Abstract
Signal transducer and activator of transcription 3 (STAT3) has oncogenic potential. The biological effects of STAT3 have not been studied extensively in the pathogenesis of colon cancer, nor has the role of Janus kinase 3 (JAK3), the physiological activator of STAT3, been evaluated. Here, we demonstrate that activated STAT3 (pSTAT3) and activated JAK3 (pJAK3) are expressed constitutively in two colon cancer cell lines, SW480 and HT29. To evaluate the significance of JAK3/STAT3 signaling, we inhibited JAK3 with AG490 and STAT3 with a dominant-negative construct. Inhibition of JAK3 down-regulated pSTAT3. The blockade of JAK3/STAT3 signaling significantly decreased viability of colon cancer cells due to apoptosis and cell-cycle arrest through down-regulation of Bcl-2, Bcl-XL, Mcl-1, and cyclin D2 and up-regulation of p21waf1/cip1 and p27kip1. We also examined histological sections from 22 tumors from patients with stage II or stage IV colon cancer and found STAT3, JAK3, and their activated forms to be frequently expressed. Furthermore, quantitative reverse transcriptase-polymerase chain reaction identified JAK3 mRNA in colon cancer cell lines and primary tumors. Our findings illustrate the biological importance of JAK3/STAT3 activation in the oncogenesis of colon cancer and provide novel evidence that JAK3 is expressed and contributes to STAT3 activation in this malignant neoplasm.
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor with known oncogenic potential.1 Activated STAT3 has been proposed recently to be a novel molecular target for therapeutic intervention in malignant neoplasms.2 STAT3 normally resides in the cytoplasm. After its activation via phosphorylation of the tyrosine705 residue, STAT3 dimerizes and translocates to the nucleus where it controls the transcription of several apoptosis- and cell cycle-regulatory proteins. The net effect of STAT3-mediated transcription directs the cells into cell survival and cell-cycle progression.3 Three major mechanisms are implicated in the activation of STAT3: cytokine stimulation of membrane receptors with innate kinase activity, such as epidermal growth factor and platelet-derived growth factor receptors; cytoplasmic kinases such as Src and Abl; and a family of receptor-associated tyrosine kinases known as Janus kinases (JAKs).4–10
Colorectal cancer is one of the leading causes of morbidity and death in the world and is the third leading cause of cancer-related deaths in North America.11 Approximately 147,500 new colon cancer cases were detected in the United States in 2003 and an estimated 57,100 patients died from this disease in the same year.11 Recent studies have shown that mechanisms involving STAT3 pathway may play a role in colon carcinogenesis. For example, a recent study showed that Src kinases play a major role in the pathogenesis of colon cancer,12 at least in part, via activation of STAT3.13 In addition, Fer was found to phosphorylate and activate STAT3 in colon cancer cells.14 However, the role of STAT3 in the pathogenesis of colon cancer has not been extensively evaluated. In addition, it is unclear as to the role of JAK3, the physiological activator of STAT3, in stimulating STAT3 in colon carcinoma cells.
In the present study, we demonstrated that STAT3 and JAK3 are constitutively activated in two human colon carcinoma cell lines, SW480 and HT29. To investigate the biological significance of JAK3/STAT3 activation in these cells, we used AG490, a pharmacological JAK3 inhibitor, as well as an adenoviral vector harboring the dominant-negative construct of STAT3 (AdSTAT3DN). We investigated changes in cell viability, occurrence of apoptotic cell death, and alterations in cell cycle progression. In addition, we evaluated changes in the protein expression level of several downstream targets of JAK3/STAT3 signaling pathway that are known to be directly related to apoptotic cell death (Bcl-2, Bcl-XL, Mcl-1, and survivin) and cell cycle regulation (cyclin D2, p21waf1/cip1, and p27kip1). We also studied the expression of STAT3 and JAK3 in a number of primary colon cancer tumors from patients.
Materials and Methods
Colon Cancer Cell Lines, Cell Culture, and Antibodies
The human colon carcinoma cell lines used in this study SW480 and HT29 were a gift from Dr. Gary Gallick (The University of Texas MD Anderson Cancer Center). The human embryonic kidney cell line 293 was purchased from American Type Culture Collection (Manassas, VA) and the anaplastic large cell lymphoma cell line Karpas 299 from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). The cell lines were cultured in Dulbecco’s modified Eagle’s medium/F-12 or RPMI (Life Technologies, Inc., Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (56°C for 30 minutes), 100 IU/ml penicillin, and 10 μg/ml streptomycin, and maintained under an atmosphere of 95% oxygen and 5% carbon dioxide in 98% humidity at 37°C. Excluding Bcl-XL (Zymed Laboratories Inc., South San Francisco, CA), common γ chain (γc; R&D Systems, Minneapolis, MN), FLAG (Abcam, Inc., Cambridge, MA), and β-actin (Sigma, St. Louis, MO), all antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies used for the detection of STAT3 were catalogue number sc-8019; lot B0703 for both Western blot and immunohistochemistry, pSTAT3Tyr705: sc-8059; lot H142 for Western blot and sc-7993-R; lot G1503 for immunohistochemistry, JAK3: sc-513; lot C3004 for both Western blot and immunohistochemistry, pJAK3Tyr980: sc-16567; lot S0702 for Western blot and sc-16567-R; lot J0702 for immunohistochemistry, and γc: BAF 284 for Western blot.
Treatment of Human Colon Carcinoma Cells with AG490
AG490 (tyrphostin; Alexis Biochemicals, San Diego, CA) was reconstituted in dimethyl sulfoxide and stored at −20°C until used. It was added in the dark at 16 to 24 hours after the cells were seeded. Dimethyl sulfoxide at a concentration equivalent to the highest concentration of the inhibitor was used as a negative control. Cell viability, determined by exclusion of staining with trypan blue dye, was greater than 90% at the beginning of all experiments.
Infecting the Human Colon Carcinoma Cells with STAT3 Dominant-Negative Adenoviral Vector (AdSTAT3DN)
The characteristics of AdSTAT3DN have been previously detailed.15 Two days after seeding, the cells were infected with AdSTAT3DN suspended in Dulbecco’s modified Eagle’s medium at different multiplicity of infection (MOI) and incubated for 2 hours. The viral suspension was removed and the colon cancer cells were cultured. GFP adenoviral vector (AdGFP) was used as a negative control.
Cell Viability by Exclusion of Staining with Trypan Blue Dye
Control and treated colon cancer cells were stained with trypan blue dye. Briefly, the cells were seeded for 18 hours and then the medium was removed and fresh medium was added with the inhibitor. At 24 hours after treatment, the cells were stained with trypan blue dye and examined using light microscopy and a hemocytometer.
Detection of Apoptosis
Morphological Changes Using Giemsa and Hoechst 33258 Staining
Cytospin slides were stained with Giemsa stain using an automatic stainer and examined using a light microscope. In addition, cell suspensions (100 μl at a concentration of 1.0 × 106 cells/ml) were incubated with 1 μl of Hoechst 33258 (1 mg/ml in distilled water) for 10 minutes at room temperature. Subsequently, cytospin slides were prepared. After air-drying, cells were examined under a fluorescence microscope. Cells were considered to be apoptotic when the nuclei showed chromatin condensation and/or fragmentation.
Detection of Annexin V Binding to the Cell Membrane by Flow Cytometry
Detection of apoptosis was studied after staining of the cells with annexin V-fluorescein isothiocyanate and propidium iodide in accordance with the manufacturer’s protocol (BD Pharmingen, San Diego, CA). Briefly, 5.0 × 105 cells were washed in ice-cold phosphate-buffered saline (PBS) without Ca2+ or Mg2+ (Life Technologies). The cells were then suspended in 100 μl of binding buffer and incubated with 2.0 μl of annexin V-fluorescein isothiocyanate and 5.0 μl of propidium iodide for 15 minutes in the dark at room temperature. After adding 400 μl of the binding buffer, analysis was immediately performed using a flow cytometer (FACscan flow cytometer; BD, San Jose, CA).
Caspase 3 Activity Assay
The activity of caspase 3 was detected by using a commercially available kit (BD Biosciences, Palo Alto, CA). Briefly, the cells were washed in PBS free of Ca2+ and Mg2+ and lysed in cell lysis buffer. The cell lysate was centrifuged at 12,000 rpm for 3 minutes at 4°C to precipitate the cellular debris. Reaction buffer containing dithiothreitol and caspase 3/CPP32 substrate (DEVD-pNA) were added to the supernatant. The samples were analyzed in a 96-well-plate at 405 nm. Both the treated samples and negative controls were run simultaneously in triplicate.
Cell Cycle Analysis
Cell-cycle analysis was performed using a bromodeoxyuridine (BrdU) kit (BD Biosciences) and analyzed using a flow cytometer. Briefly, cells were incubated with 10 μM BrdU in cell culture medium for 1 hour at 37°C, fixed with 100 μl of Cytofix/Cytoperm buffer for 15 to 30 minutes on ice, and then treated with DNase for 1 hour at 37°C. Fluorescein isothiocyanate-labeled anti-BrdU antibody was incubated with the cells for 20 minutes at room temperature. After washing, the cells were suspended with 7-AAD and analyzed by flow cytometry.
Relative Quantification of JAK3 Gene Expression by Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) in Colon Cancer Cell Lines
Total RNA was isolated from the cell lines using the RNeasy kit (Qiagen, Valencia, CA). A commercially available kit (Optimum FFPE Isolation; Ambion, Austin, TX) was used for the extraction of total RNA from formalin-fixed, paraffin-embedded tissue sections from some of the patients. DNA was removed by digestion with DNase I (Qiagen). cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) in a 20 μl reaction according to the manufacturer’s instructions. PCR was performed in a MicroAmp Optical 96-well reaction plate (Applied Biosystems, Foster City, CA) using 1.0 μl of cDNA template, 12.5 μl of 2× TaqMan Master Mix (Applied Biosystems), 1.25 μl of 20× Assay-on-Demand gene expression product (assay ID Hs00169663_m1 for JAK3 target gene, product number 4319413E for human 18s rRNA as endogenous control, Applied Biosystems). The RT reaction was performed in triplicate wells for 2 minutes at 50°C, followed by hot-start PCR (10 minutes at 95°C) and 40 cycles of 15 seconds of denaturation at 95°C and 1 minute annealing/extension at 60°C using an ABI 7900 sequence detection system (Applied Biosystems). The relative quantification of JAK3 gene expression was calculated according to the following formula: 2 −ΔCT (ΔCT = CT of JAK3 − CT of 18s rRNA); where CT is the cycle threshold. As a control of specificity, the PCR products were electrophoresed on 8% polyacrylamide gel.
Western Blot and Immunoprecipitation Studies
Immunoprecipitation was used to identify possible changes in the tyrosine phosphorylation of STAT3 and JAK3 proteins after treatment with JAK3/STAT3 inhibitor. The cell lysates were incubated with primary antibody overnight at 4°C. Agarose beads conjugated with protein A/C were then added and incubated for 2 hours at 4°C. The immunocomplexes were spun down, washed five times with cold PBS and once with lysis buffer, and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blot analysis was performed using standard techniques. Briefly, the cells were lysed in lysis buffer and centrifuged at 14,000 × g for 10 minutes at 4°C. The supernatant was collected and 50 to 80 μg of protein were electrophoresed on a 6 to 12% sodium dodecyl sulfate polyacrylamide gel. The proteins were transferred to nitrocellulose and were probed with various specific primary antibodies and then with the appropriate horseradish peroxidase-conjugated secondary antibody (Jackson Immunoresearch Laboratories, Inc., West Grove, PA). Proteins were detected using the enhanced chemiluminescence detection kit (Amersham Life Sciences, Arlington Heights, IL).
Patients, Tissue Microarray, Immunohistochemical Staining, and Relative Quantitative RT-PCR of JAK3
Archival tissue material from 22 patients with stage II or IV colon cancer was included in the study after approval of the institutional ethical research committee. Tissue microarray was prepared using standard techniques from formalin-fixed, paraffin-embedded colonic samples obtained from these patients by surgical resections. The tissue microarray included normal colonic epithelium, adenoma (if available), superficial carcinoma, deep carcinoma, and metastatic lesions (stage IV) to the liver and/or the lymph nodes from each of the patients.
Histological sections from the tissue microarray as well as from cellblocks prepared from SW480 and HT29 cells lines were used for immunohistochemical staining. The sections were first deparaffinized in xylene and rehydrated using a graded series of ethanol. A three-step streptavidin-biotin-horseradish peroxidase method was used after heat-induced epitope retrieval. Briefly, endogenous peroxidase activity was blocked for 30 minutes in 3% hydrogen peroxide and subsequently, the slides were incubated with Protein Blocking Solution (DakoCytomation, Carpinteria, CA) for 15 minutes. Thereafter, the slides were incubated overnight with the primary antibodies diluted in 0.1% bovine serum albumin, 50 mM Tris-HCl buffer, pH 7.6. The dilutions were 1:50 for STAT3, 1:200 for pSTAT3Tyr705, and 1:500 for both JAK3 and pJAK3Tyr980. Detection of the immunoreaction was achieved using the LSAB+ kit (DakoCytomation) that contains the secondary biotinylated antibody (incubation time, 20 minutes) and the streptavidin/horseradish peroxidase complex (incubation time, 20 minutes). 3,3′ Diaminobenzidine/H2O2 (DakoCytomation) was used as the chromogen and hematoxylin (DakoCytomation) as the counterstain. Sections prepared from formalin-fixed, paraffin-embedded Karpas 299 cells were used as positive controls. Application of mouse IgG1 antibody (DakoCytomation) was used as a negative control to exclude unspecific cross-reactions of the primary antibodies in all experiments. Immunohistochemical staining of paraffin-embedded sections was also performed after depletion of pSTAT3 and pJAK3 antibodies with antigen-specific peptides (Santa Cruz Biotechnology). The peptide was mixed with the specific antibody at a ratio of 5:1 (weight), and incubated for 2 hours at room temperature. Thereafter, immunohistochemical staining was performed as summarized above.
In addition, we studied the relative expression of JAK3 mRNA in frozen primary colon cancer tumors from eight patients and paraffin-embedded tissue sections from two patients using relative quantitative RT-PCR as explained previously. Corresponding benign colonic tissues were also studied when available.
Results
Detection of pSTAT3, JAK3, pJAK3, and γc in Colon Cancer Cell Lines
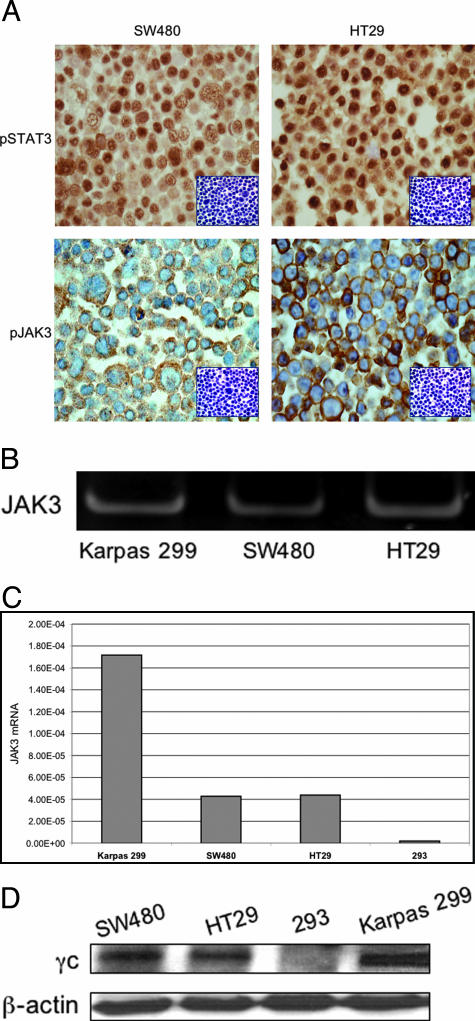
Immunohistochemical staining demonstrated the expression of pSTAT3 and pJAK3 in the two colon carcinoma cell lines, SW480 and HT29 (Figure 1A). The expression of pSTAT3 was primarily nuclear and pJAK3 was cytoplasmic and membranous. To confirm the specificity of the antibodies, immunohistochemical staining of the cell lines was also performed after depletion by antigen-specific peptides (Figure 1A, insets). Figure 1B shows PCR product for JAK3 using nondenaturing polyacrylamide gel electrophoresis after RT-PCR in SW480 and HT29 colon cancer cell lines. Signal bands are detected in the colon cancer cell lines as well as in Karpas 299 anaplastic large cell lymphoma cells that express high levels of JAK3. Figure 1C demonstrates quantitative RT-PCR of JAK3 mRNA in SW480 and HT29 cells. The cell lines 293 and Karpas 299 cells were used as negative and positive controls, respectively. Human 18s rRNA was used as endogenous control. The expression of JAK3 was ∼3.4 times higher in Karpas 299 cells compared with HT29 and SW480 cells. Our Western blot studies also showed the expression of γc, which is required for JAK3 signaling, in SW480 and HT29 cells (Figure 1D). The cells lines 293 and Karpas 299 were used as negative and positive controls, respectively.
Figure 1.
A: The colon cancer cell lines SW480 and HT29 are positive for pSTAT3 (predominantly nuclear staining) and pJAK3 (cytoplasmic and membranous staining) (immunohistochemical staining). To confirm the specificity of the antibodies, immunohistochemical staining using pSTAT3 and pJAK3 antibodies was also performed after depletion with antigen-specific peptides as shown in the insets. B: PCR product for JAK3 using nondenaturing polyacrylamide gel electrophoresis after RT-PCR in HT29 and SW480 colon cancer cell lines as well as the anaplastic large cell lymphoma cell line, Karpas 299, as positive control. C: Quantitative RT-PCR of JAK3 mRNA in SW480 and HT29 cells. The 293 and Karpas 299 cell lines were used as negative and positive controls, respectively. Human 18s rRNA was used as endogenous control. D: Western blot studies show that γc chain, which is required for JAK3-mediated signaling, is expressed in the colon cancer cells SW480 and HT29. The cell lines 293 and Karpas 299 were used as negative and positive controls, respectively. β-Actin shows equal loading of the protein. Original magnifications, ×400 (A).
AG490-Induced Down-Regulation of pJAK3
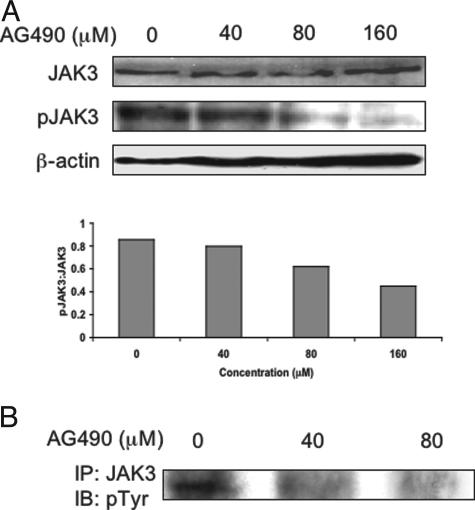
Western blot studies showed a concentration-dependent decrease in the level of pJAK3 at 24 hours after treating SW480 cells with AG490 (Figure 2A, top). pJAK3 was almost undetectable at a concentration of 160 μmol/L of AG490. No detectable changes in the JAK3 level were seen. Figure 2A (bottom) also shows densitometric analysis of the Western blot bands. There was a gradual decrease in pJAK3:JAK3 ratio with increasing the concentration of AG490. The results were confirmed by immunoprecipitation, in which JAK3 protein was pulled down with an anti-JAK3 antibody and the blots were stained with an anti-phosphorylated tyrosine antibody (Figure 2B).
Figure 2.
A, top: AG490 induced a concentration-dependent decrease in pJAK3 in SW480 cells as shown by Western blotting. pJAK3 was almost undetectable at a concentration of 160 μmol/L. There was no change in JAK3. The experiment was performed at 24 hours after treatment. β-Actin shows equal loading of the protein. A, bottom: Densitometric analysis of the Western blot bands showing a concentration-dependent decrease in pJAK3:JAK3 ratio. B: Immunoprecipitation studies confirmed that AG490 induces a concentration-dependent decrease in tyrosine-phosphorylated JAK3 in SW480 cells at 24 hours after treatment.
AG490- or AdSTAT3DN-Induced Down-Regulation of pSTAT3
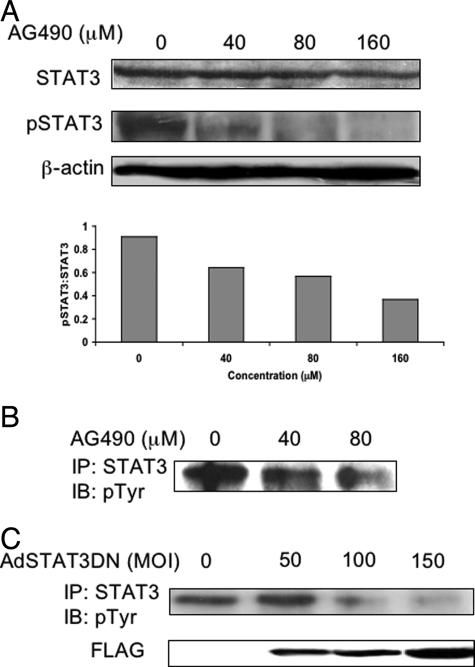
Similar to the changes in the pJAK3 level, AG490 induced concentration-dependent decreases in the pSTAT3 level in SW480 cells at 24 hours, as shown by Western blots (Figure 3A, top). AG490 (80 μM) induced nearly complete absence of pSTAT3. No changes in the STAT3 level were seen. Using densitometry, there was gradual decrease in pSTAT3:STAT3 ratio with increasing the concentration of AG490 (Figure 3A, bottom). The findings were confirmed using immunoprecipitation techniques, in which STAT3 protein was pulled down with an anti-STAT3 antibody and the blots were stained with an anti-phosphorylated tyrosine antibody (Figure 3B). Furthermore, AdSTAT3DN induced concentration-dependent decreases in pSTAT3 levels in SW480 cells as shown in the immunoprecipitation studies illustrated in Figure 3C. The level of pSTAT3 was minimal at an MOI of 150. Immunoblots stained with anti-FLAG antibody revealed the presence of FLAG only in infected cells but not the negative control. The staining intensity of FLAG increased with higher MOI.
Figure 3.
A, top: Western blot study showing that AG490 induces concentration-dependent down-regulation in pSTAT3, without significant changes in STAT3 levels in SW480 cells. pSTAT3 was almost undetectable at 160 μM of AG490. β-Actin shows equal loading of the protein. The experiments were performed at 24 hours after treating the cells with AG490. A, bottom: Densitometry of the Western blot bands demonstrating gradual decrease in pSTAT3:STAT3 with increasing the concentration of AG490. B: Immunoprecipitation studies confirmed that AG490 induces a concentration-dependent decrease in pSTAT3 in SW480 cells. The experiment was performed at 24 hours after treatment. C: AdSTAT3DN induced a concentration-dependent decrease in tyrosine-phosphorylated STAT3 as shown in SW480 cells by immunoprecipitation studies. The immunoprecipitation studies were performed at 24 hours after treating the cells with AdSTAT3DN. Infecting the cells with AdSTAT3DN was associated with a gradual increase in FLAG as shown by Western blotting.
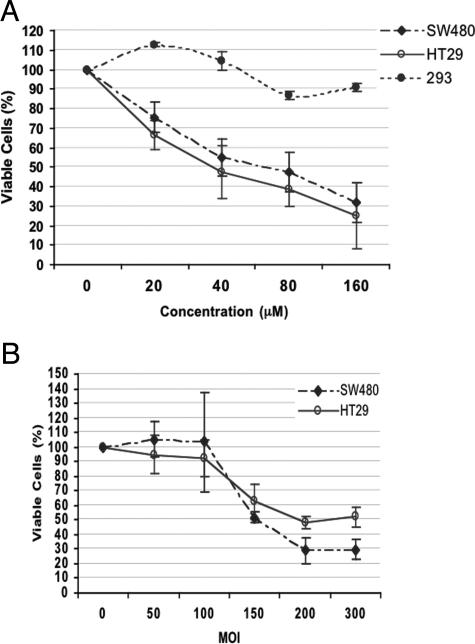
Inhibition of JAK3/STAT3 Signaling by AG490 or AdSTAT3DN Induced a Significant Decrease in the Viability of Colon Carcinoma Cells
Figure 4A shows that, after 24 hours of treatment, AG490 induced a concentration-dependent decrease in the number of viable SW480 and HT29 cells as detected by exclusion of staining with trypan blue dye. The IC50 (ie, inhibitory concentration to induce 50% reduction of viable cells) of AG490 was 40 μM for HT29 and 80 μM for SW480 cells. In contrast, a significant decrease in cell viability was not detected in the 293 cells that are lacking JAK3 (Dr. R. Kirken, personal communication). Similarly, AdSTAT3DN induced concentration-dependent decrease in the viability of SW480 and HT29 at 48 hours with IC50 of 150 and 200 MOI, respectively (Figure 4B).
Figure 4.
Treating SW480 and HT29 cells with AG490 (top) and AdSTAT3DN (bottom) induced a concentration-dependent decrease in the number of viable cells as demonstrated by the exclusion of staining with trypan blue dye. The IC50 for AG490 was 40 μM in HT29 and 80 μM in SW480 cells. AG490 did not induce a significant decrease in the viability of 293 cells that lack the expression of JAK3 (top). The IC50 for AdSTAT3DN was 150 MOI for SW480 and 200 MOI for HT29. The results represent mean ± SE of three experiments.
Inhibition of the JAK3/STAT3 Signaling Induced Caspase 3-Dependent Apoptotic Cell Death in Colon Carcinoma Cells
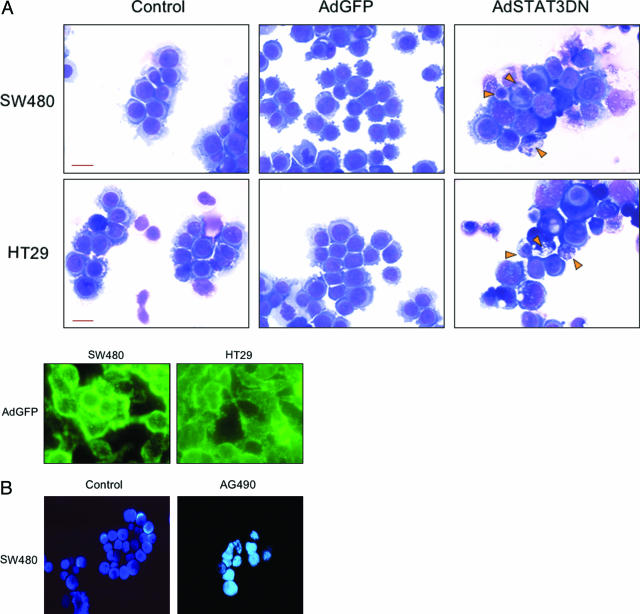
To evaluate whether the decrease in cell viability might have occurred due to apoptotic cell death, we evaluated cell morphology after staining SW480 and HT29 cells with Giemsa after treatment with AdSTAT3DN at 100 MOI for 48 hours (Figure 5A, top). Cell shrinkage and nuclear condensation and fragmentation were evident in the two cell lines after treatment. AdGFP at an MOI equivalent to AdSTAT3DN did not induce significant apoptotic cell death as shown in Figure 5A (top). Figure 5A (bottom) shows efficient infection of the cells with AdGFP. Similarly, staining SW480 cells with Hoechst 33258 showed typical morphological features of apoptosis including nuclear condensation and/or fragmentation at 72 hours after treatment with 80 μM of AG490 (Figure 5B).
Figure 5.
A, top: Morphological changes consistent with apoptosis in SW480 and HT29 cells after treatment with AdSTAT3DN (100 MOI at 48 hours). Cell shrinkage and nuclear condensation and fragmentation are seen in cells treated with AdSTAT3DN (arrowheads) compared with untreated control cells and cells treated with AdGFP at an equivalent MOI (Giemsa stain). A, bottom: Successful infection of SW480 and HT29 cells with AdGFP (fluorescence microscopy). B: Morphological changes in SW480 cells after treatment with AG490 (80 μmol/L) and staining with Hoechst 33258. Compared with control cells, treated cells showed intense fluorescent signal at 72. In addition, there were morphological features consistent with apoptosis in the form of cell shrinkage and nuclear fragmentation. Original magnifications: ×500 (A, top); ×600 (A, bottom); ×400 (B). Scale bars, 8 μm.
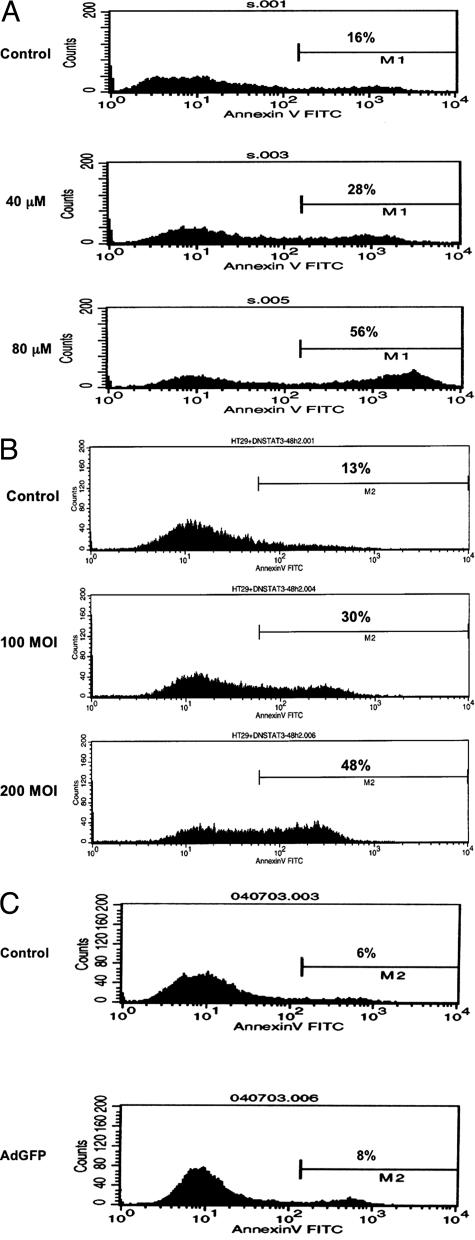
To quantify apoptotic cell death, we performed flow cytometric studies to evaluate annexin V binding to the cell surface after treating the cells with increasing concentrations of AG490. At 24 hours after treatment, annexin binding to the surface of SW480 cells increased with increasing the concentration of AG490 (Figure 6A). Our studies show that there was a 3.5-fold increase in apoptotic SW480 cells at 80 μM of AG490. Similarly, there was a 4.5-fold increase in apoptotic HT29 cells at 80 μM of AG490 after 24 hours of treatment (data not shown). AdSTAT3DN induced similar effects. At 48 hours after treating HT29 cells there was 2.3- and 3.7-fold increase in apoptotic cell death at a MOI of 100 and 200, respectively (Figure 6B). AdGFP at a MOI equivalent to AdSTAT3DN did not induce significant apoptotic cell death in HT29 at 48 hours after treatment as shown in Figure 6C.
Figure 6.
A: Flow cytometric analysis of SW480 cells showing that AG490 induces a concentration-dependent increase in annexin V-prestained apoptotic cells at 24 hours after treatment. Apoptotic cells increased from 16% in control cells to 28% and 56% at 40 μM and 80 μM respectively. The results are representative of three consistent experiments. B: AdSTAT3DN induced a concentration-dependent increase in apoptotic cell death in HT29 cells at 48 hours after treatment. AdSTAT3DN increased the apoptotic cells to 30% at 100 MOI and 48% at 200 MOI, compared with 13% in the untreated cells. The results are similar to two other experiments. C: Compared with control untreated HT29 cells, AdGFP, at a MOI equivalent to AdSTAT3DN, did not induce a significant increase in apoptotic cell death after 48 hours of treatment.
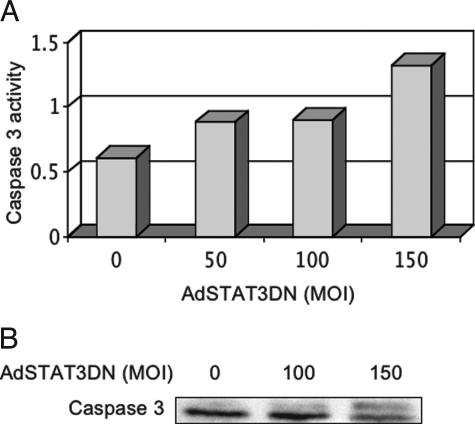
Increased caspase 3 activation in cells treated with increasing MOI of AdSTAT3DN was shown by enzymatic assay based on the cleavage of DEVD-p-nitroanilide (DEVD-pNA) peptide substrate. Figure 7A shows a gradual increase in caspase 3 activity in HT29 cells with increasing MOI of AdSTAT3DN at 16 hours after treatment. In addition, Western blot analysis demonstrated that AdSTAT3DN at an MOI of 150 induced peptide cleavage of caspase 3 in HT29 cells at 24 hours after infecting the cells (Figure 7B).
Figure 7.
A: A gradual increase in caspase 3 activity in HT29 cells was detected with increasing the MOI of AdSTAT3DN. Two consistent experiments were performed at 16 hours after treating the cells. B: Western blot studies show cleavage of caspase 3 at 24 hours after infecting HT29 cells with AdSTAT3DN. The cleavage occurred at an MOI of 150.
Inhibition of JAK3/STAT3 Signaling Induced G1 Cell Cycle Arrest in Colon Carcinoma Cell Lines
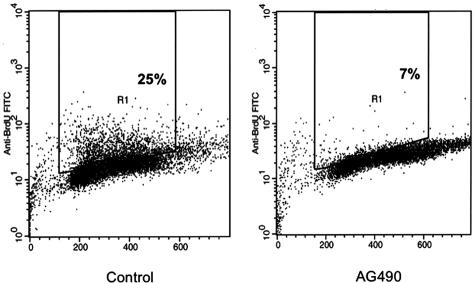
Inhibition of JAK3/STAT3 signaling induced G1 cell cycle arrest as demonstrated by the marked decrease in SW480 cells in the S phase from 25 to 7% at 6 hours after treatment with AG490 (Figure 8).
Figure 8.
Treating SW480 cells with 80 μM of AG490 induced G1 cell-cycle arrest as demonstrated by the decrease in the cells in the S phase from 25% to only 7% at 6 hours after treatment. The experiment was performed twice with consistent findings.
Effect of Inhibition of JAK3/STAT3 Signaling by AG490 or AdSTAT3DN on Downstream Targets
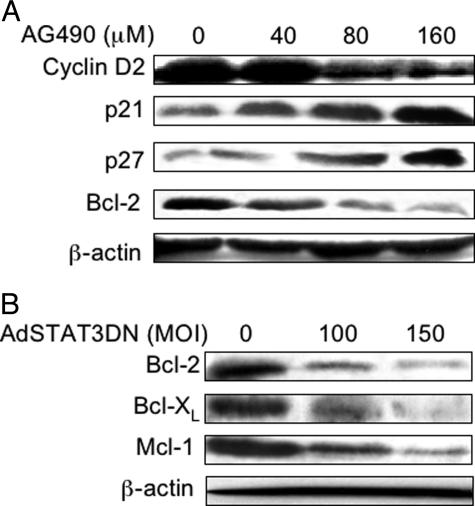
We also examined the changes in the protein levels of various apoptosis- and cell cycle-regulatory proteins that are known to be downstream targets of the JAK3/STAT3 signaling pathway. At 18 hours, increased concentrations of AG490 induced down-regulation of cyclin D2 and Bcl-2 that was simultaneously associated with up-regulation of p21waf1/cip1 and p27kip1 in SW480 cells (Figure 9A). No detectable changes at the protein level of survivin were seen (data not shown). In addition, treatment of SW480 cells with AdSTAT3DN induced concentration-dependent decreases in three major anti-apoptotic proteins, Bcl-2, Bcl-XL, and Mcl-1, which were detected at 24 hours after infection of the cells (Figure 9B).
Figure 9.
A: AG490 induced concentration-dependent alterations in downstream targets of JAK3/STAT3 signaling in SW480 cells at 18 hours after treatment. Western blot studies showed down-regulation of cyclin D2 and Bcl-2. Simultaneously, there was gradual increase in the cyclin-dependent kinase inhibitors p21waf1/cip1 and p27kip1. B: Western blot studies showing that AdSTAT3DN induced significant down-regulation of three major anti-apoptotic proteins in HT29 cells, Bcl-2, Bcl-XL, and Mcl-1, at 24 hours after infecting the cells.
Expression of STAT3, JAK3, and Their Activated Forms in Colon Cancer Primary Tumors
Characterizations of the patients included in the study are shown in Table 1. Eleven patients were in stage II and 11 in stage IV of the disease. The two groups were age- and sex-matched. Each of the two groups included six males and five females. The age of the patients in stage II group ranged between 54 and 74 years, with a means ± SE of 63.0 ± 1.8 years, whereas the age of the patients included in stage IV group ranged between 48 and 79 years, with a mean ± SE of 64.2 ± 2.6 years.
Table 1.
Characterization of Colon Carcinoma Patients Included in the Study
| Age | Sex | Anatomical site | Histology | Stage | Metastatic site | |
|---|---|---|---|---|---|---|
| 1 | 55 | M | Ascending colon | AC/MD | II | |
| 2 | 67 | F | Colon, NOS | AC/MD | II | |
| 3 | 65 | M | Rectum | AC/MD | II | |
| 4 | 62 | M | Cecum | AC/MD | II | |
| 5 | 54 | M | Sigmoid colon | AC/MD | II | |
| 6 | 65 | F | Cecum | AC/MD | II | |
| 7 | 59 | M | Rectosigmoid | AC/MD | II | |
| 8 | 69 | M | Rectum | AC/MD | II | |
| 9 | 58 | F | Cecum | AC/MD | II | |
| 10 | 65 | F | Colon, NOS | AC/MD | II | |
| 11 | 74 | F | Right transverse colon | AC/MD | II | |
| 12 | 58 | M | Cecum | AC/MD | IV | Liver, LNs |
| 13 | 59 | F | Splenic flexure | AC/MD | IV | LNs |
| 14 | 70 | M | Sigmoid colon | AC/MD | IV | Liver |
| 15 | 62 | M | Cecum | AC/PD/MU | IV | LNs |
| 16 | 64 | M | Splenic flexure | AC/MD | IV | Gerota’s fascia |
| 17 | 64 | F | Cecum | AC/MD | IV | LNs |
| 18 | 58 | M | Rectum | AC/MD | IV | LNs |
| 19 | 72 | M | Rectum | AC/MD | IV | LNs |
| 20 | 48 | F | Ascending colon | AC/MD | IV | LNs |
| 21 | 72 | F | Right transverse colon | AC/MD | IV | LNs |
| 22 | 79 | F | Cecum | AC/MD | IV | LNs |
AC, adenocarcinoma; MD, moderately differentiated; NOS, not otherwise specified; LNs, lymph nodes; PD, poorly differentiated; MU, mucinous.
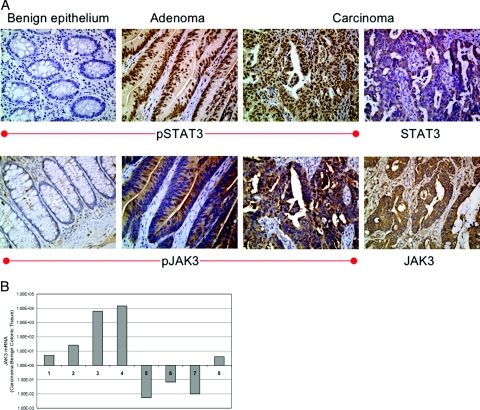
We used standard immunohistochemical staining to explore the expression of JAK3, STAT3, and their activated forms in colon carcinoma cell lines and normal epithelium, adenomas (when available), primary tumors, and metastatic lesions (stage IV patients) from each of the patients. Table 2 shows the frequency of expression of STAT3, pSTAT3, JAK3, and pJAK3 in colon carcinoma patients. STAT3 was the only protein present in normal colonic epithelium (80% of the samples), whereas all other proteins were undetectable. In addition, STAT3 was present in 62.5% of adenomas, 90.9% of stages II and IV, and 100% of metastatic colon carcinoma lesions. pSTAT3 was frequently expressed; 12.5% of adenomas, 72.7% of stage II, 63.6 of stage IV, and 77.7% of the metastatic lesions. JAK3 protein was detected in a significant number of the patients; 50% of adenomas, 81.8% of stage II, and 100% of stage IV and metastatic lesions. pJAK3 was present in 25% of adenomas, 81.8% of stage II, 90.9% of stage IV, and 100% of metastatic lesions. Figure 10A demonstrates examples of the patterns of expression of pSTAT3 and STAT3 (top panel) and pJAK3 and JAK3 (bottom panel). The sections are from the same patient and demonstrate normal colonic mucosa that was negative for pSTAT3 and pJAK3 compared with adenoma and colon carcinoma lesions that were positive for both pSTAT3 and pJAK3. STAT3, JAK3, and pJAK3 showed cytoplasmic staining, whereas pSTAT3 showed predominantly nuclear localization (Figure 10A).
Table 2.
Frequency of Expression of JAK3, STAT3, and Their Activated Forms
| Normal colonic epithelium | Adenoma | Stage II colon carcinoma | Stage IV colon carcinoma | Metastasis | |
|---|---|---|---|---|---|
| STAT3 | 16/20 (80%) | 5/8 (62.5%) | 10/11 (90.9%) | 10/1 (90.9%) | 9/9 (100%) |
| pSTAT3 | 0/21 (0%) | 1/8 (12.5%) | 8/11 (72.7%) | 7/11 (63.6%) | 7/9 (77.7%) |
| JAK3 | 0/21 (0%) | 4/8 (50%) | 9/11 (81.8%) | 11/11 (100%) | 9/9 (100%) |
| pJAK3 | 0/21 (0%) | 2/8 (25%) | 9/11 (81.8%) | 10/11 (90.9%) | 9/9 (100%) |
Figure 10.
A: Immunohistochemical staining of formalin-fixed tissue samples from one representative patient included in the tissue microarray. pSTAT3 and pJAK3 are negative in benign colonic epithelium, whereas tubular adenoma and invasive carcinoma lesions are positive for pSTAT3 (nuclear staining) and pJAK3 (cytoplasmic and membranous staining). The inactive forms, STAT3 and JAK3, show cytoplasmic expression in the same tumor (immunohistochemical staining). B: The ratio of JAK3 mRNA, as detected by quantitative RT-PCR, in primary colon cancer tumors versus benign colonic tissues (logarithmic scale). All of the eight tumors demonstrated the presence of JAK3 mRNA, with five showing higher levels in the tumors compared with benign tissues. Two other tumors not included in the figure demonstrated the presence of JAK3 mRNA, however, one of these tumors did not show JAK3 mRNA in the benign colonic tissue and the other lacked corresponding archival benign tissue. Human 18s rRNA was used as endogenous control. Original magnifications, ×200 (A).
Furthermore, we performed quantitative RT-PCR on archival tissues available from some of the patients. The studies included frozen tumors and available corresponding benign tissues from eight patients as well as from paraffin-embedded tissues from two patients after microdissection. Figure 10B shows the ratio of JAK3 mRNA in the tumors relative to its levels in the benign colonic tissues. JAK3 mRNA was detected in all of the colon cancer tumors. In five patients, the level of JAK3 mRNA was higher in the tumors compared with benign tissues, whereas in three patients JAK3 mRNA was detectable in the tumors albeit at lower levels than the benign tissues. In addition, JAK3 mRNA was present in the tumor tissue from one patient and undetectable in the corresponding benign mucosa. JAK3 mRNA was detected in another colon carcinoma tumor; however, corresponding archival benign tissue was not available from this patient.
Discussion
Whereas the pathogenetic role of JAK/STAT signaling pathway has been documented recently in several malignant neoplasms, such as carcinomas of the breast,16 prostate,17 and head and neck,18 as well as in hematopoietic neoplasms,19,20 the possible oncogenic role of JAK/STAT signaling pathway has not been extensively investigated in colon cancer. Recent studies have proposed that the STAT3 signaling pathway may be involved in colon carcinogenesis because the tyrosine kinases Src and Fer appear to induce their oncogenic effect in colon carcinoma cells, at least in part, via phosphorylation of STAT3.13,14 However, the possible role of JAK3, the physiological activator of STAT3, has not been previously investigated in human colon carcinoma cells.
Our study shows that both STAT3 and JAK3 are constitutively phosphorylated and activated in two colon cancer cell lines, SW480 and HT29. Controversy has surrounded the expression of JAK3 in epithelial tumors including colon carcinoma. Under physiological conditions, the expression of JAK3 appears to be restricted to hematopoietic and endothelial cells. Previous studies, however, showed the presence of JAK3 in lung carcinoma cell lines.21,22 Whereas some previous studies concluded that JAK3 is not expressed in HT29 cells,23,24 Lai and colleagues25 demonstrated the presence of JAK3 and two spliced variants in SW480 cells using Northern blotting and RT-PCR. We demonstrated herein the presence of JAK3 and pJAK3 in HT29 and SW480 cells using four different techniques including quantitative RT-PCR, Western blotting, immunoprecipitation, and immunohistochemical staining. Furthermore, we demonstrated the expression of the common γc that is required for JAK3 signaling, in the two cell lines, SW480 and HT29, using Western blotting. The presence of JAK3 and pJAK3 in colon carcinoma cells is further confirmed in our study by immunohistochemical staining of primary tumors from patients with colon carcinoma. Importantly, quantitative RT-PCR studies showed the presence of JAK3 mRNA in primary colon cancer tumors. It is of note that we did not detect JAK3 protein in benign colonic epithelium by immunohistochemistry, whereas JAK3 mRNA was detected by using quantitative RT-PCR in some of the benign tissues. This finding implicates that most likely the expression of JAK3 protein in colon carcinoma cells is inducible and associates the neoplastic transformation, as has been illustrated in previous in vitro studies.26 The cytokines known to induce the expression and subsequent activation of JAK3 are limited to those interacting with receptors that contain γc, including interleukin (IL)-2, IL-4, IL-7, IL-9, and IL-15.26,27 Previous studies have demonstrated the importance of some of these cytokines, particularly IL-4 and IL-15, in the pathogenesis of colon carcinoma.23,24,26,28,29 The frequent expression of JAK3 and pJAK3 in colon cancer tumors and metastatic lesions signifies an important role in mediating the effect of these cytokines and the initiation of colon cancer tumorigenesis. Despite that a majority of colon cancer tumors in our study demonstrated expression of pJAK3, the expression of pSTAT3 was less frequent. This finding implicates that pJAK3 may exert its oncogenic effects via interaction with other signal transduction pathways, for example ERK-MAP kinase pathway, or the activation of other members of STAT family of transcription proteins,30,31 and not only through the activation of STAT3. Importantly, pJAK3 and pSTAT3 were expressed in a subgroup of colonic adenomas and the frequency of their expression increased with the progression of the disease because 77.7% of the metastatic tumors expressed pSTAT3 and 100% expressed pJAK3. These findings imply that the expression of pSTAT3 and pJAK3 may be directly related to the progression of the disease. However, the number of patients included in our study is relatively small and further studies are needed to validate this hypothesis.
We sought to evaluate further the biological significance of JAK3/STAT3 activation in the pathogenesis of colon cancer cells. Our approach was to use a pharmacological JAK3 inhibitor, AG490 (tyrphostin).19,32–35 In addition, we selectively blocked STAT3 using AdSTAT3DN.15 We found that AG490 induces concentration-dependent down-regulation of pJAK3 and pSTAT3. No changes were noted in JAK3 and STAT3 proteins. Similarly, infection of colon cancer cells with AdSTAT3DN induced a concentration-dependent decrease in pSTAT3, whereas STAT3 level did not show similar changes. Down-regulation of pJAK3 and pSTAT3 was associated with a gradual decrease in the number of viable cells. The decrease in cell viability can be attributable to the significant increase in apoptotic cell death and the occurrence of cell cycle arrest after inhibition of JAK3/STAT3 signaling.
We have also investigated the molecular basis for the occurrence of apoptotic cell death and cell cycle arrest in colon cancer cells. JAK3/STAT3 signaling is known to control the transcription of key regulators of apoptosis and cell cycle progression.36–40 Treatment of colon cancer cells with AG490 or AdSTAT3DN resulted in a concentration-dependent decrease in cyclin D2, Mcl-1, Bcl-XL, and Bcl-2. Concurrently, there was an increase in p21waf1/cip1 and p27kip1.
Our findings are the first to illustrate the mechanistic role of JAK3/STAT3 signaling in colon cancer cells. They also demonstrate novel evidence that JAK3 is expressed and appears to play an important role in STAT3 phosphorylation and activation in colon carcinoma cells. Our study highlights the importance of the downstream targets of JAK3/STAT3 signaling that possess significant anti-apoptotic activity, namely Bcl-2, Bcl-XL, and Mcl-1, in colon cancer pathogenesis. Also, our findings confirmed that several downstream targets of JAK3/STAT3 signaling that are involved in cell-cycle regulation, including cyclin D2, p21waf1/cip1, and p27kip1, play a major role in cell cycle regulation in colon cancer cells. Previous studies have demonstrated the significant role that these proteins play in colonic tumorigenesis.41–47 Survivin is another downstream target of the JAK-STAT signaling pathway that possesses significant anti-apoptotic effect.48 Recently, Mahboubi and colleagues,49 have shown that IL-11-induced up-regulation of survivin expression in endothelial cells is mediated via STAT3. Previous studies have demonstrated that survivin is important in the pathogenesis of colon cancer cells.50,51 The absence of significant changes in survivin levels after inhibition of JAK3/STAT3 signaling in our study may be explained by the fact that other pathways, such as Akt, are also involved in the regulation of survivin expression.52,53
In conclusion, the present study demonstrates that JAK3/STAT3 signaling pathway plays an important role in the pathogenesis of human colon cancer by promoting cell survival and counteracting apoptotic cell death. Our study also illustrates that targeting JAK3/STAT3 signaling could represent a novel approach for therapy in this aggressive neoplasm.
Footnotes
Address reprint requests to Hesham M. Amin, M.D., M.Sc., Division of Pathology and Laboratory Medicine, Box 72, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030. E-mail: hamin@mdanderson.org.
Supported by a Career Development Award from the National Institutes of Health Specialized Programs of Research Excellence grant to MD Anderson Cancer Center (H.M.A.) and H.M.A. is a scholar of the Physician Scientist Program at MD Anderson Cancer Center.
References
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- Horvath CM. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem Sci. 2000;25:496–502. doi: 10.1016/s0968-0004(00)01624-8. [DOI] [PubMed] [Google Scholar]
- Sehgal PB. STAT-signaling through the cytoplasmic compartment: consideration of a new paradigm. Cell Signal. 2000;12:525–535. doi: 10.1016/s0898-6568(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- Rane SG, Reddy EP. JAKs, STATs and Src kinases in hematopoiesis. Oncogene. 2002;21:3334–3358. doi: 10.1038/sj.onc.1205398. [DOI] [PubMed] [Google Scholar]
- Benekli M, Baer MR, Baumann H, Wetzler M. Signal transducer and activator of transcription proteins in leukemias. Blood. 2003;101:2940–2954. doi: 10.1182/blood-2002-04-1204. [DOI] [PubMed] [Google Scholar]
- Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signaling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Parker AJ, Gallick GE. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21:7797–7807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- Laird AD, Li G, Moss KG, Blake RA, Broome MA, Cherrington JM, Mendel DB. Src family kinase activity is required for signal transducer and activator of transcription 3 and focal adhesion kinase phosphorylation and vascular endothelial growth factor signaling in vivo and for anchorage-dependent and -independent growth of human tumor cells. Mol Cancer Ther. 2003;2:461–469. [PubMed] [Google Scholar]
- Orlovsky K, Theodor L, Malovani H, Chowers Y, Nir U. Gamma interferon down-regulates Fer and induces its association with inactive Stat3 in colon carcinoma cell. Oncogene. 2002;21:4997–5001. doi: 10.1038/sj.onc.1205624. [DOI] [PubMed] [Google Scholar]
- Kunisada K, Tone E, Fujio Y, Matsui H, Yamauchi-Takihara K, Kishimoto T. Activation of gp130 transduces hypertrophic signals via STAT3 in cardiac myocytes. Circulation. 1998;98:346–352. doi: 10.1161/01.cir.98.4.346. [DOI] [PubMed] [Google Scholar]
- Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Karker A, Jove R. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. [PubMed] [Google Scholar]
- Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, Kamens J, Grandis JR. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–31583. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, Jove R, Loughran TP., Jr Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin HM, Medeiros LJ, Ma Y, Feretzaki M, Das P, Leventaki V, Rassidakis GZ, O’Conner SL, McDonnell TJ, Lai R. Inhibition of JAK3 induces apoptosis and decreases anaplastic large cell lymphoma kinase activity in anaplastic large cell lymphoma. Oncogene. 2003;22:5399–5407. doi: 10.1038/sj.onc.1206849. [DOI] [PubMed] [Google Scholar]
- Cosenza L, Gorgun G, Urbano A, Foss F. Interleukin-7 receptor expression and activation in nonhaematopoietic neoplastic cells. Cell Signal. 2002;14:317–325. doi: 10.1016/s0898-6568(01)00245-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Kern JA. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol. 2002;27:306–313. doi: 10.1165/rcmb.4850. [DOI] [PubMed] [Google Scholar]
- Murata T, Noguchi PD, Puri RK. Receptors for interleukin (IL)-4 do not associate with the common gamma chain, and IL-4 induces the phosphorylation of JAK2 tyrosine kinase in human colon carcinoma cells. J Biol Chem. 1995;270:30829–30836. doi: 10.1074/jbc.270.51.30829. [DOI] [PubMed] [Google Scholar]
- Murata T, Noguchi PD, Puri RK. IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: similarities between IL-4 and IL-13 signaling. J Immunol. 1996;156:2972–2978. [PubMed] [Google Scholar]
- Lai KS, Jin Y, Graham DK, Witthuhn BA, Ihle JN, Liu ET. A kinase-deficient splice variant of the human JAK3 is expressed in hematopoietic and epithelial cancer cells. J Biol Chem. 1995;270:25028–25036. doi: 10.1074/jbc.270.42.25028. [DOI] [PubMed] [Google Scholar]
- Verbsky JW, Bach EA, Fang YF, Yang L, Randolph DA, Fields LE. Expression of Janus kinase 3 in human endothelial and other non-lymphoid and non-myeloid cells. J Biol Chem. 1996;271:13976–13980. doi: 10.1074/jbc.271.24.13976. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Bacon CM, Finbloom DS, Rees RC, Kaplan D, Shibuya K, Ortaldo JR, Gupta S, Chen YQ, Giri JD, O’Shea JJ. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci USA. 1995;92:8705–8709. doi: 10.1073/pnas.92.19.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gri G, Chiodoni C, Gallo E, Stoppacciaro A, Liew FY, Colombo MP. Antitumor effect of interleukin (IL)-12 in the absence of endogenous IFN-gamma: a role for intrinsic tumor immunogenicity and IL-15. Cancer Res. 2002;62:4390–4397. [PubMed] [Google Scholar]
- Kuniyasu H, Ohmori H, Sasaki T, Sasahira T, Yoshida K, Kitadai Y, Fidler IJ. Production of interleukin 15 by human colon cancer cells is associated with induction of mucosal hyperplasia, angiogenesis, and metastasis. Clin Cancer Res. 2003;9:4802–4810. [PubMed] [Google Scholar]
- Wang LH, Kirken RA, Erwin RA, Yu C-R, Farrar WL. JAK3, STAT, and MAPK signaling pathways as novel molecular targets for the tyrphostin AG-490 regulation of IL-2-mediated T cell response. J Immunol. 1999;162:3879–3904. [PubMed] [Google Scholar]
- Kirken RA, Erwin-Cohen R, Behbod F, Wang M, Stepkoski SM, Kahan BD. Tyrphostin AG490 selectively inhibits activation of the JAK3/STAT5/MAPK pathway and rejection of rat heart allografts. Transplant Proc. 2001;33:95. doi: 10.1016/s0041-1345(00)02791-3. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Kaltoft K, Nordahl M, Ropke C, Geisler C, Mustelin T, Dobson P, Svejgaard A, Odum N. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci USA. 1997;94:6764–6769. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen KW, Kaltoft K, Mikkelsen G, Nielsen M, Zhang Q, Geisler C, Nissen MH, Ropke C, Wasik MA, Odum N. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia. 2001;15:787–793. doi: 10.1038/sj.leu.2402093. [DOI] [PubMed] [Google Scholar]
- Kube D, Holtick U, Vockerodt M, Ahmadi T, Haier B, Behrmann I, Heinrich PC, Diehl V, Tesch H. STAT3 is constitutively activated in Hodgkin cell lines. Blood. 2001;98:762–770. doi: 10.1182/blood.v98.3.762. [DOI] [PubMed] [Google Scholar]
- Sun X, Layton JE, Elefanty A, Lieschke GJ. Comparison of effects of the tyrosine kinase inhibitors AG957, AG490, and STI571 on BCR-ABL-expressing cells, demonstrating synergy between AG490 and STI571. Blood. 2001;97:2008–2015. doi: 10.1182/blood.v97.7.2008. [DOI] [PubMed] [Google Scholar]
- de Koning JP, Soede-Bobok AA, Ward AC, Schelen AM, Antonissen C, van Leeuwen D, Lowenberg B, Touw IP. STAT3-mediated differentiation and survival of myeloid cells in response to granulocyte colony-stimulating factor: role of the cyclin-dependent kinase inhibitor p27Kip1. Oncogene. 2000;19:3290–3298. doi: 10.1038/sj.onc.1203627. [DOI] [PubMed] [Google Scholar]
- Odajima J, Matsumura I, Sonoyama J, Daino H, Kawasaki A, Tanaka H, Inohara N, Kitamura T, Downward J, Nakajima K, Hirano T, Kanakura Y. Full oncogenic activities of v-Src are mediated by multiple signaling pathways. Ras as an essential mediator of cell survival. J Biol Chem. 2000;275:24096–24105. doi: 10.1074/jbc.m001606200. [DOI] [PubMed] [Google Scholar]
- Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene. 2000;19:5419–5427. doi: 10.1038/sj.onc.1203947. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kinoshita T, Hirano T, Yokota T, Miyauima A. STAT3 down-regulates the expression of cyclin D during liver development. J Biol Chem. 2002;277:36167–36173. doi: 10.1074/jbc.M203184200. [DOI] [PubMed] [Google Scholar]
- Barre B, Avril S, Coqueret O. Opposite regulation of myc and p21waf1 transcription by STAT3 proteins. J Biol Chem. 2003;278:2990–2996. doi: 10.1074/jbc.M210422200. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Hart J, Michelassi F, Lee JJ. Prognostic value of bcl-2 oncoprotein expression in stage II colon carcinoma. Clin Cancer Res. 1995;1:1103–1110. [PubMed] [Google Scholar]
- Koshiji M, Adachi Y, Sogo S, Taketani S, Oyaizu N, Than S, Inaba M, Phawa S, Hioki K, Ikehara S. Apoptosis of colorectal adenocarcinoma (COLO 201) by tumor necrosis factor-alpha (TNF-alpha) and/or interferon-gamma (INF-gamma), resulting from down-modulation of Bcl-2 expression. Clin Exp Immunol. 1998;111:211–218. doi: 10.1046/j.1365-2249.1998.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Pardee AB. β-Lapachone induces cell cycle arrest and apoptosis in human colon cancer cells. Mol Med. 1999;5:711–720. [PMC free article] [PubMed] [Google Scholar]
- Leschelle X, Delpal S, Goubern M, Blottiere HM, Blachier F. Butyrate metabolism upstream and downstream acetyl-CoA synthesis and growth control of human colon carcinoma cell. Eur J Biochem. 2000;267:6435–6442. doi: 10.1046/j.1432-1327.2000.01731.x. [DOI] [PubMed] [Google Scholar]
- Nita ME, Ono-Nita SK, Tsuno N, Tominaga O, Takenoue T, Sunami E, Kitayama J, Nakamura Y, Nagawa H. Bcl-XL antisense sensitizes human colon cancer cell line to 5-fluorouracil. Jpn J Cancer. 2000;91:825–832. doi: 10.1111/j.1349-7006.2000.tb01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, Roller PP, Wang S, Yang D. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cell. Biochem Pharmacol. 2003;66:93–103. doi: 10.1016/s0006-2952(03)00248-x. [DOI] [PubMed] [Google Scholar]
- Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med. 2001;7:542–547. doi: 10.1016/s1471-4914(01)02243-2. [DOI] [PubMed] [Google Scholar]
- Mahboubi K, Li F, Plescia J, Kirkiles-Smith NC, Mesri M, Du Y, Carroll JM, Elias JA, Altieri DC, Pober JS. Interleukin-11 up-regulates survivin expression in endothelial cells through a signal transducer and activator of transcription-3 pathway. Lab Invest. 2001;81:327–334. doi: 10.1038/labinvest.3780241. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001;91:2026–2032. doi: 10.1002/1097-0142(20010601)91:11<2026::aid-cncr1228>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Manome Y, Nakamura M, Tanigawa N. Downregulation of survivin expression by induction of the effector cell protease receptor-1 reduces tumor growth potential and results in an increased sensitivity to anticancer agents in human colon cancer. Eur J Cancer. 2002;38:2316–2324. doi: 10.1016/s0959-8049(02)00247-2. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Plescia J, Chheang S, Tallini G, Zhu YM, King M, Altieri DC, Languino LR. Fibronectin protects prostate cells from tumor necrosis factor-α-induced apoptosis via the AKT/survivin pathway. J Biol Chem. 2003;278:50402–50411. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]