Abstract
Pterygia are inflammatory, invasive, and proliferative lesions of the human ocular surface in which the matrix metalloproteinase (MMP) collagenase-1 (MMP-1) is highly expressed. Pterygia development may involve MMP-1 activity against interstitial fibrillar collagen, an abundant extracellular matrix component of the cornea, and its induction by ultraviolet light (UVB). We examined the pathways responsible for enhanced expression of MMP-1 in pterygium epithelial cells after UVB exposure and/or treatment with chemical inhibitors of mitogen-activated protein kinases or epidermal growth factor receptor. The induction of MMP-1 by UVB was comparable to that mediated by heparin-binding epidermal growth factor-like growth factor and epidermal growth factor. The epidermal growth factor receptor inhibitor PD153035 partially blocked the UVB-mediated induction of MMP-1 and totally abrogated its production after stimulation with either heparin-binding epidermal growth factor-like growth factor or epidermal growth factor. UVB exposure enhanced the phosphorylated form of ERK1/2 in a time-dependent manner whereas the ERK1/2 inhibitor PD98059 decreased this induction by at least fivefold. Transcripts for c-jun and c-fos were detected as early as 2 hours after UVB exposure and were suppressed by PD98059. The identification of a specific intracellular signaling pathway responsible for the enhanced production of a key enzyme that denatures intact fibrillar collagen has important implications for understanding the pathophysiology and future therapy for pterygia.
Pterygium is a condition of the ocular surface characterized by squamous cell metaplasia and goblet cell hyperplasia. The lesion consists of a wing-shaped mass of fibrovascular conjunctival tissue that invades the normal cornea. Other obvious pathological changes include activation of stromal fibroblasts, a persistent inflammatory component, elastotic degeneration, and destruction of collagenous barriers such as Bowman’s layer. Pterygia are particularly prevalent in heavily sun-exposed individuals in whom extensive epidemiological studies link this disease to excessive ultraviolet (UV) radiation.1–5 Despite this evidence, there is still controversy regarding the actual trigger for the development of pterygia and whether or not there is a genetic predisposition to this disease.6 Recently, we have identified UVB as an environmental agent likely to be responsible for initiating molecular events that lead to the formation of pterygia.7,8 From our hypothesis,9 we postulate that UVB radiation activates cells at or near the limbus. This activation may cause 1) phenotypic alterations in a distinct population of epithelial cells, 2) production of proinflammatory and angiogenic cytokines7 and growth factors,8 and 3) increased invasiveness due to enhanced production of matrix metalloproteinases (MMPs) over and above their natural tissue inhibitors (TIMPs).6,9–12
To date, we6,9–12 and others13–17 have accumulated data from in vitro culture and in vivo tissue-based studies that resemble investigations performed in human UV-exposed skin. These studies have demonstrated increased MMPs in human skin and skin-derived cells in response to UV radiation.18–21 Furthermore, photodamaged skin displays several histological features in common with pterygia, such as regions lacking intact collagen fibrils, the presence of a disrupted matrix, and large deposits of amorphous material composed of cellular debris and elastotic matrix.22
The extensive expression of MMP-1 in cultured pterygium-derived cells and pterygium tissue has been documented by several independent investigators.9–11,13,14 Interestingly, we have also noted diminished expression of MMP-1 in quiescent normal conjunctival tissue specimens.9,10 The importance of MMP-1 should not be underestimated because it can specifically denature the collagen triple helix at a specific locus, it is required for keratinocyte migration on a type I collagen matrix,23 it can promote endothelial cell migration during angiogenesis,24 and its overexpression can result in epidermal hyperplasia, and enhance cell proliferation by activating insulin-like growth factor.25 Thus determining the role and identifying the signals that regulate the expression of MMP-1 in pterygia may be critical for understanding how this disease develops in humans.
In a previous investigation we identified the extracellular signal-regulated kinase (ERK1/2) mitogen-activated protein kinase (MAPK) as the intracellular signal transduction pathway activated by UVB that was responsible for induction of MMP-1 in cultured pterygium epithelial cell (PECs). Blocking this pathway with the chemical inhibitor PD98059 resulted in a significant decrease in MMP-1 production, whereas no inhibition was observed with an inhibitor of c-Jun N-terminal kinase (JNK) and p38 (SB202190).9 In the context of pterygium development, these findings are relevant because activation of the ERK pathway can promote cell survival and proliferation, whereas activation of JNK and p38 signaling pathways can result in apop-tosis.26 Furthermore, the induction of MMP-1 by UVB,27,28 UVA,29 and possibly infrared-A radiation30 is dependent on AP-1 (c-jun/c-fos) transcriptional activity that is downstream of ERK.
An important aim of this study was to trace the intracellular signal cascade and to identify the sensor(s) for the UVB stimulus. One of the earliest events in response to UV radiation involves ligand-independent autophosphorylation of cell surface growth factor receptors such as the keratinocyte growth factor receptor (KGFR)31 and the epidermal growth factor receptor (EGFR).32 It is highly likely that stimulation of such receptors by UV contributes to the activation of MAPK pathways. Furthermore, targeting the EGFR in our model is relevant because this receptor33,34 and at least one of its ligands heparin-binding epidermal growth factor-like growth factor (HB-EGF)8,35 has been identified in pterygia. Using a pterygium-derived epithelial cell culture model, we demonstrated the partial involvement of the EGFR in transmitting an extracellular UVB signal that resulted in the activation of an intracellular signal transduction pathway. Interestingly, the same molecules and pathways were not activated by UVB in normal conjunctival or limbal8 epithelial cells and this observation is currently under investigation in our laboratory.
Materials and Methods
Surgical Tissue Specimens
Resected pterygia (n = 8) and normal conjunctiva (n = 8) were obtained from the Prince of Wales Hospital, Sydney, Australia. Autologous tissue (n = 3) that remained after grafting was also used in this study and included small segments of conjunctiva. All tissue specimens were formalin-fixed and paraffin-embedded immediately after surgery. Informed consent was obtained from each subject. All research protocols were approved by the University of New South Wales Human Ethics Committee and performed in accordance with the tenets of the World Medical Associations Declaration of Helsinki.
Immunohistochemical Analysis
Serial sections (4 μm) of pterygia, normal conjunctiva, limbus, and cornea were processed for immunohistochemistry as previously described.6–12 In brief, tissue sections were deparaffinized, hydrated, equilibrated in 0.05 mol/L Tris-buffered saline (TBS), pH 7.6, blocked with the appropriate serum (20% final) diluted in 2% bovine serum albumin/TBS, and incubated at room temperature for 30 minutes. Sections were incubated with primary antibody in 2% bovine serum albumin/TBS at the indicated dilution, temperature, and time (Table 1). A rigorous staining optimization protocol was performed for each antibody. Antibody concentration, time of application (30 minutes to 16 hours), and temperature of incubation (4 to 37°C) was predetermined in preliminary experiments. Antigen retrieval using either microwave treatment or enzymatic digestion was performed but not required for optimal staining. Sections were extensively washed in TBS before the addition of the appropriate biotinylated secondary antibody (Table 1). Sections were rinsed, then incubated with horseradish peroxidase-conjugated streptavidin (DAKO, Carpinteria, CA) for an additional hour at room temperature and the immunoreactivity developed by adding 3-amino-9-ethyl-carbazole (Sigma Aldrich, St. Louis, MO). Some sections were counterstained with hematoxylin. Immunohistochemical controls included either omitting the primary antibody or replacing the primary antibody with a mouse, rabbit, or goat isotype (IgG)-negative control antibody (Table 1). Any difference in the staining intensity between pterygia, conjunctiva, and limbus was comparable because both diseased and normal tissue were analyzed under identical conditions in the same experimental run.
Table 1.
Primary Antibodies Used for Immunohistochemistry and Western Blotting
| Antibody | Source | Catalogue no. | Application | Dilution | Temperature/time |
|---|---|---|---|---|---|
| Primary antibodies | |||||
| p-p44/p42 (RaH) | CST | 9101 | WB, IHC | 1:1000, (1:30) | 4°C/16 hours |
| p44/p42 (RaH) | CST | 9102 | WB, IHC | 1:1000, (1:30) | 4°C/16 hours |
| p-p38 (RaH) | SCB | sc-7975 | WB | 1:500 | 4°C/16 hours |
| MMP-1 (MaH) | ICN | 63-178 | WB | 1:1000 | 22°C/1 hour |
| pEGFR (MaH) | CST | 2236 | IHC | 1:400 | 4°C/16 hours |
| c-fos (RaH) | SCB | sc-52 | IHC | 1:200 | 37°C/1 hour |
| c-jun (RaH) | SCB | sc-1694 | IHC | 1:200 | 37°C/1 hour |
| Sp1 (RaH) | SCB | sc-59 | IHC | 1:200 | 37°C/1 hour |
| EGF (RaH) | SCB | sc-275 | IHC | 1:25 | 4°C/16 hours |
| HB-EGF (GaH) | R&D | AF-259-NA | IHC | 1:60 | 4°C/16 hours |
| Rabbit IgG | SCB | sc-2027 | IHC | 1:25–1:200 | 37°C/16 hours |
| Mouse IgG | Dako | X0931 | IHC | 1:400 | 4°C/16 hours |
| Goat IgG | R&D | AB-108-C | IHC | 1:60 | 4°C/16 hours |
| Secondary antibodies | |||||
| SaRHRP | Dako | P0217 | WB | 1:2000 | 22°C/1 hour |
| RaMHRP | Dako | P0260 | WB | 1:2000 | 22°C/1 hour |
| GaRb | Dako | E0466 | IHC | 1:200 | 22°C/30 minutes |
| GaMb | Dako | E0433 | IHC | 1:200 | 22°C/30 minutes |
| RaGb | Dako | E0466 | IHC | 1:200 | 22°C/30 minutes |
Abbreviations used: CST, Cell Signaling Technology (GeneSearch, Arundel, Australia); GaH, goat anti-human; GaMb, biotin-conjugated goat anti-mouse; GaRb, biotin-conjugated goat anti-rabbit; ICN, ICN Biomedicals (Sydney, Australia); IHC, immunohistochemistry; MaH, mouse anti-human; R&D, R&D Systems (Minneapolis, MN); RaGb, biotin-conjugated rabbit anti-goat; RaH, rabbit anti-human; RaMHRP, horseradish peroxidase-conjugated rabbit anti-mouse; SC, Santa Cruz Biotechnology (Santa Cruz, CA); WB, (Western blotting); SaRHRP, horseradish peroxidase-conjugated swine anti-rabbit.
For the EGFR-based studies, PECs were cultured in chamber slides (Nunc, Roskilde, Denmark),10 fixed, and stained for this receptor. These conditions ensured no physical disruption of the cell-membrane-bound receptor due to enzymatic digestion. For the EGFR ligand studies, PECs were grown (see below) in 100-mm dishes (Corning, Corning, NY), treated under control conditions or irradiated with 20 mJ/cm2 UVB, trypsin-digested, pelleted, paraffin-embedded, sectioned, and stained for epidermal growth factor (EGF) and HB-EGF (Table 1).
Cell Culture
PECs or conjunctival epithelial cells (CECs) were seeded at 1 × 106 cells in 100-mm tissue culture dishes (Corning) or seeded at 1.5 × 105 cells per well in 6-well plates (Nunc) and grown in the presence of 10% fetal bovine serum/Eagle’s minimum essential medium. Media from semiconfluent cells was aspirated, cells were washed three times with sterile phosphate-buffered saline (PBS), and synchronized in G0 or quiescence by withdrawal of serum growth factors for 16 hours as previously described.6–9 This media was replaced with 5 ml of PBS and the monolayer irradiated with 20 mJ/cm2 UVB using TL 20 W/12 RS bulbs (Philips, Sydney, Australia) as previously reported.6–9 This amount of radiation equates to a 3-minute exposure and was an amount that did not affect cell viability or cell morphology. Moreover, it represents the dose that resulted in maximal expression of MMP-1 in PECs and pterygium tissue.9 UVB light intensity was monitored and calibrated before each experiment with the aid of a radiometer (IL1400A; International Light, Newburyport, MA). Cells were then rinsed once with PBS and placed in fresh serum-free medium. In some experiments, cells were treated with chemical MAPK inhibitors. The specific ERK1/2 inhibitor PD98059 (Calbiochem, La Jolla, CA) was used at 25 μmol/L, while the JNK and p38 inhibitor SB203580 (Calbiochem) was added to a final concentration of 10 μmol/L for the specified times. For inhibition of EGFR-mediated signal transduction, quiescent PECs were pretreated with PD153035 (0.1 to 5 μmol/L final, Calbiochem) for 1 hour before and for the specified times after irradiation or stimulated with either recombinant human EGF (Sigma) or HB-EGF (R&D Systems, Minneapolis, MN) to a final concentration of 100 ng/ml. PD153035 is an extremely potent and specific inhibitor of the tyrosine kinase activity of the EGFR and rapidly suppresses autophosphorylation at low nanomolar concentrations. In other experiments, cells were treated with either 100 ng/ml of pertussis toxin (Calbiochem) because this agent is a known desensitizer of G-proteins or with 0.1 to 1.0 μmol/L AG1295 (Calbiochem), a selective inhibitor of the PDGFR kinase activity. Supernatants, cell lysates, and RNA samples were harvested at defined time points after treatment and stored at −70°C.
Enzyme-Linked Immunosorbent Assay for MMP-1
Supernatants derived from control, growth factor-stimulated, or UVB-irradiated cells were analyzed by commercial enzyme-linked immunosorbent assay (Biotrak; Amersham Pharmacia Biotech, Sydney, Australia) for MMP-1,9 precisely as described by the manufacturer. The optical density of each 96-well plate was read at 450 nm using a microplate reader (Spectramax Plus; Molecular Devices, Sunnyvale, CA).
RNA Extraction and Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR)
Total RNA was extracted (RNAgents Total RNA extraction kit; Promega, Sydney, Australia) from control, PD98059-treated, and UVB-exposed PECs at the designated time points. Reverse transcription was performed using the Preamplification System for First-Strand cDNA Synthesis kit (Invitrogen, Groningen, The Netherlands) as previously described.10 Aliquots (1 μl) of cDNA were amplified by PCR after the addition of 100 nmol/L each of the forward and reverse primers (Table 2). A 2-minute hot start at 95°C was performed to denature the double-stranded cDNA, followed by 30 cycles of PCR (each cycle: 95°C, 30 seconds; 55°C, 30 seconds; 72°C, 30 seconds) and the reactions terminated with a 2-minute extension at 72°C. For the amplification of c-jun (315 bp) and c-fos (431 bp) transcripts, it was necessary to set the annealing temperature to 63°C. Cycle number was predetermined so that the products formed fell within the linear portion of the amplification curve. Products were visualized on 1.2% agarose gels, precast with ethidium bromide, and semiquantified with the Gel Doc 2000 and the Quantity One program (Bio-Rad, Sydney, Australia). Products were sequenced (Supamac, Sydney, Australia) to confirm identity. A 100-bp ladder (Invitrogen) was run in adjacent lanes.
Table 2.
Primer Pairs Used for PCR Analysis
| MMP-19,10 | F | 5′-GGT GAT GAA GCA GCC CAG-3′ |
| R | 5′-CAG TAG AAT GGG AGA GTC-3′ | |
| TIMP-19,10 | F | 5′-TGC ACC TGT GTC CCA CCC CAC CCA CAG ACG-3′ |
| R | 5′-GGC TAT CTG GGA CCG CAG GGA CTG CCA GGT-3′ | |
| c-jun36 | F | 5′-GAA ACG ACC TTC TAT GAC GAT GCC CTC AA-3′ |
| R | 5′-GAA CCC CTC CTG CTC ATC TGT CAC GTT CTT-3′ | |
| c-fos37 | F | 5′-CTA CGA GGC GTC ATC CTC CCG-3′ |
| R | 5′-AGC TCC CTC CGG TTG CGG CAT-3′ | |
| GAPDH9,10 | F | 5′-TGA TGA CAT CAA GAA GGT GGT GAA G-3′ |
| R | 5′-TCC TTG GAG GCC ATG TGG GCC AT-3′ |
Previous studies (referenced) have successfully used these primer sets to amplify the respective gene products. GAPDH, glyceraldehyde 3 phosphate dehydrogenase; F, forward primer; R, reverse primer.
Preparation of Total Cell Lysates
Culture medium was aspirated from monolayers and each dish was washed twice with PBS. Cells were lysed by adding 100 μl (per well for a six-well plate) or 500 μl (per 100-mm2 dish) of lysate sample buffer [62.5 mmol/L Tris-HCl (pH 6.8), 2% w/v sodium dodecyl sulfate (SDS), 10% glycerol, 0.01% w/v bromophenol blue, 50 mmol/L dithiothreitol] then scraped, transferred to a 1.5-ml microfuge tube, and left on ice. Lysates were sonicated for 10 to 15 seconds to shear the DNA and reduce sample viscosity, centrifuged at 13,000 rpm at 4°C for 10 minutes to remove cell debris, aliquoted, and stored at −70°C. Before loading, each sample was heated to 95°C for 5 minutes, cooled on ice, then analyzed by Western blotting.
Western Blotting
Western blotting was performed as previously described.6,9,10,12 Conditioned media or total cell lysates from control, UVB-irradiated, growth factor-stimulated, or MAPK inhibitor-treated PECs were used as the antigenic source. Conditioned media [25 μl plus 8 μl of zymography sample buffer (see below)] or total cell lysates [25 μl total volume (see sample preparation above) were electrophoretically separated on SDS-polyacrylamide gel electrophoresis (PAGE) using a 4% stacking and 10% resolving gels for MMP-1 (performed under nondenaturing conditions) or 12% resolving gel for ERK1/2 (performed under reducing conditions). Proteins contained within the conditioned media or total cell lysates were transferred to PolyScreen polyvinylidene difluoride transfer membranes (Perkin-Elmer Life Sciences, Boston, MA) as previously described.6,9,10,12 After transfer, the membranes were blocked in 5% skim milk powder in TBST for 1 hour, washed briefly in TBST, then probed with a predetermined concentration of primary antibody directed against human antigens (Table 1) diluted in 5% bovine serum albumin/TBST. Membranes were extensively washed in TBST (3 × 5 minutes) and incubated with a 1:2000 dilution of the appropriate horseradish peroxidase-conjugated secondary antibody (Table 1) for 1 hour at room temperature. Membranes were again washed (3 × 5 minutes) in TBST, then placed in chemiluminescent reagent for 1 minute (Western Lightning, Perkin Elmer Life Science) and exposed to Hyperfilm MP (Amersham Pharmacia Biotech). All immunoreactive bands were semiquantified with the Gel Doc 2000 and the Quantity One programs (Bio-Rad). A prestained low-molecular weight protein ladder (Bio-Rad) was run in adjacent lanes. Total cell lysates were standardized for protein content (Pierce, Rockford, IL) before immunoblotting. Coomassie Blue-stained (Bio-Rad) SDS-PAGE gels also confirmed equal loading.
Gelatin-Substrate Zymography
Zymography was performed as previously described.9–11 Supernatants (25 μl), standardized for cell number were diluted with 8 μl of nonreducing sample buffer (0.25 mol/L Tris-HCl, pH 6.8, 10% SDS, 4% sucrose, 0.1% bromophenol blue) and loaded without boiling into 10% SDS-PAGE gels containing 1 mg/ml gelatin (Sigma). After electrophoresis, the gels were rinsed twice for 50 minutes each in 2.5% Triton X-100 (Sigma), incubated overnight at 37°C in substrate buffer (50 mmol/L Tris-HCl, pH 7.4, 10 mmol/L CaCl2, and 0.02% NaN3), stained with Coomassie Blue R-250 (Bio-Rad) for 1.5 hours, then destained (3 × 50 minutes) to expose gelatinolytic bands. A low-range molecular weight protein ladder (Bio-Rad) was run in adjacent lanes.
Statistical Analysis
MMP-1 levels in the culture supernatants were expressed as mean ± SD. Differences in the level of MMP-1 between control, cytokine, and UV dose-treated cells were assessed by one-way analysis of variance, followed by the Newman-Keuls test for multiple comparisons of treatment groups with the control group. Comparison between UVB-exposed and mock-irradiated PECs at different time points was made with an unpaired Student’s t-test. A commercial software package (Prism; GraphPad Software, San Diego, CA) was used for all data analysis.
Results
UVB-Mediated Induction of MMP-1 Is Abrogated by PD98059
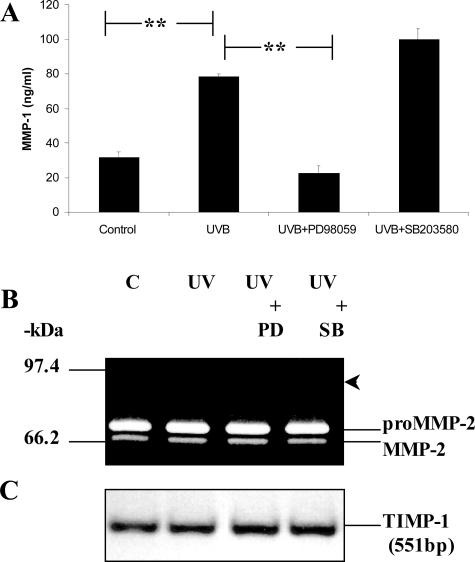
PECs were exposed to 20 mJ/cm2 of UVB, which was the amount of radiation previously delivered to obtain maximum MMP-1 production without any obvious death or change in cell morphology.9 Using this dose, PECs were irradiated and/or treated with chemical inhibitors directed against selective MAPK pathways for 48 hours after UVB exposure. This was regarded as a conservative approach because high levels of MMP-1 could still be detected as late as 72 hours after UVB.9 MMP-1 production significantly increased to 78.3 ng/ml (P < 0.001) after UVB compared to 31.9 ng/ml in mock-irradiated control cells (Figure 1A). This induction was totally abrogated by PD98059 (22.7 ng/ml, P < 0.001) compared to UVB alone but increased moderately in response to SB203580 (99.9 ng/ml, P < 0.01). Despite the enhanced expression of MMP-1, only constitutive MMP-2 activity (Figure 1B) and TIMP-1 transcripts (Figure 1C) were detected after UVB or MAPK inhibitor treatment. Furthermore, MMP-9 protein was not detected after any of the treatments. It is conceivable that the expression of other MMPs was affected by this exposure,6 however in the current study, no additional investigations were initiated to disclose them.
Figure 1.
Inhibition of MMP-1 by PD98059. PECs were cultured under control conditions, irradiated with 20 mJ/cm2 UVB, or treated with either PD98059 (25 μmol/L) or SB203580 (10 μmol/L). Cell supernatants and total RNA were harvested after 48 hours (A and B) or 24 hours (C) and analyzed by enzyme-linked immunosorbent assay (A), gelatin zymography (B), or RT-PCR (C). The arrowhead in B points to the region of gelatinolytic activity expected for latent MMP-9. The bars in A represent the mean ± STD of triplicate experiments. **Significance set at P < 0.001.
UVB Induces ERK1/2 Phosphorylation in a Time-Dependent Manner
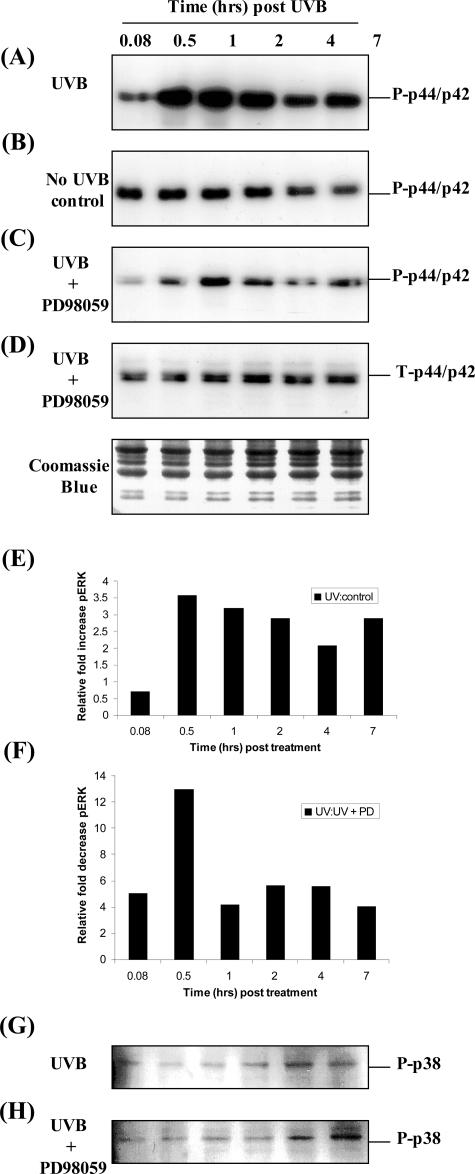
Our previous9 and current results (Figure 1) indicate that the induction of epithelial cell-derived MMP-1 involved the activation of the ERK1/2 MAPK pathway. In this arm of the study we further elucidated the specific role of the ERK1/2 MAPK signaling pathway in the regulation of MMP-1 expression. Initially, PECs were exposed to 20 mJ/cm2 of UVB and the activation of ERK1/2 was determined by Western blot analysis using antibodies directed against the phosphorylated species of ERK1/2. Exposure of PECs to UVB resulted in a rapid and transient activation of phospho-ERK1/2, that increased between 30 minutes to 2 hours after UV and returned to low basal levels after 4 hours (Figure 2A). In contrast, phosphorylated ERK1/2 was not changed significantly throughout the same time course in cells that were not irradiated (Figure 2B). This induction was semiquantified by comparing the ratio of phospho-ERK1/2 after UVB to phospho-ERK1/2 under control conditions in which phospho-ERK1/2 was increased at least 3.5-fold 30 minutes after UVB (Figure 2E). In addition, PD98059 significantly reduced phospho-ERK1/2 levels in UVB-exposed cells as demonstrated by the reduction in the intensity of the immunoreactive band (Figure 2, compare A with C). The most obvious inhibition (12-fold) was observed after 30 minutes (Figure 2F). UVB also enhanced phosphorylated p38 protein slightly above constitutive levels particularly late (4 to 7 hours) after exposure (Figure 2G). This induction was not inhibited by PD98059 (Figure 2H), suggesting that this pathway is unlikely to be involved in modulating MMP-1 production. A representative Coomassie Blue-stained gel demonstrated equal loading in each lane (Figure 2).
Figure 2.
PD98059 inhibited the UVB-induced ERK1/2 activation. PECs were seeded at equal density and cultured until 95% confluence was established. Cells were serum starved overnight then either irradiated with 20 mJ/cm2 UVB (A and G) or mock irradiated (B), and immediately treated with the ERK inhibitor PD98059 (25 μmol/L final) (C, D, and H). Total cell lysates were prepared at the indicated times ranging from 5 minutes to 7 hours. Western blot analysis was performed (A–D, G, and H) with phospho (P)-specific P-p44/p42 (A–C) and phospho-specific P-p38 (G and H) antibodies or antibodies directed against total (T)-p44/p42 (ERK) (D). Equal protein loading was confirmed by staining the SDS-PAGE gels with Coomassie Blue. The Western blot results were further semiquantified and comparisons made between UVB-irradiated and mock-irradiated PECs (E) and UVB-exposed and PD98059-treated PECs (F) with respect to phospho-ERK (pERK) expression. These results are representative of three different cell lines analyzed.
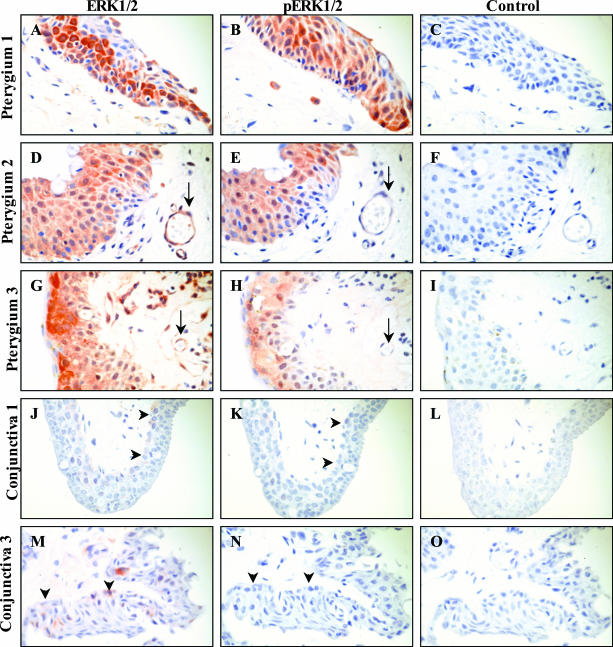
Localization of Phospho-ERK1/2 in Pterygium Specimens
As the ERK1/2 MAPK signaling pathway was shown to play a role in the UVB-mediated induction of MMP-1 in cultured PECs, we attempted to examine the cellular distribution and relative abundance of phospho-ERK1/2 in pterygium specimens. Total ERK1/2 expression was extensive and expressed in all (n = 8) pterygium specimens analyzed (Figure 3; A, D, G). ERK1/2 was predominantly localized to the pterygium epithelium, vascular endothelium (Figure 3, D and G; arrow), some stromal fibroblasts, and inflammatory leukocytes. The expression of phospho-ERK1/2 (Figure 3; B, E, and H) was relatively similar to that of total ERK1/2, predominantly confined to the pterygium epithelium and the occasional inflammatory cell, whereas the vascular endothelium was generally void of any reactivity (Figure 3, E and H; arrow). In contrast, relatively fewer cells expressed total ERK1/2 in normal conjunctiva (Figure 3, J and M; arrowheads) and the intensity of staining was generally diminished when compared to pterygium specimens. Furthermore, reactivity for phospho-ERK1/2 was rarely observed in normal conjunctival tissue (Figure 3, K and N; arrowheads). Control reactions performed in pterygium (Figure 3; C, F, I) and conjunctival (Figure 3, L and O) sections were generally void of immunoreactivity.
Figure 3.
Localization of ERK1/2 in pterygia. Pterygia (A–I) and normal conjunctiva (J–O) were serially sectioned and stained for total ERK (A, D, G, J, and M) or pERK (B, E, H, K, and N). Control reactions included omitting the primary antibody (C and F) or applying a relevant isotype control IgG antibody (I, L, and O). Tissue sections in A to C and J to L were derived from one subject and tissue in G to I and M to O were derived from another patient. Pterygium tissue in D to F was obtained from a third donor. Red staining denoted positive immunoreactivity. Arrows in D and G indicate a positively stained blood vessel. Whereas the arrows in E and H point to the same unstained vessel. Arrowheads in J and M illustrate mild reactivity for total ERK in the basal conjunctival epithelium, whereas the arrowheads in K and N indicate the absence of any reactivity for phospho ERK (pERK) in the same region of the conjunctiva. This pattern of expression was representative of all diseased and normal tissue specimens analyzed. Original magnifications, ×500.
c-jun and c-fos Expression in Cultured PECs and Pterygium Tissue
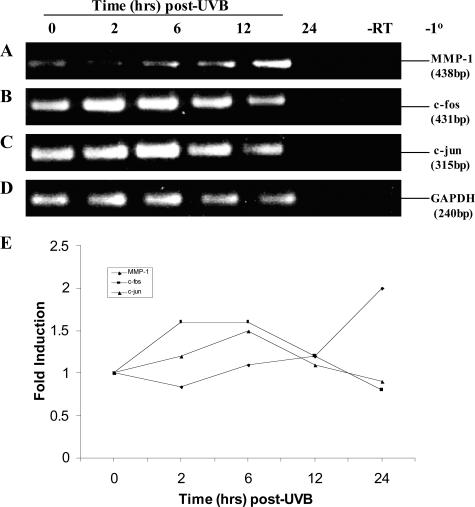
The increased expression of MMP-1 mRNA has been shown to involve the activation of AP-1.38 AP-1 is a protein dimer that consists of c-jun and c-fos. Increased c-fos gene expression correlated with induced AP-1 activation in UVB-exposed keratinocytes and is directed by specific MAPK pathways.38 Given this background, PECs were irradiated with 20 mJ/cm2 UVB, RNA was harvested, and analyzed by RT-PCR (Figure 4; A to D). Note the intermediate-early induction (2 to 6 hours) of both c-fos and c-jun transcripts after UVB (Figure 4, B and C, respectively). Whereas MMP-1 mRNA steadily increased between 12 to 24 hours after UVB (Figure 4A). Although MMP-1 transcripts increased, c-jun and c-fos mRNA expression fell to near background levels (Figure 4E).
Figure 4.
The intermediate-early genes c-jun and c-fos are induced after UVB. Semiconfluent cultures of PECs were exposed to 20 mJ/cm2 UVB. Total RNA was isolated at 0 to 24 hours after UVB and analyzed by RT-PCR to determine MMP-1 (A), c-fos (B), c-jun (C), and GAPDH (D) mRNA expression using gene-specific primers. Control reactions included RNA that was not reverse transcribed (−RT) or reactions in which the primer pairs were omitted (−1°). No products formed in reactions in which a DNA template was not included (not shown). Molecular weight of the products was estimated by comparison to a 100-bp ladder. These results were further semiquantified as fold-increase that was standardized to GAPDH levels (E). These results are representative of three different cell lines analyzed.
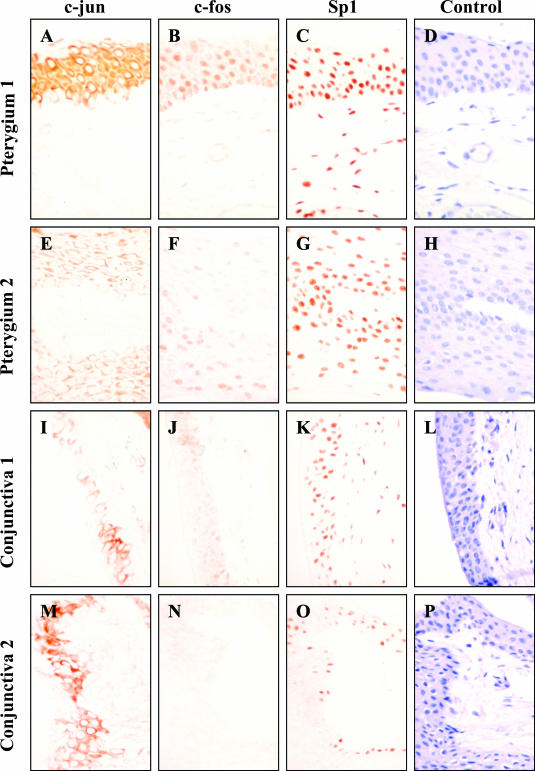
Autologous tissue samples from pterygia and normal conjunctiva were analyzed by immunohistochemistry to determine the distribution of c-jun and c-fos. c-jun staining was extensive and localized predominantly to the pterygium (Figure 5, A and E) and conjunctival epithelium (Figure 5, I and M). Likewise, staining for c-fos was identified on the pterygium epithelium (Figure 5, B and F) but fewer cells expressed this transcription factor in the normal conjunctiva (Figure 5, J and N). The relative expression of these proteins was comparable between diseased and normal tissue, as sections were stained in the one experimental run under identical conditions. Furthermore, the absence of any significant staining for c-fos in the conjunctiva was not attributable to the absence of antigen as these specimens stained positively for the transcription factor Sp1 (Figure 5, K and O) that is regarded as a housekeeping-like gene. Sp1 was not only found in the pterygium epithelium, but also stained conjunctival epithelium and pterygium fibroblasts (Figure 5; C, G, K, and O). Sections incubated with an isotype control antibody (Figure 5, D and L) or in the absence of a primary antibody (Figure 5, H and P) developed no reactivity.
Figure 5.
Localization of c-jun and c-fos in ocular tissue. Pterygia (A–H) and normal conjunctiva (I–P) were serially sectioned and stained for c-jun (A, E, I, and M), c-fos (B, F, J, and N), and Sp1 (C, G, K, and O). Control reactions included omitting the primary antibody (H and P) or applying a relevant isotype control IgG antibody (D and L). Tissue sections in A to D and I to L were derived from one donor and tissue in E to H and M to P were derived from a second patient. Red staining denoted positive immunoreactivity. To illustrate the extent and location of the immunoreactivity, sections in A to C, E to G, I to K, and M to O were not counterstained. Because no reactivity was observed in any of the control reactions (D, H, L, and P), these sections were counterstained with hematoxylin. This pattern of expression is representative of all diseased and normal tissue specimens analyzed. Original magnifications, ×500.
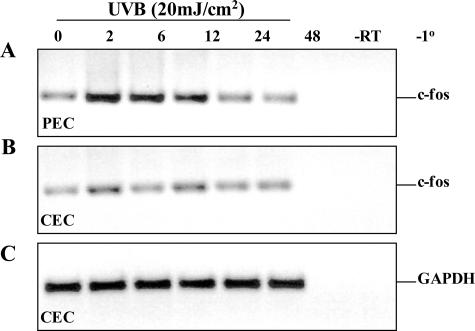
In our previous studies9,10 we noted the diminished expression of MMP-1 in normal human conjunctival tissue compared to the abundant expression of this protease in pterygium specimens. Interestingly, MMP-1 could not be induced after UVB radiation in cultured CECs.9 Similarly, in the current investigation we noted the lack of c-fos expression in conjunctival specimens (Figure 5, J and N). Because c-fos expression is downstream of ERK1/2 and upstream from MMP-1 induction, we investigated whether UVB radiation could modulate c-fos expression in CECs. Indeed c-fos mRNA expression was not significantly altered when CECs were exposed to UVB (Figure 6B). In contrast, c-fos transcripts were transiently induced in a time-dependent manner in the corresponding PECs (Figure 6A).
Figure 6.
Differential expression of c-fos in epithelial cells. Semiconfluent cultures of PECs (A) and CECs (B, C) were exposed to 20 mJ/cm2 UVB. Total RNA was isolated at 0 to 48 hours after UVB and analyzed by RT-PCR to determine c-fos (A, B) and GAPDH (C) mRNA levels using gene-specific primers. Control reactions included RNA that was not reverse transcribed (−RT) or reactions in which the primer pairs were omitted (−1°). No products formed in reactions that did not include a DNA template (not shown). Molecular weight of the products was estimated by comparison to a 100-bp ladder.
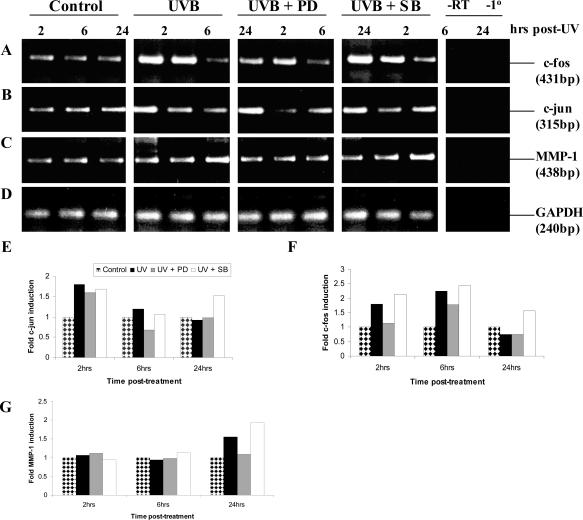
Having identified the involvement of the ERK1/2 MAPK signaling pathway in the UVB-mediated induction of MMP-1 in PECs (Figures 1 and 2), it was important to establish whether c-jun and c-fos expression was affected by inhibiting specific MAPK pathways as other investigators have shown in human keratinocytes.39 In this set of experiments, PECs were cultured under control conditions or irradiated and treated with either the ERK inhibitor (PD98059) or the JNK/p38 inhibitor (SB203580) for the times indicated, after which total RNA was extracted and analyzed by RT-PCR. Early (2 to 6 hours) and late (24 hours) time points of harvest were chosen because c-jun and c-fos are intermediate/early-induced genes whereas MMP-1 mRNA is induced later after UVB exposure9 (Figure 4). Although the data for the control and UVB-exposed PECs corroborated those in Figure 4 with respect to c-jun, c-fos, and MMP-1 mRNA expression, obvious changes in the expression of these genes were noted on incubation with the specific MAPK inhibitors (Figure 7). The expression profile for MMP-1 was not altered at the early time points (2 to 6 hours) irrespective of the treatment but increased at least 1.5-fold 24 hours after UV, was inhibited to background levels by PD98058, and was slightly induced by SB203580 to levels above those seen in UVB-exposed cells (Figure 7, C and G). These mRNA data paralleled those for MMP-1 protein production displayed in Figure 1B. In contrast to the late enhanced expression of MMP-1 mRNA after UVB, c-jun (Figure 7, B and E) and c-fos (Figure 7, A and F) mRNAs were increased relatively early (2 to 6 hours) but the increased expression of both genes was abrogated by the addition of PD98059, and like MMP-1 mRNA, they were moderately induced by SB203580.
Figure 7.
Inhibition of c-jun, c-fos, and MMP-1 mRNA by PD98059. Semiconfluent PECs were exposed to 20 mJ/cm2 UVB. Total RNA was isolated at intermediate (2 to 6 hours) and at a late (24 hours) time point after UVB and analyzed by RT-PCR to determine c-fos (A), c-jun (B), MMP-1 (C), and GAPDH (D) mRNA expression using gene-specific primers. Control reactions included RNA that was not reverse transcribed (−RT) or reactions in which the primer pairs were omitted (−1°). Molecular weight of the products was estimated by comparison to a 100-bp ladder. These results were semiquantified and graphically represented as fold-induction for c-jun (E), c-fos (F), and MMP-1 (G) that was standardized to GAPDH levels (D).
Activation, Phosphorylation, and Internalization of the EGFR after UVB
Our previous study suggested that the increased production of MMP-1 was not due to an autocrine mechanism involving a soluble protein (a ligand-dependent event) instead a direct (a ligand-independent event) effect was likely.9 This arm of the study was designed to address whether or not UVB radiation was capable of activating the EGFR. This receptor was targeted because it has previously been identified in pterygia33,34 and at least one of its ligands, HB-EGF,8,35 has been linked with the pathogenesis of this disease. In addition, the EGFR is activated in the human skin in response to UVB,27 and both HB-EGF and EGF can activate ERK1/2.40,41
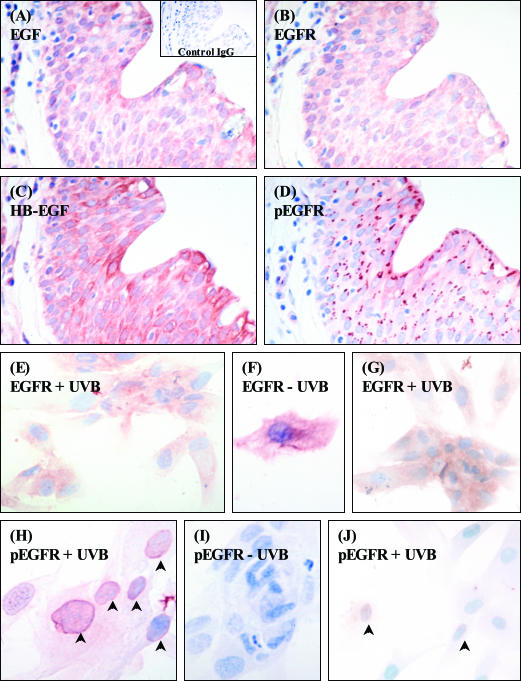
Initially, the expression of the EGFR was confirmed in several pterygium specimens (n = 5). This receptor was heavily expressed by most PECs, in a pattern typical of cytoplasmic and membranous staining (Figure 8B). Furthermore, both HB-EGF (Figure 8C) and EGF (Figure 8A) were identified in serial pterygium sections. Interestingly, the active phosphorylated form of the EGFR was localized to the perinuclear space with a distinct polarized staining pattern (Figure 8D). Next, the activation status of the EGFR was determined in cultured epithelial cells. EGFR expression in nonirradiated PECs (Figure 8F) was abundant as shown by the moderate-to-intense immunoreactivity and was comparable to the expression on nonirradiated CECs (data not shown). Also, when both PECs (Figure 8E) and CECs (Figure 8G) were irradiated, no significant change in the distribution of receptor staining was noted. Moreover, little or no phospho-EGFR was detected in nonexposed PECs (Figure 8I), and CECs (data not shown). But obvious internalization and translocation of this receptor to the nucleus region of the PEC was noted after UVB (Figure 8H, arrowheads), in a pattern similar to that disclosed in vivo (Figure 8D). In-terestingly, only a small portion of UV-irradiated CECs demonstrated staining for phospho-EGFR (Figure 8J, arrowheads)
Figure 8.
Expression of the EGFR and its ligands in pterygia and cultured PECs. Serial sections of pterygium (A–D), cultured PECs (E, F, H, I), or CECs (G and J) were stained for the EGFR (B, E–G), the active phosphorylated EGFR (D, H–J), HB-EGF (C), and EGF (A). Control reactions included omitting the primary antibody (not shown) or applying a relevant isotype control IgG antibody (A, inset). Some cells were mock irradiated [−UVB, (F and I)] or exposed to 20 mJ/cm2 UVB [+UVB (E, G, H, J)]. Red staining denoted positive immunoreactivity. All tissue sections and cultured cells were counterstained with hematoxylin. Arrowheads in H and J point to perinuclear immunoreactivity for the phospho-EGFR. This pattern of expression was representative of all diseased tissue specimens and cultured cells analyzed. Original magnifications: ×500 (A–D, E, G, I, J); ×640 (F and H).
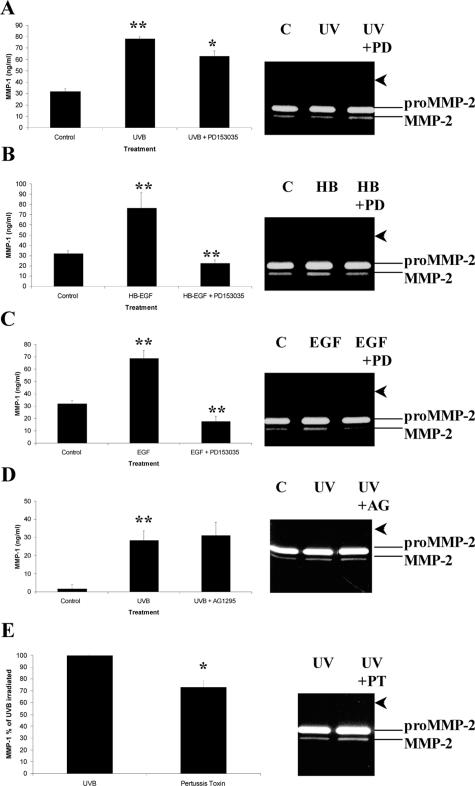
Increasing evidence from keratinocyte cultures32 and human skin41 indicates that ligand-independent activation of the EGFR involves a variety of extracellular stimuli including UV irradiation. To address whether the UVB-mediated induction of MMP-1 involved the activation of the EGFR, serum-starved PECs were preincubated with PD153035. This agent is an extremely potent and specific inhibitor of the tyrosine kinase activity of the EGFR. The results showed that MMP-1 levels were moderately abrogated (P < 0.01) by PD153035 (Figure 9A). This represented a 19.4% inhibition compared to UVB alone and was further abrogated by the addition 5 μmol/L PD153035 to 28% (data not shown). MMP-2 levels were not altered by the same treatment (Figure 9A, zymogram). Interestingly, both recombinant-human HB-EGF (Figure 9B) and EGF (Figure 9C) significantly (P < 0.001) enhanced MMP-1 to the levels observed after UVB irradiation. However, the growth factor-mediated induction of MMP-1 was totally abolished by PD153035 (Figure 9, B and C). Again no modulation of MMP-2, and no induction of MMP-9 was observed by either recombinant human HB-EGF or EGF (Figure 9, B and C; zymograms). Furthermore, desensitization of G-proteins with pertussis toxin significantly reduced the UVB-mediated induction of MMP-1 by at least 27% (Figure 9E), suggesting that the increased MMP-1 production was partially due to G-protein coupled receptors. Importantly, when a selective inhibitor of the tyrosine kinase activity of the PDGFR (AG1295) was co-incubated with the same cells, no significant reduction in MMP-1 levels were observed (Figure 9D).
Figure 9.
Abrogation of MMP-1 production by PD153035. PECs were cultured under control conditions, irradiated with 20 mJ/cm2 UVB (A, D, E), stimulated with either recombinant human HB-EGF (B) or EGF (C), and then treated with the EGFR inhibitor PD153035 (A–C), the PDGFR inhibitor AG1295 (D), or the G-protein desensitizer pertussis toxin (E). Supernatants were harvested after 48 hours and analyzed by enzyme-linked immunosorbent assay (A–E) or gelatin zymography (gel insets). Each bar represents the mean ± STD of triplicate experiments. *P < 0.01 or **P < 0.001. The arrowheads point to the region of gelatinolytic activity expected for MMP-9.
Discussion
The UV spectrum is subdivided into three ranges: short (UVC, 200 to 280 nm), mid-range (UVB, 280 to 320 nm), and long (UVA, 320 to 400 nm). UVC is absorbed by the atmosphere before reaching the earth’s surface and therefore is not likely to cause pathological changes. However, exposure to UVA and UVB is associated with disorders of the skin18–22 and the eye,1,5,6,42 which is not surprising because both organs are continuously exposed to the phototoxic effects of this agent. Exposure of mammalian cells to UV can cause cell death due to apoptosis or cell proliferation as in skin cancer. One of the initial cellular events after UV radiation is activation of cell-surface receptors such as the EGFR27,32,43 and KGFR.31
In the current investigation we provide evidence that implicates cell-surface receptors (eg, EGFR) as potential transmitters of the extracellular UV signal and we refer to this as a ligand-independent event. Although the binding of EGFR ligands is also a likely mechanism of enhancing MMP-1 expression in our cell culture model, we provide convincing preliminary evidence that argue against a ligand-dependent event. For example, our previous study demonstrated that conditioned media from UVB-irradiated PECs (known to contain several UVB-inducible cytokines and growth factors)7,8 was unable to stimulate the production of MMP-1 in quiescent PECs9 and the induction of MMP-1 was partially blocked (Figure 9) by a specific EGFR inhibitor (PD153035) as well as a nonspecific G-protein receptor inhibitor (pertussis toxin). This partial blockade may indicate the activation of other receptor-mediated signaling pathways and this is the topic of ongoing investigations in our laboratory. Furthermore, HB-EGF stimulated the expression of TIMP-16 (and MMP-1), while UVB irradiation had a negligible effect on the expression of this inhibitor (Figure 1C).9
Several studies have shown that extracellular signals in the form of UV light or osmotic stress can activate intracellular MAPK cascades through the phosphorylation of growth factor and cytokine receptors such as the EGFR, TNFR, and IL-1R.32,43,44 This is known to occur via receptor clustering and internalization to endosomal compartments.44 Although EGFR has been identified in pterygia,33,34 to our knowledge, we are the first group to demonstrate not only the presence of EGFR ligands such as HB-EGF8,35 (Figure 8C) and EGF (Figure 8A) but more importantly the active phosphorylated form of this receptor in pterygium specimens (Figure 8D) and UVB-exposed PECs (Figure 8G) in a pattern not too dissimilar to that observed in a previous report.44 Interestingly, AG1295 (a selective inhibitor of the PDGFR tyrosine kinase activity) was unable to reduce MMP-1 in the same experimental setting (Figure 9D). These results are relevant because the PDGFR has been identified in pterygium cells.45
In many regards our data are in direct agreement with studies performed in human skin or skin-derived keratinocytes in which MMP-1 has been widely reported as the major enzyme sensitive to the effects of UVA and UVB. However, the cascade of extra- and intracellular signaling events that result in the induction of MMP-1 after UV stimulation in the normal and diseased eye are scant. Stress-induced activation of ERK1/2 has been demonstrated in glaucomatous human eyes where widespread and excessive immunoreactivity for phospho-ERK was observed in diseased compared with few positively stained cells in normal retinas.46 Other studies have demonstrated ERK1/2 activation in proliferating retinal pigment epithelial cells,47 in the VEGF-induced retinal microvascular endothelial cell proliferation,48 and in cytokine-stimulated trabecular meshwork cells.49 Common to these three investigations was the potent pharmacological inhibition of ERK1/2 and cell proliferation by PD98059. These data suggest that MAPK inhibitors may provide useful therapeutic intervention in ocular disorders such as pterygia that are characterized by extensive cellular proliferation. Using normal autologous conjunctival tissue, we were able to detect faint reactivity for total ERK1/2 and rarely was phospho-ERK1/2 observed (Figure 3; J to O). These results suggest that the pterygium epithelium may receive continuous stimulatory signals, either environmental or physiological, that may be responsible for the extensive ERK1/2 expression in situ.
Several reports have established the effect of UVB on the induction of c-fos and c-jun protein/mRNA in vitro using the human keratinocyte line HaCaT or the transfected derivative cell line FL30.32,39,50–52 In those investigations it was generally observed that both c-fos and c-jun were maximally increased by 1 to 4 hours after UVB and that after the addition of the ERK inhibitor (PD98059), the expression of both genes was significantly suppressed. These data are in direct agreement with our results that demonstrated an early induction of both gene transcripts that preceded an increase in MMP-1 expression (Figures 4, 6, and 7). Furthermore, the authors were able to demonstrate increased immunoreactive staining for c-jun protein in UVB-exposed human skin predominantly in the epithelial layers.27 In contrast, c-jun was rarely detected in nonirradiated skin and was significantly reduced by pretreatment with all-trans retinoic acid. c-fos levels were similar in UV-exposed compared to nonirradiated skin.27 This contrasted slightly with our study in that c-jun staining was demonstrable, but little or no reactivity for c-fos was found in the normal conjunctiva (Figure 5, J and N). Interestingly, c-fos mRNA was constitutively expressed in nonirradiated CECs, but not significantly induced after UVB exposure (Figure 6B). These data may explain why MMP-1 protein was not induced in CECs after the same treatment9 and provide further clues as to the pathway most likely activated in our model.
An important question that still remains unanswered is why normal CECs are less responsive to the effects of UVB. One theory is that these cells have developed mechanisms to combat the phototoxic effects of UVB and this may explain the relatively rare occurrence of conjunctival neoplasms.53 Moreover, differences between CECs and PECs in response to UV may be at the level of cellular differentiation. Sesto and colleagues54 used an oligonucleotide microarray approach and demonstrated the overexpression of keratins K5 and K14 (markers of epithelial cell proliferative capacity) compared to keratins K1 and K10 (typical of differentiated phenotype) in UVB-irradiated primary human keratinocytes. Also expressed at late time points were markers of terminal differentiation and components of the cornified envelope such as involucrin.55 The authors stated that this could be functionally beneficial because enhanced cornification may provide additional protection to the cell from the effects of UV. Although speculative, similar mechanisms may explain the UV-protective phenotype of the conjunctival-derived epithelial cell described in the present study. Alternatively, it is well documented that cells of different lineage respond to different UV wavelengths, although this generally applies to epithelial verses fibroblastic cell lines.19,54,56 Finally, it has been suggested that cell surface receptor density (such as EGFR) may play a critical role in transmitting the initial stress signal.57 In the current investigation, we did not quantify receptor density on the different epithelial cells used, however, it is not unreasonable to propose that this may be another likely explanation for the absence of a UV response in cultured normal CECs and limbal-derived epithelial cells.8 Significant staining differences were indeed noted for pEGFR between UVB-exposed PECs and CECs and this could be one explanation for the reduction in sensitivity toward UV radiation. However, these results should be interpreted with caution as different ocular surface epithelial cells can undergo phenotypic and functional changes under culture conditions.6
Pterygia are tumor-like lesions that consist of a fibrovascular proliferative tissue mass (nonclonal in origin) that invade the cornea and are analogous to a rheumatoid synovial pannus. Although the human tissue specimens used in the current investigation do not clearly demonstrate basophilic elastotic degeneration, this is indeed a classical feature of pterygia (see figures within Di Girolamo and colleagues9,12) that resemble the pathological changes found in photodamaged skin.22 Whether elastosis occurs as a direct consequence of UV-induced damage or indirectly via the induction of inflammatory mediators such as cytokines and MMPs, are mechanisms yet to be elucidated. The natural history of pterygia is not one of progression to malignancy. Interestingly, conjunctival neoplasms and pterygia arise within the same limbal region, are triggered by UV radiation, and both conditions can occur together or contralaterally.58 The mechanism by which UV radiation triggers the development of a pterygium separate from a conjunctival neoplasm is also not known.
The data presented in this study form the basis of a preliminary investigation to map the intracellular pathway(s) likely activated in response to UVB radiation in a reliable in vitro culture model. Importantly, these data provide clues as to potential therapeutic agents that could be used either pre- or postoperatively. It would not be unreasonable to target cell-surface molecules such as the EGFR, as others have done in the setting of neoplasia.59 Not only is this receptor present in pterygia and pterygium-derived epithelial cells (Figure 8),33,34 it is phosphorylated by UVB, EGF, and HB-EGF, and is activated by viruses such as hepatitis B, Epstein-Barr, and Pox viruses.59 This, too, is relevant to the development of pterygia because there is on-going debate as to whether there is a viral component to this disease.60–62 Alternatively, specific blockade of intracellular pathways downstream of EGFR activation such as targeting MAPK has been performed with some success in reducing disease severity63 and inhibiting tumor cell invasiveness.64 Finally, retinoic acid has been shown to inhibit the UVB-mediated induction of c-jun protein in human skin.27 The mechanism by which retinoic acid acts on epithelial cells has not been fully elucidated but this agent has been successfully used in the clinic to treat limbal dysplasia.65
Footnotes
Address reprint requests to Dr. Nick Di Girolamo, Inflammatory Diseases Research Unit, Department of Pathology, School of Medical Sciences, Faculty of Medicine, The University of New South Wales, Sydney, 2052, Australia. E-mail: n.digirolamo@unsw.edu.au.
Supported by the National Health and Medical Research Council.
References
- Coroneo MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993;77:734–739. doi: 10.1136/bjo.77.11.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroneo MT, Di Girolamo N, Wakefield D. The pathogenesis of pterygia. Curr Opin Ophthalmol. 1999;10:282–288. doi: 10.1097/00055735-199908000-00011. [DOI] [PubMed] [Google Scholar]
- Hilgers JHC. Pterygium: its incidence, heredity and etiology. Am J Ophthalmol. 1960;50:635–644. doi: 10.1016/0002-9394(60)90245-2. [DOI] [PubMed] [Google Scholar]
- Karai I, Horiguchi S. Pterygium in welders. Br J Ophthalmol. 1984;68:347–349. doi: 10.1136/bjo.68.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall TJ, English DR. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol. 1999;128:280–287. doi: 10.1016/s0002-9394(99)00161-0. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Chui J, Coroneo MT, Wakefield D. The pathogenesis of pterygia: role of cytokines, growth factors, metalloproteinases, and ultraviolet light. Prog Ret Eye Res. 2004;23:195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Coroneo MT, Kumar RK, Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3430–3437. [PubMed] [Google Scholar]
- Nolan T, Di Girolamo N, Sachdev N, Hampartzoumian T, Coroneo MT, Wakefield D. The role of UV irradiation and heparin-binding epidermal growth factor-like growth factor (HB-EGF) in the pathogenesis of pterygium. Am J Pathol. 2003;162:567–574. doi: 10.1016/S0002-9440(10)63850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo N, Coroneo MT, Wakefield D. UVB-elicited induction of MMP-1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPK-dependent pathway. Invest Ophthalmol Vis Sci. 2003;44:4705–4714. doi: 10.1167/iovs.03-0356. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, McCluskey P, Lloyd A, Coroneo MT, Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:671–679. [PubMed] [Google Scholar]
- Di Girolamo N, Wakefield D, Coroneo MT. Differential expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Invest Ophthalmol Vis Sci. 2000;41:4142–4149. [PubMed] [Google Scholar]
- Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001;42:1963–1968. [PubMed] [Google Scholar]
- Li D-Q, Lee S-B, Gunja-Smith Z, Liu Y, Solomon A, Meller D, Tseng SCG. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by cultured pterygium head fibroblasts. Arch Ophthalmol. 2001;119:71–80. [PubMed] [Google Scholar]
- Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2002;119:695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- Seifert P, Eckert J, Spitznas M. Topological-histological investigation of the pterygium. Graef’s Arch Clin Exp Ophthalmol. 2001;239:288–293. doi: 10.1007/s004170100262. [DOI] [PubMed] [Google Scholar]
- Wang I-J, Hu F-R, Chen P-J, Lin C-T. Mechanism of abnormal elastin gene expression in the pinguecula part of pterygia. Am J Pathol. 2000;157:1269–1276. doi: 10.1016/S0002-9440(10)64642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin P, Jakobiec FA, Iwamoto T. Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia and pinguecula. Ophthalmology. 1983;90:96–109. doi: 10.1016/s0161-6420(83)34594-2. [DOI] [PubMed] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung J-H, Wang Z-Q, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Choi H-C, Bata-Csorgo Z, Shao Y, Datta S, Wang Z-Q, Kang S, Voorhees JJ. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J Invest Dermatol. 2001;117:219–226. doi: 10.1046/j.0022-202x.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang Z-Q, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin aging and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Bernstein EF, Chen YQ, Kopp JB, Fisher L, Brown DB, Hahn PJ, Robey FA, Lakkakorpi J, Uitto J. Long-term sun exposure alters the collagen of the papillary dermis: comparison of sun-protected and photoaged skin by Northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J Am Acad Dermatol. 1996;34:209–218. doi: 10.1016/s0190-9622(96)80114-9. [DOI] [PubMed] [Google Scholar]
- Pilcher BK, Dumin J-A, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partrige CR, Hawker JR, Jr, Forough R. Overexpression of a secretory form of FGF-1 promotes MMP-1-mediated endothelial cell migration. J Cell Biochem. 2000;78:487–499. [PubMed] [Google Scholar]
- D’armiento J, DiColandrea T, Dalal SS, Okada Y, Huang M-T, Conney AH, Chada K. Collagenase expression in transgenic mouse skin causes hyperkeratosis and acanthosis and increases susceptibility to tumorigenesis. Mol Cell Biol. 1995;15:5732–5739. doi: 10.1128/mcb.15.10.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1329. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Talwar HS, Lin J, Lin P, McPhillips F, Wang Z, Li X, Wan Y, Kang S, Voorhees JJ. Retinoic acid inhibits induction of c-jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J Clin Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneisen P, Wenk J, Klotz LO, Wlaschek M, Briviba K, Krieg T, Sies H, Scharffetter-Kochanek K. Central role of ferrous/ferric iron in the ultraviolet B irradiation-mediated signaling pathway leading to increased interstitial collagenase matrix-degrading metalloproteinase (MMP)-1) and stromelysin (MMP-3) mRNA levels in cultured human dermal fibroblasts. J Biol Chem. 1998;273:5279–5287. doi: 10.1074/jbc.273.9.5279. [DOI] [PubMed] [Google Scholar]
- Wenk J, Brenneisen P, Wlaschek M, Poswig A, Briviba K, Oberley TD, Scharfetter-Kochanek K. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1 mediated induction of matrix-degrading metalloproteinase-1. J Biol Chem. 1999;274:25869–25876. doi: 10.1074/jbc.274.36.25869. [DOI] [PubMed] [Google Scholar]
- Schieke SM, Stege H, Kurten V, Grether-Beck S, Sies H, Krutmann J. Infrared-A radiation-induced matrix metalloproteinase 1 expression is mediated through extracellular signal-regulated kinase 1/2 activation in human dermal fibroblasts. J Invest Dermatol. 2002;119:1323–1329. doi: 10.1046/j.1523-1747.2002.19630.x. [DOI] [PubMed] [Google Scholar]
- Marchese C, Maresca V, Cardinali G, Belleudi F, Ceccarelli S, Bellocci M, Frati L, Torrisi M-R, Picardo M. UVB-induced activation and internalization of keratinocyte growth factor receptor. Oncogene. 2003;22:2422–2431. doi: 10.1038/sj.onc.1206301. [DOI] [PubMed] [Google Scholar]
- Wan YS, Wang ZQ, Voorhees J, Fisher G. EGF receptor crosstalks with cytokine receptors leading to the activation of c-jun kinase in response to UV irradiation in human keratinocytes. Cell Signal. 2001;13:139–144. doi: 10.1016/s0898-6568(00)00146-7. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xei Y, Zhang M. Overexpression of type 1 growth factor receptors in pterygium. Chin Med J. 2002;115:418–421. [PubMed] [Google Scholar]
- Maini R, Collison DJ, Maidment JM, Davies PD, Wormstone IM. Pterygial derived fibroblasts express functionally active histamine and epidermal growth factor receptors. Exp Eye Res. 2002;74:237–244. doi: 10.1006/exer.2001.1116. [DOI] [PubMed] [Google Scholar]
- Nolan TM, Di Girolamo N, Coroneo MT, Wakefield D. Proliferative effects of heparin-binding epidermal growth factor-like growth factor on pterygium epithelial cells and fibroblasts. Invest Ophthalmol Vis Sci. 2004;45:110–113. doi: 10.1167/iovs.03-0046. [DOI] [PubMed] [Google Scholar]
- Irving J, Feng J, Wistrom C, Pikaart M, Villeponteau B. An altered repertoire of fos/jun (AP-1) at the onset of replicative senescence. Exp Cell Res. 1992;202:161–166. doi: 10.1016/0014-4827(92)90415-5. [DOI] [PubMed] [Google Scholar]
- Sun HB, Yokota H. Messenger-RNA expression of matrix metalloproteinases, tissue inhibitors of metalloproteinases, and transcription factors in rheumatic synovial cells under mechanical stimuli. Bone. 2000;28:303–309. doi: 10.1016/s8756-3282(00)00454-3. [DOI] [PubMed] [Google Scholar]
- Tower GB, Coon CC, Benbow U, Vincenti MP, Brinckerhoff CE. Erk 1/2 differentially regulates the expression from the 1G/2G single nucleotide polymorphism in the MMP-1 promoter in melanoma cells. Biochim Biophys Acta. 2002;1586:265–274. doi: 10.1016/s0925-4439(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Chen W, Bowden GT. Activation of p38 MAP kinase and ERK are required for the ultraviolet-B induced c-fos gene expression in human keratinocytes. Oncogene. 1999;18:7469–7476. doi: 10.1038/sj.onc.1203210. [DOI] [PubMed] [Google Scholar]
- Cummins AB, Palmer C, Mossman BT, Taatjes DJ. Persistent localization of activated extracellular signal-regulated kinases (ERK1/2) is epithelial cell-specific in an inhalation model of asbestosis. Am J Pathol. 2003;162:713–720. doi: 10.1016/S0002-9440(10)63867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll SW, Kansra S, Elder JT. Metalloproteinases stimulate ErbB-dependent ERK signaling in human skin organ culture. J Biol Chem. 2002;277:26839–26845. doi: 10.1074/jbc.M201108200. [DOI] [PubMed] [Google Scholar]
- Kwok LS, Kuznetsov VA, Ho A, Coroneo MT. Prevention of the adverse photic effects of peripheral light-focusing using UV-blocking contact lenses. Invest Ophthalmol Vis Sci. 2003;44:1501–1507. doi: 10.1167/iovs.02-0380. [DOI] [PubMed] [Google Scholar]
- Blaudschun R, Brenneisen P, Wlaschek M, Meewes C, Scharffetter-Kochanek K. The first peak of the UVB irradiation-dependent biphasic induction of vascular endothelial growth factor (VEGF) is due to phosphorylation of the epidermal growth factor receptor and independent of autocrine transforming growth factor α. FEBS Lett. 2000;474:195–200. doi: 10.1016/s0014-5793(00)01605-7. [DOI] [PubMed] [Google Scholar]
- Rossette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–334. [PubMed] [Google Scholar]
- Tezel G, Chauban BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3025–3033. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- Hecquet C, Lefevre G, Valtink M, Engelmann K, Mascarelli F. Activation and role of MAP kinase-dependent pathways in retinal pigment epithelial cells: ERK and RPE cell proliferation. Invest Ophthalmol Vis Sci. 2002;43:3091–3098. [PubMed] [Google Scholar]
- Bullard LE, Qi X, Penn JS. Role for extracellular signal-responsive kinase-1 and -2 in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2003;44:1722–1731. doi: 10.1167/iovs.01-1193. [DOI] [PubMed] [Google Scholar]
- Alexander JP, Ascott TS. Involvement of the Erk-MAP kinase pathway in TNFα regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci. 2003;44:164–169. doi: 10.1167/iovs.01-1201. [DOI] [PubMed] [Google Scholar]
- Chen W, Bowden GT. Role of p38 mitogen-activated protein kinases in ultraviolet-B irradiation-induced activator protein 1 activation in human keratinocytes. Mol Carcinog. 2000;28:196–202. doi: 10.1002/1098-2744(200008)28:4<196::aid-mc2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Gonzales M, Bowden GT. The role of PI-3-kinase in the UVB-induced expression of c-fos. Oncogene. 2002;21:2721–2728. doi: 10.1038/sj.onc.1205366. [DOI] [PubMed] [Google Scholar]
- Isoherranen K, Westermarck J, Kahari V-M, Jansen C, Punnonen K. Differential regulation of the AP-1 family members by UV irradiation in vitro and in vivo. Cell Signal. 1998;10:191–195. doi: 10.1016/s0898-6568(97)00100-9. [DOI] [PubMed] [Google Scholar]
- Seregard S, Kock E. Squamous spindle cell carcinoma of the conjunctiva. Acta Ophthalmol Scand. 1995;73:464–466. doi: 10.1111/j.1600-0420.1995.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Sesto A, Navarro M, Burslem F, Jorcano JL. Analysis of the ultraviolet B response in primary human keratinocytes using oligonucleotide microarrays. Proc Natl Acad Sci USA. 2002;99:2965–2970. doi: 10.1073/pnas.052678999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Turi TG, Schuck A, Freedberg IM, Khitrov G, Blumenberg M. Rays and arrays: the transcriptional program in the response of human epidermal keratinocytes to UVB illumination. FASEB J. 2001;15:2533–2535. doi: 10.1096/fj.01-0172fje. [DOI] [PubMed] [Google Scholar]
- Otto AI, Riou L, Marionnet C, Mori T, Sarasin A, Magnaldo T. Differential behaviors towards ultraviolet A and B of fibroblasts and keratinocytes from normal and DNA-repair-deficient patients. Cancer Res. 1999;59:1212–1218. [PubMed] [Google Scholar]
- Sawano A, Takayama S, Matsuda M, Miyawaki A. Lateral propagation of EGF signaling after local stimulation is dependent on receptor density. Dev Cell. 2002;3:245–257. doi: 10.1016/s1534-5807(02)00224-1. [DOI] [PubMed] [Google Scholar]
- Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–450. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- Yarden Y. The EGFR family and its ligands in human cancer: signaling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37:S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- Gallagher MJ, Giannoudis A, Herrington CS, Hiscott P. Human papillomavirus in pterygium. Br J Ophthalmol. 2001;85:782–784. doi: 10.1136/bjo.85.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F, Moore PS, Ugalde J, Perra MT, Scarpa A, Sirigu P. Detection of human papillomavirus DNA in pterygia from different geographical regions. Br J Ophthalmol. 2003;87:864–866. doi: 10.1136/bjo.87.7.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid TW, Dushku N. Does human papillomavirus cause pterygium? Br J Ophthalmol. 2003;87:806–808. doi: 10.1136/bjo.87.7.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Kumar S, Griswold DE, Underwood DC, Votta BJ, Adams JL. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology. 2000;47:185–201. doi: 10.1016/s0162-3109(00)00206-x. [DOI] [PubMed] [Google Scholar]
- Tanimura S, Asato K, Fujishiro S-H, Kohno M. Specific blockade of the ERK pathway inhibits the invasiveness of tumor cells: down-regulation of matrix metalloproteinase-3/-9/-14 and CD44. Biochem Biophys Res Comm. 2003;304:801–806. doi: 10.1016/s0006-291x(03)00670-3. [DOI] [PubMed] [Google Scholar]
- Wright P. Topical retinoid acid therapy for disorders of the outer eye. Trans Ophthalmol Soc UK. 1985;104:869–874. [PubMed] [Google Scholar]