Abstract
Placental trophoblast cells form a cellular barrier between the potentially immunogenic fetus and maternal leukocytes. Trophoblasts subvert maternal immunity by producing surface-bound and soluble factors that interact with maternal leukocytes. Here, we describe the distribution of three members of the expanding family of B7 immunomodulatory molecules: B7-DC, B7-H2, and B7-H3. B7-DC and B7-H3 inhibit antigen-stimulated lymphocyte activation while B7-H2 serves in a regulatory capacity, often promoting a Th2 immunophenotype. First trimester and term placentas, purified trophoblast cells, choriocarcinoma cell lines, and human umbilical vein endothelial cells were analyzed for B7 family RNA and protein expression. Transcripts and proteins for all three B7s were present throughout gestation but were differentially expressed within the trophoblast and the stroma. Whereas B7-DC was prominent on the syncytiotrophoblast of early placenta, it was absent from the trophoblast at term. In contrast, B7-H2 and B7-H3 were prominent on the extravillous trophoblast throughout gestation. Lastly, stromal cells, including macrophages and endothelial cells, differentially expressed B7-DC, B7-H2, and B7-H3, depending on gestational age. Thus, all three of these newly discovered B7 proteins are differentially positioned at the maternal-fetal interface such that they could steer maternal leukocytes away from a harmful immune response and toward a favorable one.
Genetic disparity between the fetus and the mother necessitates mechanisms to avoid, suppress, or redirect the maternal immune system such that the fetus does not elicit a potentially hazardous immune response. A number of mechanisms for fetal subversion of the maternal immune system have been proposed, and the placenta is an important source of soluble and cell-associated factors that control immune responses of the mother through direct or indirect cell-to-cell communication. Trophoblast cells, various populations of which completely surround fetal tissues, constitute the interface between the fetus and maternal blood and tissues, and as such, have a major role in cellular alterations of the maternal immune system.1
Members of the B7 family of immunomodulatory proteins mediate cell-to-cell interaction by ligating surface-associated receptors belonging to the CD28 family, which are typically expressed by lymphocytes. Both the B7 and CD28 families are subclasses of the immunoglobulin (Ig) superfamily of type I transmembrane proteins. The best-described B7 proteins are B7-1 and B7-2, both of which interact with the co-stimulatory receptor CD28 and the inhibitory receptor CTLA-4. B7-1 and B7-2 are expressed mainly by professional antigen-presenting cells; interaction with these proteins plays a decisive role in determining lymphocyte responses to foreign antigen.2,3
Recently, interest in the B7 and CD28 families’ immunomodulatory proteins has surged due to the identification of five new B7 proteins. Like B7-1 and B7-2, these new members either co-stimulate or inhibit lymphocytes, depending on the receptor-ligand combination. The new B7 proteins are thought to be critical regulators of immunity within peripheral organs. We have mapped one of the new members, B7-H1, in the placenta.4 B7-H1 (also called PD-L1) binding to its receptor, PD-1, results in inhibition of antigen-induced T-cell activation. Targeted deletion of PD-1 causes severe autoimmune disease in organs expressing B7-H1 or its sister ligand, B7-DC (also called PD-L2), suggesting a critical role in maintenance of self-tolerance.5,6 In the placenta, B7-H1 is highly expressed on all trophoblast cells, suggesting that its immunoevasive role in protection of peripheral organs is paralleled in the placenta.4,7
Other new members of the B7 family include B7-DC, B7-H2, and B7-H3. B7-DC shares the PD-1 receptor with B7-H1, which, as described above, is believed to mediate immunological self-tolerance. B7-DC can inhibit lymphocyte activation, or, like B7-H1, co-stimulate lymphocyte function through an as yet ill-understood mechanism.8,9 B7-H2, which was identified independently by several investigators and thus has several aliases, is constitutively expressed by B lymphocytes and is induced by tumor necrosis factor-α on nonlymphoid cells.10–13 B7-H2 is the ligand for ICOS (inducible co-stimulator), which is expressed by activated and resting memory T cells.12 Several reports suggest that their interaction is particularly important in regulating Th2-type immunity.14 Lastly, B7-H3 was identified first as a co-stimulatory molecule that enhances lymphocyte activity.15,16 Further investigation revealed that B7-H3 can also down-modulate T-cell function, particularly under Th1-polarizing conditions.17–19 Although this dual functionality is reminiscent of that of other B7 family molecules, its molecular basis is also not well understood. A receptor for B7-H3 is expressed on activated, but not resting T cells.15,20
Studies using Northern or reverse transcriptase (RT)-polymerase chain reaction (PCR) screening have identified these newly discovered B7 family members in several tissues, including the placenta.17,21,22 However, nothing is known about spatial or temporal expression of the genes in the placenta. In that the collective function of the B7 family members is to regulate immune responses and direct immunological tolerance, expression of these regulatory proteins on trophoblast cells is of great interest in determining the mechanisms of immunological acceptance of pregnancy. In this report, we report detailed analyses of RNA and protein expression for B7-DC, B7-H2, and B7-H3 in the human placenta.
Materials and Methods
Tissues
Human placental samples were obtained under protocols approved by the institutional review boards at the University of Kansas Medical Center and at Shawnee Mission Medical Center in Merriam, Kansas. Term placentas were obtained from uncomplicated pregnancies after Caesarian sections at both institutions, and first (gestational weeks 6 to 13) trimester tissues were collected from patients undergoing elective pregnancy terminations. Amniochorion membrane and placenta (including the basal plate) were dissected from term placentas, and placental villi with or without associated decidua were collected from first trimester tissue. Tissues were excised and fixed in 4% paraformaldehyde overnight, dehydrated, and embedded in paraffin. For RNA and protein isolation, villous tissue excluding the basal and chorionic plates was collected and flash-frozen in liquid nitrogen.
Term Villous Cytotrophoblast Isolation
Villous cytotrophoblast cells were collected for analysis of endogenous protein and RNA as described.23 Briefly, basal plate tissue was removed and ∼40 g of villous tissue was collected, rinsed with phosphate-buffered saline (PBS), and finely minced. The tissue was dissociated using a mixture of trypsin (Invitrogen, Carlsbad, CA) and DNase (Sigma, St. Louis, MO), and the resulting cell suspension was layered over a 5 to 70% Percoll gradient for density gradient centrifugation. The enriched cytotrophoblasts were then subjected to immunopurification using magnetic microbead technology (Miltenyi Biotec, Auburn, CA). Enriched trophoblast cells were depleted of HLA class I-positive cells by consecutive labeling with W6/32 antibody (American Type Culture Collection, Manassas, VA) and anti-mouse IgG conjugated to magnetic microsphere beads. Viability was determined by trypan blue dye exclusion, and purity, which was routinely greater than 98%, was evaluated by flow cytometry as described using a cytokeratin-7-specific antibody (DAKO, Carpinteria, CA).24
Human Umbilical Vein Endothelial Cell (HUVEC) Isolation
HUVECs were isolated from a 6-inch section of umbilical cord from uncomplicated term pregnancies as previously described.25 Isolated HUVECs were plated in a 25-cm2 flask precoated with 2% gelatin. Cells were expanded to 80% confluence for two passages in RPMI containing 10% fetal calf serum and frozen for protein and RNA collection before analysis.
Isolation of Human Peripheral Blood Mononuclear Cells (PBMCs)
Peripheral blood was drawn from healthy volunteers, diluted 1:2 in PBS, and layered over an equal volume of Histopaque (Sigma). The blood was then centrifuged for 30 minutes at 400 × g, and cells at the serum-Histopaque interface were collected, washed, and counted by trypan blue dye exclusion. For analysis of RNA, cells (8.5 × 106) were plated into 100-mm culture dishes for 20 hours. For isolation of protein, cells (15 × 106) were plated into 100-mm culture dishes for 72 hours, and either treated with medium alone or treated with 10 μg/ml of phytohemagglutinin (PHA, Sigma).
RNA Extraction and RT-PCR
Expression of mRNA for B7 family proteins was evaluated in placental tissue, purified primary cells, cell lines, and PBMCs. First trimester (n = 4) and term (n = 4) placental RNA was extracted from cryosectioned tissue samples placed in 1.5-ml tubes while adjacent cryosections were placed on slides and evaluated for tissue composition. Only tissues that were found to be free of maternal decidua were used for analysis. RNA was extracted using Trizol according to the manufacturer’s protocol (Invitrogen), and 150 ng were reverse-transcribed using murine Moloney leukemia virus reverse transcriptase and oligo-dT12–18 primers (Invitrogen) in 40-μl reaction volumes. Two μl of the reverse transcription reactions were subjected to PCR for 35 cycles using Taq polymerase (Invitrogen) in 25-μl reactions. Primers, listed in Table 1, were purchased from Integrated DNA Technologies (Coralville, IA), and sequences were obtained either from previously published reports or were designed using Primer3 software (http://frodo.wi.mit.edu).26 Negative controls included omission of the reverse transcriptase enzyme from the reverse transcription reactions and omission of cDNA template from the PCR reactions. Reaction products (20 μl) were resolved by electrophoresis on 2% 3:1 agarose gels (Amresco, Solon, OH) for verification of product size, and were excised and cloned into the pGEM T-Easy vector (Promega, Madison, WI) for sequencing. Sequencing was performed using the Ready Reaction mix (Applied Biosystems, Foster City, CA) at the DNA Sequencing Core within the Center for Reproductive Sciences at the University of Kansas Medical Center.
Table 1.
Primer Sequences for B7 Family Members
| Primer pairs | Primer sequence | Annealing temperature | Product size | Reference |
|---|---|---|---|---|
| B7-H2 Fwd (VL141) | cgt gta ctg gat caa taa gac gg | 60°C | 417 bp | 22 |
| B7-H2 Rev (VL274) | tga gct ccg gtc aaa cgt ggc c | |||
| B7-DC Fwd | gta cat aat aga gca tgg cag ca | 55°C | 101 bp | 9 |
| B7-DC Rev | cca cct ttt gca aac tgg ctg t | |||
| B7-DC/2 Fwd | ggc ctc gtt cca cat acc tca agt | 55°C | 372 bp | |
| B7-DC/2 Rev | gga act tac ttt ggc cag cat tga | |||
| B7-H3 Fwd | ctg agg tgt tct ggc agg at | 60°C | 362 bp | |
| B7-H3 Rev | cac cag ctc ttt ggt gtc tg | |||
| G3PDH Fwd | cca tgt tcg tca tgg gtg tga acc a | 57°C | 272 bp | |
| G3PDH Rev | gcc agt aca ggc agg gat gat gtt c |
Immunoblot Analysis
Term villous placental tissue (n = 3) and cultured PBMCs were lysed in RIPA buffer containing detergents (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) and protease inhibitors (100 μg/ml phenylmethylsulfonic acid, 10 μg/ml aprotinin, 10 μg/ml leupeptin). Protein concentration was determined using the DC assay (Bio-Rad, Hercules, CA), and 10 to 25 μg of protein were electrophoresed under denaturing conditions on a Criterion gel apparatus (Bio-Rad). Proteins were transferred to a supported nitrocellulose membrane (Schleicher & Schuell, Keene, NH), blocked in 3% nonfat milk in Tris-buffered saline, and probed with goat anti-human B7-H2 (1 μg/ml; R&D Systems, Minneapolis, MN), goat anti-B7-H3 (1 μg/ml, R&D Systems), or rabbit anti-actin (1:5000 dilution, Sigma). To confirm specificity of the B7-H2 antibody, protein lysates from B7-H2-transfected or mock-transfected CHO cells11 were electrophoresed in parallel with the placental tissue and cell lysates. Blots were also incubated with nonimmune goat or rabbit IgG to verify the absence of nonspecific immunoglobulin binding. Incubations with primary antibodies were performed in 3% milk/TBS-T overnight at 4°C. After washing and probing with the corresponding horseradish peroxidase-labeled anti-goat or anti-rabbit secondary antibody (40 ng/ml; Jackson ImmunoResearch, West Grove, PA), bound antibodies were detected using the enhanced chemiluminescent detection system (Pierce Biotechnology, Rockford, IL) and autoradiography.
Immunohistochemistry
Paraffin-embedded tissues were cut into 5- to 8-μm sections and rehydrated. For detection of B7-H2 and B7-H3, antigen retrieval was performed by heating the slides in 1× Reveal buffer (Biocare Medical, Walnut Creek, CA) to 185°F for 20 minutes in a microwave oven. Tissues were allowed to cool for an additional 20 minutes and blocked against nonspecific antibody binding with 10% (v/v) horse or goat serum in PBS for goat or rabbit primary antibodies, respectively. Primary antibodies [rabbit anti-human B7-H2, 8 to 10 μg/ml (Amgen, Thousand Oaks, CA); goat anti-B7-DC, 2 μg/ml; or goat anti-B7-H3, 5 μg/ml] or nonimmune control rabbit or goat IgG (Sigma) were applied at similar concentrations to sections, which were then incubated in a 4°C humidified chamber overnight. After washing, secondary antibody (biotinylated horse anti-goat or goat anti-rabbit IgG; Vector Laboratories, Burlingame, CA) was applied at a concentration of 10 μg/ml for 25 minutes. Endogenous peroxidase was depleted after binding of the secondary antibody using 0.5% (v/v) H2O2 in methanol. Streptavidin-horseradish peroxidase conjugate (Zymed, South San Francisco, CA) was applied to the tissue sections, and antibody binding was detected using aminoethyl carbazole for color development (Zymed). Color development was performed for ∼5 (B7-DC and B7-H3) or 10 minutes (B7-H2).
Results
RNA for B7 Family Members in First Trimester and Term Placenta
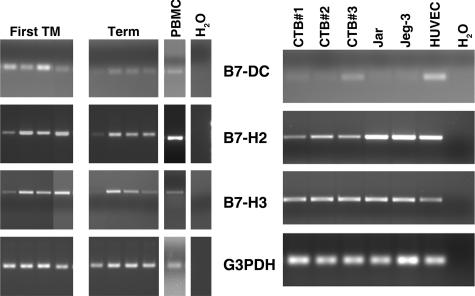
We have previously reported that two B7 family proteins, B7-2 and B7-H1, are transcribed and translated in the human placenta.4 To determine whether mRNAs for other newly discovered B7 proteins are present in the placenta, total RNA was extracted from first trimester and term placenta. Using primers specific for B7-DC, B7-H2, and B7-H3 (Table 1), reverse-transcribed RNA was amplified by PCR. As shown in Figure 1, mRNA was detected in all first trimester and term placenta samples analyzed using each of these primer sets, although sample-to-sample variation was evident. B7-DC mRNA was consistently faint in the term placenta as compared to first trimester samples; a second set of primers for B7-DC (B7-DC/2; Table 1) showed a similar pattern between gestational ages. Among term placentas, one sample showed only faint, but visible product for all three B7 members, despite having adequate G3PDH mRNA. In addition, control human PBMCs contained detectable amounts of mRNA for all three B7s. Sequencing of the products confirmed their identity, and omission of the reverse transcriptase enzyme verified that products did not originate from contaminating genomic DNA.
Figure 1.
Analysis of B7 transcripts in placentas and leukocytes. Ten-μm cryosections of placental tissue were used as a source of RNA so that nearby tissue sections could be counterstained and analyzed microscopically to verify that tissue included solely placental villi. RNA was subjected to RT-PCR using primers specific for B7-DC, B7-H2, B7-H3, or the housekeeping gene, G3PDH. Left: Ethidium bromide-stained agarose gel of RT-PCR products from total RNA of four first trimester placentas, four term placentas, and human PBMCs. H2O: water was substituted for cDNA as a negative control. Right: RT-PCR products of RNA from three different term villous cytotrophoblast preparations (CTB nos. 1, 2, and 3), Jar, JEG-3, and HUVECs.
To determine whether mRNA expression was from trophoblast cells or another population of placental cells, we next examined cytotrophoblast cells derived from term villous tissue, cytotrophoblast cell lines, and HUVECs for mRNA of each of the B7 family members (Figure 1). Only faint B7-DC reaction products were detected in cDNA from primary trophoblast cells and trophoblast cell lines in comparison to mRNA HUVECs. The alternative primer pair specific for B7-DC yielded identical results (not shown). B7-H2 and B7-H3, on the other hand, were readily amplified from both cytotrophoblast cells and HUVECs.
The above data indicate that the genes for three newly discovered B7 family members, B7-DC, B7-H2, and B7-H3, are transcribed in both first trimester and term placenta. Although B7-H2 and B7-H3 mRNA are transcribed by cytotrophoblast cells, B7-DC mRNA appears to be only minimally present or absent from these cells.
Analysis of B7 Proteins in First Trimester and Term Placenta
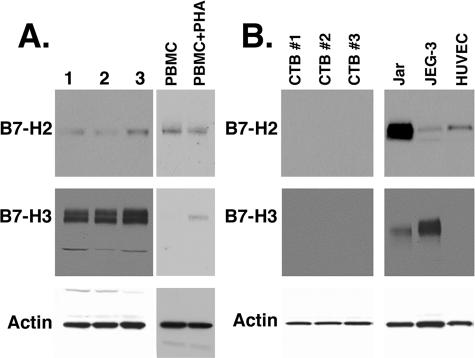
Having determined that B7-DC, B7-H2, and B7-H3 mRNAs are present in the placenta, we next asked whether the proteins are also present by immunoblot analysis for B7-H2 and B7-H3, and immunohistochemistry for all three proteins. Using immunoblot analysis on term placental protein lysates, we detected a single band corresponding to ∼60 kd using goat anti-human B7-H2 (Figure 2A). This antibody also detected a similarly sized band in B7-H2-transfected, but not untransfected, CHO cells (not shown), as well as in resting and PHA-activated human PBMCs. B7-H3 protein was also detected in placental lysates with a dominant band of ∼110 kd. Additionally, a smaller band of ∼60 kd was detected in the placental samples using the B7-H3 antibody (Figure 2A). Finally, B7-H3 protein was detected in PHA-activated, but not resting, PBMCs.
Figure 2.
Immunoblot analysis of term placenta and cells. A: Protein lysates from three different term placentas and from resting and PHA-activated human PBMCs were electrophoresed and subjected to immunoblot analysis and chemiluminescence detection for B7-H2, B7-H3, and actin. B: Protein from three different preparations of term villous cytotrophoblast cell preparations, Jar, JEG-3, or HUVECs were analyzed for these proteins.
We next examined purified primary villous trophoblast cells, choriocarcinoma cell lines, and HUVECs by immunoblot analysis for two of the B7 family proteins, B7-H2 and B7-H3. Whereas B7-H2 was undetectable in trophoblast cells, the protein was present in HUVECs, JEG-3, and Jar cells. B7-H3 protein was detected only in the JEG-3 and Jar protein lysates, in which only the 110-kd band and not the 60-kd band was observed.
Immunohistochemistry of B7 Proteins in Placentas
To define more precisely the location of B7 proteins in first trimester and term placentas, we performed immunohistochemistry using B7 family-specific antibodies. Figures 3 and 4 show the results for first trimester and term placentas, respectively.
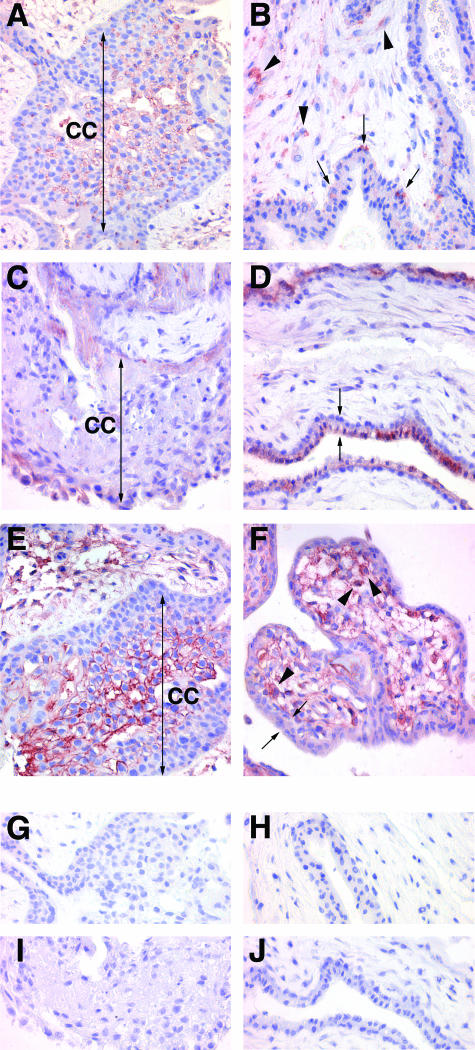
Figure 3.
Immunohistochemical localization of B7 family proteins in the first trimester placenta. A, C, E, G, and I: Extravillous trophoblast cell columns. B, D, F, H, and J: Villous portions of the placenta. A, B: B7-H2; C, D: B7-DC; E, F: B7-H3; G, H: rabbit IgG control; I, J: goat IgG control. Two-headed arrows in A, C, and E bracket cell columns. Arrowheads in B and F show positively staining stromal cells. Arrows in B show positively stained villous cytotrophoblast cells. Double arrows in D and F bracket the villous cyto- and syncytiotrophoblast. CC, cell column. Original magnifications, ×400.
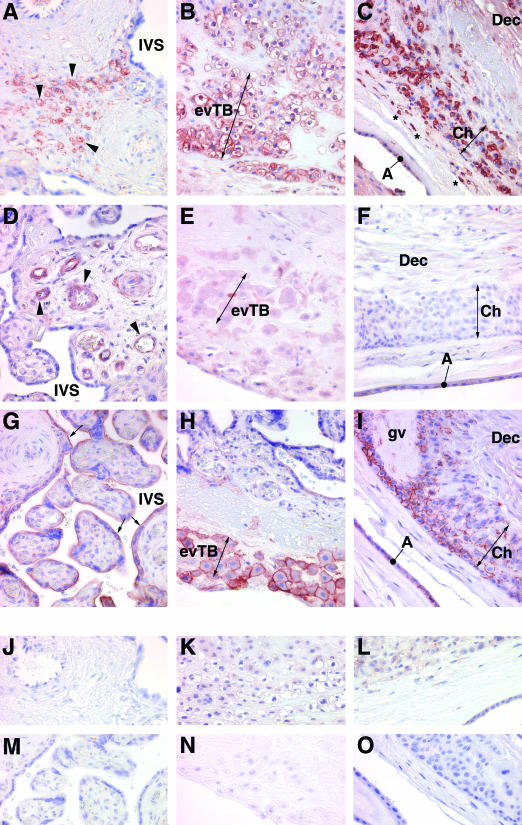
Figure 4.
Immunohistochemical localization of B7 family proteins in the term placenta. A, D, G, J, and M: Villous placenta; B, E, H, K, and N: basal plate placenta; C, F, I, L, and O: amniochorion membrane. A–C: B7-H2; D-F: B7-DC; G--I: B7-H3; J--L: rabbit IgG control; M–O: goat IgG control. Arrowheads in A and D show positively stained villous stromal cells; two-headed arrows in B, E, H and C, F, I bracket clusters of extravillous trophoblast cells of the basal plate and chorion membrane, respectively; asterisks in C denote positively stained cells within the connective tissue between the amnion and chorion; arrows in G show the syncytiotrophoblast. The amnion membrane in I has been separated from the chorion due to processing artifact, and has doubled back on itself. A, amnion epithelium; Ch, chorion; Dec, decidua; evTB, extravillous trophoblast; IVS intervillous space; gv, ghost villus. Original magnifications, ×400.
B7-H2
In first trimester placentas, extravillous trophoblast cells within cell columns, particularly those cells distal to the villi, and cell islands bound the B7-H2 antibody (Figure 3A). Villous trophoblast cells and syncytiotrophoblast of the first trimester placenta exhibited only occasional expression of this protein (Figure 3B). Stromal cells, most likely macrophages and fibroblasts, also showed some B7-H2 immunoreactivity.
In the term placenta, stromal cells of large stem villi were the predominant cells expressing B7-H2 (Figure 4A). B7-H2 was only occasionally found in cells underlying the syncytiotrophoblast; but as in the first trimester, and consistent with the absence of signal from immunoblot analysis, unstained cells at this location far outnumbered antibody-stained cells. Flow cytometry and immunocytochemistry of cytocentrifuge preparations of term villous trophoblast cells yielded similar results in that only a small percentage (<5%) of trophoblast cells bound B7-H2 antibody (data not shown). In contrast to the very limited expression of B7-H2 by villous cytotrophoblast cells, term extravillous cytotrophoblast cells of the basal plate and chorion membrane, like first trimester extravillous trophoblast cells, showed marked, consistent reaction with the B7-H2 antibody (Figure 4, B and C). Additionally, cells, most likely macrophages,4 within the connective tissue between the amnion and chorion membranes strongly expressed the B7-H2 protein (Figure 4C).
B7-DC
In the first trimester placenta (Figure 3, C and D) B7-DC was primarily localized to the syncytiotrophoblast. In one 12-week placental sample, B7-DC was, interestingly, found primarily on the basolateral surface of the syncytiotrophoblast (not shown). In all other first trimester samples (7 to 10 weeks) the protein was focused at the apical surface. Underlying villous trophoblast cells exhibited very little or no B7-DC immunoreactivity, even in the 12-week sample. Similarly, extravillous trophoblast cells of the first trimester placenta showed minimal staining with this antibody (Figure 3C). Hofbauer cells and fibroblasts also showed faint but detectable immunoreactivity, and endothelial cells occasionally expressed the protein.
The term placenta exhibited a strikingly different pattern of B7-DC localization. B7-DC expression was prominently expressed on endothelial cells of many, but not all, placental blood vessels (Figure 4D). In the larger stem villi, smooth muscle cells surrounding arterioles as well as fibroblasts also expressed B7-DC. Hofbauer cells seldom stained positively. B7-DC immunoreactivity was virtually absent on trophoblast populations, with the exception of low reactivity on basal plate extravillous trophoblast cells, and in one sample, rare and patchy appearance on the syncytiotrophoblast.
B7-H3
B7-H3 expression in the first trimester placenta is shown in Figure 3, E and F. Extravillous trophoblast cells showed striking expression of the B7-H3 protein, with clear association with the cell surface (Figure 3E). This was particularly true of column extravillous trophoblast cells distal to the placental villi. Villous cytotrophoblast and syncytiotrophoblast cells exhibited only limited B7-H3 expression in the early placenta, but fibroblasts and Hofbauer cells clearly showed B7-H3 expression (Figure 3F).
In the term placenta (Figure 4, G–I), stromal cells continued to express some B7-H3, but often to a lesser extent than was seen in the first trimester placenta. The syncytiotrophoblast exhibited light B7-H3 staining at its apical surface, but in agreement with the negative result of immunoblot analysis of purified villous cytotrophoblast cells, staining of villous cytotrophoblast cells immediately underlying the syncytiotrophoblast layer was not apparent (Figure 4G). On the other hand, extravillous trophoblast cells of the basal plate and many of those in the chorion membrane also robustly expressed the protein (Figure 4, H and I). Finally, amnion epithelial cells also expressed the B7-H3 protein in a manner consistent with cell surface expression.
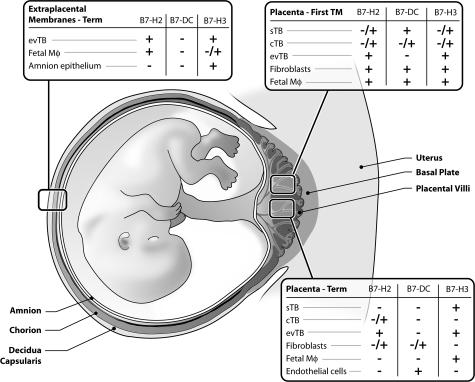
Figure 5 summarizes our findings of B7 family expression in the placenta. B7-DC, although abundant in many cell types of the first trimester placenta, was restricted primarily to endothelial cells of the term placenta. B7-DC was not observed in term extraplacental membranes. Stromal cells of the placental villi and extravillous trophoblast cells are the predominant source of the B7-H2 protein. Extravillous trophoblast cell expression of B7-H2 was consistent throughout gestation, including those cells of the chorion membrane. Finally, the B7-H3 protein is expressed by Hofbauer cells in first trimester and term placentas, and is especially prominent in extravillous trophoblast cells of first trimester placenta, term placenta, and extraplacental membranes.
Figure 5.
Summary of immunohistochemical results in first trimester and term placentas and extraplacental membranes. +, Presence of the protein; +/−, variable presence of the protein; −, the absence of the protein. cTB, cytotrophoblast; evTB, extravillous trophoblast; IVS, intervillous space; Mϕ, macrophages; sTB, syncytiotrophoblast.
Discussion
One of the functions of the placenta is to establish a haven in which the fetus can flourish despite intimate contact with an immunologically semiforeign environment. In this task, the trophoblast is the principal player. We report for the first time the spatial and temporal expression of the newly discovered B7 family proteins, B7-DC, B7-H2, and B7-H3 in the human placenta. Transcripts encoding all three immunomodulatory proteins were identified in the first trimester and term placenta and purified subpopulations of placental cells. Immunoblot and immunohistochemistry provided a detailed analysis of the exact localization patterns of each.
Although we acknowledge that the methods of RT-PCR analysis used in this study are not quantitative, we consistently found that B7-DC mRNA was easily detected in first trimester, but less readily identifiable in term placenta. Using two different primer sets, HUVECs yielded the strongest B7-DC signal, suggesting that endothelial cells may be a principal source of the protein. These data corresponded with immunohistochemical analysis, in which it was found that B7-DC was abundant in first trimester placenta, and that it was localized almost exclusively, although not ubiquitously, to endothelial cells of the term placenta. The expression of B7-DC on endothelial cells in the term placenta agrees with the results of Brown and colleagues,21 who in a survey of multiple tissues, found B7-DC expression on placental endothelial, but not trophoblast cells.
The abundance of B7-DC in the first trimester syncytiotrophoblast and its absence in the term syncytiotrophoblast complements the pattern of staining of the sister ligand to B7-DC, B7-H1, which was found in lower levels in the first trimester as compared to term placenta.4 Thus, the presence of PD-1 ligands is constant throughout pregnancy. On the other hand, it is clear from the reciprocity of expression that the two PD-1 ligands are differentially regulated.
B7-H2 mRNA was identified in all placenta, trophoblast, and cell line samples analyzed, consistent with the widespread expression of this gene seen in other studies. Western blot analysis of placental proteins confirmed that B7-H2 protein is expressed as a 60-kd band, in agreement with the size of the protein detected within B7-H2-transfected CHO cells. Previous studies revealed that B7-H2 is expressed by endothelial cells of the umbilical cord, which we confirmed by Western blot (Figure 2B) and by immunohistochemistry (not shown). However, immunohistochemistry did not reveal prominent staining of the placental endothelial cells in this study, suggesting a functional difference between cord and placental endothelial cells. Hofbauer cells of the first trimester and term placenta reacted with the B7-H2 antibody, as did extravillous trophoblast cells. The pattern of staining of extravillous trophoblast cells distal, but not proximal to the villi suggests that acquisition of B7-H2 protein is associated with development of the invasive phenotype. However, neither villous trophoblast cells underlying the syncytiotrophoblast, nor the syncytiotrophoblast itself exhibited strong staining with this antibody. Thus, the primary source of B7-H2 protein in the placenta may be stromal cells, including macrophages.
B7-H2 interactions with its receptor, ICOS, is associated with Th2 cell effector function in terms of cell surface expression, differentiation, and cytokine production.27–29 Some evidence, particularly in the murine model, suggest that Th2 immunity is beneficial to pregnancy while Th1 immunity is harmful. B7-H2 expression in the murine embryonic yolk sac was suggested to serve in a Th2-related capacity during pregnancy.10 Our findings of B7-H2 expression by extravillous trophoblast cells suggest the possibility that it plays a role in driving or maintaining the immunophenotype of decidual T cells, which is skewed toward Th2.30 Although the data for the Th1/Th2 hypothesis of pregnancy is far from clear-cut,31 it is intriguing that, in addition to B7-H2, other Th2 immunomodulators seem to be preferentially expressed by human trophoblast cells.32
Multiple tissue Northern blot and RT-PCR analyses have identified B7-H3 in several peripheral (nonlymphoid) tissues.15,17,20,33 However, little is known about the expression of the protein outside the immune system. We have now confirmed that B7-H3 mRNA is expressed in the term placenta, and extended the observations by showing that B7-H3 mRNA is also found in the first trimester placenta, and more specifically, in purified cytotrophoblast cells and HUVECs.
By immunoblot analysis of placenta lysates, we detected a band of ∼110 kd, which is consistent with the expected size of the isoform of B7-H3 containing four extracellular immunoglobulin domains.17,33 We also detected a smaller band of ∼60 kd; it is possible that this band represents a shorter isoform of B7-H3 containing two immunoglobulin domains.17,33 Further studies will be required to discern the identity of this band.
Villous cytotrophoblast cells and HUVECs either failed to express the protein or expressed the protein at a level below the detection limit of the assay. Because the RNA could be detected in both of these cell types, the data suggest that posttranscriptional and/or translational regulation is a critical control point of B7-H3 gene expression. Immunohistochemistry of early placenta revealed that B7-H3 is expressed by mesenchymal cells within placental villi, but much less so by villous trophoblast cells or syncytiotrophoblasts. On the other hand, B7-H3 was strongly expressed by extravillous trophoblast cells within first trimester cell columns. Like B7-H2, B7-H3 was found most often in the extravillous trophoblast cells distal to the villi, again suggesting developmentally regulated expression. In the term placenta, the protein continued to be highly expressed on extravillous trophoblast cells of both the basal plate and chorion membrane. Chorion trophoblast cells exhibited variable staining, reminiscent of the heterogeneous nature of this population of cells.34 Additionally, B7-H3 was faintly present in the syncytiotrophoblast of the term placenta. Consistent with the immunoblot analysis, cells underlying the syncytiotrophoblast were not found to express the protein by immunohistochemistry.
The choriocarcinoma cell lines Jeg-3 and Jar expressed both B7-H2 and B7-H3 mRNA and proteins. In addition, these cells express B7-H1.4 Whether these cells acquired B7-H2 and B7-H3 expression before or after carcinomic transformation is impossible to say; however, it is entirely feasible that B7 proteins on choriocarcinoma cells influence immunological recognition by maternal lymphocytes. B7-H1 is often found on transformed cells while absent on their nontransformed counterparts, and is thought to facilitate immunological escape by these cells.35 In contrast, both B7-H2 and B7-H3 have been shown to enhance tumor immunity.36–38 Further studies will be required to elucidate the relative contribution of these molecules in modulating lymphocyte responses to choriocarcinoma cells.
The results described in this study are the first to show protein expression of three newly discovered B7 family members in the placenta, and are summarized in Figure 5. The differential expression of these proteins in the villous cytotrophoblast underlying the syncytiotrophoblast, in the syncytiotrophoblast, and in the extravillous cytotrophoblast suggests that their expression is tied to their stage of differentiation. Changes in expression of the B7 proteins after the first trimester may be of functional significance relating to the exposure of trophoblast cells to maternal blood and lymphocytes. Further studies will be required clarify the role of B7 family proteins in the placenta; however, we suggest that these powerful immunomodulators are key in providing the placenta and fetus with a suitable immunological environment throughout pregnancy.
Acknowledgments
We thank Miao Chang and Kathy Hucker for isolating HUVECs; Dr. Lieping Chen for the kind gift of B7-H2-transfected and mock-transfected CHO cells; Amgen Inc. for their donation of the rabbit anti-human B7-H2 antibody; Dr. Joan Hunt for assistance in collection of first trimester placenta; Drs. Elizabeth Wickstrom and Lynn Rasmussen for assistance in obtaining placentas and informed consent at Shawnee Mission Medical Center. The University of Kansas Center for Reproductive Sciences Specialized Cooperative Center Program in Reproduction Research, NICHD provided a U54 flow cytometry, DNA sequencing, and graphics support services.
Footnotes
Address reprint requests to Margaret G. Petroff, Department of Anatomy and Cell Biology, University of Kansas Medical Center, 3901 Rainbow Blvd., Kansas City, KS 66160-7400. E-mail: mpetroff@kumc.edu.
Supported by the National Institutes of Health (grant R01 HD045611 to M.G.P.) and the Kansas City Area Life Sciences Institute (to M.G.P.).
References
- Petroff MG, Hunt JS. Immunity at the maternal-fetal interface. Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, editors. San Diego: Elsevier, Inc.,; Mucosal Immunology. 2005 [Google Scholar]
- June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68:1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1-deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Chen L, Phillips TA, Hunt JS. B7 family molecules: novel immunomodulators at the maternal-fetal interface. Placenta. 2002;23(Suppl A):S95–S101. doi: 10.1053/plac.2002.0813. [DOI] [PubMed] [Google Scholar]
- Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- Ling V, Wu PW, Finnerty HF, Bean KM, Spaulding V, Fouser LA, Leonard JP, Hunter SE, Zollner R, Thomas JL, Miyashiro JS, Jacobs KA, Collins M. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J Immunol. 2000;164:1653–1657. doi: 10.4049/jimmunol.164.4.1653. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J, Chen L. Costimulation of T cells by B7–H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–2813. [PubMed] [Google Scholar]
- Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol. 2002;14:384–390. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7–H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- Sun X, Vale M, Leung E, Kanwar JR, Gupta R, Krissansen GW. Mouse B7–H3 induces antitumor immunity. Gene Ther. 2003;10:1728–1734. doi: 10.1038/sj.gt.3302070. [DOI] [PubMed] [Google Scholar]
- Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, Collins M. Duplication of primate and rodent B7–H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82:365–377. doi: 10.1016/s0888-7543(03)00126-5. [DOI] [PubMed] [Google Scholar]
- Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, Mak TW. The B7 family member B7–H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C. Murine B7–H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7–H3 genes. J Immunol. 2002;168:6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- Ling V, Wu PW, Miyashiro JS, Marusic S, Finnerty HF, Collins M. Differential expression of inducible costimulator-ligand splice variants: lymphoid regulation of mouse GL50-B and human GL50 molecules. J Immunol. 2001;166:7300–7308. doi: 10.4049/jimmunol.166.12.7300. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS: Isolation and culture of term human trophoblast cells. Placenta Methods and Protocols. Edited by Soares MJ. Totowa, Humana Press, Inc. (in press) [DOI] [PubMed] [Google Scholar]
- Potgens AJ, Gaus G, Frank HG, Kaufmann P. Characterization of trophoblast cell isolations by a modified flow cytometry assay. Placenta. 2001;22:251–255. doi: 10.1053/plac.2000.0597. [DOI] [PubMed] [Google Scholar]
- Arroyo J, Torry RJ, Torry DS. Deferential regulation of placenta growth factor (PlGF)-mediated signal transduction in human primary term trophoblast and endothelial cells. Placenta. 2004;25:379–386. doi: 10.1016/j.placenta.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Krawetz S, Misener S, editors. Totowa: Humana Press,; Bioinformatics Methods and ProtocolsMethods in Molecular Biology. 2000:pp 365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Lohning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, Hamelmann E, Kroczek RA. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, Ling V, Collins M, Sharpe AH, Freeman GJ. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. Reproductive immunology 2003: reassessing the Th1/Th2 paradigm? Immunol Lett. 2004;92:207–214. doi: 10.1016/j.imlet.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Phillips TA, Ni J, Hunt JS. Cell-specific expression of B lymphocyte (APRIL, BLyS)- and Th2 (CD30L/CD153)-promoting tumor necrosis factor superfamily ligands in human placentas. J Leukoc Biol. 2003;74:81–87. doi: 10.1189/jlb.0103033. [DOI] [PubMed] [Google Scholar]
- Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, Knapp W. Molecular characterization of human 4Ig-B7–H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172:2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P. New York: Springer-Verlag,; Pathology of the Human Placenta. 2000 [Google Scholar]
- Dong H, Chen L. B7–H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- Wallin JJ, Liang L, Bakardjiev A, Sha WC. Enhancement of CD8+ T cell responses by ICOS/B7h costimulation. J Immunol. 2001;167:132–139. doi: 10.4049/jimmunol.167.1.132. [DOI] [PubMed] [Google Scholar]
- Liu X, Bai X-F, Wen J, Gao J-X, Liu J, Lu P, Wang Y, Zheng P, Liu Y. B7H costimulates clonal expansion of, and cognate destruction of tumor cells by, CD8+ T lymphocytes in vivo. J Exp Med. 2001;194:1339–1348. doi: 10.1084/jem.194.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, Lau JS, Dong H, Tamada K, Flies AS, Liu Y, Chen L. B7–H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. 2004;173:5445–5450. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]