Abstract
The glomeruli of postmenopausal C57BL6 mice, and age-matched males, show progressive hypertrophy and glomerulosclerosis. We asked whether this was a multistage process, was due to alterations in glomerular progenitors, and was reversible in female mice. Using cross bone marrow transplants (BMT) between young and old females, we found that BMT delivered a phenotype that was donor age-specific. The fact that lesions in young recipients were more severe if the donors were in late rather than early menopause suggested that new progenitor phenotypes had appeared. Postmenopausal recipients of BMT from young donors had reduced glomerular hypertrophy and sclerosis, implying that the aging lesions in females were reversible and that progenitors, rather than the local environment, determined the glomerular profile. The altered phenotype included increased extracellular matrix synthesis and decreased matrix metalloproteinase-2 levels as well as cell hypertrophy. The mechanism of the cellular hypertrophy was due to uncoupling of hypertrophy from proliferation, resulting from elevated p27 levels. Thus, glomerular hypertrophy and sclerosis in aging females is a multistage process, is reversible, and may be determined by the phenotype of bone marrow-derived progenitor cells.
The frequency of chronic kidney disease is increased in the otherwise healthy, aging population and glomerulosclerosis is one major cause.1,2 We studied female C57BL6 (B6) mice because they are resistant to glomerulosclerosis in the premenopausal period, but develop progressive glomerular hypertrophy and sclerosis shortly after menopause.3 This model was also chosen because it resembles the increase in renal disease found in some groups of women.2 We examined the phenotype of mesangial cells, and the changes induced by the administration of bone marrow, which contains glomerular progenitors. This approach was used because we previously showed that glomerulosclerosis is often associated with hypertrophy and that these changes are due to phenotypic changes in mesangial cell progenitors resident in the bone marrow.4,5 For instance, bone marrow transplants (BMTs) from ROP Os/+ mice that had glomerular hypertrophy and advanced sclerosis, transmitted both hypertrophy and sclerosis to the glomeruli of naïve ROP+/+ mice.4 We considered glomerular hypertrophy to be an important variable in the pathogenesis of glomerulosclerosis because this change is present in a variety of other models of glomerulosclerosis.6–9 Mesangial cells isolated from aged female mice expressed stable phenotypic changes in cell size and extracellular matrix turnover.3 Thus, it seemed quite likely that bone marrow mesangial cell progenitor abnormalities may also play an important role in glomerular changes in aging females. The second question addressed in this study was whether the changes in aging were due to an accumulation of sclerotic changes with aging and whether they were reversible if normal progenitors were provided via BMT. We also asked if the changes in aging were due to a single event that occurred shortly after estrogen lack was established and progressively worsened, or whether the changes were due to multiple events. Finally, we examined age-matched males, to determine whether the histological and phenotypic changes were restricted to female mice.
The mechanisms of glomerular hypertrophy in aging are incompletely understood. However, because the total number of glomerular cells was not increased and there was not obvious mesangial matrix expansion in early postmenopausal mice,3 an increase in the size of individual glomerular cells may be a main contributor. Cell growth, increased cell size, usually occurs early in the cell cycle, a pivotal period for protein synthesis. We speculated that cell hypertrophy in aging glomeruli could be due to abnormalities in early cell-cycle regulatory molecules. Cyclin-dependent kinase inhibitors, p27 and p21 are actively involved in the regulation of G1 → S cell-cycle transition.10 Increased p27 and p21 have been shown to play important role in glomerular hypertrophy in diabetic nephropathy.11–13 Thus, we examined p27 and p21 levels in mesangial cells and glomeruli of aged mice and recipients of BMT from either pre- or postmenopausal mice.
Materials and Methods
Mice
Female C57BL/6 (B6) mice 4, 18, and 30 months old were purchased from the National Institute on Aging, National Institutes of Health, Bethesda, MD. Four-month-old female GFP (green fluorescent protein) transgenic mice (B6 background) were purchased from Jackson Laboratories (Bar Harbor, ME). Female p27 knockout and wild-type mice (5 months old) were obtained from a colony developed at the Department of Molecular Biology, Memorial Sloan-Kettering Cancer Institute, New York, NY.10 Because B6 mice older than 28 months of age may develop malignancies, we examined all organs and excluded mice with tumors from the study. Experiments were conducted in accordance with the guidelines for the care and use of animals approved by Institution Animal Care and Use Committee, University of Miami School of Medicine, Miami, FL. Two mice died during the follow-up period (one naïve control and one postmenopausal recipient of a BMT from a postmenopausal donor). To determine whether the glomerular lesions and mesangial cell phenotypic changes found in postmenopausal B6 mice were also present in aged male mice, we examined 22- and 30-month-old male B6 mice (n = 3/age).
Bone Marrow Transplantation (BMT)
Recipients received lethal irradiation (950 cGy) and were intravenously infused with 1 × 107 donor BM cells obtained from the femur and tibia of donors (Table 1).4,5 Recipients were followed for 4 months after transplantation and compared to age-matched transplants or naïve controls. Biweekly urine samples were collected during this period and body weight was measured every 2 weeks.
Table 1.
Experimental Design
| Groups | Age
|
||
|---|---|---|---|
| Donors (n = 2) | Recipients (n = 6) | Sacrifice | |
| 8 months | 8 months | ||
| 4 months into 4 months | 4 months | 4 months | 8 months |
| (4 months→4 months) | |||
| 18 months into 4 months | 18 months | 4 months | 8 months |
| (18 months→4 months) | |||
| 30 months into 4 months | 30 months | 4 months | 8 months |
| (30 months→4 months) | |||
| 22 months | 22 months | ||
| 18 months into 18 months | 18 months | 18 months | 22 months |
| (18 months→18 months) | |||
| 4 months into 18 months | 4 months | 18 months | 22 months |
| (4 months→18 months) | |||
| 30 months | 30 months | ||
Specimen Preparation
At sacrifice urine and serum were collected and body, heart, kidney, and uterus weights were recorded. Urinary albumin excretion rate and blood urea nitrogen levels were determined as previously described.3 The abdominal aorta was exposed and the kidneys were perfused with saline solution at normal aortic pressure.3 The left kidney was removed, weighed, and the upper and lower poles were incubated in 0.1% collagenase at 37°C for glomerular microdissection.3,5,14 The remainder of the kidney was snap-frozen. The right kidney was fixed by in situ perfusion with 4% paraformaldehyde. Fragments were either embedded in low melting point paraffin for immunohistochemical studies or in methacrylate for histological and morphometric analysis.3,7
Morphometry
Methacrylate-embedded tissue was stained with periodic acid-Schiff (PAS). Fifty or more glomeruli, sequentially selected in each section as they were encountered, were recorded with an Olympus BH-2 microscope and Micro Image A209RGB color video camera (Olympus America Inc., Melville, NY). Because subcapsular and juxtamedullary glomeruli differ in size, the method of scanning the slides for glomeruli consisted of moving the stage of the microscope to scan from the capsule to the juxtamedullary region, and returning, until at least 50 glomeruli were recorded. In most sections, all glomeruli in a section were included. Glomerular tuft and mesangial area were measured as described.3,7
Immunohistochemical Staining
Type IV collagen and laminin were evaluated as previously described using frozen sections.3 Macrophages were identified using acetone-fixed, frozen sections that were coated with rat anti-CD68 antibody (Serotec Inc., Raleigh, NC) followed by biotin-conjugated goat anti-rat IgG (Serotec Inc.) and streptavidin-conjugated fluorescein isothiocyanate (Zymed Laboratories, Inc., South San Francisco, CA).5 Adjacent sections, exposed only to the second antibody, served as negative controls and spleen sections were used as a positive control. For p27 and p21 immunostaining, sections of paraffin-embedded tissue were deparaffinized and processed using the automatic system Nex-Es HIS (Ventana, Tucson, AZ). The primary p27 and p21 antibodies (Santa Cruz Biotechnologies Inc., Santa Cruz, CA) were used at a concentration of 1:50 with an incubation time of 2 hours. The number of cells staining positively using these antibodies was counted in 10 to 15 successively encountered glomeruli in each section.
Isolation of Glomerular Mesangial Cells
Microdissected glomeruli were placed into fibronectin-coated wells of tissue culture plates and the outgrowing mesangial cells were characterized as described.3,9 We obtained at least two cell lines from each experimental group and age-matched naïve mice. Mesangial cells were also isolated from p27 knockout mice and their littermate controls, as well as from the glomeruli of 22-month-old male B6 mice. Mesangial cells between passage 4 to 11 were used for all experiments.
Western Blots
Two mesangial cell lines isolated from each experimental group and age-matched naïve mice were allowed to grow to 70% confluency in 75-mm2 culture flask in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium containing 2% fetal bovine serum (FBS). Cellular proteins were electrophoresed, transferred to nitrocellulose gels, and identified using antibodies against p27 or p21 (Santa Cruz Biotechnologies Inc.).
Flow Cytometry
To determine cell size, 1 × 105 cells were seeded in each well of a six-well plate and incubated with DMEM/F12 medium containing 10% FBS for 24 hours. After three washes with phosphate-buffered saline, DMEM/F12 medium containing 2% FBS was added to cells. The cell layers were trypsinized 48 hours later for flow cytometry analysis. Bio-beads of standard size from 5 to 75 μm were used to control for cell size.
p27 Studies
Mesangial cells (2 × 105) isolated from p27−/− and wild-type mice (two lines each) were seeded in each well of six-well plates. Transfection was performed in mesangial cells using Transfast according to the manufacturer’s protocol (Promega, Madison, WI).3,15 Cells were transfected with a mouse p27 cDNA expression vector (2 μg) and a GFP expression vector (200 ng). Controls consisted of cells transfected with an empty vector (2 μg) plus a GFP vector (200 ng), those transfected with sperm DNA, and untransfected cells. We found that the high ratio of p27 cDNA expression vector to GFP cDNA expression vector used for transfection ensured co-localization of p27 with GFP (data not shown). After transfection, cells were first incubated with DMEM/F12 medium containing 10% FBS for 24 hours and then with 0.1% FBS medium for an additional 24 hours. Cell size was measured by flow cytometry. GFP-positive and -negative cells were separated and their size was determined. The experiments were repeated three times.
Mesangial Cell Type I and Type IV Collagen Production and Matrix Metalloproteinase 2 (MMP-2)
Type I and type IV collagen synthesis by mesangial cells from 5-, 22-, and 28-month-old naïve mice were measured by enzyme-linked immunosorbent assay, and MMP-2 activity was measured by zymography.3–5 Type IV collagen produced by mesangial cells from 5- and 22-month-old male mice was also measured.
Statistical Analysis
The data were compared by analysis of variance with Bonferroni’s correction. All data were expressed as mean ± SD. A value of P < 0.05 was considered to be significant.
Results
Experimental Design and General Health Status
There were at least six mice in each group (Table 1). The contribution of BM-derived progenitors to glomerular lesions and the phenotype of mesangial cells was examined as follows: B6 mice received BM cells pooled from GFP B6 mice, and GFP B6 mice received BM cells pooled from B6 mice. Glomeruli were isolated by microdissection and explanted. The initial outgrowth contained mainly GFP-positive cells in B6 recipients of BM from GFP donors and contained mainly GFP-negative cells in GFP recipients of BM from B6 donors. These results were consistent with our previous findings, and that of others, that the bone marrow contains mesangial cell progenitors that deliver their genotype to the glomerulus.4,5,16–18 Three groups served as controls for irradiation and other experimental conditions. The first consisted of 4-month-old and 18-month-old B6 recipients that received BM pooled from age-matched donors (Table 1). The second consisted of three 4-month-old and three 18-month-old mice that were irradiated, but did not receive BMT. All six died within 2 weeks, as expected. The third control group consisted of 4-month-old and 18-month-old mice that did not receive irradiation or BMT (nontreated controls) and were followed to the same age as those that received BMT. All transplanted mice were followed for 4 months after BMT. No changes in general health status of recipients were noted except that the coat color of irradiated mice gradually turned to gray. There were no differences in body, heart, or kidney weight and no differences in either the blood urea nitrogen levels or the urine albumin/creatinine ratio between any of the groups. Uterus weight was significantly reduced in naïve postmenopausal B6 as well as in all of the recipients of BMT (data not shown).
Transfer of the Aging Phenotype to Premenopausal Recipients
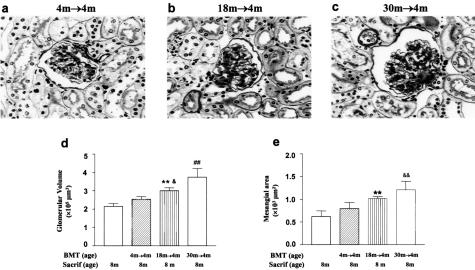
One aim of the study was to determine whether phenotypic changes in progenitors underlay the lesions found in aged glomeruli. We chose the BM as a source of progenitors, based on our previous data that the BM is a repository of glomerular progenitors and that these progenitors were able to transfer a disease genotype and phenotype to normal glomeruli.4,5 Four-month-old B6 recipients received pooled BM from 18-month-old B6 (18 months → 4 months), from 30-month-old B6 (30 month → 4 months), or from 4-month-old donors (4 months → 4 months), and all were followed for 4 months after transplantation and compared to naïve, age-matched controls. The glomeruli of 30 months → 4 months recipients were histologically enlarged and sclerotic (Figure 1). They resembled that of the donors.3 Both glomerular size (Figure 1d) and mesangial area (Figure 1e) were increased. Parallel, but less marked changes were also present in 18 months → 4 months recipients (Figure 1b). Namely, the glomerular size was larger than in the 4 months → 4 months controls (Figure 1, a and d), but not as large as the 30 months → 4 months recipients. Similarly, the mesangial area was increased, compared to the 4 months → 4 months controls, but was less than that of the 30 months → 4 months recipients (Figure 1e). As expected from the light microscopic findings, there was an increase in the amount of type IV collagen and laminin B1 in the expanded mesangial areas by immunofluorescence microscopy of both the 18 months → 4 months and 30 months → 4 months recipients, being more pronounced in the latter (data not shown). Finally, the glomeruli of 4-month-old recipients of BMT from 4-month-old donors were indistinguishable from those of naïve 8-month-old normal controls.
Figure 1.
Transfer of the aging lesions after BMT. a: The glomeruli of 4-month-old recipients of 4-month-old BMT (4 months → 4 months, age at BMT = 4 months; age at sacrifice = 8 months) were normal by light microscopy. Sacrif, sacrifice; m, month. PAS stain. b: The mesangial areas, especially at the vascular poles, were enlarged in 4-month-old recipients of 18-month-old BMT (18 months → 4 months). c: Diffuse expansion of the mesangial areas was present in 4-month-old recipients of 30-month-old BMT (30 months → 4 months). d: The glomerular volume was similar in 4 months → 4 months and 8-month-old naïve controls. The glomerular volume in 18 months → 4 months was larger than that in 8 months (**P < 0.01) and in 4 months → 4 months (&P < 0.05). The glomerular volume in 30 months → 4 months, compared with either young controls (8 months and 4 months → 4 months) (##P < 0.01) or with 18 months → 4 months, was further increased (##P < 0.01). e: The mesangial areas in 4 months → 4 months and 8 months were similar. However, mesangial area was increased in 18 months → 4 months compared with 8 months (**P < 0.01), and was further increased in 30 months → 4 months as compared with 4 months → 4 months (&&P < 0.01). The mesangial area did not differ between 4 months → 4 months and 18 months → 4 months. Original magnification, ×400 (a–c).
Reduction of Lesions in Postmenopausal Recipients of BMT from Premenopausal Donors
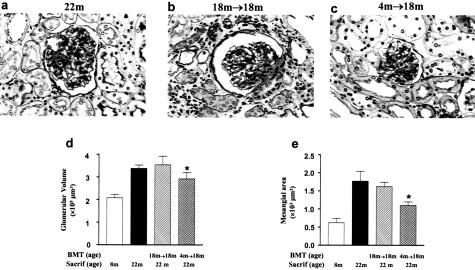
The reversibility of the aging phenotype was examined using BMT between 4-month-old donors and 18-month-old B6 recipients (4 months → 18 month) (Table 1). The 18-month-old recipients of BMT from 4-month-old donors had smaller glomeruli and less glomerulosclerosis than age-matched controls (Figure 2). Because glomerular lesions were more prominent in these recipients than in age-matched controls, we concluded that progenitors from young donors were capable of reducing, but did not abolish, renal lesions in postmenopausal recipients within the 4-month follow-up period. A further decrease may have been noted if the follow-up period had been extended. Nonetheless, these data demonstrate that the progenitor phenotype, rather than the state of the target tissue determine glomerular scarring and hypertrophy. The recipients also had a reduction in tubulointerstitial lesions. There were no histological differences between 18-month-old recipients of BMT from 18-month donors and age-matched controls.
Figure 2.
Modulation of glomerular structure after BMT. a: Glomerular hypertrophy and expansion of the extracellular matrix, tubular atrophy, thickened tubular basement membranes, and interstitial inflammatory cell infiltration were present in 22-month-old naïve mice (22 months). PAS stain. b: Glomerular and tubulointerstitial lesions were prominent in 18-month-old recipients of BMT from 18-month-old mice (18 months → 18 months, age at BMT = 18 months; age at sacrifice = 22 months), changes similar to that of 22-month-old naïve mice. Sacrif, sacrifice; m, month. c: Glomerular hypertrophy and extracellular matrix expansion were less evident, and tubulointerstitial lesions were less severe, in 18-month-old recipients of BMT from 4-month-old mice (4 months → 18 months). d: Glomerular hypertrophy was significantly decreased in 4 months → 18 months compared to 22 months or 18 months → 18 months (*P < 0.05). e: Mesangial area was decreased in 4 months → 18 months compared with the 22 months or 18 months → 18 months (*P < 0.05). The mesangial areas of 22 months and 18 months →18 months were similar. Original magnification, ×400 (a–c).
Relationship between Glomerular Cell Number and Glomerular Aging Lesions
Glomerular cell number did not differ among the recipients of BMT from either 4- or 18-month-old donors or among the naïve controls, except for those at 30 months of age. We previously showed that the increased glomerular cell number in 30-month-old mice appeared to be primarily due to a prominent macrophage infiltration.3 A small increase in glomerular cell number was found in 4-month-old recipients of BMT from 30-month-old donors (30.05 ± 0.46/glomerulus versus 8-month-old naïve controls, 27.3 ± 0.47/glomerulus, P < 0.05). This may be partially due to macrophage infiltration (0.8/glomerulus in 30 months → 4 months versus 0.3/glomerulus in 8-month-old naïve controls, P < 0.05).
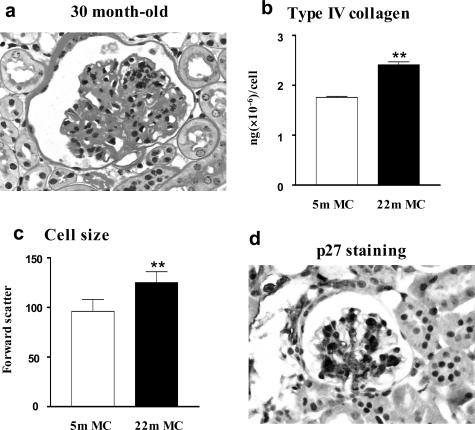
Progressive Changes in Mesangial Cell Phenotype: Extracellular Matrix
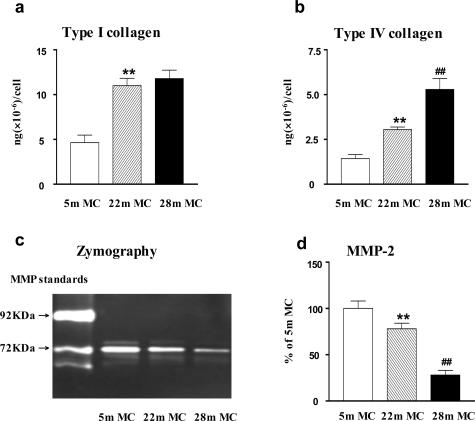
We investigated mesangial cells to determine whether there was an aging-related change in ECM synthesis and/or degradation. Mesangial cell type IV collagen production in vitro correlated directly with the degree of glomerulosclerosis in vivo. The changes consisted of a progressive increase in the amount of type IV collagen released into the medium and deposited on the culture dish, which correlated directly with the age of the mouse from which the mesangial cells were isolated. Namely, it was higher in both aging groups than in young mice, but less in those from 22-month-old mice than in those from 28-month-old mice (Figure 3b). Type I collagen production was increased in mesangial cells from female mice shortly after menopause and the levels were similar in cells from mice at 28 months of age. Thus, although the synthesis of both types I and IV collagen was increased in the two postmenopausal ages shown, type IV collagen production correlated best with the progressive evolution of the histological changes. MMP-2 was decreased in mesangial cells isolated from 22-month-old naïve mice and was further decreased at 28 months (Figure 3, c and d). The differences in type IV collagen production and decreased MMP-2 expression between early and late menopause support the postulate that aging is a multistage process.
Figure 3.
Progressive phenotypic changes in ECM synthesis and degradation in mesangial cells from aging females. a: Mesangial cells isolated from 22- and 28-month-old mice (22-month MC, 28-month MC) produced significantly more type I collagen than mesangial cells isolated from 5-month-old mice (5-month MC). **P < 0.01. b: Progressive increase in type IV collagen production in mesangial cells with aging (**P < 0.01 versus 5-month MC; ##P < 0.01 versus 22-month MC). c: MMP-2 activity was reduced in mesangial cells from 22-month-old mice (22-month MC) and it was further decreased in mesangial cells from 28-month-old mice. d: Quantitation of three independent experiments reveals that the production of MMP-2 activity by mesangial cells progressively decreased with aging (**P < 0.01 versus 5-month MC; ##P < 0.01 versus 22-month MC).
Progressive Changes in Mesangial Cell Phenotype: Cell Size
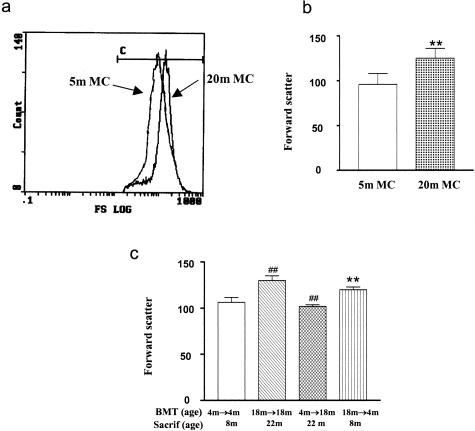
The glomeruli were enlarged, but not hypercellular in 18- to 22-month-old mice, suggesting that the hypertrophy was most likely a result of changes in the size of glomerular cells. We examined the size of mesangial cells because they represent ∼40% of the population of glomerular cells, and we reasoned that changes in the size of cells in a population this large could influence overall glomerular size. Mesangial cells of 20-month-old postmenopausal mice were larger than those of premenopausal mice (Figure 4, a and b). Mesangial cells from 18-month-old recipients of BMT from 18-month-old donors (18 months → 18 months, age at sacrifice = 22 months) were larger than those from 4-month-old recipients of BMT from 4-month-old donors (4 months → 4 months, P < 0.01) (age at sacrifice = 8 months). Mesangial cells from 4-month-old recipients of BMT from 18-month-old donors (18 months → 4 months, age at sacrifice = 8 months) were similar to 18-month-old recipients of BMT from 18-month-old donors and larger than 4-month-old recipients of BMT from 4-month-old donors (**P < 0.01, Figure 4c) (age at sacrifice = 8 months). On the other hand, mesangial cells from 18-month-old recipients of BMT from 4-month-old donors (4 months → 18 months, age at sacrifice = 22 months) were reduced in size compared to 18-month-old recipients of BMT from 18-month-old donors (##P < 0.01, age at sacrifice = 22 months).
Figure 4.
Analysis of mesangial cell size by flow cytometry. a: Forward scatter, an index of cell size, revealed that mesangial cells from 20-month-old mice (20-month MC) were larger than mesangial cells from 5-month-old mice (5-month MC). b: Analyses of three independent measurements of two cell lines from 5-month-old mice and two cell lines from 20-month-old mice confirm the increase in cell size. **P < 0.01. c: Mesangial cells from 18-month-old recipients of BMT from 18-month-old mice donors (18 months → 18 months, age at BMT = 18 months; age at sacrifice = 22 months) were larger than those from 4-month-old recipients of BMT from 4-month-old donors (4 months → 4 months, P < 0.01). Mesangial cells from 4-month-old recipients of BMT from 18-month-old donors (18 months → 4 months) were larger than 4-month-old recipients of BMT from 4-month-old donors (**P < 0.01). On the other hand, mesangial cells from 18-month-old recipients of BMT from 4-month-old donors (4 months → 18 months) were reduced in size compared to 18-month-old recipients of BMT from 18-month-old donors (##P < 0.01).
p27 in Mesangial Cell Growth and Glomerular Hypertrophy
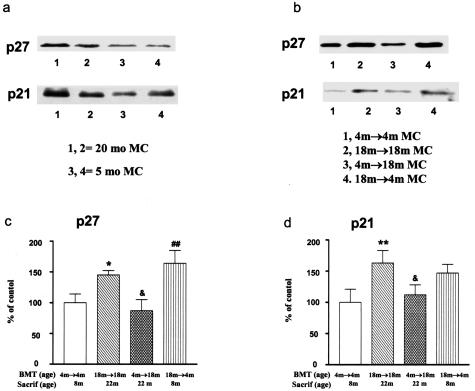
Cell growth is usually tightly associated with proliferation. The uncoupling of these two processes may occur due to a dysfunction of cell-cycle regulation. We found that p27 levels were higher in mesangial cells isolated from 20-month-old mice than in those isolated from 5-month-old mice (Figure 5a). Interestingly, higher p27 levels were also found in mesangial cells isolated from 4-month-old recipients of BMT from 18-month-old donors (Figure 5, b and c) (P < 0.05), whereas mesangial cells from 18-month-old recipients of BMT from 4-month-old donors had reduced p27 levels (P < 0.05). Thus, the p27 levels in mesangial cells were directly correlated with cell size. An increase in p21 levels was also found in mesangial cells isolated from 20-month-old mice (Figure 5a). Finally, p21 levels were increased in mesangial cells isolated from premenopausal recipients of postmenopausal donor BM and decreased in mesangial cells isolated from 18-month-old recipients of BMT from 4-month-old donors (Figure 5, b and d). Thus, the levels of p21 generally followed the same pattern as p27 (Figure 5, b and d).
Figure 5.
p27 and p21 and mesangial cell size in aging. a: A representative Western blot of two cell lines demonstrating higher p27 levels in mesangial cells isolated from 20-month-old mice (20-month MC) (lanes 1 and 2) compared to mesangial cells isolated from 5-month-old mice (5-month MC) (lanes 3 and 4). There is also a slight increase in p21 levels. b: Representative Western blot gels demonstrating that p27 levels were increased in mesangial cells from 4-month-old recipients of BMT from 18-month-old donors (18 month → 4-month MC, age at BMT = 4 months; age at sacrifice = 8 months). However, p27 levels were decreased in mesangial cells isolated from 18-month-old recipients of BMT from 4-month-old donors (4 month → 18 month MC), compared to their respective age-matched transplant controls (18 month → 18 month MC or 4 month → 4 month MC). Note that p21 levels follow p27 levels, but are less pronounced. c: Densitometry scanning of Western blot gels from three experiments revealed that p27 levels were increased in mesangial cells from 18-month-old recipients of BMT from 18-month-old donors (18 month → 18 month MC) and were decreased in 18-month-old recipients of BMT from 4-month-old donors (4 month → 18 month MC). Data obtained from 4 month → 4 month MC were defined as 100% (control). *P < 0.05 versus 4 month → 4 month MC; &P < 0.05 versus 18 month → 18 month MC; ##P < 0.01 versus 4 month → 4 month MC. d: Densitometry scanning of Western blot gels from three experiments revealed that p21 levels were increased in mesangial cells from 18-month-old recipients of BMT from 18-month-old donors and were decreased in 18-month-old recipients of BMT from 4-month-old donors. Data obtained from 4 month → 4 month MC were defined as 100% (control). **P < 0.01 versus 4 month → 4 month MC; &P < 0.05 versus 18 month → 18 month MC.
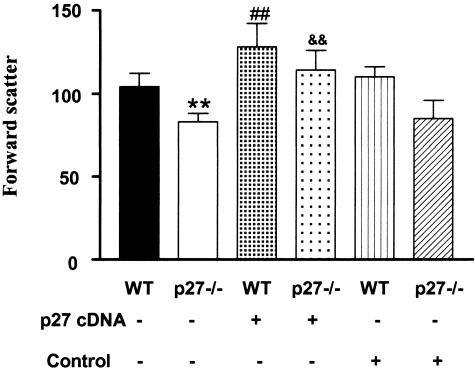
Because there was a direct relationship between cell size and p27 levels, we examined mesangial cells from premenopausal p27−/− and wild-type mice. We found that two different lines of cells isolated from p27−/− mice were significantly smaller than those isolated from their wild-type controls (P < 0.01, Figure 6). Restoration of the expression of p27 in p27−/− mesangial cells by transfection with a plasmid containing a p27 cDNA expression vector returned them to a size comparable to that in wild-type mesangial cells. An increase in cell size was found in wild-type mesangial cells in which p27 levels were increased by transfection with a plasmid containing a p27 cDNA expression vector. On the other hand, p27−/− or wild-type mesangial cells transfected with an empty vector did not result in a change in cell size (Figure 6).
Figure 6.
Relationship between p27 and mesangial cell size, general aspects. Cell size analyzed by flow cytometry revealed that mesangial cells isolated from p27 knockout mice were smaller than those isolated from wild-type mice (WT, **P < 0.01). Transfection of p27−/− mesangial cells with a p27 cDNA expression vector restored cells to a size comparable with that of wild-type (WT) mesangial cells (&&P < 0.01 versus p27−/−). Wild-type mesangial cells transfected with the p27 cDNA expression vector were also increased in size (##P < 0.01 versus WT or WT transfected with an empty vector). No change in cell size was found in mesangial cells transfected with an empty vector.
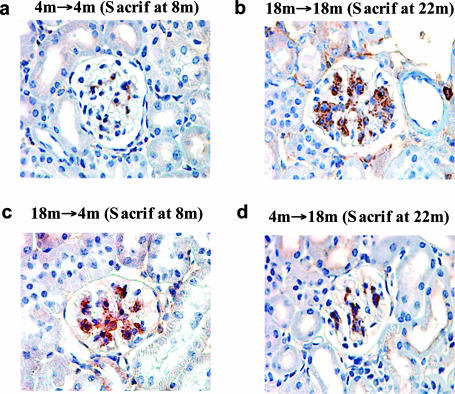
The in vivo relevance of these findings was addressed in kidney sections. Weak to moderate p27 staining was found in the glomeruli of 4-month-old recipients of BMT from 4-month-old donors (Figure 7), a finding similar to that in the glomeruli of age-matched controls. Both the extent and the intensity of p27 staining were significantly increased in the glomeruli of 18-month-old recipients of BMT from 18-month-old donors, and this staining pattern was indistinguishable from age-matched controls. In concert with the observation that glomerular hypertrophy was transferred from postmenopausal donors to premenopausal recipients by BMT (Figure 1), p27 staining was increased in 4-month-old recipients of BMT from 18-month-old donors (Figure 7c). In addition, p27 staining was diminished in 18-month-old recipients of BMT from 4-month-old donors, a finding concordant with the observed decrease in glomerular hypertrophy (Figure 7d). No change in p21 glomerular staining was found between 4- and 22-month-old mice (data not shown), consistent with the relatively small changes noted in vitro.
Figure 7.
In vivo correlations between p27 immunostaining and glomerular size. a: There is a small, scattered amount of p27 (brown staining material) in this glomerulus from a 4-month-old recipient of a BMT from 4-month-old donors (4 months → 4 months). b: A glomerulus from an 18-month-old recipient of a BMT from 18-month-old donors (18 months → 18 months) contains a relatively large amount of stainable p27. c: A glomerulus from a 4-month-old recipient of a BMT from 18-month-old donors (18 months → 4 months) shows a similar amount of stainable p27 to that in b. d: A glomerulus from an 18-month-old recipient of BMT from 4-month-old donors (4 months → 18 months) has less stainable p27 than in either b or c.
Glomerular and Mesangial Cell Changes in Aging Male B6 Mice
Glomerular lesions characterized by hypertrophy and moderate mesangial matrix expansion were also seen in 22-month-old male B6 mice, which were associated with increased p27 staining (Figure 8). Glomerular lesions were significantly worsened in 30-month-old mice (Figure 8a). Mesangial cells isolated from 22-month-old male B6 mice exhibited increased type IV collagen production, increased cell size (Figure 8c), as well as elevated p27 levels (data not shown).
Figure 8.
Glomerular lesions and phenotypic changes in mesangial cells from aging males. a: Glomerular size, mesangial area, and cell number were increased in 30-month-old male B6 mouse (PAS). b: Mesangial cells isolated from 22-month-old male B6 mouse produced significantly more type IV collagen than mesangial cells isolated from 5-month-old male B6 mouse (**P < 0.01). c: Mesangial cells isolated from 22-month-old male B6 mouse were larger than mesangial cells isolated from 5-month-old male B6 mouse (**P < 0.01). d: A glomerulus from a 22-month-old male B6 mouse shows a large amount of p27 staining. Original magnification ×400 (a).
Discussion
The questions we addressed were the molecular nature of the changes in glomerular aging in mice, their cellular source, and reversibility of the lesions. We found an aging phenotype in both glomeruli and mesangial cells of postmenopausal females. Similar changes were also found in aged male B6 mice. An aging phenotype has also been found in the mesangial cells of Fischer 344 rats.19 The changes in aged B6 mesangial cells consisted of increased size and synthesis of extracellular matrix, coupled with decreased MMP-2 levels. These alterations paralleled the degree of glomerular hypertrophy and sclerosis. Furthermore, the phenotype differed between early and late menopause and was not due to a change in either the number of glomeruli or the cells contained therein.3 These data suggested that the changes were due to changes in the phenotype of glomerular cells. Thus, we used mesangial cells to investigate the mechanism(s) underlying both the glomerular hypertrophy and sclerosis.
We found that mesangial cells from aging mice had increased cell growth in the absence of proliferation, and this was associated with increased p27 levels. Because p27 has been shown to directly increase mesangial cell growth while suppressing cell proliferation,11 elevated p27 levels may contribute to mesangial cell hypertrophy in aging. The significance of this in vitro finding was validated by the fact that glomeruli in vivo also had increased p27 by immunostaining. Furthermore, mesangial cells from p27−/− mice were smaller, and their small size could be corrected by transfecting them with a plasmid containing a p27 cDNA expression vector. The induction of increased p27 levels in wild-type mice mesangial cells, with a plasmid containing a p27 cDNA expression vector, also resulted in increased cell size. Thus, the levels of p27 in mesangial cells were directly correlated with cell size. Additional confirmation of the importance of the p27 in mesangial cell size control was obtained by transferring BM from a young donor, with normal p27 levels, into aging mice. The glomeruli in these aging recipients were reduced in size.
The fact that cell size was increased in mesangial cells derived from premenopausal recipients of BMT from postmenopausal donors suggests that BM-derived progenitors were large. This interpretation is supported by the finding that mesangial cell size was smaller in 18-month-old recipients of BMT from 4-month-old donors than the mesangial cells of naïve 20-month-old mice. These data suggest that progenitor size determines glomerular size. An artifact introduced by the BMT procedure can be excluded by the finding that cell size was identical in 4-month-old recipients of BMT from 4-month-old donors or 18-month-old recipients of BMT from 18-month-old mice, compared to their respective age-matched controls. Thus, progenitors may be actively involved in glomerular mesangial cell size regulation.
To determine whether the progressive increase in glomerular size was due to a single event we used BMT. Although glomerular size was significantly increased in young recipients of BM from old mice, if the donors were in the early postmenopausal period glomerular size in the recipient was smaller than if the donor was in a later postmenopausal period. This suggested that the underlying causes of glomerular hypertrophy present in early menopause were different from that present in late menopause. Thus, glomerular hypertrophy may have more than one underlying defect, with p27 abnormalities being an early and persistent event. The role of elevated p21 levels in mesangial cell hypertrophy in aging was not clear, because we found an increase in p21 in mesangial cells in vitro, but not in the glomeruli of aged mice.
The sclerosis was found to be more pronounced with time after the onset of menopause.3 The fact that both the degree of glomerular hypertrophy and sclerosis continued to increase after menopause could be interpreted as a single molecular event, which simply took a period of time to become manifest. Arguing against this interpretation is the fact that the lesions in BMT recipients mirrored the sclerotic lesions in the donors. Namely, BMT from a donor in the early period transferred mild sclerosis to glomeruli, and if the donor was in the late phases of aging the transferred sclerotic lesions were more severe, even though all recipients were followed for a similar length of time after BMT. Further support for this interpretation was found in analysis of mesangial cells isolated from donors and recipients. Namely, the histological changes were reflected in the differing molecular profiles in mesangial cells isolated from mice in early or late menopause. There was a more marked increase in synthesis of collagen type IV and a decrease in MMP-2 in mesangial cells isolated from mice in the late postmenopausal, compared to young controls or mice early in the postmenopausal period. Thus, as was noted with respect to glomerular hypertrophy, the sclerosis appeared to be a multistage process. The conclusion that the aging process may result from several individual insults, was consistent with our previous observations. We have shown that an injury in the early postmenopausal period precipitated the development of glomerular lesions characteristic of a much more advanced age.3 The nature of underlying molecular defect(s) remains unclear, and studies to clarify this issue are ongoing.
Because kidney disease in aging women is much more common than previously thought and the population is aging,1,2 the reversibility of aging lesions is of importance. Based on our earlier observations, and those of many others, that the adjacent matrix has a strong effect on stromal cell behavior in vitro,20–23 we anticipated that the delivery of normal progenitors to aging glomeruli might not be beneficial. However, we found that the glomeruli of aged recipients of BMT from young donors had a substantial reduction in both overall size and sclerosis. This finding is the first demonstration of reduced glomerular size and sclerosis mediated by glomerular progenitors derived from an extrarenal source. However, circulating factor(s) derived from the young cells contained in the BMT may also have played a role in reducing the aging lesions, as suggested in a recent study by Conboy and colleagues.24
Macrophages have been thought to either cause or potentiate many forms of glomerular and vascular in-jury.25,26 Thus, the presence of an increased number of macrophages in the glomeruli in the late aging period suggested that they might play a role in the development of the lesions. Their contribution to the glomerular lesions of aging was investigated using BMT. Young recipients of BMT from aging mice, at two different times after the onset of menopause, developed both glomerular hypertrophy and sclerosis, which mirrored lesions specific to the age of the donor. However, the number of macrophages in young recipients of BMT from an old donor was only marginally increased. Thus, macrophage infiltration does not seem to be a major contributor to the development of the either the increased size or sclerosis in aging glomeruli. Although the causes and consequences of increased localization of macrophages to glomeruli in late aging is not understood, our recent data suggests that other aspects of the glomerular phenotype in aging, ie, an increase in circulating tumor necrosis factor-α and in the expression by mesangial cells of chemokine and adhesion molecules such as RANTES and VCAM-1, could be important.27
The underlying cause(s) of phenotypic changes in bone marrow mesangial cell progenitors in postmenopausal B6 mice is not clear. Although the loss of estrogen and its action(s) may play a role, the fact that aged male B6 mice also develop progressive glomerulosclerosis and mesangial cell phenotypic changes argues against this concept. Furthermore, young female recipients of a BMT from aged male mice donors developed glomerular lesions characterized by increased extracellular matrix.28 In addition, because pretransplant irradiation causes ovarian atrophy and estrogen levels are low in all recipients, the normal phenotype of young recipients of young BM and the reduction of lesions in aged recipients of BMT from a young donor cannot be solely attributed to an estrogen effect.
In summary, the aging phenotype consists of elevated p27 levels, which contribute to glomerular hypertrophy, and an increased net deposition of extracellular matrix. The aging phenotype in females appears to be a multistage process and may be delivered to naïve glomeruli by glomerular progenitors, which may be derived from an extrarenal source. Furthermore, the delivery of progenitors with a normal phenotype to aged mice with glomerular lesions, results in a decrease in both glomerular size and sclerosis. Thus, the aging lesions are reversible and changes in the composition of the local extracellular matrix do not appear to interfere with the ability of newly arriving normal progenitors or factor(s) to return the architecture to a more normal state.
Footnotes
Address reprint requests to Feng Zheng, M.D., Division of Experimental Diabetes and Aging, Department of Geriatrics, Mount Sinai School of Medicine, Box 1640, One Gustave L. Levy Place, New York, NY 10029. E-mail: feng.zheng@mssm.edu.
Supported by the National Institutes of Health (National Institutes of Diabetes and Digestive and Kidney Diseases grant DK64118-01 to F.Z. and National Institute on Aging grants AG-19366-04 to G.E.S. and AG-17170-04 to L.J.S.), the National Kidney Foundation (fellowship to A.R.P.), and Genzyme (to F.Z.).
Z.F. and A.R.P. contributed equally to this work.
L.J.S. is deceased.
References
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- US Renal Data System Washington, DC: The National Institutes of Health, NIDDK; USRDS 1999 Annual Report. 1999 [Google Scholar]
- Zheng F, Plati AR, Potier M, Schulman Y, Berho M, Banerjee A, Leclercq B, Zisman A, Striker LJ, Striker GE. Resistance to glomerulosclerosis in B6 mice disappears after menopause. Am J Pathol. 2003;162:1339–1348. doi: 10.1016/S0002-9440(10)63929-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornacchia F, Fornoni A, Plati AR, Thomas A, Wang Y, Inverardi L, Striker LJ, Striker GE. Glomerulosclerosis is transmitted by bone marrow-derived mesangial cell progenitors. J Clin Invest. 2001;108:1649–1656. doi: 10.1172/JCI12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Cornaccia F, Schulman I, Banerjee A, Cheng QL, Potier M, Plati AR, Berho M, Elliot SJ, Li J, Fornoni A, Zang YJ, Zisman A, Striker LJ, Striker GE. Development of albuminuria and glomerular lesions in normoglycemic B6 recipients of db/db mice bone marrow: the role of mesangial cell progenitors. Diabetes. 2004;53:2420–2427. doi: 10.2337/diabetes.53.9.2420. [DOI] [PubMed] [Google Scholar]
- Doi T, Striker LJ, Quaife C, Conti FG, Palmiter R, Behringer R, Brinster R, Striker GE. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulin-like growth factor-1. Am J Pathol. 1988;131:398–403. [PMC free article] [PubMed] [Google Scholar]
- Doi T, Hattori M, Agodoa LY, Sato T, Yoshida H, Striker LJ, Striker GE. Glomerular lesions in nonobese diabetic mouse: before and after the onset of hyperglycemia. Lab Invest. 1990;63:204–212. [PubMed] [Google Scholar]
- He C, Esposito C, Phillips C, Zalups RK, Henderson DA, Striker GE, Striker LJ. Dissociation of glomerular hypertrophy, cell proliferation, and glomerulosclerosis in mouse strains heterozygous for a mutation (Os) which induces a 50% reduction in nephron number. J Clin Invest. 1996;97:1242–1249. doi: 10.1172/JCI118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacot TA, Striker GE, Stetler-Stevenson M, Striker LJ. Mesangial cells from transgenic mice with progressive glomerulosclerosis exhibit stable, phenotypic changes including undetectable MMP-9 and increased type IV collagen. Lab Invest. 1996;75:791–799. [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Awazu M, Omori S, Ishikura K, Hida M, Fujita H. The lack of cyclin kinase inhibitor p27(Kip1) ameliorates progression of diabetic nephropathy. J Am Soc Nephrol. 2003;14:699–708. doi: 10.1097/01.asn.0000051726.41601.c0. [DOI] [PubMed] [Google Scholar]
- Wolf G, Schroeder R, Zahner G, Stahl RA, Shankland SJ. High glucose-induced hypertrophy of mesangial cells requires p27(Kip1), an inhibitor of cyclin-dependent kinases. Am J Pathol. 2001;158:1091–1100. doi: 10.1016/S0002-9440(10)64056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan CJ, al Douahji M, Shankland SJ. The cyclin kinase inhibitor p21WAF1, CIP1 is increased in experimental diabetic nephropathy: potential role in glomerular hypertrophy. J Am Soc Nephrol. 1998;9:986–993. doi: 10.1681/ASN.V96986. [DOI] [PubMed] [Google Scholar]
- Peten EP, Garcia-Perez A, Terada Y, Woodrow D, Martin BM, Striker GE, Striker LJ. Age-related changes in alpha 1- and alpha 2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol. 1992;263:F951–F957. doi: 10.1152/ajprenal.1992.263.5.F951. [DOI] [PubMed] [Google Scholar]
- Zheng F, Fornoni A, Elliot SJ, Guan Y, Breyer MD, Striker LJ, Striker GE. Upregulation of type I collagen by TGF-beta in mesangial cells is blocked by PPARgamma activation. Am J Physiol. 2002;282:F639–F648. doi: 10.1152/ajprenal.00189.2001. [DOI] [PubMed] [Google Scholar]
- Imasawa T. Roles of bone marrow cells in glomerular diseases. Clin Exp Nephrol. 2003;7:179–185. doi: 10.1007/s10157-003-0248-9. [DOI] [PubMed] [Google Scholar]
- Ito T, Suzuki A, Imai E, Okabe M, Hori M. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol. 2001;12:2625–2635. doi: 10.1681/ASN.V12122625. [DOI] [PubMed] [Google Scholar]
- Masuya M, Drake CJ, Fleming PA, Reilly CM, Zeng H, Hill WD, Martin-Studdard A, Hess DC, Ogawa M. Hematopoietic origin of glomerular mesangial cells. Blood. 2003;101:2215–2218. doi: 10.1182/blood-2002-04-1076. [DOI] [PubMed] [Google Scholar]
- Ruiz-Torres P, Lucio J, Gonzalez-Rubio M, Rodriguez-Puyol M, Rodriguez-Puyol D. Oxidant/antioxidant balance in isolated glomeruli and cultured mesangial cells. Free Radic Biol Med. 1997;22:49–56. doi: 10.1016/s0891-5849(96)00239-0. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Kaplan DL, Volloch V. Matrix-mediated retention of osteogenic differentiation potential by human adult bone marrow stromal cells during ex vivo expansion. Biomaterials. 2004;25:3233–3243. doi: 10.1016/j.biomaterials.2003.10.005. [DOI] [PubMed] [Google Scholar]
- He CJ, Striker LJ, Tsokos M, Yang CW, Peten EP, Striker GE. Relationships between mesangial cell proliferation and types I and IV collagen mRNA levels in vitro. Am J Physiol. 1995;269:C554–C562. doi: 10.1152/ajpcell.1995.269.3.C554. [DOI] [PubMed] [Google Scholar]
- Santos OF, Nigam SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev Biol. 1993;160:293–302. doi: 10.1006/dbio.1993.1308. [DOI] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Nikolic-Paterson DJ, Atkins RC. The role of macrophages in glomerulonephritis. Nephrol Dial Transplant. 2001;16(Suppl 5):3–7. doi: 10.1093/ndt/16.suppl_5.3. [DOI] [PubMed] [Google Scholar]
- Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- Zheng F, Cheng QL, Plati AR, Ye SQ, Berho M, Banerjee A, Potier M, Jaimes EA, Guan Y, Hao C, Yu H, Striker LJ, Striker GE. The glomerulosclerosis of aging in females: contribution of the proinflammatory mesangial cell phenotype to macrophage infiltration. Am J Pathol. 2004;165:1789–1798. doi: 10.1016/S0002-9440(10)63434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Rossini M, Pozzi A, Ma LJ, Fogo AB. Aging bone marrow augments mesangial collagen IV deposition in young mice. J Am Soc Nephrol Renal Week. 2004:SU-PO790. [Google Scholar]