Abstract
Sepsis is associated with bacterial translocation (BT) and changes in colonic paracellular permeability (CPP), but the link between these effects is unknown. The present study aimed to identify whether changes in CPP after lipopolysaccharide (LPS) administration triggers BT, colonic inflammation, visceral pain, and sickness behavior and to evaluate the role of myosin light chain kinase (MLCK) in colonocyte cytoskeleton contraction. Rats received the MLCK inhibitor ML-7 alone or combined with LPS. CPP was measured for 6 hours after administration. Visceral pain, food intake, BT, electron microscopy of tight junctions of colonocytes, cytokine levels, and Western blotting of phosphorylated MLC from colonic mucosa were assessed in a time range of 0 to 3 hours after treatment. Sepsis increased CPP at 0 to 6 hours after LPS and associated with tight junction morphological changes, increased MLC phosphorylation, and mucosal release of proinflammatory cytokines. Massive BT, visceral hyperalgesia, and reduced food intake were also observed. Addition of ML-7 prevented all LPS-induced effects, except for changes in food intake. In conclusion, LPS-mediated effects on CPP include gut inflammation, BT, and visceral hyperalgesia. Inhibition of MLCK-dependent colonocyte cytoskeleton contraction by ML-7 prevents the LPS-induced alterations of CPP and its subsequent effects.
The gastrointestinal mucosal barrier plays a pivotal role in the body’s protection against luminal pathogens and antigenic molecules. The impairment of the gastrointestinal barrier function with massive bacterial translocation (BT) occurs during sepsis, both in human and in animal models.1–5 In these circumstances, bacteria and their lysis products, such as lipopolysaccharide (LPS), gain access to portal and systemic circulations, producing critical deleterious effects. Sepsis is the most common cause of death for hospitalized patients.6
The gastrointestinal barrier includes secreted mucus and the epithelial cell layer itself, which prevent BT of intestinal flora through both transcellular and paracellular pathways.7 Tight junction (TJ) opening is the major limiting factor of the paracellular pathway. Examination of isolated human and rodent small intestine cells and cell lines have shown that TJ opening is driven by myosin light chain (MLC) phosphorylation, which depends on myosin light chain kinase (MLCK) activation.8,9 MLC phosphorylation occurs within the perijunctional actomyosin ring and co-localizes with the TJ.9 Studies using an inducible active MLCK have shown that this activity is sufficient to activate the downstream events necessary for TJ regulation.10
Moreover MLCK-mediated regulation of TJ opening appears to be a common intermediate in a variety of physiological and pathophysiological pathways related to altered paracellular permeability both in vitro and in vivo.10–12 For example, enteropathogenic Escherichia coli infection causes diarrhea in children. An in vitro model has demonstrated that enteropathogenic E. coli dramatically increases TJ permeability, accompanied by TJ opening, reorganization of the actin cytoskeleton, redistribution of TJ proteins, and increased MLC phosphorylation, all of which can be reversed by inhibition of MLCK.13,14 These data suggest that enteropathogenic E. coli utilizes an intrinsic pathway of MLCK-mediated TJ regulation to alter barrier function.
Increased epithelial permeability is caused not only by exogenous factors such as infection but also by the local immune system, which plays an important role in modulating intestinal permeability. Indeed, cytokines such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-6 have also been found to decrease barrier function of cultured intestinal epithelial cell monolayers.13,15–18 Incubation of such cultures with both TNF-α and IFN-γ leads to reorganization of many TJ proteins, including zonula occludens 1, occludin, and claudin-1.15 The resulting changes in paracellular permeability are associated with marked increases in MLC phosphorylation and can be reversed using a specific membrane permeant inhibitor of MLCK, indicating that TNF-α and IFN-γ also use the MLCK-driven pathway to increase TJ permeability.13
Furthermore, in in vivo rodent models, data show the pivotal role of cytoskeleton contractions, through MLC phosphorylation, in the alterations of colonic paracellular permeability (CPP) consecutive to a stress session19 or inflammation induction.20 The prevention of stress-induced increase of CPP, using a MLCK inhibitor, reduces stress-induced visceral hypersensitivity.21 However, in vivo studies have not investigated the mechanisms at the origin of the increased CPP in sepsis or whether preventing epithelial barrier dysfunction may reduce the symptoms currently reported for this pathophysiological situation, namely fever,22 altered feeding behavior,23 and visceral hypersensitivity.24
Consequently, the purpose of the present study was to evaluate the changes of CPP associated with E. coli LPS-induced sepsis in rats. We then investigated the specific role of myosin light chain kinase (MLCK) in colonocytes and the corresponding alteration of TJ. Finally, we measured the consequences of CPP changes on classical endpoints characterizing sepsis, such as BT, feeding behavior, and visceral sensitivity.
Materials and Methods
Animals and Surgery
This study was performed on male Wistar rats weighing 200 to 225 g (Janvier, S.A., Le Genest St. Isle, France). Rats were individually housed in polypropylene cages in a light- (12/12 hours cycle) and temperature-controlled room (20°C ± 2°C). They were allowed free access to water and food (UAR pellets; UAR, Epinay-sur-orge, France). Experimental animal procedures were approved by the local animal care and use committee of Institut National de la Recherche Agronomique.
Rats were anesthetized by intraperitoneal injection of acepromazine (Calmivet; Vetoquinol, Lure, France) and ketamine (Imalgene 1000; Rhône-Mérieux, Lyon, France) at 0.6 and 120 mg/kg, respectively. An intracolonic polyethylene catheter (40 cm length, 0.3 mm diameter) was implanted into the proximal colon (1 cm from the cecocolonic junction), attached to the abdominal muscle wall, and exteriorized at the back of the neck. Rats were also surgically fitted with three groups of three NiCr electrodes (60 cm length, 0.8 mm diameter) for recording abdominal striated muscle contractions. Electrodes were exteriorized on the back of the neck and protected by a glass tube attached to the skin.
CPP Measurement
Assessment of CPP to large molecules was performed by intracolonic injection of 51Cr-ethylendiaminetetraacetic acid (51Cr-EDTA; Perkin Elmer Life Science, Paris, France), a selective marker of CPP. 51Cr-EDTA (0.7 μCi) was diluted in 250 μl of sterile NaCl 0.9% and was injected intracolonically through the catheter. Animals were placed in metabolic cages and urine was collected. Total radioactivity found in urine was measured with a γ-counter (Cobra II; Packard, Meriden, CT). Permeability to 51Cr-EDTA was expressed as the percentage of the total radioactivity collected in the urine.
Bacterial Translocation
To evaluate BT, bacteria cultures were performed according to previously described techniques.25 Briefly, after animal sacrifice, abdomens were shaved, cleaned with iodine tincture, and opened via a midline incision with sterile scissors. Under aseptic conditions, mesenteric lymph nodes, spleen, and liver were removed and weighed. Each organ was homogenized, and serial dilutions of aliquots were plated onto blood agar to count total anaerobic bacteria. Duplicate cultures were made for each specimen. All specimens were incubated at 37°C for 24 to 48 hours. Cultures were considered positive when more then 100 colonies per g of tissue were found. Results of BT experiments were expressed as number of positive organs with translocation.
Electron Microscopy of Tight Junction
The method used to measure the frequency of junctional dilatations was adapted from Soderholm and colleagues.26 Briefly, segments of proximal colon (2 cm) were collected at 1 cm from the cecocolonic junction, fixed in 2.5% glutaraldehyde-2% paraformaldehyde solution (2 hours; +4°C), rinsed in 0.1 mol/L cacodylate buffer, and postfixed for 1 hour at +4°C in 1% osmium tetroxide. Subsequently, tissue samples were dehydrated in graded ethanol and embedded in Epon-Araldite resin. Ultra-thin sections (70 nm) were obtained with an ultramicrotome system (Reichert OMU2) and collected on copper/palladium grids after staining with 4% uranyl acetate and 0.4% lead citrate. To evaluate changes in TJ morphology, the junctional regions of two randomly selected sections of longitudinally sectioned villi were examined for each specimen. All sectioned TJ on the top half of villi were assessed. Sections were examined with a Hitachi HU11C electron microscope. In colonic epithelial cells, open tight junctions (TJs) appeared as a dilatation or a gap in the apical space of the junctional complex. Examinations were performed on a total of ∼50 TJs per specimen. TJs were considered open when the size of dilatation was greater than 20 nm. Results were expressed as percentage of open TJs.
Proinflammatory Cytokine Assay in Colonic Mucosa
The cytokines IL-1β, IL-6, TNF-α, and IFN-γ were assayed in colonic mucosa with the Bio-Plex suspension array system (Bio-Rad, Marnes-la-Coquette, France). After rats were sacrificed, 2 cm of proximal colon were collected at 1 cm from the cecocolonic junction, washed with saline, and scraped to remove mucosa. Then, proteins were extracted with RIPA buffer. Protein concentration was assessed using the DC protein assay kit (Bio-Rad). Cytokine levels of supernatants were measured with Bio-Plex rat cytokine assays. Beads coated with capture antibodies (5000 beads per cytokine) were incubated with premixed standards or sample supernatants (50 μl) in 96-well filter plates. Plates were shaken for 30 seconds at 1000 rpm and then incubated at room temperature for 30 minutes at 300 rpm. After incubation, premixed detection antibodies (1 μg/ml) were added, and plates were shaken and incubated as before. After washing, streptavidin-phycoerythrin (2 μg/ml) was added to the wells, and the plates were incubated for 10 minutes at room temperature with shaking. After washing, beads were resuspended in 125 μl of Bio-Plex cytokine assay buffer and read by the Bio-Plex array reader. Data were analyzed with Bio-Plex Manager software version 2.0. Results were expressed as pg of cytokines per mg of protein.
Western Blotting of Phosphorylated MLC
Colonic mucosa (2 cm) was collected as described above, and proteins were extracted with RIPA buffer. Protein concentration was assessed using the DC protein assay kit (Bio-Rad). For phosphorylated MLC (pMLC) determination, equal amounts of each extract were electrophoresed in 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electrotransferred onto 0.2-mm nitrocellulose membrane. Membrane was blocked for 1 hour with Tris-buffered saline/milk 6% and then incubated for 1 hour at room temperature with primary antibody. We used a 1/1000 dilution for primary antibody anti-phospho-MLC (Santa Cruz Biotechnology, Santa Cruz, CA). After washing, peroxidase-labeled G-protein (Sigma) was added for 1 hour at room temperature. The membrane was developed by incubating for 1 to 5 minutes with SuperSignal Reagent (Pierce, Bezons, France). Band intensities were quantified by densitometry and expressed as mean area density using Quantify One 4.1.1 software. For total protein blots, mean area density was expressed relative to actin expression.
Electromyographic Recording
For rectal sensitivity assessment, rats were placed in plastic tunnels (6 cm diameter and 25 cm length) in which they could not move, escape, or turn around. The balloon used for distension was an arterial embolectomy catheter (Fogarty; Edwards Laboratories, Santa Ana, CA). Rectal distension was performed by insertion of the balloon into the rectum, up to 1 cm of the anus, with the catheter fixed at the tail with adhesive tape. The balloon was inflated progressively, by 0.4-ml steps from 0 to 1.2 ml, each step of inflation lasting 5 minutes. Abdominal striated muscle contractions were recorded with an electromyograph (Mini VIII; Alvar, Paris, France) using a short time constant (0.03 second), to remove low-frequency signals (<3 Hz), and a paper speed of 3.6 cm/minute. The number of spike bursts for each period of distension was used as an index of pain.27
Food Intake and Diarrhea Assessment
Two weeks before the beginning of tests, rats received food pellets only between 11:00 a.m. and 2:00 p.m. daily. Food intake was assessed by weighing the remaining food pellets after this 3-hour period. On the day of the experiment, rats received their treatments, and food intake was quantified as previously described. For measurement of diarrhea after LPS administration with or without ML-7 pretreatment, animals were placed in an individual cage without litter. The feces were collected every 30 minutes for 3 hours after LPS administration. The fresh weight of the feces was measured at 3 hours after LPS. For dehydration, the feces were placed at 100°C for 24 hours and the water content of the feces was determined and expressed as percentage of water for 100 g of fresh feces.
Experimental Protocol
In all experiments, LPS from E. coli was injected intraperitoneally at the dose of 1 mg/kg in 250 μl of sterile saline (NaCl 0.9%). This dose has been previously described to provoke sepsis in rats.24 ML-7 (1 mg/kg), a selective MLCK inhibitor, or its vehicle (NaCl 0.9%) was administered by intraperitoneal route (250 μl) 24, 12, and 1 hour before LPS injection. ML-7 was used at a dose previously described in two distinct rodent models to inhibit both stress- and PAR2-activating peptide-induced increase in CPP.19,20
In a first series of experiment, CPP was measured in four groups of 10 rats receiving NaCl 0.9% (LPS vehicle + ML-7 vehicle), LPS + NaCl 0.9%, NaCl 0.9% + ML-7, or LPS + ML-7. For all groups, the CPP to 51Cr-EDTA was measured 6 hours after treatment, which is the minimal duration required to collect sufficient urine for radioactivity counting. BT and electron microscopy of colonic epithelial cells were performed on four additional groups of six rats, as described above. Liver, spleen, and mesenteric lymph nodes were removed to evaluate BT in these organs. All rats were sacrificed 3 hours after treatment.
Cytokine concentrations in colonic mucosa were measured on six groups of five rats. First, two groups of rats were treated with LPS vehicle combined with ML-7 or its vehicle. Second, two other groups were treated with LPS combined with ML-7 or its vehicle, and sacrificed 1 hour after endotoxin injection. Another two groups were similarly treated but sacrificed 3 hours after LPS administration. Additional 18 groups of three rats were used for Western immunoblotting of phospho-myosin light chain (pMLC). First, six groups of rats were sacrificed respectively at 0, 0.5, 1, 2, 3, and 6 hours after LPS administration. Second, a similar kinetic study was conducted in six similar groups treated by ML-7 before LPS administration. Third, control pMLC levels were also determined in rats pretreated with ML-7 alone and sacrificed at 0, 0.5, 1, 2, 3, and 6 hours after treatment.
Two other groups of 10 rats were used for electromyographic recording of abdominal striated muscle contractions in response to rectal distension. Food intake was assessed on three groups of eight rats that received either LPS vehicle, LPS, or LPS + ML-7. LPS was injected 1.5 hours before feeding, corresponding to time 0 of the experiment. Lastly, diarrheic effect of LPS administration was measured on three groups of eight rats receiving LPS, vehicle + ML-7, or double-vehicle treatment. LPS administration corresponded to time 0 of experiment.
Chemicals
LPS (from E. coli, serotype 0111:B4; L-3024, lot no. 111K4046) was purchased from Sigma-Aldrich (St. Quentin Fallavier, France) and dissolved in sterile saline (NaCl 0.9%). ML-7 [1-(5-Iodonaphthalene-1-sulonyl)-1H-hexahydro-1, 4-diazepine, HCl] was obtained from Sigma-Aldrich and dissolved in saline.
Data and Statistical Analysis
Colonic permeability, colonic mucosal cytokine concentration, diarrhea assessment, food intake, and number of abdominal contractions were expressed as means ± SEM, and data were compared by analysis of variance followed by a Tukey test. Electron microscopy results were expressed as the percentage of open TJs ± SEM, and values were compared using Student’s t-test. Differences were considered significant for P < 0.05.
Results
CPP Measurement
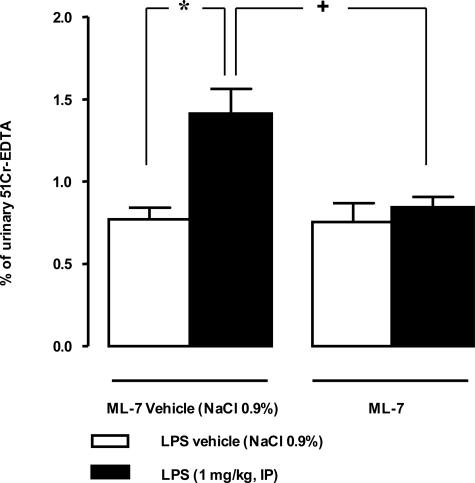
Compared with vehicle treatment, LPS significantly increased the percentage of 51Cr-EDTA crossing the epithelial barrier from 0.77% ± 0.07% to 1.42% ± 0.14% during 6 hours after its administration (Figure 1). Prior intraperitoneal treatment with the selective MLCK inhibitor ML-7 prevented the LPS-induced increase of CPP and restored 51Cr-EDTA permeability to control values (0.84% ± 0.06% versus 0.77% ± 0.07%). ML-7 alone had no effect on this parameter.
Figure 1.
Influence of MLCK inhibitor (ML-7) on LPS-induced increase in CPP to 51Cr-EDTA from 0 to 6 hours after LPS administration. LPS injection (1 mg/kg, intraperitoneally) significantly increased 0- to 6-hour CPP. ML-7 injections (3 × 1 mg/kg/48 hours, intraperitoneally) reversed LPS-induced increase in permeability for the 0- to 6-hour period. ML-7 had no effect per se on CPP. Values are means ± SEM. *Significantly different (P < 0.05) from corresponding control values. +Significantly different (P < 0.05) from LPS values.
Bacterial Translocation
In control rats, no BT was detected in the liver, spleen, or mesenteric lymph nodes. Three hours after LPS administration, we observed a significant BT of aerobic bacteria into the liver, spleen, and mesenteric lymph nodes (Table 1). During this same time, no anaerobic BT was detected in these three organs. Prior MLCK inhibition significantly reduced the incidence of LPS-induced BT.
Table 1.
Bacterial Translocation in the Liver, Spleen, and MLN 3 Hours after LPS Administration
| Groups | Number of animals with/without translocation at 3 hours after LPS injection
|
|||||
|---|---|---|---|---|---|---|
| Aerobic bacteria
|
Anaerobic bacteria
|
|||||
| Liver | Spleen | MLN | Liver | Spleen | MLN | |
| Control | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 |
| LPS | 4/2* | 4/2* | 5/1* | 0/6 | 0/6 | 0/6 |
| LPS + ML-7 | 1/5† | 0/6† | 2/4† | 0/6 | 0/6 | 0/6 |
No cases of translocation were found in control groups. Bacterial translocation of aerobic bacteria was frequent in LPS groups but was reduced by pretreatment with ML-7. No cases of anaerobic bacteria translocation were observed. (n = 6 for each group).
P < 0.05 versus control;
P < 0.05 versus LPS.
Electron Microscopy of Tight Junctions
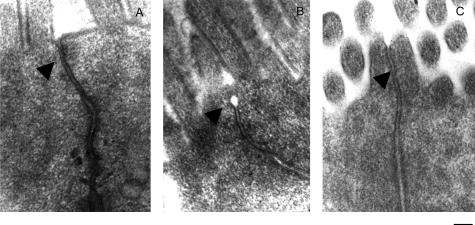
Transmission electron microscopy of the TJ region in proximal colon enterocytes illustrated that under physiological conditions only a low percentage of open TJs was observed (1.7% ± 1.1%) in control rats whereas this percentage was significantly higher 3 hours after LPS injection (16.7% ± 3.1%, P < 0.05; Figure 2). MLCK inhibition significantly reduced the LPS-induced TJ opening observed at 3 hours after LPS injection (3.7% ± 2.9% versus 16.7% ± 3.1%, P < 0.05; Figure 2).
Figure 2.
Transmission electron micrographs of the TJ region of colonic enterocytes. A: In controls, TJs of cell membranes of adjacent cells are in close opposition. B: Typical TJ dilatations (arrows) observed 3 hours after LPS exposure. C: This effect was prevented by ML-7 pretreatment. Scale bar, 0.2 μm.
Proinflammatory Cytokine Assay in Colonic Mucosa
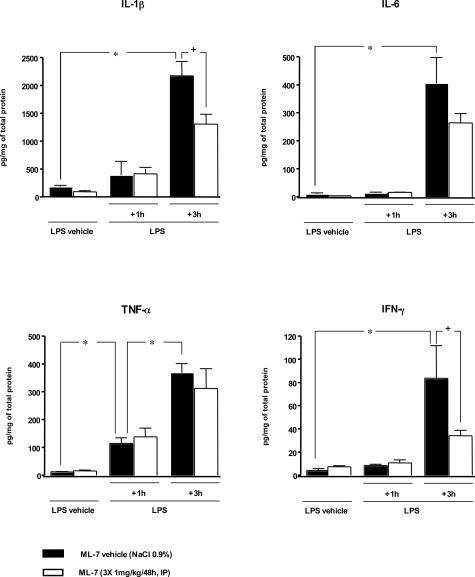
Intraperitoneal injection of LPS was responsible for colonic mucosal inflammation characterized by an increase of proinflammatory levels in colonic mucosa (Figure 3). Indeed, compared with control rats, rats receiving LPS displayed a significant (P < 0.05) increase of TNF-α levels in colonic mucosa at 1 hour after its administration. At this time, however, the levels of IL-1β, IL-6, and IFN-γ remained statistically identical to those observed in control rats (Figure 3). Three hours after LPS administration levels of all proinflammatory cytokines, TNF-α, IL-1β, IL-6, and IFN-γ, were significantly (P < 0.05) increased in colonic mucosa of septic rats compared with controls. ML-7 alone did not modify the basal cytokine levels in colonic mucosa compared to those in control rats. Similarly, ML-7 had no effect on the early (1 hour) LPS-induced TNF-α release in colonic mucosa. In contrast ML-7 significantly diminished (P < 0.05) the late release (3 hours) of IL-1β and IFN-γ in colonic mucosa but failed to diminish LPS-induced release of IL-6 and TNF-α (Figure 3).
Figure 3.
Proinflammatory cytokine levels in colonic mucosa 3 hours after LPS administration. Compared with corresponding control rats (LPS vehicle + M-7 vehicle), injection of LPS (1 mg/kg, intraperitoneally) significantly increased the level of TNF-α in colonic mucosa 1 hour after its administration but had no effect on IL-1β, IL-6, or IFN-γ release. Three hours after LPS injection, a significant increase in TNF-α, IL-1β, IL-6, and IFN-γ levels was detected in colonic mucosa of septic rats compared with corresponding controls. Compared with corresponding LPS groups, prior treatment with ML-7 had no effect on cytokine release 1 hour after LPS administration but had significantly reduced the IL-β and IFN-γ release 3 hours after LPS. However, MLCK inhibition failed to reduce the release of IL-6 and TNF-α. ML-7 had no effect per se on proinflammatory cytokine release. Values are means ± SEM. *Significant difference (P < 0.05) from corresponding control values. +Significant difference (P < 0.05) from corresponding LPS values.
Western Blotting of Phosphorylated MLC
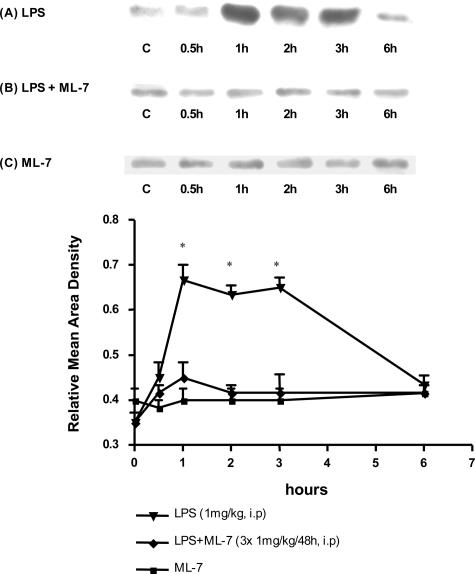
As shown in Figure 4A, LPS administration triggered an increase of pMLC levels in colonocytes from 1 hour up to 3 hours after LPS administration. Prior inhibition of MLCK completely prevented this LPS-induced increase of phosphorylation of MLC; indeed, the levels of pMLC remained unchanged after LPS administration (Figure 4B). ML-7 administered alone had no effect on the basal level of pMLC during the course of experiments (Figure 4C).
Figure 4.
Representative Western immunoblots of phosphorylated MLC (pMLC). Results are representative of three animals per group. A: Time course pMLC levels were measured at 0.5, 1, 2, 3, and 6 hours after LPS challenge. pMLC levels increased 1 hour after LPS exposure and were maintained throughout 3 hours. B: ML-7 inhibited the increase of pMLC levels consecutive to LPS treatment. C: ML-7 had no effect per se on pMLC levels in basal conditions. The graphs represent relative mean area density of the total protein blots ± SEM.
Rectal Sensitivity, Food Intake, and Diarrhea
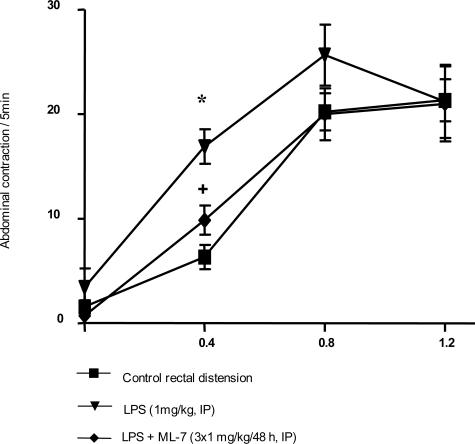
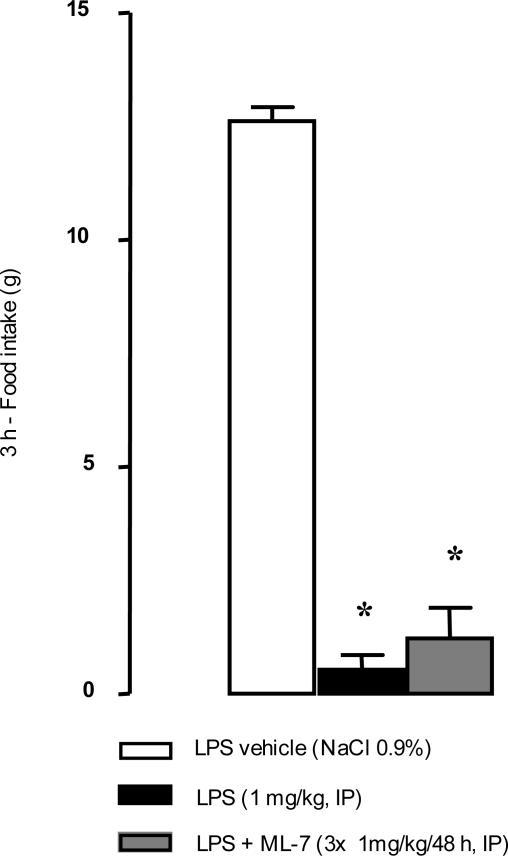
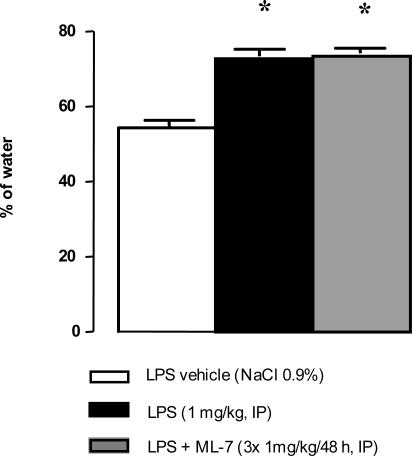
Under basal conditions, rectal distension increased the total number of abdominal contractions for distending volumes of 0.8 and 1.2 ml but not for 0.4 ml (Figure 5). Three hours after LPS administration (1 mg/kg), the number of abdominal contractions in response to rectal distension was significantly increased (16.9 ± 1.6 versus 6.3 ± 1.2 contractions/5 minutes, P < 0.05; Figure 5) for the lowest (0.4 ml) volume of distension compared with vehicle. ML-7 significantly reduced the LPS-induced increase of abdominal muscle contractions in response to the lowest volume of distension (0.4 ml): 9.8 ± 1.4 versus 16.9 ± 1.7 contractions/5 minutes (P < 0.05; Figure 5). LPS injection significantly reduced food intake (P < 0.05) from 12.0 ± 0.4 g in controls to 0.5 ± 0.3 g in treated rats (Figure 6). However, ML-7 treatment failed to prevent LPS-induced decrease of food intake (1.2 ± 0.7 versus 0.5 ± 0.3 g, P > 0.05). LPS administration resulted in a significant emission of watery feces compared with control rats (73.5% ± 1.9% versus 54.3% ± 2.1% water, P < 0.05; Figure 7). Prior ML-7 treatment failed to inhibit the LPS-induced diarrhea (74.3% ± 1.3% versus 73.5% ± 1.9% water, P > 0.05).
Figure 5.
Effect of ML-7 on LPS-induced visceral hypersensitivity in response to different volumes of rectal distension at 3 hours after LPS. LPS increased the abdominal response to distension at 3 hours after LPS but only for the lowest volume of distension (0.4 ml). ML-7 reduced the LPS-induced allodynia. ML-7 had no effect per se on visceral sensitivity. Values are means ± SEM. *Significantly different (P < 0.05) from corresponding control values. +Significantly different (P < 0.05) from LPS values.
Figure 6.
Effect of intraperitoneal administration of LPS on food intake with or without ML-7 pretreatment. Food intake was assessed 1.5 hours after LPS (1 mg/kg) administration during a 3-hour period. ML-7 pretreatment (3 × 1 mg/kg/48 hours, intraperitoneally) failed to reverse the LPS-induced effects on food intake. Results are expressed as means ± SEM. *Significantly different (P < 0.05) from corresponding control values (saline).
Figure 7.
Diarrheic effect of LPS administration with or without ML-7 pretreatment. LPS administration (1 mg/kg, intraperitoneally) resulted in a significant emission of watery feces compared with controls. Prior treatment with ML-7 (3 × 1 mg/kg/48 hours, intraperitoneally) did not prevent the LPS-induced diarrheic effects. Results are expressed as means ± SEM. *P < 0.05 versus control group.
Discussion
Our study shows that CPP increases after intraperitoneal LPS administration and is associated with a massive BT. We also demonstrate that this LPS-induced failure of the epithelial barrier is associated with a release of proinflammatory cytokines in colonic mucosa and morphological alterations of the TJs of colonocytes and that the deleterious effects of LPS are subsequent to increased CPP, which depends on epithelial cell cytoskeleton contraction after MLCK activation. However, blockage by ML-7 of the increase in CPP induced by LPS, and subsequent colonic inflammation response, does not attenuate its effects on diarrhea or feeding behavior.
Bacterial Translocation
LPS increases CPP and BT in less than 6 hours. Previous data, both in animals and humans, have already described increased gut permeability after the injection of viable E. coli3 or after a single administration of E. coli endotoxin.2 A failure of the intestinal barrier, which results in an increase of mucosal permeability, has been hypothesized to be a major promoter of BT;28 however, the mechanisms underlying the process of BT are poorly defined. Possible routes for transmucosal passage of bacteria include both transcellular and paracellular pathways. Transcellular migration of bacteria across the bowel wall may occur by pinocytosis in epithelial cells and has been proposed as the main translocation mechanism in the presence of an intact mucosal barrier.29 However, the hypothesis of a BT through paracellular pathways is supported by recent data. Indeed, latex particles of similar diameter to E. coli translocated with efficiencies similar to those of viable indigenous bacteria.30 Moreover, previous in vitro and in vivo data have already report a link between the degree of paracellular permeability and the BT through the intestinal epithelium.19,31 Herein, we described for the first time that a reduction of LPS-induced alteration of CPP by a MLCK inhibitor is associated with a reduction of BT.
Colonic Inflammation
In this study we demonstrate that intraperitoneal LPS induces a massive release of proinflammatory cytokines in colonic mucosa. Similar results have been obtained in rats, in which a significant colonic production and expression of IL-6 was observed 3 hours after intraperitoneal administration of LPS from E. coli.32 It seems that circulating LPS from E. coli injected by the intraperitoneal route can induce the local release of proinflammatory cytokines in the colonic mucosa. At 3 hours after LPS injection, a prior intraperitoneal MLCK inhibitor administration significantly reduced the LPS-induced release of IL-1β and IFN-γ whereas the release of TNF-α and IL-6 was unaffected.
In our experiment only TNF-α levels are increased 1 hour after LPS injection. This early increase of TNF-α after LPS administration has already been described in rodent models and may play a role in the increase of the CPP.33 In contrast, the level of other proinflammatory cytokines is increased only 3 hours after LPS injection, as previously reported for IL-1β and IL-633 and IFN-γ.34 This late increase may be a consequence of increased CPP. Indeed, pMLC levels increased from 1 to 3 hours after LPS simultaneously with the CPP. Therefore, we can conclude that the early cytokine release (TNF-α) in colonic mucosa observed within 1 hour after LPS injection is only due to circulating LPS and that late cytokine release (IL-1β, IL-6, IFN-γ) between 1 and 3 hours after LPS results from concerted action of circulating LPS and altered CPP. This last hypothesis is supported by the observation that prior MLCK inhibition, with ML-7, diminishes the IL-1β and IFN-γ levels without affecting the levels of TNF-α. The lack of effects of ML-7 on IL-6 could be explained by the high degree of TNF-α dependency of this cytokine. Indeed, in septic mice TNF-α has been shown to regulate IL-6 production both in vivo and in vitro.35
In conclusion, we hypothesize the following mechanisms. Circulating LPS from E. coli initiates an early proinflammatory response with cytokine release in colonic mucosa. This early phase is followed by a change in gut permeability with a massive BT, which prolongs release of proinflammatory cytokines such as IL-1β and IFN-γ. Prevention of alterations in CPP and BT by ML-7 blocks the second activation of gut immune cells by luminal contents and reduces gut inflammation after LPS challenge.
Visceral Hypersensitivity to Distension, Food Intake, and Diarrhea
Visceral hypersensitivity to distension 6 to 12 hours after LPS administration has been shown to be linked to the central release of IL-1β and/or TNF-α.36 In our experiments visceral hypersensitivity was present 3 hours after LPS administration, and suppressing CPP increase could prevent this increase in sensitivity. This supports the hypothesis that increase of colonic permeability to antigens and allergens is responsible for visceral hypersensitivity to mechanical stimuli such as gut contractions seen 3 hours after LPS. This hypothesis is also supported by previous work showing a decrease in stress-induced visceral hypersensitivity to distension by preventing alterations of CPP using 2,4,6-triaminopyrimidine, a chemical that binds TJs and prevents the entry of pathogens.21
The alteration of food intake after LPS administration was not prevented by ML-7 (MLCK inhibitor), suggesting that this effect is not related to alterations in gut mucosal barrier. These results are in agreement with previous data suggesting that LPS-induced inhibition of feeding behavior involves a direct activation of vagal afferent fibers by cytokines, such as IL-1β.37 Such cytokines are released from peritoneal LPS-stimulated macrophages directly or by immune cells located on the vagus nerve. Results of diarrhea experiments illustrate that prevention of LPS-induced increases in CPP by MLCK inhibitor, ML-7, failed to prevent the diarrheic effect of LPS. This result contradicts the hypothesis that changes in paracellular pathways influence water secretion. In conclusion, prevention of altered CPP by MLCK inhibitor in septic rats reduces peripheral deleterious effects of sepsis such as colonic inflammation, visceral hypersensitivity to distension, and BT but fails to reduce the diarrhea and the central effect of sepsis on food intake.
Origin of the LPS-Induced CPP Alterations
The mechanisms by which LPS enhances in vivo intestinal permeability are unknown. Possible explanations include direct and/or indirect effect of endotoxin to stimulate cytokines released from activated immunocytes within the gut. In our study, we showed that circulating LPS induced the release of proinflammatory cytokines in colonic mucosa. Several cytokines, such as IL-6, TNF-α, and IFN-γ, are known to modulate in vitro TJ opening. Addition of IFN-γ to the basolateral surface of cultured T84 colonocyte monolayer causes a significant increase in paracellular permeability accompanied by disruption of perijunctional F-actin.23,38 Furthermore, the ability of TNF-α and IFN-γ to disrupt intestinal barrier function, by a proapoptotic-independent mechanism, has also been described.15 Lastly, in a co-culture study of T84 cells and endotoxin-activated monocytes, a decrease in transepithelial resistance was observed within 24 hours of co-culture.39 The changes in epithelial permeability caused by TNF-α and IFN-γ are associated with marked increases in MLC phosphorylation,13 and recent data has shown that IFN-γ primes intestinal epithelia to respond to TNF-α.40 However, the mechanisms at the origin of this phosphorylation are unknown. In our model, MLC phosphorylation occurred at 1 hour after LPS administration; at this time only TNF-α was significantly increased, and this pathway appears to be a possible candidate for phosphorylation of MLC.
Also, molecules other than cytokines may contribute to signaling cascades that result in a rapid alteration of epithelial permeability. For example, activation of colonic proteinase-activated receptor-2 (PAR2) by intracolonic infusion of the PAR2-activating peptide SLIGRL causes a rapid (2 to 4 hours) increase of CPP in mice, a process that depends on the activation of MLCK.20 In all cases of increased CPP by cytokines and/or proteases, MLCK appeared to be a key enzyme in mediating the LPS-induced signal at the origin of TJ dilatation and alteration of colonic epithelial barrier.
In conclusion, our data show that LPS increases gut paracellular permeability after the activation of epithelial cell MLCK and contraction of the cytoskeleton. This alteration of the colonic epithelial barrier is associated with BT to liver, spleen, and mesenteric lymph nodes and colonic mucosal inflammation and is responsible for visceral hyperalgesia but not for sickness behavior or diarrhea. Inhibition of MLCK, which catalyzes the MLC phosphorylation responsible for the increased permeability, appears to be a promising approach to prevent the deleterious effects of sepsis on the mucosal barrier and some of its consequences.
Footnotes
Address reprint requests to Dr. Lionel Bueno, INRA, Neuro-Gastroenterology and Nutrition Unit, 180 Chemin de Tournefeuille, BP.3, 31931 Toulouse Cedex 9, France. E-mail: lbueno@toulouse.inra.fr.
This work was supported by a grant from the Institut National de la Recherche Agronomique, Toulouse, France.
References
- O’Dwer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459–1464. doi: 10.1001/archsurg.1988.01400360029003. [DOI] [PubMed] [Google Scholar]
- Wang Q, Pantzar N, Jeppsson B, Westrom BR, Karlsson BW. Increased intestinal marker absorption due to regional permeability changes and decreased intestinal transit during sepsis in rat. Scand J Gastroenterol. 1994;29:1001–1008. doi: 10.3109/00365529409094877. [DOI] [PubMed] [Google Scholar]
- O’Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35. doi: 10.1136/gut.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–228. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaber P, Smidt I, Tamme K, Liigant A, Tapfer H, Mikelsaar M, Talvik R. Translocation of indigenous microflora in an experimental model of sepsis. J Med Microbiol. 2000;49:431–439. doi: 10.1099/0022-1317-49-5-431. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Madara JL. Pathobiology of the intestinal epithelial barrier. Am J Pathol. 1990;137:1273–1281. [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, Turner JR. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na(+)-glucose cotransport. Am J Physiol. 2001;281:G1487–G1493. doi: 10.1152/ajpgi.2001.281.6.G1487. [DOI] [PubMed] [Google Scholar]
- Turner JR. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation. Semin Cell Dev Biol. 2000;11:301–308. doi: 10.1006/scdb.2000.0180. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, Van Eldik LJ, Watterson DM. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Tazuke Y, Drongowski RA, Teitelbaum DH, Coran AG. Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr Surg Int. 2003;19:321–325. doi: 10.1007/s00383-003-1003-8. [DOI] [PubMed] [Google Scholar]
- Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology. 1998;114:657–668. doi: 10.1016/s0016-5085(98)70579-7. [DOI] [PubMed] [Google Scholar]
- Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel JF, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- Cenac N, Garcia-Villar R, Ferrier L, Larauche M, Vergnolle N, Bunnett NW, Coelho AM, Fioramonti J, Bueno L. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol. 2003;170:4296–4300. doi: 10.4049/jimmunol.170.8.4296. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Bueno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005;113:141–147. doi: 10.1016/j.pain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Dogan MD, Ataoglu H, Akarsu ES. Effects of different serotypes of Escherichia coli lipopolysaccharides on body temperature in rats. Life Sci. 2000;67:2319–2329. doi: 10.1016/s0024-3205(00)00821-3. [DOI] [PubMed] [Google Scholar]
- Aubert A, Kelley KW, Dantzer R. Differential effect of lipopolysaccharide on food hoarding behavior and food consumption in rats. Brain Behav Immun. 1997;11:229–237. doi: 10.1006/brbi.1997.0503. [DOI] [PubMed] [Google Scholar]
- Coelho AM, Fioramonti J, Bueno L. Systemic lipopolysaccharide influences rectal sensitivity in rats: role of mast cells, cytokines, and vagus nerve. Am J Physiol. 2000;279:G781–G790. doi: 10.1152/ajpgi.2000.279.4.G781. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm JD, Olaison G, Peterson KH, Franzen LE, Lindmark T, Wiren M, Tagesson C, Sjodahl R. Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn’s disease. Gut. 2002;50:307–313. doi: 10.1136/gut.50.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morteau O, Hachet T, Caussette M, Bueno L. Experimental colitis alters visceromotor response to colorectal distension in awake rats. Dig Dis Sci. 1994;39:1239–1248. doi: 10.1007/BF02093789. [DOI] [PubMed] [Google Scholar]
- Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol. 1999;473:11–30. doi: 10.1007/978-1-4615-4143-1_2. [DOI] [PubMed] [Google Scholar]
- Alexander JW, Boyce ST, Babcock GF, Gianotti L, Peck MD, Dunn DL, Pyles T, Childress CP, Ash SK. The process of microbial translocation. Ann Surg. 1990;212:496–510. doi: 10.1097/00000658-199010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–154. doi: 10.1016/s0966-842x(00)88906-4. [DOI] [PubMed] [Google Scholar]
- Kops SK, Lowe DK, Bement WM, West AB. Migration of Salmonella typhi through intestinal epithelial monolayers: an in vitro study. Microbiol Immunol. 1996;40:799–811. doi: 10.1111/j.1348-0421.1996.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Adams JK, Tepperman BL. Colonic production and expression of IL-4, IL-6, and IL-10 in neonatal suckling rats after LPS challenge. Am J Physiol. 2001;280:G755–G776. doi: 10.1152/ajpgi.2001.280.4.G755. [DOI] [PubMed] [Google Scholar]
- Blanque R, Meakin C, Millet S, Gardner CR. Selective enhancement of LPS-induced serum TNF-alpha production by carrageenan pretreatment in mice. Gen Pharmacol. 1998;31:301–306. doi: 10.1016/s0306-3623(97)00434-5. [DOI] [PubMed] [Google Scholar]
- Beno DW, Uhing MR, Goto M, Chen Y, Jiyamapa-Serna VA, Kimura RE. Staphylococcal enterotoxin B potentiates LPS-induced hepatic dysfunction in chronically catheterized rats. Am J Physiol. 2001;280:G866–G872. doi: 10.1152/ajpgi.2001.280.5.G866. [DOI] [PubMed] [Google Scholar]
- Engelberts I, von Asmuth EJ, van der Linden CJ, Buurman WA. The interrelation between TNF, IL-6, and PAF secretion induced by LPS in an in vivo and in vitro murine model. Lymphokine Cytokine Res. 1991;10:127–131. [PubMed] [Google Scholar]
- Coelho AM, Fioramonti J, Bueno L. Brain interleukin-1beta and tumor necrosis factor-alpha are involved in lipopolysaccharide-induced delayed rectal allodynia in awake rats. Brain Res Bull. 2000;52:223–228. doi: 10.1016/s0361-9230(00)00269-0. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchon SM, Martins CA, Guerrant RL, Roche JK. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153:5730–5739. [PubMed] [Google Scholar]
- Zareie M, McKay DM, Kovarik GG, Perdue MH. Monocyte/macrophages evoke epithelial dysfunction: indirect role of tumor necrosis factor-alpha. Am J Physiol. 1998;275:C932–C939. doi: 10.1152/ajpcell.1998.275.4.C932. [DOI] [PubMed] [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]