Abstract
Decreased phagocytotic ability of macrophages has been reported to be associated with the severity of endometriosis, although the underlying mechanism remains uncharacterized. Expression and secretion of matrix metalloproteinase (MMP)-9 by macrophages is a means to degrade the extracellular matrix of cells that are designated for phagocytosis. Here, we describe the regulation of MMP-9 expression and activity in peritoneal macrophages of women with endometriosis. Results demonstrated that peritoneal macrophages isolated from women with endometriosis have decreased levels of protein and enzyme activity of MMP-9. Treatment of macrophages with peritoneal fluid obtained from patients with severe endometriosis inhibited MMP-9 expression and gelatinase activity. Further investigation identified prostaglandin (PG) E2 as the major factor in the peritoneal fluid that inhibited MMP-9 activity. The inhibitory effect of PGE2 was mediated via the EP2/EP4-dependent PKA pathway. Furthermore, expression of tissue inhibitor of metalloproteinase-1, tissue inhibitor of metalloproteinase-2, and RECK in macrophages was not affected by treatment with PGE2, indicating the effect of PGE2 on suppressing MMP-9 activity was not mediated by up-regulation of its inhibitor. Our results suggest that decreased phagocytotic capability of peritoneal macrophage in patients with endometriosis may be caused by PGE2-mediated decreases in MMP-9 expression.
Endometriosis is a common gynecological disorder with a complex, multifactorial etiology that causes chronic pelvic pain, dysmenorrhea, and even infertility. The prevalence of this disease is ∼10 to 15% among women of reproductive age. The underlying pathophysiological mechanism is still enigmatic. Although retrograde menstruation has been suggested to be the crucial constituent in the development of endometriosis,1 factors allowing the implantation and propagation of endometriotic lesions are primarily unclear. Aberrant production of steroids by ectopic endometriotic lesions and alteration/dysfunction of the immune system may lead to the development of endometriosis.2–5 During the development of endometriosis, immune cells are recruited into the peritoneal cavity. Among these immune cells, macrophages are the dominant cell type in the peritoneal cavity and are involved in phagocytosis and inflammation, especially in cleaning the retrograded endometrial debris.6,7 Peritoneal macrophages isolated from patients with endometriosis were found to have phenotypic and functional alterations leading to poor phagocytotic capacity, which is highly associated with severity of endometriosis.4,8 Nevertheless, the mechanism of suppressed phagocytotic capability of macrophages in endometriosis is poorly understood.
Matrix metalloproteinases (MMPs), also called matrixins, are proteinases that participate in extracellular matrix degradation.9 Based on substrate specificity, sequence similarity, and domain organization, vertebrate MMPs can be divided into six groups such as collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs.9 Under normal physiological conditions, the activities of MMPs are precisely regulated at the level of transcription, of activation of the precursor zymogens, of interaction with specific extracellular matrix components, and of inhibition by endogenous inhibitors.9
Gelatinases including MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are further distinguished by the insertion of three head-to-tail cysteine-rich repeats within their catalytic domain. These inserts resemble the collagen-binding type II repeats of fibronectin and are required to bind and cleave collagen and elastin.10,11 MMP-9 is the largest and most complex family member for the remodeling of extracellular matrix components at various physiological and pathological processes, such as development and angiogenesis. MMP-9-deficient mice were found to result in subfertility and immune dysfunction.12 MMP-9 is also considered as an important factor in the pathogenesis of endometriosis during the ectopic implantation and development of endometriotic tissue. Increased MMP-9 but not MMP-2 expression by eutopic and ectopic endometrial tissue in women with endometriosis was noted and was associated with the severity of endometriosis.13–15
Macrophages can secrete MMP-2, -7, -9, and -12 to degrade elastin and have been implicated to play an important role in the pathogeneses of emphysema and aortic aneurysm.16–19 Several studies also suggested a role for MMP-9 in cell migration, leukocyte infiltration, and tissue remodeling.12,20–22 In addition, MMP-9 can facilitate the destruction of the type IV collagen-containing basement membrane, which separates the epithelial and stromal compartment.23 We hypothesize that the decreased phagocytotic capability of peritoneal macrophages in women with endometriosis may be due to inhibition of MMP-9 expression and activity by unidentified factors in the peritoneal fluid (PF) of women with endometriosis. In this study, we aim to investigate the expression level and enzymatic activity of MMP-9 secreted by peritoneal macrophages derived from normal women and women with endometriosis. The effects of PF from endometriotic patients in the regulation of MMP-9 secreted by macrophages are also evaluated.
Prostaglandins (PGs) are known, for many decades, to play pivotal roles in many physiological and pathological processes including modulation of immune responses.24 We have previously found that concentrations of PGE2 are elevated in the PF of women with endometriosis25 owing to aberrant expression of COX-1 and COX-2 in peritoneal macrophages25 and COX-2 in endometriotic stromal cells.26 Thus, we seek to examine whether PGE2 could be an active ingredient in endometriotic PF in suppressing MMP-9 activity. In addition, regulation of MMP-9 activity by proinflammatory cytokines such as tumor necrosis factor-α, interleukin (IL)-1β, interferon (IFN)-γ, and leptin, which play important roles in modulating behaviors of macrophages, are also investigated.
Materials and Methods
Patients
Patients were grouped by their endometriosis grade according to revised classification of the American Society of Reproductive Medicine.27 All endometriosis samples were histologically confirmed. Peritoneal macrophage specimens were obtained from patients scheduled for laparotomy or laparoscopy at the Department of Obstetrics and Gynecology, National Cheng Kung University Hospital. In the control group, women undergoing laparoscopy for benign gynecological conditions (uterine myoma and tubal factor) were recruited. All patients were of reproductive age with normal menstrual cycles. The patients were not receiving any endocrine therapy, such as GnRH analog, danazol, or pseudopregnancy therapy. The following cases were excluded from the study: malignant neoplasms other than cervical carcinoma in situ, ovarian neoplasms, pelvic inflammation, and pregnancy. The experimental procedure was approved by the Clinical Research Ethics Committee at the National Cheng Kung University Medical Center and informed consent was obtained from each patient.
Isolation of Peritoneal and Peripheral Macrophages
PF was collected using an aspiration needle under direct vision, before the commencement of any pelvic surgery. The PF of each individual, collected in a sterile manner at the time of laparoscopy, was subjected to macrophage isolation as previously described.25 In brief, the PF collected from individual patients was centrifuged for 10 minutes at 500 × g and the cell-free supernatant was transferred to a new centrifuge tube and stored at −80°C for later use. The resulting pellet was resuspended with 6 ml of phosphate-buffered saline (PBS) and then slowly layered onto 4 ml of Ficoll-Paque (Promega, Madison, WI) solution. This was centrifuged at 700 × g for 30 minutes at 4°C. The mononuclear cell layer was transferred to a clean centrifuge tube and washed twice with PBS. The pellet was resuspended with Dulbecco’s modified Eagle’s medium/F12 and cell number was counted using a hemacytometer under a light microscope. The cell viability was determined by 0.025% trypan blue dye exclusion. Mononuclear cells were allowed to adhere onto 30-mm Petri dish for 30 minutes and unattached cells were washed off using PBS. The cells attached were primary CD14-positive cells as determined by fluorescent immunostaining followed by flow cytometry (data not shown). The cell viability was determined by 0.025% trypan blue dye exclusion. Live mononuclear cells (2 × 105) were allowed to adhere onto a 30-mm Petri dish for 30 minutes (or 16 hours in some experiments). For isolation of mononuclear cells from peripheral blood, heparinized venous blood was centrifuged at 1000 × g for 10 minutes and subsequently at 1800 × g for 12 minutes. After removal of plasma, the cell pellet was then resuspended in PBS in 1:2 ratio (v/v; pellet:PBS) and then had mononuclear cells isolated as described.25 Macrophages obtained from different individuals were not pooled and were treated as different batches of cells.
Cell Culture and Treatment
Purified peritoneal mononuclear cells (2 × 105 cells) were allowed to adhere onto the surface of 30-mm Petri dishes for 30 minutes (or 16 hours) in the presence of 1 ml of Dulbecco’s modified Eagle’s medium/F12 supplemented with 10% fetal bovine serum and antibiotics (100 IU/ml penicillin, 100 μg/ml streptomycin sulfate, and 0.625 μg/ml fungizone) and cultured in a humidified atmosphere with 5% CO2 at 37°C. To determine the time effect of MMP-9 activity, macrophages were allowed to attach for 30 minutes and subsequently were cultured in a fresh medium for the indicated time. In all other experiments, the macrophages were allowed to attach for 16 hours as determined from the first experiment, and then treated with selected cytokine or pooled PF from normal (or endometriotic) patients for 24 hours. The media were collected and subjected to zymography and protein analyses, while the cells were then directly lysed in specific lysis buffer and subjected to mRNA or protein analyses.
Zymography
MMP-9 activity in the media was analyzed by zymography as described by Ma and colleagues28 with modification. Ten μl of collected media were mixed with 3 μl of sodium dodecyl sulfate (SDS) sample buffer without reducing agent, and proteins were subjected to be dissolved on 8% polyacrylamide gels that were co-polymerized with 1 mg/ml of gelatin. After electrophoresis, the gels were washed six times with 2.5% Triton X-100 for 1 hour at room temperature to remove the SDS, then incubated with a buffer containing 5 mmol/L CaCl2 and 1 μmol/L ZnCl2 for 8 hours at 37°C. Gels were stained with Coomassie blue (0.5%) for 10 minutes and destained by a solution of acetic acid and methanol (v/v; methanol:water:acetic acid = 5:4:1) for 30 minutes. Proteolytic activity was detected by gelatin lysis, as evidenced as clear bands against the blue background of stained gelatin after incubating for 10 to 16 hours in the presence of activation buffer. The gels were analyzed using AlphaImager software (Alpha Innotech Corp, San Leandro, CA) to quantify the optic densities of clear bands.
Western Blot
Cells were directly lysed in Tris-sucrose-EDTA buffer (10 mmol/L Tris, 250 mmol/L sucrose, and 0.1 mmol/L EDTA, pH 7.4) and cellular protein concentrations were determined. Equal amounts of proteins were boiled in 6× SDS sample buffer (125 mmol/L Tris-HCl, 10% 2-mercaptoethanol, 4% SDS, 20% glycerol, 0.01% bromophenol blue) and subjected to SDS-polyacrylamide gel electrophoresis separation. Subsequently, the gels were transferred onto a polyvinylidene difluoride membrane and nonspecific binding was blocked by immersing the membrane in 5% nonfat milk for 1 hour at room temperature. Membrane was then incubated with goat anti-human MMP-9, tissue inhibitor of metalloproteinase (TIMP)-1, or TIMP-2 antibody at a 1: 1000 dilution at 4°C overnight. After washing with PBS plus 0.05% Tween-20 (PBS-T) three times for 10 minutes each, membrane was further incubated with horseradish peroxidase-conjugated rabbit anti-goat IgG at a 1: 2000 dilution for 1 hour at room temperature. The membrane was then washed with PBS-T for 1 hour and detected by enhanced chemiluminescence. The X-ray films were analyzed using AlphaImager software to quantify band intensity.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Expression of MMP-9, TIMP-1, TIMP-2, and the reversion-inducing cysteine-rich protein with Karzal motifs (RECK) mRNA was determined by RT-PCR. In brief, total RNA (200 ng) was reverse-transcribed at 42°C for 60 minutes and 5 μl of RT products were subjected for 30 cycles of PCR amplification using standard procedures as described previously.3 The primer sequences used for PCR were: 5′-GAGACCGGTGAGCTGGATAG-3′ and 5′-TCGAAGATGAAGGGGAAGTG-3 for MMP-9 (467 bp), 5′-TGACATCCGGTTCGTCTACA-3′ and 5′-TGATGTGCAAGAGTCCATCC-3′ for TIMP-1 (122 bp), 5′-AAGCGGTCA-GTGAGAAGGAA-3′ and 5′-TCTCAGGCCCTTTGAAC-ATC-3′ for TIMP-2 (108 bp), and 5′-GATGTGTTTGGATACT-TCAGCA-3′ and 5′-AATGATGAGGGCAGAGAGAG-3′ for RECK (180 bp). Primer sequences for GAPDH, EP1, EP2, EP3, and EP4 were reported previously.29,30
Statistical Analysis
Results were expressed as mean ± SEM. Differences among disease groups or treatment groups were analyzed by one-way analysis of variance using Prism statistical software version 4.02 (GraphPad Software Inc., San Diego, CA). Posttest multiple comparisons were performed using Tukey’s procedure and significant differences were accepted when two-tailed analysis yielded P < 0.05.
Results
Expression and Enzymatic Activity of MMP-9 in Peritoneal Macrophages
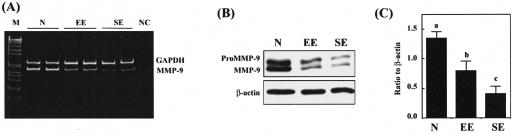
Peritoneal macrophages obtained from patients of endometriosis-free (defined as normal herein, n = 7), early endometriosis (n = 8), and severe endometriosis (n = 10) were purified and levels of mRNA encoding for MMP-9 were determined by RT-PCR. Macrophages derived from women with endometriosis expressed less MMP-9 mRNA than those isolated from normal controls (Figure 1A). Although the RT-PCR results were not quantitative, levels of MMP-9 mRNA tended to be inversely correlated with the severity of the disease (Figure 1A). To obtain further information, we examined levels of MMP-9 protein in peritoneal macrophages isolated from normal or endometriosis patients. Consistent with the mRNA data, Western blot analysis revealed that levels of MMP-9 protein were also decreased along with the severity of endometriosis (Figure 1, B and C). These results indicate that peritoneal macrophages in patients with endometriosis might be less active in phagocytosis as has been reported.8
Figure 1.
Distinct MMP-9 expression patterns in peritoneal macrophages of women with or without endometriosis. A: A representative gel picture showing RT-PCR result of levels of MMP-9 mRNA in macrophages isolated from PF of normal (N), early-stage endometriosis (EE), and severe-stage endometriosis (SE) women. GAPDH was amplified to serve as an internal control. NC, negative control omitting reverse transcriptase. B: A representative Western blot result demonstrates that expression of MMP-9 protein is decreased along with the severity of endometriosis. The band intensities of MMP-9 and β-actin were quantified using AlphaImager software and analyzed. C: The means and SE of ratios of MMP-9 to β-actin obtained from 7 normal patients (N), 8 early-stage endometriosis (EE), and 10 severe-stage endometriosis (SE) patients. Different letters denote significant differences between groups when Tukey’s analysis yields P < 0.05.
To correlate the levels of MMP-9 expression with enzymatic activities, culture media were subjected to zymographic analysis. A time course experiment showed that MMP-9 activities secreted by peritoneal macrophages were greatly elevated during 12 to 24 hours after adherence on solid surface (Figure 2, A and B). In concordance with the mRNA and protein data, the gelatinase activity of MMP-9 in cultured medium of peritoneal macrophages collected from severe-stage endometriosis was the weakest compared with those from normal patient and early-stage endometriosis (Figure 2C). In contrast, the enzymatic activity of MMP-2 was not different among the groups (Figure 2C). On the other hand, the gelatinase activity of peripheral monocytes/macrophages was minimal and was not different among normal, early-stage endometriosis, and severe-stage endometriosis (Figure 2D).
Figure 2.
Gelatinase activity of MMP-9 but not MMP-2 was decreased in peritoneal macrophages isolated from patients with endometriosis. A and B: Analysis of the time effect on the activation of peritoneal macrophages in terms of gelatinase activity. Macrophages (2 × 105 cells/well) isolated from patients with endometriosis were plated in 24-well plates for the indicated time and culture media were collected for zymographic analysis. A: A representative zymographic picture revealed that the enzymatic activity of MMP-9 secreted by peritoneal macrophages was elevated along with time of culture. B: The means of relative intensity units of MMP-9 obtained from four different samples. Because the gelatinase activity proportionally increases between 12 and 24 hours after attachment, we thus empirically chose 16 hours as the time for all of the following experiments. C: A representative zymographic picture shows a decrease in MMP-9 but not MMP-2 enzymatic activity along with the severity of endometriosis. Six batches of peritoneal macrophages isolated from normal (N), early-stage endometriosis (EE), and severe-stage endometriosis (SE) women, respectively, were subjected to zymographic analyses and the results were similar. D: A representative zymographic picture shows gelatinase activity of MMP-9 in cultured media of macrophages isolated from PF and peripheral blood (PB). Three batches of macrophages isolated from peripheral blood of normal (N), early-stage endometriosis (EE), and severe-stage endometriosis (SE) women were subjected to zymographic analyses and the results were similar.
Suppression of MMP-9 Activity by Endometriotic PF
To explore the mechanisms responsible for inhibition of MMP-9 activity derived from peritoneal macrophages at different stages of endometriosis, pooled PF collected from patients with severe endometriosis (endometriotic PF) was used to treat peritoneal macrophages for 24 hours. Treatment of peritoneal macrophages obtained from normal women with endometriotic PF markedly suppressed MMP-9 activities (Figure 3, A and B). Suppression of MMP-9 activity was most evident by 1:1 dilution of endometriotic PF. The suppressive capability was reduced because endometriotic PF was more diluted and was totally lost when endometriotic PF was diluted 100 times (Figure 3, A and B; and data not shown). The inhibitory ability was only observed in endometriotic PF as evident by that PF collected from normal patients failed to exert such effect (Figure 3, A and C). Furthermore, the effect of PF on MMP-9 activity was not due to levels of MMP-9 present in the PF because the original PF, regardless from normal or endometriosis patients, exerted minimal or no MMP-9 activity when macrophages were omitted from the culture dish (Figure 3, A and C). Unlike MMP-9, the gelatinase activity of MMP-2 is mainly contributed by proteins present in PF and/or culture medium (Figure 3, A and B).
Figure 3.
Endometriotic PFs suppressed gelatinase activities of MMP-9 in peritoneal macrophages. A and C: Gelatinase activity of normal and endometriosis peritoneal macrophages treated by normal peritoneal fluids, PF(N), and endometriotic peritoneal fluids, PF(E). Macrophages (2 × 105 cells/well) isolated from patients were plated in 24-well plates and treated by PF(N) and PF(E) with 1:1, 1:5, and 1:10 dilution. Representative zymographic pictures show that enzymatic activity of MMP-9 secreted by normal (A) or endometriotic (C) peritoneal macrophages treated with PF obtained normal (N) and/or endometriosis (Endo) patients. B and D: Means and SE of relative gelatinase activities of MMP-9 obtained from five and six experiments using different batches of cells obtained from normal and endometriosis patients, respectively. Asterisks denote significant difference from the control group.
In peritoneal macrophages obtained from early-stage endometriotic patients (defined as endometriosis in Figure 3), the basal enzymatic activity of MMP-9 is weaker than that obtained from normal women (compare the control group in Figure 3, B and D). Nevertheless, the responsiveness to endometriotic PF was the same. Treatment with endometriotic PF in 1:1 dilution markedly inhibited MMP-9 activity (Figure 3, C and D). The inhibitory effect was still evident when endometriotic PF was diluted to 1/10th (Figure 3, C and D). Similar results were also obtained when severe-stage peritoneal macrophages were used (data not shown).
Effects of PF on Inhibiting MMP-9 Expression in Peritoneal Macrophages
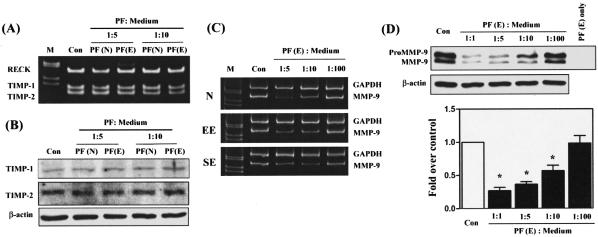
To characterize whether suppression of MMP-9 activity is due to an increased amount of MMP inhibitors, such as TIMP-1, TIMP-2, and RECK produced by macrophages, mRNA and/or protein levels of TIMP-1, TIMP-2, and RECK were evaluated. Western blot and RT-PCR analyses revealed no significant difference in TIMP-1, TIMP-2, and RECK levels when peritoneal macrophages of normal patients were treated with normal PF or endometriotic PF (Figure 4, A and B). In peritoneal macrophages isolated from patients with endometriosis, treatment with PF also had no substantial effect on TIMP-1, TIMP-2, or RECK expression (data not shown).
Figure 4.
The effect of PFs on expression of MMP-9 and MMP inhibitors in peritoneal macrophages. Macrophages were plated for 16 hours and then treated with peritoneal fluids from normal, PF(N), or endometriotic, PF(E), patients for 24 hours as described in the Materials and Methods. A: A representative gel picture showing RT-PCR result of levels of TIMP-1, TIMP-2, and RECK mRNA in macrophages. This experiment was repeated four times and the results were similar. B: A representative Western blot result demonstrates the expression of TIMP-1, TIMP-2, and β-actin protein. This experiment was repeated three times and the results were similar. C: A representative gel picture showing RT-PCR result of levels of MMP-9 mRNA in endometriotic PF-treated macrophages. Expression of MMP-9 mRNA was decreased by treatment with endometriotic PF in normal (N, n = 3), early-stage endometriosis (EE, n = 3), and severe-stage endometriosis (SE, n = 6) patients, respectively. D: A representative Western blot shows a significant decrease in expression of MMP-9 protein in peritoneal macrophages obtained from normal women treated with endometriotic PF (top). Endometriotic PF (without macrophages) was used as a negative control to demonstrate the presence of MMP-9 that was produced by macrophage but not from PF. The bottom panel shows means and standard errors of four experiments using different batches of cells. Asterisks denote results significantly different from the control group.
To determine whether the inhibitory effect of endometriotic PF on macrophage MMP-9 activity was due to a decrease in de novo synthesis of MMP-9, the mRNA encoding for MMP-9 and levels of MMP-9 protein were evaluated. Semiquantitative RT-PCR demonstrated that expression of MMP-9 transcript was reduced by treatment with endometriotic PF irrespective of the source of peritoneal macrophages (Figure 4C). Western blot analysis revealed that MMP-9 proteins were decreased in peritoneal macrophages treated with endometriotic PF (Figure 4D). The inhibitory effect diminished when the endometriotic PF was diluted to 1/100 (Figure 4, C and D). As internal controls, there was no significant difference in GAPDH and β-actin expression in macrophages treated with either normal or endometriotic PFs (Figure 4, C and D). Because peritoneal macrophages of normal women and women with endometriosis respond to treatments with the same pattern, in terms of gene expression and MMP-9 activity, we thus performed all subsequent experiments using peritoneal macrophages obtained from women with endometriosis, unless otherwise mentioned, due to ethical concerns.
Effects of Cytokines and Proinflammatory Agents on MMP-9 Activity
To further identify the potential candidates that regulate MMP-9 expression and activity, several known cytokines and/or proinflammatory factors were administered to treat peritoneal macrophages. Results indicated that IL-1β slightly increased MMP-9 activity (Figure 5A) whereas high-dose IFN-γ partially inhibited peritoneal macrophage-derived MMP-9 activity (Figure 5A). Treatment with other cytokines such as tumor necrosis factor-α and leptin showed no substantial effects on MMP-9 activity (Figure 5A and data not shown). In contrast, treatment of peritoneal macrophages with PGE2 significantly inhibited MMP-9 activity in a dose-dependent manner (Figure 5B).
Figure 5.
Influence of gelatinase activity of MMP-9 by cytokines and proinflammatory agents. A: Zymographic pictures showing gelatinase activity of MMP-9 secreted by peritoneal macrophages. MMP-9 activities were slightly increased by IL-1β treatment, whereas IFN-γ treatment exhibited inhibition at high dose (200 ng/ml). Treatment with leptin revealed no significant difference in MMP-9 activity. All of the experiments were repeated at least four times and the results were similar. B: A representative zymographic picture showing PGE2 treatment significantly inhibited MMP-9 activity in a dose-dependent manner. The experiment was repeated four times and the results were similar.
PGE2 Suppresses MMP-9 Activity by Inhibiting Its Expression
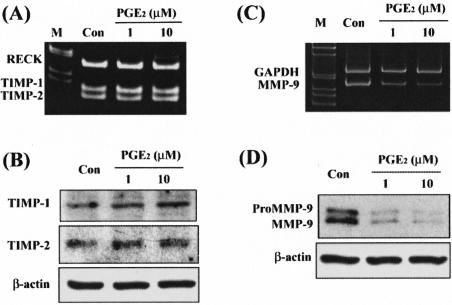
To determine whether suppression of MMP-9 activity by PGE2 could be due to an increase in MMP-9 inhibitors, expression of TIMP-1, TIMP-2, and RECK was determined by RT-PCR and/or Western blot. Treatment with PGE2 had no substantial effect on levels of mRNA encoding for TIMP-1, TIMP-2, or RECK (Figure 6A). Western blot analysis also demonstrated that TIMP-1 and TIMP-2 proteins were not affected by PGE2 (Figure 6B) suggesting the effect of PGE2 on decreased MMP-9 activity is not mediated via up-regulation of its inhibitors. We next examined whether inhibition of MMP-9 activity is due to a decrease in its expression. RT-PCR results demonstrated that PGE2 significantly inhibited MMP-9 mRNA expression (Figure 6C), which was mirrored by a decrease in cellular MMP-9 protein (Figure 6D). To further determine the mechanisms responsible for inhibition of MMP-9 expression by PGE2, selective pharmacological agonists and/or antagonists of EP receptors were used to treat peritoneal macrophages. Treatment with EP2 agonist (butaprost, 10 μmol/L) exerted a similar effect as those treated with PGE2 whereas treatment with EP3 agonist (sulprostone, 10 μmol/L) failed to inhibit MMP-9 expression (Figure 7A). Co-treatment with PGE1OH (EP2/EP4 agonist, 10 μmol/L) and AH6809 (EP1/EP2 antagonist, 80 μmol/L) also inhibited MMP-9 expression indicating that effect of PGE2 may also be mediated via EP4 receptor (Figure 7A). To test whether the lack of effect of EP3 agonist was due to lack of EP3 receptor expression, RT-PCR was used to evaluate the presence or absence of different EP receptor isoforms in peritoneal macrophages. The result demonstrated that three EP receptor isoforms, EP2, EP3, and EP4 were expressed in macrophages whereas EP1 receptor was undetectable (Figure 7B). The downstream signaling of EP2/EP4 was mediated via protein kinase A (PKA) pathway. Thus, a PKA inhibitor, H89 was used to block actions of PGE2. Pretreatment with H89 (10 μmol/L) reversed PGE2-mediated inhibition of MMP-9 expression (Figure 7C) and activity (Figure 7D). Concordantly, H89 also relieved butaprost-suppressed MMP-9 protein expression (Figure 7D).
Figure 6.
Effect of PGE2 on expression of MMP-9 and MMP inhibitors in peritoneal macrophages. Peritoneal macrophages were treated with 1 or 10 μmol/L PGE2. A: A representative gel picture showing RT-PCR result of levels of TIMP-1, TIMP-2, and RECK mRNA in macrophages. The experiment was repeated four times and the results were similar. B: A representative Western blot result demonstrates the expression of TIMP-1, TIMP-2, and β-actin protein. The experiment was repeated three times and the results were similar. C: A representative gel picture showing RT-PCR result of levels of MMP-9 mRNA in PGE2-treated macrophages. Expression of MMP-9 mRNA was decreased by treatment with 1 and 10 μmol/L PGE2, respectively. The experiment was repeated four times and the results were similar. D: Western blot analyses revealed a significant decrease in expression of MMP-9 protein with PGE2 treatment in peritoneal macrophages. The experiment was repeated four times and the results were similar.
Figure 7.
Effect of PGE2 receptor agonist and/or antagonist treatment on gelatinase activity and expression of MMP-9 in peritoneal macrophages. A: A representative Western blot shows expression of MMP-9 protein in macrophages treated with PGE2 receptor agonist and/or antagonist. Macrophages were treated with EP2 agonist (butaprost, buta), EP3 receptor agonist (sulprostone, Sul), or a combination of EP2/EP4 receptor agonist, PGE1OH, and EP1/EP2 receptor antagonist, AH6809 (E1OH+AH), and expression of MMP-9 protein was analyzed. The bottom panel shows means and standard errors obtained from four experiments in which different batches of cells were used. Asterisks denote significant difference from control group (P < 0.05). B: A representative gel picture shows RT-PCR results of levels of EP2, EP3, EP4, and GAPDH mRNA in peritoneal macrophages (labeled as 1 to 4). C: A representative Western blot result demonstrating the expression of MMP-9 protein in macrophages treated with different EP receptor agonists and/or antagonist. This experiment was repeated three times and the results were similar. D: Zymographic picture shows gelatinase activity of MMP-9 secreted by peritoneal macrophages. Pretreatment with H89 reversed PGE2- and butaprost-suppressed MMP-9 gelatinase activity. This experiment was repeated three times and the results were similar.
Discussion
In the current study, we found a decrease in expression and enzymatic activity of MMP-9 in peritoneal macrophages isolated from patients with endometriosis. Decreased MMP-9 expression and activity in macrophages is due, at least in part, to exposure to factors present in the PF of women with endometriosis (Figure 8). The conclusion is based on results obtained in experiments in which enzymatic activity of MMP-9 is inhibited by treatment of macrophages with the PF isolated from endometriotic but not normal patients. Inhibition of MMP-9 activity by endometriotic PF is not due to increased expression of its cognate inhibitors such as TIMP-1, TIMP-2, or RECK but to down-regulation of MMP-9 protein expression. In concordance with this result, PGE2, which is aberrantly elevated in PF of women with endometriosis, potently inhibits MMP-9 expression thus suppresses its enzymatic activity. The inhibitory effect of PGE2 is mediated via EP2/EP4-coupled PKA signaling pathway and is independent of expression levels of TIMPs. Our current findings may explain the underlying mechanism responsible for the long-standing notion that peritoneal macrophage of endometriosis patients is less active in phagocytosis compared to that of normal patients.8
Figure 8.
The schematic drawing indicates expression of MMP-9 by macrophages was suppressed by factors such as PGE2 present in the PF of women with endometriosis.
Endometriosis is a chronic inflammation that recruits many immune cells, especially macrophages, to the peritoneum. An increased number of active macrophages has been found in PF of patients with endometriosis.25 Infiltrated macrophages are supposed to function as scavengers, which help removing peritoneal debris. Nevertheless, it has been found that peritoneal macrophages isolated from patients with endometriosis have poor phagocytotic capacity.8 It is not clear whether decreased phagocytotic capability of peritoneal macrophages in patients with endometriosis is the cause or effect of endometriosis. Regardless, dysfunction of peritoneal macrophages is a severe impairment of the defense system and an important factor leading to the development of endometriosis. In the current study, we demonstrate that expression of total and active MMP-9 in peritoneal macrophages is reduced in patients with endometriosis. Expression and secretion of MMP-9 by macrophages is a means to degrade the extracellular matrix of cells that are designated for phagocytosis. Decreased expression and activity of MMP-9 secreted by macrophages results in reducing phagocytotic ability, which may, at least in part, account for the loss of function in peritoneal macrophages in endometriosis.
Tissue inhibitors for MMPs are major cellular inhibitors of the MMP family and RECK has modest MMP inhibitory activity.31–33 It is possible that decreased MMP-9 expression and activity in peritoneal macrophage of women with endometriosis may be due to an elevation of these MMP inhibitors. However, in the current study, we found that levels of TIMPs and RECK do not contribute to the decreased phagocytosis of peritoneal macrophages in patients with endometriosis. Recently, it has been reported that concentrations of TIMP-1 are decreased in endometriotic PF over a normal control,14 which provides indirect evidence to support our current results that suppression of MMP-9 activity by endometriotic PF appears to be independent of the endogenous MMP inhibitors.
The biochemical natures of PF between patients with and without endometriosis are quite different. Distinct activities of MMP-9 in patients with or without endometriosis may be caused by factors that are differentially present in the PF. To explore such a possibility, pooled PF collected from endometriosis-free or severe endometriosis patients were used to treat peritoneal macrophages. Our results showing that the PF from patients with endometriosis suppresses MMP-9 expression and activity provide a likely mechanism to explain the notion that macrophages isolated from women with endometriosis are less active in phagocytosis.8
It is known that several cytokines and proinflammatory agents, such as IL-1, tumor necrosis factor-α, leptin, and PGE2 are elevated in the PF of patients with endometriosis.25,34–36 These agents may act as paracrine and/or autocrine signals to regulate immune response and inflammation. For example, the immune regulatory role of leptin has been documented37 and numerous studies also showed that MMP-9 is regulated by many cytokines.28,38–40 Thus, we examined several candidate cytokines or hormones that have been demonstrated to play roles in the pathogenesis of endometriosis. Our results implicated that none of these agents except PGE2 could be the factor in endometriotic PF that exerts the inhibitory effect. At its first glance, repression of MMP-9 activity by high-dose (200 ng/ml) IFN-γ may implicate a role for IFN-γ in reducing phagocytotic capability of macrophages in endometriosis patients. Nevertheless, the pathophysiological significance of IFN-γ in vivo is still uncertain because the inhibitory effect is very low and the dose for IFN-γ to exert such effect is too high. All these reasonable doubts argue against the notion that IFN-γ could be the key player regulating the decreased phagocytotic ability of macrophages in women with endometriosis.
In contrast, our results clearly demonstrate that PGE2 plays a critical role in suppressing MMP-9 activity in peritoneal macrophage culture medium. The mode of actions exerted by PGE2 is similar to that by endometriotic PF in many ways. First, both inhibit MMP-9 expression rather than activation. Second, both have no effect on inducing MMP-9 inhibitors, such as TIMPs and RECK. And third, both affect only MMP-9 but not MMP-2 (compare Figures 3 and 5). Furthermore, the effective dose of PGE2 (100 nmol/L and it might be lower) is physiological (kd of PGE2 is between 30 to 100 nmol/L) and is within the concentration range of endometriotic PF.41 In concordance with our current result, it has recently been reported that PGE2 is able to suppress IL-1β-induced MMP-3 expression in human gingival fibroblasts.42 Together, these data provide evidence to support the immune modulatory role of PGE2. Obviously, PGE2 is not the only suppressor in endometriotic PF and more effort is needed to identify factors that may play important roles in suppressing MMP-9 expression and activity.
There are four different receptors (EP1, EP2, EP3, and EP4) with various alternative splicing variants for PGE2.43 The signaling pathways coupled to these distinct EP receptors are quite different and complicated. By using pharmacological agonists and antagonist, we found that both EP2 and EP4 are important for exerting inhibitory effect of PGE2. Because the downstream signaling pathway of both EP2 and EP4 is mediated by PKA, we used H89, a selective PKA inhibitor, to block PGE2 action. Administration of H89 significantly reverses PGE2 and butaprost-inhibited MMP-9 expression and activity provides another line of evidence to support the action of PGE2 be mediated by EP2/EP4-coupled PKA signaling pathway. Further investigation is warranted to unravel mechanism responsible for PGE2-mediated MMP-9 suppression at the molecular level.
In summary, our current data provide strong evidence to support previous findings that peritoneal macrophages isolated from patients with endometriosis are less active in phagocytosis8 and that local immune dysfunction is a critical factor leading to the development of endometriosis.4,5 Although we have not directly demonstrated that PGE2 or endometriotic PF treatment could reduce phagocytotic ability of macrophages, a plethora of evidence in the literature has implicated that MMP-9 plays a pivotal role in the macrophage’s ability to degrade basement membrane and thus its capability of phagocytosis.16,17,20–22,44 Considering that PGE2 can effectively suppress MMP-9 expression and activity, our current results may shed light on developing new strategies against endometriosis by trying to restore the intact phagocytotic capability of peritoneal macrophages of patients with endometriosis. For example, developing more long-lasting and effective COX inhibitors cannot only reduce the pain associated with this disease but also be beneficial by inhibiting PGE2 production and thus relieving PGE2-suppressed phagocytotic ability of macrophages.
Footnotes
Address reprint requests to Shaw-Jenq Tsai, Ph.D., Department of Physiology, College of Medicine, National Cheng Kung University, 1 University Rd., Tainan 70101 Taiwan, Republic of China. E-mail: seantsai@mail.ncku.edu.tw.
Supported by grants from National Science Council of the Republic of China (grants NSC-92-2312-B-006-019 and NSC-92-2314-B-006-050).
M.-H.W. and Y.S. contributed equally to this study.
References
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–425. [Google Scholar]
- Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Sebastian S. Estrogen production and metabolism in endometriosis. Ann NY Acad Sci. 2002;955:75–85. doi: 10.1111/j.1749-6632.2002.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wu MH, Lin CC, Sun HS, Chen SM. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab. 2001;86:5765–5773. doi: 10.1210/jcem.86.12.8082. [DOI] [PubMed] [Google Scholar]
- Dmowski WP, Braun D, Gebel H. Endometriosis: genetic and immunologic aspects. Prog Clin Biol Res. 1990;323:99–122. [PubMed] [Google Scholar]
- Dmowski WP, Gebel HM, Braun DP. The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet Gynecol Scand Suppl. 1994;159:7–14. [PubMed] [Google Scholar]
- Dunselman GA, Hendrix MG, Bouckaert PX, Evers JL. Functional aspects of peritoneal macrophages in endometriosis of women. J Reprod Fertil. 1988;82:707–710. doi: 10.1530/jrf.0.0820707. [DOI] [PubMed] [Google Scholar]
- Haney AF, Muscato JJ, Weinberg JB. Peritoneal fluid cell populations in infertility patients. Fertil Steril. 1981;35:696–698. doi: 10.1016/s0015-0282(16)45567-6. [DOI] [PubMed] [Google Scholar]
- Raiter-Tenenbaum A, Baranao RI, Etchepareborda JJ, Meresman GF, Rumi LS. Functional and phenotypic alterations in peritoneal macrophages from patients with early and advanced endometriosis. Arch Gynecol Obstet. 1998;261:147–157. doi: 10.1007/s004040050214. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Murphy G, Nguyen Q, Cockett MI, Atkinson SJ, Allan JA, Knight CG, Willenbrock F, Docherty AJ. Assessment of the role of the fibronectin-like domain of gelatinase A by analysis of a deletion mutant. J Biol Chem. 1994;269:6632–6636. [PubMed] [Google Scholar]
- Shipley JM, Doyle GA, Fliszar CJ, Ye QZ, Johnson LL, Shapiro SD, Welgus HG, Senior RM. The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. Role of the fibronectin type II-like repeats. J Biol Chem. 1996;271:4335–4341. doi: 10.1074/jbc.271.8.4335. [DOI] [PubMed] [Google Scholar]
- Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- Chung HW, Wen Y, Chun SH, Nezhat C, Woo BH, Lake Polan M. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: a rationale for endometriotic invasiveness. Fertil Steril. 2001;75:152–159. doi: 10.1016/s0015-0282(00)01670-8. [DOI] [PubMed] [Google Scholar]
- Szamatowicz J, Laudanski P, Tomaszewska I. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1: a possible role in the pathogenesis of endometriosis. Hum Reprod. 2002;17:284–288. doi: 10.1093/humrep/17.2.284. [DOI] [PubMed] [Google Scholar]
- Collette T, Bellehumeur C, Kats R, Maheux R, Mailloux J, Villeneuve M, Akoum A. Evidence for an increased release of proteolytic activity by the eutopic endometrial tissue in women with endometriosis and for involvement of matrix metalloproteinase-9. Hum Reprod. 2004;19:1257–1264. doi: 10.1093/humrep/deh290. [DOI] [PubMed] [Google Scholar]
- Dhami R, Gilks B, Xie C, Zay K, Wright JL, Churg A. Acute cigarette smoke-induced connective tissue breakdown is mediated by neutrophils and prevented by alpha1-antitrypsin. Am J Respir Cell Mol Biol. 2000;22:244–252. doi: 10.1165/ajrcmb.22.2.3809. [DOI] [PubMed] [Google Scholar]
- Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Cury JD, Shapiro SD, Goldberg GI, Welgus HG. Neutral proteinases of human mononuclear phagocytes. Cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol. 1991;146:1286–1293. [PubMed] [Google Scholar]
- Welgus HG, Campbell EJ, Cury JD, Eisen AZ, Senior RM, Wilhelm SM, Goldberg GI. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990;86:1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91:852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- Ratzinger G, Stoitzner P, Ebner S, Lutz MB, Layton GT, Rainer C, Senior RM, Shipley JM, Fritsch P, Schuler G, Romani N. Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J Immunol. 2002;168:4361–4371. doi: 10.4049/jimmunol.168.9.4361. [DOI] [PubMed] [Google Scholar]
- McMillan JI, Weeks R, West JW, Bursten S, Rice GC, Lovett DH. Pharmacological inhibition of gelatinase B induction and tumor cell invasion. Int J Cancer. 1996;67:523–531. doi: 10.1002/(SICI)1097-0215(19960807)67:4<523::AID-IJC11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Habu Y, Fukada-Tanaka S, Hisatomi Y, Iida S. Amplified restriction fragment length polymorphism-based mRNA fingerprinting using a single restriction enzyme that recognizes a 4-bp sequence. Biochem Biophys Res Commun. 1997;234:516–521. doi: 10.1006/bbrc.1997.6666. [DOI] [PubMed] [Google Scholar]
- Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, Tsai SJ. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod. 2002;8:1103–1110. doi: 10.1093/molehr/8.12.1103. [DOI] [PubMed] [Google Scholar]
- Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ. Distinct regulation of cyclooxygenase-2 by interleukin-1beta in normal and endometriotic stromal cells. J Clin Endocrinol Metab. 2005;90:286–295. doi: 10.1210/jc.2004-1612. [DOI] [PubMed] [Google Scholar]
- ASRM The American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis:1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- Ma Z, Qin H, Benveniste EN. Transcriptional suppression of matrix metalloproteinase-9 gene expression by IFN-gamma and IFN-beta: critical role of STAT-1alpha. J Immunol. 2001;167:5150–5159. doi: 10.4049/jimmunol.167.9.5150. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wu MH, Chuang PC, Chen HM. Distinct regulation of gene expression by prostaglandin F2α (PGF2α) is associated with PGF2α resistance or susceptibility in human granulosa-luteal cells. Mol Hum Reprod. 2001;7:415–423. doi: 10.1093/molehr/7.5.415. [DOI] [PubMed] [Google Scholar]
- Sun HS, Hsiao KY, Hsu CC, Wu MH, Tsai SJ. Transactivation of steroidogenic acute regulatory protein in human endometriotic stromal cells is mediated by the prostaglandin EP2 receptor. Endocrinology. 2003;144:3934–3942. doi: 10.1210/en.2003-0289. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Coussens LM. RECKing MMP function: implications for cancer development. Trends Cell Biol. 2002;12:209–211. doi: 10.1016/s0962-8924(02)02280-8. [DOI] [PubMed] [Google Scholar]
- Noda M, Oh J, Takahashi R, Kondo S, Kitayama H, Takahashi C. RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev. 2003;22:167–175. doi: 10.1023/a:1023043315031. [DOI] [PubMed] [Google Scholar]
- Cheong YC, Shelton JB, Laird SM, Richmond M, Kudesia G, Li TC, Ledger WL. IL-1, IL-6 and TNF-alpha concentrations in the peritoneal fluid of women with pelvic adhesions. Hum Reprod. 2002;17:69–75. doi: 10.1093/humrep/17.1.69. [DOI] [PubMed] [Google Scholar]
- Keenan JA, Chen TT, Chadwell NL, Torry DS, Caudle MR. IL-1 beta, TNF-alpha, and IL-2 in peritoneal fluid and macrophage-conditioned media of women with endometriosis. Am J Reprod Immunol. 1995;34:381–385. doi: 10.1111/j.1600-0897.1995.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Wu MH, Chuang PC, Chen SM, Lin CC, Tsai SJ. Increased leptin expression in endometriosis cells is associated with endometrial stromal cell proliferation and leptin gene-upregulation. Mol Hum Reprod. 2002;8:456–464. doi: 10.1093/molehr/8.5.456. [DOI] [PubMed] [Google Scholar]
- Lord G. Role of leptin in immunology. Nutr Rev. 2002;60:S35–S85. doi: 10.1301/002966402320634913. [DOI] [PubMed] [Google Scholar]
- Sanceau J, Boyd DD, Seiki M, Bauvois B. Interferons inhibit tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 activation via interferon regulatory factor-1 binding competition with NF-kappa B. J Biol Chem. 2002;277:35766–35775. doi: 10.1074/jbc.M202959200. [DOI] [PubMed] [Google Scholar]
- Yoo HG, Shin BA, Park JS, Lee KH, Chay KO, Yang SY, Ahn BW, Jung YD. IL-1beta induces MMP-9 via reactive oxygen species and NF-kappaB in murine macrophage RAW 264.7 cells. Biochem Biophys Res Commun. 2002;298:251–256. doi: 10.1016/s0006-291x(02)02431-2. [DOI] [PubMed] [Google Scholar]
- Xie B, Dong Z, Fidler IJ. Regulatory mechanisms for the expression of type IV collagenases/gelatinases in murine macrophages. J Immunol. 1994;152:3637–3644. [PubMed] [Google Scholar]
- Dawood MY, Khan-Dawood FS, Wilson L., Jr Peritoneal fluid prostaglandins and prostanoids in women with endometriosis, chronic pelvic inflammatory disease, and pelvic pain. Am J Obstet Gynecol. 1984;148:391–395. doi: 10.1016/0002-9378(84)90713-0. [DOI] [PubMed] [Google Scholar]
- Ruwanpura SM, Noguchi K, Ishikawa I. Prostaglandin E2 regulates interleukin-1beta-induced matrix metalloproteinase-3 production in human gingival fibroblasts. J Dent Res. 2004;83:260–265. doi: 10.1177/154405910408300315. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]