Abstract
Niemann-Pick type C (NPC) disease is caused by mutations to genes that encode proteins critical to intracellular lipid homeostasis. The events underlying NPC progressive neurodegeneration are poorly understood but include neurofibrillary tangles of the type found in Alzheimer’s disease. Here we investigated possible contributions of a phosphatidylinositol-3 kinase cascade [PI3K, Akt, glycogen synthase kinase-3β (GSK-3β)] that is linked to apoptosis and various degenerative conditions. Brain concentrations of phosphorylated Akt, which phosphorylates and inactivates GSK-3β, were significantly elevated in Npc1−/− mice relative to Npc1+/+ mice. Accordingly, levels of inactive GSK-3β were 50 to 100% higher in mutant brains than in controls. Increases in inactive GSK-3β occurred early in postnatal development, well before neuronal loss, and were most prominent in structures with intracellular cholesterol accumulation, suggesting a contribution to subsequent degeneration. Perturbations of nuclear factor (NF)-κB, which is regulated by GSK-3β, occurred in Npc1−/− mouse brains. Nuclear concentrations and DNA binding activity of NF-κB’s transactivation subunit, p65, were significantly reduced in Npc1−/− mice compared to Npc1+/+ mice. Cytoplasmic levels of the p50 subunit and its precursor, p105, were higher in Npc1−/− mice. These results suggest that excessive activity in the PI3K-Akt pathway depresses GSK-3β, thereby disrupting the formation and/or nuclear import of p50/p65 NF-κB dimers and contributing to neuronal degeneration.
Niemann-Pick type C (NPC) disease is a fatal neurovisceral storage disorder caused by mutations to the NPC1 gene1 or, much less frequently, to the NPC2 gene.2 A striking feature of NPC pathology is the presence in brain of neurofibrillary tangles that, by an array of measures, are indistinguishable from those found in Alzheimer’s disease.3–7 Tangles in the latter case are thought to arise from excessive activation of a set of kinases, including glycogen synthase kinase (GSK-3β), resulting in the hyperphosphorylation of the microtubule crosslinking protein tau and its assembly into helical filaments. Several in vitro and in vivo studies have implicated GSK-3β in tangle formation;8–10 moreover, recent work indicates that the kinase co-localizes with intraneuronal tangles and probably accumulates in neurons before the onset of pathology.11 Given these results, it is of interest to ask if regional and temporal changes in levels of activated GSK-3β are linked with the onset of degeneration in NPC or in mouse models of the disease; however, mapping studies appropriate to this question have not been reported.
In addition to its potential role in tangle formation, GSK-3β is also implicated in neurodegenerative diseases because it is a target of several anti-apoptotic signaling pathways,12–15 eg, the phosphatidylinositol-3 kinase (PI3K) signaling cascade.16 PI3K phosphorylates plasma membrane inositol lipids that then recruit pleckstrin homology domain proteins including Akt/protein kinase B and phosphoinositide-dependent protein kinase; the latter enzyme then phosphorylates the former at Ser473 and Thr308. Activation of Akt promotes cell survival by phosphorylating and thus inactivating a number of proapoptotic proteins including GSK-3β, BAD, caspase-9, and Forkhead transcription factors.17–19 However, genetic deletion of GSK-3β results in fetal death associated with massive hepatocyte apoptosis, similar to that observed after the knockout of the p65 subunit of nuclear factor (NF)-κB.20 The unexpected prosurvival effect of GSK-3β, possibly via regulation of NF-κB, has recently been confirmed by several studies using a variety of preparations.21–26
NF-κB consists of a group of dimeric proteins that bind to a common DNA sequence motif; dimers consisting of p65 and p50 are the most common forms.27–30 GSK-3β regulates NF-κB by phosphorylating its p65 transactivation subunit25 and by phosphorylating and stabilizing p105, the precursor of its p50 subunit.24
In all, then, GSK-3β is of interest to the understanding of NPC both as a potential contributor to the tauopathy that characterizes the disease and as a pivotal step in a signaling cascade implicated in a variety of degenerative conditions. In the present study, we tested for spatio-temporal correlations between the activity of Akt, GSK-3β, and NF-κB and cholesterol deposits or neuropathology in a mouse model of NPC. We found evidence for increased levels of Akt activation and GSK-3β inactivation, and for NF-κB deregulation; moreover, these changes occur early in postnatal development and in a regionally selective manner. Taken together, the results provide the first evidence that NPC is accompanied by a profound disturbance of the PI3K/Akt-GSK-3β-NF-κB signaling pathway, and strongly suggest that such disturbance is a contributor to, rather than a consequence of, degeneration.
Materials and Methods
Mice
Heterozygous breeding pairs of BALB/cNctr-npc1N mice (Npc1+/−) were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained in our animal facility in accordance with National Institutes of Health guidelines and protocols approved by the Institutional Animal Care and Use Committee with care to minimize distress to the animals. Mouse breeding and genotyping were as previously described.31 Animals were killed at postnatal weeks 1, 2, 4, and 8 (four to eight animals for each age group) under deep anesthesia (200 mg/kg sodium pentobarbital) by perfusion for immunohistochemical and histological studies or by decapitation for biochemical analyses. For histological studies, animals were perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Brains were removed and incubated with 15% sucrose followed by 30% sucrose before being sectioned at 25 μm with a freezing microtome. Coronal sections were stored in a cryoprotective solution (30% ethylene glycol, 20% glycerol in 0.025 mol/L phosphate buffer, pH 7.3) at −20°C before being processed for histological and immunohistochemical studies.
Western Blots
Brain regions [cerebellum, brainstem (including diencephalon, midbrain, pons, and medulla), hippocampus, and cortex] from Npc1−/− and Npc1+/+ mice were dissected in ice-cold artificial cerebrospinal fluid, and homogenized in 50 mmol/L Tris-HCl (pH 7.4) buffer containing 1 mmol/L EDTA, 1 mmol/L EGTA, and a protease inhibitor cocktail (Sigma, St. Louis, MO). Electrophoresis and immunoblotting were performed following conventional procedures. Briefly, proteins (40 to 60 μg) from each sample were denatured by boiling for 5 minutes in a sample buffer [2% sodium dodecyl sulfate, 50 mmol/L Tris-HCl (pH 6.8), 10% 2-mercaptoethanol, 10% glycerol, and 0.1% bromophenol blue], and separated by electrophoresis on sodium dodecyl sulfate-polyacrylamide gels (12%), after which proteins were transferred to nitrocellulose membranes. Nitrocellulose membranes were incubated with primary antibodies for 12 to 16 hours at room temperature; immunoreactivity was visualized by using enhanced chemiluminescence (ECL Plus kit and reagents; Amersham Pharmacia Biotech). Antibodies used were: anti-Akt1 (1:10,000), anti-Akt1-pThr308 (1:1000; both from Upstate, Charlottesville, VA), anti-Akt1-pSer473 (1:500; Cell Signaling Technology, Beverly, MA), and anti-GSK-3βpSer9, (which also recognizes GSK-3α phosphorylated at Ser21, 1:1000; Cell Signaling Technology); anti-GSK-3βpTyr216 (1:10,000; Upstate), anti-GSK-3β (1:1000), anti-NF-κB p50 (1:5000), anti-p65 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), and anti-actin (1:10,000; Sigma). Levels of different bands were analyzed by using the National Institutes of Health Image program. Statistical significance was determined by two-tailed Student’s t-test.
NF-κB Binding Assay
DNA binding activity of NF-κB (p65/p50) was determined by using the enzyme-linked immunosorbent assay-based nonradioactive NF-κB p65/p50 transcription factor assay kit following the manufacturer’s protocol (Chemicon, Temecula, CA). Nuclear extraction was done as previously described.32 Briefly, brain tissues were homogenized in ice-cold PBS with a Dounce homogenizer (10 strokes). Homogenates were centrifuged at 4°C for 30 seconds at 12,000 × g, and the supernatants discarded. Pellets were resuspended in lysis buffer (10 mmol/L HEPES, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.5 mmol/L dithiothreitol, 0.5 mmol/L phenylmethyl sulfonyl fluoride, 2 μg/ml antipain, 2 μg/ml leupeptin) and incubated on ice for 10 minutes with 10% Nonidet P-40 solution added. The mixtures were mixed vigorously for 30 seconds and recentrifuged for 30 seconds at 14,000 × g. Pelleted nuclei were resuspended in extraction buffer (20 mmol/L HEPES, 25% glycerol, 1.5 mmol/L MgCl2, 300 mmol/L NaCl, 0.25 mmol/L EDTA, 0.5 mmol/L dithiothreitol, 0.5 mmol/L phenylmethyl sulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml antipain), incubated on ice for 20 minutes, then centrifuged again for 20 minutes at 14,000 × g. The supernatants containing the nuclear proteins were incubated with biotinylated-specific DNA probes for 1 hour at room temperature. The mixture was then transferred to the streptavidin-coated 96-well plates. Binding of active NF-κB with DNA was revealed by incubation with anti-p50/p65 antibodies, followed by the horseradish peroxidase-conjugated secondary antibodies and a colorimetric reaction, which was analyzed by a microplate reader (Molecular Devices, Sunnyvale, CA). Results obtained from brains of Npc1−/− and Npc1+/+ mice were analyzed by one-way analysis of variance and P values smaller than 0.05 were considered significant.
Filipin Staining
Filipin has been demonstrated to specifically stain free cholesterol because treatment with cholesterol oxidase results in a complete loss of fluorescence.33 Brain tissue sections were washed with phosphate-buffered saline and incubated in the dark with 50 μg/ml filipin in PBS for 3 hours under agitation at room temperature. After washing in PBS, some sections were further processed for immunostaining with anti-GSK-3pβSer9 antibody (see below); others were mounted on slides and coverslipped with Vectashield mounting media (Vector Laboratories, Burlingame, CA). Filipin fluorescence was detected by a Zeiss Axiovert epifluorescence microscope.
Fluoro-Jade B (FJB) Staining
FJB is a fluorochrome that is a selective marker for neurodegeneration.34,35 Sections were mounted on pretreated slides (Superfrost/plus; Fisher, Pittsburgh, PA), air-dried, and rehydrated in distilled water for 3 minutes. Then, slides were incubated sequentially in 1) 1% sodium hydroxide in 80% ethanol for 5 minutes, 2) 70% ethanol for 2 minutes, 3) 0.06% potassium permanganate for 10 minutes, and 4) FJB staining solution (Histo-Chem Inc., Jefferson, AZ), prepared according to the manufacturer’s instructions for 20 minutes. Some sections were further processed for immunostaining with anti-GSK-3βpSer9 antibody. Slides were then washed with water, dehydrated in graded ethanol, and coverslipped with DPX mounting solution (BDH Laboratory Supplies, Poole, UK).
Immunohistochemistry
Sagittal sections from cerebellum and coronal sections from the rest of the brains of Npc1+/+ and Npc1−/− mice at different ages were simultaneously processed for immunostaining. Immunohistochemistry was performed using the avidin-biotin horseradish peroxidase complex (ABC) method. Briefly, free-floating sections were first incubated in 3% normal goat serum diluted in PBS with 0.1% Triton X-100 for 1 hour at room temperature, followed by incubation with anti-GSK-3βpSer9 (1:500; Cell Signaling Technology) or anti-p65 (1:1000; Santa Cruz) overnight at 4°C. After three washes in PBS, sections were incubated with corresponding biotinylated secondary antibodies (1:400; Vector Laboratories) in 1.5% normal goat serum solution for 2 to 3 hours, then in ABC diluted in PBS for 45 minutes. Peroxidase reaction was performed with 3,3′-diaminobenzidine tetrahydrochloride (0.05% in 50 mmol/L Tris-HCl buffer, pH 7.4) as chromogen and 0.03% H2O2 as oxidant. Free-floating sections were mounted on precoated slides and air-dried. Sections were then dehydrated in graded ethanol and finally covered with Permount. For double staining, rabbit anti-GSK-3βpSer9 and mouse anti-cathepsin D antibodies (Santa Cruz Biotechnology) were used; binding of antibodies to antigens was revealed by Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 555 goat anti-mouse antibodies (Molecular Probes, Eugene, OR). For tissues that were first stained by filipin or FJB followed by anti-GSK-3βpSer9 antibody, Alexa Fluor 555 goat anti-rabbit IgG was used, and slides were coverslipped with Vectashield mounting media.
Results
Akt Phosphorylation in Npc1−/− Mouse Brain during Postnatal Development
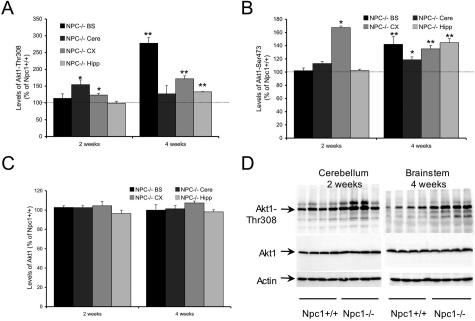
To test the status of the PI3K-Akt pathway in the developing central nervous system of Npc1−/− mice, immunoblotting analysis was performed with anti-Akt1-pThr308, anti-Akt1-pSer473, or anti-Akt1 antibodies. At postnatal week 2, significant increases in levels of Akt1-pThr308 were found in cerebellum and neocortex (155% and 124% of values found in Npc1+/+ mice, respectively), whereas at postnatal week 4 a marked increase was found in brainstem (278% of Npc1+/+ mice) and cerebral cortex (172% of Npc1+/+ mice) and a moderate increase in hippocampus (133% of Npc1+/+ mice; Figure 1A). Immunoblots labeled with anti-Akt1-pSer473 antibodies indicated that marked increases (167% of Npc1+/+ mice) in active Akt1 were present in cerebral cortex at postnatal week 2; this effect expanded to all four brain regions analyzed by postnatal week 4 (Figure 1B). Levels of total Akt1 estimated from Western blots labeled with anti-Akt1 antibodies did not differ between the two types of mice [Figure 1, C and D (bottom)]. The ratios of Akt1-pThr308/Akt1 and Akt1-pSer473/Akt1 in Npc1−/− mice were significantly elevated in all brain regions at postnatal week 4 and in some brain regions at postnatal week 2 (Tables 1 and 2). Furthermore, in vitro experiments indicated that phosphorylation of GSK-3 fusion proteins mediated by Akt1 immunoprecipitated from Npc1−/− mouse brains using an anti-Akt1 immobilized antibody was 125% higher than by that from Npc1+/+ mouse brains (n = 3, P < 0.05). Together, these results clearly indicate that levels of active Akt1 were increased in Npc1−/− mouse brain during postnatal development as compared to those found in Npc1+/+ mice.
Figure 1.
Increased Akt1 phosphorylation in developing brains of Npc1−/− mice. Samples from different brain regions (brainstem, cerebellum, neocortex, hippocampus) were collected at postnatal weeks 2 and 4 and subjected to immunoblotting analyses using anti-Akt1-Thr308, anti-Akt1-Ser473, and anti-Akt1 antibodies. The blots were then analyzed by using NIH image analysis software. A, B, and C are quantitative results of blots labeled with anti-Akt1-Thr308, anti-Akt1-Ser473, or anti-Akt1 antibodies, respectively. Data are presented as percent of values from Npc1+/+ mice; values shown in C are normalized to actin. *P < 0.05 and **P < 0.01. D: Representative images of blots labeled with anti-Akt1-Thr308 (top), anti-Akt1 (middle), or anti-actin (bottom) antibodies.
Table 1.
Ratio of Akt1-Thr308/Akt1 (Percentage of Npc1+/+)
| NPC−/− BS | NPC−/− Cere | NPC−/− CX | NPC−/− Hipp | |
|---|---|---|---|---|
| 2 weeks | 110.7 ± 12.8 | 150.7 ± 13.6* | 118.2 ± 5.2* | 102.7 ± 6.2 |
| 4 weeks | 278.1 ± 16.5† | 125.4 ± 23.8 | 160.3 ± 7.9† | 135.5 ± 0.8† |
P < 0.05,
P < 0.01.
Table 2.
Ratio of Akt1-Ser473/Akt1 (Percentage of Npc1+/+)
| NPC−/− BS | NPC−/− Cere | NPC−/− CX | NPC−/− Hipp | |
|---|---|---|---|---|
| 2 weeks | 99.3 ± 4.0 | 110.1 ± 3.0 | 160.2 ± 2.3* | 106.1 ± 2.1 |
| 4 weeks | 142.2 ± 11.4† | 116.9 ± 4.4* | 125.9 ± 4.9† | 147.2 ± 6.1† |
P < 0.05,
P < 0.01.
Levels of GSK-3 in Npc1−/− Mouse Brain during Postnatal Development
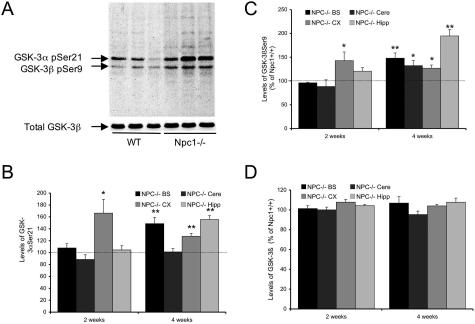
As noted, GSK-3β is inactivated via phosphorylation at its Ser9 residue by the PI3K-Akt system; the results described above would thus predict increases in the inactive form of GSK-3β in Npc1−/− mice. Brain homogenates prepared from 2- to 4-week-old animals were subjected to Western blot analyses using a polyclonal antibody that recognizes the inactive (phosphorylated) forms of GSK-3α and -3β, which migrate as two separate bands with an apparent molecular weight of 51 and 46 kd (Figure 2A). Levels of both GSK-3α-pSer21 and GSK-3βpSer9 were significantly increased in Npc1−/− compared to Npc1+/+ mice (Figure 2A). Quantitative analysis showed that at postnatal week 2, increases in phosphorylated GSK-3α (166 ± 23%, P < 0.05; Figure 2B) and GSK-3β (142 ± 18%, P < 0.05; Figure 2C) were statistically significant in cerebral cortex, but not in other brain areas. By 4 weeks however, significant increases in levels of phosphorylated GSK-3α were observed in brainstem, cerebral cortex, and hippocampus but not in cerebellum (Figure 2B). Levels of phosphorylated GSK-3β were higher in all four brain regions of Npc1−/− as compared to those in Npc1+/+ mice (Figure 2C). Levels of total GSK-3β (Figure 2D) and of the active isoforms of GSK-3 (GSK-3βpTyr216, not shown) did not differ between the two genotypes.
Figure 2.
Changes in GSK-3 levels in brains of Npc1−/− mice during postnatal development. A, top: Representative immunoblots labeled with anti-GSK-3βSer9 antibodies (that recognize GSK-3αSer21, too) from brain tissues of 2- to 3-week-old wild-type (WT) or Npc1−/− mice. A, bottom: Representative immunoblots labeled with an anti-GSK-3β antibody that recognizes both active and inactive forms of GSK-3β (total GSK-3β). Levels of total GSK-3β were not different between wild-type and Npc1−/− mice. B–D: Quantitative results of phosphorylated GSK-3α (B) and GSK-3β (C) and total GSK-3β (D, values normalized to actin). Data are expressed as percentage of control and represent means ± SEM (n = 4 to 5 animals; **P < 0.01).
Immunohistochemical Localization of GSK-3βpSer9 in Npc1−/− Mouse Brain
Week 1
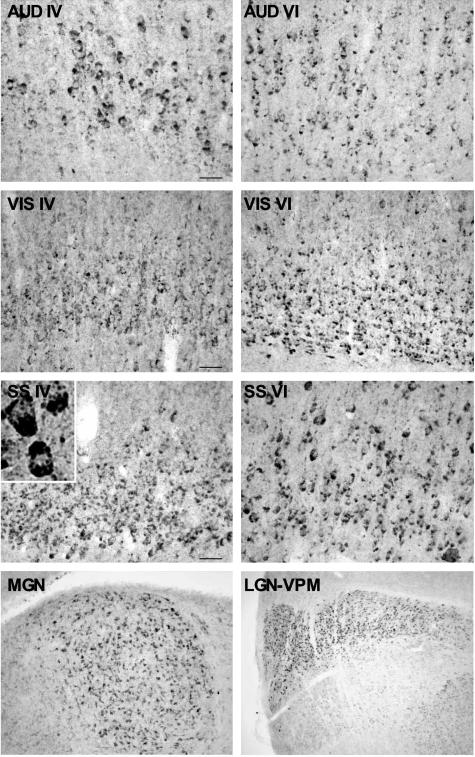
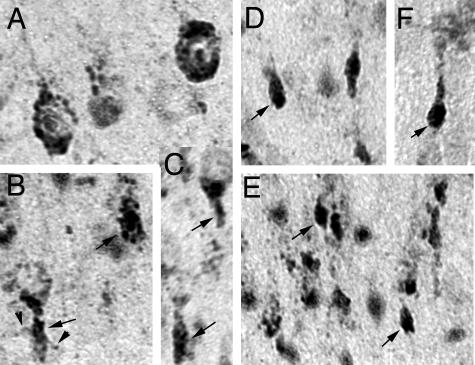
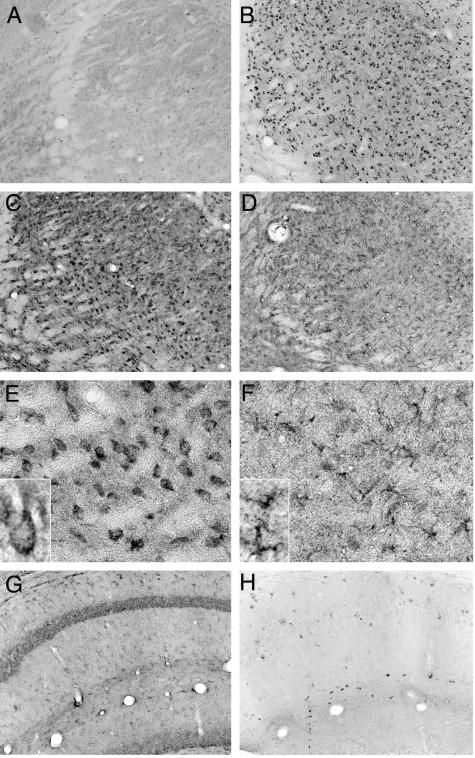
Immunohistochemical studies revealed that there was virtually no detectable staining of GSK-3βpSer9 in Npc1+/+ mouse brains at postnatal week 1, whereas high levels of the phosphorylated kinase were present in Npc1−/− mouse brains at this age (Figure 3). Interestingly, the highest levels of staining were mostly distributed along the major sensory projection systems, including the medial geniculate nucleus and primary auditory cortex, the lateral geniculate nucleus and primary visual cortex, and the lateral thalamic complex and primary somatosensory cortex (Figure 3). In neocortex, high levels of GSK-3βpSer9 staining occurred mainly in neurons of layers IV and VI. Evident GSK-3βpSer9 staining was also observed in deep layers of primary motor cortex (not shown). High magnification (Figure 3, inset; and Figure 4) indicated that GSK-3βpSer9-immunopositive elements were mostly granules with different sizes, which were located in cell bodies around nuclei. From their morphology and subcellular location, these elements resembled endosomes/lysosomes. In some cells, these elements were either clustered at the polar end of cell bodies or accumulated in the initial segments of axons forming meganeurite-like structures (Figure 4, arrows). Occasionally, spines budding from meganeurites were observed (Figure 4, arrowheads), a peculiar feature known as ectopic dendritogenesis in lysosomal storage diseases.36
Figure 3.
Distribution of GSK-3βSer9 in thalamus and neocortex of Npc1−/− mice at postnatal week 1. Coronal brain sections were prepared from 1-week-old Npc1−/− mice and immunostained with anti-GSK-3βSer9 antibodies. Staining was mainly present in layers IV and VI of auditory (AUD), visual (VIS), and somatosensory (SS) cortex, medial geniculate (MG), lateral geniculate (LG), and ventral posteromedial nucleus (VPM) of the thalamus. Inset: A higher magnification image of GSK-3βSer9-immunopositive cortical neurons. Scale bars, 40 μm.
Figure 4.
Subcellular localization of GSK-3βSer9 in developing neocortex of Npc1−/− mice. GSK-3βSer9 in auditory (A–C) and motor cortex (D–F) of Npc1−/− mice at postnatal week 1 (A–E) and week 2 (F). Arrows indicate meganeurite-like structures; arrowheads indicate spines growing out of a meganeurite (B).
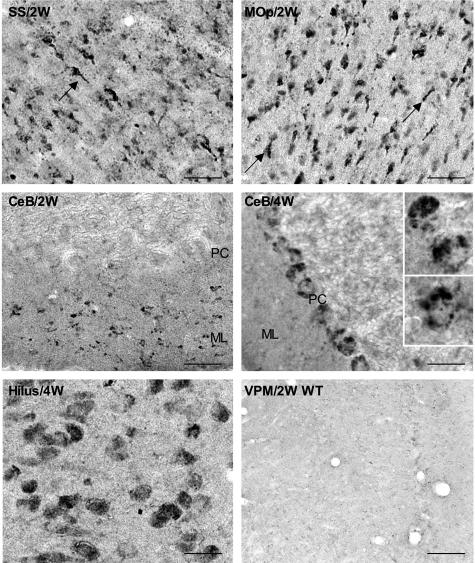
Weeks 2 to 4
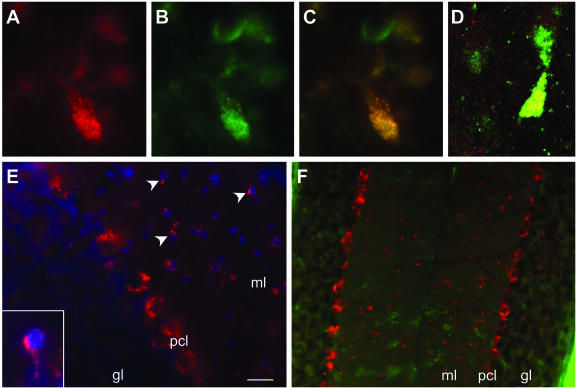
By postnatal week 2, levels of GSK-3βpSer9 immunoreactivity decreased in layer IV of neocortex, but moderate levels of staining remained in layer VI (Figure 5, SS/2W). Staining for GSK-3βpSer9 also became obvious in several other brains areas, being particularly dense in several thalamic nuclei and in the molecular layer of cerebellum (Figure 5). By 4 weeks, immunoreactivity for GSK-3βpSer9 further increased in the aforementioned noncortical regions and expanded to other brain structures. Dense GSK-3βpSer9 immunoreactivity appeared inside Purkinje cells (Figure 5, PC). GSK-3βpSer9-immunoreactive cells were also evident in the hilus region of hippocampus (Figure 5). In most cases, the staining was clearly granular and double-immunostaining results showed that GSK-3βpSer9-immunopositive granules were also labeled with anti-cathepsin D antibodies, in particular in meganeurite-like structures (Figure 6; A to D). In the molecular layer of cerebellar cortex, stained granules were located in cell bodies, around 4,6-diamidino-2-phenylindole-labeled nuclei in small-sized cells (Figure 6E, arrowheads and inset). These cells were not labeled with antibodies against F4/80 antigen, a marker of microglia (Figure 6F). GSK-3βpSer9 immunostaining became weaker by 8 to 10 weeks, when numerous neurons were dying or had died (not shown). No obvious GSK-3βpSer9 immunostaining was found in brains of Npc1+/+ mice at any time during postnatal development (an example is shown in Figure 5).
Figure 5.
GSK-3βSer9 immunoreactivity in brains of 2- and 4-week-old Npc1−/− and wild-type mice. SS/2W: Deep layers of somatosensory cortex from a 2-week-old Npc1−/− mouse; arrow indicates a labeled axonal swelling resembling meganeurites found in human NPC neocortex. Mop/2W: Deep layers of primary motor cortex from a 2-week-old Npc1−/− mouse; arrows indicate labeled meganeurites. CeB/2W: Cerebellar cortex from a 2-week-old Npc1−/− mouse; note that numerous small cells were stained in the molecular layer (ML), but Purkinje cells (PC) were not stained. CeB/4W: GSK-3βSer9 staining in cerebellar cortex of a 4-week-old Npc1−/− mouse was located mainly in Purkinje cells. Insets show clusters of GSK-3βSer9-immunoreactive products. Hilus/4W: Npc1−/− hilar neurons at 4 weeks. VPM/2W WT, ventrolateral thalamic nuclei from a 2-week-old Npc1+/+ mouse (representative images from four 2- and 4-week-old Npc1+/+ and Npc1−/− mice). Scale bars, 40 μm.
Figure 6.
Subcellular and cellular localization of GSK-3βSer9 in brains of 4-week-old Npc1−/− mice. A–C: GSK-3βSer9 (A) and cathepsin D (B) were co-localized (C) in meganeurite-like structures in cortical neurons of Npc1−/− mice. D: Confocal microscopic image showing the co-localization (yellow granules) of GSK-3βSer9 (red) and cathepsin D (green). E: GSK-3βSer9 immunoreactivity in cerebellar cortex where it was localized in small-sized cells (arrowheads and inset). F: GSK-3βSer9 (red)-immunopositive cells were not labeled by a microglial marker, F4/80 antigen (green). gl, granular layer; ml, molecular layer; pcl, Purkinje cell layer. Scale bar = 20 μm in E, 40 μm in F.
Co-Localization of Inactive GSK-3β with Intracellular Accumulation of Cholesterol in Degenerating Brain Regions
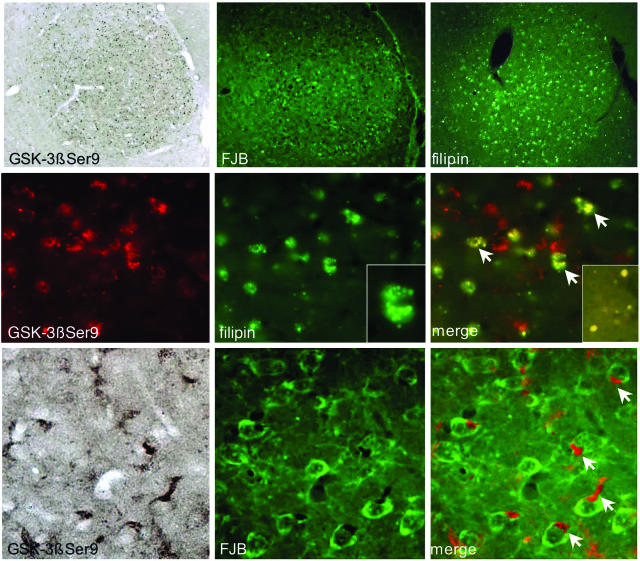
Intracellular accumulation of cholesterol and glycolipids is the characteristic pathology of NPC.36–38 Cholesterol clusters were visualized by staining brain tissue sections with filipin, a natural fluorescent antibiotic that has a high affinity for cholesterol. In agreement with the literature, accumulation of cholesterol assessed with filipin staining was obvious only in axonal spheroids scattered along the corpus callosum at postnatal week 2, whereas clear somatic staining was seen in brain sections from animals older than 4 weeks of age; spatial distribution of intraneuronal filipin staining matched that of GSK-3βpSer9 immunostaining and of Gallyas silver staining (not shown). As shown in Figure 7, GSK-3βpSer9 immunostaining was specifically localized in filipin-labeled brain structures from 4-week-old Npc1−/− mice. Closer examination showed that inactive GSK-3β was located in cholesterol-enriched vesicles in filipin-labeled structures, eg, in the ventral lateral thalamic complex (Figure 7, middle). Double staining indicated that GSK-3βpSer9-immunopositive structures were also labeled with FJB, a dye widely used to label degenerating neurons. Images taken at higher magnification showed that GSK-3βpSer9-immunoreactive products often clustered at one pole of the cell body or accumulated in structures previously described as “meganeurites”36 in FJB-labeled neurons (Figure 7, arrows in bottom panels).
Figure 7.
Co-localization of GSK-3βSer9 with cholesterol and FJB in degenerating brain areas. Brain tissue sections from 4-week-old Npc1−/− mice were first stained with filipin or FJB (both green), then processed for immunostaining with anti-GSK-3βSer9 antibody. GSK-3βSer9 immunoreactivity was revealed with Alexa Fluor 594-conjugated anti-rabbit IgG (red; for double staining with filipin) or with ABC method (for double staining with FJB). Top: Lower power images of medial geniculate nucleus. Middle and bottom: Higher power images of ventral lateral thalamic nuclei. GSK-3βSer9 is co-localized with filipin-labeled vesicles and in FJB-labeled neurons. GSK-3βSer9-immunopositive products often accumulated in meganeurite-like structures (arrows at bottom). Inset in right middle panel shows co-localization of GSK-3βSer9 with cholesterol in axonal spheroids in corpus callosum.
Changes in NF-κB in Npc1−/− Mouse Brains
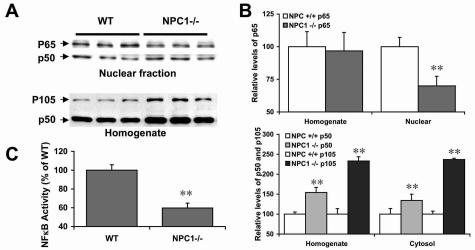
In vitro studies have shown that GSK-3β phosphorylates NF-κB subunit p65.25 A more recent study showed that GSK-3β can also affect nuclear levels of NF-κB by directly participating in nuclear transport of p65.22 Western blots labeled with anti-p65 antibodies showed that nuclear p65 levels were significantly lower in brains of 2- to 3-week-old Npc1−/− mice as compared to Npc1+/+ mice of the same age (70 ± 7% of Npc1+/+; mean ± SEM; P < 0.01); in contrast, whole homogenate p65 levels were not modified (Figure 8, A and B), suggesting that nuclear import of the factor might be impaired. GSK-3β can also phosphorylate and stabilize p105, the precursor of the p50 subunit.24 Immunoblots labeled with anti-p105/p50 antibodies indicated that levels of both p50 and its precursor were significantly higher in homogenate (154 ± 13% and 233 ± 11%, respectively) and cytosol fractions (134 ± 16% and 237 ± 3%, respectively) from brains of Npc1−/− mice as compared to Npc1+/+ mice (Figure 8, A and B). Finally, p50 levels in nuclear fractions from Npc1−/− mice were similar to those in Npc1+/+ mice. To further determine whether changes in levels of NF-κB proteins also affected the transcriptional activity of this nuclear factor, an enzyme-linked immunosorbent assay-based kit was used to compare the DNA binding ability of NF-κB in brain nuclear fractions from Npc1−/− and Npc1+/+ mice. A very large decrease in DNA binding activity of p65/p50 dimers was observed in nuclear fractions from Npc1−/− mouse brains as compared to those from Npc1+/+ mice [optical density = 344 ± 20 in Npc1+/+ and 205 ± 18 in Npc1−/− mice (mean ± SEM); n = 6 mice and P < 0.002; Figure 8C].
Figure 8.
Changes in NF-κB in Npc1−/− mouse brain. A: Representative Western blots of nuclear fractions and whole homogenates prepared from brains of 2- to 3-week-old Npc1+/+ and Npc1−/− mice; blots were labeled with anti-p65 and anti-p50 antibodies. B: Quantification of Western blots labeled with anti-p65 and anti-p50 antibodies. There was a 30% decrease in levels of nuclear p65 in brains of Npc1−/− mice but not in whole homogenates (top). Levels of both p50 and its precursor (p105) increased in the cytosolic fractions and whole homogenates (bottom). **P < 0.01, n = 4 ∼ 5. No significant difference was observed with levels of nuclear p50. C: DNA binding activity assay showed a marked decrease in p65/p50 binding activity in nuclear fractions from brains of Npc1−/− mice. **P < 0.01, n = 5.
Survey images from immunohistochemical studies indicated that in 2- to 4-week-old Npc1−/− mouse brains, p65 immunoreactivity was particularly low in the most vulnerable regions (Figure 9D), where levels of GSK-3βpSer9 were markedly elevated (Figure 9B). Closer examination revealed that in these areas, p65 immunoreactivity in neurons was markedly lower, whereas that in glia (Figure 9F, inset) was higher as compared to that in Npc1+/+ mice (Figure 9E). Interestingly, less vulnerable neurons, eg, pyramidal neurons in the hippocampus, exhibited no detectable levels of GSK-3βpSer9 (Figure 9H) and did not show significant decrease in NF-κB (Figure 9G).
Figure 9.
Negative correlation between p65 and GSK-3βSer9 immunoreactivity in brains of 4-week-old Npc1+/+ and Npc1−/− mice. Adjacent tissue sections were immunostained with anti-GSK-3βSer9 (A, B, H) or anti-p65 subunit of NF-κB (C–G). A, C, E: Tissue sections from ventral-posteromedial nuclei of thalamus (VPM) from Npc1+/+ mice. B, D, F: Tissue sections from VPM in Npc1−/− mice. G and H: Hippocampus from Npc1−/− mice. Note the loss of neuronal staining and the appearance of p65 immunoreactivity in glia in VPM from Npc1−/− mice. Note also that the loss of p65 immunostaining was obvious in VPM that exhibited high levels of GSK-3βSer9 immunoreactivity, but not in pyramidal neurons of hippocampus that showed very low levels of inactive GSK-3β.
Discussion
Pathology and Abnormal Activity in the PI3K Signaling Cascade
The results of the present study indicate that the PI3K signaling cascade exhibits major disturbance in the brains of Npc1−/− mice. Changes in each of three links in the pathway were evident in immunoblots by postnatal week 2 and in immunocytochemical analyses of phosphorylated GSK-3β staining by week 1. It is noteworthy that the degree of changes in levels of active Akt and inactive GSK-3 estimated by Western blots might be underestimated because immunohistochemical results indicated that only selective cell populations were GSK-3βpSer9-immunopositive in brains of Npc1−/− mice. The earliest signs of axonal degeneration described for the Npc1−/− mouse occur at postnatal day 9 in the corpus callosum and in the white matter of the cerebellum.39 Similarly, we found here that cholesterol deposits, as detected by filipin staining, were infrequent at postnatal week 2 and only became widespread at postnatal week 4, as did FluroJade-labeled neurons. The changes in the PI3K pathway seen here thus occurred concurrently with disturbances in lipid storage and well in advance of widespread neuronal degeneration. Sustained increases in levels of inactive GSK-3β were most evident in a subset of brain structures, including thalamic sensory relay nuclei and cerebellar cortex, known from the literature39–41 and confirmed here, to be vulnerable to NPC. These temporal and regional results suggest that the observed perturbations of the PI3K cascade are contributors to the brain pathology that characterizes the disease. On the other hand, the apparent inactivation of GSK-3β does not seem to accord with the idea that hyperphosphorylation of tau protein by this enzyme is an important step in the process leading to neurofibrillary tangles found in NPC patients, although we cannot exclude the possibility that tau is associated with levels of active GSK-3β beyond the sensitivity of our assays, which could then participate in tau phosphorylation in Npc1−/− mice. Activated GSK-3β has been localized to tangles in Alzheimer’s disease brains, leading to the hypothesis that the enzyme, through tau hyperphosphorylation, contributes critically to the assembly of helical filaments. Several kinases other than GSK-3β can phosphorylate tau and have been implicated in tangle formation; as previously reported, MAP kinase42,43 and cdk544 are likely to drive the process of tangle formation in NPC.
Elevated levels of phosphorylated Akt strongly suggest that PI3K is unusually active in Npc1−/− mouse brain. The NPC proteins are normally involved in transporting cholesterol from late endosomes/lysosomes to the plasma membrane and, related to this, NPC cells differ from controls with regard to membrane cholesterol content.45 Cholesterol levels, in turn, potently modulate the activation of PI3K by endogenous triggering agents and this is reflected in the phosphorylation of Akt.46 The disturbed cholesterol transport that characterizes NPC, though undetectable by filipin staining, could thus account for the altered signaling cascade found in the present study. Alternatively, because glia-mediated inflammatory reactions start early in Npc1−/− mouse brains,5,31,47 increases in Akt phosphorylation could result from up-regulation of microglial tumor necrosis factor (TNF)-α and interleukin (IL)-1β, two recognized activators of PI3K.48–52 Activation of Akt could also be mediated by MAPK because activity of this kinase is increased in Npc1−/− mouse brain.42,43
Abnormal Distribution of GSK-3β
The immunocytochemical studies showed that disturbances of the PI3K pathway go beyond abnormal levels of activity to include abnormal distribution. Specifically, the phosphorylated (inactive) form of GSK-3β found in Npc1−/− mouse brains, but uncommonly in controls, was concentrated in granules surrounding nuclei. The size, number, and distribution of these puncta indicated that they were related to late endosome-lysosomes, a point that was confirmed by co-localization studies indicating that inactive GSK-3β accumulated in cholesterol- and cathepsin D-positive structures. Although the subject has not been extensively studied, it is reasonable to assume that glycolipid and cholesterol cellular accumulation substantially impairs lysosomal functioning, resulting in increased aberrant autophagocytosis. Indeed, the conversion of LC3-I to LC3-II was significantly higher in Npc1−/− mouse brains (X. Bi et al, unpublished results). Although GSK-3β, a cytoplasmic kinase, would not normally be a target for lysosomal processing, the enzyme is found in mitochondria, and is inactivated there by phosphorylated Akt transported from the plasma membrane.53 Mitochondria are a principle target of macroautophagy, and thus could be the source of the lysosomal accumulation of inactive GSK-3β; the persistence of the kinase, or at least of its pertinent epitopes, would then reflect the compromised internal environment of the organelles and consequent reduced rates of proteolysis.
Interestingly, GSK-3β immunoreactivity was found in meganeurites, massive swellings located at the base of the cell body or in the initial segment of the axon. These structures are a concomitant of various storage diseases including NPC,54 and have been described in animal models of NPC.36 They are composed of large numbers of individual and fused lysosomes and can be experimentally induced, along with lysosomal proliferation, by inhibitors of select proteases.55,56 Collectively, these results raise the possibility that the large expansion of the endosome/lysosome system associated with storage diseases creates a sink for Akt and GSK-3β that, in part at least, is removed by autophagy and leads to the formation of pathological structures of the meganeurite type.
Inactivation of GSK-3β Is Associated with Deregulation of NF-κB
Accumulating evidence indicates that GSK-3β regulates the activity of NF-κB, although the mechanisms involved are not clear, nor is it known whether the kinase increases or decreases the activity of this transcription factor. NF-κB-activating stimuli such as growth factors57–59 or cytokines48–52 trigger the PI3K/Akt system and thereby inactivate GSK-3β. Moreover, suppression of GSK-3β with lithium or more specific inhibitors enhanced the transcriptional activity of NF-κB in renal medullary interstitial cells.60 In contrast, genetic depletion of GSK-3β resulted in embryonic fatality with similar pathological features as those produced by gene targeting of IkB kinase-β, an enzyme critical to NF-κB activation, or of the p65 subunit of NF-κB itself.20 These results strongly suggested that GSK-3β activates the transcription factor. The confusion between these opposite results suggests that GSK-3β interacts in multiple, and in some cases opposing, ways with NF-κB. Four potential GSK-3β phosphorylation sites have been identified on the transactivation domains of the p65 subunit and, as predicted from this, recombinant GSK-3β phosphorylated a fusion protein containing the relevant segments.25 Subsequent work indicated that phosphorylation of p65 at Ser536 increased NF-κB activity,61 whereas phosphorylation at Ser486 decreased basal NF-κB activity.62 GSK-3β can also affect nuclear levels and activity of NF-κB by directly affecting nuclear import of p65.22 In addition, GSK-3β phosphorylates the p105 precursor of the p50 member of the NF-κB dimer; this has the dual effect of slowing constitutive processing to p50 and accelerating the degradation of p50 on stimulation with TNF-α.24 Indeed, knocking out GSK-3β increases constitutive processing of p105 to p50 and leads to accumulation of p50. Knocking out GSK-3β also prevents the degradation of p105 in response to TNF-α and results in increased levels of p105 as GSK-3β phosphorylated p105 could be further phosphorylated by IKK, which targets p105 to proteasome-mediated degradation.24 It has been proposed that the absence of GSK-3β results in the accumulation of p50 and the formation of p50/p50 homodimers that compete with p50/p65 and inhibit NF-κB-mediated transcription.24,63
Results from the present study showed that nuclear p65 levels were reduced as was DNA binding activity of the transcription factor, which suggests an impaired nuclear import of NF-κB heterodimers from the cytoplasm. We also found that cytoplasmic concentrations of p105 and p50 were significantly elevated in the mutants, as would presumably occur under conditions in which GSK-3β-mediated phosphorylation of the subunit was deficient and TNF-α stimulation was also increased.64
Deregulation of NF-κB in NPC Disease: Involvement in Trophic Signaling Impairment and Inflammation?
NF-κB is constitutively active in hippocampus, neocortex, and other central nervous system structures; activation of NF-κB leads to transcription of many genes, such as growth factors, the anti-apoptotic factor Bcl-2, and the antioxidant enzyme Mn-SOD, which in turn promote neurite growth during development, and provide neuroprotection against various insults in adult central nervous system.65 Interestingly, a recent study has revealed a candidate NF-κB site in promoter 3 of the BDNF gene and showed that N-methyl-d-aspartate receptor-induced BDNF expression was mediated by NF-κB.66 Thus, activation of NF-κB by trophic factors would enhance BDNF expression, thereby providing a prosurvival-positive feedback loop. Disruption of this loop resulting from deregulation of NF-κB could be a molecular basis for impairment in trophic signaling in NPC disease as reported in embryonic striatal neurons from Npc1−/− mice.67 Lack of BDNF responsiveness seems to be a common feature of NPC, as embryonic striatal neurons prepared from Npc2-deficient mice also failed to respond to BDNF.68 Although this could be due to changes in the lipid environment of trophic factor receptors because depletion of membrane cholesterol interferes with NGF-mediated responses in primary neuronal cultures69 and PC12 cells,70,71 our results point to deregulation of NF-κB as an additional contributor.
Unlike neurons, glia in vulnerable regions of Npc1−/− mice brains exhibited increased expression of p65. Besides GSK-3β, activity of NF-κB in glia is regulated by multiple factors. For instance, extracellular ATP released from injured neurons is known to activate NF-κB in microglia via P2Y receptors that are only expressed in certain types of immune cells.72 Cytokines, such as TNF-α and IL-1β, activate NF-κB in glia,73,74 via an IκB kinase-dependent pathway.65,75 Activation of this transcriptional factor in turn activates microglia. Thus, both neuronal and glial changes could contribute to neurodegeneration in NPC disease: decreases in NF-κB activity in neurons, possibly induced by inactivation of GSK-3β-dependent pathway, would reduce the expression of neuroprotective factors, whereas increases in glial NF-κB, induced by ATP, TNF-α, or IL-1β, would induce inflammatory responses and promote neurodegeneration (see Figure 10 for a summary of the potential mechanisms of neurodegeneration in NPC).
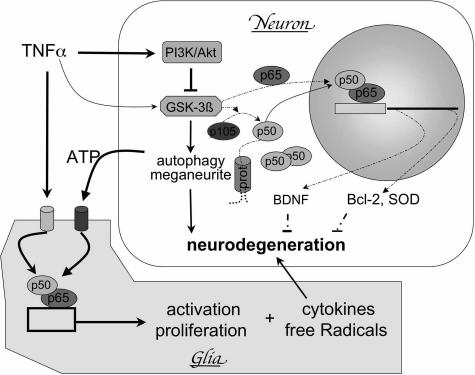
Figure 10.
Deregulation of the PI3K-Akt-GSK-3β pathway and neurodegeneration in NPC: a hypothesis. Activation of PI3K/Akt by the cytokine TNF-α or other factors, induces the phosphorylation and inactivation of GSK-3β in neurons, which in turn 1) impairs nuclear import of p65, 2) decreases the processing of p105 to p50, and 3) reduces proteasome (prot)-mediated degradation of p50. The net result would be a deficiency in p65/p50 nuclear dimers of NF-κB and decreased transcription of trophic factors, anti-apoptotic factors, and anti-oxidant protein/enzymes, which would lead to neurodegeneration. In addition, inactivation of GSK-3β may also be involved in autophagy, impairment in axonal trafficking, and formation of dystrophic neurites. On the other hand, cytokines and ATP released from damaged neurons bind to receptors that are specifically expressed in glia and activate NF-κB, which would induce glial proliferation and production of cytokines and free radicals that further facilitate neurodegeneration. Arrows indicate stimulating and ⊥ signs indicate inhibitory effects; thicker solid lines indicate enhanced, whereas broken lines indicate decreased levels of activation in NPC.
Together, our results showed that the PI3K-Akt-GSK-3β signaling system was markedly disturbed during early postnatal development in Npc1−/− mouse brains, especially in regions that exhibit early neurodegeneration. Changes in GSK-3β were closely associated with accumulation of cholesterol and deregulation of NF-κB. Furthermore, the appearance of NF-κB in microglia was temporally and spatially associated with microglia activation, a result which suggests that deregulation of the GSK-3β/NF-κB pathway contributes to neurodegeneration in Npc1−/− mice not only by directly affecting neuronal function but also indirectly through microglia activation. Results from the present study could therefore provide novel therapeutic targets to develop new approaches for treating this devastating disease.
Acknowledgments
We thank Lawrence Fu, Yvette Gonzales, Kevin Lee, and Carmen Rivera for excellent technical support.
Footnotes
Address reprint requests to Dr. Xiaoning Bi, Department of Psychiatry and Human Behavior, 101 Theory Dr., #250, UC Irvine, Irvine, CA 92617. E-mail: xbi@uci.edu.
Supported by grants from University of California at Irvine, National Institute of Neurological Disorders and Stroke, and the National Institutes of Health (to X.B.).
References
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O’Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- Auer IA, Schmidt ML, Lee VM, Curry B, Suzuki K, Shin RW, Pentchev PG, Carstea ED, Trojanowski JQ. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer’s disease. Acta Neuropathol (Berl) 1995;90:547–551. doi: 10.1007/BF00318566. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Parker CC, Pentchev PG, Katz D, Ghetti B, D’Agostino AN, Carstea ED. Neurofibrillary tangles in Niemann-Pick disease type C. Acta Neuropathol (Berl) 1995;89:227–238. doi: 10.1007/BF00309338. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sakiyama T, Harada N, Abe M, Tadokoro M. Pathologic changes of glial cells in murine model of Niemann-Pick disease type C: immunohistochemical, lectin-histochemical and ultrastructural observations. Pediatr Int. 2003;45:1–4. doi: 10.1046/j.1442-200x.2003.01651.x. [DOI] [PubMed] [Google Scholar]
- Love S, Bridges LR, Case CP. Neurofibrillary tangles in Niemann-Pick disease type C. Brain. 1995;118:119–129. doi: 10.1093/brain/118.1.119. [DOI] [PubMed] [Google Scholar]
- Patterson MC, Pentchev PG. Niemann-Pick; type C. Neurology. 1996;46:1785–1786. doi: 10.1212/wnl.46.6.1785-b. [DOI] [PubMed] [Google Scholar]
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Xiong S, Xie C, Davies P, Markesbery WR. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimers Dis. 2004;6:659–671. doi: 10.3233/jad-2004-6610. [DOI] [PubMed] [Google Scholar]
- Brion JP, Anderton BH, Authelet M, Dayanandan R, Leroy K, Lovestone S, Octave JN, Pradier L, Touchet N, Tremp G. Neurofibrillary tangles and tau phosphorylation. Biochem Soc Symp. 2001;67:81–88. doi: 10.1042/bss0670081. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur Neuropsychopharmacol. 2002;12:13–25. doi: 10.1016/s0924-977x(01)00131-6. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H. Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med. 2002;8:126–132. doi: 10.1016/s1471-4914(01)02266-3. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Beffert U, Busciglio J, Brady ST. Fast axonal transport misregulation and Alzheimer’s disease. Neuromol Med. 2002;2:89–99. doi: 10.1385/NMM:2:2:089. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer’s disease. Brain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- Vantler M, Caglayan E, Zimmermann WH, Baumer AT, Rosenkranz S. Systematic evaluation of anti-apoptotic growth factor signaling in vascular smooth muscle cells: only phosphatidylinositol 3′-kinase is important. J Biol Chem. 2005;280:14168–14176. doi: 10.1074/jbc.M413310200. [DOI] [PubMed] [Google Scholar]
- Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- De Ketelaere A, Vermeulen L, Vialard J, Van De Weyer I, Van Wauwe J, Haegeman G, Moelans I. Involvement of GSK-3beta in TWEAK-mediated NF-kappaB activation. FEBS Lett. 2004;566:60–64. doi: 10.1016/j.febslet.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Deng J, Xia W, Miller SA, Wen Y, Wang HY, Hung MC. Crossregulation of NF-kappaB by the APC/GSK-3beta/beta-catenin pathway. Mol Carcinog. 2004;39:139–146. doi: 10.1002/mc.10169. [DOI] [PubMed] [Google Scholar]
- Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3 beta regulates NF-kappa B1/p105 stability. J Biol Chem. 2003;278:39583–39590. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol. 2002;283:G204–G211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- Bournat JC, Brown AM, Soler AP. Wnt-1 dependent activation of the survival factor NF-kappaB in PC12 cells. J Neurosci Res. 2000;61:21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene. 1999;18:6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol. 2002;64:963–970. doi: 10.1016/s0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12:73–90. doi: 10.1016/s1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- Baudry M, Yao Y, Simmons D, Liu J, Bi X. Postnatal development of inflammation in a murine model of Niemann-Pick type C disease: immunohistochemical observations of microglia and astroglia. Exp Neurol. 2003;184:887–903. doi: 10.1016/S0014-4886(03)00345-5. [DOI] [PubMed] [Google Scholar]
- Rong Y, Baudry M. Seizure activity results in a rapid induction of nuclear factor-kappa B in adult but not juvenile rat limbic structures. J Neurochem. 1996;67:662–668. doi: 10.1046/j.1471-4159.1996.67020662.x. [DOI] [PubMed] [Google Scholar]
- Bornig H, Geyer G. Staining of cholesterol with the fluorescent antibiotic “filipin.”. Acta Histochem. 1974;50:110–115. [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol Pathol. 2000;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker WJ. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685:48–62. doi: 10.1016/j.bbalip.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Vincent I, Bu B, Erickson RP. Understanding Niemann-Pick type C disease: a fat problem. Curr Opin Neurol. 2003;16:155–161. doi: 10.1097/01.wco.0000063764.15877.1c. [DOI] [PubMed] [Google Scholar]
- Liscum L, Sturley SL. Intracellular trafficking of Niemann-Pick C proteins 1 and 2: obligate components of subcellular lipid transport. Biochim Biophys Acta. 2004;1685:22–27. doi: 10.1016/j.bbalip.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Ong WY, Kumar U, Switzer RC, Sidhu A, Suresh G, Hu CY, Patel SC. Neurodegeneration in Niemann-Pick type C disease mice. Exp Brain Res. 2001;141:218–231. doi: 10.1007/s002210100870. [DOI] [PubMed] [Google Scholar]
- German DC, Quintero EM, Liang C, Xie C, Dietschy JM. Degeneration of neurons and glia in the Niemann-Pick C mouse is unrelated to the low-density lipoprotein receptor. Neuroscience. 2001;105:999–1005. doi: 10.1016/s0306-4522(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Yamada A, Saji M, Ukita Y, Shinoda Y, Taniguchi M, Higaki K, Ninomiya H, Ohno K. Progressive neuronal loss in the ventral posterior lateral and medial nuclei of thalamus in Niemann-Pick disease type C mouse brain. Brain Dev. 2001;23:288–297. doi: 10.1016/s0387-7604(01)00209-1. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Gong JS, Garver WS, Heidenreich RA, Ninomiya H, Ohno K, Yanagisawa K, Michikawa M. Site-specific phosphorylation of tau accompanied by activation of mitogen-activated protein kinase (MAPK) in brains of Niemann-Pick type C mice. J Biol Chem. 2001;276:10314–10319. doi: 10.1074/jbc.M009733200. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Gong JS, Chang TY, Yanagisawa K, Michikawa M. Promotion of tau phosphorylation by MAP kinase Erk1/2 is accompanied by reduced cholesterol level in detergent-insoluble membrane fraction in Niemann-Pick C1-deficient cells. J Neurochem. 2003;84:1086–1096. doi: 10.1046/j.1471-4159.2003.01596.x. [DOI] [PubMed] [Google Scholar]
- Bu B, Li J, Davies P, Vincent I. Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model. J Neurosci. 2002;22:6515–6525. doi: 10.1523/JNEUROSCI.22-15-06515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtanik KM, Liscum L. The transport of low density lipoprotein-derived cholesterol to the plasma membrane is defective in NPC1 cells. J Biol Chem. 2003;278:14850–14856. doi: 10.1074/jbc.M300488200. [DOI] [PubMed] [Google Scholar]
- Peres C, Yart A, Perret B, Salles JP, Raynal P. Modulation of phosphoinositide 3-kinase activation by cholesterol level suggests a novel positive role for lipid rafts in lysophosphatidic acid signalling. FEBS Lett. 2003;534:164–168. doi: 10.1016/s0014-5793(02)03832-2. [DOI] [PubMed] [Google Scholar]
- German DC, Liang CL, Song T, Yazdani U, Xie C, Dietschy JM. Neurodegeneration in the Niemann-Pick C mouse: glial involvement. Neuroscience. 2002;109:437–450. doi: 10.1016/s0306-4522(01)00517-6. [DOI] [PubMed] [Google Scholar]
- Dahle MK, Overland G, Myhre AE, Stuestol JF, Hartung T, Krohn CD, Mathiesen O, Wang JE, Aasen AO. The phosphatidylinositol 3-kinase/protein kinase B signaling pathway is activated by lipoteichoic acid and plays a role in Kupffer cell production of interleukin-6 (IL-6) and IL-10. Infect Immun. 2004;72:5704–5711. doi: 10.1128/IAI.72.10.5704-5711.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivarelli MS, McDonald D, Miller M, Cusson N, Kelliher M, Geha RS. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp Med. 2004;200:399–404. doi: 10.1084/jem.20040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Culmsee C, Klumpp S, Krieglstein J. Neuroprotection by transforming growth factor-beta1 involves activation of nuclear factor-kappaB through phosphatidylinositol-3-OH kinase/Akt and mitogen-activated protein kinase-extracellular-signal regulated kinase1,2 signaling pathways. Neuroscience. 2004;123:897–906. doi: 10.1016/j.neuroscience.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22:RC216:1–7. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Goebel HH. Isocortical pathology in type C Niemann-Pick disease. A combined Golgi-pigmentoarchitectonic study. J Neuropathol Exp Neurol. 1983;42:671–687. doi: 10.1097/00005072-198311000-00007. [DOI] [PubMed] [Google Scholar]
- Bednarski E, Ribak CE, Lynch G. Suppression of cathepsins B and L causes a proliferation of lysosomes and the formation of meganeurites in hippocampus. J Neurosci. 1997;17:4006–4021. doi: 10.1523/JNEUROSCI.17-11-04006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Zhou J, Lynch G. Lysosomal protease inhibitors induce meganeurites and tangle-like structures in entorhinohippocampal regions vulnerable to Alzheimer’s disease. Exp Neurol. 1999;158:312–327. doi: 10.1006/exnr.1999.7087. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Kar S, Dore S, Quirion R. Insulin-like growth factor-1 (IGF-1): a neuroprotective trophic factor acting via the Akt kinase pathway. J Neural Transm Suppl. 2000;60:261–272. doi: 10.1007/978-3-7091-6301-6_17. [DOI] [PubMed] [Google Scholar]
- Rodgers EE, Theibert AB. Functions of PI 3-kinase in development of the nervous system. Int J Dev Neurosci. 2002;20:187–197. doi: 10.1016/s0736-5748(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Rao R, Hao CM, Breyer MD. Hypertonic stress activates glycogen synthase kinase 3beta-mediated apoptosis of renal medullary interstitial cells, suppressing an NFkappaB-driven cyclooxygenase-2-dependent survival pathway. J Biol Chem. 2004;279:3949–3955. doi: 10.1074/jbc.M309325200. [DOI] [PubMed] [Google Scholar]
- Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004;279:55633–55643. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- Buss H, Dorrie A, Schmitz ML, Frank R, Livingstone M, Resch K, Kracht M. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem. 2004;279:49571–49574. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- Haefner B. A model for NF-kappa B regulation by GSK-3 beta. Drug Discov Today. 2003;8:1062–1063. doi: 10.1016/s1359-6446(03)02898-8. [DOI] [PubMed] [Google Scholar]
- Wu YP, Mizukami H, Matsuda J, Saito Y, Proia RL, Suzuki K. Apoptosis accompanied by up-regulation of TNF-alpha death pathway genes in the brain of Niemann-Pick type C disease. Mol Genet Metab. 2005;84:9–17. doi: 10.1016/j.ymgme.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini AM, Jiang X, Wu X, Tian F, Zhu D, Okagaki P, Lipsky RH. Role of brain-derived neurotrophic factor and NF-kappaB in neuronal plasticity and survival: from genes to phenotype. Restor Neurol Neurosci. 2004;22:121–130. [PubMed] [Google Scholar]
- Henderson LP, Lin L, Prasad A, Paul CA, Chang TY, Maue RA. Embryonic striatal neurons from Niemann-Pick type C mice exhibit defects in cholesterol metabolism and neurotrophin responsiveness. J Biol Chem. 2000;275:20179–20187. doi: 10.1074/jbc.M001793200. [DOI] [PubMed] [Google Scholar]
- Paul CA, Boegle AK, Maue RA. Before the loss: neuronal dysfunction in Niemann-Pick type C disease. Biochim Biophys Acta. 2004;1685:63–76. doi: 10.1016/j.bbalip.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Guirland C, Suzuki S, Kojima M, Lu B, Zheng JQ. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- Peiro S, Comella JX, Enrich C, Martin-Zanca D, Rocamora N. PC12 cells have caveolae that contain TrkA. Caveolae-disrupting drugs inhibit nerve growth factor-induced, but not epidermal growth factor-induced, MAPK phosphorylation. J Biol Chem. 2000;275:37846–37852. doi: 10.1074/jbc.M000487200. [DOI] [PubMed] [Google Scholar]
- Huang CS, Zhou J, Feng AK, Lynch CC, Klumperman J, DeArmond SJ, Mobley WC. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J Biol Chem. 1999;274:36707–36714. doi: 10.1074/jbc.274.51.36707. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17 beta-estradiol. J Neurochem. 2001;78:1315–1324. doi: 10.1046/j.1471-4159.2001.00511.x. [DOI] [PubMed] [Google Scholar]
- Rosenstiel P, Lucius R, Deuschl G, Sievers J, Wilms H. From theory to therapy: implications from an in vitro model of ramified microglia. Microsc Res Tech. 2001;54:18–25. doi: 10.1002/jemt.1116. [DOI] [PubMed] [Google Scholar]