Abstract
Differentiation of adult bone marrow (BM) cells into nonhematopoietic cells is a rare phenomenon. Several reports, however, suggest that human umbilical cord blood (hUCB)-derived cells give rise to hepatocytes after transplantation into nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice. Therefore, we analyzed the hepatic differentiation potential of hUCB cells and compared the frequency of newly formed hepatocyte-like cells in the livers of recipient NOD-SCID mice after transplantation of hUCB versus murine BM cells. Mononuclear cell preparations of hUCB cells or murine BM from enhanced green fluorescent protein transgenic or wild-type mice were transplanted into sublethally irradiated NOD-SCID mice. Liver regeneration was induced by carbon tetrachloride injury with and without sub-sequent hepatocyte growth factor treatment. By immunohistochemistry and reverse transcriptasepolymerase chain reaction, we detected clusters of hepatocyte-like cells in the livers of hUCB-transplanted mice. These cells expressed human albumin and Hep Par 1 but mouse CK18, suggesting the formation of chimeric hepatocyte-like cells. Native fluorescence microscopy and double immunofluorescence failed to detect single hepatocytes derived from transplanted enhanced green fluorescent protein-transgenic mouse BM. Fluorescent in situ hybridization rarely revealed donor-derived hepatocyte-like cells after cross-gender mouse BM transplantation. Thus, hUCB cells have differentiation capabilities different from murine BM cells after transplantation into NOD-SCID mice, demonstrating the importance of further testing before hUCB cells can be used therapeutically.
Understanding the mechanisms of transdifferentiation and plasticity would provide important clues for the use of stem cells in organ repopulation and regeneration. Whether transdifferentiation and plasticity of adult stem cells exist at all, has recently become a controversially debated issue. Several reports have either favored or opposed the concept of differentiation of bone marrow (BM) cells into many different types of tissue cells.1–15 Petersen and colleagues,1 Alison and colleagues,2 and Theise and colleagues14 were among the first to show in rats, mice, as well as in humans that hepatocytes and cholangiocytes could be derived from BM. With Y-chromosome staining and liver-specific markers they detected BM-derived hepatocytes in the liver of irradiated mice and humans after gender-mismatched BM transplantation indicating participation of BM in liver regeneration. Lagasse and colleagues16 have shown that highly purified stem cells isolated from the BM of adult mice rescued the liver defect in the fumaryl acetoacetate hydrolase FAH(−/−) mouse, an animal model of tyrosinemia type I, by restoring the biochemical function of its liver. The transplanted BM cells were able to protect the mice from lethal irradiation and to generate functional hepatocytes in the liver. The generation of hepatocytes, however, was not the result of direct differentiation, but occurred by fusion of hematopoietic cells with recipient hepatocytes under the high selection pressure in this model.17,18
Krause and colleagues3 and Harris and colleagues5 injected highly purified BM cells into irradiated mice and obtained engraftment in several organs, including skin, lung, and liver without apparent signs of cell fusion. In contrast to these experiments, other groups including ours failed to show a significant contribution of BM-derived cells in liver regeneration of mice. After reconstitution with either enhanced green fluorescent protein (EGFP) or β-galactosidase-transgenic hematopoietic stem cells only a very few marker gene-positive non-hematopoietic cells were detected in the recipient livers.6,19,20
It has been reported that intravenous administration of human umbilical cord blood (hUCB) in the mouse model of amyotrophic lateral sclerosis may replace damaged neurones21 and also can produce primitive neuropoietic progenitors.22 Transplantation of hUCB into fetal sheep resulted in human hepatocyte formation in a noninjury animal model without signs of fusion.23 Umbilical cord blood contains hematopoietic stem cells, which differ in some aspects from BM hematopoietic stem cells.24 One study has shown expression of a variant AFP transcript in hUCB cells that might suggest the presence of some nonhematopoietic primitive progenitors that might have the potential to differentiate into cells of hepatic as well as hematopoietic phenotype.25,26
hUCB is highly enriched for hematopoietic stem cells and can partially repopulate the BM of NOD-SCID mice. A few recent articles have highlighted the differentiation potential of human cord blood cells and the generation of human hepatocytes from transplanted cord blood cells in NOD-SCID mice.27–29 As of now, behavior of mouse BM cells in response to liver injury in NOD-SCID mice has not been shown and also human cord blood-derived liver cells are needed to be further characterized. In the present study we aimed to analyze whether differences exist in hepatic differentiation capabilities of mouse BM cells and human cord blood cells after transplantation into NOD-SCID mice under identical experimental conditions. Here we report that human cord blood cells give rise to hepatocyte-like cells after transplantation into NOD-SCID mice in response to carbon tetrachloride (CCl4)-induced liver injury, whereas mouse BM cells rarely formed hepatocyte-like cells in similar experimental conditions. To the best of our knowledge we show for the first time that human cord blood cell-derived hepatocyte-like cells expressing human albumin and human hepatocyte-specific antigen Hep Par 1 do not express human cytokeratins (CKs) 8 and 18, but express their murine counterpart as demonstrated for CK18 in this study.
Materials and Methods
Animals
Six- to eight-week-old EGFP-transgenic C57BL/6 mice [C57BL/6-TgN (Actb EGFP) 1Osb], 8- to 14-week-old NOD-SCID mice, and 8- to 12-week-old C57BL/6 mice were obtained from The Jackson Laboratories (Bar Harbor, ME) and held under germ-free conditions. All animals were maintained and handled in accordance with institutional guidelines. Recipient NOD-SCID mice were divided into five groups: group A (n = 10) received human UCB cells and later CCl4 treatment, group B (n = 6) received human UCB cells without CCl4 treatment, group C (n = 10) and group D (n = 6) received mouse EGFP-positive BM mononuclear cells with and without CCl4 treatment, respectively, and group E (n = 3) was sham-transplanted with subsequent treatment of CCl4. In addition, a group of female mice was transplanted with male EGFP-positive BM mononuclear cells for subsequent fluorescence in situ hybridization (FISH) detection of Y-chromosome-positive cells in the recipient liver after CCl4 damage (six NOD-SCID recipient mice) or with BM cells derived from 3-, 2-, and 1-week-old donor mice (three NOD-SCID recipient mice, each).
Isolation of Mouse Bone Marrow Cells and Human Cord Blood Cells
Bone marrow was harvested from the femurs and tibias of the EGFP-transgenic mice. Mononuclear cells were isolated by Histopaque-1077 density gradient centrifugation and collected in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum (all from Sigma, Munich, Germany). The hUCB from full-term deliveries was obtained from the University Hospital Hannover in accordance with the guidelines of the institutional review of the Hannover Medical School after informed consent had been obtained. Mononuclear cells were isolated by density gradient centrifugation (P = 1.077 × g/ml).30 Briefly, mononuclear cells were layered over the Ficoll solution and centrifuged at 400 × g for 20 minutes. Mononuclear cells were collected from the interface and washed twice in phosphate-buffered saline (PBS). Cell viability was more than 95% by trypan blue exclusion.
Transplantation
Animal experiments with NOD-SCID mice have been summarized in Table 1. The animals were irradiated with a single dose of 250 rads 2 to 4 hours before cell transplantation. Human cord blood-derived mononuclear cells (1 × 106) (groups A and B) or 1 × 106 murine EGFP-positive BM mononuclear cells (groups C and D) or were infused by lateral tail vein injection. At 3 weeks after transplantation, mice were screened for cell engraftment by detection of EGFP-positive cells or human leukocyte antigen-expressing cells in peripheral blood using fluorescence-activated cell sorting analysis. Four weeks after transplantation NOD-SCID mice were injected intraperitoneally with a single dose of 0.4 ml of CCl4/kg body weight carbon tetrachloride (CCl4) diluted to 100 μl with olive oil to induce acute liver damage. To confirm CCl4-induced liver injury aspartate and alanine aminotransferases (AST and ALT) were measured in peripheral blood (from group E) with an autoanalyzer. In each group (except group E) half of the mice were additionally treated three times with 1.5 μg of hepatocyte growth factor (HGF) 48, 60, and 72 hours after CCl4 application in an attempt to increase regeneration and hepatocyte differentiation. Four weeks after induction of liver injury and 8 weeks after cell transplantation the recipient NOD-SCID mice were sacrificed. Peripheral blood and BM from all mice were analyzed for donor-derived EGFP-positive blood cells or for human cell engraftment as described above. Before removal of liver, pancreas, heart, intestine, and spleen each mouse was perfused with saline by cardiac puncture under anesthesia to wash out circulating blood cells in the organs.
Table 1.
Summary of the Experimental Data
| Group | n | Transplanted cells, 0 day | Liver injury, day 28 | HGF application (48, 60, and 72 hours) after CCl4 application | Detection of donor-derived liver cells* |
|---|---|---|---|---|---|
| A | 10 | 1 × 106Human umbilical cord blood cells | CCl4 | HGF (n = 5) | Present 30, 42, 48, 58, 34 |
| PBS (n = 5) | Present 32, 17, 40, 12, 22 | ||||
| B | 6 | 1 × 106Human umbilical cord blood cells | NA | HGF (n = 3) | Present 4, 0, 0 |
| PBS (n = 3) | Present 2, 1, 0 | ||||
| C | 10 | 1 × 106Adult mouse bone marrow | CCl4 | HGF (n = 5) | Absent |
| mononuclear cells | PBS (n = 5) | Absent | |||
| D | 6 | 1 × 106Adult mouse bone marrow | NA | HGF (n = 3) | Absent |
| mononuclear cells | PBS (n = 3) | Absent | |||
| E | 3 | Only PBS | CCl4 | NA | Absent |
NA, not applied.
These numbers represent cells found in 20 sections (4 μm) analyzed from each mouse. These numbers are based on human hepatocyte-specific antigen (Hep-par 1)-positive cells for human UCB-derived hepatocyte-like cells while criteria for mouse bone marrow-derived hepatocyte-like cells was immunodetection for GFP and DPPIV markers.
Histology and Immunohistochemistry
Liver, pancreas, heart, intestine, and spleen from all recipient mice were dissected and either immediately frozen in liquid nitrogen and stored at −80°C or fixed in 4% formalin and embedded in paraffin. For native EGFP fluorescence analysis, 10-μm frozen sections were analyzed with an Olympus BX40 microscope (Olympus, Hamburg, Germany) equipped with appropriate filter combinations. Autofluorescence was excluded by parallel examination of the red (617 nm) and green emission (528 nm). One hundred representative tissue sections were analyzed from each mouse and organ. For immunofluorescence staining frozen liver sections were fixed in acetone at −20°C for 10 minutes, followed by rehydration in PBS, pH 7.4. EGFP-protein (anti-EGFP-Alexa Fluor 488; Molecular Probes, Leiden, The Netherlands) and the canalicular hepatocyte marker protein dipeptidyl peptidase (DPPIV) (rat anti-mouse-CD26; BD Pharmingen, Heidelberg, Germany) were co-stained at a dilution of 1:200 followed by application of the secondary Cy3-labeled goat anti-rat IgG antibody (Jackson ImmunoResearch/Dianova, Hamburg, Germany) at a dilution of 1:400. The nuclei were counterstained with 4′,6-diamidino-2-phenylidole (DAPI, Sigma Taufkirchen, Germany).
For hematoxylin and eosin (H&E) staining sections were stained with hematoxylin for 3 minutes, washed, and stained with 0.5% eosin for an additional 3 minutes. After an additional washing step with water the slides were dehydrated in 70%, 96%, and 100% ethanol, and in xylene before they were embedded in DPX (Polysciences, Eppelheim, Germany).
For detection of human liver-specific antigens albumin, Hep Par 1, CK18, and CK8 formalin-fixed and paraffin-embedded tissues were cut into 4-μm sections and deposited on Super Frost Plus glass slides (Menzel-Glaser, Germany). Slides were dried completely and deparaffinized, and rehydrated in xylene and grades of alcohol. The sections were exposed to epitope retrieval by placing them in boiling water containing 0.01 mol/L sodium citrate buffer (pH 6) for 15 minutes followed by quenching of endogenous peroxides with 1% hydrogen peroxide (H2O2) for 10 minutes. Nonspecific staining was eliminated by an additional blocking step with a protein block solution (X0909; DAKO, Glostrup, Denmark) by incubating sections for 30 minutes in a humid chamber. The primary antibodies were diluted in PBS, pH 7.4, and applied at a dilution of 1:100 for the anti-human hepatocyte antibody (Hep Par 1, M 7158; DAKO) at 1:50 for the anti-human albumin (F0117, DAKO), anti-human CK18 antibody (M 7010, DAKO), and anti-human CK8 (M 0631, DAKO). After application of the secondary antibody (K 5007, DAKO) color development was performed with the 3-amino-9-ethylcarbazole chromogen system (Zymed, Germany) for Hep Par 1 and with diaminobenzidine (DAKO) for albumin, CK18, and CK8. The incubation times were 60 minutes and 30 minutes for primary and secondary antibodies, respectively.
For immunofluorescence staining on paraffin sections, anti-human hepatocyte antibody (Hep Par 1) and human CK18 antibody were applied at a dilution of 1:100 and 1:50, respectively. To detect mouse CK18, a monoclonal antibody to CK18 (BM2275P, Acris, Hidden Hausen, Germany) was used at a dilution of 1:100. Secondary antibodies (Alexa Fluor 488 and Alexa 350, Molecular Probes; and Cy-3 conjugated) were all used at a dilution of 1:500.
Fluorescent in Situ Hybridization (FISH)
After deparaffinization and rehydration of sections FISH was performed to detect Y-chromosome in NOD-SCID mice that were transplanted with BM mononuclear cells of male transgenic EGFP mice. FISH was performed by using a fluorescein isothiocyanate-labeled murine Y-chromosome-specific probe (Cambio, Cambridge, UK) according to the protocol provided by Cambio with minor modifi-cations. Evaluation of signals was performed using an epifluorescence microscope (Axio-skop 2; Zeiss, Oberkochen, Germany) equipped with a fluorescein, Cy3, and DAPI filter set and a 100-W mercury lamp.
Detection of mRNA by Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
To reconfirm the findings of immunohistochemistry RT-PCR analysis was performed. Total RNA from liver tissue was isolated by using the RNeasy mini kit (Qiagen, Hilden, Germany) and digested with DNase 1 (Invitrogen). Equal amounts of RNA were used for the synthesis of cDNA. Human-specific primers were designed to detect expression of human albumin, AFP, cytochrome P-450, CK8, CK18, and CK19 mRNA in the mouse liver tissues. Expression of β-tubulin mRNA was used as a housekeeping gene. The primer sequences are listed as human albumin forward primer 5′-CCAGGAAGACATCCTTTGC-3′, reverse primer 5′-CCTGAGCCAGAGATTTCC-3′, human CK19 forward primer 5′-AGGTGGATTCCGCTCCGGGCA-3′, reverse primer 5′-ATCTTCCTGTC-CCTCGAGCA-3′, human CK18 forward primer 5′-TG-GTCACCACACAGTCTGCT-3′, reverse primer 5′-CCAA-GGCATCACCAAGATTA-3′, human CK8 forward primer 5′-TGAGGTCAAGGCACAGTACG-3′ reverse primer 5′-TGATGTTCCGGTTCATCTCA-3′ human P-450 forward primer 5′-CAGAGATGGAGAAGGCCAAG-3′ reverse primer 5′-CCCTATCACGTCGTCGATCT-3′ human AFP forward primer 5′-ACCATGAAGTGGGTGGAATC-3′, reverse primer 5′-ATTTAAACTCCCAAAGCAGCAC-3′, mouse β-tubulin forward primer 5′-GGCTTTCCTCCACTGGTACA-3′, reverse primer 5′ CACCATTTACCCCCAATGAG-3′.
The PCR conditions for human albumin were 30 seconds at 94°C, 45 seconds at 55°C, 45 seconds at 72°C, and for human CK19 1 minute at 94°C, 1 minute at 64°C, 2 minutes at 72°C. The PCR conditions for CK-18 were: 95°C for1 minute, 50°C for 1 minute, and 72°C for 1 minute. For detection of human AFP samples were heated to 94°C for 3 minutes followed by amplification cycles of 2 minutes at 94°C, 2 minutes at 62°C, and 3 minutes at 72°C. For human CK8, human cytochrome P450 mouse and β-tubulin PCR conditions were: 95°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. After 35 cycles final extension was performed at 72°C for 7 minutes in all PCR reactions. PCR products obtained with albumin primers were isolated and sequenced (GATC Biotech AG, Germany) to show homology of the sequence with published data for human albumin.
CCl4 Exposure of Cultured Hepatocytes
Mouse and human hepatocytes were prepared by a two-step collagenase perfusion method and cultured on a rat collagen matrix in hepatocyte culture medium (Gibco/BRL) containing 10% fetal calf serum. Twenty-four hours after plating the medium was removed and replaced by hepatocyte culture medium containing 15 mmol/L CCl4 for 45 minutes. AST and ALT levels were measured by an autoanalyzer according to standard protocols. The cell survival rate was estimated by the trypan blue exclusion test.
Results
Hematopoietic Reconstitution
After sublethal irradiation of the recipient NOD-SCID mice transplantation of the EGFP-positive murine mononuclear cells resulted in effective BM repopulation. Eight weeks after transplantation flow cytometry showed that 35 to 67% of the BM cells originated from the transplanted EGFP-positive cells. In 14 of 16 animals of the hUCB transplant group human leukocyte antigen-1-positive cells were detected by flow cytometry in the BM at the end of the study ranging from 0.61 to 32% of the total cells analyzed.
Liver Injury
Transaminases are released into bloodstream from damaged hepatocytes and represent an index of liver injury. Twenty-four hours after intraperitoneal application of CCl4 AST and ALT were elevated to 2254 U/L and 2423 U/L, respectively, indicating severe hepatocellular damage in the liver.
Analysis of Tissues from Mice Transplanted with EGFP-Positive BM Cells
Native fluorescence microscopy of liver (also pancreas, intestine, heart, and spleen) sections from BM cell recipient NOD-SCID mice (groups C and D) showed single fluorescent cells or aggregates of small fluorescent cells (Figure 1a), which corresponded to areas of mostly portal vein-associated cell infiltrates in H&E-stained tissue sections (Figure 1b). These cells were smaller in size than the surrounding hepatocytes and showed a higher nucleus:cytoplasm ratio. None of the single cells or aggregates of cells showed a typical staining pattern for BM-derived liver epithelial cells. In a double-immunofluorescence analysis EGFP antigen-positive cells did not stain with an antibody against DPPIV, located on canalicular membranes of hepatocytes (Figure 1; c to e). DAPI staining of rare EGFP-positive and DPPIV-negative cells showed the distinct pattern of the nuclei compared to the surrounding endogenous hepatocytes (Figure 1f). Absence of EGFP and DPPIV-positive cells indicated that BM cells did not give rise to hepatocytes after transplantation into NOD-SCID mice (groups C and D).
Figure 1.
Analysis of donor-derived epithelial cells in the livers of NOD-SCID mice transplanted with EGFP-positive BM mononuclear cells. a: Native fluorescence of one rare EGFP-positive nodule in an untreated tissue section. b: H&E staining of a representative section that was positive for EGFP in the native fluorescence microscopy. The EGFP-positive cells (arrow) are smaller in size and do not show morphological characteristics of hepatocytes. c–f: A rare GFP-positive nodule in one section was stained with GFP antibody, DPPIV antibody, and with DAPI together. Immunofluorescence staining for EGFP (c) and the canalicular marker DPPIV (d). e: The merged figure clearly demonstrates that this rarely found EGFP-positive nodule is not positive for DPPIV supporting the absence of hepatocyte-like cells in this nodule. f: DAPI staining of this nodule shows the distinct pattern of the nuclei in the EGFP-positive nodule compared to the surrounding endogenous hepatocytes. Original magnifications: ×20 (a, c–f); ×10 (b).
A group of female NOD-SCID mice (n = 6) was transplanted with BM mononuclear cells derived from male EGFP-positive and normal C57BL/6 mice. FISH analysis was performed to detect Y-chromosome in the recipient female mouse liver. In male mouse liver, which served as positive control, most of the cells stained positive for Y-chromosome with fluorescein isothiocyanate signals at the edge of nuclei (Figure 2a). Female nuclei (negative control) showed complete absence of Y-chromosome fluorescein isothiocyanate signals (Figure 2c). In the group of transplanted animals we rarely found Y-chromosome-positive nuclei in analyzed sections of recipient livers (Figure 2b). Co-staining of Y-chromosome FISH and either CK18 or albumin rarely detected a single hepatocyte-like cell (1 cell per 10 sections) with typical hepatocyte morphology in transplanted animals (data not shown).
Figure 2.
Mouse Y-chromosome FISH in gender-mismatched transplanted mice. a: Normal male mouse liver showing successful detection of Y-chromosome-positive nuclei. b: A rare Y-chromosome-positive nucleus in the mouse liver, transplanted with bone morrow cells of transgenic GFP mice (arrow). c: Normal female mouse liver showing complete absence of Y-chromosome. Original magnifications: ×63 (a); ×100 (b and c).
The results shown for transplanted adult BM mononuclear cells were similar in mice that have been transplanted with BM cells derived from 3-, 2-, and 1-week-old young mice with and without CCl4 damage of the liver or with HGF and without HGF treatment after induction of the liver damage. As expected, there were many EGFP-positive cells in the spleen, demonstrating the contribution of the transplanted cells to the hematopoietic system outside the BM. We were, however, not able to detect any EGFP-positive parenchymal cell in other organs such as pancreas, heart, or intestine by native fluorescence and immunofluorescence analysis.
To rule out the possibility of a differential toxic effect of CCl4 on murine and human hepatocytes, cell culture experiments with primary cells were performed. Both types of cells were treated with CCl4 for 45 minutes and AST as well as ALT concentrations were measured in the supernatant (Figure 3a). There was no significant difference in the amount of ALT and AST released into the medium and also no difference in overall survival of the cells as measured by the trypan blue exclusion test (Figure 3b).
Figure 3.
Effect of CCl4 on human and mouse hepatocytes. a: AST and ALT were measured in supernatant by biochemical analyzer. Detoxification by CCl4 was found to be similar because there is no significant difference between values of AST and ALT. b: Percentage survival of human and mouse hepatocytes by trypan blue exclusion test also demonstrates similar detoxification effect of CCl4 on both cell types.
Tissue Analysis after Xenogenic Transplantation
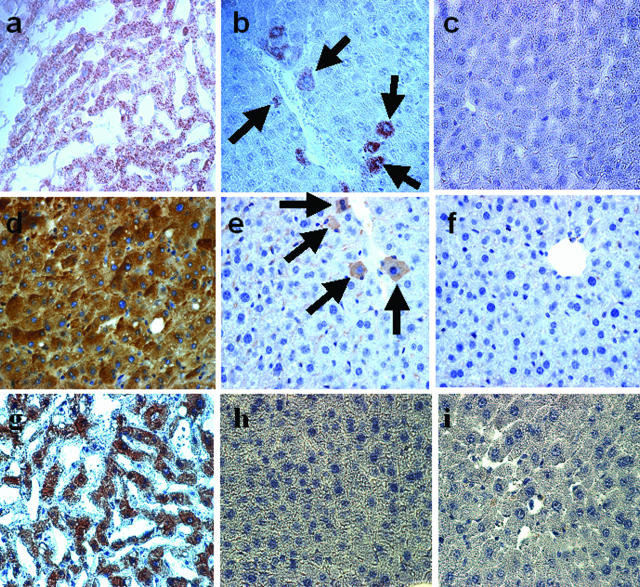
Four weeks after application of CCl4, cells of human origin were detected in the livers of recipient NOD-SCID mice. By staining of liver sections with the Hep Par 1 antibody, which specifically stains human hepatocytes (Figure 4a), single cells and small groups of cells mostly located in close vicinity to vascular structures were detected (Figure 4b). Liver sections from sham-transplanted NOD-SCID mice were found to be completely negative for Hep Par 1 staining (Figure 4c). Tissue from transplanted animals was further analyzed for expression of human albumin, which is produced exclusively by hepatocytes. Cells expressing human albumin were observed by immunohistochemistry in human liver sections (Figure 4d) and in hUCB-transplanted (Figure 4e) but not in sham-transplanted animals (Figure 4f). The frequency of hUCB-derived hepatocyte-like cells varied from section to section and mouse to mouse as well (Table 1). The Hep Par 1-positive, albumin-producing cells, which were detected after hUCB transplantation, however, did not stain for the human CK18 antigen (Figure 4h), as shown in human liver sections used as a positive control (Figure 4g). In addition human CK8, an important marker for hepatocyte development, was also tested by immunohistochemistry. We were, however, not able to find even a single human CK8-expressing cell in the human UCB-transplanted NOD-SCID mice (data not shown).
Figure 4.
a–c: Detection of hUCB-derived cells in the liver of a NOD-SCID mouse after CCl4 injury and HGF treatment by immunohistochemistry with the HepPar1 antibody. a: Human liver section was used as a positive control. b: Liver section from hUCB-transplanted NOD-SCID mice: single cells and small clusters of donor-derived cells stained positive for the human hepatocellular antigen Hep Par1 in hUCB-transplanted NOD-SCID mice (arrows). c: Sham-transplanted NOD-SCID liver ensures the complete absence of HepPar1-positive cells. d–f: Immunohistochemistry for human albumin in liver sections of NOD-SCID mice after hUCB BM reconstitution. d: Human liver sections were used as positive control. e: Single and small clusters of cells stained positive for human albumin in hUCB-transplanted NOD-SCID mice (arrows). f: Sham-transplanted NOD-SCID mouse liver. No human albumin-positive cell was detectable demonstrating the specificity of the antibody staining. g–i: Immunohistochemistry for CK18 expression in hUCB-transplanted NOD-SCID mice. g: In human liver the epithelial marker CK18 is strongly expressed by hepatocytes. h: Notably, CK18 expression was absent in hUCB-transplanted NOD-SCID mice suggesting an immature phenotype of the donor-derived cells. i: Liver sections of sham-transplanted NOD-SCID mice served as negative control. Original magnifications, ×40.
We next prepared RNA from the liver of hUCB-transplanted mice and performed RT-PCR for human liver-specific genes. Liver tissues from transplanted animals showed the presence of human albumin mRNA indicating the presence of hUCB-derived albumin-producing cells in the liver (Figure 5). A 329-bp albumin band was detected in 8 of 10 human UCB-transplanted NOD-SCID mice, which were injected with CCl4 (group A). This 329-bp product was eluted from the gel for sequencing and found to be human albumin. Human CK18 and CK19 (a cholangiocyte marker) and α-1 fetoprotein (marker for hepatic progenitor cells) were not detectable by RT-PCR in the liver from hUCB-transplanted mice and confirms the results obtained by immunohistochemistry. Although we have detected hepatocyte-like cells by immunohistochemistry in the livers of human UCB-transplanted NOD-SCID mice, which have not been treated with CCl4 (group B), the 329-bp human albumin band was not detectable. Again, the human CK8 and cytochrome P450 mRNAs were not detected in livers from human UCB-transplanted mice (not shown).
Figure 5.
RT-PCR of human hepatocellular genes in hUCB-transplanted NOD-SCID mice after CCl4 and HGF treatment. Lane 1: Liver tissue of sham-transplanted NOD-SCID mice served as negative control. Lane 2: Positive controls: cDNA from human liver for alb, CK18, and CK19, from HUH-7 human hepatoma cells for AFP. Lane 3: Liver tissue of hUCB-transplanted NOD-SCID mice. Specific PCR products were obtained with cDNA of transplanted NOD-SCID mouse liver for human albumin but not for CK18, CK19, and AFP. In addition to these markers, human CK8 and cytochrome P450 could not be detected in cDNA of transplanted NOD-SCID mouse liver (not shown). No PCR product was detected in the absence of reversed transcriptase (not shown). hALB, human albumin; hAFP, human α-fetoprotein; hCK18, human CK18; hCK19, human CK19; mβ-tub, mouse β-tubulin.
We further studied whether hUCB-derived hepatocyte-like cells in the transplanted NOD-SCID mice could have resulted from fusion with mouse hepatocytes. In serial sections Hep Par 1-positive cells were stained for human and mouse CK18 (Figure 6). Serial sections with a Hep Par 1-positive cell (Figure 6a) were stained with human CK and mouse CK (Figure 6, b and c). Immunofluorescence analysis revealed that the Hep Par 1-positive cell was also stained by mouse CK18, but not with a human-specific CK18 antibody. Staining of cytoplasmic mouse CK18 and absence of human CK18 in serial sections would indicate a fusion origin of these Hep Par 1-positive cells. Similar results were obtained with immunohistochemical staining based on the horseradish peroxidase-diaminobenzidine detection for human CK18 (Figure 6g).
Figure 6.
Immunostaining of serial sections reveals that hepatocyte-like cells were result of fusion (green, Hep Par 1; blue, human CK18; and red, mouse CK18). a–c: Serial sections of liver from hUCB-transplanted mouse. a: First serial section shows presence of donor-derived cells stained positive (arrow) for the human hepatocellular antigen Hep Par1, detected by Alexa Fluor 488 (green). b: Next section was stained for human CK18, which shows complete absence of human CK18. c: In third serial section all cells were found to be positive for mouse CK18. d: Human liver section stained for Hep Par 1 as a positive control. e: Human liver section stained for human CK18 as a positive control. f: DAPI staining of first serial section. g: Serial section was stained for human CK18, which also shows complete absence of human CK18, by immunohistochemical staining. h: H&E staining of one of these serial section. Original magnifications: ×10 (a–c, f–h); ×20 (d, e and insets in a–c).
Discussion
We have tested the hypothesis that the selection of cell sources and differences in transplantation models and mouse backgrounds may explain discrepancies observed in the ability of hematopoietic stem cells to differentiate into cells of endodermal origin in vivo. Recently, Newsome and colleagues,27 Wang and colleagues,28 and Ishikawa and colleagues29 were able to show that mononuclear cell preparations of hUCB give rise to hepatocytes after transplantation into sublethally irradiated NOD-SCID mice. With similar hematopoietic stem cell preparations derived from murine BM we and other groups could not show evidence for plasticity or direct differentiation in BM reconstitution experiments or after direct transplantation of cells into the liver.6,19,20 Several reasons could potentially explain these conflicting results. First, umbilical cord blood-derived cells may be more plastic in vivo than cells derived from mouse BM. Second, transplantation of cells into mice with a NOD-SCID immunodeficient background may facilitate the differentiation of stem cells into hepatocytes and third, neonatal hUCB cells may have a reservoir of preformed hepatic progenitors, which may not be present in mouse BM preparations.25,26
In our experiments we did not find evidence that the NOD-SCID immunodeficient background of the recipient animals facilitated the in vivo differentiation of murine hematopoietic stem cells into hepatocytes. We used EGFP transgenic murine hematopoietic cells for the transplantation study to unequivocally detect BM-derived cells in the liver. To rigorously control our experiments, we always included analysis of liver sections from mice that have been successfully transplanted with fetal hepatoblasts derived from the same EGFP transgenic donor mouse strain31 and also of liver sections from sham-transplanted mice. Although rare single cells and aggregates of BM-derived cells were present in the livers of murine BM cell-transplanted NOD-SCID mice, none of these cells met the criteria of a differentiated liver cell.
It has been argued that transgene extinction could lead to the failure to detect transdifferentiated cells in the liver.32 To address this issue Y-chromosome FISH analysis was performed in gender-mismatched transplanted animals. Although we were able to detect a few single Y-chromosome-positive cells in recipient livers, their frequency (2 to 3 per 10 sections; 1 per 10 sections meets criteria of co-staining with albumin or CK18) was very low.
In comparison to these results we infused mononuclear human cord blood cells into the NOD-SCID mice and followed the same transplantation protocol as applied for the murine hematopoietic cells. In the livers of these animals we were able to detect cells reacting specifically with the Hep Par 1 antibody, which exclusively stains human hepatocytes, as previously reported.33 Because the distinct antigen for the Hep Par 1 antibody is not known, we tested for additional markers of human liver cells in the hUCB-transplanted mouse liver and detected human albumin protein-producing cells by immunohistochemistry and human albumin mRNA expression by RT-PCR.
The number of Hep Par 1, albumin-positive cells in hUCB-transplanted mouse liver was higher than the frequency of hepatocyte-like cells derived from transplanted mouse BM in the livers of recipient mice. Compared to the hUCB cells, which were harvested immediately after birth, the applied adult mouse BM cells may have lost most of their capacity to differentiate into hepatocytes. We therefore transplanted mouse BM cells isolated from 1-, 2-, and 3-week-old EGFP-transgenic mice into NOD-SCID recipients followed by CCl4 application. We were, however, not able to detect a higher frequency of BM-derived hepatocytes in the livers of recipient animals either by native fluorescence, immunofluorescence, or FISH analysis compared to adult BM recipients (data not shown).
A characteristic phenotype of adult parenchymal liver cells is the expression of human CK8 and CK18 (hepatocytes) and CK19 (cholangiocytes). To our surprise the human CKs 8, 18, and 19 were neither detected by immunohistochemistry nor by RT-PCR. The lack of human CKs 8 and 18 could be explained by an immature phenotype of the Hep Par 1-positive, albumin-producing cells. We therefore analyzed the expression of α-fetoprotein, which is a marker of immature liver progenitor cells. In none of the livers from hUCB recipient mice was α-fetoprotein detected by RT-PCR. Although earlier reports have shown Hep Par 1-positive cells in the liver of hUCB-recipient NOD-SCID mice similar to ours, we were thus not able to show full evidence for the formation of mature human hepatocytes. We conclude from our results that the cells detected in the liver of hUCB-transplanted NOD-SCID mice show some specific human hepatic markers but do not fulfil all criteria of a human hepatocyte. This may be due to the fact that fusion results in a cell type characterized by chimeric gene and protein expression.
Liver damage has been proposed as a stimulus for differentiation of extra hepatic stem cells into hepatocytes.14,34 Therefore we have chosen a liver injury model and injected the liver toxin CCl4 4 weeks after cell transplantation to induce a temporary liver failure. In our model the CCl4 toxin may have shown differential toxicity on mouse and human liver cells. In cell culture experiments performed with primary human and mouse hepatocytes similar toxicity was observed (Figure 3). From these experiments we conclude that the hUCB-derived cells in the recipient liver may not have received a survival advantage by the application of the CCl4 toxin. Some animals also received HGF to stimulate hepatic regeneration after the injury. HGF has also been shown to be essential for the induction of a hepatic phenotype in hUCB in vitro.35 No effect was observed on the frequency of hepatic differentiation of murine BM-derived cells. In the hUCB recipient animals there were more Hep Par 1-positive and albumin-producing cells in CCl4- and HGF-injected animals. The differences, however, did not reach significance levels. Staining of serial sections with human- and mouse-specific antibodies as shown in Figure 6 favors fusion rather than direct differentiation, because Hep Par 1-positive cells showed a murine CK expression profile. Although the presence of mouse and human markers in hUCB-derived cells supports fusion as the main mechanism in the analyzed tissue sections, we cannot completely rule out the possibility of incomplete direct differentiation.
We conclude, that in the chosen experimental setting human cord blood cells more frequently contribute to the formation of hepatocyte-like cells than adult or neonatal mouse BM cells after transplantation into NOD-SCID mice. Molecular analysis, however, suggests that these cord blood-derived cells in the liver represent human/mouse chimera that do not show a mature or complete human hepatocyte phenotype due to lack of appropriate mRNA and protein markers. The adult mouse regenerating liver environment does not provide the necessary molecular signals for full differentiation of hUCB into mature human hepatocytes because it was demonstrated after transplantation of these cells into fetuses of sheep.23 Detailed molecular analysis of the chimeric cells and further transplantation studies including animal disease models are necessary to investigate the therapeutic potential of hUCB cells.
Footnotes
Address reprint requests to Michael Ott, M.D., Department of Gastroenterology, Hepatology, and Endocrinology, Hannover Medical School, Carl-Neuberg-Str. 1, 30623, Hannover. Germany. E-mail: ott-mhh@gmx.de.
Supported by the Deutsche Forschungsgemeinschaft (grant KF0110/1Ot) and the Bundesministerium für Bildung und Forschung (grant FK0312683).
Present address of T.C.: Max-Planck-Institute for Molecular Biomedicine, Department of Cell and Developmental Biology, Muenster, Germany; present address of L.A.: Cytonet Hannover GmbH, Hannover, Germany.
References
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Alison MR, Poulsom R, Jeffrey R, Dhillon AP, Qualglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci USA. 2003;100:1364–1369. doi: 10.1073/pnas.0336479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- Theise ND, Nimmakayalu M, Gardner R, Illei P, Morgan G, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Charlton CA, Brazelton TR, Hackman RC, Blau HM. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc Natl Acad Sci USA. 2003;100:2088–2093. doi: 10.1073/pnas.0337659100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- Kanazawa Y, Verma IM. Little evidence of bone marrow-derived hepatocytes in the replacement of injured liver. Proc Natl Acad Sci USA. 2003;100:11850–11853. doi: 10.1073/pnas.1834198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantz T, Sharma AD, Jochheim-Richter A, Arseniev L, Klein C, Manns MP, Ott M. Reevaluation of bone marrow derived cells as a source for hepatocyte regeneration. Cell Transpl. 2004;13:659–666. doi: 10.3727/000000004783983521. [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Willing AE, Zigova T, Saporta S, Justen EB, Lane JC, Hudson JE, Chen N, Davis CD, Sanberg PR. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J Hematother Stem Cell Res. 2003;12:255–270. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Allouard Q, Pettengell R. Umbilical cord blood stem cells can expand hematopoietic and neuroglial progenitors in vitro. Exp Cell Res. 2004;295:350–359. doi: 10.1016/j.yexcr.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004;104:2582–2590. doi: 10.1182/blood-2004-01-0259. [DOI] [PubMed] [Google Scholar]
- Mayani H, Lansdorp PM. Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells. 1998;16:153–165. doi: 10.1002/stem.160153. [DOI] [PubMed] [Google Scholar]
- Kubota H, Storms RW, Reid LM. Variant forms of α-fetoprotein transcripts expressed in human hematopoietic progenitors. J Biol Chem. 2002;277:27629–27635. doi: 10.1074/jbc.M202117200. [DOI] [PubMed] [Google Scholar]
- Wernet P, Fischer J, Caplan A, Zanjani E, Muller HWH, Knipper A, Kogler G. Isolation of non-hematopoietic stem cells from umbilical cord blood. Biol Blood Marrow Transpl. 2004;10:738–739. [Google Scholar]
- Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC, Harrison DJ, Bickmore WA, Plevris JN. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891–1900. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201–4208. doi: 10.1182/blood-2002-05-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F, Drake CJ, Yang S, Fleming P, Minamiguchi H, Visconti RP, Crosby CV, Argraves WS, Harada M, Key LL, Jr, Livingston AG, Wingard JR, Ogawa M. Transplanted human cord blood cells give rise to hepatocytes in engrafted mice. Ann NY Acad Sci. 2003;996:174–185. doi: 10.1111/j.1749-6632.2003.tb03245.x. [DOI] [PubMed] [Google Scholar]
- Boeyum A. Isolation of leucocytes from human blood: further observations—methylcellulose, dextran, and Ficoll as erythrocyte aggregating agents. Scand J Clin Lab Invest. 1968;21:31–50. [PubMed] [Google Scholar]
- Cantz T, Zuckerman DM, Burda MR, Dandri M, Göricke B, Thalhammer S, Heckl WM, Manns MP, Petersen J, Ott M. Quantitative gene expression analysis reveals transition of fetal liver progenitor cells to mature hepatocytes after transplantation in uPA/RAG-2 mice. Am J Pathol. 2003;162:37–45. doi: 10.1016/S0002-9440(10)63796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theise ND. Restoring balance to liver stem cell research. J Hepatol. 2004;41:673–676. doi: 10.1016/j.jhep.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Wennerberg AE, Nalesnik MA, Coleman WB. Hepatocyte paraffin 1: a monoclonal antibody that reacts with hepatocytes and can be used for differential diagnosis of hepatic tumors. Am J Pathol. 1993;143:1050–1054. [Google Scholar]
- Grompe M, Lindstedt S, al-Dhalimy M, Kennaway NG, Papaconstantinou J, Torres-Ramos CA, Ou CN, Finegold M. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1995;10:453–460. doi: 10.1038/ng0895-453. [DOI] [PubMed] [Google Scholar]
- Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K, Yasumizu T, Teraoka H. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217–227. doi: 10.1634/stemcells.21-2-217. [DOI] [PubMed] [Google Scholar]