Abstract
A major challenge in reconstructive surgery is flap ischemia, which might benefit from induction of therapeutic angiogenesis. Here we demonstrate the effect of an adeno-associated virus (AAV) vector delivering vascular endothelial growth factor (VEGF)165 in two widely recognized in vivo flap models. For the epigastric flap model, animals were injected subcutaneously with 1.5 × 1011 particles of AAV-VEGF at day 0, 7, or 14 before flap dissection. In the transverse rectus abdominis musculocutaneous flap model, AAV-VEGF was injected intramuscularly. The delivery of AAV-VEGF significantly improved flap survival in both models, reducing necrosis in all treatment groups compared to controls. The most notable results were obtained by administering the vector 14 days before flap dissection. In the transverse rectus abdominis musculocutaneous flap model, AAV-VEGF reduced the necrotic area by >50% at 1 week after surgery, with a highly significant improvement in the healing process throughout the following 2 weeks. The therapeutic effect of AAV-VEGF on flap survival was confirmed by histological evidence of neoangiogenesis in the formation of large numbers of CD31-positive capillaries and α-smooth muscle actin-positive arteriolae, particularly evident at the border between viable and necrotic tissue. These results underscore the efficacy of VEGF-induced neovascularization for the prevention of tissue ischemia and the improvement of flap survival in reconstructive surgery.
Partial necrosis of skin flaps represents a major clinical problem in patients undergoing reconstructive procedures, with significant morbidity and no effective therapy available. The lack of oxygen and nutrients in the distal part of the flap strongly compromises skin viability, resulting in flap necrosis, which often requires secondary reconstructive interventions.1 The recent concept of therapeutic angiogenesis by local administration of angiogenic growth factors has emerged as an attractive approach to enhance blood supply and perfusion in compromised tissues, thus improving flap survival.
The angiogenic response to tissue ischemia is a complex process involving the coordinated interplay of a variety of soluble factors, controlling new blood vessel formation. One of the fundamental molecules that are required in the angiogenic process is the vascular endothelial growth factor (VEGF). Four different isoforms of VEGF have been described, with 121, 165, 189, and 206 amino acids, arising by differential splicing of the primary VEGF gene transcript. VEGF165 is the predominant form in all cells and tissues and its expression is promptly induced by tissue hypoxia. The main activity of VEGF is to stimulate the proliferation and migration of endothelial cells, with the constitution of a primitive capillary network, which subsequently matures to form larger vessels.
Research on therapeutic angiogenesis relating to plastic and reconstructive surgery is in its early stages. Several laboratories have explored the possibility of promoting skin flap neovascularization by using recombinant VEGF proteins. In most of these studies, with some exceptions,2 recombinant VEGF provided a beneficial effect on the flap survival,3–9 although the exact mechanisms have not always been specifically addressed. Despite these encouraging findings, the use of recombinant proteins in a clinical setting is hampered by several factors, such as their short half-lives, poor bioavailability, and consequent need for frequent administrations to sustain long-lasting effects. Delivery of therapeutic genes to induce neo-angiogenesis represents an appealing alternative strategy that might overcome most of these problems. Moreover, the easy accessibility of the flaps makes them an ideal target for an in vivo gene transfer approach.
A variety of techniques allow for a coding DNA to be taken up by host cells, which then express the respective protein. Many of them have been used for gene delivery to the muscle and skin, including the use of naked plasmid DNA and viral vectors.10–18 The use of viral vectors presents a notable advantage over nonviral systems, by providing a higher rate of transduction and expression. Among the novel approaches that hold promise to become a relevant therapeutic modality in humans is the use of vectors based on the adeno-associated virus (AAV), a nonpathogenic and widespread parvovirus, incapable of autonomous replication. Vectors based on AAV are able to transduce both dividing and nondividing cells and show a specific tropism for postmitotic cells, including skeletal and cardiac muscle,10,19 neurons,20 and liver.21,22 Because these vectors do not contain any viral genes—which are transiently transfected in trans for the packaging process—they elicit virtually no inflammatory or immune response.23,24 As a consequence, transgene expression from these vectors persists for several months in a variety of animal tissues in vivo.25
Besides being able to transduce human keratinocytes in vitro,26 we and others have observed that the subcutaneous delivery of AAV vectors results in the efficient transduction of hair follicles, sweat gland ducts,27 and the panniculus carnosus (the skeletal muscle layer within the dermal sheet in rodents).28,29 To our knowledge, the data reported in this study are the first demonstration that AAV can be successfully used in the plastic surgery field to deliver the VEGF165 gene as a tool to promote therapeutic angiogenesis and skin flap survival in two different in vivo flap models.
Materials and Methods
Recombinant AAV Vector Preparation and Characterization
Two recombinant AAV vectors were obtained in this study, expressing the LacZ reporter gene and the cDNA for the 165 amino acid isoform of VEGF (VEGF165) under the control of the constitutive cytomegalovirus immediate early promoter. Infectious vector stocks were generated in 293 cells and titrated by a competitive polymerase chain reaction procedure, as already described.30
Animals and Experimental Protocols
Animal care and treatment were conducted in conformity with institutional guidelines in compliance with national and international laws and policies (European Economic Community Council Directive 86/609, OJL 358, December 12th, 1987). A total of 88 adult male Wistar rats weighting 250 to 300 g were used for this study and housed under controlled environmental conditions. After general anesthesia with Avertin 2% (10 ml/kg), abdominal hair was removed and skin washed with sterile water. A flap measuring 5 × 8 cm was outlined on the skin, extending distally from the xiphoid process and bilaterally from the midline (Figure 2).
Figure 2.
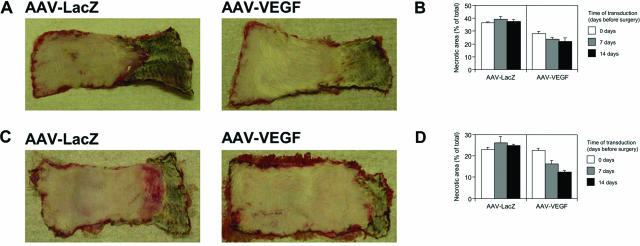
Effect of VEGF on skin flap survival at different times of administration. At postoperative day 7, the regions of survival and necrosis were clearly defined in all of the flaps: the surviving skin appeared pink and tender, whereas the distal necrotic portion was black and rigid. A: The pictures show two selected epigastric flaps, treated with AAV-LacZ (left) and AAV-VEGF (right), 14 days before flap elevation. The viability of the skin appears clearly improved after AAV-VEGF administration. B: The effect of AAV-VEGF on epigastric flap survival was assessed by injecting the vector at different time points before surgery (0, 7, and 14 days). The histograms represent the mean values and SD of the necrotic area relative to the total flap area, as measured by digital planimetry, for each experimental group. C: The pictures show two selected TRAM flaps, treated with AAV-LacZ (left) and AAV-VEGF (right), 14 days before flap elevation. Also in this model, the treatment with AAV-VEGF resulted in a significant improvement in tissue viability. D: The effect of AAV-VEGF on TRAM flap survival was assessed by injecting the vector at different time points before surgery (0, 7, and 14 days). The histograms represent the mean values and SD of the necrotic area relative to the total flap area, as measured by digital planimetry, for each experimental group.
In the first model, the skin flap was raised from the fascia on the inferior epigastric artery, by cutting all of the vessels from the left side of the abdomen, all of the perforators, and the right lateral thoracic artery. At the end of the procedure, the skin was immediately resutured in place. In this model, 150 μl of either AAV-VEGF or placebo were injected at 10 equally spaced subcutaneous sites along the midline of the flap.
For the TRAM model, the entire left part of the flap was raised on a plane between the panniculus carnosus and the abdominal fascia, but on the right side the medial portion of the flap was left attached to the anterior rectus sheet. In this way, the rectus abdominis represents the muscular component of the flap, providing the blood supply to the cutaneous component through the perforator arteries. This time, AAV-VEGF or placebo was injected intramuscularly in the same region where each perforator artery arises from the rectus abdominis.
Each of the 12 experimental groups described in Figure 2 was initially composed of four rats (an overview of the experimental groups is provided in Table 1). Rats in groups 1, 2, 3, 7, 8, and 9 received AAV-VEGF at 0, 7, and 14 days before surgery, respectively, whereas groups 4, 5, 6, 10, 11, and 12 were control groups, in which the same volume of AAV-LacZ or saline was administered at the same time points (in every control group, two animals received AAV-LacZ and the other two received saline). Based on the results of this first set of experiments, 40 additional rats were treated with the TRAM flap and injected with AAV-VEGF or AAV-LacZ (n = 20 per group) vectors 14 days before surgery. Flap survival was first assessed at day 7 after surgery by taking digital images of the flaps using a Nikon E995 camera followed by the measurement of the necrotic area using the UTHSCSA Image Tool software. At the same time point, 12 animals per group were sacrificed for histological assessment. Flaps were harvested after the scars, as described in Figure 4A, and tissue biopsies were fixed overnight in 10% buffered formalin. The other rats (n = 8 per group) were followed for an additional 2 weeks at 3-day intervals, by measuring the extent of the necrotic area to assess the efficacy of the healing process.
Table 1.
Experimental Groups
| Treatment | Time of vector delivery | Group no. |
|---|---|---|
| Epigastric flap (n = 24) | ||
| AAV-VEGF | During surgery | 1 |
| 7 days before surgery | 2 | |
| 14 days before surgery | 3 | |
| AAV-LacZ | During surgery | 4 |
| 7 days before surgery | 5 | |
| 14 days before surgery | 6 | |
| TRAM flap (n = 24) | ||
| AAV-VEGF | During surgery | 7 |
| 7 days before surgery | 8 | |
| 14 days before surgery | 9 | |
| AAV-LacZ | During surgery | 10 |
| 7 days before surgery | 11 | |
| 14 days before surgery | 12 |
Figure 4.
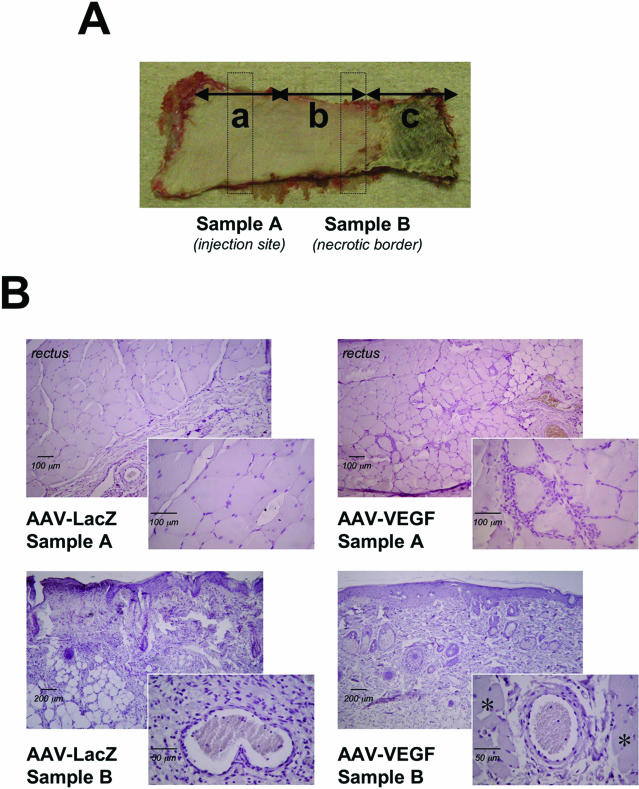
Histological sampling and assessment of TRAM flap viability. A: Each flap presented three distinguishable zones, according to their distance from the vascular pedicle: a survived zone (a), an intermediate zone (b), and a necrotic zone (c). To histologically examine flap viability, we harvested the skin and the muscular sheet from the injection site (sample A), as well as a more distal cutaneous sample (sample B, at ∼3 cm from sample A) from the intermediate zone. B: Shown are representative sections of samples A and B from AAV-VEGF-treated (right) and control (left) animals. At the injection site (sample A), a massive cellular infiltration appeared as a consequence of AAV-VEGF treatment (top). More notably, sample B of VEGF-treated flaps showed an intact and viable epithelial layer with conserved tissue architecture, whereas, in control flaps, the epithelium was thin and discontinuous, with massive inflammation and adipose substitution. Myonecrosis was detected only in LacZ-treated flaps, as indicated by the disappearance of the panniculus carnosus (shown by asterisks in the VEGF sample). Note the presence of circulating inflammatory cells in the arterial lumen in the insets, more abundant in the LacZ-treated as compared to the VEGF-treated samples.
Histological and Immunohistochemical Evaluation
Fixed samples were dehydrated with graded ethanol and embedded in paraffin. Five-μm sections were stained with hematoxylin for morphological analysis of tissues. For the histological score assignment, six slides of each biopsy were independently examined by three researchers without knowledge of the previous treatment; biopsies were harvested from at least six different animals per experimental group. The inflammatory and adipose areas were calculated by digital planimetry of tissue sections.
To visualize blood vessels by immunohistochemistry, rehydrated serial paraffin sections were subjected to antigen retrieval procedures. After inactivation of endogenous peroxidase with 3% hydrogen peroxide, samples were rinsed in phosphate-buffered saline (PBS) and blocked with nonimmune horse serum followed by incubation with an anti-CD31 antibody (Santa Cruz) or an anti-α-smooth muscle actin (α-SMA) antibody (Sigma-Aldrich, St. Louis, MO). Slides were rinsed in PBS and then incubated with biotinylated horse secondary antibody (Vector Laboratories, Burlingame, CA). After an additional washing in PBS, slides were incubated in the presence of an avidin-biotin complex and developed with 3,3′-diaminobenzidine (Lab Vision Corporation, Fremont, CA).
Statistical Analysis
The comparison of the efficacy of AAV vector delivery at different time points with respect to flap surgery was performed by two-way factorial analysis of variance, followed by appropriate one-way analysis of variance and posthocs. Posthoc analysis was performed with Bonferroni/Dunn. Pair-wise comparison between groups was performed using the Student’s t-test.
Results
Efficacy of AAV-VEGF Gene Transfer in Two Skin Flap Models in the Rat
This study is based on the use of two AAV vectors (AAV2 serotype), delivering either the LacZ reporter gene (AAV-LacZ) as a control, or the 165 amino acid isoform of human VEGF (AAV-VEGF) under the control of the constitutive human cytomegalovirus immediate early promoter. Both vectors were obtained by standard co-transfection procedures at a titer of 1 × 1012 viral genome particles/ml in the International Centre for Genetic Engineering and Biotechnology AAV Vector Unit facility. We have already described the angiogenic effect of the AAV-VEGF vector in vivo after intramuscular injection in the rat skeletal muscle, in conditions of both normal perfusion30 or after ischemia,31 as well as in a rat skin wound model.29
Considering the strong angiogenic effect exerted throughout time by the AAV-VEGF vector, we wanted to assess its therapeutic potential to improve skin flap survival. Two different in vivo flap models in the rat were developed, the first one involving only the epidermis and dermis (cutaneous), while the second one also included a muscular layer (musculocutaneous). A total number of 48 animals were involved in a first set of experiments, aimed at exploring the optimal timing of AAV vector delivery; half of the animals were treated with the cutaneous flap and half with the musculocutaneous flap. In both cases, a rectangular skin paddle was raised on the epigastrium, an area having a predictable axial vascular system consisting of the lateral thoracic artery (bilaterally), of the inferior superficial epigastric artery (bilaterally), and of four musculocutaneous perforator branches, arising from the rectus abdominis on both sides (Figure 1A).
Figure 1.
Schematic representation of the skin flaps and their vascular components, with the indication of the vector injection sites. A: The surgical models of skin flap used in this study are based on a rectangular skin paddle measuring 5 × 8 cm, drawn on the abdomen of the animals. The predictable vascular system of the flap is composed of the lateral thoracic arteries, the inferior epigastric arteries, and the musculocutaneous perforator arteries arising from the rectus abdominis (usually four vessels on each side). B and C: The pictures schematically show the vascular component providing the blood supply to each flap and the injection sites. In particular, the skin flap (B) was raised from the fascia on the inferior epigastric artery, and the vector injected at 10 equally spaced subcutaneous sites along the midline (inset in the top left part). The TRAM flap (C) was raised on a plane between the panniculus carnosus and the abdominal fascia, with the rectus abdominis as the only source of blood supply through the perforator arteries; in this model, the vector was administered by intramuscular injection in the region where each perforator artery arises from the rectus sheet (inset in the top left part).
In the animals treated with the cutaneous flap (n = 24), a skin flap was raised from the muscular fascia, on the inferior epigastric artery (epigastric flap); a solution containing the AAV vectors was injected at 10 equally spaced subcutaneous sites along the midline of the flap (Figure 1B). In the other group of animals (n = 24) a musculocutaneous flap was obtained by raising the entire left part of the skin paddle on a plane between the panniculus carnosus (which is equivalent to the subcutaneous fat in humans) and the abdominal fascia, while on the right side the medial portion of the flap was left attached to the anterior rectus sheet (transverse rectus abdominis musculocutaneous, TRAM, flap). In this way, the rectus abdominis represented the muscular component of the flap, providing the blood supply to the cutaneous component through the perforator arteries. In the TRAM-treated animals, the viral vector preparations were injected intramuscularly in the same region where each perforator artery arises from the rectus abdominis (Figure 1C).
As described in Table 1, each group of animals was further divided into six subgroups (12 in total; n = 4 per group), according to the vector they received (either AAV-LacZ or AAV-VEGF) and the time of vector administration (0, 7, or 14 day before surgery). The overall results of these experiments are presented in Figure 2, A and B, for the epigastric flap and Figure 2, C and D, for the TRAM flap; these figures show the extent of flap necrosis at day 7 after surgery. In both flap models, a notable beneficial effect of AAV-VEGF injection on flap survival was observed. The flaps treated with AAV-LacZ predictably underwent necrosis in their distal portion, similar to the untreated flaps, in which the extent of the necrotic area reached 37.4 ± 1.8% and 24.4 ± 2.3% of the total flap surface for the cutaneous and the TRAM flaps, respectively (data not shown). As shown in Figure 2B, the treatment with AAV-VEGF significantly reduced epigastric flap necrosis in all of the three paired experimental groups (P < 0.01 by two-way analysis of variance). The reduction in the necrotic area was 23.0% when the vector was injected during surgery, and 40.0% and 41.7% when injected 7 or 14 days before surgery, respectively. Similar results were also obtained for the TRAM model (P < 0.01), in which the reduction of flap necrosis in the VEGF-treated animals versus LacZ controls was not evident when the vector was injected during surgery, and it was 38.1% and 50.0% when AAV-VEGF was injected at 7 or 14 days before surgery, respectively (Figure 2D).
Taken together, these results indicate that the injection of AAV-VEGF significantly increases flap survival. In particular, posthoc statistical analysis in the TRAM flap model indicated that statistical significance was achieved when the vector was administered at 14 days before surgery (P < 0.05). Representative pictures of flaps treated with AAV-LacZ or AAV-VEGF 14 days before surgery are shown in Figure 2, A and C, for the epigastric and TRAM flap models, respectively.
AAV-VEGF Significantly Improves Flap Survival, Vascularization, and Healing in the TRAM Model
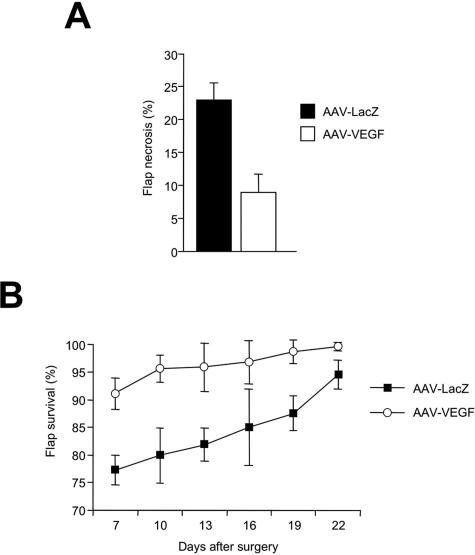
According to the results obtained by this first set of in vivo experiments, a larger number or animals was used for a subsequent experiment in which the efficacy of AAV-VEGF delivery was assessed in the TRAM flap model, followed by histological and immunohistochemical evaluation of the injected tissues. Rats received either AAV-LacZ or AAV-VEGF (n = 20 per group) 14 days before surgery, followed by the measurement of the necrotic area at day 7 after surgery. The results of this experiment are shown in Figure 3A. The percentage of the necrotic area was 22.8 ± 2.7% and 8.9 ± 2.8% in the animals treated with AAV-LacZ and AAV-VEGF, respectively, resulting in almost 60% improvement in flap viability (P < 0.01).
Figure 3.
Pretreatment with AAV-VEGF significantly improves TRAM flap survival and healing. A: The histogram shows the mean and SD of percent flap necrosis measured in 40 rats (20 per group) that were treated with the TRAM flap and injected with either AAV-LacZ or AAV-VEGF at day 14 before flap elevation. Measurements were performed at day 7 after surgery. B: The healing process was monitored throughout time after surgery up to 22 days, by measuring the extent of flap survival in animals treated with AAV-LacZ or AAV-VEGF (n = 8 per group). The means and SD of survived flap areas are shown at each time point (expressed as a percentage of the total flap area).
Flap healing was further assessed for an additional 15 days by measuring the extension of the viable area of the flaps (n = 8 for each group). As shown in Figure 3B, treatment with AAV-VEGF determined a marked improvement in the healing process, resulting in >95% flap viability as early as 10 days after surgery and in the almost complete disappearance of the necrotic area at day 22. In contrast, healing was remarkably delayed in the rats treated with AAV-LacZ (P < 0.01 by one-way analysis of variance).
To determine whether the improved tissue viability was actually due to a sustained angiogenic response induced by VEGF, representative sections from flap biopsies harvested at day 7 after surgery were examined by histology and immunohistochemistry. All flaps were designed to have three zones (indicated as a, b, and c in Figure 4A), according to their distance from the vascular pedicle: a survived zone (a), from which we took sample A, corresponding to the injection site; an intermediate zone (b), from which we took sample B at ∼3 cm from sample A, a region corresponding to the border between viable and necrotic skin; and a necrotic zone (c). Marked differences were observed by histological analysis between AAV-VEGF- and AAV-LacZ-treated animals (Figure 4B). In sample A, the animals injected with AAV-VEGF showed massive cellular infiltration close to the injection site. This finding is consistent with our previous study, in which we injected the same AAV-VEGF vector in the normoperfused skeletal muscle, and observed the recruitment of a large number of proliferating cells in close proximity to the newly formed blood vessels, suggesting an important role of these cells in VEGF-induced neoangiogenesis.30 In sample B, a remarkable difference was also observed in terms of tissue viability. In the control, AAV-LacZ-treated animals, the epithelial layer was thin and immature, with evidence of acute inflammation, adipose substitution, and myonecrosis (as shown by the almost complete disappearance of the panniculus carnosus); the infiltrating inflammatory cells, mostly monocytes and neutrophils, were dispersed throughout the skin layers, concomitant with a severe disruption of the tissue architecture. In contrast, the morphology was preserved in the AAV-VEGF-treated flaps, as revealed by the presence of an intact epithelial layer and of skin appendages, with only mild and focal inflammation, and poor accumulation of adipose tissue.
To quantify the main differences between the two experimental groups observed by histological examination, three independent researchers blindly assigned a quantitative score for several parameters, including epidermal integrity, inflammation, adipose substitution, and tissue architecture, according to the criteria reported in Table 2. The overall scores obtained from all of the analyzed parameters clearly indicated a better viability of AAV-VEGF-treated flaps, with preservation of tissue architecture very similar to that of the normal skin.
Table 2.
Evaluation of Control Flaps and Flaps Treated with Either AAV-VEGF or AAV-LacZ Using a Quantitative Score Assessing the Indicated Histological Parameters
| Histological score (sample B) | Normal skin | AAV-LacZ | AAV-VEGF |
|---|---|---|---|
| Epidermal integrity (mean number of discontinuities per ×40 microscopic field) 3 = ≤1, 2 = 2 to 4, 1 = ≥5 | 3.0 | 1.2 | 2.9 |
| Skin appendages (mean number of hair follicles, sebaceous glands, and sweat glands per ×40 microscopic field) 3 = ≥15, 2 = 14 to 4, 1 = ≤5 | 2.8 | 1.1 | 1.9 |
| Inflammation (mean cross-sectional area infiltrated by leukocytes per ×40 microscopic field) 3 = ≤10%, 2 = 11 to 49%, 1 = ≥50% | 3.0 | 1.2 | 2.2 |
| Adipose substitution (mean cross-sectional area infiltrated by adipocytes per ×40 microscopic field) 3 = ≤10%, 2 = 11 to 49%, 1 = ≥50% | 3.0 | 1.8 | 2.8 |
| Tissue architecture 3 = preserved, 2 = moderate structural changes, 1 = grossly altered | 3.0 | 1.0 | 2.1 |
| Total score | 14.8 | 6.3 | 11.9 |
Finally, in the same groups of animals we also assessed the effect of vector transduction on the extent of new blood vessel formation. By using an antibody specific for the endothelial marker CD31, we could detect a massive formation of new capillaries (shown in Figure 5 at different magnifications for one selected animal), both in the proximity of the injection site (sample A) and in the skin of the more distal intermediate zone (sample B). This was most apparent deep to the panniculus carnosus layer, as shown by the insets, resulting in a more than twofold increase in the number of capillaries detectable in the dermal sheet (P < 0.05) (Figure 5B).
Figure 5.
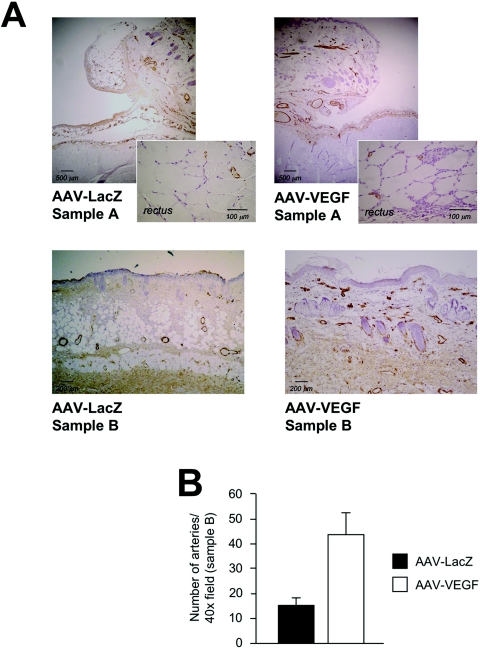
VEGF induces endothelial cell proliferation and angiogenesis. A: The presence of endothelial cells in control (left) and VEGF-treated (right) flaps was detected by immunohistochemistry using an anti-CD31 antibody. AAV-VEGF induced the proliferation of endothelial cells at the injection site (sample A), in which several CD31-positive cells infiltrated the interstitial spaces between the fibers of the rectus abdominis muscle (inset on the right), as well as in the more distal sample B. This endothelial cell proliferation was paralleled by the formation of a great number of new capillaries, most evident at the level of the panniculus carnosus (p.c.), as shown in the bottom panels at a higher magnification. B: Quantification of capillaries in treated flaps. Counts were obtained from samples B of both AAV-LacZ- and AAV-VEGF-treated flaps. Shown are the means and SD of the counts, expressed as number of capillaries per ×40 field, assuming statistical significance at P < 0.05 using a two-tailed t-test.
Even more evident were the results obtained by immunostaining the same samples with an antibody that specifically recognizes the smooth muscle-specific isoform of α-actin (α-SMA), and thus highlights the presence of arterial vessels. As shown in Figure 6A an increase in the number of arteriolae was detected at the level of the injection site (sample A) and, more evidently, in the dermis of the intermediate zone (sample B) in the AAV-VEGF-treated animals (more than threefold increase in the latter area; P < 0.05; quantified in the histogram shown in Figure 6B). Several of these arterial vessels, with a diameter in the 20- to 120-μm range, were filled with erythrocytes (not shown), indicating their functional connection to the systemic circulation.
Figure 6.
VEGF induces the formation of α-SMA-positive arteriolae. A: The property of VEGF to sustain the formation of arterial vessels was assessed by immunohistochemistry using an antibody against the α-actin isoform specific for the smooth muscle cells (α-SMA). A modest effect was detected in sample A, with a greater reactivity in the AAV-VEGF-injected muscle (inset on the right), whereas a remarkable increase in the number of arteriolae was observed after VEGF treatment in the more distal sample B (bottom), approximately corresponding to the border between viable and necrotic skin. B: Quantification of α-SMA-positive vessels in samples B. Presentation of the data and statistics are as in Figure 5B.
Discussion
The use of biological agents to promote new blood vessel formation represents an exciting field of research and has recently aroused considerable interest as an innovative approach to treat tissue ischemia in a wide variety of clinical settings. To date, most of the studies have concerned a possible application of therapeutic angiogenesis for the treatment of cardiovascular disorders, such as myocardial infarction and lower limb ischemia. Nevertheless, the same strategy might result of equal interest in many plastic surgery techniques, in which ischemia is often a contributing cause of surgical failure. Tissue necrosis at the distal part of the flaps is generally attributed to deficient blood perfusion, and thus should benefit from the induction of therapeutic angiogenesis. Among the number of molecules involved in the angiogenic process, VEGF has been shown to play a central role during the early phases of angiogenesis, promoting endothelial cell migration and proliferation, and preventing their apoptosis.32
Several studies have already provided the proof of concept that the administration of exogenous recombinant VEGF enhances the survival of the distal portion of the flap to a certain extent.3–9 These studies, however, also highlighted an important limitation of such an approach, mainly related to the short half-life of recombinant proteins in vivo. In contrast, gene transfer can maintain the production and secretion of the growth factors for prolonged periods, thus sustaining the proangiogenic stimulus throughout time.
Among the vectors currently considered for gene transfer in plastic surgery, those based on adenovirus can mediate high levels of protein expression, but are associated with the disadvantage of producing strong inflammatory and immune reactions, which, on one side, can further compromise tissue viability while, on the other side, lead to the early loss of transgene expression.33 The drawbacks of adenoviral vectors have already emerged in a recent study, which evaluated the effect of VEGF gene transfer on the survival of epigastric skin flaps.13
In contrast, a constantly increasing number of preclinical and clinical gene therapy studies exploit vectors based on AAV. We and others have previously demonstrated the exquisite and still unexplained tropism of AAV for muscle cells,29–31 which suggested that AAV might be the ideal vector to target the muscular component of the musculocutaneous flaps. Starting from these considerations, our study was initially designed as a preliminary control trial to identify the optimal site and time for vector administration. Our results show that the greatest improvement in flap survival can be obtained after intramuscular injection of the vector 14 days before surgery, further strengthening the concept of AAV as an ideal vector to transduce muscle cells for long periods of time. Expression of VEGF significantly improved flap survival, with a macroscopically evident reduction of the necrotic portion, as well as with a significant improvement in tissue viability at histological examination. The beneficial effect of AAV-VEGF on flap survival well correlated with an impressive formation of new blood vessels—both capillaries and arteriolae. At least for the TRAM flap, the clear improvement in tissue viability might be ascribed to a better perfusion through the muscular layer due to a local angiogenic effect of VEGF. Alternatively, secreted VEGF might have diffused from the muscle to the skin layer of the more distal portion of the flap, thus promoting angiogenesis within the derma, in agreement with a series of recent reports showing that AAV vectors delivered to the skeletal muscle are able to drive the expression and secretion of different molecules into the circulation.24,28 The increase in the density of arteriolae in the skin distal to the injection site (sample B) in the TRAM model is strongly in favor of the second hypothesis, although we cannot exclude an indirect effect of VEGF through an enhancement of the blood supply.
In accordance with the marked improvement in flap viability after AAV-VEGF transduction, our time course experiment clearly indicates a persistent therapeutic effect of the vector up to 3 weeks after surgery. Although the extent of flap necrosis is usually assessed at no longer that 7 days after flap elevation, reflecting the timing by which the problem appears to be clinically relevant, an increased vascularization driven by VEGF might provide an additional benefit in the following healing process. Our results strongly support this hypothesis, and reinforce the notion that a delivery system able to sustain a prolonged expression of the therapeutic gene is extremely desirable for this kind of therapeutic application.
The observation that the administration of AAV-VEGF is particularly effective when performed preoperatively also supports the usefulness of AAV-mediated therapeutic angiogenesis in elective surgery conditions. Considering that the problem of flap breakdown is especially prevalent in patients who have diabetes, obesity, and peripheral vascular disease,34 as well as in smokers,35 it seems reasonable that AAV-VEGF administration during the preoperative phase might represent a useful therapeutic method to enhance blood flow in skin areas undergoing elevation and transposition in high-risk patients. In terms of clinical applications, the observation that the efficacy of AAV-mediated gene transfer is more evident in the TRAM model rather than in the epigastric flap model, renders AAV-VEGF gene transfer even more attractive. In fact, the results obtained from the epigastric flap model, in which the panniculus carnosus (present in rodents but not in humans) represents the major target of AAV vector transduction, cannot be directly extrapolated to the human skin. In contrast, the TRAM flap, including a muscular component in both species, is much more representative of the human condition.
Taken together, our results reinforce the notion that adequate blood supply is an essential requisite for flap survival, and indicate the feasibility of an angiogenic gene therapy approach in plastic and reconstructive surgery. In addition, considering the prolonged gene expression driven by AAV vectors, this delivery system might become a novel and powerful experimental tool to investigate the biological role of other relevant growth factors, as well as their combinations, to find new therapeutic approaches for the treatment of tissue ischemia in plastic surgery. Few recent studies have reported about the angiogenic potential of other factors, such as angiopoietin-1,36–38 platelet-derived growth factor,39 and fibroblast growth factor,40–42 in different animal models of skin flaps. Because AAV vectors enter the cells at high multiplicity of infection, an attractive approach could be the simultaneous administration of different vector preparations, expressing different proteins involved in the angiogenic process.30
Although it is the myocardium and the muscles of the lower limb that have been the intended sites of angiogenesis in the majority of studies to date, there is optimism that plastic and reconstructive surgery and its patients could be major beneficiaries of the knowledge currently being accumulated. The various types of flaps, from local skin flaps to complex free flaps could be helped by reinforcement of the natural angiogenic process occurring in the microvasculature. One of the major potential applications of a therapeutic angiogenesis approach using gene therapy will be the design of larger flaps than would otherwise be possible, able to cover tissue defects previously considered too large or complex.
Acknowledgments
We thank Marina Dapas and Maria Elena Lopez for excellent technical support in AAV vector production, Maria Cristina Prati and Matteo Dell’Omodarme for statistical consultancy, and Suzanne Kerbavcic for editorial assistance.
Footnotes
Address reprint requests to Mauro Giacca, M.D. Ph.D., Director, ICGEB Trieste, Padriciano, 99, 34012 Trieste, Italy. E-mail: giacca@icgeb.org.
Supported by grants from the Progetto Finalizzato “Genetica Molecolare” of the “Consiglio Nazionale delle Ricerche,” Italy; the Fondo Integrato per la Ricerca di Base program of the “Ministero dell’Istruzione, Universita’ e Ricerca,” Italy; the “Fondazione Cassa di Risparmio” of Trieste, Italy; and the “Fondo Trieste,” Italy.
S.Z. and G.P. contributed equally to this work.
References
- Hallock GG. Physiological studies using laser Doppler flowmetry to compare blood flow to the zones of the free TRAM flap. Ann Plast Surg. 2001;47:229–233. doi: 10.1097/00000637-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Machens HG, Salehi J, Weich H, Munch S, Siemers F, Krapohl BD, Herter KH, Kruger S, Reichert B, Berger A, Vogt P, Mailander P. Angiogenic effects of injected VEGF165 and sVEGFR-1 (sFLT-1) in a rat flap model. J Surg Res. 2003;111:136–142. doi: 10.1016/s0022-4804(03)00084-2. [DOI] [PubMed] [Google Scholar]
- Padubidri A, Browne E., Jr Effect of vascular endothelial growth factor (VEGF) on survival of random extension of axial pattern skin flaps in the rat. Ann Plast Surg. 1996;37:604–611. doi: 10.1097/00000637-199612000-00006. [DOI] [PubMed] [Google Scholar]
- Li QF, Reis ED, Zhang WX, Silver L, Fallon JT, Weinberg H. Accelerated flap prefabrication with vascular endothelial growth factor. J Reconstr Microsurg. 2000;16:45–49. doi: 10.1055/s-2000-7540. [DOI] [PubMed] [Google Scholar]
- Kryger Z, Dogan T, Zhang F, Komorowska-Timek E, Shi DY, Cheng C, Lineaweaver WC, Buncke HJ. Effects of VEGF administration following ischemia on survival of the gracilis muscle flap in the rat. Ann Plast Surg. 1999;43:172–178. [PubMed] [Google Scholar]
- Kryger Z, Zhang F, Dogan T, Cheng C, Lineaweaver WC, Buncke HJ. The effects of VEGF on survival of a random flap in the rat: examination of various routes of administration. Br J Plast Surg. 2000;53:234–239. doi: 10.1054/bjps.1999.3315. [DOI] [PubMed] [Google Scholar]
- Banbury J, Siemionow M, Porvasnik S, Petras S, Browne E. Improved perfusion after subcritical ischemia in muscle flaps treated with vascular endothelial growth factor. Plast Reconstr Surg. 2000;106:1541–1546. doi: 10.1097/00006534-200012000-00015. [DOI] [PubMed] [Google Scholar]
- Zhang F, Fischer K, Komorowska-Timek E, Guo M, Cui D, Dorsett-Martin W, Buncke HJ, Lineaweaver WC. Improvement of skin paddle survival by application of vascular endothelial growth factor in a rat TRAM flap model. Ann Plast Surg. 2001;46:314–319. doi: 10.1097/00000637-200103000-00019. [DOI] [PubMed] [Google Scholar]
- Zhang F, Richards L, Angel MF, Zhang J, Liu H, Dorsett-Martin W, Lineaweaver WC. Accelerating flap maturation by vascular endothelium growth factor in a rat tube flap model. Br J Plast Surg. 2002;55:59–63. doi: 10.1054/bjps.2001.3704. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Spratt SK, Lagarde C, Bohl D, Kaspar B, Sloan B, Cohen LK, Danos O. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]
- Greenhalgh DA, Rothnagel JA, Roop DR. Epidermis: an attractive target tissue for gene therapy. J Invest Dermatol. 1994;103:63S–69S. doi: 10.1111/1523-1747.ep12399070. [DOI] [PubMed] [Google Scholar]
- Cui L, Li FC, Zhang Q, Qian YL, Guan WX. Effect of adenovirus-mediated gene transfection of vascular endothelial growth factor on survival of random flaps in rats. Chin J Traumatol. 2003;6:199–204. [PubMed] [Google Scholar]
- Gurunluoglu R, Ozer K, Skugor B, Lubiatowski P, Carnevale K, Siemionow M. Effect of transfection time on the survival of epigastric skin flaps pretreated with adenovirus encoding the VEGF gene. Ann Plast Surg. 2002;49:161–169. doi: 10.1097/00000637-200208000-00009. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Taichman LB. Virus-mediated gene transfer for cutaneous gene therapy. Hum Gene Ther. 2000;11:2247–2251. doi: 10.1089/104303400750035771. [DOI] [PubMed] [Google Scholar]
- O’Toole G, MacKenzie D, Lindeman R, Buckley MF, Marucci D, McCarthy N, Poole M. Vascular endothelial growth factor gene therapy in ischaemic rat skin flaps. Br J Plast Surg. 2002;55:55–58. doi: 10.1054/bjps.2001.3741. [DOI] [PubMed] [Google Scholar]
- Neumeister MW, Song YH, Mowlavi A, Suchy H, Mathur A. Effects of liposome-mediated gene transfer of VEGF in ischemic rat gracilis muscle. Microsurgery. 2001;21:58–62. doi: 10.1002/micr.1010. [DOI] [PubMed] [Google Scholar]
- Lubiatowski P, Goldman CK, Gurunluoglu R, Carnevale K, Siemionow M. Enhancement of epigastric skin flap survival by adenovirus-mediated VEGF gene therapy. Plast Reconstr Surg. 2002;109:1986–1993. doi: 10.1097/00006534-200205000-00031. [DOI] [PubMed] [Google Scholar]
- Liu PY, Tong W, Liu K, Han SH, Wang XT, Badiavas E, Rieger-Christ K, Summerhayes I. Liposome-mediated transfer of vascular endothelial growth factor cDNA augments survival of random-pattern skin flaps in the rat. Wound Repair Regen. 2004;12:80–85. doi: 10.1111/j.1067-1927.2004.012114.x. [DOI] [PubMed] [Google Scholar]
- Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci USA. 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O’Malley KL, During MJ. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- Nakai H, Herzog RW, Hagstrom JN, Walter J, Kung SH, Yang EY, Tai SJ, Iwaki Y, Kurtzman GJ, Fisher KJ, Colosi P, Couto LB, High KA. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver. Blood. 1998;91:4600–4607. [PubMed] [Google Scholar]
- Xiao W, Berta SC, Lu MM, Moscioni AD, Tazelaar J, Wilson JM. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998;72:10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Monahan PE, Samulski RJ. AAV vectors: is clinical success on the horizon? Gene Ther. 2000;7:24–30. doi: 10.1038/sj.gt.3301109. [DOI] [PubMed] [Google Scholar]
- Descamps V, Blumenfeld N, Beuzard Y, Perricaudet M. Keratinocytes as a target for gene therapy. Sustained production of erythropoietin in mice by human keratinocytes transduced with an adenoassociated virus vector. Arch Dermatol. 1996;132:1207–1211. doi: 10.1001/archderm.132.10.1207. [DOI] [PubMed] [Google Scholar]
- Hengge UR, Mirmohammadsadegh A. Adeno-associated virus expresses transgenes in hair follicles and epidermis. Mol Ther. 2000;2:188–194. doi: 10.1006/mthe.2000.0118. [DOI] [PubMed] [Google Scholar]
- Donahue BA, McArthur JG, Spratt SK, Bohl D, Lagarde C, Sanchez L, Kaspar BA, Sloan BA, Lee YL, Danos O, Snyder RO. Selective uptake and sustained expression of AAV vectors following subcutaneous delivery. J Gene Med. 1999;1:31–42. doi: 10.1002/(SICI)1521-2254(199901/02)1:1<31::AID-JGM3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Deodato B, Arsic N, Zentilin L, Galeano M, Santoro D, Torre V, Altavilla D, Valdembri D, Bussolino F, Squadrito F, Giacca M. Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther. 2002;9:777–785. doi: 10.1038/sj.gt.3301697. [DOI] [PubMed] [Google Scholar]
- Arsic N, Zentilin L, Zacchigna S, Santoro D, Stanta G, Salvi A, Sinagra G, Giacca M. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol Ther. 2003;7:450–459. doi: 10.1016/s1525-0016(03)00034-0. [DOI] [PubMed] [Google Scholar]
- Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10:844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- Yang Y, Haecker SE, Su Q, Wilson JM. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- Hultman CS, Daiza S. Skin-sparing mastectomy flap complications after breast reconstruction: review of incidence, management, and outcome. Ann Plast Surg. 2003;50:249–255. doi: 10.1097/01.sap.0000046784.70583.e1. [DOI] [PubMed] [Google Scholar]
- Craig S, Rees TD. The effects of smoking on experimental skin flaps in hamsters. Plast Reconstr Surg. 1985;75:842–846. doi: 10.1097/00006534-198506000-00015. [DOI] [PubMed] [Google Scholar]
- Gurunluoglu R, Lubiatowski P, Goldman CK, Carnevale K, Siemionow M. Enhancement of muscle flap hemodynamics by angiopoietin-1. Ann Plast Surg. 2002;48:401–409. doi: 10.1097/00000637-200204000-00011. [DOI] [PubMed] [Google Scholar]
- Lubiatowski P, Gurunluoglu R, Goldman CK, Skugor B, Carnevale K, Siemionow M. Gene therapy by adenovirus-mediated vascular endothelial growth factor and angiopoietin-1 promotes perfusion of muscle flaps. Plast Reconstr Surg. 2002;110:149–159. doi: 10.1097/00006534-200207000-00026. [DOI] [PubMed] [Google Scholar]
- Jung H, Gurunluoglu R, Scharpf J, Siemionow M. Adenovirus-mediated angiopoietin-1 gene therapy enhances skin flap survival. Microsurgery. 2003;23:374–380. doi: 10.1002/micr.10140. [DOI] [PubMed] [Google Scholar]
- Carroll CM, Carroll SM, Schuschke DA, Barker JH. Augmentation of skeletal muscle flap survival using platelet derived growth factor. Plast Reconstr Surg. 1998;102:407–415. doi: 10.1097/00006534-199808000-00018. [DOI] [PubMed] [Google Scholar]
- Rashid MA, Akita S, Razzaque MS, Yoshimoto H, Ishihara H, Fujii T, Tanaka K, Taguchi T. Coadministration of basic fibroblast growth factor and sucrose octasulfate (sucralfate) facilitates the rat dorsal flap survival and viability. Plast Reconstr Surg. 1999;103:941–948. doi: 10.1097/00006534-199903000-00026. [DOI] [PubMed] [Google Scholar]
- Khouri RK, Brown DM, Leal-Khouri SM, Tark KC, Shaw WW. The effect of basic fibroblast growth factor on the neovascularisation process: skin flap survival and staged flap transfers. Br J Plast Surg. 1991;44:585–588. doi: 10.1016/0007-1226(91)90094-z. [DOI] [PubMed] [Google Scholar]
- Bayati S, Russell RC, Roth AC. Stimulation of angiogenesis to improve the viability of prefabricated flaps. Plast Reconstr Surg. 1998;101:1290–1295. doi: 10.1097/00006534-199804050-00020. [DOI] [PubMed] [Google Scholar]