Abstract
Alcohol-induced cardiomyopathy including fibrosis has been recognized clinically for a long time, but its pathogenesis is incompletely understood. Studies using experimental animals have not fully duplicated the pathological changes in humans, and animal models of alcoholic cardiac fibrosis are not available. In the present study, we have developed a mouse model in which cardiac hypertrophy and fibrosis were produced in metallothionein-knockout (MT-KO) mice fed an alcohol-containing liquid diet for 2 months. The same alcohol feeding did not produce cardiac fibrosis in the wild-type (WT) control mice, although there was no difference in the alcohol-induced heart hypertrophy between the WT controls and the MT-KO mice. Zinc supplementation prevented cardiac fibrosis but did not affect heart hypertrophy in the alcohol-fed MT-KO mice, suggesting a specific link between zinc homeostasis and cardiac fibrosis. Serum creatine phosphokinase activity was significantly higher in the alcohol-administered MT-KO mice than in the WT mice, and zinc supplementation decreased serum creatine phosphokinase activities and eliminated the difference between the groups. Thus, disturbance in zinc homeostasis due to the lack of MT associates with alcohol-induced cardiac fibrosis and more severe cardiac injury, making the MT-KO mouse model of alcohol-induced cardiac fibrosis a useful tool to investigate specific factors involved in the alcoholic cardiomyopathy.
Myocardial fibrosis resulting from excessive fibrillar collagen deposition makes a critical contribution to left ventricular remodeling and heart failure in alcoholic patients. However, this typical cardiomyopathy has not been duplicated in animal models, being an obstacle for experimental studies of the pathogenesis of alcoholic cardiomyopathy (ACM). Zinc is a critical element that is involved in the regulation of fibrillar collagen metabolism and deposition. In general, zinc suppresses tissue collagen deposition through at least in part the inhibition of proline hydroxylase activity,1 which is activated by alcohol-induced oxidative stress.1–3 Zinc also is an essential element of matrix metalloproteinases, a group of zinc-containing enzymes functioning in fibrillar collagen degradation. However, the role of zinc in the regulation of collagen synthesis and degradation in the heart has not been understood. On the other hand, many studies have explored the inhibitory effect of zinc supplementation on fibrogenesis in the liver.4–6 It has been well known that a long-term consumption of excessive alcohol leads to zinc depletion in the liver of alcoholic patients.7 Many experimental studies have shown that supplementation with zinc inhibits liver fibrosis in animal models.4–6
Zinc deficiency is an important feature found in dilated cardiomyopathy in patients.8–11 A recent study examining serum zinc concentrations from patients with idiopathic dilated cardiomyopathy has shown that lower serum zinc occurred in the patients than in healthy subjects.8 The same findings have been reported in earlier studies; serum zinc and selenium levels are lower in patients with dilated cardiomyopathy.9–11 The zinc levels in the heart of ACM patients and animal models have not been determined. However, it has been well known that zinc levels in the liver of alcoholics and alcoholic liver disease animal models are lower than healthy subjects.7,12–14 An important question is whether zinc deficiency is involved in the pathogenesis of ACM.
Zinc metabolism and homeostasis in the cell are related to metallothionein (MT), which under physiological conditions binds seven atoms of zinc.15 The role of MT in the regulation of zinc homeostasis has been revealed only very recently,16 although such a role of MT has been suggested for a long time due to its zinc-binding capacity.15 MT is a small protein rich in cysteine and containing no aromatic amino acids or histidine.15 MT has a dumbbell-shaped structure with a thiolate α-domain M4IICys11 and a β-domain M3IICys9.17 This unique structure and high cysteine content confer MT a substantial capacity of zinc binding; four zinc atoms in the α-domain and three in the β-domain.18 The most important feature of MT is that under oxidative stress conditions, it releases zinc.18–20 The primary mechanism of regulation of zinc binding to and release from MT is likely that cysteine residues within MT can be converted between reduced and oxidized forms.16,20
A metallothionein-knockout (MT-KO) mouse model, in which MT-I and -II isoforms are genetically disrupted, has been produced.21 These mice have not shown deleterious phenotypes under physiological conditions.21 Their growth and reproductive characteristics have not been altered.22,23 Because of the requirement of MT in zinc homeostasis and the regulatory role of zinc in collagen metabolism and deposition, this MT-KO mouse model provides a useful tool to address the link between zinc deficiency and ACM. Thus, we took advantage of this unique mouse model to develop alcohol-induced cardiac fibrosis and to examine the effect of altered zinc homeostasis on pathogenesis of ACM.
Materials and Methods
Animals
Homozygous MT-KO mice (20 to 25 g, 8 to 10 weeks of age) produced on the 129/Sv genetic background were obtained from Jackson Laboratories (Bar Harbor, ME). MT-KO mice are phenotypically identical to their wild-type (WT) counterparts under normal conditions.21 In addition, reproductive characteristics, growth, and development are unaltered by the MT-null mutation.22,23 The mice were housed in the animal quarters at the University of Louisville Research Resources Center. They were maintained at 22°C with a 12-hour light/dark cycle and had free access to rodent chow and water. The experimental procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association for Accreditation of Laboratory Animal Care.
Modified Chronic Alcohol Feeding Protocol
An alcohol-containing Lieber and DeCarli liquid diet has been widely used to produce animal models for alcoholics. According to our preliminary experiments, long-term feeding with the alcohol-containing Lieber and DeCarli liquid diet causes decreases in food intake and body weight gain in mice. To eliminate the possible interference of malnutrition caused by decreased food intake, we established an improved alcohol-feeding protocol for mice. In this protocol, a 1-day-stop on the last day of each week was introduced in the feeding schedule by replacing alcohol diet with control diet. The content (%, w/v) of alcohol in the diet was gradually increased, starting at 3.6, increasing 0.2 every week, and reaching to 5.0% at the end of the 2-month feeding. Accordingly, the calorie contribution of alcohol started at 26% of total calories and reached 36% at the end of 2 months of feeding. This improved alcohol-feeding protocol produced a food intake of 445 ml/kg/day, ethanol intake of 19 g/kg/day.
Animal Treatments
Ten-week-old male MT-KO and WT mice (25 to 27 g body weight) were used. Animals were pair-fed with the Lieber and DeCarli liquid diet (Bio-Serv, Frenchtown, NJ), containing either alcohol or isocaloric dextrose. Animals were randomly assigned to eight groups following a 2 × 2 × 2 factorial design, MT-null × alcohol × zinc. Zinc supplementation was done by adding zinc sulfate to the liquid diet at 100 mg zinc atom/L, with a daily intake ∼33 mg zinc atom/kg. Food intake was recorded daily and body weight was monitored weekly. At the end of the experiment, the mice were anesthetized with sodium pentobarbital (0.05 mg/g body weight). Blood was drawn using a heparinized syringe from the dorsal vena cava, and plasma was obtained by centrifugation. The heart was perfused with saline and tissue samples were processed for both pathological and biochemical analysis.
Examination of Blood Alcohol Levels
Blood alcohol levels were examined at 9:00 a.m. on the last day of alcohol feeding. Blood samples were taken from the tail vein and immediately deproteinized with 6.25% trichloroacetic acid solution. Alcohol concentrations were determined by using a Sigma Diagnostics alcohol kit (procedure no. 332-UV; Sigma, St. Louis, MO) according to the manufacturer’s instructions.
Zinc and MT Assays
Zinc concentrations in the liver as an index of zinc status in the body were determined by inductively coupled argon plasma emission spectroscopy (model 1140; Jarrel-Ash, Waltham, MA) after lyophilization and digestion with nitric acid and hydrogen peroxide.24 MT concentrations in the heart were determined by a cadmium-hemoglobin affinity assay.25
Assessment of Heart Morphological Changes and Tissue Injury
At the time of harvesting, the heart weight was measured after perfusion and the tissue blocks for histological and ultrathin sections were prepared according to a procedure described previously.26 Histopathological and ultrastructural changes in the heart were examined by light and electron microscopy as described previously.26 Blood creatine phosphokinase (CPK) activities were colorimetrically measured by using a Sigma CK-20 kit (Sigma) following the instructions provided by the manufacturer.
Myocardial Fibrosis
Heart tissue sections were stained with 0.1% Sirius red for detecting collagen accumulation.27 The Sirius red-stained sections were assessed for the proportion of collagen accumulation in the heart tissues using a computer-assisted image analyzer (SigmaScan Pro5.0; SPSS Inc, Chicago, IL). There were five mice in each treatment group, in which five slides obtained from each sample (mouse) and seven random fields of each 1.2 mm2 at ×130 magnification per slide were examined. The ratio of the total collagen accumulation area to the whole area of the tissue section examined was calculated and the total collagen content in each heart was expressed as percent of collagen accumulation in the heart.
Statistics
All data are expressed as mean ± SE (n = 4 to 6). The data were analyzed according to a 2 × 2 × 2 factorial design. After a significant interaction was detected by the multiway analysis of variance, the significance of the main effects was further determined. The level of significance was considered at P < 0.05.
Results
Characterization of Alcohol Liquid Diet Feeding in Mice
To monitor alcohol intake, daily food intake was recorded in the alcohol-fed WT and MT-KO mice. The pair-fed mice were adjusted for their food supply accordingly. The dietary alcohol levels were increased by 0.2% (w/v) every week and the daily food intake showed an increase during the 2-month feeding period with an average of 445 ml/kg/day in WT and 434 ml/kg/day in MT-KO mice. The calculated daily alcohol intake (g/kg/day) was 19.2 in WT and 18.7 in MT-KO mice. The average daily zinc intake (mg/kg/day) was 33.4 in WT and 32.6 in MT-KO mice. The blood alcohol levels measured at 9:00 a.m. on the last day of feeding were 221 ± 45 mg/dl in WT and 210 ± 30 mg/dl in MT-KO mice. There were no significant differences in any of the parameters measured above between the MT-KO and WT mice with or without zinc supplementation.
Zinc Supplementation on Zinc Concentrations in the Liver and MT Levels in the Heart
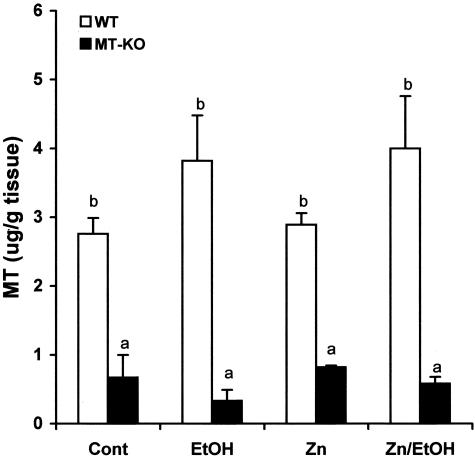
Zinc concentrations in the liver were measured as an overall indication of systemic zinc status. As shown in Table 1, chronic alcohol feeding caused a significant decrease in the hepatic zinc concentration in both WT and MT-KO mice. Dietary zinc supplementation did not significantly increase the basal level of hepatic zinc concentrations, but prevented the alcohol-induced decrease in both WT and MT-KO mice. MT concentrations in the heart of WT mice were much higher than in MT-KO-mice (Figure 1). Alcohol feeding slightly but not statistically significantly increased MT concentrations in the hearts of WT mice and zinc supplementation did not cause significant changes. MT levels in the hearts of MT-KO mice were at the margin of the detection limitation of the assay, and neither alcohol nor zinc supplementation altered MT concentrations in the MT-KO mice.
Table 1.
Effect of Zinc Supplementation on Zinc Concentrations in the Liver
| Control | EtOH | Zinc | Zn/EtOH | |
|---|---|---|---|---|
| WT | 86.7 ± 5.23a | 66.4 ± 3.60b | 90.3 ± 4.01a | 84.6 ± 4.98a |
| MT-KO | 83.7 ± 4.66a | 60.4 ± 3.57b | 85.5 ± 10.23a | 74.8 ± 4.97c |
n = 6, the values that do not share the same letter differ significantly from each other (P < 0.05).
Figure 1.
MT concentrations in the hearts of WT and MT-KO mice treated with alcohol, zinc, or alcohol plus zinc. The hearts were obtained 2 months after the alcohol-containing liquid diet feeding with or without zinc supplementation. Data were obtained from four animals in each group and the values that do not share the same letter are significantly (P < 0.05) different from each other.
Alcohol-Induced Myocardial Morphological Alterations
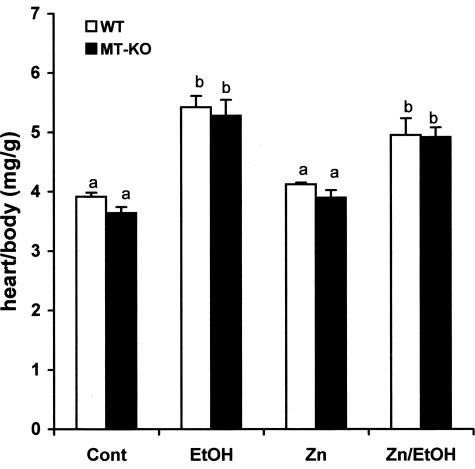
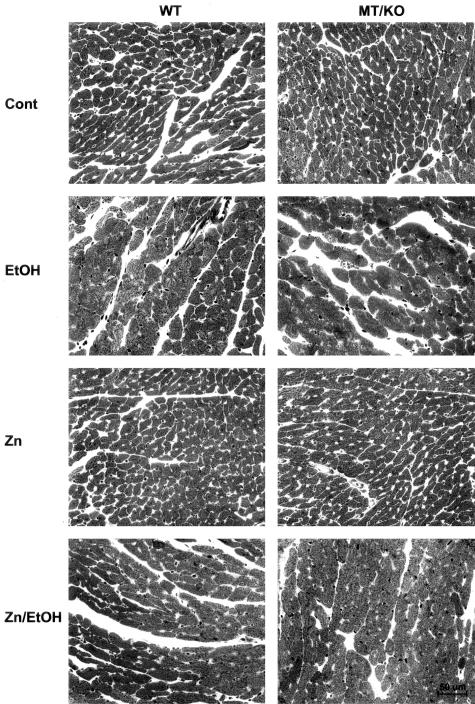
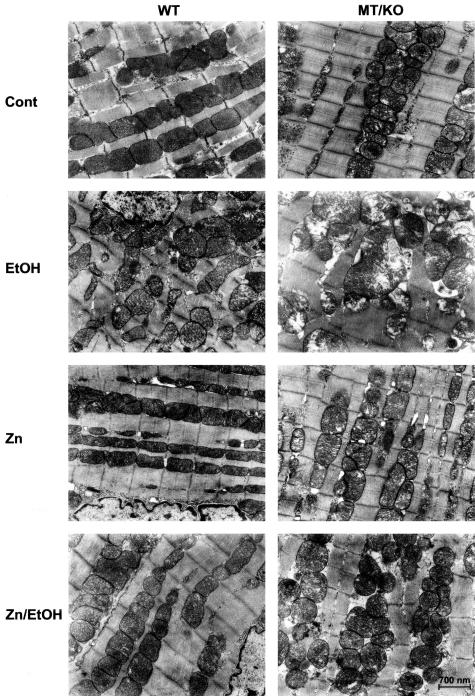
The results presented in Figure 2 show that alcohol induced cardiac hypertrophy as measured by the heart weight normalized to body weight. Zinc supplementation did not change the hypertrophic effect of alcohol on the heart. Histological examination identified hypertrophic cardiomyocytes in the alcohol-treated myocardium from both WT and MT-KO mice (Figure 3). Zinc supplementation did not suppress alcohol-induced cardiac hypertrophy. Ultrastructural examination by electron microscope revealed alcohol-induced disruption of the mitochondrial structure, including alterations in the integrity of the mitochondrial membrane, disorganized cristae in the mitochondria, mitochondrial swelling, and overall disorganization of mitochondria in the myocardium (Figure 4). These damages were more severe in the myocardium of MT-KO mice than in the WT mice. Zinc supplementation appeared to suppress some of the changes and to be more effective in the WT mice. In the zinc-supplemented MT-KO mice, alcohol-induced mitochondrial swelling and disorganization remained observable.
Figure 2.
Alcohol-induced increase in the heart weight normalized to body weight in WT and MT-KO mice. Each value was obtained from four to six mice and the values that do not share the same letter differ significantly (P < 0.05) from each other.
Figure 3.
Alcohol-induced histopathological changes in the myocardium and the effect of zinc supplementation on the changes. Alcohol (EtOH) feeding markedly increased the size of cardiomyocytes in comparison with the controls (Cont) in both WT and MT-KO mice. Zinc (Zn) supplementation did not alter the morphology of the myocardium or affect the alcohol-induced hypertrophy of the cardiomyocytes (Zn/EtOH). Degenerative morphological changes including myocyte necrosis were identified in the alcohol-treated myocardium.
Figure 4.
Alcohol-induced ultrastructural changes in the myocardium and the effect of zinc supplementation. Alcohol (EtOH) feeding induced marked organelle changes in both WT and MT-KO myocardium. More damages, in particular mitochondrial swelling and cristae disorganization, were found in MT-KO mice. Zinc (Zn) supplementation greatly improved the ultrastructural changes in both WT and MT-KO mice (Zn/EtOH).
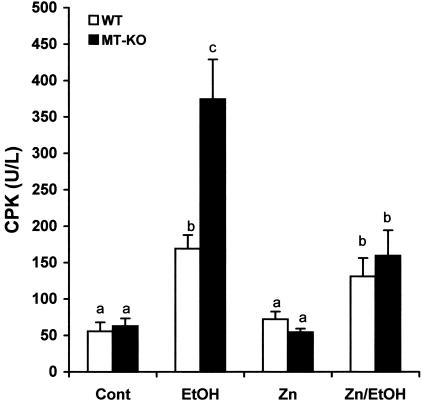
Zinc Supplementation Prevented Alcohol-Induced Cardiac Fibrosis
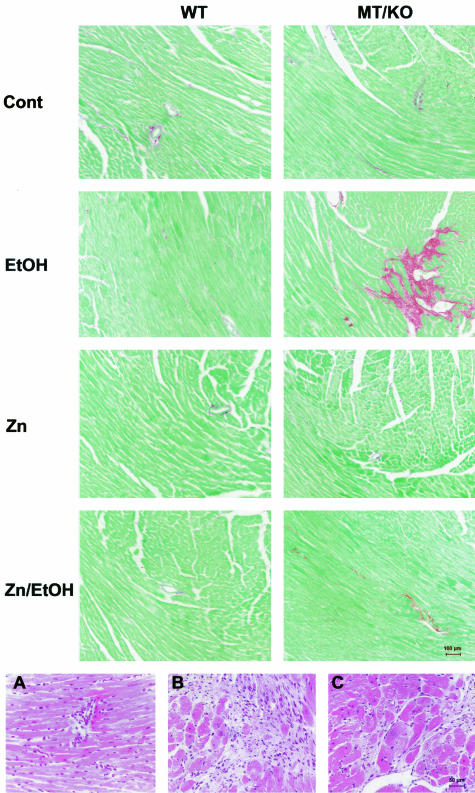
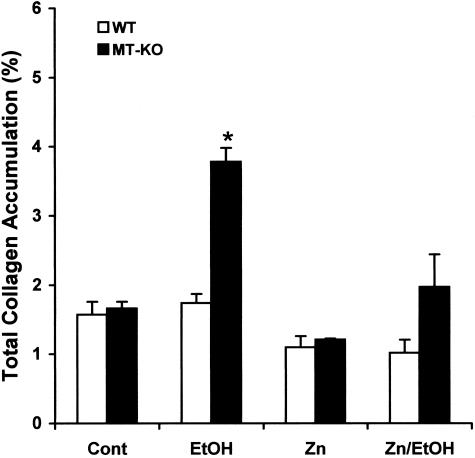
The morphological analyses above indicate that alcohol would induce myocardial injury differently between WT and MT-KO mice. Serum activities of CPK were measured as a marker of myocardial injury. The results presented in Figure 5 show that alcohol significantly elevated serum CPK activities in both WT and MT-KO mice. However, the elevation in the MT-KO mice was significantly higher than in the WT mice. Zinc supplementation suppressed the overt increase in serum CPK activities in the MT-KO mice and eliminated the difference between WT and MT-KO mice. We next examined whether alcohol induces myocardial fibrosis and the effect of zinc supplementation. As shown in Figure 6, under control conditions, there was some detectable collagen accumulation visualized by the Sirius red staining and most of the collagen accumulation was perivascular. Alcohol did not change the perivascular collagen accumulation in the WT mice, but increased the accumulation in the MT-KO mice. However, the most predominant change in the MT-KO mouse myocardium was the interstitial collagen accumulation, or replacement fibrosis. In the fibrotic MT-KO myocardium, perivascular inflammatory infiltration was observed and the fibrotic area was associated with a patchy interstitial neutrophil infiltration (Figure 6). It was also observed that the fibrotic area was surrounded by hypertrophic cardiomyocytes. Zinc supplementation completely prevented the replacement fibrosis and inhibited the excessive perivascular collagen accumulation in the alcoholic MT-KO mice. Although excessive collagen accumulation occurred, ventricular dilation was not observed in the MT-KO mouse heart (data not shown). Semiquantitative analysis using a computer-assisted morphometric analyzer system revealed that there were greater than twofold more collagen accumulations in the alcohol-treated MT-KO mouse myocardium than in the WT mice (Figure 7). Zinc supplementation caused an observable, but not statistically significant, decrease in collagen accumulation in both WT and MT-KO controls and completely eliminated alcohol-elevated collagen accumulation in the MT-KO mice.
Figure 5.
Effects of alcohol feeding on serum CPK activities in WT and MT-KO mice with or without zinc supplementation. Each value was obtained from four to eight mice and the values that do not share the same letter differ significantly (P < 0.05) from each other.
Figure 6.
Top: Alcohol-induced myocardial fibrosis and the effect of zinc supplementation. Alcohol (EtOH) feeding induced collagen deposition in MT-KO myocardium and zinc supplementation prevented the fibrotic effect (Zn/EtOH). Alcohol did not cause the same fibrotic change in WT mice and zinc supplementation per se did not induce fibrotic response in either WT or MT-KO myocardium. Bottom: Perivascular inflammatory infiltration (A), patchy interstitial neutrophil infiltration in fibrotic area (B), and hypertrophic cardiomyocytes surrounding fibrotic area (C) in alcoholic MT-KO myocardium.
Figure 7.
Semiquantitative analysis of the proportion of fibrotic area in the whole heart, expressed as percentage of the heart. Each data point was obtained from five mice, and five slides from each mouse and seven random fields (1.2 mm2 per field) per slide were examined and digitalized using a computer-assisted morphometric imaging analyzer system. The data presented are mean ± SEM and an asterisk indicates a significant difference from the rest of the groups (P < 0.05).
Discussion
The results obtained from the present study demonstrate that in the MT-KO mouse model a relative short-term (2 months) alcohol-containing liquid diet feeding can produce myocardial injury that simulates clinical ACM, including myocardial fibrosis that has never been produced in animal models before. An important observation of the present study is that alcohol-induced myocardial fibrosis is related to MT regulation of zinc homeostasis. That myocardial fibrosis was observed only in the MT-KO mice and zinc supplementation completely prevented the fibrotic effect strongly suggests the crucial role of zinc depletion or disturbance in zinc homeostasis in the pathogenesis of alcohol-induced myocardial fibrosis. Both WT and MT-KO mice developed cardiac hypertrophy equally and zinc supplementation did not prevent the hypertrophic effect of alcohol, indicating changes in zinc status may not be associated with alcohol-induced cardiac hypertrophy. These results suggest that the signaling pathways and molecular mechanisms by which alcohol causes myocardial fibrosis would differ from those by which alcohol induces cardiac hypertrophy.
Alcoholic heart muscle disease has been recognized for a long time28 and the prevalence of alcoholic heart muscle disease in selected populations ranges from 23 to 40%.29,30 Alcohol is believed to be one of the major causal agents for patients with nonischemic, dilated cardiomyopathy, referred to as ACM.31 The exact pathogenesis of ACM has not been completely understood. However, it is clear that the duration of heavy alcohol use in patients is a critical factor. Clinical data have shown that ACM typically is seen after a long term of consistent consumption of at least 80 g of alcohol per day.32,33 In general, asymptomatic ACM patients with changes in cardiac structure and function had a history of consuming >90 g/day of alcohol for more than 5 years.33–36 In terms of the amount and duration of alcohol consumption required to produce symptomatic ACM, some limited data have shown that more than 10 years of excessive alcohol consumption in alcoholics produces congestive heart failure.37 The daily amount of alcohol consumption is essentially the same between the asymptomatic and symptomatic patients.37 Therefore, the key factor affecting the development of heart failure, in particular the transition from asymptomatic to symptomatic, is the duration of heavy daily alcohol consumption, although other undefined factors may also make critical contributions.
Several rodent models of alcohol-induced heart injury have been tested.38–43 Most approaches used in these studies involve alcohol-containing liquid diet feeding for several weeks to several months. However, the simulation of the daily excessive amount of alcohol consumption in rodents without disturbance in the food intake to produce nutritional deficiency is a constant challenge. One of the most difficulties in using rodent models is that the short life span of mice or rats does not allow a sufficient long period of alcohol exposure to produce some of the critical pathological changes such as myocardial fibrosis observed in humans.
The unique MT-KO/ACM mouse model produced in the present study is of a great promise for experimental approaches to the understanding of the pathogenesis of ACM. In this model, the disturbance in food intake of the animals was minimized but the duration of the feeding for 2 months was sufficient to produce both cardiac hypertrophy and fibrosis, which closely simulate what were found in alcoholic patients. In particular, blood alcohol levels in these mice were 210 to 220 mg/dl. This range of blood alcohol levels was comparable to what was found in the alcoholic patients. One study from 153 alcoholic patients has found that the blood alcohol levels ranged from 109 to 558 mg/dl, averaged 245 mg/dl.44 Another study from 117 hospitalized male alcoholic patients have found that the blood alcohol levels ranged from 29 to 577 mg/dl in all patients and from 200 to 299 mg/dl in 48 of them.45 A study on 24 emergency department patients has found that the blood alcohol levels in these patients ranged from 58 to 447 mg/dl, averaged 249 ± 109 mg/dl.46 Therefore, the blood alcohol levels in the mouse model presented here were closely mimicking what were found in the alcoholic patients, further indicating the clinical relevance of the present finding. Importantly, the myocardial fibrosis occurred only in the MT-KO mice and there were no differences in the blood alcohol levels and the alcoholic cardiac hypertrophy between WT and MT-KO mice, providing a unique experimental model to study specifically the pathogenesis of alcoholic myocardial fibrosis. However, the time course of this study (2 months) has limitations in terms of the allowance for full expression of ACM in the mouse model. For instance, we have not observed ventricular dilation in the MT-KO/ACM mice, suggesting a requirement of a prolonged observation for ventricular dilation to occur.
Understanding the molecular mechanism of ACM is a critical task in the clinical and experimental studies of the important problem of alcoholics. The results produced from the present study indicated the importance of zinc in myocardial fibrogenesis. Clinical data have shown that depressed zinc concentrations in the blood of alcoholics and patients with dilated cardiomyopathy have been a consistent defect.8–11 Zinc depletion is thus a critical detrimental consequence of a long-term consumption of excessive alcohol and would play an important role in the pathogenesis of ACM. A significant finding of the present study is that zinc depletion is related more to myocardial fibrosis than to cardiac hypertrophy. Alcohol-induced zinc depletion would take a long time to occur in animal model, which may explain why myocardial fibrosis has never been observed in alcoholic rodent models. In the MT-KO mice, due to the lack of zinc regulatory protein MT, zinc depletion would be accelerated; thus the fibrotic effect of alcohol can be readily expressed, as shown in the present study. In this context, the prevention by zinc supplementation from alcohol-induced myocardial fibrosis in the MT-KO mice confirmed the involvement of zinc depletion in the pathogenesis of myocardial fibrosis. In a resent study,27 we have observed that MT gene therapy reversed an established liver fibrosis induced by carbon tetrachloride in mice, further demonstrating the cause-and-effect relationship between disturbance in zinc homeostasis and fibrogenesis.
The role of MT in the regulation of zinc homeostasis has been recognized recently and many studies have produced exciting new insights into the interaction between zinc and MT and the biological significance.16,18–20,47 In general, Zn-MT is an important storage form of zinc in the cell. Under oxidative stress conditions, zinc is released from MT for biological activities including regulation of transcription factors and gene expression. In the absence of MT, endogenous zinc storage would be easily depleted and the zinc availability under stress conditions would be limited. Therefore, unless another source of zinc is available such as supplementation with exogenous zinc in the present study, the consequence of zinc depletion would occur, such as myocardial fibrosis observed in the present study. In this context, the MT-KO mice would have a wide range of applications in the study of dilated cardiomyopathy, as suggested by previous studies that zinc depletion occurs in patients with dilated cardiomyopathy.8–10 On the other hand, MT has been shown to play an important role in cytoprotection from oxidative stress in the heart.25,48–52 Therefore, the possibility of a direct involvement of MT as a potent antioxidant in preventing alcohol-induced oxidative heart injury, thereby myocardial fibrosis, cannot be excluded.
Myocardial fibrosis plays a key role in myocardial remodeling and heart failure. The MT-KO/ACM mouse model thus can help to dissect the subsequent molecular and cellular events of alcohol-induced myocardial fibrosis. This animal model provides not only a unique tool to study the pathogenesis of alcoholic myocardial fibrosis but also an alternative approach to the understanding of the development and progression of ACM.
Acknowledgments
We thank Xinggu Sun for technical assistance.
Footnotes
Address reprint requests to Dr. Y. James Kang, Department of Medicine, University of Louisville School of Medicine, 511 S. Floyd St., MDR 530, Louisville, KY 40202. E-mail: yjkang01@louisville.edu.
Supported in part by the National Institutes of Health (grants HL59225 and HL63760 to Y.J.K. and AA13601 to Z.Z.).
Y.J.K. is a distinguished university scholar of the University of Louisville.
References
- Camps J, Bargallo T, Gimenez A, Alie S, Caballeria J, Pares A, Joven J, Masana L, Rodes J. Relationship between hepatic lipid peroxidation and fibrogenesis in carbon tetrachloride-treated rats: effect of zinc administration. Clin Sci (Lond) 1992;83:695–700. doi: 10.1042/cs0830695. [DOI] [PubMed] [Google Scholar]
- Mezey E, Potter JJ, Maddrey WC. Hepatic collagen proline hydroxylase activity in alcoholic liver disease. Clin Chim Acta. 1976;68:313–320. doi: 10.1016/0009-8981(76)90397-1. [DOI] [PubMed] [Google Scholar]
- Mann SW, Fuller GC, Rodil JV, Vidins EI. Hepatic prolyl hydroxylase and collagen synthesis in patients with alcoholic liver disease. Gut. 1979;20:825–832. doi: 10.1136/gut.20.10.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttinen H, Ryhanen L, Puistola U, Arranto A, Oikarinen A. Decrease in liver collagen accumulation in carbon tetrachloride-injured and normal growing rats upon administration of zinc. Gastroenterology. 1984;86:532–539. [PubMed] [Google Scholar]
- Cabre M, Camps J, Ferre N, Paternain JL, Joven J. The antioxidant and hepatoprotective effects of zinc are related to hepatic cytochrome P450 depression and metallothionein induction in rats with experimental cirrhosis. Int J Vitam Nutr Res. 2001;71:229–236. doi: 10.1024/0300-9831.71.4.229. [DOI] [PubMed] [Google Scholar]
- Gimenez A, Pares A, Alie S, Camps J, Deulofeu R, Caballeria J, Rodes J. Fibrogenic and collagenolytic activity in carbon-tetrachloride-injured rats: beneficial effects of zinc administration. J Hepatol. 1994;21:292–298. doi: 10.1016/s0168-8278(05)80304-6. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Su LC. Zinc deficiency in the alcoholic: a review. Alcohol Clin Exp Res. 1983;7:5–10. doi: 10.1111/j.1530-0277.1983.tb05402.x. [DOI] [PubMed] [Google Scholar]
- Topuzoglu G, Erbay AR, Karul AB, Yensel N. Concentrations of copper, zinc, and magnesium in sera from patients with idiopathic dilated cardiomyopathy. Biol Trace Elem Res. 2003;95:11–17. doi: 10.1385/bter:95:1:11. [DOI] [PubMed] [Google Scholar]
- Martin-Lagos F, Navarro-Alarcon M, Terres-Martos C, Lopez G, de la Serrana H, Lopez-Martinez MC. Serum copper and zinc concentrations in serum from patients with cancer and cardiovascular disease. Sci Total Environ. 1997;204:27–35. doi: 10.1016/s0048-9697(97)00163-0. [DOI] [PubMed] [Google Scholar]
- Oster O. Trace element concentrations (Cu, Zn, Fe) in sera from patients with dilated cardiomyopathy. Clin Chim Acta. 1993;214:209–218. doi: 10.1016/0009-8981(93)90112-h. [DOI] [PubMed] [Google Scholar]
- Bogden JD, Al-Rabiai S, Gilani SH. Effect of chronic ethanol ingestion on the metabolism of copper, iron, manganese, selenium, and zinc in an animal model of alcoholic cardiomyopathy. J Toxicol Environ Health. 1984;14:407–417. doi: 10.1080/15287398409530589. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Antonow DR, Cohen DA, Shedlofsky SI. Zinc metabolism in alcoholic liver disease. Alcohol Clin Exp Res. 1986;10:582–589. doi: 10.1111/j.1530-0277.1986.tb05149.x. [DOI] [PubMed] [Google Scholar]
- Cook CC, Walden RJ, Graham BR, Gillham C, Davies S, Prichard BN. Trace element and vitamin deficiency in alcoholic and control subjects. Alcohol Alcohol. 1991;26:541–548. doi: 10.1093/oxfordjournals.alcalc.a045157. [DOI] [PubMed] [Google Scholar]
- Loguercio C, De Girolamo V, Federico A, Feng SL, Cataldi V, Del Vecchio Blanco C, Gialanella G. Trace elements and chronic liver diseases. J Trace Elem Med Biol. 1997;11:158–161. doi: 10.1016/s0946-672x(97)80045-4. [DOI] [PubMed] [Google Scholar]
- Kagi JH. Overview of metallothionein. Methods Enzymol. 1991;205:613–626. doi: 10.1016/0076-6879(91)05145-l. [DOI] [PubMed] [Google Scholar]
- Maret W. The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr. 2000;130(Suppl 5):1455S–1458S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- Otvos JD, Armitage IM. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci USA. 1980;77:7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee BL. The function of metallothionein. Neurochem Int. 1995;27:23–33. doi: 10.1016/0197-0186(94)00165-q. [DOI] [PubMed] [Google Scholar]
- Maret W, Vallee BL. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci USA. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc Natl Acad Sci USA. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA. 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeebu SS, Liu J, Liu Y, Klaassen CD. Metallothionein-null mice are more sensitive than wild-type mice to liver injury induced by repeated exposure to cadmium. Toxicol Sci. 2000;55:223–232. doi: 10.1093/toxsci/55.1.223. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Habeebu SS, Klaassen CD. Metallothionein (MT)-null mice are sensitive to cisplatin-induced hepatotoxicity. Toxicol Appl Pharmacol. 1998;149:24–31. doi: 10.1006/taap.1997.8325. [DOI] [PubMed] [Google Scholar]
- Nielsen FH, Zimmerman TJ, Shuler TR. Interactions among nickel, copper and iron in rats: liver and plasma contents of lipids and trace elements. Biol Trace Elem Res. 1982;4:125–143. doi: 10.1007/BF02783253. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest. 1997;100:1501–1506. doi: 10.1172/JCI119672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhou Z, Kang YJ. Attenuation of doxorubicin chronic toxicity in metallothionein-overexpressing transgenic mouse heart. Cancer Res. 2001;61:3382–3387. [PubMed] [Google Scholar]
- Jiang Y, Kang YJ. Metallothionein gene therapy for chemical-induced liver fibrosis in mice. Mol Ther. 2004;10:1130–1139. doi: 10.1016/j.ymthe.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Alcohol and cardiovascular diseases: a historical overview. Novartis Found Symp. 1998;216:2–12. [PubMed] [Google Scholar]
- Fauchier L, Babuty D, Poret P, Casset-Senon D, Autret ML, Cosnay P, Fauchier JP. Comparison of long-term outcome of alcoholic and idiopathic dilated cardiomyopathy. Eur Heart J. 2000;21:306–314. doi: 10.1053/euhj.1999.1761. [DOI] [PubMed] [Google Scholar]
- McKenna CJ, Codd MB, McCann HA, Sugrue DD. Alcohol consumption and idiopathic dilated cardiomyopathy: a case control study. Am Heart J. 1998;135:833–837. doi: 10.1016/s0002-8703(98)70042-0. [DOI] [PubMed] [Google Scholar]
- Gavazzi A, De Maria R, Parolini M, Porcu M. Alcohol abuse and dilated cardiomyopathy in men. Am J Cardiol. 2000;85:1114–1118. doi: 10.1016/s0002-9149(00)00706-2. [DOI] [PubMed] [Google Scholar]
- Regan TJ. Alcohol and the cardiovascular system. JAMA. 1990;264:377–381. [PubMed] [Google Scholar]
- Lazarevic AM, Nakatani S, Neskovic AN, Marinkovic J, Yasumura Y, Stojicic D, Miyatake K, Bojic M, Popovic AD. Early changes in left ventricular function in chronic asymptomatic alcoholics: relation to the duration of heavy drinking. J Am Coll Cardiol. 2000;35:1599–1606. doi: 10.1016/s0735-1097(00)00565-9. [DOI] [PubMed] [Google Scholar]
- Kupari M, Koskinen P, Suokas A, Ventila M. Left ventricular filling impairment in asymptomatic chronic alcoholics. Am J Cardiol. 1990;66:1473–1477. doi: 10.1016/0002-9149(90)90537-b. [DOI] [PubMed] [Google Scholar]
- Askanas A, Udoshi M, Sadjadi SA. The heart in chronic alcoholism: a noninvasive study. Am Heart J. 1980;99:9–16. doi: 10.1016/0002-8703(80)90309-9. [DOI] [PubMed] [Google Scholar]
- Silberbauer K, Juhasz M, Ohrenberger G, Hess C. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in young alcoholics. Cardiology. 1988;75:431–439. doi: 10.1159/000174413. [DOI] [PubMed] [Google Scholar]
- Mathews EC, Jr, Gardin JM, Henry WL, Del Negro AA, Fletcher RD, Snow JA, Epstein SE. Echocardiographic abnormalities in chronic alcoholics with and without overt congestive heart failure. Am J Cardiol. 1981;47:570–578. doi: 10.1016/0002-9149(81)90540-3. [DOI] [PubMed] [Google Scholar]
- Regan TJ, Morvai V. Experimental models for studying the effects of ethanol on the myocardium. Acta Med Scand Suppl. 1987;717:107–113. doi: 10.1111/j.0954-6820.1987.tb13047.x. [DOI] [PubMed] [Google Scholar]
- Capasso JM, Li P, Guideri G, Malhotra A, Cortese R, Anversa P. Myocardial mechanical, biochemical, and structural alterations induced by chronic ethanol ingestion in rats. Circ Res. 1992;71:346–356. doi: 10.1161/01.res.71.2.346. [DOI] [PubMed] [Google Scholar]
- Chan TC, Sutter MC. The effects of chronic ethanol consumption on cardiac function in rats. Can J Physiol Pharmacol. 1982;60:777–782. doi: 10.1139/y82-108. [DOI] [PubMed] [Google Scholar]
- Thomas AP, Rozanski DJ, Renard DC, Rubin E. Effects of ethanol on the contractile function of the heart: a review. Alcohol Clin Exp Res. 1994;18:121–131. doi: 10.1111/j.1530-0277.1994.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Kim SD, Beck J, Bieniarz T, Schumacher A, Piano MR. A rodent model of alcoholic heart muscle disease and its evaluation by echocardiography. Alcohol Clin Exp Res. 2001;25:457–463. [PubMed] [Google Scholar]
- Kim SD, Bieniarz T, Esser KA, Piano MR. Cardiac structure and function after short-term ethanol consumption in rats. Alcohol. 2003;29:21–29. doi: 10.1016/s0741-8329(02)00296-3. [DOI] [PubMed] [Google Scholar]
- Lowenstein SR, Weissberg MP, Terry D. Alcohol intoxication, injuries, and dangerous behaviors and the revolving emergency department door. J Trauma. 1990;30:1252–1258. doi: 10.1097/00005373-199010000-00010. [DOI] [PubMed] [Google Scholar]
- Adachi J, Mizoi Y, Fukunaga T, Ogawa Y, Ueno Y, Imamichi H. Degrees of alcohol intoxication in 117 hospitalized cases. J Stud Alcohol. 1991;52:448–453. doi: 10.15288/jsa.1991.52.448. [DOI] [PubMed] [Google Scholar]
- Brennan DF, Betzelos S, Reed R, Falk JL. Ethanol elimination rates in an ED population. Am J Emerg Med. 1995;13:276–280. doi: 10.1016/0735-6757(95)90199-X. [DOI] [PubMed] [Google Scholar]
- St.Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am J Physiol. 2002;282:L185–L192. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]
- Kang YJ. The antioxidant function of metallothionein in the heart. Proc Soc Exp Biol Med. 1999;222:263–273. doi: 10.1046/j.1525-1373.1999.d01-143.x. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Zhou ZX, Wang GW, Buridi A, Klein JB. Suppression by metallothionein of doxorubicin-induced cardiomyocyte apoptosis through inhibition of p38 mitogen-activated protein kinases. J Biol Chem. 2000;275:13690–13698. doi: 10.1074/jbc.275.18.13690. [DOI] [PubMed] [Google Scholar]
- Wang GW, Klein JB, Kang YJ. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J Pharmacol Exp Ther. 2001;298:461–468. [PubMed] [Google Scholar]
- Kang YJ, Li Y, Sun X, Sun X. Antiapoptotic effect and inhibition of ischemia/reperfusion-induced myocardial injury in metallothionein-overexpressing transgenic mice. Am J Pathol. 2003;163:1579–1586. doi: 10.1016/S0002-9440(10)63514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhou Z, Kang YJ. Attenuation of doxorubicin chronic toxicity in metallothionein-overexpressing transgenic mouse heart. Cancer Res. 2001;61:3382–3387. [PubMed] [Google Scholar]