Abstract
Overexpression of Cripto-1 has been reported in several types of human cancers including breast cancer. To investigate the role of human Cripto-1 (CR-1) in mammary gland development and tumorigenesis, we developed transgenic mice that express the human CR-1 transgene under the regulation of the whey acidic protein (WAP) promoter in the FVB/N mouse background. The CR-1 transgene was detected in the mammary gland of 15-week-old virgin WAP-CR-1 female mice that eventually developed hyperplastic lesions. From mid-pregnancy to early lactation, mammary lobulo-alveolar structures in WAP-CR-1 mice were less differentiated and delayed in their development due to decreased cell proliferation as compared to FVB/N mice. Early involution, due to increased apoptosis, was observed in the mammary glands of WAP-CR-1 mice. Higher levels of phosphorylated AKT and MAPK were detected in mammary glands of multiparous WAP-CR-1 mice as compared to multiparous FVB/N mice suggesting increased cell proliferation and survival of the transgenic mammary gland. In addition, more than half (15 of 29) of the WAP-CR-1 multiparous female mice developed multifocal mammary tumors of mixed histological subtypes. These results demonstrate that overexpression of CR-1 during pregnancy and lactation can lead to alterations in mammary gland development and to production of mammary tumors in multiparous mice.
The human Cripto-1 (CR-1) gene was initially isolated from a human teratocarcinoma cDNA library and was identified as a potential oncogene.1 CR-1 encodes a 28-kd glycoprotein of 188 amino acids that contains three intradisulfide bonds encompassing a modified epidermal growth factor-like consensus sequence of ∼37 amino acids and a cysteine-rich cripto-Frl-cryptic (CFC) domain.1 Cripto-1 can function as a dominant transforming gene in vitro in several different mammary epithelial cell lines.2,3 In the mouse, cripto-1 (Cr-1) is expressed in cap cells and subtending ductal epithelial cells of the growing terminal end buds in the virgin mammary gland and expression increases in the ductal epithelium during pregnancy and lactation.4 Although a detailed stage-by-stage analysis of CR-1 expression in human breast has not been performed, CR-1 protein has been detected by enzyme-linked immunosorbent assay in human milk suggesting that it may be produced and secreted from normal human mammary epithelium during pregnancy and lactation.5 An increase in lateral side-branching was observed in several different sublines of MMTV-CR-1 postpubescent transgenic virgin mice.6 This agreed with previous in vivo data using primary mouse mammary cells overexpressing a transduced Cr-1 transgene that have been transplanted into the cleared mammary fat pad of syngeneic virgin mice.7 In addition, overexpression of cripto-1 (Cr-1 or CR-1) in different normal mouse mammary epithelial cell lines enhanced branching morphogenesis of these cell lines in type I collagen gels or Matrigel and facilitated cellular migration, invasion, and epithelial-mesenchymal transition in vitro and in vivo.3,8 High levels of Cr-1 have also been detected in mammary hyperplasias and mammary tumors that arise in transgenic mice expressing different oncogenes such as transforming growth factor (TGF)-β, erbB-2, Int-3, SV40 large T antigen, and polyoma middle T antigen.9,10
Cripto-1 mRNA and protein are expressed in ∼75 to 80% of primary human breast, colon, and lung tumors as well as in 50 to 60% of testicular, stomach, pancreatic, and ovarian cancers.11,12 Anti-sense inhibition of CR-1 expression in human cancer cells blocks their ability to grow in soft agar or in vivo in nude mice.13 This suggests that inappropriate expression of CR-1 can contribute to tumorigenesis. Similarly, functional blockade of the CFC or epidermal growth factor-like domains of CR-1 using specific blocking antibodies inhibit tumor growth by ∼70% in two testicular and colon xenograft models as well as other carcinoma cell lines as a result of induction of apoptosis or inhibition of AKT phosphorylation.14,15
Multiparous MMTV-CR-1 transgenic mice developed papillary adenocarcinomas at a frequency of ∼35% after 15 to 18 months.6 In addition to the lactating mouse mammary gland, the MMTV promoter is active in the virgin mammary gland suggesting lactation-independent activity for this promoter.16 To investigate the role of CR-1 on normal mammary gland development and/or tumorigenesis during pregnancy and lactation, we generated several FVB/N transgenic mouse lines in which mammary gland expression of the CR-1 transgene was induced using the whey acidic protein (WAP) promoter that is maximally expressed at mid-pregnancy.17,18 Three WAP-CR-1 transgenic lines were established that express the CR-1 transgene during pregnancy and early lactation at low, intermediate, and high levels. The high-level expressing subline F7 was selected as representative of the other WAP-CR-1 transgenic mice and used in all analysis throughout this study. The present data provide the first evidence that mammary-specific overexpression of CR-1 using the WAP promoter results in delayed mammary lobulo-alveolar development during late pregnancy and early lactation, accelerated apoptosis during early involution, induction of mammary hyperplastic lesions in nulliparous female WAP-CR-1 mice, and development of mammary tumors containing different histological subtypes in aged multiparous WAP-CR-1 female mice.
Materials and Methods
Generation of WAP-CR-1 Transgenic Mice
To generate the expression vector, a 890-bp fragment containing the entire open-reading frame of the human CR-1 cDNA flanked by 220 bp of 5′- and 110 bp of 3′- untranslated regions was cloned into the NotI site of the pEF/myc/cyto vector after removal of the EF1α promoter yielding plasmid 2B3.6 Transgenic FVB/N mice expressing the CR-1 transgene under the control of the WAP promoter were generated by pronuclear injection of the 4990-bp 2B3-digested DNA fragment into oocytes of FVB/N mice (Laboratory Animal Sciences Program, SAIC, Frederick, MD). Three female transgenic founder animals were identified and germ-line transmission of the transgene was demonstrated for the three female founders.
Analysis of the Transgene Integration
The integration of the CR-1 construct was verified by polymerase chain reaction (PCR) and by restriction mapping of genomic DNA extracted from tail biopsies.19 PCR was performed using primers specific for the human CR-1 cDNA sequence (HCR-F1: 5′-CAG GAA TTT GCT CGT CCA TCT CGG-3′ and HCR-R1: 5′-TAG TAC GTG CAG ACG GTG GTA GTT-3′). The reaction conditions and cycling parameters were, with minor modifications, as described by the Taq polymerase manufacturer (Promega, Madison, WI). Southern blot analysis was used to determine the copy number of the integrated transgene. Briefly, genomic tail DNA was digested with EcoRI and fragments were separated on a 0.8% agarose gel, denatured with 0.5 mol/L NaOH, 1.0 mol/L NaCl for 30 minutes, and blotted on nylon membrane by overnight capillary transfer in denaturation solution. Transgene copy numbers were estimated by comparing the hybridization signal to the signal obtained with a known amount of purified plasmid DNA of the CR-1 transgene mixed with genomic tail DNA from nontransgenic FVB/N mice.
Semiquantification of mRNA expression was determined by densitometric reading of the bands obtained by reverse transcriptase (RT)-PCR and using the public domain National Institutes of Health Image program developed at the US National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/. Final densitometric readings were normalized against GAPDH and expressed as optical densitometric (OD) units. Mean values were calculated from the multiple results and compared using t-test. Statistical significance was determined if the P value was <0.05.
Tissue Specificity of Transgene Expression and Western Blot Analysis
Protein extracts were prepared by grinding tissues on dry ice and then lysing in 20 mmol/L Tris-HCl (pH 7.5) containing 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 5 mmol/L MgCl2, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L sodium orthovanadate, and 20 mmol/L sodium fluoride. Crude protein lysates (50 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 4 to 20% polyacrylamide gels (Novex, San Diego, CA), transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA), and blocked for 1 hour with 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween 20. For the detection of CR-1 protein, a mouse monoclonal anti-human CR-1 antibody (A10.B2.18, 1:5000; kindly provided by Biogen Inc., Cambridge, MA) raised against the N terminal domain of human CR-114 was used. The following commercial antibodies were also used for Western blot analysis: rabbit anti-CCAAT/enhancer-binding protein delta (CEBPD) (SC-636, 1:250; Santa Cruz Biotechnology, Inc, Santa Cruz, CA); rabbit anti-Stat3, rabbit anti-mitogen activated protein kinase (MAPK), anti-phospho-MAPK, anti-AKT, and anti-phospho-AKT (1:1000; Cell Signaling, Beverly, MA); mouse monoclonal anti-dephosphorylated β-catenin (DP-β-cat, 1:5; generously provided by Dr. Hans Cleaver, University of Utrecht, Utrecht, The Netherlands); mouse anti-α-tubulin (1:1000; Sigma, St. Louis, MO). Goat anti-β-casein (1:500) was a generous gift from Dr. Barbara Vonderhaar (National Cancer Institute/Mammary Biology and Tumorigenesis Laboratory, Bethesda, MD) and Dr. Lothar Hennighausen (National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) kindly provided the rabbit anti-WAP (1:1000). Appropriate anti-rabbit, anti-mouse, or anti-goat horseradish peroxidase-conjugated secondary antibodies (1:5000; Amersham, Piscataway, NJ) were used. Immunoreactive bands were detected by enhanced chemiluminescence (Amersham). Quantification of Western blot expression was determined by densitometric analysis of the bands as described above. Final densitometric readings of the bands from Western blot were normalized against α-tubulin.
Whole Mount Fixation
Mammary glands from selected mice were dissected, spread out, and fixed on glass slides with Carnoy’s solution (glacial acetic acid:ethanol, 1:3) for 60 minutes at room temperature. The glands were rehydrated before overnight staining in aluminum carmine [1 g carmine, 2.5 g AlK(SO4)2 boiled for 20 minutes in distilled H2O, filtered, and brought to a final volume of 500 ml]. The glands were then dehydrated, cleared with xylene, and mounted. Photographs were taken using a Polaroid DMC-1 digital camera (Polaroid, Cambridge, MA) mounted on a Leica MZ125 microscope (Leica, Wetzlar, Germany). Areas of interest and suspect lesions were microdissected and removed from the whole mount, embedded in paraffin, and processed for histology.
Histology and Immunohistochemistry
Macroscopically identifiable areas suggestive of hyperplasias or tumors were isolated and fixed in 4% phosphate-buffered paraformaldehyde overnight at 4°C. Paraffin sections were prepared by American HistoLabs (Gaithersburg, MD) and stained with hematoxylin and eosin (H&E) for subsequent histological analysis. For immunohistochemistry mammary tissue fixed in freshly prepared 4% paraformaldehyde and embedded in paraffin was cut in 5-μm sections and subsequently deparaffinized in xylene and rehydrated in a series of graded ethanol. Endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 10 minutes. Antigen retrieval was performed by incubating slides with ready-to-use pepsin solution (Digest-all; Zymed Laboratories Inc., San Francisco, CA) for 6 minutes at 37°C and washed several times in phosphate-buffered saline. Immunostaining with rabbit polyclonal primary antibodies was performed using the appropriate Vectastain ABC kit and following the manufacturer’s instructions. Immunostaining with mouse monoclonal primary antibodies was performed using the ARK peroxidase kit (DakoCytomation, Carpinteria, CA) and following the manufacturer’s instructions. The following primary antibodies were used: mouse anti-CR-1 (A10.B2.18, 1:100; Biogen); rabbit anti-cytokeratin-1 (CK1) and anti-CK6 (1:100, (DakoCytomation); rabbit anti-β-catenin (1:100; Upstate, Lake placid, NY); mouse anti-keratin AE-13 (1:100; ImmuQuest Ltd., UK). Sections were counterstained with hematoxylin.
To evaluate proliferation of mammary epithelial cells between FVB/N and WAP-CR-1 mice, sections for immunohistochemistry were stained with rabbit anti-proliferating cell nuclear antigen (PCNA) (SC-7907, 1:200; Santa Cruz, CA). PCNA-stained cells were identified at ×63 magnification and counted in at least four different fields in sections from four different mammary glands per group. The mean value was then calculated from each group of mice. To identify apoptotic cells, enzymatic in situ fluorescent labeling of apoptosis-induced DNA strand breaks (terminal dUTP nick-end labeling assay) was performed on mammary sections from four different WAP-CR-1 and four different FVB/N mice during day 3 of involution using the In Situ Cell Death Detection kit (Roche, Indianapolis, IN) and following the manufacturer’s instructions. The mean value of fluorescent apoptotic bodies for the group of FVB/N and the group of WAP-CR-1 mice was quantified as described for PCNA quantification.
Results
Generation of WAP-CR-1 Transgenic Mice and Expression of the CR-1 Transgene
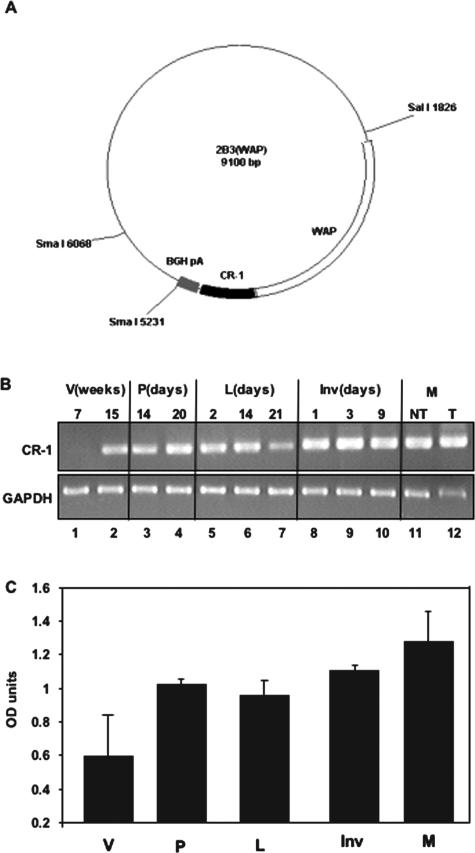
To investigate the consequence of overexpression of human CR-1 in the mouse mammary gland, we generated three FVB/N mouse lines overexpressing the WAP promoter-driven transgene encoding the human CR-1 cDNA (Figure 1A). Expression of the CR-1 transgene in the mammary gland of the F7 subline was demonstrated by reverse transcriptase (RT)-PCR analysis (Figure 1B). CR-1 mRNA expression was first detected at 15 weeks (Figure 1B, lane 2) in mammary glands of WAP-CR-1 virgin mice. During pregnancy, CR-1 mRNA was expressed between days 14 and 20 (Figure 1B, lanes 3 and 4) and during lactation and involution (Figure 1B, lanes 5 to 10). Cripto-1 mRNA was also detected in mammary glands (Figure 1B, lane 11) and mammary tumors (Figure 1B, lane 12) of multiparous transgenic mice. Densitometric readings of the bands obtained by RT-PCR showing expression of CR-1 in the mammary glands of the WAP-CR-1 transgenic mouse at the different stages analyzed is graphically represented in Figure 1C.
Figure 1.
A: Schematic representation of the WAP promoter-human Cripto-1 (CR-1) expression vector 2B3 is shown. B: RT-PCR analysis using primers specific for CR-1 shows expression of the CR-1 transgene, relative to GAPDH, in the mammary gland obtained at different developmental stages from virgin (V), pregnant (P), lactating (L), and involuting (Inv) WAP-CR-1 transgenic mice. Mammary tissues from multiparous (M) WAP-CR-1 transgenic mice without (NT) or with mammary tumors (T) were also assessed. C: Quantification of RT-PCR by densitometry and normalization for GAPDH expression is shown.
Mammary Gland Development and Differentiation Is Impaired in WAP-CR-1 Transgenic Mice during Pregnancy and Lactation
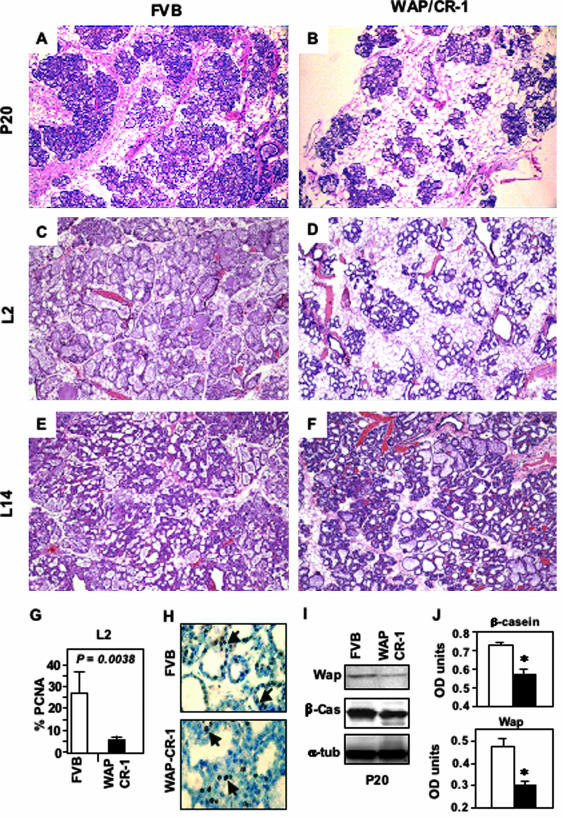
No significant alterations in allometric ductal morphogenesis or growth including lateral secondary or tertiary side-branching were observed in the mammary gland of either prepubescent or postpubescent virgin 5- to 13-week-old virgin WAP-CR-1 mice as compared to age-matched FVB/N mice (not shown). To compare possible differences of development and differentiation of mammary tissue between FVB/N and WAP-CR-1 mice during pregnancy and lactation, we analyzed four mice from each group at different stages of pregnancy and lactation. Histological analysis demonstrated that there was a significant reduction in epithelial content and fewer lobular-alveolar structures formed and relative increase in adipose tissue in the mammary glands of WAP-CR-1 transgenic mice as compared to the mammary glands of FVB/N female mice at all stages of late pregnancy (Figure 2, A versus B) and during early lactation at day 2 (Figure 2, C versus D). In contrast, histological analysis of mammary glands from day 14 lactating FVB/N mice and WAP-CR-1 transgenic mice showed no evident difference in epithelial content and lobular-alveolar formation (Figure 2, E versus F). These results suggest a delay in morphological differentiation in mammary glands overexpressing CR-1 during late pregnancy and early stages of lactation. Further support for this possibility is evidenced by a significant reduction in the percentage of proliferating mammary epithelial cells detected in mammary glands of day 2 lactating WAP-CR-1 transgenic mice (5.8% ± 1.7%, n = 4) as compared to matched FVB/N mammary glands (27.4% ± 9.3%, n = 4; P = 0.0038), which was determined by quantification of PCNA-stained mammary epithelium (Figure 2, G and H). Reduced expression of the milk proteins β-casein and WAP in late-pregnant (P20) mammary gland tissue extracts was detected by Western blot analysis in the WAP-CR-1 transgenic mice as compared to β-casein and WAP levels in tissue extracts of mammary glands from FVB/N mice (Figure 2I). These data were confirmed and summarized by the results from densitometric analysis of Western blots of mammary gland extracts from additional FVB/N and WAP-CR-1 transgenic mice (Figure 2J). The relative low levels of milk proteins reflects the reduced number of lobulo-alveolar structures and suggests a delay in morphological development in the mammary glands of the CR-1 transgenic mice as compared to the matched FVB/N mice.
Figure 2.
A to F: H&E-stained sections of mouse mammary tissue from FVB/N control (A, C, E) and WAP-CR-1 transgenic (B, D, F) obtained at pregnancy day 20 (P20), lactation day 2 (L2), and lactation day 14 (L14). Reduced formation of lobulo-alveolar structures in WAP-CR-1 transgenic compared to FVB/N control mice is detected at P20 and L2 (A versus B and C versus D). Differences in epithelial content and alveolar development is no longer apparent in mammary glands of FVB/N control (E) and WAP-CR-1 transgenic (F) in later stages of lactation (L14). G and H: A significant reduction in proliferation rate in the WAP-CR-1 transgenic mammary epithelial cells can be detected at lactation day 2 (L2) as compared to FVB/N as demonstrated by the percentage (G) of epithelial cells staining positive for PCNA (H, arrows). Sections are counterstained with hematoxylin. I and J: Reduced synthesis of the milk proteins WAP and β-casein in mammary glands from 20-day pregnant (P20) WAP-CR-1 transgenic as compared to mammary glands from matched FVB/N mice is demonstrated by representative Western blot (I) and summary of densitometric analysis of Western blots from repeat experiments (J). *P < 0.05. Original magnifications: ×20 (A–F); ×63 (H).
WAP-CR-1 Mice Exhibit an Increase in Apoptosis during Involution
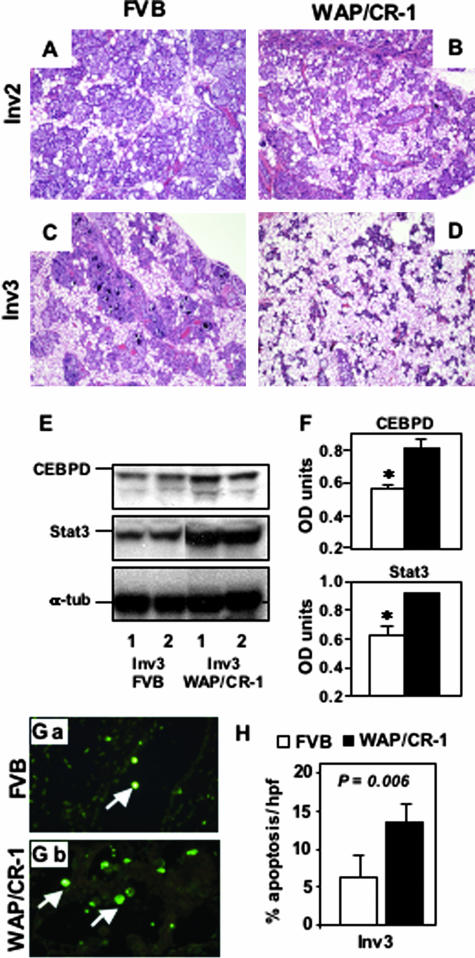
After weaning, epithelial cells of the mammary gland undergo a coordinated apoptotic process during early involution.20 To determine whether CR-1 overexpression during involution can modify this response, we assessed tissue morphology and apoptotic activity in mammary glands from FVB/N and WAP-CR-1 mice at day 2 and day 3 of involution. Comparison of H&E-stained mammary gland sections of day 2 and day 3 involuting mammary glands (Figure 3, A and B) showed a reduction in epithelial content in the WAP-CR-1 mice as compared to FVB/N mice (Figure 3, C and D). Analysis of CEBPD and Stat3 protein expression, two early biochemical markers of glandular involution,21,22 in mammary tissue from mice during day 3 of involution demonstrates that involution was accelerated in the mammary glands of WAP-CR-1 transgenic mice relative to FVB/N mice as shown in a representative Western blot (Figure 3E) with the relative densitometric quantification after normalization against α-tubulin expression (Figure 3F). Terminal dUTP nick-end labeling assay was used to identify and quantify apoptotic cells in mammary gland sections from FVB/N mice and WAP-CR-1 transgenic mice (Figure 3, Ga and Gb). This assay showed a significant increase in the apoptotic rate in mammary glands of WAP-CR-1 transgenic mice at day 3 of involution (14.3% ± 2.5%, n = 4) as compared to aged-matched mammary glands in FVB/N mice (5.5% ± 3.5%, n = 4; P = 0.006) (Figure 3H).
Figure 3.
A–D: H&E-stained sections of mouse mammary tissue from FVB/N control (A, C) and WAP-CR-1 transgenic mice (B, D) obtained during days 2 and 3 of involution (respectively, Inv2 and Inv3) show reduction in mammary epithelial content in the WAP-CR-1 mice as compared to FVB/N control mice, which is accentuated during Inv3 (C versus D). E and F: Expression of markers for involution, CEBPD, and Stat3, is detected by Western blot (E) of lysates from representative mammary glands of two FVB/N and WAP-CR-1 mice and quantified by densitometric analysis (F). G and H: Immunofluorescent terminal dUTP nick-end labeling assay of mammary glands from FVB (Ga) and WAP/CR-1 (Gb) shows a significant increase in the percentage of apoptotic cells in mammary tissue from WAP-CR-1 during Inv3 compared to matched FVB/N (H). Original magnifications: ×20 (A–D); ×63 (G).
Nulliparous and Multiparous WAP-CR-1 Mice Develop Mammary Hyperplasias and Tumors, Respectively
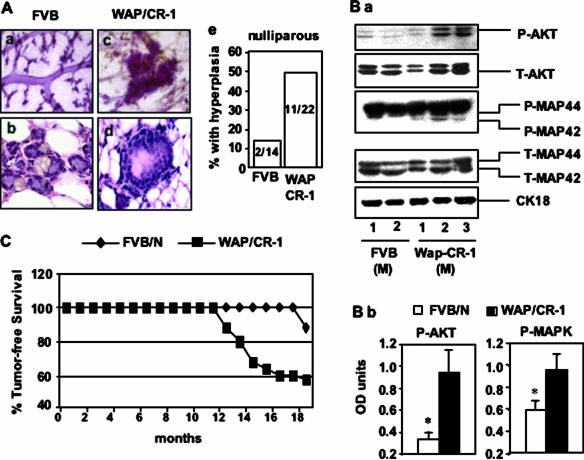
We assessed mammary gland morphology in FVB/N and WAP-CR-1 nulliparous mice after 14 to 18 months. Compared to FVB/N nulliparous mice, mammary glands from WAP-CR-1 nulliparous mice exhibited an increase in the number of foci, identified by whole mount morphology, that suggested hyperplastic changes (Figure 4, Aa versus Ac). H&E-stained histological sections of normal ductal structures in nulliparous FVB/N mice (Figure 4Ab) as compared to the lesions identified in the mammary glands of WAP-CR-1 transgenic mice, shows multilayering of atypical intraductal epithelium (Figure 4Ad). This is similar to what has been described as mammary intraepithelial neoplasia (MIN).23 Moreover, these lesions were identified histologically in 11 of 22 (50%) mammary glands from WAP-CR-1 nulliparous mice as compared to only 2 of 14 (14.3%) mammary glands from FVB/N nulliparous mice (Figure 4Ae). This result suggests that overexpression of CR-1 can induce hyperplastic changes in mammary epithelium of aged nulliparous female mice.
Figure 4.
A: Mammary tissue of 15-month-old nulliparous FVB/N mice (Aa, Ab) and nulliparous WAP-CR-1 transgenic mice (Ac, Ad) as compared by whole mount morphology (Aa and Ac, Carnoy stain) and histological analysis (Ab and Ad, H&E). Ductal structures with increased layers of atypical epithelial cells suggestive of MIN were identified more frequently in mammary tissue from ≥15-month-old WAP-CR-1 transgenic nulliparous mice (50%) as compared to the number of similar lesions found in age-matched nulliparous FVB/N mice (14.2%) (Ae). B: Mammary tissue from multiparous (M) WAP-CR-1 mice as compared to multiparous FVB/N expressed increased levels of the active, phosphorylated forms of AKT and MAPK as shown by a representative Western blot analysis (Ba) of the respective mammary tissue lysates and confirmed by densitometric quantification of the Western blot expression bands after normalization against total expression (T-) of the respective signaling molecule (Bb). CK-18 controls for epithelial content in tissues analyzed. C: Mammary gland tumor-free survival is greater in multiparous FVB/N mice compared to multiparous WAP-CR-1 transgenic mice. Overall, ∼50% of WAP-CR-1 transgenic mice developed mammary tumors throughout 13 months. Original magnifications: ×5 (Aa, Ac); ×40 (Ab, Ad).
To determine whether CR-1 overexpression could affect the activation of proteins that are involved in inducing cell proliferation and/or cell survival, the levels of phosphorylated AKT and MAPK were assessed in the mammary glands of several wild-type FVB/N and WAP-CR-1 multiparous mice (three to five pregnancies) aged 14 to 16 months. As shown by the representative Western blots (Figure 4Ba) and by the densitometric analysis of the bands normalized against total AKT and MAPK, respectively (Figure 4Bb), increased levels of phosphorylated AKT and p42 MAPK were detected in mammary tissue lysates from multiparous WAP-CR-1 as compared to FVB/N mice providing indirect evidence of increased cell proliferation and survival in WAP-CR-1 mammary glands. In addition, we found that 6 of 9 (66.7%) multiparous heterozygous WAP-CR-1 mice and 9 of 20 (45%) of the homozygous WAP-CR-1 mice developed mammary tumors within a time frame that ranged from 12 to 20 months (Table 1). In all, more than half (15 of 29) of the multiparous WAP-CR-1 transgenic mice, compared to only 2 of 16 of the multiparous FVB/N mice, analyzed between 12 and 20 months developed mammary tumors (Figure 4C).
Table 1.
WAP-CR-1 Tumor Incidence and Latency
| Founder | Multiparous mice (n) | Mice with tumors (%) | Latency in months (range) |
|---|---|---|---|
| F7 WAP-CR-1 heterozygous | 9 | 6 (66.7) | 17 ± 4.2 (13 to 20) |
| F7 WAP-CR-1 homozygous | 20 | 9 (45) | 15 ± 4.2 (12 to 19) |
Histology of WAP-CR-1 Mammary Tumors
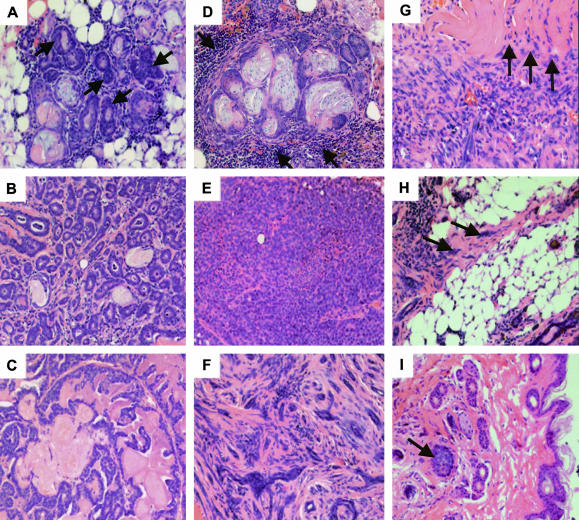
The histological criteria used as a guideline to classify the mammary tumors in the present study have been previously described.23 Histological analysis of the tumor sections obtained from the WAP-CR-1 transgenic mice did not reveal any predominant histotype. Each sample contained multifocal areas containing a mixture of different histological characteristics as summarized in Table 2. Within most of the tumor sections it was possible to identify scattered foci of duct-like structures composed of multiple layers of compact epithelial cells with relatively large pleomorphic and hyperchromic nuclei and classified as MIN (Figure 5A). Some areas showed cells with relatively small nuclei clustered around a central lumen containing secretory material, and forming small glandular structures surrounded by stroma and classified as glandular carcinoma (Figure 5B). Epithelial structures with uniform nuclei lining multiple, thin stromal axes and forming delicate branching frond-like structures jutting into larger lumens were also identified and classified as papillary carcinoma (Figure 5C). Occasionally, foci surrounded by a predominant mononuclear infiltrate were identified. The inflammatory cells appeared as forming a boundary around areas composed of rare neoplastic glands mixed with stratified squamous epithelium that surrounded ghost cells trapped in laminar keratin, resembling to what has been described as “pilar tumors.”24 These were classified as adenosquamous carcinomas (Figure 5D). Some tumor areas showed epithelial cells forming cord-like structures separated by thin stroma or tight sheet-like structures with small uniform nuclei and almost no stroma. These lesions were classified as solid carcinomas (Figure 5E). Also observed within the tumor sections were areas composed of swirls of spindle-shaped cells with flat hyperchromic nuclei intermixed with nests of epithelial cells containing irregular hyperchromic nuclei forming small glandular structures. These were classified as myoepitheliomas (Figure 5F). In comparison, of the only two mammary tumors found in the multiparous FVB/N mice (not shown), one consisted of areas with aspects of papillary and solid adenocarcinoma and the other was characterized by the presence of poorly differentiated cells with large nuclei containing prominent nucleoli arranged in a disorganized pattern. Neither of these tumors showed the mixed characteristics of the mammary tumors that were identified in the multiparous WAP-CR-1 mice. To evaluate the malignant potential of these tumors, histological evidence of invasive behavior was assessed.23 Tumor margin was readily found compressing the surrounding tissue and foci of tumor cells were occasionally found infiltrating adjacent muscle (Figure 5G) and adipose tissue (Figure 5H). In one sample, relatively large tumor epithelial cells with dark, irregular nuclei were found forming colonies of small solid nests or glandular-like foci surrounded by loose stroma within the subepidermal tissue (Figure 5I).
Table 2.
Characteristics Detected within WAP-CR-1 Mammary Tumor Sections
| No. | MIN | Gln | Pap | Solid | Adenosq | Myoep | Infl | Invasion | Necrosis |
|---|---|---|---|---|---|---|---|---|---|
| 12073 hm | + | + | + | + | |||||
| 12204 hm | + | + | + | ||||||
| 11706 hm | + | + | + | + | + | ||||
| 12710 hm | + | + | + | ||||||
| 12181 hm | + | + | + | Muscle | + | ||||
| 12492 hm | + | + | + | Adipose | + | ||||
| 12032 hm | + | + | + | + | |||||
| 12029 hm | + | + | + | + | + | + | Muscle | + | |
| 12706 hm | + | + | |||||||
| 10995 ht | + | + | + | + | + | Dermis | |||
| 11900 ht | + | + | + | + | + | + | |||
| 11841 ht | + | + | + | + | Muscle, adipose | ||||
| 11756 ht | + | + | + | ||||||
| 11621 ht | + | + | + | + | + | ||||
| 11695 ht | + | + | + | + |
Abbreviations: hm, homozygous; ht, heterozygous; HP/MIN, hyperplasia/mammary intraepithelial neoplasia; Gln, glandular; Pap, papillary; Adenosq, adenosquamous; Myoep, myoepithelioma; Infl, inflammation.
Figure 5.
Representative H&E-stained sections illustrate the major histological subtypes identified in mammary tumors from WAP-CR-1 transgenic mice: A: Mammary intraepithelial neoplasia (arrows); B: glandular; C: papillary; D: adenosquamous lesions surrounded by inflammatory infiltrates (arrows); E: solid; F: myoepithelial. Occasionally, surrounding muscle (G), adipose (H), or subepidermal (I) tissue showed local invasion by tumor cells (arrows). Original magnifications, ×20.
Immunohistochemistry of Sections from WAP-CR-1 Mammary Tumors
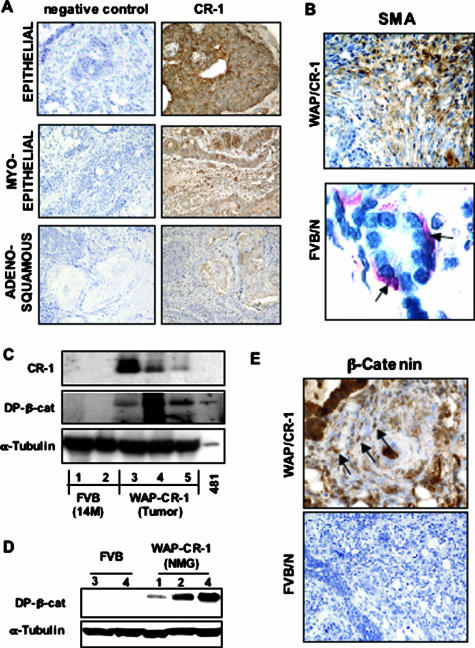
Immunohistochemistry showed positive staining for human CR-1 in all major histological subtypes identified within the WAP-CR-1 mammary tumors (Figure 6A). Staining for smooth muscle actin of the spindle-shaped cells composing the swirls of tumor tissue within the areas of adenomyoepithelioma in mammary tumors of the WAP-CR-1 transgenic mouse confirmed the myoepithelial origin of these tumor cells (Figure 6B). Typical staining for smooth muscle actin was observed in myoepithelial cells surrounding the glandular acinar unit of normal mammary glands from FVB/N mice (Figure 6B). Western blot analysis of lysates that were prepared from mammary tumor tissue of WAP-CR-1 transgenic mice showed increased expression of activated dephosphorylated (DP) form of β-catenin as compared to lysates of mammary tissue from multiparous FVB/N mice (Figure 6C). DP-β-catenin was also expressed in nontumor mammary tissue from WAP-CR-1 transgenic mice (Figure 6D). Immunohistochemistry of WAP-CR-1 mammary gland tumor sections using an antibody against β-catenin showed positive nuclear staining of tumor cells (Figure 6E) suggesting that accumulation and subsequent nuclear translocation of β-catenin is occurring in the cells of mammary gland tumors in WAP-CR-1 transgenic mice. In contrast, little or no staining for β-catenin was observed in sections from a rare spontaneous mammary tumor in FVB/N mouse that developed secondary to pituitary adenoma (Figure 6E).
Figure 6.
A: Immunohistochemistry results show positive staining for human CR-1 in the different subtypes identified in mammary tumors from WAP-CR-1 transgenic mice. B, top: Staining for smooth muscle actin (SMA) suggests myoepithelial origin of the cells composing myoepithelial-type WAP/CR-1 mammary tumor. B, bottom: In normal FVB/N mammary gland, smooth muscle actin stains the myoepithelial cells (red) (AEC chromogen used) surrounding the acinar unit. C: Western blot analysis of mammary gland tissue lysates demonstrates expression of human CR-1 and expression of DP-β-cat in mammary tumors from WAP-CR-1 multiparous mice as compared to almost no expression in mammary glands from multiparous FVB/N mice. D: Expression of DP-β-cat is also detected in nontumor mammary glands (NMG) of WAP-CR-1 transgenic mice. E: Representative immunohistochemistry shows positive nuclear staining for β-catenin (top, arrows) in sections of mammary tumors from WAP-CR-1 transgenic mice as compared to negative staining in mammary tumor section from FVB/N mouse (bottom). All sections are counterstained with hematoxylin. Original magnifications: ×20 (A; E, bottom); ×40 (B, top; E, top); ×63 (B, bottom).
Mammary Tumors of WAP-CR-1 Transgenic Mice Show Signs of Epidermal Transdifferentiation
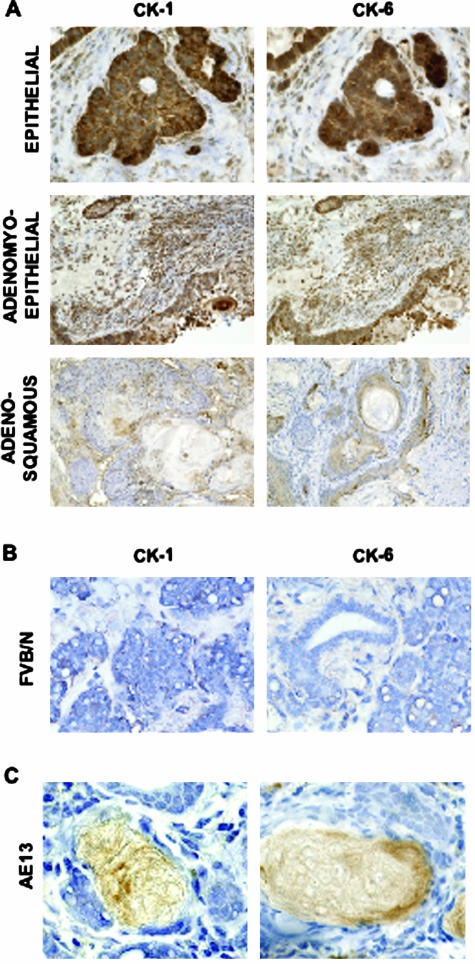
Areas of adenosquamous carcinoma detected in sections of mammary gland samples from the WAP-CR-1 transgenic mice were similar to lesions reported in mammary tumors from transgenic mice with an activated canonical Wnt/β-catenin/Lef-1 signaling pathway.24 This type of mixed histology with areas of squamous differentiation suggested that a canonical Wnt/β-catenin/Lef-1 signaling pathway might be activated in the mammary tumors from the multiparous WAP-CR-1 transgenic mice. Because stabilization of β-catenin, an essential initiating step for activation of the Wnt/β-catenin/Lef-1 signaling pathway, has been associated with epidermal transdifferentiation, including squamous metaplastic changes of the mammary epithelium,24,25 we investigated whether a similar process was occurring in mammary tumors from the WAP-CR-1 transgenic mice. Positive immunostaining for CK-1 and CK-6 in mammary tumor sections was detected in areas of different histological differentiation (Figure 6D) suggesting that epidermal transdifferentiation is an event that occurs in mammary epithelium of WAP-CR-1 tumors because CK-1 and CK-6 are normally expressed, respectively, in the spinous layer of the epidermis26 and in proliferating epidermal cells in hair follicles27 and are only rarely expressed in normal glands,28 as confirmed by the poor staining for CK-1 and CK-6 in mammary gland sections from FVB/N mice (Figure 7B). Positive immunostaining for AE-13 was found in the keratin layers associated with squamous metaplastic foci in areas of adenosquamous carcinoma in WAP-CR-1 mammary tumors (Figure 7C). These data also suggest that epidermal transdifferentiation was occurring in these lesions because AE-13 staining is specific for hair and nail keratin.25
Figure 7.
A: Immunohistochemistry shows positive staining for cytokeratin-1 (CK-1) and cytokeratin-6 (CK-6) detected in the different subtypes in mammary tumors from WAP-CR-1 transgenic mice. B: Little or no staining was observed for either CK-1 or CK-6 in sections of mammary gland from FVB/N mice. C: Positive immunostaining for hair and nail cytokeratin, AE-13, is shown in two squamous metaplastic lesions of the adenosquamous subtype in the WAP-CR-1 mammary tumors. All sections are counterstained with hematoxylin. Original magnifications: ×20 (A, B); ×63 (C).
Discussion
The epidermal growth factor-CFC gene family, which includes mouse and human cripto-1, oep, Frl-1, and cryptic are important embryonic morphogens.29 During embryogenesis, cripto-1 functions as a co-receptor for Nodal, which is a TGF-β-related protein, by facilitating the binding of Nodal to the type I Alk4 Activin receptor. This receptor can then activate an intracellular canonical Smad2/Smad3/Smad4 signaling pathway.30 In adult mammary epithelial cells, cripto-1 can also function through a Nodal and Alk4-independent signaling pathway that involves binding to glypican-1. This results in the subsequent activation of c-src, MAPK, phosphatidylinositol 3-kinase (PI-3 kinase), and AKT and reduction in the activity of glycogen synthase kinase-3β (GSK-3β).8,31,32 The present study has used a transgenic mouse line in which the CR-1 transgene has been expressed under the transcriptional control of the WAP promoter to maximize expression of the transgene during pregnancy and lactation and to determine the potential developmental and/or oncogenic effects of CR-1 overexpression in the mammary gland at these stages in female FVB/N mice. FVB/N mice were selected because they have a low incidence of less than 1 to 5% of spontaneous mammary tumors in either nulliparous or 1- to 2-year-old multiparous female mice.33–35
We demonstrate that nulliparous aged WAP-CR-1 mice develop mammary intraductal hyperplasias that correlates with the expression of the human CR-1 transgene mRNA. This phenotype resembles the phenotype in the aged mammary glands of nulliparous MMTV-CR-1 mice that also develop mammary hyperplasias but in addition exhibit enhanced ductal side branching6 that is not observed in the virgin WAP-CR-1 mammary glands (Table 3). This effect of the transgene in virgin WAP-CR-1 mice may not be unexpected since endogenous WAP expression has been detected in the mammary glands of 2-month-old virgin ICR mice. In addition, transgene expression from the WAP promoter has been reported during estrous in the mammary epithelium of 6-week-old WAP-TGF-β1 and WAP-LacZ/FVB/N mice. This suggests that the WAP promoter can be activated, albeit at low levels, in the nonpregnant mammary gland in a population of mammary epithelial cells that are committed to a secretory/alveolar cell fate.24,36 Elevated expression of CR-1 using WAP promoter may also explain the observation that in WAP-CR-1 female mice during late pregnancy and early lactation, lobulo-alveolar development, and the expression of endogenous WAP and β-casein in the mammary gland were either significantly delayed or reduced. The inhibitory effects on alveolar development and milk protein expression may be due in part to a reduction in the proliferation rate of mammary epithelial cells that was observed during early lactation in the WAP-CR-1 mice. Although there was no significant effect on the ability of female WAP-CR-1 mice to lactate and to nurse their pups, several WAP-CR-1 pups were ∼40% lower in body weight than comparably aged FVB/N pups (not shown) suggesting a reduction in milk protein content and/or milk production. These in vivo data are in accord with the ability of CR-1 to significantly inhibit lactogenic hormone-induced expression of β-casein and WAP in vitro in primary mouse mammary epithelial cells or in mouse mammary HC-11 and CID-9 epithelial cells.37,38 Delayed development in mammary gland of WAP-CR-1 mice is similar histologically to that observed in the mammary glands of WAP-TGF-β1 and Activin-null female mice during late pregnancy and early lactation.36,39 More specifically, Activin B was shown to regulate ductal morphogenesis and mammary epithelial cell differentiation.39 In this regard, the mammary phenotype observed in the WAP-CR-1 mice during pregnancy and lactation may in part be due to the ability of Cr-1 to bind Activin B and to block the activity of this growth factor.14,40 The inhibitory effects of CR-1 overexpression on lobulo-alveolar development during pregnancy and lactation in the WAP-CR-1 mice were not observed in MMTV-CR-1 mice.6 In fact, a similar divergence in mammary phenotypes was observed between MMTV-TGF-β141 and WAP-TGF-β142 mice, in which ductal morphogenesis was impaired in the MMTV-TGF-β1 mice but not alveolar development as was found in the WAP-TGF-β1 mice.
Table 3.
Stage-by-Stage Comparison of Mammary Gland Phenotype between MMTV-CR-1 and WAP-CR-1 Transgenic Mice
| Stage | MMTV-CR-1 transgenic mouse | WAP-CR-1 transgenic mouse |
|---|---|---|
| Virgin (4–12 weeks) | Speculated side branches in mammary ductal tree of MMTV-CR-1 transgenic mice not present in mammary glands of FVB/N normal mice | Mammary glands of WAP-CR-1 transgenic mice are indistinguishable histologically when compared to mammary glands of matched wild type FVB/N mice |
| Significant increase in secondary and tertiary side branching in MMTV-CR-1 transgenic mice compared to mammary glands of FVB/N normal mice | ||
| Increased presence of dilated ducts with intraductal epithelial proliferation in MMTV-CR-1 transgenic mice surrounded by condensed stroma as compared to mammary glands of FVB/N normal mice | ||
| Virgin (6–18 months) | Foci of intraductal hyperplasia and hyperplastic alveolar nodules (HANS) appear in mammary glands of 6-month-old virgin MMTV-CR-1 mice. No such lesions were detected matched FVB/M mammary glands | Hyperplasias develop in mammary glands of older virgin WAP-CR-1 (14–18 months). No such lesions were detected matched FVB/N mammary glands |
| Pregnancy and lactation | No differences between FVB/N and MMTV-CR-1 were observed with respect to epithelial content, lobulo-alveolar development or in the level of expression of milk proteins such as β-casein or WAP | Reduced lobulo-alveolar development and reduced expression of the milk proteins, β-casein and WAP in WAP-CR-1 transgenic mice compared to matched FVB/N mice |
| Early involution | No significant differences in the timing or rates of involution in MMTV-CR-1 mice as compared to FVB/N female mice | Accelerated involution in mammary glands of WAP-CR-1 mice as compared to FVB/N mice |
| No difference in apoptotic rates as determined by TUNEL assay | Significnat increase in expression Stat3 and CEBPD in mammary glands of WAP-CR-1 mice as compared to FVB/N mice | |
| Significant increase in the apoptotic rate in WAP-CR-1 transgenic mice as compared to mammary glands of matched FVB/N mice | ||
| Multiparous (>12 months) | 33% of MMTV-CR-1 transgenic mice develop mammary gland adenocarcinomas almost exclusively of the papillary subtype | 45–65% of WAP-CR-1 transgenic mice develop tumors with a mixture of different histological characteristics (see Table 2) |
A reduction in milk protein expression, such as β-casein and WAP, was observed in the WAP-CR-1 mice. This might lead to a precocious onset or accelerated rate of involution. In fact, during early involution, there was an enhanced level of apoptosis that was observed in the WAP-CR-1 mammary epithelial cells. In addition, the up-regulated expression of CEBPD and Stat3, which are two biochemical markers that are expressed during the early stages of mammary gland involution,21,22 would be in accord with the reduced epithelial content that was histologically detected in the WAP-CR-1 mammary glands during early involution as compared to matched mammary glands from FVB/N mice. The ability of CR-1 overexpression in vivo to enhance apoptosis during early involution in the mammary gland is in agreement with the capacity of recombinant CR-1 to induce apoptosis through a caspase-3-dependent pathway in mouse mammary HC-11 epithelial cells in vitro under conditions in which β-casein expression was also inhibited.43
Expression of the CR-1 transgene in multiparous WAP-CR-1 mice that are 14 to 16 months old leads to the activation of MAPK and AKT. Activation of these proteins has been implicated in the stimulation of mammary epithelial cell proliferation and survival, respectively, in other transgenic mice that develop mammary tumors.44,45 Although there was a tendency for the multiparous homozygous WAP-CR-1 mice to develop mammary tumors earlier than in the heterozygous transgenic mice, ∼45% of the homozygous multiparous WAP-CR-1 transgenic mice versus 65% of the heterozygous multiparous WAP-CR-1 mice developed mammary tumors. However, this difference in incidence of tumor formation between homozygous and heterozygous WAP-CR-1 transgenic mice is most likely an artifact due to the smaller number of heterozygous animals that were available for this study rather than to a gene dose effect. The tumors contained foci of MIN mixed with glandular, papillary, and solid carcinomas. In addition, areas of adenosquamous and myoepithelial differentiation were also identified within these tumors. The tumors had pushing borders and occasionally showed signs of local invasion. These tumors were histologically distinct from the mammary tumors that developed in the MMTV-CR-1 transgenic mouse that developed exclusively papillary adenocarcinomas6 but similar to the mixed histotype of mammary tumors that arise in MMTV-Wnt1 mice or mammary tumors that develop in mice expressing stabilized mutant forms of β-catenin with either the MMTV or WAP promoters.24,25,46–48 In fact, increased expression of stable, dephosphorylated β-catenin along with positive nuclear immunostaining for β-catenin in the WAP-CR-1 mammary tumors suggest that a canonical Wnt signaling pathway may be activated in these tumors. In addition, active Wnt signaling pathway in the mammary gland has been associated with epidermal transdifferentiation characterized by expression of epidermal cytokeratins normally not found in the mammary epithelium.25 Signs of epidermal transdifferentiation were found in WAP-CR-1 mammary tumors since positive immunostaining for the epidermal cell cytokeratins CK-1 and CK-626 and for hair and nail cytokeratin, AE-13,27 was detected. A functional connection between Wnt signaling and CR-1 is further supported by the recent observation that mouse Cr-1 is a target gene in a canonical Wnt3a/β-catenin/Lef-1 signaling pathway during mouse embryogenesis, in mouse Apc Min (−/−) colon adenomas and in human carcinoma cells.49 This suggests that CR-1 overexpression in the mammary gland using the WAP promoter can lead to mimicry of the Wnt1 or activated β-catenin phenotype. In this respect, an increase in expression of Cr-1 has recently been detected by immunohistochemistry in the MMTV-Wnt1 mammary tumors, which also indirectly supports a similar connection.6 Finally, we have recently found that MMTV-CR-1 mammary tumors express high levels of dephosphorylated β-catenin and phosphorylated, inactive GSK-3β. GSK-3β is a downstream negative effector in the canonical Wnt signaling pathway.8 Although the biological interactions between CR-1 and a specific Wnt family member in the development of the mammary gland warrants further exploration, collectively, these data suggest that CR-1 may be a significant mediator of some of the oncological effects of an activated canonical Wnt signaling pathway in the mammary gland.
Acknowledgments
We thank Brenda Wallace-Jones for her excellent technical assistance; Dr. Barbara Vonderhaar (National Cancer Institute/Mammary Biology and Tumorigenesis Laboratory, Bethesda, MD) who kindly provided the antibody against β-casein; and Dr. Lothar Hennighausen (National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) who kindly provided the antibody against WAP.
Footnotes
Address reprint requests to David S. Salomon, Ph.D., National Cancer Institute, 10 Center Dr., Building 37, Room 1106, Bethesda, MD 20892. E-mail: salomond@mail.nih.gov.
Y.S. and L.S. contributed equally to this study.
References
- Ciccodicola A, Dono R, Obici S, Simeone A, Zollo M, Persico MG. Molecular characterization of a gene of the ‘EGF family’ expressed in undifferentiated human NTERA2 teratocarcinoma cells. EMBO J. 1989;8:1987–1991. doi: 10.1002/j.1460-2075.1989.tb03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello F, Dono R, Kim N, Persico MG, Salomon DS. Expression of Cripto, a novel gene of the epidermal growth factor gene family, leads to in vitro transformation of a normal mouse mammary epithelial cell line. Cancer Res. 1991;51:1051–1054. [PubMed] [Google Scholar]
- Wechselberger C, Ebert AD, Bianco C, Khan NI, Sun Y, Wallace-Jones B, Montesano R, Salomon DS. Cripto-1 enhances migration and branching morphogenesis of mouse mammary epithelial cells. Exp Cell Res. 2001;266:95–105. doi: 10.1006/excr.2001.5195. [DOI] [PubMed] [Google Scholar]
- Kenney NJ, Huang RP, Johnson GR, Wu JX, Okamura D, Matheny W, Kordon E, Gullick WJ, Plowman G, Smith GH, Salomon DS, Adamson ED. Detection and location of amphiregulin and Cripto-1 expression in the developing postnatal mouse mammary gland. Mol Reprod Dev. 1995;41:277–286. doi: 10.1002/mrd.1080410302. [DOI] [PubMed] [Google Scholar]
- Bianco C, Wechselberger C, Ebert A, Khan NI, Sun Y, Salomon DS. Identification of Cripto-1 in human milk. Breast Cancer Res Treat. 2001;66:1–7. doi: 10.1023/a:1010648923432. [DOI] [PubMed] [Google Scholar]
- Wechselberger C, Strizzi L, Kenney N, Hirota M, Sun Y, Ebert A, Orozco O, Bianco C, Khan N, Wallace-Jones B, Normanno N, Adkins H, Sanicola M, Salomon D: Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene (in press) [DOI] [PubMed] [Google Scholar]
- Kenney N, Smith G, Johnson M, Rosemberg K, Salomon DS, Dickson R. Cripto-1 activity in the intact and ovariectomized virgin mouse mammary gland. Pathogenesis. 1997;1:57–71. [Google Scholar]
- Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- Kenney NJ, Smith GH, Maroulakou IG, Green JH, Muller WJ, Callahan R, Salomon DS, Dickson RB. Detection of amphiregulin and Cripto-1 in mammary tumors from transgenic mice. Mol Carcinog. 1996;15:44–56. doi: 10.1002/(SICI)1098-2744(199601)15:1<44::AID-MC7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Niemeyer CC, Spencer-Dene B, Wu JX, Adamson ED. Preneoplastic mammary tumor markers: Cripto and amphiregulin are overexpressed in hyperplastic stages of tumor progression in transgenic mice. Int J Cancer. 1999;81:588–591. doi: 10.1002/(sici)1097-0215(19990517)81:4<588::aid-ijc14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Qi CF, Liscia DS, Normanno N, Merlo G, Johnson GR, Gullick WJ, Ciardiello F, Saeki T, Brandt R, Kim N, Kenney N, Salomon DS. Expression of transforming growth factor alpha, amphiregulin and Cripto-1 in human breast carcinomas. Br J Cancer. 1994;69:903–910. doi: 10.1038/bjc.1994.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson ED, Minchiotti G, Salomon DS. Cripto: a tumor growth factor and more. J Cell Physiol. 2002;190:267–278. doi: 10.1002/jcp.10072. [DOI] [PubMed] [Google Scholar]
- Ciardiello F, Tortora G, Bianco C, Selvam MP, Basolo F, Fontanini G, Pacifico F, Normanno N, Brandt R, Persico MG, Salomon DS, Bianco RA. Inhibition of CRIPTO expression and tumorigenicity in human colon cancer cells by antisense RNA and oligodeoxynucleotides. Oncogene. 1994;9:291–298. [PubMed] [Google Scholar]
- Adkins HB, Bianco C, Schiffer SG, Rayhorn P, Zafari M, Cheung AE, Orozco O, Olson D, De Luca A, Chen LL, Miatkowski K, Benjamin C, Normanno N, Williams KP, Jarpe M, LePage D, Salomon D, Sanicola M. Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. J Clin Invest. 2003;112:575–587. doi: 10.1172/JCI17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing PX, Hu XF, Pietersz GA, Hosick HL, McKenzie IF. Cripto: a novel target for antibody-based cancer immunotherapy. Cancer Res. 2004;64:4018–4023. doi: 10.1158/0008-5472.CAN-03-3888. [DOI] [PubMed] [Google Scholar]
- Mok E, Golovkina TV, Ross SR. A mouse mammary tumor virus mammary gland enhancer confers tissue-specific but not lactation-dependent expression in transgenic mice. J Virol. 1992;66:7529–7532. doi: 10.1128/jvi.66.12.7529-7532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhappan C, Gallahan D, Stahle C, Chu E, Smith GH, Merlino G, Callahan R. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 1992;6:345–355. doi: 10.1101/gad.6.3.345. [DOI] [PubMed] [Google Scholar]
- Wen J, Kawamata Y, Tojo H, Tanaka S, Tachi C. Expression of whey acidic protein (WAP) genes in tissues other than the mammary gland in normal and transgenic mice expressing mWAP/hGH fusion gene. Mol Reprod Dev. 1995;41:399–406. doi: 10.1002/mrd.1080410402. [DOI] [PubMed] [Google Scholar]
- Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde CJ, Knight CH, Flint DJ. Control of milk secretion and apoptosis during mammary involution. J Mammary Gland Biol Neoplasia. 1999;4:129–136. doi: 10.1023/a:1018717006152. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Sharan S, Sterneck E. Comparison of mammary gland involution between 129S1 and C57BL/6 inbred mouse strains: differential regulation of Bcl2a1, Trp53, Cebpb, and Cebpd expression. Oncogene. 2004;23:2548–2553. doi: 10.1038/sj.onc.1207363. [DOI] [PubMed] [Google Scholar]
- Chapman RS, Lourenco P, Tonner E, Flint D, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. The role of Stat3 in apoptosis and mammary gland involution. Conditional deletion of Stat3. Adv Exp Med Biol. 2000;480:129–138. doi: 10.1007/0-306-46832-8_16. [DOI] [PubMed] [Google Scholar]
- Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Shillingford JM, Le Provost F, Gounari F, Bronson R, von Boehmer H, Taketo MM, Cardiff RD, Hennighausen L, Khazaie K. Activation of beta-catenin signaling in differentiated mammary secretory cells induces transdifferentiation into epidermis and squamous metaplasias. Proc Natl Acad Sci USA. 2002;99:219–224. doi: 10.1073/pnas.012414099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Rosner A, Nozawa M, Byrd C, Morgan F, Landesman-Bollag E, Xu X, Seldin DC, Schmidt EV, Taketo MM, Robinson GW, Cardiff RD, Hennighausen L. Activation of different Wnt/beta-catenin signaling components in mammary epithelium induces transdifferentiation and the formation of pilar tumors. Oncogene. 2002;21:5548–5556. doi: 10.1038/sj.onc.1205686. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Byrne C. The epidermis: rising to the surface. Curr Opin Genet Dev. 1994;4:725–736. doi: 10.1016/0959-437x(94)90140-x. [DOI] [PubMed] [Google Scholar]
- Weiss RA, Eichner R, Sun TT. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984;98:1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GH, Mehrel T, Roop DR. Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ. 1990;1:161–170. [PubMed] [Google Scholar]
- Shen MM, Schier AF. The EGF-CFC gene family in vertebrate development. Trends Genet. 2000;16:303–309. doi: 10.1016/s0168-9525(00)02006-0. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, Sun Y, Khan N, Kenney N, Ebert A, Williams KP, Sanicola M, Salomon DS. Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial Cells. Mol Cell Biol. 2002;22:2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, Sun Y, Khan N, Hirota M, Adkins H, Williams K, Margolis RU, Sanicola M, Salomon DS. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63:1192–1197. [PubMed] [Google Scholar]
- Smith GH, Gallahan D, Diella F, Jhappan C, Merlino G, Callahan R. Constitutive expression of a truncated INT3 gene in mouse mammary epithelium impairs differentiation and functional development. Cell Growth Differ. 1995;6:563–577. [PubMed] [Google Scholar]
- Nieto AI, Shyamala G, Galvez JJ, Thordarson G, Wakefield LM, Cardiff RD. Persistent mammary hyperplasia in FVB/N mice. Comp Med. 2003;53:433–438. [PubMed] [Google Scholar]
- Raafat A, Bargo S, Anver MR, Callahan R. Mammary development and tumorigenesis in mice expressing a truncated human Notch4/Int3 intracellular domain (h-Int3sh). Oncogene. 2004;23:9401–9407. doi: 10.1038/sj.onc.1208187. [DOI] [PubMed] [Google Scholar]
- Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168:47–61. doi: 10.1006/dbio.1995.1060. [DOI] [PubMed] [Google Scholar]
- De Santis ML, Kannan S, Smith GH, Seno M, Bianco C, Kim N, Martinez-Lacaci I, Wallace-Jones B, Salomon DS. Cripto-1 inhibits beta-casein expression in mammary epithelial cells through a p21ras-and phosphatidylinositol 3′-kinase-dependent pathway. Cell Growth Differ. 1997;8:1257–1266. [PubMed] [Google Scholar]
- Niemeyer CC, Persico MG, Adamson ED. Cripto: roles in mammary cell growth, survival, differentiation and transformation. Cell Death Differ. 1998;5:440–449. doi: 10.1038/sj.cdd.4400368. [DOI] [PubMed] [Google Scholar]
- Robinson GW, Hennighausen L. Inhibins and activins regulate mammary epithelial cell differentiation through mesenchymal-epithelial interactions. Development. 1997;124:2701–2708. doi: 10.1242/dev.124.14.2701. [DOI] [PubMed] [Google Scholar]
- Gray PC, Harrison CA, Vale W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc Natl Acad Sci USA. 2003;100:5193–5198. doi: 10.1073/pnas.0531290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DF, Jr, Johnson MD, Matsui Y, Robinson SD, Gold LI, Purchio AF, Daniel CW, Hogan BL, Moses HL. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 1993;7:2308–2317. doi: 10.1101/gad.7.12a.2308. [DOI] [PubMed] [Google Scholar]
- Jhappan C, Geiser AG, Kordon EC, Bagheri D, Hennighausen L, Roberts AB, Smith GH, Merlino G. Targeting expression of a transforming growth factor beta 1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. EMBO J. 1993;12:1835–1845. doi: 10.1002/j.1460-2075.1993.tb05832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis ML, Martinez-Lacaci I, Bianco C, Seno M, Wallace-Jones B, Kim N, Ebert A, Wechselberger C, Salomon DS. Cripto-1 induces apoptosis in HC-11 mouse mammary epithelial cells. Cell Death Differ. 2000;7:189–196. doi: 10.1038/sj.cdd.4400588. [DOI] [PubMed] [Google Scholar]
- Amundadottir LT, Leder P. Signal transduction pathways activated and required for mammary carcinogenesis in response to specific oncogenes. Oncogene. 1998;16:737–746. doi: 10.1038/sj.onc.1201829. [DOI] [PubMed] [Google Scholar]
- Hutchinson J, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol Cell Biol. 2001;21:2203–2212. doi: 10.1128/MCB.21.6.2203-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner A, Miyoshi K, Landesman-Bollag E, Xu X, Seldin DC, Moser AR, MacLeod CL, Shyamala G, Gillgrass AE, Cardiff RD. Pathway pathology: histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am J Pathol. 2002;161:1087–1097. doi: 10.1016/S0002-9440(10)64269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuliere J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze’ev A, Thiery JP, Glukhova MA. Targeted activation of {beta}-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005;132:267–277. doi: 10.1242/dev.01583. [DOI] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, Tan LK, Rosen JM, Varmus HE. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkel M, Huelsken J, Wakamiya M, Ding J, van de Wetering M, Clevers H, Taketo MM, Behringer RR, Shen MM, Birchmeier W. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]