Abstract
Allograft inflammatory factor (AIF)-1 is a cytoplasmic, calcium-binding protein whose expression in transplanted human hearts correlates with rejection and development of coronary artery vasculopathy (CAV). AIF-1 is constitutively expressed in monocytes/macrophages, but its expression in human lymphocytes has not been described. After immunohistochemical analysis of human coronary arteries with CAV, we identified AIF-1 expression in CD3-positive lymphocytes. AIF-1 was differentially expressed in peripheral blood mononuclear cells and in the T-lymphoblastoid MOLT-4 cell line exposed to various cytokines, suggesting a role for AIF-1 in T-lymphocyte activation. To determine AIF-1 function, MOLT-4 cells were stably transduced by AIF-1 retrovirus. Overexpression of AIF-1 in these cells led to a 238% increase in cell number compared to empty vector controls. AIF-1 polymerized nonmuscle actin and MOLT-4 cells overexpressing AIF-1 migrated 95% more rapidly than empty vector controls. Primary human vascular smooth muscle cells cultured in conditioned media from AIF-1-transduced MOLT-4 cells proliferated 99% more rapidly than vascular smooth muscle cells cultured in conditioned media from empty vector-transduced MOLT-4 cells. These data indicate that AIF-1 is expressed in activated T lymphocytes, that its expression enhances activation of lymphocytes, and that AIF-1 expression in activated lymphocytes may have important ramifications for activation of adjacent arterial vascular smooth muscle cells and development of CAV.
Although progress in immunosuppressive therapy has been successful in reduction of graft rejection, the vascular narrowing indicative of graft vascular disease remains the major complication that limits long-term survival of solid organ transplantation.1 In the case of cardiac allografts, this disease is characterized by a diffuse, concentric intimal hyperplasia extending throughout the coronary arterial tree, veins, and capillaries. Consequently, interventions useful for patients with conventional coronary artery disease are not applicable to the majority of patients with coronary artery vasculopathy (CAV) because of its extensive nature. The pathogenesis of CAV is believed to involve a chronic immune response of the recipient to the donor vasculature in which activated recipient immune cells damage the endothelium, resulting in the production of cytokines that elicit activation and proliferation of medial vascular smooth muscle cells (VSMCs).2 The activation of VSMCs is responsible for most of the obliterative arterial intimal thickening present in solid organ allografts as well as in CAV.3,4
The important role of T lymphocytes in promotion of CAV, even in the absence of rejection, has been well established. Activated T lymphocytes, in addition to macrophages and VSMCs, make up much of the cellular content of the neointima in CAV vessels.5 Products of activated T cells, granzyme A and perforin, have been identified as markers of rejection and compromised cardiac function in transplanted human hearts.6 In animals, it has been shown that increasing the frequency of donor-reactive T cells did not mediate acute rejection but did increase the rate and severity of transplant vasculopathy.7 Several studies have demonstrated that T-helper cells play an essential function in development and severity of neointimal thickening associated with allograft vasculopathy,8–10 suggesting a fundamental role in activation of vascular cells. Consequently, identification and characterization of proteins that link vascular/immune communication may elucidate the molecular mechanisms of many vascular diseases.
Allograft inflammatory factor-1 (AIF-1) is a 143-amino acid, cytoplasmic, evolutionarily conserved, calcium-binding protein. AIF-1 is constitutively expressed in macrophages and glial cells, and has been implicated in the inflammatory process of several cell types. Data from several groups in diverse systems advocate an important role for AIF-1 in inflammatory processes ranging from expression in infiltrating macrophages in rat cardiac allografts,11 in microglial injury and activation,12 and in the allograft response of phylogenetically distant species such as carp and marine sponges.13,14 In humans, the AIF-1 gene maps to a the major histocompatibility complex class III region on chromosome 6p21.3, which is known for clusters of genes involved in the inflammatory response. Although constitutively expressed in lymphoid tissue, we have determined that AIF-1 is not expressed in VSMCs, but can be induced by injury and inflammatory cytokines.15 Overexpression of AIF-1in VSMCs leads to increased proliferation and migration, and expression of AIF-1 in cardiac allografts is associated with the severity of CAV.16–18
The function of AIF-1 in T lymphocytes has not been reported, nor have the functional consequences of lymphocyte expression on local VSMC pathophysiology been explored. In this study we determine that AIF-1 is expressed in infiltrating T lymphocytes in human coronary arteries with CAV as well as in cytokine-activated cultured lymphocytes. The purpose of this study is to determine the functional significance of AIF-1 expression in lymphocytes, and determine how this expression might impact development of CAV. In this article, we describe AIF-1 expression in CD3-positive lymphocytes in human coronary arteries with CAV, and characterize the effects of AIF-1 overexpression on T-lymphocyte migration and proliferation. We also determine the effects of AIF-1-activated lymphocytes on human VSMC function.
Materials and Methods
Immunohistochemistry
Coronary arteries from transplanted hearts with CAV were used in this study. All tissue procurement protocols were approved by the Institutional Review Board for Human Studies at Temple University. Tissue sections were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 5 μm, deparaffinized in xylene, and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked with 1.5% hydrogen peroxide in methanol for 15 minutes. After blocking with normal serum, sections were incubated with primary antibodies for 1 hour at room temperature. Antibody for CD3 (T-lymphocyte marker) was used at a concentration of 2 μg/ml. AIF-1 antibody, which has been previously described, was used at 1.0 μg/ml.18 Sections were then incubated with biotinylated secondary antibody (1:200) followed by avidin-biotin-peroxidase complex in the Vectastain Elite kit (both from Vector Laboratories, Burlingame, CA). The reaction product was visualized using 3,3′-diaminobenzidine (Vector Laboratories) as the chromogenic substrate, producing a reddish-brown stain. The sections were counterstained with hematoxylin.
Cells and Culture
Primary human coronary VSMCs were obtained as cryopreserved secondary culture from Cascade Corp. (Portland, OR) and subcultured as described.17 Cells from passage 3 to 6 were used in the described studies. All cytokines and phytohemagglutinin A (PHA) were purchased from Sigma (St. Louis, MO). MOLT-4 cells were purchased from American Type Culture Collection (Rockville, MD) and cultured in RPMI medium supplemented with 10% fetal calf serum. Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy human donors by venipuncture, isolated by Ficoll-Hypaque gradient centrifugation, and cultured as for MOLT-4 cells.
Retroviral Stable Transduction and Proliferation Assay
AIF-1 retrovirus (AIF-1RV) was constructed using a kit from Clontech (La Jolla, CA) according to the manufacturer’s instructions as previously described.17 Briefly, the protein-coding region of the AIF-1 cDNA was inserted into the retroviral packaging vector pLXSN containing the gentimycin resistance gene. For some samples, cDNA coding for the 9-amino acid hemagglutinin antigen (HA) tag was inserted 3′ to the AIF-1 sequence. This was transfected into an ectotropic 293-derived packaging cell line.19 Media was then collected from these cells and used to infect an amphitropic second packaging cell line, PT-67, according to the manufacturer’s instructions. This supernatant containing recombinant high-titer virus was then used to infect MOLT-4 cells in two 4-hour exposures of viral supernatant in the presence of 8 μg/ml of Polybrene (∼40 to 50% stable transduction was achieved). Stably transduced G418-resistant cells were pooled from each transduction rather than individual clones to avoid the effects of clonal variation. For proliferation assays, equal numbers of stable transfectants were seeded onto 12-well plates at a density of 7500 cells/ml. Medium was changed on the 4th day, and after 1, 4, and 7 days, viable, trypan blue-excluding cells were counted using a standard hemocytometer. Statistical analyses from at least three independent experiments were performed by paired sample t-test.
Migration and Chemotaxis
For analysis of chemotaxis, 6.5-mm diameter transwell Boyden chamber plates (Costar, Cambridge, MA) with 8-μm polycarbonate membrane pore size were seeded with stably transduced MOLT-4 cells (50,000 cells per membrane) in 600 μl of medium containing 0.5% fetal calf serum as described.20 Sixty μl of fetal calf serum was placed in the lower chamber and cells were incubated for the indicated times at 37°C, at which time cells in the lower chamber were recovered and counted in a hemocytometer. Experiments were performed in triplicate from three independently derived stably transduced groups of MOLT-4 cells.
Western Blotting
MOLT-4 cell extracts were prepared and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and protein transfer were performed as described.2 Membranes were incubated with a 1:2000 dilution of AIF-1 primary antibody, and a 1:2000 dilution of secondary antibody. Equal protein concentrations of cell extracts were determined by Bradford assay and equal loading on gels were verified by Ponceau S staining of the membrane. Reactive proteins were visualized using enhanced chemiluminescence.
Actin Interaction and Polymerization
The AIF-1 protein-coding region was cloned into the pGEX vector and expressed in Escherichia coli as a glutathione S-transferase (GST) fusion protein according to the manufacturer’s protocol (Pharmacia, Inc., Uppsala, Sweden). The affinity pull-down and actin polymerization assays were performed as described.15 Briefly, extracts from MOLT-4 cells were first precleared with GST Sepharose beads, and then incubated with GST Sepharose beads (lane 3), or with AIF-1/GST Sepharose beads (lane 4). Pellets were washed and interacting proteins separated by SDS-PAGE and identified by Western blot with anti-actin antibody. In vitro nonmuscle actin polymerization was determined by differential centrifugation in a kit purchased from Cytoskeleton, Inc. (Denver, CO), according to the manufacturer’s instructions. Briefly, 23 μmol/L of AIF-1 and nonmuscle actin were incubated together for 30 minutes in F-actin buffer containing ATP and Ca2+, then centrifuged at 8000 × g, the speed at which only cross-linked actin will pellet. Protein in the pellets and supernatants were analyzed by SDS-PAGE, Coomassie staining, and densitometry using NIH Image software.
Results
AIF-1 Is Expressed in CD3-Positive Lymphocytes
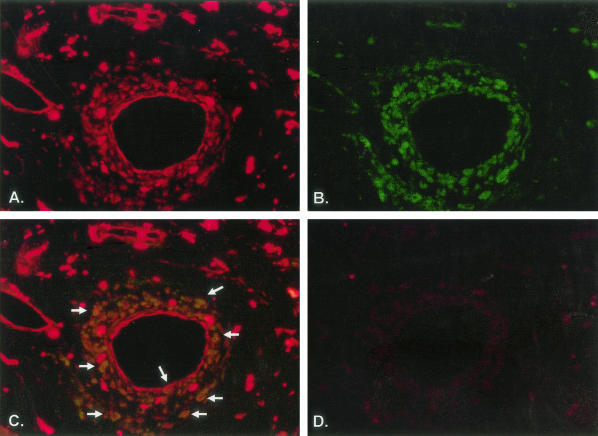
Several different cell types are involved in the pathogenesis and progression of CAV.1 Because AIF-1 expression has been linked to the development and severity of CAV, it was important to determine which cell populations expressed this protein. AIF-1 expression in activated VSMCs and monocytes has been previously reported. In this study, immunohistochemical analysis of human coronary arteries with CAV determined AIF-1 expression in CD3-positive lymphocytes (Figure 1A). Expression of AIF-1 in human lymphocytes has not been described, and subsequent experiments were designed to characterize this expression and its functional implications in T-lymphocyte pathophysiological processes.
Figure 1.
Immunohistochemical analysis of AIF-1 expression in a human coronary artery. A to D: Sections from a human artery with transplant arteriopathy. A: Expression of AIF-1 in red-staining cells; B: CD3-positive cells are in green; C: merged images showing co-expression of AIF-1- and CD3-positive cells. Arrows depict examples of co-staining. D: Negative control consisting of secondary antibody only.
AIF-1 Is Differentially Expressed by Cytokine Stimulation
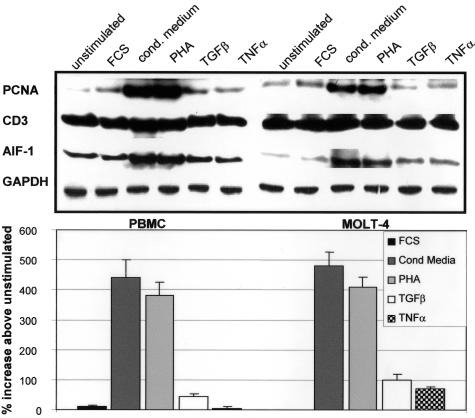
Differential cytokine induction of AIF-1 has been described in monocytes and VSMCs.11,15 To determine whether AIF-1 expression could be modulated by cytokines in lymphocytes, we challenged normal, human PBMCs and the human T-lymphoblastoid cell line MOLT-4 to various cytokines, and examined AIF-1 expression by Western blot. Several similarities in sensitivity to stimuli and degree of AIF-1 expression by these stimuli were noted between these cells. Figure 2 shows a constitutive expression of AIF-1 in both unstimulated PMBCs and MOLT-4 cells. T-lymphocyte-conditioned medium and PHA elicited the strongest induction of AIF-1 expression, with mean increases of 4.4- and 4.8-fold for conditioned medium, and 3.8- and 4.1-fold for PHA, in PBMCs and MOLT-4 cells, respectively. It is interesting to note that AIF-1 expression paralleled proliferation of these cells, as both of these stimuli induced proliferating cell nuclear antigen to the greatest degree. Although both of these cell populations express AIF-1 and displayed a similar response to stimuli, MOLT-4 cells were used as a surrogate to further characterize the role of AIF-1 expression in T-lymphocyte activation because they are a homogenous cell population and responses to stimuli would not vary from experiment to experiment, and more importantly, because they are immortalized, which facilitates stable transduction for long-term functional studies.
Figure 2.
Expression of AIF-1 protein in activated human lymphocytes. Human PBMCs and the human T-myeloblast cell line, MOLT-4, were serum-starved for 48 hours, and protein from 1) serum-starved, and cells treated for 40 hours with 2) 15% fetal calf serum; 3) T-cell conditioned media; 4) PHA; 5) transforming growth factor-β; and 6) tumor necrosis factor-α. Extracts from these cells were subjected to Western blot with anti-AIF-1, proliferating cell nuclear antigen, CD3, and GAPDH antibody. Bands were quantitated by densitometry, and normalized to GAPDH. Blot shown is representative of three performed from different groups of PMBC and MOLT-4 cells. Bar graph indicates percent expression above unstimulated cells for three groups of experiments.
AIF-1 Expression Enhances T-Lymphocyte Proliferation
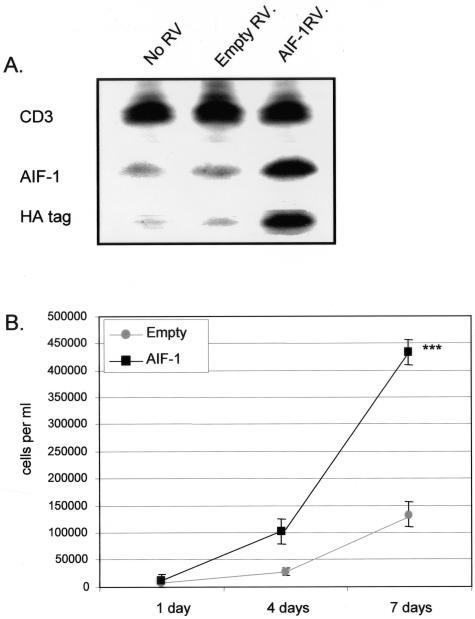
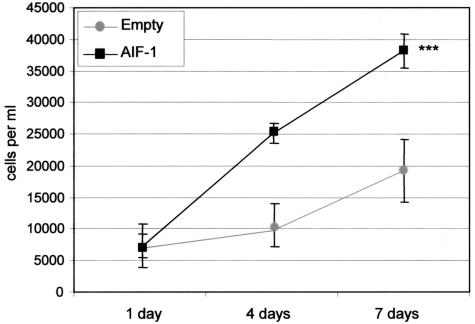
To characterize the functional effects of AIF-1 expression in lymphocytes, we overexpressed AIF-1 by retroviral (RV) gene transduction in MOLT-4 cells and examined various parameters of T-lymphocyte activation including proliferation and migration. MOLT-4 cells were transduced with AIF-1 RV containing the protein-coding region of AIF-1 cDNA (AIF-1-RV) tagged with the hemagglutinin epitope (HA) or with vector alone (empty RV), and stable transductants were selected with antibiotic and pooled. Constitutive and retroviral delivered AIF-1 protein expression was verified by Western blot (Figure 3A). To examine effects on proliferation, equal numbers of stable transductants were seeded into 12-well plates, and after 1, 4, and 7 days, viable, trypan blue-excluding cells were trypsinized and counted. Figure 3B shows that MOLT-4 cells that constitutively overexpress AIF-1 demonstrate a mean 238% increase in proliferation compared with control cells (4.3 × 105 versus 1.2 × 105, for AIF-1 and empty vector, respectively) (P < 0.001). Table 1 demonstrates the population doubling times for four separate experiments that were calculated and overall, AIF-1-expressing MOLT-4 cells have a 41% decrease in doubling time (47.8 ± 4.3 hours for empty vector, and 28.2 ± 0.8 hours for AIF-1, respectively). These differences are not due to an increase in viability of AIF-1-expressing cells because both control and AIF-1-overexpressing cells had less than 5% trypan blue-positive cells. Together, these results indicate that increased expression of AIF-1 leads to increased proliferation of MOLT-4 cells.
Figure 3.
AIF-1 mediated proliferation of MOLT-4 cells. A: Stable expression of AIF-1 protein in MOLT-4 cells. The human T-myeloblast cell line, MOLT-4, was transduced with retrovirus containing AIF-1 tagged with the hemagglutinin (HA) or empty vector retrovirus, and selected with antibiotic. Resistant populations were pooled and subjected to Western blot with anti-AIF-1 to determine endogenously expressed, or HA tag antibody to identify virally transduced AIF-1. CD3 antibody was used as a loading control. Blot shown is representative of three performed from different groups of stably transduced MOLT-4 cells. B: Overexpression of AIF-1 results in enhanced MOLT-4 proliferation. Equal numbers of pooled MOLT-4 cells stably transduced with AIF-1 retrovirus or empty vector were seeded into 12-well plates and grown in growth medium. After 1, 4, and 7 days, viable trypan blue-excluding cells were harvested and counted in triplicate. Viability was greater than 95% for both empty vector and AIF-1-transduced cells. Numbers on the y-axis indicate cells per well. Values are means of three independent transfections with similar results (P < 0.001). Error bars are SEM from three independent transfections each performed in triplicate.
Table 1.
Calculated Doubling Times of Stably Transduced MOLT-4 Cells
| Experiment | 1 | 2 | 3 | 4 | Mean±SD |
|---|---|---|---|---|---|
| Empty vector | 49.7 | 43.6 | 43.9 | 54.1 | 47.8 ± 4.3 |
| AIF-1 | 27.0 | 28.8 | 28.0 | 29.1 | 28.2 ± 0.8 |
Equal numbers of pooled, stably transduced MOLT-4 were seeded into 12-well plates. Media was changed on the 4th day, and after 1, 4, and 7 days, cells were trypsinized and counted using a standard hemocytometer. Doubling times were calculated as described in Materials and Methods.
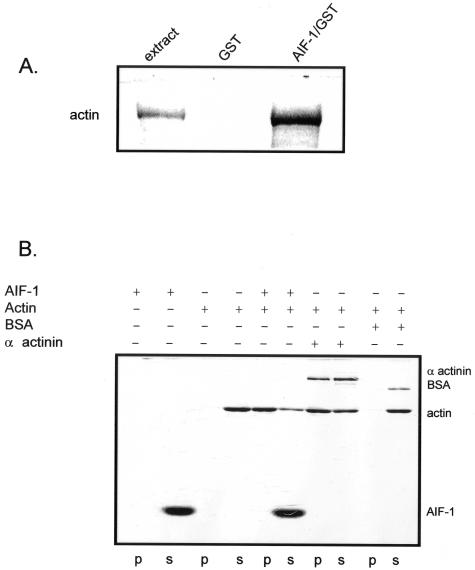
AIF-1 Interacts with and Polymerizes Nonmuscle Actin
In human VSMCs, we previously found AIF-1 to interact with and polymerize F-actin, and we hypothesized that AIF-1 would have the same effects in lymphocytes.17 Protein extracts from MOLT-4 cells were precleared with GST protein, then incubated with an affinity resin consisting of a recombinant AIF-1-GST fusion protein coupled to glutathione Sepharose beads or GST Sepharose beads as a negative control. The beads were washed, interacting proteins were separated by SDS-PAGE, and actin interaction identified by Western blot (Figure 4A). To verify this interaction and determine the effect of AIF-1 on actin dynamics, purified nonmuscle cell F-actin was incubated with recombinant AIF-1 protein, and after incubation, F-actin was pelleted at low speed (8000 × g), which allows pelleting only of heavy, cross-linked F-actin. Controls for this experiment included α-actinin, a known actin crosslinking protein, and bovine serum albumin, which does not interact with or crosslink actin.21 This experiment demonstrates that the majority of actin was recovered in the pellet, along with a portion of interacting AIF-1 (Figure 4B). Overall, these data indicate that AIF-1 binds to and has a polymerizing effect on nonmuscle actin.
Figure 4.
AIF-1 interacts with and polymerizes nonmuscle actin. A: Extracts from MOLT-4 cells were incubated overnight with recombinant AIF-1/GST fusion protein. Beads were washed, boiled, and interacting proteins were separated by SDS-PAGE. Actin was identified by Western blotting with anti-pan actin antibody. B: AIF-1 polymerization of nonmuscle actin. Nonmuscle F-actin was incubated with 23 μg of recombinant AIF-1 protein; bovine serum albumin (BSA), which does not bind actin; and α-actinin, an actin polymerizing protein (included in the kit and were used as negative and positive controls, respectively) for 30 minutes, and then centrifuged at 8000 × g. Supernatant (s) and pellets (p) were collected and proteins separated by SDS-PAGE and identified by Coomassie blue staining. Presence of actin in the pellet indicates polymerization.
AIF-1 Expression Enhances Lymphocyte Migration
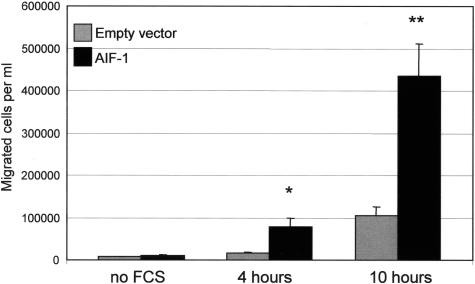
Because cytoskeletal rearrangement and actin polymerization are involved in cell motility, we examined the possibility that migration of MOLT-4 cells would be altered by AIF-1 overexpression. Figure 5 demonstrates that AIF-1-overexpressing MOLT-4 cells migrate 381% and 315% more rapidly at 4 and 10 hours, respectively, than do empty vector control cells (P < 0.05 and 0.01). This is consistent with previous studies showing VSMCs that overexpress AIF-1 migrate more rapidly in response to platelet-derived growth factor.17
Figure 5.
AIF-1 expression enhances lymphocyte chemotaxis. Stably transduced MOLT-4 cells were seeded onto Boyden chamber membranes and exposed to 10% fetal calf serum for the times indicated. MOLT-4 cells that traversed the membrane into the lower chamber were collected and counted. Values are means from experiments performed in triplicate from three independent, retrovirally-transduced groups of MOLT-4 cells (*P < 0.05, **P < 0.01).
Conditioned Media from AIF-1-Expressing Lymphocytes Can Influence VSMC Proliferation
Activated immune cells secrete many soluble factors that are known to affect VSMC phenotype and growth.22,23 We hypothesized that MOLT-4 cells that overexpress AIF-1 would also effect VSMC activation to a greater degree than empty vector control cells. For these experiments, conditioned media from stably transduced MOLT-4 cells was added to primary human VSMCs, and VSMCs counted after 4 and 7 days. Because the culture media from MOLT-4 cells contains 10% fetal calf serum, we would expect conditioned media from both empty vector and AIF-1-transduced cells to be proliferative to a degree. However, Figure 6 shows that primary human VSMCs cultured in conditioned media from AIF-1-transduced MOLT-4 cells proliferated 99% more rapidly (3.8 × 104 versus 1.9 ×× 104) than VSMCs cultured in conditioned media from empty vector transduced MOLT-4 cells (P < 0.001).
Figure 6.
Conditioned media from AIF-1-transduced lymphocytes enhances VSMC proliferation. Primary human VSMCs were seeded into 12-well plates. The next day the media was replaced with conditioned media from MOLT-4 cells stably transduced with empty vector or AIF-1. After 4 and 7 days, cells were harvested and counted in triplicate. Numbers on the y-axis indicate cells per well. Values are means from experiments performed in triplicate from supernatant from three independent retrovirally-transduced groups of MOLT-4 cells (P < 0.001).
Discussion
Cellular rejection and cardiac allograft vasculopathy (CAV) are significant causes of morbidity and mortality in patients after cardiac transplantation. Both allograft rejection and CAV are dynamic and multifactorial processes involving several different cell types in intimate proximity, including immune, vascular, and cardiac myocytes. There is extensive literature on the role of T lymphocytes in this process. T lymphocytes infiltrate allografts and are abundant in human arteries with CAV.1–4 In well-controlled animal studies, it was found that increasing the frequency of donor-reactive T cells accelerated the pace and severity of transplant vasculopathy.7 In rat cardiac allografts, the earliest and most robustly expressed genes are T-cell activation genes, in contrast to macrophage activation genes, suggesting that the initial T-cell activation is required for development of transplant vasculopathy.24 Nevertheless, the molecular mediators of T-lymphocyte activation have yet to be well defined. We have previously reported that AIF-1 is expressed in macrophages and activated VSMCs in coronary arteries with CAV, and persistent expression of AIF-1 in the cardiac allograft is associated with development of CAV.18 Although several investigators have shown a relationship between AIF-1 expression and monocyte/macrophage activation, very little has been reported on AIF-1 expression and function in T lymphocytes.
Co-staining of arterial sections from patients with CAV using AIF-1 and CD3 antibodies identified AIF-1 expression in T lymphocytes. We have determined that AIF-1 plays an important part of VSMC activation.15–17 Because immune cells in these vessels are in an activated state, we hypothesized that AIF-1 expression would play a role in activation of T cells as well. A basal level of AIF-1 protein is detectable in unstimulated PBMCs and MOLT-4 cells. Both of these cell populations respond to strongly proliferative stimuli such as T-cell conditioned media and PHA in a very similar manner. AIF-1 expression is not strongly induced by less proliferative factors such as transforming growth factor-β and tumor necrosis factor-α, and provided the impetus to explore the role of AIF-1 in cell proliferation.
Cell migration requires actin polymerization at the leading edge of the cell, and continuous remodeling of actin at the cell periphery is necessary to drive cell locomotion. AIF-1 interaction with and polymerization of nonmuscle actin prompted us to determine whether AIF-1 overexpression promoted MOLT-4 cell migration. Similar to what we previously reported in human VSMCs, overexpression of AIF-1 increases MOLT-4 cell migration in response to fetal calf serum. The increase in AIF-1 expression in stimulated cells also led us to investigate if AIF-1 expression would influence T-lymphocyte proliferation. Compared to empty vector controls, overexpression of AIF-1 in MOLT-4 cells also increased their proliferation. This is the first determination of a mechanistic role for AIF-1 in lymphocyte activation and pathophysiology. When taken together with previous work indicating an important role for AIF-1 in VSMC activation, this also points to AIF-1 as a potential therapeutic target to limit development of CAV.
Early events in allograft recognition are primarily inflammatory in nature and are initiated by T lymphocytes and macrophages that secrete various cytokines and growth factors seminal to the local inflammatory response. Despite immunosuppressive therapy, persistent immune cell infiltration and chronic vascular inflammation results in endothelial cell damage and low levels of VSMC activation even in the absence of rejection. Progression of CAV in this way is often refereed to as smoldering rejection, and is likely the result of soluble factors released from primed lymphocytes that stimulate medial VSMCs. Indeed, it has been demonstrated that infiltration of the artery wall by T lymphocytes induces a change in VSMC phenotype, which is mediated by inflammatory, mitogenic, and proliferative factors.23,25,26 Further, the immediate VSMC response to injury is initiated by multiple immune cell factors, and soluble factors from activated lymphocytes stimulate VSMC proliferation,22,23,25,26 which prompted us to determine whether conditioned medium from AIF-1-overexpressing T cells could influence VSMC growth. In this study, conditioned media from AIF-1-overexpressing MOLT-4 cells significantly increased VSMC proliferation compared with control cells. Considering that AIF-1 expression increases MOLT-4 activation, and soluble products from activated T cells are known to stimulate VSMC proliferation, we can conclude that the amount or possibly differences in the repertoire of cytokines produced by AIF-1-expressing MOLT-4 cells are responsible for the increased effects on VSMC proliferation. Identification and quantification of those cytokines induced by AIF-1 expression will be important to determine the precise mechanism by which AIF-1-mediated T-cell activation can induce VSMC proliferation. Other investigators have found that overexpression of AIF-1 leads to induction of cytokine expression. Watano and colleagues27 found that transfection of AIF-1 into a mouse macrophage cell line results in production of interleukin-6, -10, and -12 in response to lipopolysaccharide stimulation. Further, an AIF-1 transgenic mouse has been produced in which expression is restricted to lymphoid tissue.28 The investigators observed an exacerbation of experimental inflammatory colitis in those mice, with an augmentation of inflammatory cytokines. Although a mechanism was not elucidated, it is clear from both of these studies that AIF-1 expression influences lymphocyte activation.
We have shown that VSMCs normally do not express AIF-1, but incubation of cultured VSMCs with T-cell-conditioned medium induces AIF-1 expression.15 We have also previously determined that expression of AIF-1 increases VSMC activation, and that persistent expression of AIF-1 in the cardiac allograft is associated with development of CAV.16,17 Consequently, this experiment may represent an ex vivo surrogate whereby AIF-1 expression in injured arterial VSMCs is induced by activated lymphocytes, and how AIF-1 expression in VSMCs contributes to progression of restenosis.
In this study, we identify AIF-1 expression in T lymphocytes in human coronary arteries with CAV. We determine that AIF-1 expression increases when T lymphocytes are activated, and that overexpression of AIF-1 increases lymphocyte migration and proliferation, both hallmarks of activated lymphocytes, and part of the pathogenesis of CAV. This study also demonstrates that conditioned media from AIF-1-activated lymphocytes can also promote VSMC proliferation. What emerges from these studies is that AIF-1 expression in activated lymphocytes may have important ramifications for activation of arterial VSMCs and progression of vascular proliferative diseases, including CAV.
Acknowledgments
We thank Ms. Betty Ann Rogers for technical support.
Footnotes
Address reprint requests to Michael Autieri, Ph.D., Department of Physiology, Cardiovascular Research Center, Temple University School of Medicine, Room 810, MRB, 3420 N. Broad St., Philadelphia PA 19140. E-mail: mautieri@temple.edu.
Supported by the National Heart, Lung, and Blood Institute (grant HL-63810 to M.V.A.).
References
- Weis M, von Scheidt W. Cardiac allograft vasculopathy. Circulation. 1997;96:2069–2077. doi: 10.1161/01.cir.96.6.2069. [DOI] [PubMed] [Google Scholar]
- Barnhart G, Pascoe E, Mills S. Accelerated coronary arteriosclerosis in cardiac transplant recipients. Transplant Rev. 1987;1:31–46. doi: 10.1016/s0955-470x(87)80004-6. [DOI] [PubMed] [Google Scholar]
- Libby P, Salomon R, Payne D, Schoen FJ, Pober JS. Functions of vascular wall cells related to development of transplantation-associated coronary arteriosclerosis. Transplant Proc. 1989;21:3677–3684. [PubMed] [Google Scholar]
- Ventura HO, Mehra MR, Smart FW. Cardiac allograft vasculopathy: current concepts. Am Heart J. 1995;129:791–798. doi: 10.1016/0002-8703(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Dong C, Redenbach D, Wood S, Battistini B, Wilson JE, McManus BM. The pathogenesis of cardiac allograft vasculopathy. Curr Opin Cardiol. 1996;11:183–190. doi: 10.1097/00001573-199603000-00012. [DOI] [PubMed] [Google Scholar]
- Alpert S, Lewis NP, Ross H, Fowler M, Valantine HA. The relationship of granzyme A and perforin expression to cardiac allograft rejection and dysfunction. Transplantation. 1995;60:1478–1485. doi: 10.1097/00007890-199560120-00019. [DOI] [PubMed] [Google Scholar]
- He C, Schenk S, Zhang Q, Valujskikh A, Bayer J, Fairchild RL, Heeger PS. Effects of T cell frequency and graft size on transplant outcome in mice. J Immunol. 2004;172:240–247. doi: 10.4049/jimmunol.172.1.240. [DOI] [PubMed] [Google Scholar]
- Yun JJ, Fischbein MP, Whiting D, Irie Y, Fishbein MC, Burdick MD, Belperio J, Strieter RM, Laks H, Berliner JA, Ardehali A. The role of MIG/CXCL9 in cardiac allograft vasculopathy. Am J Pathol. 2002;161:1307–1313. doi: 10.1016/S0002-9440(10)64407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Laufer TM, Gerth AJ, Chase CM, Colvin RB, Russell PS, Sayegh MH, Auchincloss H., Jr Further analysis of the T-cell subsets and pathways of murine cardiac allograft rejection. Am J Transplant. 2003;3:23–27. doi: 10.1034/j.1600-6143.2003.30105.x. [DOI] [PubMed] [Google Scholar]
- Szeto WY, Krasinskas AM, Kreisel D, Krupnick AS, Popma SH, Rosengard BR. Depletion of recipient CD4+ but not CD8+ T lymphocytes prevents the development of cardiac allograft vasculopathy. Transplantation. 2002;73:1116–1122. doi: 10.1097/00007890-200204150-00019. [DOI] [PubMed] [Google Scholar]
- Utans U, Arceci R, Yamashita Y, Russell ME. Cloning and characterization of allograft inflammatory factor-1: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. J Clin Invest. 1995;95:2954–2962. doi: 10.1172/JCI118003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluesener HJ, Seid K, Meyermann R. Effects of autoantigen and dexamethasone treatment on expression of endothelial-monocyte activating polypeptide II and allograft-inflammatory factor-1 by activated macrophages and microglial cells in lesions of experimental autoimmune encephalomyelitis, neuritis, and uveitis. Acta Neuropathol. 1999;97:119–126. doi: 10.1007/s004010050964. [DOI] [PubMed] [Google Scholar]
- Fukiki K, Shin DH, Nakao M, Yano T. Molecular cloning of carp CC chemokine CXC, chemokine receptors, allograft inflammatory factor-1, and natural killer cell enhancing factor by use of suppression subtractive hybridization. Immunogenetics. 1999;49:909–914. doi: 10.1007/s002510050573. [DOI] [PubMed] [Google Scholar]
- Kruse M, Steffen R, Batel R, Muler I, Muller W. Differential expression of allograft inflammatory factor 1 and of glutathione peroxidase during auto-and allograft response in marine sponges. J Cell Sci. 1999;112:4305–4313. doi: 10.1242/jcs.112.23.4305. [DOI] [PubMed] [Google Scholar]
- Autieri MV, Mu A, Carbone C. Expression of allograft inflammatory factor-1 (AIF1) is a marker of activated human VSMC and arterial injury. Arterioscler Thromb Vasc Biol. 2000;20:1737–1744. doi: 10.1161/01.atv.20.7.1737. [DOI] [PubMed] [Google Scholar]
- Autieri MV, Carbone CM. Over expression of allograft inflammatory factor-1 promotes proliferation of vascular smooth muscle cells by cell cycle deregulation. Arterioscler Thromb Vasc Biol. 2001;21:1421–1426. doi: 10.1161/hq0901.095566. [DOI] [PubMed] [Google Scholar]
- Autieri MV, Kelemen SE, Wendt KW. AIF-1 is an actin-polymerizing and Rac1-activating protein that promotes vascular smooth muscle cell migration. Circ Res. 2003;92:1107–1114. doi: 10.1161/01.RES.0000074000.03562.CC. [DOI] [PubMed] [Google Scholar]
- Autieri MV, Kelemen SE, Thomas BA, Feler ED, Goldman BI, Eisen HJ. Allograft inflammatory factor-1 (AIF-1) expression correlates with cardiac rejection and development of cardiac allograft vasculopathy. Circulation. 2002;106:2218–2223. doi: 10.1161/01.cir.0000035652.71915.00. [DOI] [PubMed] [Google Scholar]
- Yee JK, Friedmann T, Burns JC. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol. 1994;43:99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]
- Grabski R, Dewit J, De Braekeleer J, Malicka-Blaskiewicz M, De Baetselier P, Verschueren H. Inhibition of T-cell invasion across cultured fibroblast monolayers by phenothiazine-related calmodulin inhibitors: impairment of lymphocyte motility by trifluoperazine and chlorpromazine, and alteration of the monolayer by pimozide. Biochem Pharmacol. 2001;61:1313–1317. doi: 10.1016/s0006-2952(01)00585-8. [DOI] [PubMed] [Google Scholar]
- Dubreuil RR. Structure and evolution of the actin crosslinking proteins. BioEssays. 1991;13:219–226. doi: 10.1002/bies.950130504. [DOI] [PubMed] [Google Scholar]
- Wang W, Chen HJ, Giedd KN, Schwartz A, Cannon PJ, Rabbani LE. T-cell lymphokines, interleukin-4 and gamma interferon, modulate the induction of vascular smooth muscle cell tissue plasminogen activator and migration by serum and platelet-derived growth factor. Circ Res. 1995;77:1095–1106. doi: 10.1161/01.res.77.6.1095. [DOI] [PubMed] [Google Scholar]
- Artigas S, Blaes N, Garcia V, Caspar-Bauguil S, Benoist H, Thomsen M. Modulation of the proliferation and gene expression of vascular smooth muscle cells induced by lymphocyte culture supernatants. Transplant Proc. 2000;32:364–366. doi: 10.1016/s0041-1345(99)00981-1. [DOI] [PubMed] [Google Scholar]
- Tori M, Kitagawa-Sakakida S, Li Z, Izutani H, Horiguchi K, Ito T, Matsuda H, Shirakura R. Initial T-cell activation required for transplant vasculopathy in retransplanted rat cardiac allografts. Transplantation. 2000;70:737–746. doi: 10.1097/00007890-200009150-00005. [DOI] [PubMed] [Google Scholar]
- Rolfe BE, Campbell JH, Smith NJ, Cheong MW, Campbell GR. T lymphocytes affect smooth muscle cell phenotype and proliferation. Arterioscler Thromb Vasc Biol. 1995;15:1204–1210. doi: 10.1161/01.atv.15.8.1204. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Watano K, Iwabuchi K, Fujii S, Ishimori N, Mitsuhashi S, Ato M, Kitabatake A, Onoe K. Allograft inflammatory factor-1 augments production of interleukin-6, -10 and -12 by a mouse macrophage line. Immunology. 2001;104:307–316. doi: 10.1046/j.1365-2567.2001.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi T, Iwabuchi K, Watano K, Dashtsoodol N, Mishima T, Nakai T, Shimada S, Mishida R, Fujii S, Onoe K. Allograft inflammatory factor-1 regulates trinitrobenzene sulphonic acid-induced colitis. Immunology. 2003;110:112–119. doi: 10.1046/j.1365-2567.2003.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]