Abstract
Transforming growth factor (TGF)-β1 is a potent inhibitor of mammary epithelial proliferation. In human breast, estrogen receptor (ER)-α cells rarely co-localize with markers of proliferation, but their increased frequency correlates with breast cancer risk. To determine whether TGF-β1 is necessary for the quiescence of ER-α-positive populations, we examined mouse mammary epithelial glands at estrus. Approximately 35% of epithelial cells showed TGF-β1 activation, which co-localized with nuclear receptor-phosphorylated Smad 2/3, indicating that TGF-β signaling is autocrine. Nuclear Smad co-localized with nuclear ER-α. To test whether TGF-β inhibits proliferation, we examined genetically engineered mice with different levels of TGF-β1. ER-α co-localization with markers of proliferation (ie, Ki-67 or bromodeoxyuridine) at estrus was significantly increased in the mammary glands of Tgfβ1 C57/bl/129SV heterozygote mice. This relationship was maintained after pregnancy but was absent at puberty. Conversely, mammary epithelial expression of constitutively active TGF-β1 via the MMTV promoter suppressed proliferation of ER-α-positive cells. Thus, TGF-β1 activation functionally restrains ER-α-positive cells from proliferating in adult mammary gland. Accordingly, we propose that TGF-β1 dysregulation may promote proliferation of ER-α-positive cells associated with breast cancer risk in humans.
The pluripotent cytokine, transforming growth factor (TGF)-β1 has been widely implicated in mammary epithelial growth,1,2 in cancer,3 and in response of breast cancer cells to estrogen and progesterone.4 TGF-β family members can modify cell behaviors via autocrine, paracrine, and endocrine mechanisms of action.5 A primary control of TGF-β1 activity is its secretion as a latent complex, which consists of the 24-kd cytokine and a 80-kd dimer of its pre-pro region containing the signal sequence for secretion.6 Extracellular events release TGF-β from the latent complex so that it can bind to ubiquitously expressed cell surface receptors that then initiate signaling cascades that modulate gene expression. To our knowledge, all cells secrete latent TGF-β and all cells express TGF-β receptors, underscoring the necessity of identifying the cell and tissue conditions leading to release of TGF-β as an essential control of its bioactivity.
To evaluate cause-and-effect relationships between TGF-β1 activation and epithelial cell proliferation in the mammary gland, we characterized spatial and temporal patterns of TGF-β1 activation using immunostaining in conjunction with functional assessment using Tgfβ1 knockout mice.7 Immunolocalization revealed that TGF-β1 production and activity are differentially regulated during mammary gland development, such that periods of proliferation were accompanied by decreased TGF-β1 activation in most cells. TGF-β1 depletion results in significantly accelerated morphogenesis during puberty and pregnancy and increased mammary epithelial proliferation during proliferative stages. However, TGF-β depletion is insufficient to initiate proliferation because ovariectomized Tgfβ1 heterozygote mice did not show a phenotype, although proliferation increased more than 10-fold compared to wild types after administration of estrogen and progesterone. These data indicate that TGF-β specifically inhibits the proliferative potential of mammary epithelial cells in response to ovarian steroids.
Although estrogen and progesterone are critical for mammary epithelial proliferation, it is clear that cells differ in their ability to respond to these signals. During both ductal and lobular-alveolar mammary growth, the distribution of proliferating cells is heterogeneous, suggesting the involvement of local factors in dictating the specific response to systemic hormones.8–10 Studies in estrogen receptor (ER)-α knockout mouse mammary outgrowths indicate that both stromal and epithelial ER-α, in cooperation with epithelial progesterone receptor (PR), govern mammary gland development in adult mice.11 Ovarian steroid hormone receptors are also heterogeneously expressed by a subpopulation of the mammary epithelium, but these receptors rarely co-localize with markers of proliferation in human cells.12–17 Interestingly, increased proliferation in the hormone receptor-positive population is associated with breast cancer risk,18 while a transgenic mammary model of dysregulated ER-α expression results in hyperplasia and ductal carcinoma in situ.19
TGF-β immunolocalization revealed striking epithelial heterogeneity during mammary stages characterized by proliferation.7 Although, during estrus many cells down-regulate TGF-β atestrus, consistent with its action as a growth inhibitor, some mammary epithelial cells maintain prominent TGF-β1 activation. This observation suggested to us the hypothesis that TGF-β acts to restrain such cells from proliferating particularly in the presence of ovarian steroids. Therefore, in this study we first sought to determine the relationship between the TGF-β1-positive and steroid hormone receptor epithelial subpopulations using dual immunolocalization, and second, to determine whether TGF-β depletion specifically affects proliferation of hormone receptor-positive cells.
Materials and Methods
Mice
All experiments were conducted with Lawrence Berkeley National Laboratory institutional review and approval. Animals were killed by CO2 inhalation and cervical dislocation at the indicated times in accordance with AAALAC guidelines. Mammary glands were collected from Tgfβ1 heterozygote and wild-type mice bred in-house in the C57BL/6–129SV mixed background unless otherwise noted. In some experiments, Tgfβ1 knockouts crossed back to FvB or BALB/c background mice were used, which were obtained, respectively, from Drs. Lalage Wakefield and Adam Glick at the National Cancer Institute. FvB MMTV-Tgfβ223–225 transgenic mice were previously described.20 All specimens were collected from animals reared and housed at Lawrence Berkeley National Laboratory. Estrus was determined by cytological characteristics of vaginal smears and confirmed postmortem by uterine wet weight. Nulliparous animals ∼10 weeks of age were killed in estrus. Ovariectomy and estradiol and progesterone treatment of ovariectomized mice was performed as previously described.7 At least three animals were used for each treatment group. The inguinal mammary glands were dissected free of the skin and embedded in OCT compound (Miles Inc., Elkhart, IN). Frozen OCT-embedded tissue blocks were stored at −70°C until the time of sectioning.
Antibodies
TGF-β1 was detected using polyclonal, affinity-purified chicken anti-TGF-β1 (AF-101-NA, lot FS08; R&D Systems, Minneapolis, MN), which preferentially reacts with the active form of TGF-β1. We used monoclonal antibody NCL-ER-6F11 to ER-α and rabbit polyclonal antibody to Ki-67 both from Novocastra (Newcastle, UK). Antibody FL-425 (no. SC-8332; Santa Cruz Biotechnology, Santa Cruz, CA) recognizes receptor-phosphorylated Smad proteins. The rabbit polyclonal antibody to PR was developed in-house by G.S.21 For secondary antibodies, Alexa 488-labeled goat anti-mouse IgG (Molecular Probes, Eugene, OR), fluorescein isothiocyanate-labeled anti-mouse (Pierce, Rockford, IL), Texas Red-labeled goat anti-rabbit IgG (Molecular Probes) and Texas Red-labeled rabbit anti-chicken IgY (Sigma, St. Louis, MO) were used.
Immunohistochemstry
Frozen OCT-embedded mammary glands were sectioned onto gelatin-coated coverslips, then fixed using 2% buffered paraformaldehyde for 20 minutes at room temperature, followed by 0.1 mol/L of glycine in phosphate-buffered saline (PBS) washes. For ER-α co-localization with Ki-67, Smad, or PR, sections were treated with prewarmed 0.1% Triton X-100 in PBS at 37°C for 20 minutes followed by PBS washes. Nonspecific sites were blocked using the supernatant from a 0.5% casein/PBS solution (pH 7.4) for 60 minutes. Endogenous mouse IgG was blocked using Mouse-on-Mouse blocking agent (Vector Laboratories, Burlingame, CA) diluted 50% in 0.5% casein/PBS solution for 4 hours at room temperature. Sections were incubated in primary antibodies diluted in 0.5% casein/PBS solution and incubated overnight at 4°C. After washes, each primary was detected by sequential incubations with species-specific secondary antibodies. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). The sections were mounted in Vectashield (Vector Laboratories) and stored at −20°C until evaluated. For PR co-localization with active TGF-β1, sections were blocked with 0.5% casein/PBS solution, reacted with primary antibodies overnight at 4°C. After washes, each primary was detected by sequential incubations with species-specific secondary antibodies.
Microscopy and Image Analysis
Immunofluorescence images were obtained and processed as previously described.7 Color images of ducts were split into their constituent channels and scored for total and positive cells using the text annotation feature in Corel Photopaint 7 (Corel, Dallas, TX) without knowledge of tissue source of images. Three animals per genotype/treatment and at least 300 cells per animal were scored for presence of the marker. Prism 3 (GraphPad Inc., San Diego, CA) was used to conduct two-tailed Student’s t-test to evaluate whether mean frequency differed significantly between treatment groups.
Results
Autocrine TGF-β1 Action in the Mammary Epithelium
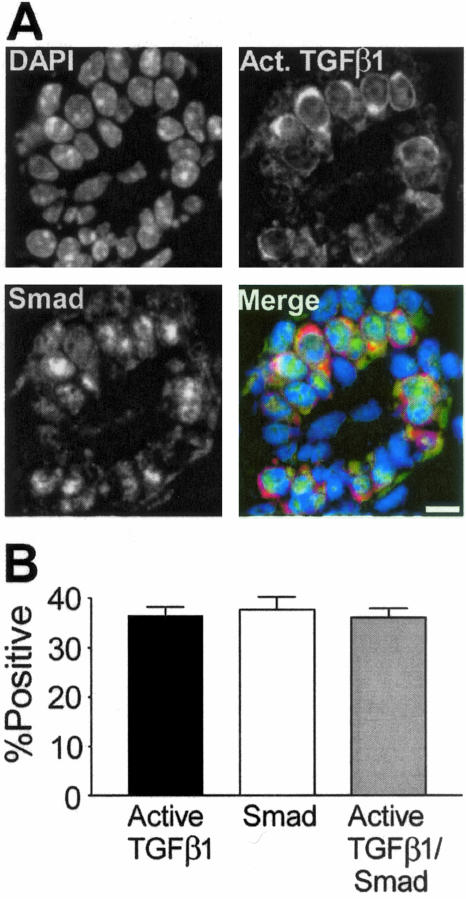
Mammary epithelial proliferation is dictated by the endogenous ovarian steroid milieu such that it is maximal at estrus. At estrus activation state-specific TGF-β1 immunoreactivity is reduced in most cells,7 but ∼35% of luminal epithelial cells show prominent immunoreactivity (Figure 1). All cells secrete latent TGF-β and the extracellular matrix is a reservoir of latent protein, thus it is difficult to ascertain its original cellular source. Further, TGF-β1 activity is controlled by extracellular activation and depending on the mode of latent TGF-β1 activation, TGF-β1 can signal in both an autocrine22 and paracrine23 manner. Thus, it is important to determine when TGF-β1 is actively signaling and to which cells. On ligand binding, TGF-β type I receptor phosphorylates Smad 2 and 3 (R-Smad), which leads to nuclear translocation and initiation of transcription.24 We asked whether TGF-β was acting in an autocrine or paracrine manner by using dual immunofluorescence to localize both TGF-β and nuclear R-Smad. The frequencies of cells positive for active TGF-β1 and nuclear R-Smad were similar. Figure 1A shows single fluorochrome images and a merged image of a mammary duct containing cells positive for active TGF-β1 and nuclear Smad. Co-localization of active TGF-β1/nuclear R-Smad cells showed nearly perfect correspondence (Figure 1B). Most cells positive for active TGF-β were Ki-67-negative (91%, n = 2 mice). These data indicated that cell-restricted TGF-β1 activation during estrus results in triggering of the TGF-β signaling pathway in the same cells.
Figure 1.
Nuclear R-Smad protein co-localizes in mammary epithelial cells that are positive for active TGF-β1. A: Individual channel images of DAPI-stained nuclei (DAPI), R-Smad, and TGF-β1 immunostaining in a transverse section of a duct. Merged image shows R-Smad immunoreactivity as green and active TGF-β1 as red. B: Active TGF-β1 and nuclear R-Smad are markers of the same cell population in the mammary epithelium at estrus. Shown are means ± SEM from three C57BL/6–129SV mixed background animals. At least 250 cells were scored per animal. Scale bar, 20 μm.
Ovarian Hormone Receptor-Positive Cells Are Positive for Active TGF-β1 and Exhibit Nuclear R-Smad
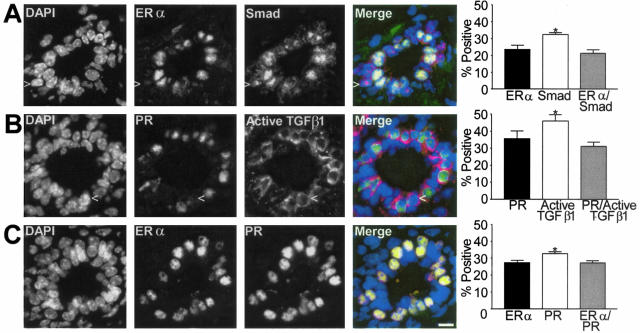
It is well established that estrogen action is mediated through its cognate receptors, ER-α and ER-β, and that ER-α is essential for mammary epithelial cell proliferation.25 ER-α localization by immunohistochemistry is heterogeneous within the mammary epithelium.26,27 To determine the relationship between TGF-β activation and the ER-α-positive cell populations, we used dual immunofluorescence to determine co-localization of ER-α and R-Smad. Almost all ER-α-positive cells were positive for nuclear R-Smad (Figure 2A). The same was true when ER-α was co-localized with active TGF-β1 (data not shown). Dual immunofluorescence of PR and active TGF-β1 (Figure 2B) also demonstrated that most PR-positive cells were positive for active TGF-β1. ER-α co-localized with PR (Figure 2C), as is found in human and rat mammary gland.27 These data indicated that most cells that are ER-α- and PR-positive maintain TGF-β activation during estrus, suggesting that TGF-β may inhibit their ability to respond to ovarian hormone-induced proliferation.
Figure 2.
Subpopulations of the mammary epithelium of the C57BL/6–129SV mixed background mouse at estrus. A: Example of dual-immunofluorescence localization of ER-α and R-Smad immunoreactivity shows the gray scale individual images of DAPI-stained nuclei, ER-α, R-Smad, and a merged color image showing ER-α immunoreactivity as green and nuclear Smad as red which makes nuclei positive for both appear yellow/orange. Column graph shows mean co-localization frequency ± SEM (n = 3). B: Gray scale images of DAPI-stained nuclei, PR, and active TGF-β1 immunostaining in ductal epithelium. The merged color image shows that most PR-positive cells (green nuclei) are also positive for cytoplasmic active TGF-β1 (red). Column graph shows mean co-localization frequency ± SEM (n = 3). C: Individual channels and merged image of ER-α and PR immunoreactivity in a transverse section of a duct. ER-α-positive cells all exhibit PR and the nuclei appear orange in the merged image. Column graph shows mean co-localization frequency ± SEM (n = 3). Scale bar, 20 μm.
Proliferation of ER-α-Positive Mammary Epithelial Cells Is Increased in Tgfβ1 Heterozygote Mice
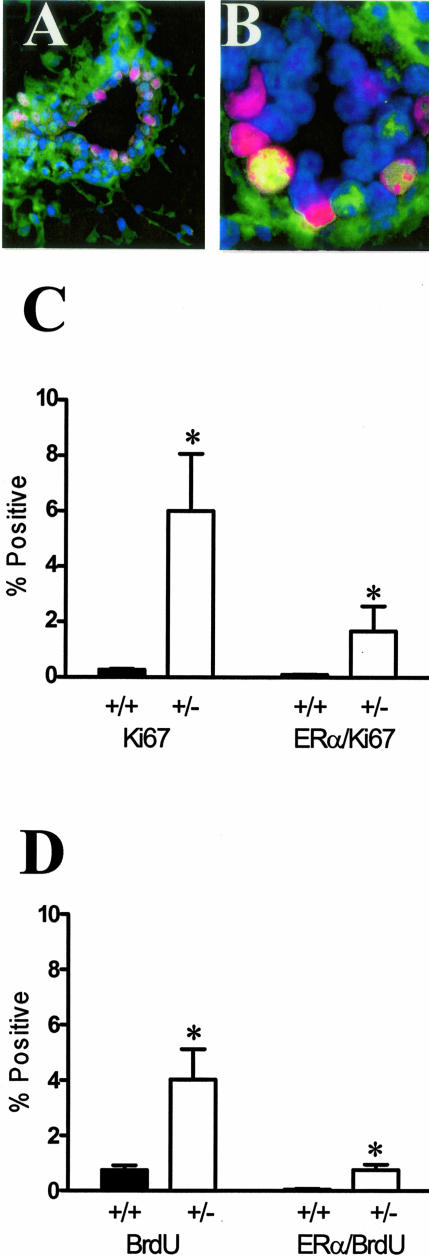
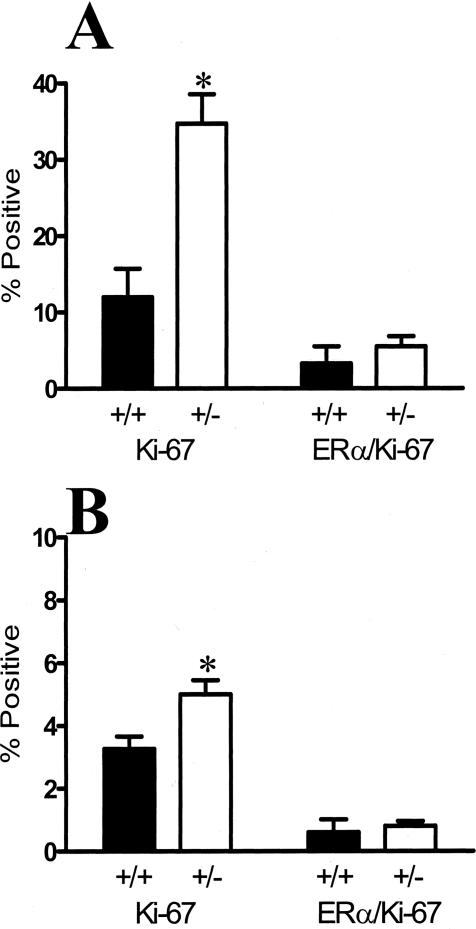
The infrequent co-localization of proliferation markers with ER-α-positive cells, despite the presence of estradiol, raises two possibilities: either the inability to proliferate is intrinsic to these cells independent of TGF-β, or that TGF-β1 selectively restrains ER-α-positive cells from proliferating. If it is the latter, then it is reasonable to expect that changes in the endogenous levels of TGF-β would lead to changes in the proliferation of ER-α-positive cells. TGF-β1 protein is reduced by more than 90% in mice lacking one Tgfβ1 allele, whether measured by immunoreactivity or by quantifying TGF-β biological activity in tissue extracts.7 Therefore, to determine the relationship between TGF-β and proliferation of ER-α-positive cells, we used dual immunofluorescence localization of nuclear ER-α and the cell-cycle marker Ki-67 and scored dual-labeled epithelial cells in Tgfβ1 heterozygote and wild-type mammary glands (Figure 3, A and B). The overall frequency of Ki-67-marked cells at estrus was increased 24-fold in Tgfβ1 heterozygote mammary epithelium compared to wild-type mice, consistent with our previous results using proliferating cell nuclear antigen as a marker of cell cycle.7 The frequency of cells exhibiting dual localization of Ki67 and ER-α increased 16-fold in Tgfβ1 heterozygotes (Figure 3C). Although the degree of response was modified by genetic background, the consequence of TGF-β1 depletion was similar in two other mouse strains (ie, BALB/c and FvB, data not shown).
Figure 3.
TGF-β1 depletion results in increased frequency of ER-α-positive mammary epithelial cells in cycle. A: Dual-immunofluorescence localization of ER-α (green; nonnuclear staining is nonspecific) and Ki67 (red) in mammary epithelium. Nuclei are counterstained with DAPI (blue). B: Dual-immunofluorescence localization of ER-α and Ki67, as in A, of higher magnification of a mammary duct with an example of double-labeled nucleus (yellow). C: Ki-67 and ER-α/Ki-67 co-localization frequency in mammary epithelium of nulliparous Tgfβ1 heterozygote (+/− in figure) and Tgfβ1 wild-type (+/+) C57BL/6–129SV mice at estrus. Three animals per genotype and at least 300 cells per animal were scored for presence of ER-α and Ki-67 immunoreactivity. Asterisks indicate significant difference from Tgfβ1 wild-type mean frequency (P < 0.01; t-test). D: BrdU and ER-α/BrdU co-localization frequency in mammary epithelium of the same nulliparous Tgfβ1 heterozygote (+/−) and Tgfβ1 wild-type (+/+) C57BL/6–129SV mice at estrus. Asterisks indicate significant difference from Tgfβ1 wild-type mean frequency (P < 0.01; t-test).
To confirm that these ER-α-positive cells were actively undergoing DNA synthesis, animals were injected with bromodeoxyuridine (BrdU) 1 hour before death. The frequency of BrdU-labeled cells in the ER-α-positive subpopulation was also significantly increased in Tgfβ1 heterozygote mice (Figure 3D), in a manner similar to what was observed with the Ki-67 labeling index. The relative frequency of labeled cells is less than that for Ki-67 because BrdU marks a narrower window of the cell cycle (ie, S phase). Thus, these studies indicated that ER-α-positive cells are capable of entering the cell cycle and undertaking DNA synthesis during estrus, but that concomitant TGF-β activation functionally restrains them.
ER-α is found in both stromal and epithelial cells of the mammary gland. The role of stromal versus epithelial ER-α was first addressed by Cooke and colleagues28 who used recombined mammary stromal and epithelial tissue from neonatal BALB/c and ERKO mice transplanted under the renal capsule of intact athymic nude mice. Their results indicated that stromal, but not epithelial, ER-α is required for ductal growth of immature mammary epithelium. Subsequent studies by Mueller and colleagues11 in a mature mouse demonstrated the lack of outgrowth of ER-α-deficient epithelial cells injected into an ER-α-positive stroma, which suggests that compartment-specific requirement for ER-α is dependent on maturation. To examine whether regulation of proliferation of ER-α-positive cells was intrinsic to the Tgfβ1 heterozygote epithelium or is mediated by depletion of TGF-β1 in the stroma, we conducted similar transplant studies of Tgfβ1 BALB/c heterozygote or wild-type fragments to wild-type stroma. We found that the frequency of cells dual labeled for ER-α and Ki67 obtained at 8 weeks after transplantation was fivefold greater in Tgfβ1 heterozygote outgrowths (2.5 ± 1.06, n = 5; P < 0.05, Mann-Whitney U-test) compared to wild type (0.47 ± 0.19, n = 4). Thus depletion of TGF-β1 in the epithelium was sufficient to significantly increase proliferation of ER-α-positive cells at estrus.
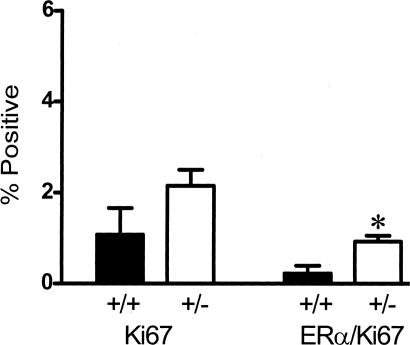
Next we examined if proliferation of ER-α-positive cells in the Tgfβ1 heterozygotes was regulated by ovarian steroids. Accordingly, we depleted endogenous levels of estrogen and progesterone by ovariectomy and examined the proliferative status of ER-α-positive cells in these mammary glands. ER-α/Ki67 dual-labeled cells were not evident in the ovariectomized mammary gland. Daily treatment of ovariectomized mice with estrogen and progesterone for 3 days resulted in 17-fold higher frequency of double-labeled ER-α/Ki-67 mammary epithelial cells in Tgfβ1 heterozygote mammary epithelium versus wild-type epithelium (2.2 ± 0.35 SEM versus 0.13 ± 0.07 SEM, P = 0.02; unpaired t-test with Welch’s correction). Thus, as with ER-α-negative cells, TGF-β depletion promotes proliferation in ER-α-positive cells only in response to estrogen and progesterone.
Decreased Proliferation of ER-α-Positive Mammary Epithelial Cells in MMTV-TGFβ223–225 Gain of Function Mice
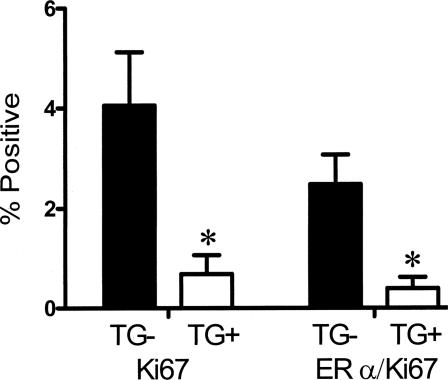
If TGF-β1 depletion results in greater proliferation of ER-α-positive mammary epithelial cells, then one would predict that increased TGF-β1 activity might suppress the ability of ER-α-positive cells to enter the cell cycle. To test this, we examined FvB MMTV-Tgfβ223–225 transgenic mice that express constitutively active TGF-β1 in the mammary epithelium.20 Mice were studied at 12 weeks of age to ensure fully developed nulliparous tissue, which was confirmed by whole mount. The frequency of dual-localization of Ki67 and ER-α was decreased sixfold in MMTV-Tgfβ223–225 transgenic mice compared to estrus-stage wild-type mice (Figure 4), which indicates that expression of constitutively active TGF-β1 can override hormone-induced proliferation of these cells.
Figure 4.
Expression of constitutively active TGF-β1 results in decreased mammary epithelial co-localization of ER-α and proliferation. Ki-67 and ER-α/Ki-67 co-localization frequency in mammary epithelium of 12-week-old nulliparous MMTV-TGFβ223–225 FvB transgenic mice (TG+) and age-matched wild-type mice (TG−) mice at estrus. Asterisks indicate significant difference from Tgfβ1 wild-type mean frequency (P < 0.05; t-test).
Parous TGF-β1 Heterozygote Mice Exhibit Increased Frequency of ER-α-Positive Cells in Cycle
Parous women have fewer proliferating ER-α-positive mammary epithelial cells compared with nulliparous women.29 Similarly, parous rats30 and rats exposed to a hormone regime that mimics pregnancy and subsequent involution31 exhibit fewer proliferating ER-α-positive mammary epithelial cells than age-matched nulliparous rats. Thus, ER-α-positive mammary epithelial cells in the parous gland appear to be either less susceptible to hormonal signals to proliferate or are more stringently restrained from proliferation than in the nulliparous condition. To test the latter possibility, we determined the frequency of proliferating ER-α-positive mammary epithelial cells in TGF-β1-depleted C57/BL parous animals (Figure 5). The frequency of dual ER-α/Ki67-positive mammary epithelial cells in estrus-stage parous Tgfβ1 wild-type mice was similar to that in estrus-stage, nulliparous Tgfβ1 wild-type mice, which is already low (ie, 0.1%). However, the frequency of ER-α co-localization with Ki-67 in parous mammary epithelial cells was fourfold higher in Tgfβ1 heterozygote mice than in Tgfβ1 wild-type mice. As we reported for mammary proliferation overall, these data indicate that ER-α-positive cells are restrained from proliferating in the parous gland through mechanism(s) mediated by TGF-β1. Consistent with this conclusion, conditional deletion of Smad4 in the mammary gland as a function of pregnancy leads to hyperplastic foci with increased cell proliferation.32
Figure 5.
TGF-β1 depletion results in increased frequency of ER-α-positive mammary epithelial cells in cycle in parous mice. Ki-67 and ER-α/Ki-67 co-localization frequency in mammary epithelium of parous Tgfβ1 heterozygote (+/−) and Tgfβ1 wild-type (+/+) C57BL/6–129SV mice at estrus. These animals were sacrificed 3 weeks after weaning. Four animals per genotype and at least 250 cells per animal were scored for presence of ER-α and Ki-67 immunoreactivity. Asterisk indicates significant difference from Tgfβ1 wild-type mean frequency (P < 0.05; t-test).
Regulation of Proliferation by TGF-β1 in ER-α-Positive Cells Is Absent at Puberty
In contrast to the foregoing data showing that TGF-β1 depletion increased proliferation overall and of ER-α-positive mammary epithelial cells at estrus, in hormone-treated ovariectomized mice, and after pregnancy, TGF-β1 depletion did not increase the proliferation of ER-α-positive cells that are present in the pubertal mammary gland. The frequency of Ki-67-positive cells was significantly higher in Tgfβ1 heterozygote endbuds and ducts than in Tgfβ1 wild type, as expected from our previous analysis,7 but the frequency of ER-α-positive co-localization with Ki-67 in either structure was not significantly affected by TGF-β depletion (Figure 6). These data suggest that proliferation of ER-α-positive cells is differentially regulated during puberty compared to adults.
Figure 6.
TGF-β1 depletion in pubertal glands results in increased frequency of cells in cycle but not in proliferating ER-α-positive mammary epithelial cells. A: Ki-67 and ER-α/Ki-67 co-localization frequency in endbud mammary epithelium of TGF-β1 heterozygote (+/−) and TGF-β1 wild-type (+/+) C57BL/6–129SV mice at 6 weeks of age. Three animals per genotype and at least 300 cells per animal were scored for presence of ER-α and Ki-67 immunoreactivity. B: Ki-67 and ER-α/Ki-67 co-localization frequency in the mammary epithelium of subtending ducts in the same tissues. Asterisks indicate significant difference from Tgfβ1 wild-type mean frequency (P < 0.01; t-test).
Discussion
The developmentally discrete patterns of TGF-β1 activation during mammary gland maturation underscores the exquisite context and cell-dependent regulation of TGF-β1 activation and activity. It also raises the question of which of its many roles TGF-β1 plays at different stages of mammary morphogenesis and differentiation. The feature that is most striking about the immunostaining for active TGF-β1 in the mammary gland at estrus is that certain cells activate more TGF-β1 at estrus than at any other time in the estrus cycle.7 As we show here, those cells are almost exclusively ER-α-positive. Based on this we infer that the TGF-β1 activity is greater in the ER-α-positive cells than in the ER-α-negative cells, and thus its ability to block proliferation is likely greater. As shown in our previous study, TGF-β1 is severely depleted in the heterozygote so that the observed effect is the result of the relative reduction of TGF-β1 on each population.7 Thus, we have determined that TGF-β1 at estrus is stringently restraining ER-α-positive cells from proliferating in response to hormonal stimulation.
TGF-β appears to be acting in an autocrine manner because mammary epithelial cells positive for TGF-β1 by immunostaining also exhibit nuclear R-Smad protein indicative of TGF-β signaling. The idea of autocrine action within the epithelium is further supported by the observation that fivefold more ER-α cells proliferated in Tgfβ1 heterozygote epithelium transplanted to wild-type stroma than similarly treated wild-type epithelium. The question of whether TGF-β activation in these cells arose stochastically or is associated with known heterogeneity of epithelial cell types was resolved by the demonstration that nearly all ER-α-positive mammary epithelial cells exhibited TGF-β1 activation and nuclear R-Smad immunoreactivity. The functional significance of the correlation between ER-α and TGF-β was evident on examination of the Tgfβ1 transgenic mouse mammary glands. We found that, with the important exception of pubertal mammary gland, TGF-β1 depletion resulted in increased frequency of co-localization of ER-α-positive epithelial cells with markers of cell cycle traversal in adult tissue. Even though the original cellular source of latent TGF-β is obscure because it is a secreted molecule produced by essentially all cells, it is clear from our transplantation study that epithelial depletion of TGF-β1 is sufficient for increased proliferation of the ER-α-positive cells. Analysis of ovariectomized Tgfβ1 heterozygote mice demonstrated that an increase in proliferation of the ER-α-positive subpopulation requires estradiol and progesterone under the protocol of daily injections. Conversely, transgenic overexpression of active TGF-β1 resulted in reduced co-localization of ER-α and markers of proliferation. Observations in human breast that epithelial cells expressing either ER-α or PR rarely co-localize with makers of proliferation led to the proposal by Clarke and colleagues12 and Shoker and colleagues18 that these cells are actively restrained from proliferation by a growth inhibitor. Based on our present studies we propose that TGF-β1 is the growth inhibitor that restrains ER-α-positive cells in adult mammary gland from responding to signals to proliferate.
The validity of extending our observations in the rodent to the human breast is based on the following salient features shared between mouse mammary gland and human breast. 1) ER-α-positive cells are almost all PR-positive (reviewed in Anderson et al26 for human); 2) ER-α-positive cells are heterogeneously distributed;26,27 3) ER-α-positive cells rarely proliferate;12,18 and 4) estrogen up-regulates PR expression.26,33 This accord supports the use of mouse models to understand the regulation and responses of ER-α-positive mammary epithelial cells. Anderson and Clarke proposed that ER-α-positive cells are sensors that indirectly, via growth factors, regulate proliferation in ER-α-negative effector cells.34 Reproductive history with an early first pregnancy is a strong factor in assessing breast cancer risk.35 Parity in women or rodents results in less proliferation of ER-α-positive mammary epithelial cells.29,30 We found that the frequency of dual ER-α/Ki67-positive cells was increased in TGF-β-depleted mice, which suggests that TGF-β1 also restrains proliferation of ER-α-positive mammary epithelial cells in parous mice. Likewise, conditional deletion of Smad 4 by a pregnancy-induced promoter leads to increased proliferation, alveolar hyperplasia, and after repeated pregnancies, squamous cell carcinoma.32 Gene expression microarray analysis suggests that other members of the TGF-β family may also have a role in restricting proliferation after parity because TGF-β3 and its transcriptional targets are up-regulated in parous glands.36
Our novel finding that adult mammary epithelial ER-α-positive cells are restrained by TGF-β1 from proliferating in the presence of estrogen also has implications for understanding the biology of ER-α-positive cells in human breast cancers. The frequency of ER-α-positive cells increases with age in human breast, which parallels increased breast cancer risk.18 Lawson and colleagues37 have shown that increased frequency of ER-α-positive cells is associated with increased breast cancer risk. Women at higher risk of breast cancer have more ER-α-positive cells compared with those women in a low-risk population. Japanese women living in Hawaii have more ER-α cells than those in Japan, which parallels cancer risk.38 ER-α-positive cells are increased in normal tissue of tumor-bearing breasts,39 in postmenopausal women,18 and in postmenopausal women using hormone replacement therapy.40 Consistent with the human data, a mouse mammary model of dysregulated ER-α predisposes to hyperplasia and ductal carcinoma in situ.19 Together these data indicate that the size of the ER-α-positive subpopulation is modulated by tissue or host factors associated with age and environment, although they have yet to be identified specifically. Based on our present observations it is reasonable to suggest that decreased responsiveness to, or activation of, TGF-β1 may be one of the earliest events in dysregulating ER-α cells. Consistent with this is the finding that ER-α-positive breast cancer lines are less sensitive to TGF-β1-mediated growth inhibition than are ER-α-negative breast cancer cell lines.41
We suggest that these data support a model in which the ER-α-positive population represents a distinct mammary lineage. Stem cell behaviors, such as cell cycle entry, regeneration, and formation of niches, have been postulated to involve regulation by TGF-β1.42–44 Boulanger and Smith45 have shown that ectopic expression of constitutively active TGF-β1 in mammary gland leads to decreased serial transplantation capacity, which they hypothesize is due to premature stem cell senescence. TGF-β1 has been implicated in telomerase expression,46,47 which is thought to be characteristic of stem cells, and, interestingly, telomerase has been implicated in TGF-β1 insensitivity of human mammary epithelial cells.48 Recently, Tumbar and colleagues49 used a novel label-retaining approach in a transgenic mouse model to mark epidermal stem cells in the follicle bulge; these cells highly express TGF-β-regulated proteins and are more likely than progeny to exhibit phosphorylated Smad proteins indicative of TGF-β signaling. These examples of early progenitors regulated by TGF-β1 suggest the possibility that the ER-α-positive population may represent a similar mammary gland progenitor population; indeed, their low proliferation frequency is consistent with that of progenitor cells in other tissues. Tissue-specific stem cells or early progenitors are thought to be the critical cellular target in carcinogenesis based on their ability to produce unlimited progeny.50–55 Dysregulation of stem cell populations in transgenic mouse models of breast cancer can predispose the mammary gland to carcinogenesis,56 thus dysregulation of ER-α progenitors may be one route to ER-α-positive breast tumors.
Acknowledgments
We thank Ms. Rosie Chau and Mr. William Chou for technical assistance in the execution of these studies and Dr. Paul Williams for statistical consultation.
Footnotes
Address reprint requests to M.H. Barcellos-Hoff, Life Sciences Division, Bldg. 74-355, 1 Cyclotron Rd., Lawrence Berkeley National Laboratory, Berkeley CA 94720. E-mail: mhbarcellos-hoff@lbl.gov.
Supported by the California Breast Cancer Research Program (grant 4BP-0136), the National Institutes of Health (National Institute of Aging grant RO1 AG022413), Department of Defense Breast Cancer Research Program (fellowship grant DAMD-17-99-1-9169 to H.A.O.-R.), and the Office of Health and Environmental Research, Health Effects Division, United States Department of Energy (contract no. 03-76SF00098).
Present address of K.B.R.E.: Cardiff School of Biosciences, University of Cardiff, Cardiff, CF10 3US, Wales, UK; and the present address of H.L.M.: Vanderbilt-Ingram Cancer Center, Nashville, TN.
References
- Daniel CW, Robinson SD. Regulation of mammary growth and function by TGF-β. Mol Reprod Dev. 1992;32:145–151. doi: 10.1002/mrd.1080320210. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Ewan KB. TGF-β and mammary gland development. Breast Cancer Res. 2000;2:92–100. doi: 10.1186/bcr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Ackhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Wakefield L, Colletta AA, McCune BK, Sporn MB. Roles for transforming growth factors-β in the genesis, prevention and treatment of breast cancer. Dickson RB, Lippman ME, editors. Boston: Kluwer Academic Publishers,; Genes, Oncogens, and HormonesAdvances in Cellular and Molecular Biology of Breast Cancer. 1991:pp 97–136. [Google Scholar]
- Smith GH. TGF-β and functional differentiation. J Mammary Gland Biol Neoplasia. 1996;1:343–352. doi: 10.1007/BF02017390. [DOI] [PubMed] [Google Scholar]
- Lawrence DA, Pircher R, Jullien P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Comm. 1985;133:1026–1034. doi: 10.1016/0006-291x(85)91239-2. [DOI] [PubMed] [Google Scholar]
- Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst RJ, Wakefield L, Barcellos-Hoff MH. Latent TGF-β activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002;160:2081–2093. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresciani F. Topography of DNA synthesis in the mammary gland of the C3H mouse and its control by ovarian hormones: an autoradiographic study. Cell Tissue Kinet. 1968;1:51–63. [Google Scholar]
- Daniel CW, Silberstein GB, Strickland P. Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res. 1987;47:6052–6057. [PubMed] [Google Scholar]
- Christov K, Swanson SM, Guzman RC, Thordarson G, Jin E, Talamantes F, Nandi S. Kinetics of mammary epithelial cell proliferation in pituitary isografted BALB/c mice. Carcinogenesis. 1993;14:2019–2025. doi: 10.1093/carcin/14.10.2019. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Clark JA, Myers PH, Korach KS. Mammary gland development in adult mice requires epithelial and stromal estrogen receptor {alpha}. Endocrinology. 2002;143:2357–2365. doi: 10.1210/endo.143.6.8836. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- Russo IH, Russo J. Role of hormones in mammary cancer initiation and progression. J Mammary Gland Biol Neoplasia. 1998;3:49–61. doi: 10.1023/a:1018770218022. [DOI] [PubMed] [Google Scholar]
- Zeps N, Bentel JM, Papadimitriou JM, D’Antuono MF, Dawkins HJ. Estrogen receptor-negative epithelial cells in mouse mammary gland development and growth. Differentiation. 1998;62:221–226. doi: 10.1046/j.1432-0436.1998.6250221.x. [DOI] [PubMed] [Google Scholar]
- Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA. Estrogen receptors alpha and beta in the rodent mammary gland. Proc Natl Acad Sci USA. 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon JL, Shyamala G, Waring DW. PR localization and anterior pituitary cell populations in vitro in ovariectomized wild-type and pr-knockout mice. Endocrinology. 2001;142:4479–4485. doi: 10.1210/endo.142.10.8425. [DOI] [PubMed] [Google Scholar]
- Grimm SL, Seagroves TN, Kabotyanski EB, Hovey RC, Vonderhaar BK, Lydon JP, Miyoshi K, Hennighausen L, Ormandy CJ, Lee AV, Stull MA, Wood TL, Rosen JM. Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobulo-alveolar development. Mol Endocrinol. 2002;16:2675–2691. doi: 10.1210/me.2002-0239. [DOI] [PubMed] [Google Scholar]
- Shoker BS, Jarvis C, Clarke RB, Anderson E, Hewlett J, Davies MP, Sibson DR, Sloane JP. Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol. 1999;155:1811–1815. doi: 10.1016/S0002-9440(10)65498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, Chodosh LA, Flaws JA, Furth PA. Deregulated estrogen receptor {alpha} expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res. 2005;65:681–685. [PMC free article] [PubMed] [Google Scholar]
- Pierce DFJ, Johnson MD, Matsui Y, Robinson SD, Gold LI, Purchio AF, Daniel CW, Hogan BLM, Moses HL. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-β1. Genes Dev. 1993;7:2308–2317. doi: 10.1101/gad.7.12a.2308. [DOI] [PubMed] [Google Scholar]
- Shyamala G, Barcellos-Hoff MH, Toft D, Yang X. In situ localization of progesterone receptors in normal mouse mammary glands: absence of receptors in the connective and adipose stroma and a heterogeneous distribution in the epithelium. J Steroid Biochem Mol Biol. 1997;63:251–259. doi: 10.1016/s0960-0760(97)00128-3. [DOI] [PubMed] [Google Scholar]
- Munger J, Huang X, Kawakatsu H, Griffiths M, Dalton S, Wu J, Pittet J, Kaminski N, Garat C, Matthay M, Rifkin D, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Morris DG, Huang X, Kaminsk N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- Anderson E, Clarke RB, Howell A. Estrogen responsiveness and control of normal human breast proliferation. J Mammary Gland Biol Neoplasia. 1998;3:23–35. doi: 10.1023/a:1018718117113. [DOI] [PubMed] [Google Scholar]
- Shyamala G, Chou Y-C, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. 2002;1655:1–12. doi: 10.1016/s0960-0760(01)00182-0. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Ao X, Grill C, Russo IH. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53:217–227. doi: 10.1023/a:1006186719322. [DOI] [PubMed] [Google Scholar]
- Yang J, Yoshizawa K, Nandi S, Tsubura A. Protective effects of pregnancy and lactation against N-methyl-N-nitrosurea induced mammary carcinoma in female Lewis rats. Carcinogenesis. 1999;20:623–628. doi: 10.1093/carcin/20.4.623. [DOI] [PubMed] [Google Scholar]
- Medina D, Sivaraman L, Hilsenbeck SG, Conneely O, Ginger M, Rosem J, O’Malley BW. Mechanisms of hormonal prevention of breast cancer. Ann NY Acad Sci. 2000;120:23–35. doi: 10.1111/j.1749-6632.2001.tb02725.x. [DOI] [PubMed] [Google Scholar]
- Li W, Qiao W, Chen L, Xu X, Yang X, Li D, Li C, Brodie SG, Meguid MM, Hennighausen L, Deng C-X. Squamous cell carcinoma and mammary abscess formation through squamous metaplasia in Smad4/Dpc4 conditional knockout mice. Development. 2003;130:6143–6153. doi: 10.1242/dev.00820. [DOI] [PubMed] [Google Scholar]
- Shyamala G, Schneider W, Schott D. Developmental regulation of murine mammary progesterone receptor gene expression. Endocrinology. 1990;126:2882–2889. doi: 10.1210/endo-126-6-2882. [DOI] [PubMed] [Google Scholar]
- Anderson E, Clarke RB. Steroid receptors and cell cycle in normal mammary epithelium. J Mammary Gland Biol Neoplasia. 2004;9:3–13. doi: 10.1023/B:JOMG.0000023584.01750.16. [DOI] [PubMed] [Google Scholar]
- Sivaraman L, Conneely OM, Medina D, O’Malley BW. p53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis. Proc Natl Acad Sci USA. 2001;98:12379–12384. doi: 10.1073/pnas.221459098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz CM, Moody SE, Master SR, Hartman JL, Keiper EA, Imielinski MB, Cox JD, Wang JY, Ha SI, Keister BA, Chodosh LA. Persistent parity-induced changes in growth factors, TGF-{beta}3, and differentiation in the rodent mammary gland. Mol Endocrinol. 2002;16:2034–2051. doi: 10.1210/me.2002-0073. [DOI] [PubMed] [Google Scholar]
- Lawson JS, Field AS, Champion S, Tran D, Ishikura H, Trichopoulos D. Low oestrogen receptor alpha expression in normal breast tissue underlies low breast cancer incidence in Japan. Lancet. 1999;354:1787–1788. doi: 10.1016/s0140-6736(99)04936-3. [DOI] [PubMed] [Google Scholar]
- Lawson JS, Field AS, Tran DD, Killeen J, Maskarenic G, Ishikura H, Trichopoulos D. Breast cancer incidence and estrogen receptor alpha in normal mammary tissue—an epidemiologic study among Japanese women in Japan and Hawaii. Int J Cancer. 2002;97:685–687. doi: 10.1002/ijc.10093. [DOI] [PubMed] [Google Scholar]
- Khan SA, Rogers MA, Obando JA, Tamsen A. Estrogen receptor expression of benign breast epithelium and its association with breast cancer. Cancer Res. 1994;54:993–997. [PubMed] [Google Scholar]
- Lawson JS, Field AS, Tran DD, Houssami N. Hormone replacement therapy use dramatically increases breast oestrogen receptor expression in obese postmenopausal women. Breast Cancer Res. 2001;3:342–345. doi: 10.1186/bcr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga CL, Tandon AK, Von Hoff DD, Osborne CK. Transforming growth factor-β: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res. 1988;48:3898–3904. [PubMed] [Google Scholar]
- Booth D, Haley JD, Bruskin AM, Potten CS. Transforming growth factor-B3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem-cell cycling. Int J Cancer. 2000;86:53–59. doi: 10.1002/(sici)1097-0215(20000401)86:1<53::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- van’t Land B, Meijer HP, Frerichs J, Koetsier M, Jager D, Smeets RL, M’Rabet L, Hoijer M. Transforming growth factor-beta2 protects the small intestine during methotrexate treatment in rats possibly by reducing stem cell cycling. Br J Cancer. 2002;87:113–118. doi: 10.1038/sj.bjc.6600342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao MA, Hwa J, Nolta JA. Molecular mechanism of transforming growth factor beta-mediated cell-cycle modulation in primary human CD34+ progenitors. Blood. 2002;99:499–506. doi: 10.1182/blood.v99.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Smith GH. Reducing mammary cancer risk through premature stem cell senescence. Oncogene. 2001;20:2264–2272. doi: 10.1038/sj.onc.1204312. [DOI] [PubMed] [Google Scholar]
- Rama S, Suresh Y, Rao AJ. Regulation of telomerase during human placental differentiation: a role for TGFbeta1. Mol Cell Endocrinol. 2001;182:233–248. doi: 10.1016/s0303-7207(01)00550-0. [DOI] [PubMed] [Google Scholar]
- Yang H, Kyo S, Takatura M, Sunl L. Autocrine transforming growth factor beta suppresses telomerase activity and transcription of human telomerase reverse transcriptase in human cancer cells. Cell Growth Differ. 2001;12:119–127. [PubMed] [Google Scholar]
- Stampfer MR, Garbe J, Levine G, Lichtsteiner S, Vasserot AP, Yaswen P. Expression of the telomerase catalytic subunit, hTERT, induces resistance to transforming growth factor beta growth inhibition in p16INK4A(−) human mammary epithelial cells. Proc Natl Acad Sci. 2001;98:4498–4503. doi: 10.1073/pnas.071483998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Sell S, Pierce GB. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab Invest. 1994;70:6–22. [PubMed] [Google Scholar]
- Clifton KH, Tanner MA, Gould MN. Assessment of radiogenic cancer initiation frequency per clonogenic rat mammary cell in vivo. Cancer Res. 1986;46:2390–2395. [PubMed] [Google Scholar]
- Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- Zeps N, Dawkins HJS, Papadimitriou JM, Redmond SL, Walters MI. Detection of a population of long-lived cells in mammary epithelium of the mouse. Cell Tissue Res. 1996;286:525–536. doi: 10.1007/s004410050722. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci USA. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]