Abstract
The α-fodrin N-terminal portion (AFN) autoantigen mediates in vivo immunoregulation of autoimmune responses in primary Sjögren’s syndrome (SS). We further examined this process and found that cleavage products of AFN were frequently detected in the salivary gland duct cells of SS patients. In in vitro studies using human salivary gland HSY cells, anti-Fas-induced apoptosis resulted in specific cleavage of α-fodrin into the 120-kd fragment, in association of α-fodrin with μ-calpain, and activation of caspase 3. Significant proliferative responses against ΑFN autoantigen were observed in the peripheral blood mononuclear cells (PBMCs) from SS patients with higher pathological score (grade 4) and with short duration from onset (within 5 years). In vivo roles of AFN peptides were investigated using PBMCs from patients with SS, systemic lupus erythematosus, and rheumatoid arthritis. Significant proliferative T-cell responses of PBMCs to AFN peptide were detected in SS but not in systemic lupus erythematosus or rheumatoid arthritis. AFN peptide induced Th1-immune responses and accelerated down-regulation of Fas-mediated T-cell apoptosis in SS. Our data further elucidate the in vivo role of AFN autoantigen on the development of SS and suggest that the AFN autoantigen is a novel participant in peripheral tolerance.
Organ-specific autoimmune diseases are characterized by tissue destruction and functional decline due to autoreactive T cells that escape self-tolerance.1,2 Primary Sjögren’s syndrome (SS) is an autoimmune disorder characterized by lymphocytic infiltrates and destruction of the salivary and lacrimal glands, and systemic production of autoantibodies to the ribonucleoprotein particles SS-A/Ro and SS-B/La.3–5 SS is a T-cell-mediated autoimmune disease, and autoreactive T cells bearing CD4 molecule may recognize unknown self antigen-triggering autoimmunity in the salivary and lacrimal glands, leading to clinical symptoms of dryness of the mouth and eyes (sicca syndrome).6,7 Accumulated evidence suggest an important role of apoptosis in disease pathogenesis of SS.8 Previously we have identified a 120-kd α-fodrin autoantigen in the pathogenesis of primary SS,9 but the role of autoantigen that render in vivo immunoregulation remains unclear.
Although an important role for T cells on the development of organ-specific autoimmune disease has been argued, it is not known whether disease is initiated by a restrained inflammatory reaction to an organ-specific autoantigen. Autoreactive T cells generally respond to a limited number of immunodominant epitopes in self-antigenic proteins including myelin basic protein, thyroglobulin, and glutamic acid decarboxylase.10–12 α-Fodrin is a ubiquitous, heterodimeric calmodulin-binding protein13 found to be cleaved by calcium-activated protease (calpain) in apoptotic T cells, and by calpain and caspase family cysteine proteases14 in anti-Fas-stimulated Jurkat cells and/or neuronal apoptosis.15–17 Previous reports have demonstrated evidence that caspase 3 is required for α-fodrin cleavage during apoptosis.18–20 In Jurkat cells, caspase 3-like proteases have been reported to cleave α-fodrin and poly (ADP-ribose) polymerase but with differential sensitivity to the caspase 3 inhibitor, DEVD-fmk.20 In neuroblastoma cells, treatment with staurosporin induced cleavage of α-fodrin at both caspase 3 and calpain cleavage sites.21 Therefore, we speculate that an increase in enzymatic activity of apoptotic proteases is involved in the progression of α-fodrin proteolysis during apoptosis of human salivary gland cells. In this study, we analyzed Fas-mediated apoptosis in SS salivary glands, and the in vivo role of the autoantigen for T-cell response, cytokine production, and peripheral tolerance.
Materials and Methods
Patients with Autoimmune Diseases
Peripheral blood samples from 18 patients with primary SS, 6 systemic lupus erythematosus (SLE), and 5 rheumatoid arthritis (RA), and from age-matched healthy donors (n = 18) were obtained from the Tokushima University Hospital, Tokushima, Japan. SLE and RA patients were diagnosed based on American College of Rheumatology criteria.22,23 All patients with SS were female, had documented xerostomia and keratoconjunctivitis sicca, and fulfilled San Diego criteria for the diagnosis of SS.3 Patients with secondary SS were carefully excluded. All patients with SS had focus scores of greater than 2 in their lip biopsy and all tested positive for autoantibodies against Ro, and 15 of 18 SS patients had autoantibodies against 120-kd α-fodrin by Western blotting. Analysis was performed under the certification of the ethics board of Tokushima University Hospital.
Immunohistology
Immunohistology was performed on freshly frozen sections (4 μm in thickness) by the biotin-avidin immunoperoxidase method using ABC reagent (Vector Laboratories, Burlingame, CA). Briefly, freshly frozen sections were fixed in acetone for 10 minutes, rinsed in phosphate-buffered saline (PBS, pH 7.2), and incubated with an appropriate blocking reagent (Vector Laboratories) for 20 minutes. They were incubated for 1 hour with biotinylated mouse monoclonal antibodies (mAbs) to CD4, CD8, L26(CD20) (BD Bioscience, San Jose, CA), and to Fas and FasL (BD PharMingen, San Diego, CA). To detect the cleavage product of α-fodrin, polyclonal rabbit Abs raised against the synthetic peptide to the purified 120-kd antigen corresponding to the identified 20 amino acid residues (RQKLEDSYRFQFFQRDAEEL) were developed and used.9 Isotype-matched sera were used as controls, respectively.
Production of Recombinant α-Fodrin
Recombinant α-fodrin N-terminus (AFN) protein (JS-1), the cDNA encoding human α-fodrin (JS-1:1,1784 bp)9 was constructed by inserting cDNA into the EcoRI site of pGEX-2T. Glutathione S-transferase fusion protein was expressed and purified using a glutathione S-transferase gene fusion system (Amersham Bioscience, Piscataway, NJ).
Synthetic Peptides
AFN peptides identical to JS-1 region were synthesized using tent-butoxycarbonyl chemistry on a model 430A peptide synthesizer (Applied Biosystems, Foster City, CA). A total of 45 synthetic peptides that were designed to be 20-amino acid residues in length, overlapping by five-amino acid residues were generated. As control peptide, laminin fragment peptide 929-933 (Sigma Chemical Co., St. Louis, MO) was used.
Proliferative T-Cell Response
Freshly isolated peripheral blood mononuclear cells (PBMCs) from SS patients and age-matched controls were assayed. When necessary, isolated CD4+ and CD8+ T cells from PBMCs using magnetic beads (Dynal, Oslo, Norway) were assayed. Single cell suspensions were cultured in 96-well flat-bottom microtiter plates (5 × 105 cells/well) in RPMI 1640 containing 10% fetal calf serum, penicillin/streptomycin, and β-mercaptoethanol. Cells were cultured with 10 μg/ml JS-1 protein, 10 μg/ml AFN peptide, and 2.0 μg/ml Con A (EY Laboratories, San Mateo, CA). To confirm the immunoreactivity with the AFN protein (JS-1), 2 × 105 CD4+ and CD8+ T cells from PBMCs of SS patients and controls were co-cultured with irradiated T-cell-depleted PBMCs as antigen-presenting cells, and stimulated with 0 to 20 μg/ml JS-1 for 72 hours. During the last 8 hours of the 72-hour culture period, 1 μCi of [3H]thymidine was added per well, and the incorporated radioactivity was determined using an automated β liquid scintillation counter.
Flow Cytometric Analysis
For analysis of intracellular cytokines, monensin (Wako Pure Chemical, Osaka, Japan) was added at 2 μmol/L to isolated PBMCs (106/ml), and 2 hours later the cells were collected, labeled with anti-CD4-PE (BD PharMingen), fixed with 4% paraformaldehyde for 10 minutes at 4°C, and then permeabilized with 0.1% saponin in PBS at room temperature for 10 minutes. Cells were incubated with anti-interleukin (IL)-2-fluorescein isothiocyanate (FITC) (8 μg/ml; BD PharMingen), anti-IL-4-FITC (5 μg/ml; BD PharMingen), and anti-interferon (IFN)-γ-FITC (1 μg/ml; BD PharMingen), respectively, and analyzed on a EPICS flow cytometer (Beckman Coulter, Miami, FL). For analysis of Fas and FasL expression, isolated PBMCs from SS patients when pulsed with AFN peptide (10 μg/ml) were assayed by flow cytometry gated on CD4+ T cells, using anti-Fas and anti-FasL mAb (BD PharMingen). Mean fluorescence intensity was calculated using the fluorescence intensity of staining for mAbs to Fas or FasL and isotype-matched control measured by flow cytometry. Apoptotic cells were detected by flow cytometry with an EPICS (Beckman Coulter) using the Annexin V-FITC apoptosis detection kit (Genzyme, Cambridge, MA).
Cell Culture and Induction of Apoptosis
Human parotid salivary gland HSY cells24 were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere of 5% CO2 in air at 37°C. The cells were maintained in a logarithmic growth phase by routine passage every 2 to 3 days. Apoptosis was induced in HSY cells by anti-Fas mAb (clone CH-11; Medical and Biological Laboratories. Co., Ltd., Nagoya, Japan).
Western Blot Analysis
For detection of a cleavage product of α-fodrin, Western blot analysis was performed with anti-α-fodrin mAb (Affiniti, Mamhead, UK). To detect the apoptotic proteases in vitro, Western blot analysis was performed using mouse mAbs to μ-calpain (clone 9; Chemicon Int., Temecula, CA) specific for catalytic subunit (80 kd), calpastatin (clone 1F7E3D10; Calbiochem, San Diego, CA) specific for amino acids 543 to 673 encoding domain IV (150, 125, 90, and 70 kd), and caspase 3 (3G2; Transduction Laboratories, Lexington, KY) specific for amino acids 28 to 44 encoding large subunit (17/19 kd). The cells were homogenized in 20 mmol/L Tris-HCl buffer (pH 7.4) containing 5 mmol/L ethylene diamine tetraacetic acid, 10 μl/ml protease inhibitor cocktail (Sigma Chemical Co.) and 0.2% Triton X-100. After centrifugation for 20 minutes at 12,000 × g at 4°C, supernatant was extracted and used for cytoplasmic protein. Pellets were homogenized in 20 mmol/L Tris-HCl buffer containing 2% Triton X-100. Protein binding was visualized with ECL Western blotting reagent (Amersham Bioscience). Protease inhibitors included leupeptin, E64, pepstatin (Wako Pure Chemicals), calpain inhibitor peptide (Sigma Chemical Co.), and caspase inhibitors [Ac-YVAD-CHO (ICN, Costa Mesa, CA); Z-VAD-fmk (ICN)].
Sequential Activation of Caspase-Like Proteases
The caspase 3-like activity in anti-Fas mAb-treated HSY cell extracts was determined using fluorescent substrate.25 Cell lysates were diluted with 0.5 ml of standard buffer, and incubated at 30°C for 30 minutes with 1 μmol/L fluorescent substrate. The specific inhibitor for caspase 3 (Z-DEVD-fmk) was added to the reaction mixture at a concentration of 1 μmol/L. Specific caspase 3-like activity was determined by subtracting the values obtained in the presence of inhibitors. The fluorescent substrate, MOCAc-DEVD (dnp)-NH2 was custom-synthesized at the Peptide Institute (Osaka, Japan). The fluorescence of the cleaved substrates was determined using a spectrofluorometer set at an excitation wavelength of 328 nm and an emission wavelength of 393 nm.
Cell Transfection
cDNAs for full-length caspase 3 and μ-calpain obtained by polymerase chain reaction were subcloned into the pCRII vector (Invitrogen Co., Carlsbad, CA). All constructs were confirmed by DNA sequencing. For expression experiments, DNA fragments were subcloned into pcDNA3.1 expression vector (Invitrogen Co.). HSY cells were transfected with the pcDNA3.1 expression vectors using the Lipofectamine reagent according to the manufacturer’s instruction (Invitrogen Co.). The cells were transfected with the individual plasmid DNA and the total amount of DNA transfected was adjusted to 10 μg with pcDNA3.1 for each 100-mm dish or 3 μg for each 60-mm dish. After a 5-hour incubation with the DNA/lipid mixture, the cells were washed with PBS before replenishing with growth media. The cells were harvested 24 hours after transfection and lysed in Tris-HCl buffer.
Results
Involvement of Apoptotic Cascade in SS Salivary Glands
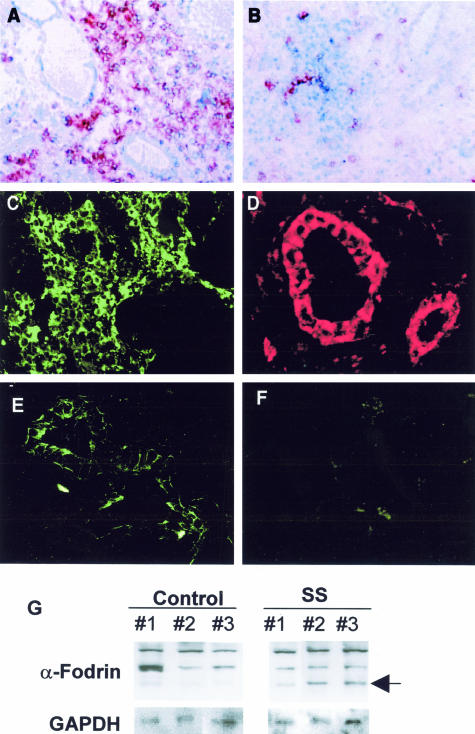
Immunohistochemical analysis revealed that a majority of infiltrating cells were CD4+, and that a small number of CD8+ cells were present in the SS salivary glands. L26+ B cells were sporadically present in the inflammatory lesions (data not shown). Shown in Figure 1, A and B, are photomicrographs taken from representative data. Immunofluorescence analysis revealed that a large number of infiltrating lymphoid cells bear FasL in SS salivary glands (Figure 1C), and epithelial duct cells stained positively with Fas on their cell surface (Figure 1D). Some acinar cells were stained negligibly positive with both anti-FasL and anti-Fas antibodies, but most acinar cells were negative. Immunofluorescence analysis using polyclonal Ab against synthetic 120-kd α-fodrin9 demonstrated that a cleavage product of α-fodrin was present in epithelial duct cells of the labial salivary gland biopsies from SS patients, but not in control glands (Figure 1, E and F). Western blot analysis confirmed the same results (Figure 1G), indicating that a cleavage product of 120-kd α-fodrin is present in the diseased glands with SS.
Figure 1.
Representative immunohistological features in the labial gland biopsies. A majority of infiltrating cells were CD4+ (A), and a small number of CD8+ T cells (B) were present in the SS salivary glands. Five samples were examined. Immunofluorescence analysis revealed that the majority of tissue-infiltrating lymphoid cells bear FasL (C), and epithelial duct cells stained positively with Fas on their cell surface (D) in SS salivary glands. Isotype-matched controls were stained negatively. Six samples for each were examined. A cleavage product of 120-kd α-fodrin was present exclusively in epithelial duct cells of the SS salivary glands (E), but not in control salivary glands (F). Six samples for each were examined. Detection of a cleavage product of 120-kd α-fodrin in the labial salivary gland biopsies with SS (no. 1, no. 2, and no. 3), but not in control individuals (no. 1, no. 2, and no. 3) on Western blotting (G). Eight samples for each were examined.
In Vitro Cleavage of α-Fodrin Induced by Apoptotic Stimuli
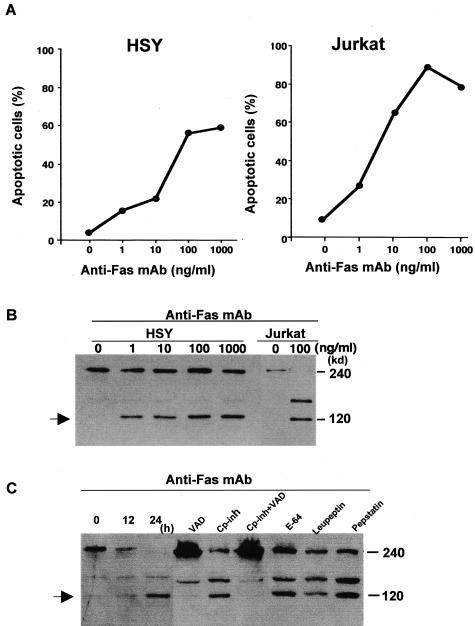
We examined the in vitro cleavage of α-fodrin using HSY and Jurkat cells induced by anti-Fas mAb (ranging from 1 to 1000 ng/ml−1) apoptotic stimuli. Anti-Fas (CH-11)-stimulated apoptosis in HSY cells was confirmed by flow cytometric analysis using an apoptosis detection kit as well as in Jurkat cells (Figure 2A). Western blot analysis demonstrated that the 240-kd α-fodrin on apoptotic HSY cells was cleaved into smaller 120-kd fragments in dose-dependent manner of anti-Fas mAb (Figure 2B). We next examined whether α-fodrin cleavage into the 120-kd fragment on apoptotic HSY cells could be blocked by preincubation with specific protease inhibitors. In apoptotic HSY cells, a combination of calpain inhibitor peptide and caspase inhibitor (Z-VAD-fmk) entirely blocked the formation of 120-kd α-fodrin, whereas calpain inhibitor peptide alone could not block 120-kd formation (Figure 2C). Caspase inhibitor alone could block considerably 120-kd formation. Cysteine protease inhibitors (E64), serine protease inhibitor (leupeptin), and acidic protease inhibitor (pepstatin) had no effect on 120-kd α-fodrin cleavage.
Figure 2.
Anti-Fas-induced apoptosis in HSY and Jurkat cells. A: The HSY and Jurkat cell apoptosis induced by anti-Fas mAb (CH-11) stimulation was determined by flow cytometry of DNA content of nuclei with PI and annexin V. B: Western blot analysis demonstrated 120-kd α-fodrin in apoptotic HSY and Jurkat cells in a dose-dependent manner. C: Effects of protease inhibitors on α-fodrin cleavage in apoptotic HSY cells. Proteolytic cleavage of α-fodrin to 120 kd in Fas-stimulated HSY cells is blocked by a combination of a calpain inhibitor peptide and caspase inhibitors (Z-VAD-fmk), but not by E64, leupeptin, and lepstatin. Calpain inhibitor peptide alone could not inhibit the 120-kd α-fodrin formation.
Calpain and Caspase Mediated α-Fodrin Cleavage
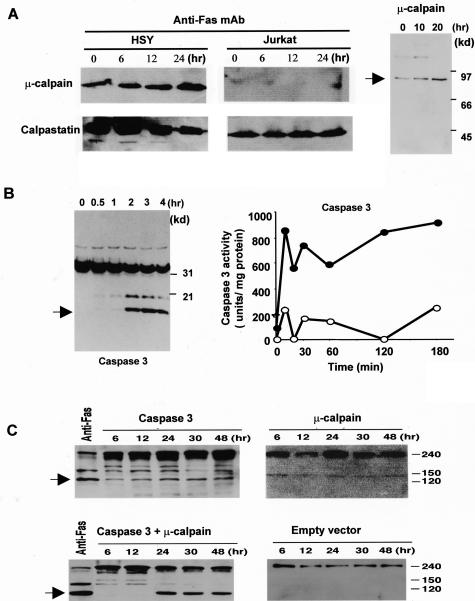
We investigated whether cysteine proteases are involved in α-fodrin cleavage on apoptotic HSY cells. We found a constitutive expression of μ-calpain, and its time-dependent increase in anti-Fas-stimulated HSY cells (Figure 3A). Of note is that abundant calpastatin activity is shown to be constitutively expressed more than calpain expression, and a time-dependent decrease of calpastatin expression was observed in apoptotic HSY cells, not in Jurkat cells (Figure 3A). It can be speculated that μ-calpain activity could be considerably affected by endogenous calpastatin during apoptosis in HSY cells. We confirmed a time-dependent increase in the active form of μ-calpain in apoptotic HSY cells (Figure 3A). Anti-Fas-stimulated HSY cells were positive for mAbs to caspase 3 in association with apoptosis (Figure 3B). Moreover, the caspase 3-like activities in anti-Fas Ab-stimulated HSY cell extracts were determined using fluorescent substrates (Figure 3B).25 To confirm the role of caspase 3 and μ-calpain proteins in induction of α-fodrin cleavage, full-length caspase 3 and μ-calpain cDNAs were transiently overexpressed in HSY cells, and cleavage product of 120-kd α-fodrin was examined by anti-α-fodrin Ab. Analysis of lysates from caspase 3 and μ-calpain cDNA co-transfected cells with Western blotting revealed a significant increase (approximately fivefold to sevenfold) of 120-kd α-fodrin in the level of expression of caspase 3 or μ-calpain in cells transfected with respective cDNA (Figure 3C).
Figure 3.
Detection of cysteine proteases in anti-Fas-induced apoptotic HSY cells. Western blot analysis of μ-calpain, and calpastatin in apoptotic HSY and Jurkat cells stimulated with anti-Fas Ab (CH-11). A constitutive expression of μ-calpain, and its time-dependent increase were observed in anti-Fas-stimulated HSY cells. Calpastatin activity is shown to be constitutively expressed more than calpain expression and a time-dependent decrease of calpastatin expression was observed in apoptotic HSY cells, not in Jurkat cells. A: Western blot analysis of the active form of μ-calpain in apoptotic HSY cells stimulated with anti-Fas mAb (CH-11). B: Western blot analysis showing a time-dependent increase in caspase 3 and sequential activation of caspase 3-like protease in anti-Fas-induced apoptotic HSY cells. The caspase 3-like activity in the lysates (100 mg protein) (filled circle) or in the presence of 50 mmol/L MOCAc-DEVD-NH2 (open circle) was determined using fluorescent substrates in apoptotic HSY cells. One unit corresponds to the activity that cleaves 1 pmol of the respective fluorescent substrate at 30°C in 30 minutes. C: Detection of cleavage product of 120-kd α-fodrin in co-transfected HSY cells overexpressed with full-length caspase 3 and μ-calpain cDNAs. Analysis of lysates from caspase 3 and μ-calpain cDNA co-transfected cells revealed a fivefold to sevenfold increase of 120-kd α-fodrin in the level of expression of caspase 3 or μ-calpain in cells transfected with each construct.
In Vivo Role of α-Fodrin in SS Patients
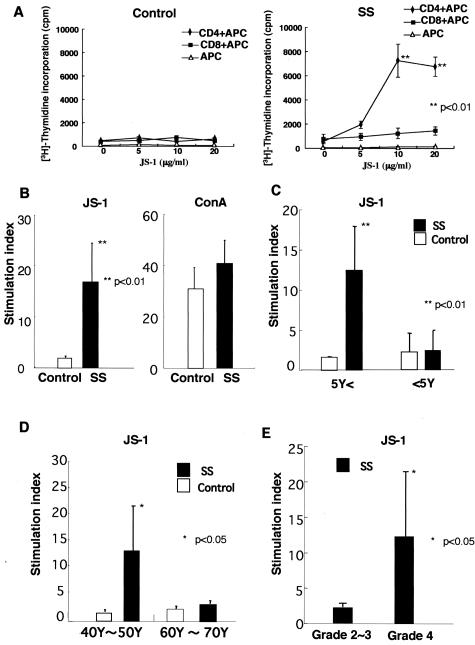
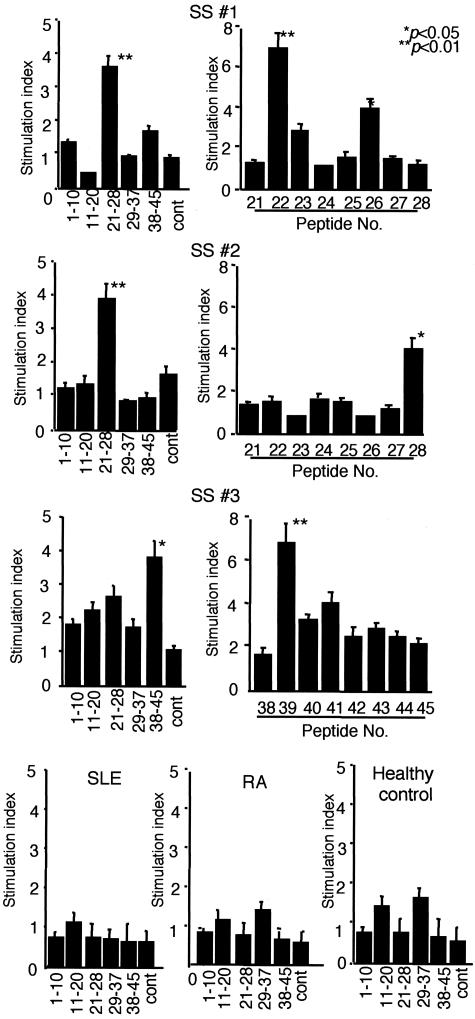
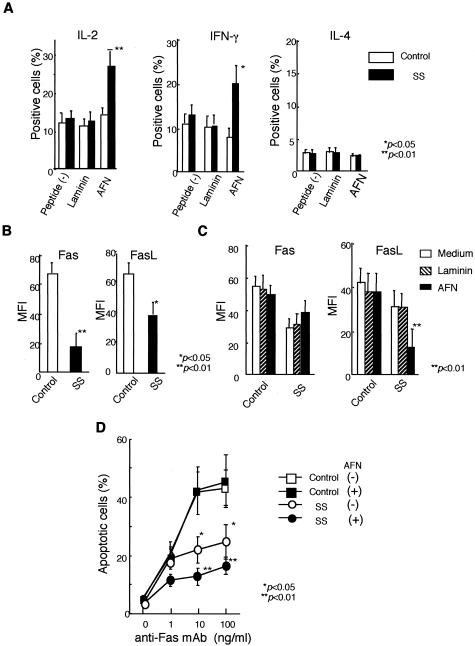
To confirm the immunoreactivity with the AFN protein (JS-1), CD4+ and CD8+ T cells were isolated from PBMCs of SS patients (n = 3) and controls (n = 2), and were co-cultured with irradiated T-cell-depleted PBMCs as antigen-presenting cells. Significant proliferative responses were observed in CD4+ T cells from SS patients, not in CD8+ T cells (Figure 4A). Moreover, it has been determined by flow cytometric analysis that the accumulated population in response to both AFN protein (JS-1) and AFN peptide among PBMCs of SS patients is CD4+ T cell (data not shown). Then, we used PBMCs for the proliferation assay. We found proliferative T-cell responses (stimulation indices > 3) to the AFN protein (JS-1) using PBMCs from 14 of 18 patients with SS, not from age-matched healthy patients (n = 11) (Figure 4B). Proliferative responses to JS-1 of SS patients with short duration (within 5 years) from the onset of disease (n = 8) were significantly higher than those with long duration (longer than 5 years) (n = 6) (Figure 4C). Proliferative responses to JS-1 autoantigen with younger SS patients (40 to 50 years of age) (n = 8) were significantly higher than those with older SS patients (60 to 70 years of age) (n = 6) (Figure 4D). Significant proliferative responses to JS-1 protein were observed in PBMCs from SS patients with higher pathological score (n = 9, grade 4) than those with lower score (n = 5, grade 2 or grade 3) (Figure 4E). Synthetic peptides of AFN were generated, and immunoregulatory roles were investigated using PBMCs from patients with SS, compared with SLE and RA. Significant proliferative T-cell responses to AFN peptide were detected in PBMCs from 9 of 18 patients with SS, but not with SLE, RA, and healthy controls (Figure 5). We next analyzed intracellular cytokines using isolated PBMCs (106/ml). CD4+ T cells from PBMCs with SS patients induce Th1 cytokine (IL-2, IFN-γ) when pulsed with AFN peptide (10 μg/ml) (Figure 6A), not with control laminin fragment peptide (10 μg/ml). We observed a significant decrease in both CD4+ Fas+ T and CD4+ FasL+ T cells in SS patients, compared with healthy controls (Figure 6B). Moreover, it was demonstrated that AFN peptide-pulsed CD4+ T cells showed a significant low intensity of FasL expression, not Fas expression (Figure 6C). Anti-Fas mAb-stimulated apoptosis showed a significant decrease in CD4+ T cells from SS patients than those from healthy control (Figure 6D). When pulsed with AFN peptide, anti-Fas mAb-stimulated apoptosis decreased more significantly in CD4+ T cells from SS patients.
Figure 4.
A: Significant proliferative CD4+ T-cell responses, not CD8+ T cells, to the AFN protein (JS-1) in the patients with primary SS (n = 3), not in age-matched control (n = 2) (**P < 0.01, Student’s t-test). B: Significant proliferative responses (stimulation indices > 3) of PBMCs to the AFN protein (JS-1) in patients with primary SS (n = 14), not in age-matched control (n = 11) (**P < 0.01, Student’s t-test). C: Proliferative responses to JS-1 of PBMCs from SS patients with short duration (within 5 years) from the onset of the disease (n = 8) were significantly higher than those with long duration (more than 5 years) (n = 6) (**P < 0.01, Student’s t-test). D: Proliferative responses to JS-1 of PBMCs from younger SS patients (40 to 50 years of age) (n = 8) were significantly higher than those with older SS patients (60 to 70 years of age) (n = 6) (*P < 0.05, Student’s t-test). E: Significant proliferative responses to JS-1 protein were observed in PBMCs from SS patients with higher pathological score (n = 9, grade 4) than those with lower score (n = 5, grade 2 or grade 3) (*P < 0.05, Student’s t-test). All data are expressed as stimulation indices ± SEM.
Figure 5.
Significant proliferative responses of PBMCs were seen with AFN peptide in the patients with primary SS. Nine of eighteen SS patients examined reacted significantly with single AFN peptide, but not in SLE (n = 6), RA (n = 5), and age-matched healthy controls (n = 6). Representative profiles in three different patients with SS (SS 1, SS 2, SS 3) indicate significant proliferative responses with peptide mixture and individual peptide of p22, p26, p28, and p39 (*P < 0.05, **P < 0.01; Student’s t-test), but not with laminin fragment peptide 929-933 as control antigen. The results are expressed as stimulation indices ± SEM.
Figure 6.
Cytokine profile and Fas-mediated apoptosis in CD4+ T cells from SS patients. A: CD4+ T cells from PBMCs with SS patients induce Th1 cytokine (IL-2, IFN-γ), not Th2 cytokine (IL-4) when pulsed with AFN peptide (10 μg/ml) by flow cytometric analysis (*P < 0.05, **P < 0.01; Student’s t-test). Laminin fragment peptide 929-933 (10 μg/ml) was used as control. Five SS patients were analyzed. B: Significant decrease in both Fas+ and FasL+ expression in CD4+ T cells from SS patients, compared with healthy controls (*P < 0.05, **P < 0.01; Student’s t-test). Five SS patients and four healthy controls were analyzed. C: AFN peptide-pulsed CD4+ T cells showing significant low intensity of FasL expression, not Fas expression, in SS patients (**P < 0.01, Student’s t-test). MFI (mean fluorescence intensity) indicates the fluorescence intensity of positively stained sample/the fluorescence intensity of its isotype control. Mean fluorescence intensity was calculated using the fluorescence intensity of staining for mAbs to Fas or FasL and isotype-matched control measured by flow cytometry. Five SS patients and four healthy controls were analyzed. D: Anti-Fas mAb-stimulated apoptosis showed significant decrease in CD4+ T cells from SS patients than those from healthy control. Moreover, anti-Fas mAb-stimulated apoptosis decreased more significantly in CD4+ T cells from SS patients pulsed with AFN peptide, than those with nonpulsed cells (*P < 0.05, **P < 0.01; Student’s t-test). Five SS patients and four healthy controls were analyzed.
Discussion
Cleavage of certain autoantigens during apoptosis may reveal immunocryptic epitopes that could potentially induce autoimmune responses in systemic autoimmune diseases.26,27 We reported previously that a cleavage product of 120-kd α-fodrin may be an important autoantigen on the development of primary SS, and anti-120-kd α-fodrin antibodies have been frequently detected in sera from patients.9 Although several candidate autoantigens besides α-fodrin have been reported in SS,28–30 the pathogenic roles of the autoantigens in initiation and progression of SS are still unclear.
The specificity of cytotoxic T-lymphocyte function has been an important issue of organ-specific autoimmune response, but little is known about the events triggering T-cell invasion of the target organs in prelude to organ-specific autoimmune diseases. In insulin-dependent diabetes mellitus, the role of environmental factors,31,32 the nature of the initiating T cell,33,34 and the identity of the inciting antigen(s)35 have all been vigorously debated. When we analyzed the mechanisms of α-fodrin cleavage in the SS salivary glands, infiltrating mononuclear cells bear a large proportion of CD4+ and Fas ligand (FasL), and the salivary gland duct cells constitutively possess Fas. In particular, cleavage products of 120-kd α-fodrin were frequently detected in the salivary gland duct cells with SS, but not in control salivary glands. Thus, we provided evidence suggesting that Fas-mediated apoptosis may be involved, in part, in in vivo α-fodrin cleavage in SS salivary glands. Moreover, it has been suggested that α-fodrin cleavage triggered by estrogen deficiency plays an important role in the development of autoimmune exocrinopathy in SS. Experimental studies of ours demonstrated a significant apoptosis associated with α-fodrin cleavage in the salivary gland cells of estrogen-deficient healthy C56BL/6(B6) mice, and inflammatory lesions developed exclusively in the salivary and lacrimal gland after the adoptive transfer with α-fodrin-reactive T cells in both ovariectomized (Ovx)-B6 and Ovx-SCID mice.36 Reduction of the intact form of α-fodrin in the affected glands suggests that elicitation of autoreactivity against α-fodrin could be the primary pathogenetic process that leads to tissue destruction. However, based on the fact that α-fodrin is a ubiquitous protein, and that the tissue destruction is confined to exocrine organs, it might be more reasonable to speculate that other undetermined tissue-specific target antigens in exocrine glands could be the primary target of the disease process mediated by pathogenic T cells. Nevertheless, production of autoantibodies and proliferative T-cell responses against cleavage product of α-fodrin, which does not take place under physiological conditions, might be an important clue that could shed light on the novel mechanisms by which tissue-specific apoptosis contributes to the disease development.
It has been reported that calpain is overactivated in autoimmune conditions, and subsequent tissue destruction.37,38 Moreover, the cascade of caspases is a critical component of the cell death pathway,39–41 and a few proteins have been found to be cleaved during apoptosis. These include poly (ADP-ribose) polymerase, a small U1 nuclear ribonucleoprotein, and α-fodrin, which were subsequently identified as substrates for caspases.27,42 Anti-Fas-induced cleavage of α-fodrin in Jurkat cells produces a predominant 120-kd fragment, and the 120-kd fragment is consistent with a previously reported caspase 3-mediated DETD cleavage site within the protein.20,21 Although the relevance of cleavage of structural proteins, including gelsolin, actins, lamins, and fodrins, is easily conceivable,43 the functional importance is not yet clear. Our data provide evidence that α-fodrin in human HSY cells is cleaved into 120-kd fragment by apoptotic proteases including calpain and caspases. When we investigated whether cysteine proteases are involved in α-fodrin cleavage, anti-Fas-treated HSY cells were positive for mAb to μ-calpain, and caspase 3 in association with apoptosis. However, we demonstrated here that calpastatin, an endogenous inhibitor of calpain, was shown to be constitutively expressed, speculating that μ-calpain activity could be considerably affected during apoptosis in HSY cells. A combination of calpain inhibitor peptide and caspase inhibitors (Z-VAD-fmk) entirely blocked the formation of 120-kd α-fodrin. When both full-length caspase 3 and μ-calpain cDNAs were transiently overexpressed in HSY cells, a cleavage product of 120-kd α-fodrin was abundantly identified than the level of expression of caspase 3 or μ-calpain in cells transfected with each construct. These data suggest that both μ-calpain and caspase 3 are required for specific α-fodrin proteolysis into the 120-kd fragment in human salivary gland cells with SS.
In this study, we detected proliferative T-cell responses to AFN protein (JS-1) of SS patients with short duration (within 5 years) from the onset of the disease were significantly higher than those with long duration (more than 5 years). Proliferative responses to autoantigen with younger SS patients (40 to 50 years of age) were significantly higher than those with older SS patients (60 to 70 years of age). Moreover, significant proliferative responses to ΑFN protein were observed in SS patients with higher pathological score (grade 4) than those with lower score (grade 2 or grade 3). These data are suggestive of clinicopathological usefulness of AFN immunoreactivity with SS patients for disease severity in addition to diagnostic significance. Because we have detected proliferative response to AFN peptides using PBMCs from SS patients, it is feasible for the future possibility that a peptide analogue of AFN could be used as a therapeutic agent. On the other hand, Fas/FasL interaction down-regulates the immune response by inducing apoptosis because activated lymphocytes express both Fas and FasL.44 CD4+ T cells from PBMCs with SS patients induce Th1 cytokine (IL-2, IFN-γ) when pulsed with each peptide, suggesting that the autoantigen peptide may play an important role in Th1/Th2 balance in vivo. Moreover, AFN peptide-pulsed CD4+ T cells down-regulate Fas-mediated apoptosis. Although antigen-induced T-cell death is known to be regulated by CD4 expression,45 molecular mechanisms responsible for T-cell death should be further elucidated. Our previous findings support the notion that Fas-mediated T-cell death is down-regulated by autoantigen stimulation in the murine SS model.46 Here we demonstrated that AFN peptide stimulation results in a significant decrease in anti-Fas-induced CD4+ T-cell apoptosis. However, it remains unclear whether T cells specific for endogenous epitopes play a significant pathological role in tissue damage during the clinical episodes.
Taken together, our results suggest that 120-kd α-fodrin, the apoptosis-associated breakdown product, may have an important role in the development of SS, and that the autoantigen is a novel participant in the regulation of Th1/Th2 balance and peripheral tolerance.
Footnotes
Address reprint requests to Yoshio Hayashi, Department of Pathology, Tokushima University School of Dentistry, 3 Kuramotocho, Tokushima 770, Japan. E-mail: hayashi@dent.tokushima-u.ac.jp.
Supported in part by the Ministry of Education, Science, and Culture of Japan (grants-in-aid for scientific research nos. 12307040 and 12557022).
References
- Gianani R, Satventnick N. Virus, cytokine, antigens, and autoimmunity. Proc Natl Acad Sci USA. 1996;93:2257–2259. doi: 10.1073/pnas.93.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M, Bennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV. Sjögren’s syndrome. Proposed criteria for classification. Arthritis Rheum. 1986;29:577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- Chan EK, Hamel JC, Buyon JP, Tan ET. Molecular definition and sequence motifs of the 52-kD component of human SS-A/Ro autoantigen. J Clin Invest. 1991;87:68–76. doi: 10.1172/JCI115003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruize AA, Smeenk RJT, Kater L. Diagnostic criteria and immunopathogenesis of Sjögren’s syndrome. Immunol Today. 1995;16:557–559. doi: 10.1016/0167-5699(95)80075-1. [DOI] [PubMed] [Google Scholar]
- Fox RI, Stern M, Michelson P. Update in Sjögren’s syndrome. Curr Opin Rheumatol. 2000;12:391–398. doi: 10.1097/00002281-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Manoussakis MN, Moutsopoulos HM. Sjögren’s syndrome: current concepts. Adv Intern Med. 2001;47:191–217. [PubMed] [Google Scholar]
- Humpherys-Beher MG, Peck AB, Dang H, Talal N. The role of apoptosis in the initiation of the autoimmune response in Sjögren’s syndrome. Clin Exp Immunol. 1999;116:383–387. doi: 10.1046/j.1365-2249.1999.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneji N, Nakamura T, Takio K, Yanagi K, Higashiyama H, Saito I, Noji S, Sugino H, Hayashi Y. Identification of α-fodrin as a candidate autoantigen in primary Sjögren’s syndrome. Science. 1997;276:604–607. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- Harrington CJ, Paez A, Hunkapiller T, Mannikko V, Brabb T, Ahearn M, Beeson C, Goverman J. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
- Dai Y, Carayanniotis KA, Eliades P, Lymberi P, Shepherd P, Kong Y, Carayanniotis G. Enhancing or suppressive effects of antibodies on processing of a pathogenic T cell epitope in thyroglobulin. J Immunol. 1999;162:6987–6992. [PubMed] [Google Scholar]
- Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto TL, Pleasic S, Forget BG, Benz EJ, Jr, Marchesi VT. Characterization of the calmodulin-binding site of nonerythroid α-spectrin. J Biol Chem. 1989;264:5826–5830. [PubMed] [Google Scholar]
- Alnemri ES, Livingstin DJ, Nicholson DW, Salvensen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Martin SD, O’Brien GA, Nishioka WK, McGahon AJ, Mahboubi A, Saido TV, Green DR. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem. 1995;270:6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- Martin SD, Finucane DM, Amarante-Mendes GP, O’Brien GA, Green DR. Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. J Biol Chem. 1996;271:28753–28756. doi: 10.1074/jbc.271.46.28753. [DOI] [PubMed] [Google Scholar]
- Vanags DM, Pörn-Ares I, Coppolaa S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB, Wang KK. Non-erythroid α-spectrin breakdown by calpain and interleukin 1β-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319:683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke RU, Sprengart ML, Porter AG. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem. 1996;273:15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- Cryns VL, Bergeron L, Zhu H, Li H, Yuan J. Specific cleavage of alpha-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1beta-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease. J Biol Chem. 1996;271:31277–31282. doi: 10.1074/jbc.271.49.31277. [DOI] [PubMed] [Google Scholar]
- Wang KW, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, Glantz, Marrow JS. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Yanagawa T, Yoshida H, Azuma M, Nishida T, Yura Y, Sato M. Expression of vasoactive intestinal polypeptide and amylase in a human parotid gland adenocarcinoma cell line in culture. J Natl Cancer Inst. 1987;79:1025–1037. [PubMed] [Google Scholar]
- Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843–854. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiano CA, Martin SJ, Green DR, Tan EM. Selective cleavage of nuclear autoantigens ensuring CD95 (Fas/APO-1)-mediated T cell apoptosis. J Exp Med. 1996;184:765–770. doi: 10.1084/jem.184.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CP, Brayer J, Yamachika S, Esch TR, Peck AB, Stewart CA, Peen R, Jonsson R, Humphreys-Beher MG. Transfer of human serum IgG to nonobese diabetic Igμnull mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjögren’s syndrome. Proc Natl Acad Sci USA. 1998;95:7538–7543. doi: 10.1073/pnas.95.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana M, Okano T, Ogawa Y, Kaburaki J, Kawakami Y. Autoantibodies to the amino terminal fragment of β-fodrin expressed in glandular epithelial cells in patients with Sjögren’s syndrome. J Immunol. 2001;167:5449–5456. doi: 10.4049/jimmunol.167.9.5449. [DOI] [PubMed] [Google Scholar]
- Winer S, Astsaturov I, Cheung R, Tsui H, Song A, Gaedigk R, Winer D, Sampson A, McKerlie C, Bookman A, Dosch HM. Primary Sjögren’s syndrome and deficiency of ICA69. Lancet. 2002;360:1063–1069. doi: 10.1016/S0140-6736(02)11144-5. [DOI] [PubMed] [Google Scholar]
- Singh B, Prange S, Jevnikar AM. Protective and destructive effects of microbial infection in insulin-dependent diabetes mellitus. Semin Immunol. 1998;10:79–86. doi: 10.1006/smim.1997.0107. [DOI] [PubMed] [Google Scholar]
- Wong FS, Janeway CA., Jr The role of CD4 and CD8 T cells in type I diabetes in the NOD mouse. Res Immunol. 1997;148:327–332. doi: 10.1016/s0923-2494(97)87242-2. [DOI] [PubMed] [Google Scholar]
- Wegmann DR. The immune response to islets in experimental diabetes and insulin-dependent diabetes mellitus. Curr Opin Immunol. 1996;8:860–864. doi: 10.1016/s0952-7915(96)80016-1. [DOI] [PubMed] [Google Scholar]
- Kay TW, Chaplin HL, Parker JL, Stephens LA, Thomas HE. CD4+ and CD8+ T lymphocytes: clarification of their pathogenic roles in diabetes in the NOD mouse. Res Immunol. 1997;148:320–327. doi: 10.1016/s0923-2494(97)87241-0. [DOI] [PubMed] [Google Scholar]
- Patel T, Gores GJ, Kaufmann SH. The role of proteases during apoptosis. FASEB J. 1996;10:587–597. doi: 10.1096/fasebj.10.5.8621058. [DOI] [PubMed] [Google Scholar]
- Ishimaru N, Arakaki R, Watanabe M, Kobayashi M, Miyazaki K, Hayashi Y. Development of autoimmune exocrinopathy resembling Sjogren’s syndrome in estrogen-deficient mice of healthy background. Am J Pathol. 2003;163:1481–1490. doi: 10.1016/S0002-9440(10)63505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menad H-A, el-Amine M. The calpain-calpastatin system in rheumatoid arthritis. Immunol Today. 1996;17:545–547. doi: 10.1016/s0167-5699(96)30064-9. [DOI] [PubMed] [Google Scholar]
- Mimori T, Suganuma K, Tanami Y, Nojima T, Matsumura M, Fujii T, Yoshizaka T, Suzuki K, Akizuki M. Autoantibodies to calpastatin (an endogenous inhibitor for calcium-dependent neutral protease, calpain) in systemic rheumatic diseases. Proc Natl Acad Sci USA. 1995;92:7267–7271. doi: 10.1073/pnas.92.16.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Deshmukh M. Caspases: a treatment target for neurodegenerative disease? Nat Med. 1997;3:954–955. doi: 10.1038/nm0997-954. [DOI] [PubMed] [Google Scholar]
- Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- Huang S, Jiang Y, Li Z, Nishida N, Mathias P, Lin S, Ulvitch RI, Nemerow GR, Han J. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Feinstein E, Kimchi A, Wallach D, Boldin M, Varfolomeev E. The death domain: a module shared by proteins with diverse cellular function. Trends Biochem Sci. 1995;20:342–344. doi: 10.1016/s0968-0004(00)89070-2. [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- Hamad AR, Schneck JP. Antigen-induced T cell death is regulated by CD4 expression. Int Rev Immunol. 2001;20:535–546. doi: 10.3109/08830180109045577. [DOI] [PubMed] [Google Scholar]
- Ishimaru N, Yanagi K, Ogawa K, Suda T, Saito I, Hayashi Y. Possible role of organ-specific autoantigen for Fas Ligand-mediated activation induced cell death in murine Sjögren’s syndrome. J Immunol. 2001;167:6031–6037. doi: 10.4049/jimmunol.167.10.6031. [DOI] [PubMed] [Google Scholar]