huang P, Caldwell RB. Normal vascular development in mice deficient in endothelial NO synthase: possible role of neuronal NO synthase. Mol Vis. 2003;9:549–558. [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- Hart BA, Simons JM. Metabolic activation of phenols by stimulated neutrophils: a concept for a selective type of anti-inflammatory drug. Biotechnol Ther. 1992;3:119–135. [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- Jacobson GM, Dourron HM, Liu J, Carretero OA, Reddy DJ, Andrzejewski T, Pagano PJ. Novel NAD(P)H oxidase inhibitor suppresses angioplasty-induced superoxide and neointimal hyperplasia of rat carotid artery. Circ Res. 2003;92:637–643. doi: 10.1161/01.RES.0000063423.94645.8A. [DOI] [PubMed] [Google Scholar]
- Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- Munzel T, Afanas’ev IB, Kleschyov AL, Harrison DG. Detection of superoxide in vascular tissue. Arterioscler Thromb Vasc Biol. 2002;22:1761–1768. doi: 10.1161/01.atv.0000034022.11764.ec. [DOI] [PubMed] [Google Scholar]
- Imada I, Sato EF, Miyamoto M, Ichimori Y, Minamiyama Y, Konaka R, Inoue M. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal Biochem. 1999;271:53–58. doi: 10.1006/abio.1999.4107. [DOI] [PubMed] [Google Scholar]
- Penn JS. Oxygen-induced retinopathy in the rat: possible contribution of peroxidation reactions. Doc Ophthalmol. 1990;74:179–186. doi: 10.1007/BF02482607. [DOI] [PubMed] [Google Scholar]
- Smith LE. Pathogenesis of retinopathy of prematurity. Semin Neonatol. 2003;8:469–473. doi: 10.1016/S1084-2756(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RD, Hancock JT, Morice AH. NADPH oxidase: a universal oxygen sensor? Free Radic Biol Med. 2000;29:416–424. doi: 10.1016/s0891-5849(00)00320-8. [DOI] [PubMed] [Google Scholar]
- Verdejo C, Marco P, Renau-Piqueras J, Pinazo-Duran MD. Lipid peroxidation in proliferative vitreoretinopathies. Eye. 1999;13:183–188. doi: 10.1038/eye.1999.48. [DOI] [PubMed] [Google Scholar]
- Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med. 2003;35:1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- Liu SX, Chiang CH, Yao QS, Chiou GC. Increase of ocular blood flow by some phytogenic compounds. J Ocul Pharmacol Ther. 1996;12:95–101. doi: 10.1089/jop.1996.12.95. [DOI] [PubMed] [Google Scholar]
- Cotter MA, Cameron NE. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci. 2003;73:1813–1824. doi: 10.1016/s0024-3205(03)00508-3. [DOI] [PubMed] [Google Scholar]
- Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci USA. 1999;96:7944–7949. doi: 10.1073/pnas.96.14.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]