Abstract
Tuberous sclerosis complex (TSC) is a tumor suppressor gene disorder characterized by mutations in the TSC1 or TSC2 genes. These mutations lead to the development of benign tumors involving smooth muscle cells, causing life-threatening lymphangioleiomyomatosis. We isolated and characterized two types of cells bearing a mutation in TSC2 exon 18 from a renal angiomyolipoma of a TSC patient: one population of α-actin-positive smooth muscle-like cells with loss of heterozygosity for the TSC2 gene (A+ cells) and another of nonloss of heterozygosity keratin 8/18-positive epithelial-like cells (R+ cells). Unlike control aortic vascular smooth muscle cells, A+ cells required epidermal growth factor (EGF) to grow and substituting EGF with insulin-like growth factor (IGF)-1 failed to increase the cell number; however, omission of EGF did not cause cell loss. The A+ cells constantly released IGF-1 into the culture medium and constitutively showed a high degree of S6K phosphorylation even when grown in serum-free medium. Exposure to antibodies against EGF and IGF-1 receptors caused a rapid loss of A+ cells: 50% by 5 days and 100% by 12 days. Signal transduction mediated by EGF and IGF-I receptors is therefore involved in A+ cell survival. These results may offer a novel therapeutic perspective for the treatment of TSC complications and lymphangioleiomyomatosis.
Tuberous sclerosis complex (TSC) is an autosomal dominant syndrome characterized by the multiorgan development of benign and occasionally malignant tumors that most frequently affect the central nervous system, kidney, and skin.1 In particular, the kidney tumors include angiomyolipomas and renal cell carcinomas, of which the former may cause renal failure as a result of the replacement of kidney parenchyma with tumoral tissue, possibly leading to life-threatening hemorrhage.2 Angiomyolipomas consist of smooth muscle cells, adipose tissue, and disorganized thick-walled vascular channels.3
TSC is characterized by mutations in the TSC1 or TSC2 tumor suppressor genes, which seem to act together as a complex of the encoded hamartin (TSC1) and tuberin (TSC2) proteins.4 Mutations in TSC1 on chromosome 9q34 and TSC2 on chromosome 16p13 lead to similar clinical phenotypes, which are more severe in the case of the TSC2 subtype.5–7 The loss of heterozygosity (LOH) for the wild-type allele corresponding to the germline TSC1 or TSC2 mutation in the TSC lesion can be explained with the two-hit tumor suppressor gene model.8 LOH of both has been documented in angiomyolipomas, astrocytomas, and rhabdomyomas from TSC patients.9,10 The frequency of LOH varies significantly among tumor types, being high in angiomyolipomas and low in central nervous system lesions.9
TSC can occur in association with pulmonary lymphangioleiomyomatosis (LAM), a progressive and often fatal interstitial lung disease characterized by the diffuse proliferation of abnormal smooth muscle cells and cystic degeneration of lung parenchyma.11,12 The smooth muscle cells in angiomyolipomas are very similar to those of pulmonary LAM, and genetic data suggest that LAM may be the result of benign cell metastases, a highly unusual disease mechanism.11 The same mutation and LOH have been found in the abnormal pulmonary smooth muscle cells and angiomyolipoma of a large percentage of LAM patients with renal angiomyolipomas,11,12 which suggests that LAM and TSC may have a common genetic origin.11,12
The TSC1/TSC2 complex negatively regulate cell size and proliferation.13,14 TSC2 is a direct target of Akt, a PI3K-regulated effector that promotes cell growth and survival by means of a mammalian target of rapamycin (mTOR)-dependent mechanism.15 Most of the extracellular and intracellular signal pathways involved in the regulation of growth factor- and nutrient-mediated cell growth are integrated by mTOR, as is suggested by the increased phosphorylation of S6 kinase (S6K) and eukaryotic initiation factor 4E binding protein-1 (4EBP1).16 Rapamycin is a microbial product that counteracts these effects by inhibiting mTOR, and Akt is a prosurvival and pro-oncogenic protein that is phosphorylated after the activation of growth factor receptors.16 Akt phosphorylation decreases the ability of TSC2 to inhibit the phosphorylation of the mTOR substrates S6K and 4EBP1,16–18 and excessive Akt, mTOR, and S6K activation causes various types of tumor, including hamartomas.1 TSC1 or TSC2 mutant cells show a high degree of S6K and 4EBP1 phosphorylation, but the overexpression of TSC1 and TSC2 inhibits the phosphorylation of both, thus suggesting that the major cell function of TSC1/TSC2 is to inhibit translation by blocking the phosphorylation of S6K and 4EBP1.16,19,20
We here describe the isolation and characterization of two cell populations from an angiomyolipoma of a TSC2 patient: actin-positive smooth muscle-like cells and keratin 8/18-positive epithelial-like cells. We identified the mutation (corresponding to a TSC2 alteration) that led to the loss of wild-type alleles in the smooth muscle cell population. The growth and proliferation of the LOH smooth muscle-like cells required epidermal growth factor (EGF) in the culture medium, and the cells released abundant insulin-like growth factor (IGF)-I into the medium. The addition of IGF-1 to the culture medium stimulated the proliferation of control smooth muscle cells from human aorta, but not that of the TSC2 LOH smooth muscle-like cells. Our experiments have been repeated several times throughout the last 2 years, thus confirming the reliability of our TSC2 human smooth muscle cells.
Materials and Methods
Establishment of the Angiomyolipoma Culture
The spontaneous renal angiomyolipoma sample (30 cm) was obtained during total nephrectomy from a 42-year-old female with a history of TSC who had given her informed consent according to the Declaration of Helsinki. The study was approved by the Institutional Review Board of Milan’s San Paolo Hospital.
The tumor tissue was dissociated using sterile filtered collagenase type II (Sigma, St. Louis, MO) in phosphate-buffered saline after manual dissociation by means of repetitive pipetting. The collagenase was neutralized with a serum-containing medium (50/50 mixture of Dulbecco’s modified Eagle’s medium/Ham F12; Euroclone, Paignton, UK) supplemented with hydrocortisone 2 × 10−7 mol/L (Sigma), EGF 10 ng/ml (Sigma), sodium selenite 5 × 10−8 mol/L (Sigma), insulin 25 μg/ml (Sigma), transferrin 10 μg/ml (Sigma), ferrous sulfate 1.6 × 10−6 mol/L (Sigma), and 15% fetal bovine serum (Euroclone) as indicated by Arbiser and colleagues.21 The CT/G human aorta vascular smooth muscle cells (VSMCs) were maintained in F12 medium containing 10% fetal bovine serum (American Type Culture Collection, Manassas, VA).
Histology and Immunohistochemistry
The tissue was frozen in liquid nitrogen-cooled isopentane, and stored at −80°C until sectioning. The angiomyolipoma was routinely stained with hematoxylin and eosin (H&E). The slides were deparaffinized and rehydrated in graded concentrations of ethanol to distilled water. Endogenous peroxide activity was blocked with 3% hydrogen peroxide for 30 minutes at room temperature, followed by a brief rinse in distilled water and a wash in phosphate-buffered saline. The tissue sections underwent pepsin enzyme digestion before antibody staining. Nonspecific background noise was inhibited by means of incubation in 5% goat serum. The primary α-actin HHF-35 (1:100; DAKO, Carpinteria, CA), HMB45 (1:100, DAKO), and RhoA antibodies (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) were incubated overnight at 4°C, and localized using the avidin-biotin complex immunoperoxidase method with the immunopure standard ABC staining (Pierce, Rockford, IL) diluted 1:100.
Cell Immunofluorescence Microscopy
The cells were cultured on glass slides, permeabilized with 70% methanol for 10 minutes, and dried in air. The primary antibodies against α-actin (1:100; Sigma), vimentin (1:70; Santa Cruz), HMB45 (1:100; DAKO), S100 (1:8000; DAKO), CD68 (1:100, DAKO), keratin 8/18 (1:100, Santa Cruz), hamartin (1:100, a gift from Dr. Nellist and Dr. Halley, Erasmus University, Rotterdam, The Netherlands), and RhoA (1:100; Santa Cruz) were applied overnight at 4°C. The samples were incubated for 3 hours at room temperature with fluorescein isothiocyanate-conjugated rabbit anti-mouse antibody (Chemicon, Temecula, CA) for α-actin, HMB45, CD68, and keratin 8/18, and fluorescein isothiocyanate-conjugated donkey anti-goat antibody (Chemicon) for vimentin and S100, and rhodamine-conjugated goat anti-rabbit antibody (Chemicon) for hamartin and RhoA. After washing, the slides were mounted in 50% glycerol with 1 μg/ml 4,6-diamidino-2-phenylindole.
Mutation Study
The DNAs were extracted from peripheral lymphocytes and cultured cells using the Wizard Genomic DNA purification kit (Promega, Madison, WI). All of the exons of TSC1 and TSC2 from the genomic DNAs were amplified by means of standard polymerase chain reaction (PCR) and previously described primers22 in a 25-μl final volume mix containing 10 to 50 ng of genomic DNA, 200 μmol/L dNTPs, 1.5 mmol/L MgCl2, 10 pmol of each primer, and 1.25 U of AmpliTaq Gold (Applied Biosystems, Foster City, CA). The TSC1 and TSC2 amplimers were divided into those that were successfully amplified at annealing temperatures of, respectively, 55°C, 60°C, and 65°C. Mutations were detected by submitting the PCR products to denaturing high-performance liquid chromatography (DHPLC) (Transgenomic, Crewe, UK). To enhance heteroduplex formation, the untreated PCR product was denaturated at 94°C for 5 minutes followed by gradual reannealing at 35°C for 1 hour. The samples were analyzed at the melt temperature determined using the DHPLC melt software. The products showing variant DHPLC melt profiles were directly sequenced using a BigDye terminator cycle sequencing kit (Applied Biosystems), and the results were analyzed using sequence analysis 3.4.1 software (Applied Biosystem). The sequencing reactions in which mutations were identified were repeated at least twice.
LOH Analysis
The panel of microsatellite markers near the TSC2 locus on chromosome 16p13.3 consisted of Kg8, D16S287, D16S291, D16S525, D16S665, D16S3024, and D16S3394. The sense primers were labeled with 6-FAM fluorescent dyes (M-Medical, Cornaredo, Italy). The primer sequences were obtained from the Genome Database (www.gdb.org). LOH was analyzed in a 25-μl final volume mix containing 10 to 50 ng of genomic DNA, 200 μmol/L dNTPs, 1.5 mmol/L MgCl2, 10 pmol of each primer, and 1.25 U of AmpliTaq Gold (Applied Biosystem). PCR amplification consisted of 94°C for 10 minutes followed by 32 cycles of 94°C for 1 minute, 60°C for 1 minute, 72°C for 1 minute, and final extension at 72°C for 15 minutes. The PCR products were analyzed using a 310 ABI prism (Applied Biosystems). All of the analyses were repeated at least twice.
Western Blotting
The tissues were homogenized using an Ultra Turrax polytron in 5 vol of a homogenization buffer consisting of 25 mmol/L Tris-HCl, pH 7.4, 0.4 mmol/L sodium azide, 0.4 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L benzamidine-HCl, 0.4 EDTA, 0.4 mmol/L EGTA, and 0.25 mol/L sucrose. The homogenate was centrifuged at 1500 × g for 10 minutes at 4°C, and the supernatant was used for Western blot analysis. The cells were lysed in lysis buffer (5 mmol/L EDTA, 100 mmol/L deoxycholic acid, 3% sodium dodecyl sulfate). The tissue and cell samples (50 μg) were boiled, electrophoretically run on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, and transferred to nitrocellulose membranes (Amersham, Arlington Heights, IL). After being blocked at room temperature for 3 hours with 5% dry milk (Merck, Darmstadt, Germany), the membranes were incubated overnight at 4°C with antibodies against tuberin (1:1000; Cell Signaling, Beverly, MA) and tuberin (C-20) (1:100; Santa Cruz), phospho-tuberin (1:1000; Cell Signaling), hamartin, the β subunit of the IGF-1 receptor (1:100; Santa Cruz), EGF receptor (1:200; Santa Cruz), phospho-Akt (1:500; Promega), Akt (1:200; Santa Cruz), phospho-S6K (1:1000; Cell Signaling), S6K1 (1:1000; Cell Signaling), phospho-S6 ribosomal protein (Ser235/236) (1:1000; Cell Signaling), phospho-4EBP1 (1:1000; Cell Signaling), 4EBP1 (1:1000; Cell Signaling), phospho-extracellular signal-regulated kinase (ERK) (1:1000; Cell Signaling), and phospho-mTOR (1:1000; Cell Signaling). The membranes were washed and incubated for 1 hour with anti-mouse antibody (1:10,000; Chemicon) for phospho-S6K and anti-rabbit antibody (1:10,000; Chemicon) for all of the other antibodies. The reaction was revealed using the SuperSignal West Pico chemiluminescent substrate (Pierce).
Evaluation of Cell Growth and Proliferation
The growth rates of the smooth muscle-like and epithelial-like cells were compared by counting at least 400 to 500 cells in an improved Neubauer chamber after 4 and 8 days of culture. The proliferation growth factor dependence of the smooth muscle-like cells and VSMCs were assayed in the presence or absence of EGF (10 ng/ml) or by replacing EGF with IGF-1 (50 and 5 ng/ml), by counting the cells after 4, 7, 10, 15, and 21 days of culture in the Neubauer chamber. The action of rapamycin was evaluated by adding 1 ng/ml to the A+ cells at plating time, with or without EGF, and measuring cell proliferation after 3, 5, and 10 days. Anti-EGF receptor (clone 225; Calbiochem, Darmstadt, Germany), anti-EGF receptor (clone EGFR.1; Calbiochem), and anti-IGF-1 receptor (clone αIR3; Calbiochem) were added at a concentration of 5 μg/ml to the complete medium and to the medium deprived of EGF, and cell proliferation was evaluated after 2, 5, 10, and 12 days of culture. The action of wortmannin (Sigma) and PD98059 (Sigma) was evaluated by adding them to the culture medium, and evaluating their effect on cell proliferation 48 hours later. Each data point of the proliferation experiments is the mean of four independent experiments.
IGF-1 Enzyme-Linked Immunosorbent Assay
The IGF-1 released by the A+ cells was assayed using an IGF-1 enzyme-linked immunosorbent assay kit in accordance with the manufacturer’s instructions (KAPB2010; Biosource Europe S.A., Nivelles, Belgium). The cells were incubated for 24 hours before the medium was collected and IGF-1 determined. These assays were performed 14 and 21 days after A+ plating.
Statistical Analysis
The data are expressed as mean values ± SEM, and were statistically analyzed using Student’s t-test; significance is indicated for P values of *<0.05 and ***< 0.001.
Results
Angiomyolipoma Immunohistochemical Staining
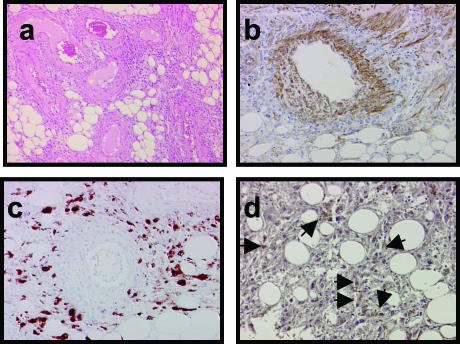
The H&E-stained sections of the angiomyolipoma (the source of the isolated cells, see below) contained three typical histological components: adipocytes, smooth muscle cells, and vascular structures (Figure 1a). The cells positive for α-actin were mainly present in the vessel walls and related surroundings (Figure 1b); the HMB-45-labeled cells23,24 were located in the outermost layer of blood vessels and throughout the angiomyolipoma (Figure 1c). The immunohistochemical evaluation of RhoA, a small GTPase that promotes the formation of stress fibers) showed several positively stained cells in the angiomyolipoma (Figure 1d). The activation of RhoA, Rac, and Cdc42 is critical for cell adhesion and motility, and their dysregulation induces cell transformation and metastasis.25
Figure 1.
a: Histological and immunohistochemical staining with α-actin-, HMB-45-, and RhoA-specific antibodies on adjacent sections of an angiomyolipoma in a TSC patient. H&E staining. b: Immunolabeling with α-actin antibody shows positive cells surrounding a blood vessel and others spread in the parenchyma. c: The HMB-45-labeled cells distributed in the periphery of a blood vessel and in the surrounding parenchyma. d: The RhoA-positive cells were small and distributed throughout the angiomyolipoma but away from blood vessels (arrows). Original magnifications: ×100 (a); ×200 (b–d).
Cellular Characterization
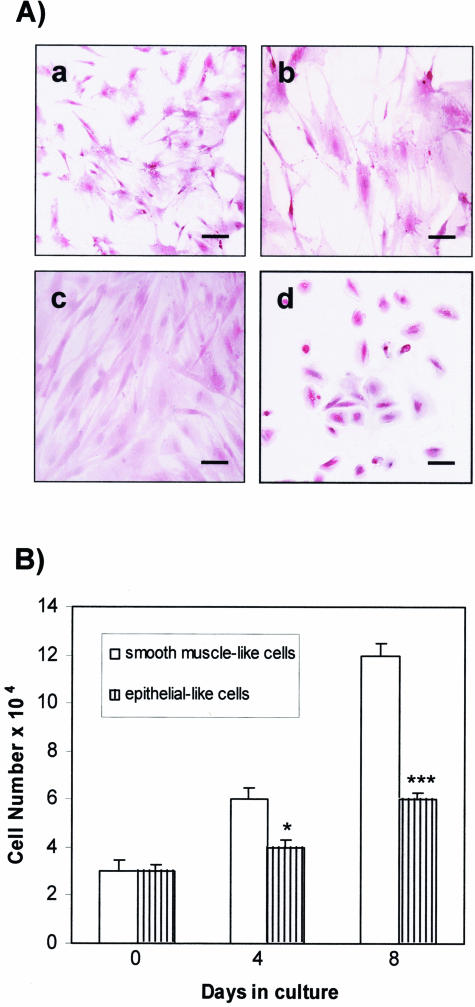
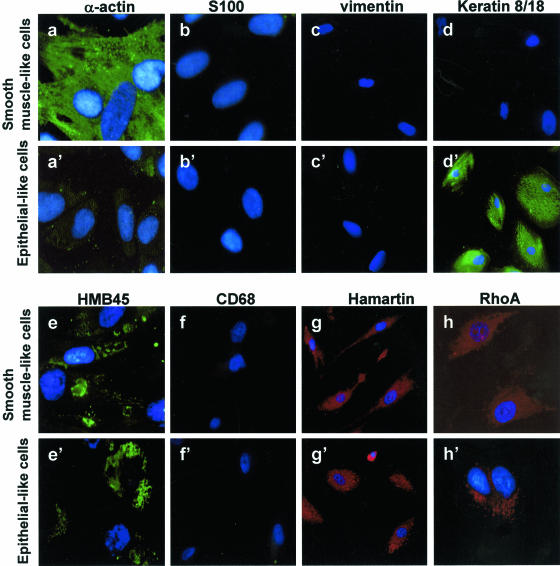
Two cell populations were isolated from the angiomyolipoma and cultured in monolayers before being separated by sequential subcloning to obtain pure homogeneous cultures. Routine H&E staining (Figure 2A) showed that one population had a characteristic flat and elongated appearance (Figure 2A; a to c) and the other had an epithelial-like morphology (Figure 2d). The growth of the flat and elongated cells was much faster than that of the epithelial-like cells (Figure 2B): from a plating density of 3 × 104 cells, they quadrupled to 12 × 104 in 8 days. All of these cells were strongly positive for smooth muscle-specific α-actin antibody (Figure 3a), with the stain being diffused throughout the cytoplasm, and are probably smooth muscle cells. They were also positive for HMB-45 antibody (Figure 3e), which is consistent with the previously described angiomyolipoma phenotype. Because they were negative for S100 (Figure 3b), vimentin (Figure 3c), keratin 8/18 (Figure 3d), and CD68 (Figure 3f), they were christened A+ because of their α-actin antibody reactivity. On the contrary, the epithelial-like cells strongly reacted with keratin 8/18 antibody (Figure 3d’) and with HMB45 (Figure 3e’), but were negative for α-actin (Figure 3a’), S100 (Figure 3b’), vimentin (Figure 3c’), and CD68 (Figure 3f’).
Figure 2.
Morphological appearance and growth rate of the two cell types isolated from the TSC human angiomyolipoma. A: H&E staining of flat-elongated smooth muscle-like cells at different magnifications (a, b) and at confluence (c), and rounder epithelial-like cells (d). B: Growth rate of smooth muscle-like and epithelial-like cells 4 and 8 days after plating. *P < 0.05 and ***P < 0.001 indicate significant differences versus smooth muscle-like cell proliferation. Scale bars: 40 μm (a, d); 20 μm (b, c).
Figure 3.
The two cell types isolated from the TSC angiomyolipoma were exposed to several antibodies to reveal their immunocytochemical characteristics. Exposure to α-actin antibody (a and a’) specific for smooth muscle cells showed that all of the flat-elongated smooth muscle-like cells were positive and the epithelial-like cells were negative; both cell types were negative for S100, a marker of lipid-containing cells (b and b’), vimentin, a marker of fibroblasts (c and c’), and CD68, a marker of macrophages, monocytes, neutrophils, basophils, and large lymphocytes (f and f’); keratin 8/18 (d and d’), and RhoA (h and h’) antibodies labeled the epithelial-like cells; the smooth muscle-like cells were negative for keratin 8/18 and slightly positive for RhoA. Both cell types were positively marked by antibodies to HMB-45 (e and e’) and hamartin (g and g’).
Hamartin and tuberin regulate the RhoA promoter of stress fiber formation, and the absence of the TSC1/TSC2 complex leads to stress fiber disassembly and focal adhesion remodeling, thus deregulating cell dynamics.26,27 Because the epithelial-like cells were strongly stained by RhoA, they were christened R+ cells (Figure 3, h and h’). Both cell types were positive for the TSC1 gene product, hamartin, which was localized in the cell cytoplasm (Figure 3, g and g’).
Mutation Analysis
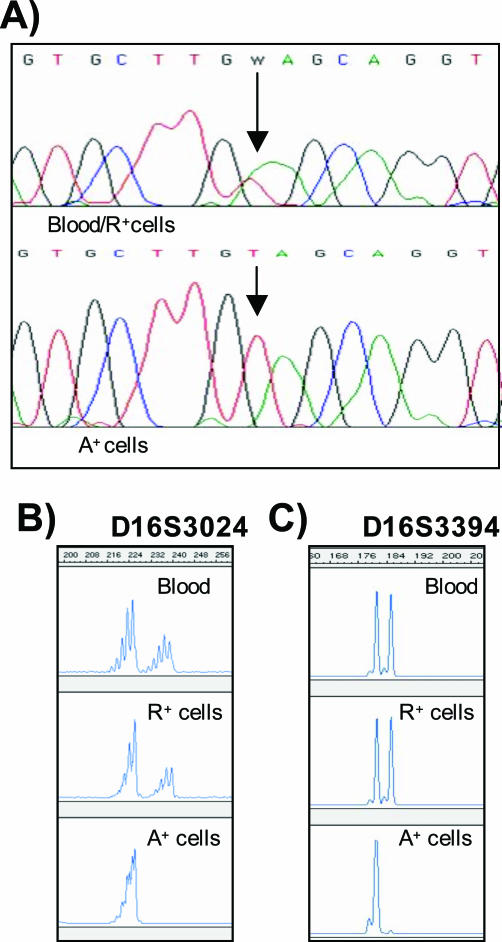
DNA sequencing has shown that blood, angiomyolipoma, and smooth muscle-like cells contained a germline TSC2 exon 18 mutation consisting of a base pair change in amino acid 698 from a lysine to a stop codon (K698X). This mutation inhibits tuberin tyrosine phosphorylation and the formation of the tuberin-hamartin complex.28 K698X-mutated tuberin should be unable to inhibit S6 kinase16 or interact with 14-3-3.29 This mutation was also heterozygously present in peripheral blood and the epithelial-like R+ cells. Sequencing of the A+ cells exclusively revealed mutant residue T at position 2110, thus indicating LOH of the TSC2 allele containing wild-type residue A (Figure 4A). The mutation detected in one copy in the A+ cells was consistent with Knudson’s two-hit tumor-suppressor gene model.30
Figure 4.
A: Determination of bi-allelic inactivation by direct sequence analysis of blood, R+ cells, and A+ cells. The mutation site is indicated by a vertical arrow. A+ cell sequencing revealed the mutation in TSC2 exon 18 at residue 2110. Representative PCR analysis of chromosome 16p13.3 microsatellite markers D16S3024 (B) and D16S3394 (C) in blood, R+ cells, and A+ cells.
LOH Analysis
We tested the blood and A+ and R+ cells of the TSC patient for LOH by means of PCR amplification using a panel of microsatellite markers near the TSC2 locus on chromosome 16p13.3. Five markers (D16S287, D16S291, D16S525, D16S665, and Kg8) were heterozygous in all of the samples (data not shown), but two (D16S3024 and D16S3394) showed LOH in the A+ cells but not in the blood and R+ cells (Figure 4, B and C).
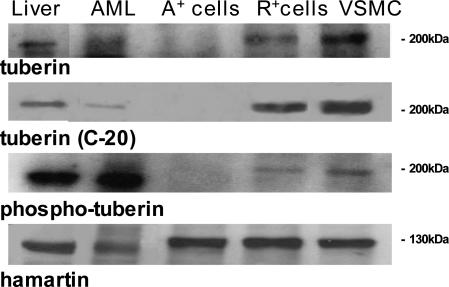
Tuberin and Hamartin Expression
The 180-kd tuberin protein is expressed in many different cell and tissue types,31 and we evaluated the expression of the TSC complex in LOH A+ cells, patient specimens, R+ cells, and VSMCs. The use of two antibodies recognizing different regions of tuberin in the catalytic domain showed that tuberin was expressed in the liver, angiomyolipoma, VSMCs, and R+ cells, but not in the A+ cells, thus confirming the results of the LOH analysis (Figure 5). In response to PI3K activation, tuberin is directly phosphorylated by Akt at Thr1462 and Ser939, and a tuberin lacking PI3K-dependent phosphorylation sites can block the activation of S6K1.18 High levels of Thr1462-phosphorylated tuberin were detected in the liver and angiomyolipoma of the TSC2 patient, but they were low in the R+ and VSMCs, and absent in the A+ cells (Figure 5). Hamartin expression was comparable in all of the tested groups (Figure 5).
Figure 5.
Expression of the TSC1/TSC2 complex in the liver and angiomyolipoma of the TSC2 patient and in A+ and VSMCs. Western blots were made using two antibodies recognizing different regions of the COOH-terminal domain (tuberin and tuberin C20), an antibody reacting with the Thr1462-phosphorylated tuberin form, and another for hamartin. The blots are representative of three separate experiments.
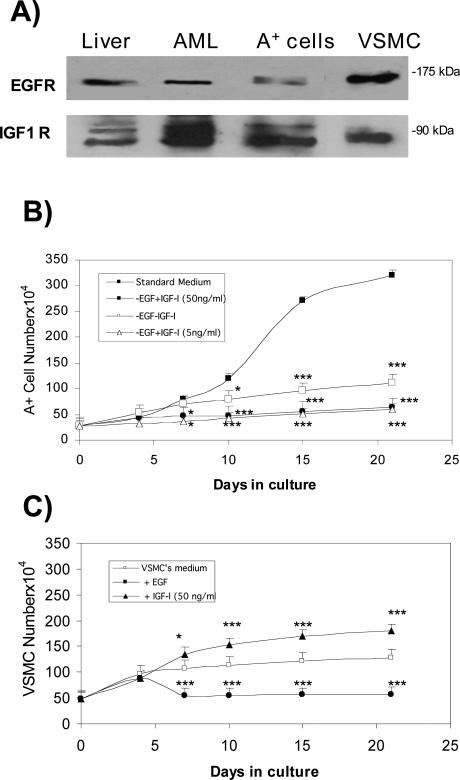
Role of EGF and IGF-1 on A+ Cell Growth and Survival
The A+ cells were isolated and cultured in a medium containing EGF at a concentration of 10 ng/ml, as indicated by Arbiser and colleagues.21 EGF and IGF-1 receptors were detected in the A+ cells, the liver, and AML of the TSC2 patient, and the VSMCs (Figure 6A). The role of EGF and IGF-1 in A+ cell growth was evaluated by eliminating EGF from the culture medium or replacing EGF with IGF-1 at concentrations of 5 or 50 ng/ml for 21 days. Under all conditions, the A+ cells survived but did not proliferate when EGF was removed from the medium or reduced to 1 ng/ml (these latter data are not shown), and so IGF-1 did not replace its proliferative action (Figure 6B). The opposite effect was observed in the VMSCs: their number markedly increased when IGF-1 was added to the culture medium, but their proliferation was quickly blocked by EGF supplementation (Figure 6C). The early slight effect of EGF on VSMC proliferation may be secondary to the reported brisk DNA synthesis with minimal cell division throughout 0 to 4 days that subsequently leads to cycle arrest.32 When rapamycin was added to the culture medium of A+ cells at plating time, their growth rate was comparable with that of the VSMCs, and the action of rapamycin was not modified by the presence or absence of EGF (Table 1).
Figure 6.
Evaluation of the role of EGF and IGF-1 on A+ cell growth. The cells were counted in a Neubauer chamber after 4, 7, 10, 15, and 21 days of culture. A: Expression of EGF receptor (EGFR) and IGF-1 receptor (IGF-1R) in the liver and angiomyolipoma of the TSC2 patient, and in A+ and VSMC cells. B: A+ cell growth in complete medium (containing 10 ng/ml EGF), in the same medium without EGF, and without EGF with the addition of IGF-1 50 or 5 ng/ml. The A+ cells proliferated and significantly increased in number only in the presence of EGF; IGF-1 failed to promote any increase. No reduction or mortality was observed under any of the experimental conditions. C: VSMC proliferation in specific standard medium, in the presence of 10 ng/ml EGF, and in the presence of 50 ng/ml IGF-1. IGF-1 supplementation led to a significant increase in cell number. Mean values ± SEM. Significant differences (*P < 0.05, ***P < 0.001) versus control were evaluated by Student’s t-test.
Table 1.
Cell Growth Rate
| Days | 3 | 5 | 10 |
|---|---|---|---|
| VSMC | 35% | 60% | 107% |
| A+ cells with RAPA and EGF | 25% | 35% | 100% |
| A+ cells with RAPA, without EGF | 24% | 36% | 100% |
| A+ cells in standard medium | 36% | 68% | 240% |
Percentage increase in cell number. Rapamycin (RAPA) at the concentration of 1 ng/ml was added at plating time. At time 0 cells (50 × 104 cells) were plated and the number of cells was evaluated at the indicated time.
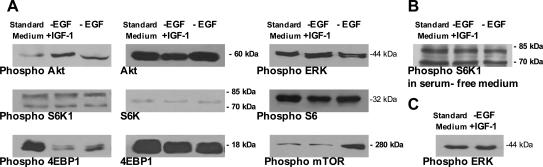
Depriving the medium of EGF for 21 days or replacing it with IGF-1 increased the phosphorylation of Akt in the A+ cells, but did not modify its expression, the phosphorylation and expression of S6K1 and the phosphorylation of its substrate ribosomal protein S6, or the phosphorylation of ERK (Figure 7A). When the A+ cells were exposed to serum-deprived medium for 24 hours, phospho-S6K1 expression was not modified (Figure 7B), and incubation with IGF-1 for 2 hours did not modify that of phospho-Erk (Figure 7C). The phosphorylation of 4EBP1 decreased when EGF was not added to the medium, even when IGF-1 was supplemented, its expression did not change under any of the culture conditions. The omission of EGF from the culture medium increased phosphorylated mTOR in comparison with the levels observed in the cells grown in complete medium; this was not affected by IGF-1 (Figure 7A). The addition of PI3K (wortmannin) or MAPK inhibitors (PD98059) to the standard medium did not influence the A+ cell growth rate: after 48 hours of exposure the A+ cells increased from 36 × 104 to 57.2 × 104 cells in standard medium, to 54.3 × 104 cells with 40 nmol/L wortmannin, to 53.2 × 104 cells with 320 nmol/L wortmannin, and to 54.4 × 104 cells with 30 μmol/L PD98059. Their average 24-hour IGF-1 secretion after 14 and 21 days in culture was respectively 9.10 ± 0.21 ng/1.5 × 105 cells and 14.21 ± 0.65 ng/1.5 × 105 cells.
Figure 7.
Western blot evaluation of the expression of Akt and its phosphorylated form on Ser473, S6K1, and its phosphorylated form on Thr389, 4EBP1, and its phosphorylated form on Thr37/46, the phosphorylated form of ERK on Thr202/Tyr204, and Ser2448-phosphorylated mTOR. A: The A+ cells were cultured for 21 days in standard medium, and in the same medium without EGF in the absence or presence of IGF-1 (50 ng/ml). The A+ cells were grown for 24 hours in serum-free medium without EGF and IGF-1 before S6K1 phosphorylation was evaluated (B), and incubated for 2 hours with IGF-1 before phospho-Erk was evaluated (C). The blots are representative of three experiments.
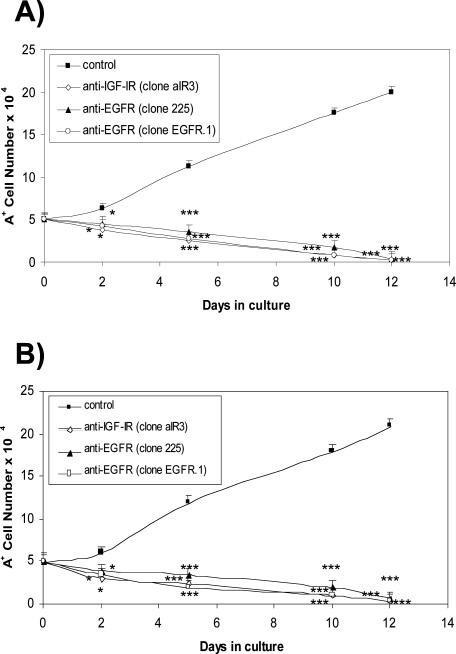
Antibodies raised against the EGF receptor (EGFR) and directed to the ligand binding site (clone 225) or the cell surface domain (clone EGFR.1), and antibody against the IGF-1 receptor (IGF-1R) were added to the A+ cell culture medium with EGF (Figure 8A) and without EGF (Figure 8B), and the evaluation of cell proliferation showed that, in both cases, antibody exposure led to a rapid decrease in cell number that reached 50% in 5 days when anti-IGF-1R and anti-EGFR clone EGFR.1 were used. It seems that both of these antibodies may be slightly more effective than anti-EGFR clone 225 (P = 0.05). Total cell loss was reached within 12 days of antibody exposure. These data suggest that IGF-1 is released autocrinally and may act on A+ cell survival, whereas EGF may act on both proliferation and survival. These cells do not release EGF (data not shown).
Figure 8.
Effect of antibodies (5 μg/ml) to EGF and IGF-1 receptors on A+ cell number. A total of 5 × 104 cells were plated and the antibodies were added to the complete medium (A) or to the same medium without EGF (B); the cells were then counted in a modified Neubauer chamber. There was a 50% reduction in cell number within 5 days, and complete cell loss in 12 days. Anti-IGF-1R and anti-EGFR clone EGFR.1 were apparently more effective than anti-EGFR clone 225, the difference being significant (P < 0.05) 5 days after plating. Mean values ± SEM. Significant differences (*P < 0.05, ***P < 0.001) versus control were evaluated by Student’s t-test.
Discussion
TSC is a tumor suppressor gene disorder associated with benign and malignant tumors. Lesions such as cortical tubers, subependymal giant cell astrocytomas, cardiac rhabdomyomas, and renal angiomyolipoma often show abnormal differentiation patterns, as well as deregulated cell growth and proliferation.1,14,15 Angiomyolipomas are uncommon renal tumors that have smooth muscle, fat, and vascular components, and belong to a group of neoplasms that co-express melanocytic and smooth muscle markers, including LAM of the lung.8
The TSC2 gene product, tuberin, functions as a renal tumor suppressor and regulates cell growth and cell cycle progression, and its loss may lead to abnormal cell proliferation.13 LOH of TSC2 mutations occurs in 60% of the angiomyolipomas taken from women with the sporadic form of LAM, specifically in smooth muscle cells and fat components.12 Cultured cells from human TSC2 angiomyolipomas may be an optimal means of studying and developing appropriate pharmacological strategies aimed at blocking the life-threatening growth of smooth muscle cells in TSC and LAM. The advantages of using human pathological TSC2 cells rather than knockout models are obvious and our results highlight some of them.
We here report the novel finding that the in vitro growth of smooth muscle-like cells derived from the renal angiomyolipoma of a TSC2 patient depends on the availability of EGF in the medium. Both the blood and angiomyolipoma cells showed a somatic TSC2 gene mutation in exon 18 consisting of a stop codon. The same gene modification was also present in isolated cells. To the best of our knowledge, only one previous study has shown that angiomyolipoma cells with TSC2 mutations can be grown in culture, although the number of such studies may have been limited by the loss of the entire primary culture.33
Our isolated angiomyolipoma cells can be grown in culture as a stabilized cell line, and so could be used as a continuous source and do not require any morphological, biochemical, and pharmacological modifications (see summary of A+ and R+ cell characterization in Table 2). They have been stored in liquid nitrogen and grown in culture for the past 2 years without any changes in their growth, pharmacological, or genetic characteristics. The subcloned smooth muscle cells (A+) are LOH and do not express tuberin, whereas the 8/18 keratin-positive cells are non-LOH and contain tuberin and its phosphorylated form. Other angiomyolipoma cell cultures have been recently reported, but the cells did not carry TSC1 or TSC2 mutations, and immortalization required the introduction of simian virus 40 large T antigen and telomerase.21
Table 2.
Summary of the Characterization of A+ and R+ Cells
| Immunocytochemistry
|
Western blotting
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| α-Actin | Keratin8/18 | HMB45 | Hamartin | RhoA | Tuberin | Phosphotuberin | Hamartin | LOH | |
| A+ cells | + | − | + | + | + | − | − | + | + |
| R+ cells | − | + | + | + | + | + | + | + | − |
Differences in protein expression between A+ and R+ cells. Immunocytochemical staining of A+ and R+ cells did not detect S100, vimentin, and CD68 proteins.
EGF transiently activates Erk, a member of the MAPK family. EGF supplementation of the culture medium is necessary to promote the proliferation and maintenance of the A+ cells, and its proliferative action cannot be replaced by the addition of IGF-1. Conversely, the proliferation rate of our control aorta smooth muscle cells (VSMCs) increased when IGF-1 was added to the growth medium and did not proliferate when exposed to EGF, thus demonstrating a clear difference between normal smooth muscle cells and our TSC2-deficient cells. The EGF-dependent growth is probably tuberin-dependent because blocking mTOR with rapamycin led to an A+ cell growth rate that was comparable with that of the VSMCs. It therefore seems that the EGF-dependent growth is triggered by mTOR activation. The requirement of EGF supplementation for A+ cell proliferation and the effect of anti-EGF-R antibodies on A+ cell survival suggest a possible new therapeutic strategy for controlling smooth muscle cell growth in angiomyolipomas and LAM.
Akt is activated in response to insulin or IGF-1 receptor activation, and is thus capable of phosphorylating tuberin.16–18 This leads to the inhibition of tuberin GTPase activity against Rheb and an increase in Rheb-GTP that activates mTOR. Activated mTOR phosphorylates p70S6K and 4EBP1, and thus enables the translational machinery and promotes cell growth.13 There was no increase in the number of A+ cells cultured for 21 days in the absence of EGF or the presence of IGF-1, but Akt phosphorylation was greater than in the A+ cells grown in a medium containing EGF.
Hyperphosphorylation of p70S6K and its ribosomal protein S6 substrate has been observed in cells lacking tuberin from the Eker rat model of TSC2,19,34 in tumor cells containing TSC2 mutations,3,33 and in cells lacking hamartin from a murine model of TSC1,35 thus demonstrating that the hamartin-tuberin complex negatively regulates p70S6K. After 21 days of culture in a standard medium containing EGF, or in a medium deprived of EGF with or without the addition of IGF-1, phosphorylation of S6K and its substrate S6 in the A+ cells were unchanged which, as recently reported,19,34,36 indicates that the loss of TSC2 function in mammalian cells leads to constitutive S6K1 activation and phosphorylation of S6. Preliminary results suggest that S6K1 is constitutively activated also in R+ cells (unpublished data).
Hyperphosphorylation of 4EBP1 reduces its affinity for eukaryotic initiation factor (eIF)4E and its subsequent dissociation from eIF4E, and leads to the promotion of translation.37 The mechanisms of this event are regulated by the PI 3-kinase pathway and Akt phosphorylation when induced by insulin,38 and by means of mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK) when promoted by Erk stimulators.39 The regulation of 4E-BP1 and eIF4F via MEK/Erk signaling may be important for the control of translation by mitogenic signals that do not activate PI 3-kinase/Akt. The growth of A+ cells requires EGF supplementation, which also markedly increased 4EBP1 phosphorylation, which should lead to dissociation from eIF4E and consequent translation. The phosphorylation of Erk was not modified by any culture condition, but mTOR phosphorylation increased in the absence of EGF and IGF-1. It is possible that A+ cells grow in the presence of EGF because the EGF-activated pathway involving Erk modulates 4EBP1 and its translational function. It is known that the Erk pathway regulates the phosphorylation of multiple 4EBP1 sites to the point that 4EBP1 is released from eIF4E, and that this activation takes place through mechanisms requiring mTOR (Figure 9).40
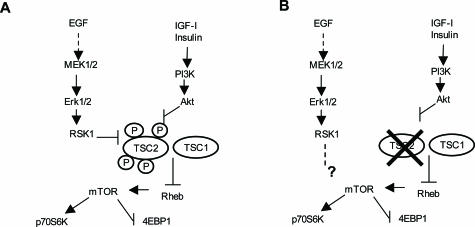
Figure 9.
Erk- and PI3K-Akt-dependent cascades regulating mTOR signaling. A: In normal condition, activation of either pathway results in the phosphorylation of both p90 ribosomal S6 kinase 1 (RSK1) and Akt that, in turn, are capable of phosphorylating tuberin directly. This inhibits tuberin function and promotes mTOR-mediated signaling. B: In TSC2 cells, the loss of tuberin releases the regulation of mTOR and, likely, may lead to the modification of ERK-RSK1 function with direct/indirect regulation of mTOR. This may explain the EGF requirement for A+ cell proliferation.
It has been reported that exposure to anti-EGF receptor39,41 and anti-IGF-1 receptor42,43 inhibits the proliferation, survival, and differentiation of various cultured malignant human cell lines and tumors. Antibodies to EGF and IGF-1 receptors were added to the culture medium in the presence or absence of EGF, and both situations led to the progressive loss of A+ cells. The activity of A+ cell EGF receptors is therefore apparently involved in both proliferation and (perhaps) survival. The simple omission of EGF from the culture medium does not cause cell loss, possibly because of the presence of serum and the autocrine release of IGF-1 by the A+ cells themselves. This differentiates A+ smooth muscle-like cells from VSMCs: the latter do not release IGF-1 and their proliferation is stimulated when it is added to the culture medium, whereas A+ cells release a substantial amount of IGF-1 but, even when added at higher concentrations, it does not promote A+ cell proliferation. However, blocking IGF-1R does cause cell loss, thus suggesting that IGF-1 may have switched from being a proliferative factor to a survival factor in A+ cells. This is confirmed by recent observations in our laboratory indicating the involvement of IGF-1 in the activation of an A+ cell anti-apoptotic pathway (unpublished data).
In conclusion, human LOH smooth muscle-like (A+) and non-LOH epithelial-like (R+) TSC2 cells from renal angiomyolipoma can be isolated, grown in culture, and indefinitely stored in liquid nitrogen. Unlike that of control aorta smooth muscle cells, the proliferation of A+ cells requires the addition of EGF to the culture medium, whereas IGF-1 is autocrinally secreted and may play a role in survival. Incubation with anti-EGFR and anti-IGF1R causes the loss of 50% of the A+ cells in 5 days and of 100% in 12 days. These effects of anti-EGFR and anti-IGF-1R on A+ cell survival may offer a new therapeutic perspective in TSC and LAM.
Footnotes
Address reprint requests to Alfredo Gorio, Laboratory of Pharmacology, Dept. of Medicine, Surgery and Dentistry, Via A. di Rudinì 8, 20142 Milano, Italy. E-mail: alfredo.gorio@unimi.it.
Supported by the FIRB2001 Programme of the Ministero Italiano dell’Università e della Ricerca (grant no. RBAU01L79J).
References
- Young J, Povey S. The genetic basis of tuberous sclerosis. Mol Med Today. 1998;4:313–319. doi: 10.1016/s1357-4310(98)01245-3. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Schwarzkopf G, Henske EP. Renal angiomyolipomas, cysts, and cancer in tuberous sclerosis complex. Semin Pediatr Neurol. 1998;5:269–275. doi: 10.1016/s1071-9091(98)80005-3. [DOI] [PubMed] [Google Scholar]
- Karbowniczek M, Yu J, Henske EP. Renal angiomyolipomas from patients with sporadic lymphangiomyomatosis contain both neoplastic and non-neoplastic vascular structures. Am J Pathol. 2003;162:491–500. doi: 10.1016/S0002-9440(10)63843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Slegtenhorst M, Nellist M, Nagelkerken B, Cheadle J, Snell R, van den Ouweland A, Reuser A, Sampson J, Halley D, van der Sluijs P. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet. 1998;7:1053–1057. doi: 10.1093/hmg/7.6.1053. [DOI] [PubMed] [Google Scholar]
- van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell R, Cheadle J, Jones A, Tachataki M, Ravine D, Sampson J, Reeve M, Richardson P, Wilmer R, Munro C, Hawkins T, Sepp T, Ali J, Ward Green A, Yates J, Kwiatkowska J, Henske E, Short M, Haines J, Jozwiak S, Kwiatkowski D. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- European Chromosome 16 Tuberous Sclerosis Consortium Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, Kasprzyk-Obara J, Domanska-Pakiela D, Kwiatkowski DJ. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henske EP, Wessner LL, Golden J, Scheithauer BW, Vortmeyer AO, Zhuang Z, Klein-Szanto AJ, Kwiatkowski DJ, Yeung RS. Loss of tuberin in both subependymal giant cell astrocytomas and angiomyolipomas supports a two-hit model for the pathogenesis of tuberous sclerosis tumors. Am J Pathol. 1997;151:1639–1647. [PMC free article] [PubMed] [Google Scholar]
- Henske EP, Scheithauer BW, Short MP, Wollmann R, Nahmias J, Hornigold N, van Slegtenhorst M, Welsh CT, Kwiatkowski DJ. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am J Hum Genet. 1996;59:400–406. [PMC free article] [PubMed] [Google Scholar]
- Sepp T, Yates JR, Green AJ. Loss of heterozygosity in tuberous sclerosis hamartomas. J Med Genet. 1996;33:962–964. doi: 10.1136/jmg.33.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henske EP. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosom Cancer. 2003;38:376–381. doi: 10.1002/gcc.10252. [DOI] [PubMed] [Google Scholar]
- Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Hengstschlager M, Rodman DM, Miloloza A, Hengstschlager-Ottnad E, Rosner M, Kubista M. Tuberous sclerosis gene products in proliferation control. Mutat Res. 2001;488:233–239. doi: 10.1016/s1383-5742(01)00058-8. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Yeung RS, Halley DJ, Nicosia SV, Pledger WJ, Cheng JQ. PI3K/AKT pathway regulates TSC tumour suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–35370. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, Panettieri RA, Krymskaya VP. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the Tsc2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbiser JL, Yeung R, Weiss SH, Arbiser ZK, Amin MB, Cohen C, Frank D, Mahajan S, Herron S, Yang J, Ond AH, Zhang HB, Bai X, Uhlmann E, Loehr A, Northrup H, Au P, Davis I, Fisher DE, Gutmann DH. The generation and characterization of a cell line derived from a sporadic renal angiomyolipoma. Am J Pathol. 2001;159:483–491. doi: 10.1016/S0002-9440(10)61720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AC, Sampson JR, Hoogendoorn B, Cohen D, Cheadle JP. Application and evaluation of denaturing HPLC for molecular genetic analysis in tuberous sclerosis. Hum Genet. 2000;106:663–668. doi: 10.1007/s004390000316. [DOI] [PubMed] [Google Scholar]
- Kaiserling E, Krober S, Xiao JC, Schaumburg-Lever G. Angiomyolipoma of the kidney. Immunoreactivity with HMB-45. Light and electron-microscopic findings. Histopathology. 1994;25:41–48. doi: 10.1111/j.1365-2559.1994.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Bjornsson J, Short MP, Kwiatkowski DJ, Henske EP. Tuberous sclerosis-associated renal cell carcinoma. Clinical, pathological, and genetic features. Am J Pathol. 1996;149:1201–1208. [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- Gonchorova E, Gonchorov D, Noonan D, Krymskaya V. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 GTPase. J Cell Biol. 2004;167:1171–1182. doi: 10.1083/jcb.200405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RF, Roy C, Diefenbach TJ, Vinters HV, Johnson MW, Jay DG, Hall A. The TSC1 tumour suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase Rho. Nat Cell Biol. 2000;2:281–287. doi: 10.1038/35010550. [DOI] [PubMed] [Google Scholar]
- Nellist M, Verhaaf V, Goedbloed MA, Reuser AJJ, van den Ouweland AMW, Halley DJJ. TSC2 missense mutations inhibit tuberin phosphorylation and prevent formation of the tuberin-hamartin complex. Hum Mol Genet. 2001;10:2889–2898. doi: 10.1093/hmg/10.25.2889. [DOI] [PubMed] [Google Scholar]
- Nellist M, Goedbloed MA, De Winter C, Verhaaf B, Jankie A, Reuser AJ, van den Ouweland AM, van der Sluijs P, Halley DJ. Identification and characterization of the interaction between tuberin and 14-3-3zeta. J Biol Chem. 2002;277:39417–39424. doi: 10.1074/jbc.M204802200. [DOI] [PubMed] [Google Scholar]
- Knudson AGJ. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienecke R, König A, DeClue JE. Identification of tuberin, the tuberous sclerosis-2 product. J Biol Chem. 1995;270:16409–16414. doi: 10.1074/jbc.270.27.16409. [DOI] [PubMed] [Google Scholar]
- Bagby SP, Kirk EA, Mitchell LH, O’Reailly MM, Holden WE, Stenberg PE, Bakke AC. Proliferative synergy of ANG II and EGF in porcine aortic vascular smooth muscle cells. Am J Physiol. 1993;265:F239–F249. doi: 10.1152/ajprenal.1993.265.2.F239. [DOI] [PubMed] [Google Scholar]
- Yu J, Astrinidis A, Howard S, Henske EP. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signalling pathways. Am J Physiol. 2004;15:L694–L700. doi: 10.1152/ajplung.00204.2003. [DOI] [PubMed] [Google Scholar]
- Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62:5645–5650. [PubMed] [Google Scholar]
- Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- Jaeschke A, Haetkamp J, Saitoh M, Roworth W, Nobukuni T, Hodges A, Sampson J, Thomas G, Lamb R. Tuberous sclerosis complex tumor suppressor-mediated S6 kinase inhibition by phosphatidylinositide-3-OH kinase is mTOR independent. J Cell Biol. 2002;159:217–224. doi: 10.1083/jcb.jcb.200206108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Takata M, Ogawa W, Kitamura T, Hino Y, Kuroda S, Kotani K, Klip A, Gingras AC, Sonenberg N, Kasuga M. Requirement for Akt (protein kinase B) in insulin-induced activation of glycogen synthase and phosphorylation of 4E-BP1 (PHAS-1). J Biol Chem. 1999;274:20611–20618. doi: 10.1074/jbc.274.29.20611. [DOI] [PubMed] [Google Scholar]
- Janmaat ML, Kruyt AEF, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003;9:2316–2326. [PubMed] [Google Scholar]
- Herbert TP, Tee AR, Proud CG. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J Biol Chem. 2002;277:11591–11596. doi: 10.1074/jbc.M110367200. [DOI] [PubMed] [Google Scholar]
- Huang S, Amstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–5362. doi: 10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- Ibrahim YH, Yee D. Insulin-like growth factor-1 and breast cancer therapy. Clin Cancer Res. 2005;11:944s–950s. [PubMed] [Google Scholar]
- Kucab JE, Dunn SE. Role of IGF-1R in mediating breast cancer invasion and metastasis. Breast Dis. 2003;17:41–47. doi: 10.3233/bd-2003-17105. [DOI] [PubMed] [Google Scholar]