Abstract
Early signs of inflammatory demyelination include entry of fibrin(ogen) into the central nervous system (CNS), which is normally excluded by the blood-brain barrier, and up-regulation of components of the plasminogen activator system. Using mice deficient in tissue-type plasminogen activator (tPA−/−) and urokinase plasminogen activator receptor (uPAR−/−), we investigated the involvement of the PA system on the clinical and pathological features of experimental allergic encephalomyelitis, an animal model of multiple sclerosis. tPA−/− mice suffered an early and a more severe acute disease characterized by incomplete recovery when compared to wild-type controls, with significantly higher CNS levels of plasminogen activator inhibitor-1. This correlated with fibrin accumulation, which co-localized with nonphosphorylated neurofilament on thickened axons in experimental allergic encephalomyelitis tissue. In contrast, uPAR−/− mice had a delayed, less acute disease reflected in delayed infiltration of inflammatory cells. These animals developed chronic disease as a result of steadily increased inflammation, increased levels of urokinase-type plasminogen activator (uPA), and greater degree of demyelination. Thus, the plasminogen activator system can modulate both inflammatory and degenerative events in the CNS through the respective effects of tPA and uPAR on fibrinolysis and cell adhesion/migration, manipulation of which may have therapeutic implications for multiple sclerosis.
Serine protease activity in multiple sclerosis (MS) is considered to play a major role in the disturbance of the blood-brain barrier and subsequent leukocyte entry leading to inflammation as well as causing myelin breakdown.1,2 Significant up-regulation of urokinase-type plasminogen activator (uPA) receptor (uPAR) and plasminogen activator inhibitor-1 (PAI-1) are among the earliest detectable signs of inflammatory demyelination.3 There is evidence that fibrin(ogen), a protein not present physiologically in the nervous tissue, enters the central nervous system (CNS) before clinical disease and demyelination.4,5
Tissue-type plasminogen activator (tPA), is constitutively expressed in the CNS by neurons and microglia and it is involved in the regulation of synaptic remodeling and neuronal activity.6 In MS lesions tPA is co-localized on demyelinated, damaged axons with nonphosphorylated neurofilament and fibrin, suggesting a role in axonal fibrin dissolution.7 However significant up-regulation of the tPA inhibitor PAI-1, and formation of tPA:PAI-1 complexes in MS tissue decreases the amount of active enzyme and fibrinolytic capacity hence contributing to fibrin deposition and axonal injury.8 Fibrin deposition has been shown to hinder axonal regeneration in a model of peripheral nerve damage,9 and removal of fibrin in experimental allergic encephalomyelitis (EAE) suppressed disease development and reduced neurological deficit.10,11
Leukocytes, including monocytes, macrophages, and activated T cells all constitutively express uPAR,12 which is an important mediator of adhesion and migration to sites of inflammation via interactions with vitronectin and integrins.13,14 Although uPAR is primarily undetectable in the normal brain, a significant increase in its expression is seen at early stages of MS lesion development.7 Co-localization of uPA and uPAR on monocytes and foamy macrophages in the perivascular zone of evolving lesions7 suggests that uPAR may facilitate cellular infiltration into the CNS via proteolysis and extracellular matrix breakdown. uPAR expression is increased on monocytes in patients with secondary progressive and relapsing-remitting MS15 and microglia isolated from MS tissue display increased levels of uPAR.16,17
In this study, the role of tPA and uPAR in myelin oligodendrocyte glycoprotein (MOG)-induced EAE, an animal model of inflammatory demyelination was investigated, in tPA−/− and uPAR−/− mice and their wild-type (WT) counterparts. This study has suggested a major role for tPA in CNS fibrinolysis and highlighted the inhibitor PAI-1 as a potential target for disease-modifying treatment. Furthermore, uPAR-mediated mononuclear cell migration appears to be important for development and progression of EAE.
Materials and Methods
Mice
tPA−/−, uPAR−/−, and WT mice (intercross of N1 generation of (C57BL/6 × 129)F1 x C57BL/6 mice) described previously,18–20 were bred at the Institute of Neurology under comparable conditions and genotyped by polymerase chain reaction. They were fed RM-1(E) diet and water ad libitum. All experiments were ethically performed according to the UK Animals (Scientific Procedures) Act (1984).
MOG-Induced EAE
Mice (6 to 8 weeks old) received 300 μg of MOG 35-55 peptide (Advanced Biotechnology Centre, Imperial College, London, UK) emulsified in 300 μl of incomplete Freund’s adjuvant (IFA) (Difco, Beckton Dickinson, Oxford, UK) supplemented with 4 mg/ml of Mycobacterium tuberculosis (H37Ra) (Difco) subcutaneously on day 0 and day 7.21,22 Mice were injected intraperitoneally with 300 ng of reconstituted lyophilized Bordetella pertussis toxin (Sigma, Dorset, UK) in 200 μl of phosphate-buffered saline (PBS). The pertussis toxin injection was repeated after 48 hours. Mice were monitored and weighed daily. Clinical disease was assessed and scored: 0, normal; 1, limp tail; 2, impaired righting reflex; 3, paresis of hindlimbs; 4, complete paralysis of hindlimbs; and 5, moribund/death. The movement activity of animals was monitored throughout 5 minutes in a 27 × 27-cm open-field activity chamber (Med Associates, Georgia, VT). For specimen collection, mice were sacrificed and immediately perfused with PBS. Spinal cords were removed and snap-frozen on dry ice and stored at −70°C until used. Control mice were taken as normal noninjected animals of each genotype.
Immunohistochemical Staining
Cryostat sections (10 μm) cut onto Vectabond-coated slides (Vector, Peterborough, UK) were immunoper-oxidase-stained overnight at 4°C or for 1 hour at room temperature with antibodies against fibrinogen (1:10,000; Sigma), myelin basic protein (MBP) (1:2000; Chemicon, Hampshire, UK), SMI32 (1:5000; Sternberger Monoclonals, Baltimore, MD), SMI35 (1:10,000, Sternberger Monoclonals), CD45 (1:2000; Serotec, Kidlington, UK), CD4 (1:1000 of ascites fluid, YTS16923,24), or F4/80 (1:50, Serotec). Sections were fixed in methanol (−20°C, 5 minutes) and stained using a three-step peroxidase method as previously described.25 Sections stained for CD45 or F4/80 were counterstained with Mayers hematoxylin (VWR, Leicestershire, UK). Omission of primary antibody, secondary antibody, or avidin-biotin complex was routinely used as controls. For semiquantitative analysis of demyelination, sections stained with anti-MBP antibody were examined using a Quantimet 500 image analyzer (Leica Microsystems Imaging Solutions Ltd., Cambridge, UK) attached to a Zeiss microscope (Hertfordshire, UK). Four random fields per section were studied with three to four sections per group. The results were expressed as the amount of light transmittance through a stained portion of section normalized to a nonstained area of the slide. Axonal pathology was assessed by counting the number of SMI32-positive axons in randomly selected areas of spinal cord white matter from control and EAE mice at ×400 magnification. Four areas were counted per section with three sections per group by two observers.
Double-immunocytochemical staining was performed with a mixture of antibodies against nonphosphorylated neurofilament (SMI32, 1:2000; Sternberger Monoclonals) and fibrinogen (1:5000, Sigma) using immunofluorescence. The staining was visualized using avidin fluorescein isothiocyanate DCS (1:200, Vector) and rabbit anti-goat IgG tetramethylrhodamine isothiocyanate (1:20, Sigma) in sodium bicarbonate buffer (100 mmol/L NaH2CO3, pH 8.5). Sections were mounted in Citifluor anti-fade reagent (University of Kent, UK) and examined by fluorescent microscopy. Single-immunofluorescence staining with each primary antibody was performed as a control.
Histopathological Evaluation
The total number of perivascular cuffs was counted in a spinal cord longitudinal section area of 4 cm2, and each cuff was given a score according to the degree of cellular infiltration; 1, perivascular inflammation three or fewer cells deep; 2, more than three cells deep; 3, parenchymal infiltrate. The average cuff count and score was taken from a total of three slides from four different mice per time point.
Protein Extraction
Snap-frozen samples of spinal cord from EAE and control mice were weighed, finely cut, and resuspended at 1:10 g wet weight/ml in 100 mmol/L Tris-HCl buffer, pH 7.4, containing 5 mmol/L ethylenediamine tetraacetic acid, 150 mmol/L NaCl, and 1% Triton X-100. Samples were homogenized by sonication and incubated on ice for 30 minutes. The tissue suspensions were spun at 13,000 × g for 60 minutes at 4°C and the supernatants collected and stored at −70°C. Protein concentrations were determined by the Lowry method.
Clot Lysis Assay
A clot lysis assay was performed as previously described.26 Briefly, spinal cord tissue protein extracts were mixed 1:50 with a reaction buffer (50 mmol/L Tris-HCl, pH 7.4) containing 7.3 μmol/L human fibrinogen (Sigma), 0.25 μmol/L human lys-plasminogen (Chromogenix, Milan, Italy), 1.7 mmol/L CaCl2, 0.7 mmol/L MgCl2, and 12.5 mmol/L NaCl. Samples were added in duplicate to 96-well microtitre plates containing 20 μl of human thrombin per well (100 U/ml) and incubated at 37°C. Absorbance was measured at 405 nm after the turbidity had reached a stable level, in 15- to 30-minute intervals throughout 5 hours then at 17 hours and 24 hours for samples from tPA−/− animals to detect fibrinolysis initiated by uPA. The absorbance data were plotted against the reaction time. Human tPA (2 mg/ml, Technoclone, Surrey, UK) was used as a positive control, while omission of sample or plasminogen in the buffer was used as a negative control.
Western Blotting
For Western blot analysis of fibrin(ogen) or plasminogen content, 40 μg of supernatant protein was resolved on a Tris-HCl sodium dodecyl sulfate-polyacrylamide (Tris-HCl SDS-PA) gel (Bio-Rad, Hertforshire, UK) and transferred to an Immobilon-P polyvinylidene difluoride membrane (Millipore, Bedford, UK). The membranes were blocked with 5% dried milk in Tris-buffered saline (T-TBS: 10 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, and 0.1% Tween-20) for 1 hour at room temperature and then incubated with anti-fibrinogen (1:1000, Sigma) or rabbit anti-plasminogen (1:1000; DAKO, Cambridgeshire, UK) antibody for 2 hours at room temperature. After washing in T-TBS, the membrane was incubated with anti-mouse (1:1000; Affinity Bioreagents, Cambridge, UK) or anti-rabbit (1:1000; Amersham Biosciences, Buckinghamshire, UK) IgG antibody conjugated to horseradish peroxidase for 1 hour at room temperature. After three final washes, the blots were developed by enhanced chemiluminescence (Amersham Biosciences). Resulting blots were analyzed using the GelPro analysis system and software (Media Cybernetics, Silver Springs, MD). To ensure equal loading of protein, membranes were stripped with Gelstrip (Chemicon) according to the manufacturer’s instructions and probed with anti-β-actin antibody (1:1000, Sigma).
Enzyme-Linked Immunosorbent Assays (ELISAs) for PAI-1, tPA, and uPA
For ELISA, 96-well plates (Costar) were coated with mouse antibodies against mouse PAI-1, tPA, or uPA, at 4 μg/ml for 48 hours at 4°C.27 The wells were blocked with 1% bovine serum albumin in 1× PBS overnight at 4°C and plates were then washed with 1× PBS Tween 80 (0.004%). Protein extract samples and standards were diluted in 1× PBS containing 0.004% Tween 80, 0.1% bovine serum albumin, and 5 mmol/L ethylenediamine tetraacetic acid, and were added at 180 μl/well and incubated overnight at 4°C. After washing, a biotinylated secondary antibody (PAI-1, tPA, or uPA) was added for 1 hour at 37°C. After addition of avidin-biotin complex (Vector) for 1 hour at room temperature, plates were developed using o-phenylenediamine, and the reaction was stopped using 4 mol/L sulfuric acid. Absorbance was read at 490 nm with a reference reading at 650 nm.
Statistical Analysis
A normality and quality of variance test was performed on all data to determine which test was appropriate. Either a t-test or a Mann-Whitney U-test was used with significance level set at P < 0.05 (Graph Pad Prism; GraphPad Software, San Diego CA). All values are indicated as the mean ± SE (SEM). The parametric Pearson’s correlation test was used for the regression analysis and the r value given where appropriate.
Results
EAE Disease in tPA−/− Mice
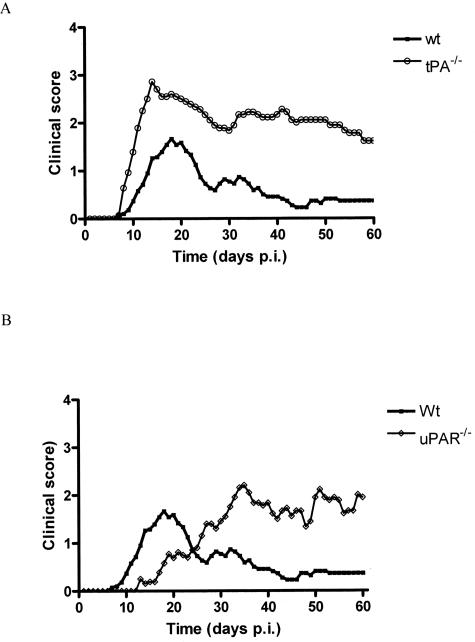
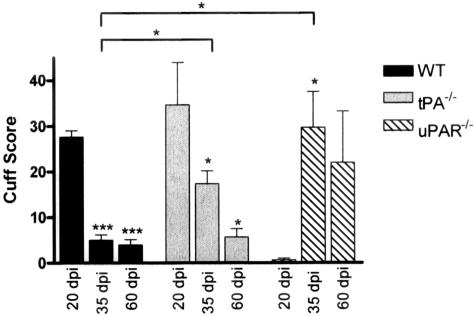
After induction of EAE with MOG 35-55 peptide WT animals exhibited signs of disease at 11.0 ± 0.5 days after disease induction (dpi) (Figure 1A), with peak of disease at 17.6 ± 0.5 dpi. The acute phase of disease lasted for ∼15 days with an average mean maximal score of 2.0 ± 0.2. Animals recovered from the attack with little residual deficit (Figure 1A) and a mean score at 60 dpi of 0.3 ± 0.1. In contrast, tPA−/− mice had a significantly earlier onset of disease, 8.9 ± 0.4 dpi (P < 0.01) with a more severe acute phase lasting ∼22 days reaching a peak significantly earlier at 14.4 ± 0.7 dpi (P < 0.01) than in the WT and with a significantly higher mean maximal score of 3.3 ± 0.2 (P < 0.001). Disease course in tPA−/− animals was characterized by an incomplete recovery (Figure 1A) with signs of residual deficit including hindlimb paresis and occasional tail spasticity. Mean scores at 40 and 60 dpi were 2.2 ± 0.2 and 1.6 ± 0.2, respectively, both significantly higher than WT mice (P < 0.001) at comparable time points. Immunohistochemical staining showed pronounced perivascular cuffing in the spinal cord in both tPA−/− and WT mice (Figure 2, A and B). Comparing F4/80 (Figure 2, A and B), CD45 (Figure 2, J and K), and CD4 (not shown) staining on serial sections of spinal cord showed that both microglia/macrophages and lymphocytes were present in large numbers in these cuffs. A few residual microglia/macrophages were still present in tPA−/− mice at 60 dpi (Figure 2H), but not in the WT animals (Figure 2G). Cuff count and score were significantly higher in tPA−/− mice than WT at 35 dpi, but at 20 and 60 dpi, these were comparable (Figure 3). Movement activity of mice was performed to investigate motor dysfunction and the degree of neurological impairment.28 tPA−/− mice were significantly less active than WT animals at 35 and 60 dpi of EAE and were also less active than the WT and tPA−/− control groups (P < 0.05, data not shown).
Figure 1.
Clinical course of EAE disease in tPA−/− and uPAR−/− mice. WT, tPA−/− (A), and uPAR−/− (B) mice were immunized with 300 μg of MOG 35-55 in complete Freud’s adjuvant on days 0 and 7 and injected with pertussis toxin on days 0 and 2 to induce EAE. All mice were scored for clinical signs of disease on a scale of 1 to 5. Results are plotted as the mean clinical score for all animals in each group; n = 19, WT (three of which failed to develop EAE); n = 14, tPA−/−; and n = 14, uPAR−/−. A: tPA−/− mice incurred a more prolonged and severe disease characterized by an incomplete recovery. B: uPAR−/− mice showed a delay in the onset and peak of disease.
Figure 2.
Infiltration of macrophages/microglia in EAE. Spinal cords were removed at 17 to 20 dpi and at day 35 dpi onwards of EAE and cut longitudinally. Frozen sections were stained with an antibody against F4/80 (A–I), CD45 (J–L), and CD4 (not shown) to assess the infiltration/migration of inflammatory mononuclear cells. In control animals there are no mononuclear cells in the spinal cord tissue (not shown). There is a delay in microglial migration and infiltration of macrophages and lymphocytes in uPAR−/− mice (C) when compared to WT and tPA−/− animals at 20 dpi (A and B). F: However, perivascular cuffs containing mononuclear cells are evident in uPAR−/− mice at the peak of disease, 35 dpi. A high degree of persisting inflammation is evident in uPAR−/− mice at 60 dpi (I) although not in WT animals (G). J–L: CD45 showed very similar patterns of staining to F4/80. Original magnifications: ×100 (A–L); ×400 (insets).
Figure 3.
Perivascular cuff scores for WT, tPA−/−, and uPAR−/− EAE mice. Spinal cords from EAE mice at 17 to 20, 35, and 60 dpi were sectioned longitudinally and stained with an antibody against CD45. Total cuffs were counted in a section area of 4 cm2, and each cuff was given a score according to the degree of infiltration; 1, perivascular inflammation fewer than three or fewer cells deep; 2, more than three cells deep; 3, parenchymal infiltrate. A total of three slides from different mice were counted per time point and the data are shown as the mean ± SEM; *P < 0.05 and ***P < 0.001, significance illustrated versus control unless illustrated by a bar. Cuff counts (not illustrated) showed a similar pattern as the cuff scores.
EAE Disease in uPAR−/− Mice
After induction of EAE, uPAR−/− mice (Figure 1B), developed signs of disease significantly later than WT mice, on 17.4 ± 1.1 dpi (P < 0.001), with peak disease occurring at 34.4 ± 3.3 dpi (P < 0.001). Histological staining of spinal cord sections taken at 20 dpi (Figure 2C) showed very few infiltrating mononuclear cells in the cords of uPAR−/− mice. However by 35 dpi a number of perivascular cuffs were identifiable with microglia/macrophages (Figure 2, F and L) and CD4-positive lymphocytes (not shown) present in the inflammatory infiltrates. Cuff count and score in uPAR−/− mice at 35 dpi were comparable with those at the peak of acute disease in WT and tPA−/− mice (20 dpi, Figure 3). Persistent inflammation in uPAR−/− mice at 60 dpi was reflected in high numbers of mononuclear cells in perivascular cuffs (Figure 2I), and a higher cuff score in comparison with WT and tPA−/− mice at 60 dpi (Figure 3). Activity of uPAR−/− mice was significantly less than WT at 35 dpi (P < 0.001), but by 60 dpi, although uPAR−/− animals had a higher mean score than WT (P < 0.05) the activity of the two groups was not significantly different (data not shown).
Demyelination and Axonal Pathology in EAE
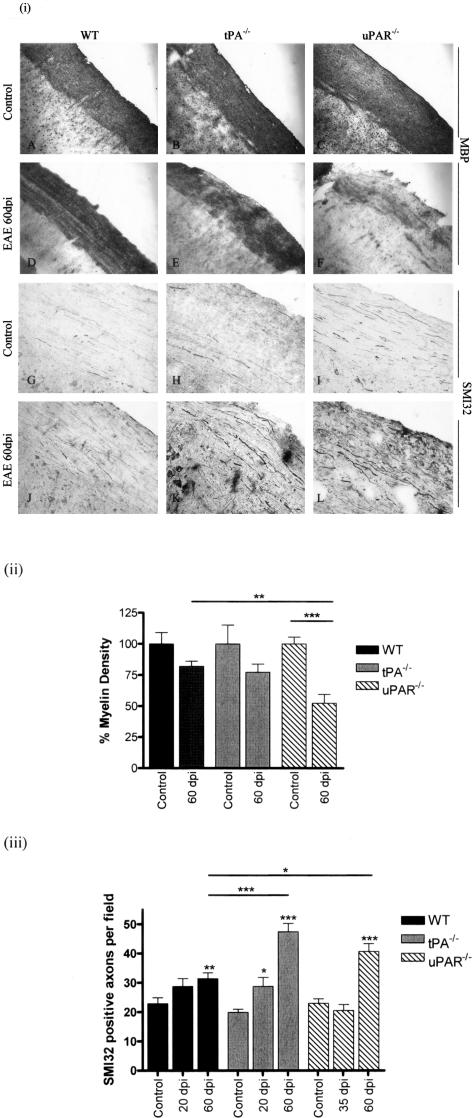
Staining with an antibody against MBP in representative sections of mouse spinal cord at specific time points during EAE development showed that demyelination was more pronounced in tPA−/− and uPAR−/− (Figure 4, E and F) mice than WT animals (Figure 4D). The highest loss of myelin was seen at 60 dpi in uPAR−/− mice. Using densitometry scanning the amount of light transmittance through sections stained with MBP was calculated to gain a semiquantitative measure of myelin density. Myelin density in uPAR−/− animals at 60 dpi was significantly reduced when compared to control animals (Figure 4ii; P < 0.001) and WT mice at the same stage of disease (P < 0.05). Data at other time points of EAE showed no significant differences between genotypes. Staining for nonphosphorylated neurofilament (SMI32) revealed very few immunopositive axons in sections from control mice (Figure 4; G to I), but large numbers of thickened SMI32-positive axons in sections from EAE mice, particularly at 60 dpi (Figure 4, J to L). These axons were counted to obtain a quantitative measure of axonal pathological changes (Figure 4iii).29 A statistically significant increase in SMI32-positive axons was found in tPA−/− mice at 20 dpi when compared to tPA−/− controls (P < 0.05). Furthermore, significant increases in SMI32-immunopositive axons were observed in all genotypes at 60 dpi when compared to relevant controls. tPA−/− and uPAR−/− mice had higher numbers of SMI32-positive axons when compared to WT mice at 60 dpi (P < 0.001 and P < 0.01, respectively). Staining for phosphorylated neurofilament (SMI35) revealed no apparent differences between different genotypes and time points (data not shown).
Figure 4.
Demyelination and axonal pathology in EAE. i: Longitudinal frozen spinal cord sections from mice at 60 dpi were stained for MBP and SMI32 to assess the degree of demyelination and changes in axonal pathology in the CNS during EAE. MBP and SMI32 staining in control untreated mice (A–C, G–I) and in EAE at 60 dpi (D–F, J–L). ii: Density of MBP staining was assessed using a Quantimet image analysis system. Density of MBP staining in uPAR−/− mice at 60 dpi was significantly less than controls and WT animals at the same stage of disease. iii: The number of SMI32-positive axons was increased in all genotypes at 60 dpi, significantly more so in tPA−/− and uPAR−/− mice. Staining for SMI35 revealed no apparent difference between different genotypes and time points (not shown). *P < 0.05, **P < 0.01, ***P < 0.001. Original magnifications, ×100.
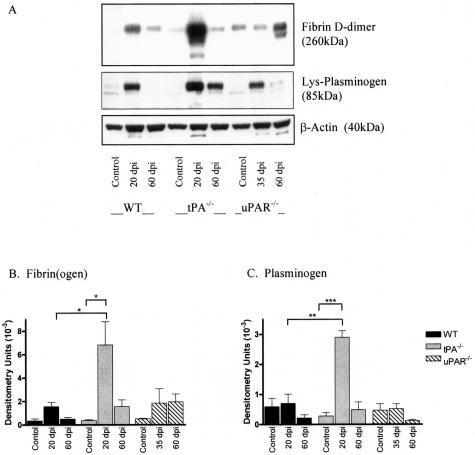
Fibrin(ogen) and Plasminogen Are Significantly Increased in tPA−/− Mice during Acute EAE
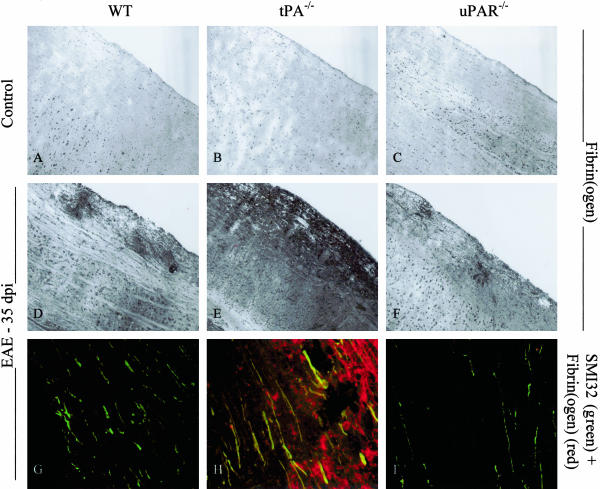
Using an antibody specific for fibrin(ogen) and fibrin degradation product D-dimer substantial extravasation and deposition of fibrin(ogen) was found surrounding perivascular cuffs in sections from EAE animals (Figure 5; D to F). In WT and uPAR−/− mice fibrin deposits were confined to areas of inflammatory infiltration, whereas in sections from tPA−/− mice, diffuse deposition was observed throughout the spinal cord parenchyma (Figure 5E). Western blotting on spinal cord protein extracts from control and EAE mice showed that levels of fibrin D-dimer, a large molecular weight fibrin degradation product, and plasminogen change throughout the course of EAE with a significant increase during acute EAE (Figure 6A). Highest accumulation of fibrin D-dimer was observed in tPA−/− mice when compared to tPA−/− controls and WT animals at the same stage of disease development (Figure 6B, P < 0.05). Plasminogen migrated as an 85-kd band that corresponds to cell-bound preactivation form of plasminogen (lysine-plasminogen). Similarly to D-dimer, the highest amount of plasminogen was measured in tPA−/− mice at the peak of acute EAE (20 dpi, P < 0.001; Figure 6C). Double-immunofluorescence staining with SMI32 and anti-fibrin(ogen) revealed a co-localization of fibrin deposits on thickened axons in spinal cord sections from tPA−/− mice during EAE (35 dpi; Figure 5H).
Figure 5.
Fibrin(ogen) localization in EAE. i: Spinal cords were removed from control animals and mice 35 dpi onwards of EAE and cut longitudinally. Frozen sections were stained with an antibody against fibrin(ogen), and double-fluorescence staining was performed with antibodies against fibrin(ogen) and SMI32, a marker of nonphosphorylated neurofilament. Fibrin(ogen) can be seen surrounding perivascular cuffs in sections from EAE animals (D–F), but in much greater amounts in tPA−/− mice. Co-localization of fibrin(ogen) on SMI32-positive axons is seen in sections from tPA−/− EAE mice (H) but not in sections from WT or uPAR−/− animals (G or I). Original magnifications: ×100 (A–F); ×400 (G–I).
Figure 6.
Western blotting of fibrin(ogen) and plasminogen. Spinal cords from control and EAE mice were homogenized for protein extraction. Levels of fibrin(ogen) and plasminogen were detected by Western blotting and were quantitatively measured by densitometry scanning (A) and results are shown as arbitrary densitometry units ± SEM. Blots were reprobed with anti-actin to ensure equal loading of proteins. Levels of fibrin(ogen) (B) and plasminogen (C) were significantly increased during the acute phase of EAE in tPA−/− mice when compared to control mice and WT mice at the same stage of disease. *P < 0.05, **P < 0.01, ***P < 0.001.
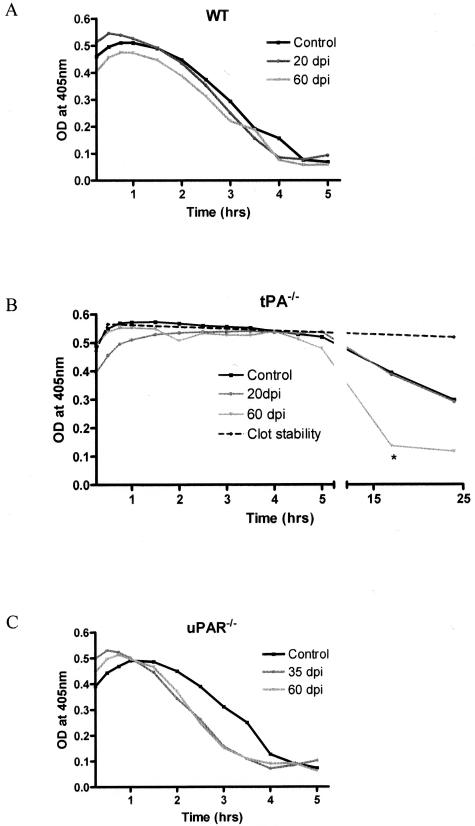
Fibrinolysis in WT, tPA−/−, and uPAR−/− Mice during EAE
Because fibrin deposition is a known feature of MS,30 it was important to determine how the lack of tPA or uPAR would affect fibrin degradation in spinal cords from control and EAE animals. A clot lysis assay was used for spinal cord protein extracts to examine fibrinolytic capacity in control and knockout mice at specific time points during EAE. Throughout 5 hours the fibrin clot degradation (measured as a decrease in OD at 405 nm) was comparable in WT and uPAR−/− mice at all time points during EAE (Figure 7, A and C). In contrast there was no clot degradation in samples from tPA−/− animals during the first 5 hours of incubation (Figure 7B). To establish whether other enzymes such as uPA can compensate for the lack of tPA, samples from tPA−/− mice were incubated for a further 17 hours or 24 hours. A significantly higher clot degradation (75.4% ± 10.0) was found in samples taken at 60 dpi in comparison to tPA−/− controls or samples taken at 20 dpi (31.38 ± 11.69% and 28.9 ± 13.0%) (P < 0.05; Figure 7B). Addition of tPA to sample buffer resulted in rapid clot degradation within the first hour of incubation, whereas omission of plasminogen in the sample buffer inhibited clot lysis.
Figure 7.
Fibrinolysis in the mouse CNS. Spinal cords from control and EAE mice at specific time points were homogenized for protein extraction. The fibrinolytic capacity was investigated using a clot lysis assay that measures the degradation of an in vitro-formed clot using spectrophotometry. Results are presented as the mean clot degradation over time for WT (A), tPA−/− (B), and uPAR−/− (C) mice. Samples from tPA−/− mice were incubated for a further 20 hours to detect fibrinolysis initiated by uPA. tPA−/− mice at 60 dpi had a significantly faster clot degradation than either control or 20 dpi mice. *P < 0.05.
Increased PAI-1 and uPA Levels during EAE
Levels of tPA and PAI-1 are increased in the cerebrospinal fluid of MS patients;31,32 PAI-1 antigen levels are also increased in MS brain tissue, whereas conflicting results are documented for tPA.7,33,34 To investigate how the levels of tPA, uPA, and PAI-1 change during the course of EAE disease, ELISAs were performed on spinal cord protein extracts. Levels of tPA did not change throughout the course of EAE disease in WT and uPAR−/− mice (data not shown). Levels of PAI-1 showed marked fluctuations and were significantly higher (P < 0.05) at the peak of disease (17 to 20 dpi for tPA−/− and WT mice and 35 dpi for uPAR−/− animals) in all three genotypes (Figure 8; A to C). This was especially pronounced in tPA−/− mice in which the PAI-1 levels were 10-fold higher in comparison with PAI levels from uPAR−/− and WT mice at 20 dpi and almost 100-fold higher than the appropriate control (Figure 8B). Statistical analysis revealed a strong positive correlation of PAI-1 with fibrin D-dimer (r = 0.836, P < 0.001), plasminogen levels (r = 0.884, P < 0.001), and clinical score (r = 0.452, P < 0.01). An accumulation of uPA was observed during the acute (20 dpi) and particularly chronic phase of EAE (60 dpi) in tPA−/− and uPAR−/− animals, which was not seen in WT mice (Figure 8; D to F). In situ, uPA was immunolocalized to inflammatory cells in perivascular cuffs (data not shown).
Figure 8.
Levels of PAI-1 and uPA during EAE. Levels of PAI-1 and uPA were determined in spinal cord homogenate samples from control and EAE mice by a modified sandwich ELISA. Results are shown as ng antigen/mg protein ± SEM. A–C: PAI-1 is significantly increased in WT, tPA−/−, and uPAR−/− mice during the acute/peak phase of EAE. D–F: In addition levels of uPA were significantly increased during the chronic phase of EAE in tPA−/− and uPAR−/− mice (E and F), which was not mirrored in WT animals (D). *P < 0.05 versus control.
Discussion
The data reported in this study highlight the important roles of tPA and uPAR in the pathogenesis of EAE by regulating fibrin deposition at sites of inflammation and cell trafficking into the CNS. A more severe and prolonged disease course was observed in tPA−/− mice characterized by an incomplete recovery and increased neurological deficit when compared to WT mice. Fibrin(ogen), which enters the CNS during the acute phase of disease and accumulates over time, was co-localized with nonphosphorylated neurofilament on compromised axons in the spinal cords of tPA−/− mice. In contrast, delayed onset and chronicity were major characteristics of EAE in uPAR−/− mice, coupled to persisting inflammatory cuffs of mononuclear cells, increased levels of uPA, and a greater degree of demyelination.
The effect of tPA deficiency on fibrinolysis in EAE was a significant impairment of in vitro clot degradation confirming tPA as the key fibrinolytic enzyme in the mouse CNS. We observed similar findings to Lu and colleagues;35 with slight differences in the time of onset of disease, which could be accounted for by strain and interbreeding variations. The neurological signs in tPA−/− mice are reflected by decreased motor activity, which was found in previous studies to be closely correlated with axonal damage.28 High levels of fibrin(ogen) deposited on vulnerable axons could represent a pathological mechanism responsible for the significantly increased neurofilament dephosphorylation and persistent neurological deficit. Fibrin deposits frequently accompany inflammatory responses,36 and fibrin(ogen) has the ability to exacerbate inflammation, acting through signaling molecules to modulate cell adhesion and migration,37,38 and increased expression of cytokines.39,40 Genetic or pharmacological depletion of fibrin(ogen) in a tumor necrosis factor transgenic mouse model of MS increased the life span of the animals as a result of decreased inflammation and a delay in demyelination.41 Furthermore, because tPA has major roles in synaptic remodeling and plasticity in the brain;6 our results could also reflect the lesser ability of tPA−/− mice to remodel synaptic connections after neuronal/axonal damage.
The expression of PAI-1, the main inhibitor of tPA and uPA, is up-regulated by proinflammatory cytokines, such as interleukin-1 and tumor necrosis factor-α.42,43 The levels of PAI-1 in EAE spinal cord correlated strongly with clinical score, the highest levels at the peak of disease in all three genotypes. The ∼10-fold higher increase in PAI-1 in tPA−/−, compared to WT mice, during the acute phase of EAE, was sufficient to completely inhibit fibrinolysis initiated by uPA, as reflected by the exceptionally high levels of fibrin(ogen) at this stage of disease. In addition, increased amounts of plasminogen in the spinal cord tPA−/− mice at the peak of disease reflect its accumulation resulting from decreased activation of the zymogen into its active form plasmin. However as levels returned to normal, during the chronic stage of disease, increased uPA in tPA−/− spinal cord could account for higher turnover of plasminogen and the fibrinolysis. A higher degree of clot degradation was observed in tPA−/− mice at 60 dpi after 17 hours of incubation suggesting that in the chronic phase of disease there was a partial compensation by uPA, a slower acting enzyme, which initiated fibrinolysis once levels of PAI-1 had decreased.
The delay in disease development in uPAR−/− mice and in cell infiltration into the spinal cord reflects a reduced adhesion and migration of inflammatory mononuclear cells into the CNS coordinated by uPAR through its interactions with integrins and vitronectin.13,44 The importance of uPAR has been documented in other in vivo models of infection or inflammation in uPAR−/− mice.45–48 However the cuff score and inflammatory histopathology in the CNS of uPAR−/− mice by 35 dpi, at the peak of disease, was comparable to that of WT and tPA−/− animals at 20 dpi, indicating that cells are migrating through the blood-brain barrier, by other mechanisms, including the production of matrix metalloproteinases (MMPs) and up-regulation of adhesion molecules.49
Persisting inflammation in the spinal cord of uPAR−/− mice, in addition to pronounced axonal impairment and demyelination with significant reduction in MBP, may account for chronicity of disease. Furthermore uPA, a stress-responsive proteinase,42 up-regulated in infiltrating inflammatory cells,44 was significantly increased in the CNS in uPAR−/− mice at 60 dpi compared to WT and tPA−/− animals. The accumulation of uPA could reflect the absence of a clearance mechanism in uPAR−/− animals as endocytosis of uPA by uPAR, low-density lipoprotein receptor-related protein and PAI-1 is a mechanism by which uPA is inactivated, cleared from the cell surface and degraded intracellularly.50 Furthermore, uPA may act independently of uPAR because uPA-catalyzed plasminogen activation in pericellular proteolysis is stimulated by cells in vitro that do not express uPAR.51 Under normal conditions uPA is expressed at low levels and kept in balance by PAI-1. In uPAR−/− mice however, uPA is present at much higher concentrations than PAI-1 at 60 dpi and levels of plasminogen are decreased at the same time point. Thus uncontrolled uPA-generated plasmin proteolysis could lead to myelin and neuronal damage in these animals.44 Plasmin and uPA are also able to cleave laminin52 and fibronectin,53 respectively, and possibly other axon-supporting extracellular matrix components. In addition, uPA-generated plasmin can activate MMPs54 that are known to have detrimental effects under neuroinflammatory conditions,55 particularly MMP-9 that can break down myelin.
In conclusion, both tPA and uPAR are implicated in the inflammation, demyelination, and neurodegeneration characteristic of EAE56,57 modulating clinical progression. Investigation into the role of tPA and uPAR during neuroinflammation has revealed potential sites for therapeutic intervention such as the inhibition of uPAR-mediated cell migration and targeting PAI-1 to promote fibrinolysis.
Acknowledgments
We thank L. Frederix and B. Van Hoef, Katholieke Universiteit Leuven, for skillful technical assistance.
Footnotes
Address reprint requests to Emma East, Department of Neuroinflammation, Institute of Neurology, University College London, 1 Wakefield St., London, WC1N 1PJ, UK. E-mail: e.east@ion.ucl.ac.uk.
Supported by Katholieke Universiteit Leuven (grant OT/03/48 to H.R.L.), AIMS2CURE, and the Multiple Sclerosis Society of Great Britain and Northern Ireland.
References
- Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- Teesalu T, Hinkkanen AE, Vaheri A. Coordinated induction of extracellular proteolysis systems during experimental autoimmune encephalomyelitis in mice. Am J Pathol. 2001;159:2227–2237. doi: 10.1016/S0002-9440(10)63073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzner ML, Gveric D, Strand C, Loughlin AJ, Paemen L, Opdenakker G, Newcombe J. The expression of tissue-type plasminogen activator, matrix metalloproteases and endogenous inhibitors in the central nervous system in multiple sclerosis: comparison of stages in lesion evolution. J Neuropathol Exp Neurol. 1996;55:1194–1204. doi: 10.1097/00005072-199612000-00002. [DOI] [PubMed] [Google Scholar]
- Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DP, Moseley IF, Rudge P, McDonald WI. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenic and clinical implications. Brain. 1990;113:1477–1489. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- Wakefield AJ, More LJ, Difford J, McLaughlin JE. Immunohistochemical study of vascular injury in acute multiple sclerosis. J Clin Pathol. 1994;47:129–133. doi: 10.1136/jcp.47.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds NW, Friedman G, Hayden S, Thewke D, Haffke S, McGuire P, Krystosek A. Plasminogen activators and their interaction with the extracellular matrix in neural development, plasticity and regeneration. Semin Neurosci. 1996;8:405–412. [Google Scholar]
- Gveric D, Hanemaaijer R, Newcombe J, van Lent NA, Sier CF, Cuzner ML. Plasminogen activators in multiple sclerosis lesions: implications for the inflammatory response and axonal damage. Brain. 2001;124:1978–1988. doi: 10.1093/brain/124.10.1978. [DOI] [PubMed] [Google Scholar]
- Gveric D. Impaired fibrinolysis in multiple sclerosis: a role for tissue plasminogen activator inhibitors. Brain. 2003;126:1590–1598. doi: 10.1093/brain/awg167. [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Kombrinck KW, Degen JL, Strickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol. 2000;149:1157–1166. doi: 10.1083/jcb.149.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Koh CS, Shimada K, Yanagisawa N, Yoshimura K. Suppression of cell-transferred experimental autoimmune encephalomyelitis in defibrinated Lewis rats. J Neuroimmunol. 1996;71:131–137. doi: 10.1016/s0165-5728(96)00150-6. [DOI] [PubMed] [Google Scholar]
- Paterson PY. Experimental allergic encephalomyelitis: a role of fibrin deposition in immunopathogenesis of inflammation in rats. Fed Proc. 1976;35:2428–2435. [PubMed] [Google Scholar]
- Garcia-Monco JC, Coleman JP, Benach JP. Soluble urokinase receptor (uPAR,CD87) is present in serum and cerebrospinal fluid in patients with neurological diseases. J Neuroimmunol. 2002;129:216–223. doi: 10.1016/s0165-5728(02)00186-8. [DOI] [PubMed] [Google Scholar]
- Chapman HA. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol. 1997;9:714–724. doi: 10.1016/s0955-0674(97)80126-3. [DOI] [PubMed] [Google Scholar]
- Tarui T, Mazar AP, Cines DB, Takada Y. Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J Biol Chem. 2001;276:3983–3990. doi: 10.1074/jbc.M008220200. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Lisak D, Beaumont T, Lisak RP, Dore-Duffy P. Expression of urokinase plasminogen activator receptor on monocytes from patients with relapsing-remitting multiple sclerosis: effect of glatiramer acetate (copolymer 1). Clin Diag Lab Immunol. 2001;8:1196–1203. doi: 10.1128/CDLI.8.6.1196-1203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington RA, Becher B, Balabanov R, Antel J, Dore-Duffy P. Expression of the activation marker urokinase plasminogen activator receptor in cultured human central nervous system microglia. J Neurosci Res. 1996;45:392–399. doi: 10.1002/(SICI)1097-4547(19960815)45:4<392::AID-JNR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Schluesener HJ, Nguyen TD, Magdolen V, Luther T, Pedel I, Mattern R, Meyermann R, Schwab JM. Lesion-associated accumulation of uPAR/CD87-expressing infiltrating granulocytes, activated microglial cells/macrophages and upregulation by endothelial cells following TBI and FCI in humans. Neuropathol Appl Neurobiol. 2000;26:522–527. doi: 10.1046/j.0305-1846.2000.287.x. [DOI] [PubMed] [Google Scholar]
- Dewerchin M, Van Nuffelen A, Wallays G, Bouché A, Moons L, Carmeliet P. Generation and characterisation of urokinase receptor-deficient mice. J Clin Invest. 1996;97:870–878. doi: 10.1172/JCI118489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord JJ, Collen D, Mulligan RC. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Bouché A, De Clercq C, Janssen S, Pollefeyt S, Wyns S, Mulligan RC, Collen D. Biological effects of disruption of the tissue-type plasminogen activator, urokinase-type plasminogen activator, and plasminogen activator inhibitor-1 genes in mice. Ann NY Acad Sci. 1995;17:367–381. doi: 10.1111/j.1749-6632.1994.tb17333.x. [DOI] [PubMed] [Google Scholar]
- Bernard CC, Johns TG, Slavin AJ, Ichikawa M, Ewing C, Liu J, Bettadapura J. Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis. J Mol Med. 1997;75:77–88. doi: 10.1007/s001090050092. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Okuda M, Bernard CC. Gender does not influence the susceptibility of C57BL/6 mice to develop chronic experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein. Immunol Lett. 2002;81:25–29. doi: 10.1016/s0165-2478(01)00339-x. [DOI] [PubMed] [Google Scholar]
- Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- O’Neill JK, Baker D, Davison AN, Allen SJ, Butter C, Waldmann H, Turk JL. Control of immune-mediated disease of the central nervous system with monoclonal (CD4-specific) antibodies. J Neuroimmunol. 1993;45:1–14. doi: 10.1016/0165-5728(93)90157-t. [DOI] [PubMed] [Google Scholar]
- Gveric D, Cuzner ML, Newcombe J. Insulin-like growth factors and binding proteins in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1999;25:215–225. doi: 10.1046/j.1365-2990.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- Urano T, Takada Y, Ihara H, Takada A. Re-examination of the extent of the activation of Lys(78) plasminogen by tissue plasminogen activator in the presence of polymerised fibrin. Haemostasis. 1996;26:220–227. doi: 10.1159/000217211. [DOI] [PubMed] [Google Scholar]
- Declerck PJ, Verstreken M, Collen D. Immunoassay of murine t-PA, u-PA and PAI-1 using monoclonal antibodies raised in gene-inactivated mice. Thromb Haemost. 1995;74:1305–1309. [PubMed] [Google Scholar]
- Pryce G, Ahmed Z, Hankey DJ, Jackson SJ, Croxford JL, Pocock JM, Ledent C, Petzold A, Thompson AJ, Giovannoni G, Cuzner ML, Baker D. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain. 2003;126:2191–2202. doi: 10.1093/brain/awg224. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Claudio L, Raine CS, Brosnan CF. Evidence of persistent blood-brain barrier abnormalities in chronic progressive multiple sclerosis. Acta Neuropathol. 1995;30:228–238. doi: 10.1007/BF00296505. [DOI] [PubMed] [Google Scholar]
- Akenami FO, Siren V, Koskiniemi M, Siimes MA, Teravainen H, Vaheri A. Cerebrospinal fluid activity of tissue plasminogen activator in patients with neurological diseases. J Clin Pathol. 1996;49:577–580. doi: 10.1136/jcp.49.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akenami FO, Koskiniemi M, Farkkila M, Vaheri A. Cerebrospinal fluid plasminogen activator inhibitor-1 in patients with neurological disease. J Clin Pathol. 1997;50:157–160. doi: 10.1136/jcp.50.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akenami FO, Siren V, Wessman M, Koskiniemi M, Vaheri A. Tissue plasminogen activator gene expression in multiple sclerosis brain tissue. J Neurol Sci. 1999;165:71–76. doi: 10.1016/s0022-510x(99)00080-5. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Gveric D, Pryce G, Baker D, Leonard JP, Cuzner ML, Diemel LT. Myelin/axonal pathology in interleukin-12 induced serial relapses of experimental allergic encephalomyelitis in the Lewis rat. Am J Pathol. 2001;158:2127–2138. doi: 10.1016/s0002-9440(10)64684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Bhasin M, Tsirka SE. Involvement of tissue plasminogen activator in onset and effector phases of experimental allergic encephalomyelitis. J Neurosci. 2002;22:10781–10789. doi: 10.1523/JNEUROSCI.22-24-10781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K, Strickland S. Nervous system pathology: the fibrin perspective. Biol Chem. 2002;383:37–45. doi: 10.1515/BC.2002.004. [DOI] [PubMed] [Google Scholar]
- Flick MJ, Du X, Witte DP, Jiroušková M, Soloviev DA, Busuttil SJ, Plow EF, Degen J. Leukocyte engagement of fibrin(ogen) via the integrin receptor aMb2/Mac-1 is critical for host inflammatory response in vitro. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by intracellular adhesion molecule 1-fibrinogen recognition. Proc Natl Acad Sci USA. 1995;92:1505–1509. doi: 10.1073/pnas.92.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RL, Ritzenhaler JD, Roman J. Transcriptional regulation of the interleukin-1b promoter via fibrinogen engagement of the CD18 intergin receptor. Am J Respir Cell Mol Biol. 1999;20:1059–1066. doi: 10.1165/ajrcmb.20.5.3281. [DOI] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, Strickland S. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumour necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci USA. 2004;101:6698–6703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Kasza A, Kowanetz M, Poslednik K, Witek B, Kordula T, Koj A. Epidermal growth factor and pro-inflammatory cytokines regulate the expression of components of the plasminogen activator system in U373-MG astrocytoma cells. Cytokine. 2001;16:187–190. doi: 10.1006/cyto.2001.0957. [DOI] [PubMed] [Google Scholar]
- Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- May AE, Kanse SM, Lund LR, Gisler RH, Imhof BA, Preissner KT. Urokinase receptor (CD87) regulates leukocyte recruitment via b2 integrins in vivo. J Exp Med. 1998;188:1029–1037. doi: 10.1084/jem.188.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyetko MR, Sud S, Kendall T, Fuller JA, Newstead MW, Standiford TJ. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary Psuedomonas aeruginosa infection. J Immunol. 2000;165:1513–1519. doi: 10.4049/jimmunol.165.3.1513. [DOI] [PubMed] [Google Scholar]
- Gyetko MR, Sud S, Sonstein J, Polak T, Sud A, Curtis JL. Antigen-driven lymphocyte recruitment to the lung is diminished in the absence of urokinase-type plasminogen activator (uPA) receptor, but is independent of uPA. J Immunol. 2001;167:5539–5542. doi: 10.4049/jimmunol.167.10.5539. [DOI] [PubMed] [Google Scholar]
- Rijneveld AW, Levi M, Florquin S, Speelman P, Carmeliet P, van der Poll T. Urokinase receptor is necessary for adequate host defense against Pneumococcal pneumonia. J Immunol. 2002;168:3507–3511. doi: 10.4049/jimmunol.168.7.3507. [DOI] [PubMed] [Google Scholar]
- Sellebjerg F, Sørensen TL. Chemokines and matrix metalloproteinase-9 in leukoctye recruitment to the central nervous system. Brain Res Bull. 2003;61:347–355. doi: 10.1016/s0361-9230(03)00097-2. [DOI] [PubMed] [Google Scholar]
- Irigoyen JP, Muñoz-Cánoves P, Montero L, Koziczak M, Nagamine Y. The plasminogen activator system: biology and regulation. Cell Mol Life Sci. 1999;56:104–132. doi: 10.1007/PL00000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstaff C, Merton RE, Fabregas P, Felez J. Characterization of cell-associated plasminogen activation catalyzed by urokine-type plasminogen activator, but independent of urokinase receptor (uPAR, CD87). Hemost Thromb Vasc Biol. 1999;93:3839–3846. [PubMed] [Google Scholar]
- Chen Z, Strickland S. Neuronal death in the hippocampus is promoted by plasmin catalysed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Gold LI, Rostagno A, Frangione B, Passalaris T. Localization of the cleavage sites on fibronectin following digestion by urokinase. J Cell Biol. 1992;50:441–452. doi: 10.1002/jcb.240500412. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Lijnen HR, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17:439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- Cuzner ML, Opdenakker G. Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–171. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Neumann H. Molecular mechanisms of axonal damage in inflammatory central nervous system diseases. Curr Opin Neurol. 2003;16:267–273. doi: 10.1097/01.wco.0000073926.19076.29. [DOI] [PubMed] [Google Scholar]