Abstract
Transforming growth factor-β (TGF-β), a multifunctional growth factor, represents three mammalian isoforms, TGF-β1, TGF-β2, and TGF-β3. In cutaneous wound healing, combined neutralization of TGF-β1 and -β2 or addition of TGF-β3 reduces scar formation. Here, we investigated whether experimental manipulation of TGF-β isoforms reduced adhesion formation after injury to the peritoneum. Adhesions were produced in mice by surgical abrasion of adjacent serosa followed by close apposition. In the first part of this study, a detailed analysis of TGF-β isoform distribution was performed through immunolocalization. TGF-β isoforms clearly showed a unique temporal and spatial pattern of expression after peritoneal wounding. Based on this pharmacokinetic data, we next administered neutralizing antibodies to TGF-β1 and -β2 or exogenous TGF-β3 peptide by local application and intraperitoneal injection at various times before and after surgery. At day 7 after surgery, addition of neutralizing antibodies to both TGF-β1 and -β2 significantly reduced the number and size of adhesions (P < 0.05) compared with the vehicle control. By contrast, exogenous addition of TGF-β3 either had no effect or increased adhesion formation compared to the vehicle control. In conclusion, these results show that by blocking both TGF-β1 and TGF-β2 using neutralizing antibodies, it is possible to prevent abdominal adhesion formation.
Peritoneal adhesions are well vascularized and innervated fibrous bands of tissue, which join together previously separated intra-abdominal organs. They commonly occur after surgical trauma, developing in more than 90% of patients undergoing laparotomy.1 A third of intestinal obstructions and nearly a quarter of female infertility cases are a consequence of adhesion formation, with surgical lysis resulting in a high recurrence rate.2 This widespread condition therefore represents a tremendous financial burden to health services, in terms of time to re-enter a patient’s abdomen for further surgery and the cost of treating adhesion complications. For instance, the cost of adhesiolysis alone in the United States in 1994 amounted to $1.3 billion.3 However, despite their clinical importance, information regarding the molecular and cellular events regulating adhesion formation is sparse, and current prevention is based on careful surgery and the occasional use of physical barriers that are effective in only a proportion of patients.
Peritoneal adhesions form when closely apposed visceral and/or parietal peritoneal surfaces are damaged due to surgical trauma, ischemic injury, inflammation, or a foreign body reaction. The protective mesothelial layer is disrupted and a fibrinous exudate is deposited between the damaged surfaces. These fibrinous adhesions are transient and degraded by proteases of the fibrinolytic system within a few days of injury, leading to restoration of the normal peritoneal surface.4 However, if there is insufficient peritoneal fibrinolytic activity, the fibrinous scaffold persists, becomes organized by invading fibroblasts and endothelial cells, and with subsequent collagen deposition forms a permanent fibrous adhesion within 1 week of surgery.5 Although serosal hypofibrinolysis is thought to be a major pathogenic factor in adhesion formation,6,7 the mechanisms modulating peritoneal fibrinolytic activity are not well understood. Peritoneal injury initiates a proinflammatory response, with the release of cytokines such as tumor necrosis factor-α, interleukin-1, interleukin-6, and transforming growth factor-β1 (TGF-β1).8–12 In vitro studies using human mesothelial cells,12–14 have shown that these proinflammatory cytokines, individually and synergistically, stimulate the production of plasminogen activator inhibitor-1 (PAI-1) and reduce the synthesis of tissue plasminogen activator (tPA). Therefore, it is likely that increased cytokine levels after injury lead to a reduction in peritoneal fibrinolytic capacity and an increase in adhesion formation.
TGF-β family members are key molecular mediators of pathological tissue fibrosis. This cytokine family is represented by three mammalian isoforms, TGF-β1, TGF-β2, and TGF-β3, which are thought to have distinct functions in vivo.15 TGF-β1 is the principal isoform implicated in fibrotic disorders in most organ systems.16–19 In the eye however, it is TGF-β2 rather than TGF-β1 that is believed to be the predominant isoform mediating fibrosis.20 The role of TGF-β3 in tissue repair is less well documented, with a number of groups demonstrating either a pro- or anti-fibrotic effect, depending on the body site investigated.21–25 Exogenous addition of TGF-β3 to rat incisional wounds led to an overall reduction in scarring, comparable with the effects observed after the combined neutralization of TGF-β1 and TGF-β2. Levels of TGF-β1 are apparently increased in adhesion tissue and associated peritoneal fluid of both human patients26,27 and surgically induced experimental models of adhesion formation.9,10,28 However, TGF-β1 and -β2 manipulation studies aimed at decreasing peritoneal adhesion formation have led to apparently conflicting results. After surgical injury to the uterine horns in rats, Williams and colleagues29 found that intraperitoneal injection of TGF-β1 for 5 days led to significantly more adhesions forming compared with the saline control. However, using the same model, the addition of an antibody against both TGF-β1 and TGF-β2 showed no effect. In another study, using a rat peritoneal resection model, Lucas and colleagues30 showed that intraperitoneal injection of neutralizing TGF-β1 antibody for 3 days reduced adhesion formation, although no effect was observed when rats were treated with either an anti-TGF-β2 or a pan-specific neutralizing antibody to all three isoforms. Krause and colleagues31 investigated adhesion formation in the TGF-β1 (+/−) heterozygous mouse. Contrary to expectation, these animals showed an increased severity of adhesions compared to the wild type, despite a reduction in TGF-β1 in the peritoneal fluid. A role for TGF-β3 in peritoneal adhesion formation has not been evaluated.
The objective of the current study was to investigate the role of TGF-β isoforms in peritoneal adhesion formation through pharmacological manipulation. We hypothesized that adhesions could be reduced either by the addition of neutralizing antibodies to TGF-β1 and TGF-β2 or exogenous addition of TGF-β3, based on the concept from cutaneous wound healing studies that a reduction in scarring depends on increasing the ratio of TGF-β3 relative to TGF-β1/TGF-β2.22 We initially demonstrate through immunolocalization that TGF-β isoform expression is altered after peritoneal injury with each isoform showing a unique temporal and spatial pattern. We then validate the importance of individual isoforms by the addition of neutralizing antibodies to TGF-β1 and TGF-β2 or the exogenous addition of TGF-β3 peptide in a murine surgical model of adhesion formation. In the final set of experiments, the administration schedule was altered based on a kinetic study that investigated the clearance of neutralizing antibodies in the peritoneal cavity. Furthermore, the inflammatory cell infiltrate in peritoneal fluid was assessed after these manipulations to establish a relationship between the changing profile of TGF-β isoforms, corresponding inflammation, and adhesion formation.
Materials and Methods
Animals
C57BL/6J adult male mice age 10 to 12 weeks with a weight of 25 to 30 g were used in all experiments and purchased from Harlan Ltd., Bicester, UK. All mice were maintained under standard conditions of food and water ad libitum on a 12-hour day-night cycle according to Home Office regulations.
Experimental Models of Adhesion Formation
Adhesions were induced in mice based on a procedure developed by Sulaiman and colleagues.32 Briefly, mice were anesthetized with a mixture of inhaled isoflurane and oxygen, and a midline incision was made through the abdominal wall and peritoneum. A standard site (6 mm diameter and 1 mm depth), midway and ∼0.5 cm lateral to the midline incision on the left abdominal wall, was injured using a trauma instrument developed by Dr. Mark Eastwood (Department of Biomedical Sciences, University of Westminster, London, UK). This comprised a clamping device that allowed the trauma to be size- and site-specific, and an abrading rod with collar that restricted the depth of insertion and hence pressure applied. The rod was rotated three times to produce a consistent lesion, verified histologically. The cecum was isolated and scraped 30 times on its lateral aspect with a scalpel blade, after first irrigating with 0.9% sterile saline. Hemorrhage was induced by lacerating a small blood vessel on the medial surface of the cecum with a hypodermic needle. The two injured surfaces were then apposed by placing two horizontal mattress sutures (8/0 Ethilon; Ethicon, Berkshire, UK) 1.2 cm apart. The midline incision was closed in two layers; the linea-alba with a continuous suture, and then the skin using interrupted sutures (6/0 Ethilon, Ethicon).
TGF-β Isoform Expression
Animals (n = 6/group) were randomly assigned to surgical or nonsurgical control (NS) groups and adhesions induced as described above. From 12 hours to 14 days after surgery, animals were harvested and adhesion tissue processed for immunocytochemical analysis. Sections were immunostained with primary rabbit antibodies against TGF-β1, TGF-β2, and TGF-β3 (catalog nos. sc-146, sc-90, and sc-82, respectively; Santa Cruz, Wiltshire, UK) using the immunoperoxidase technique. Briefly, after rehydrating 7 μm frozen sections with 10 mmol/L phosphate-buffered saline (PBS), pH 7.2, endogenous peroxidase activity was blocked by incubation in 0.3% (v/v) hydrogen peroxide in methanol for 30 minutes. Sections were then blocked with 1.5% donkey serum in PBS/0.1% bovine serum albumin and incubated overnight at 4°C with the primary antibody diluted to 1 μg/ml in PBS/0.1% bovine serum albumin. Sections were washed three times with PBS/Triton X-100, incubated with anti-rabbit biotinylated secondary antibody (Amersham Int. Plc, Bucks, UK) at a concentration of 2.5 μg/ml for 30 minutes, washed as above omitting the Triton X-100, and then treated for 30 minutes with peroxidase ABC reagent (Vectastain Elite ABC kit: Vector Laboratories, Burlingame, CA). After a final wash of PBS, sections were developed using a dilute solution of Vector Nova Red substrate (Vector Laboratories) and then counterstained with Gill’s hematoxylin (Vector Laboratories), dehydrated, and mounted in pertex mounting medium (CellPath, Powys, UK). Primary antibody specificity was determined by replacing it with a preabsorbed control; the antibody in question incubated with excess blocking peptide (Santa Cruz) before use. To ascertain potential nonspecific staining from the secondary antibody, the primary antibody was replaced with nonimmune serum from the species in which the secondary antibody was raised. In all cases immunostaining was eliminated.
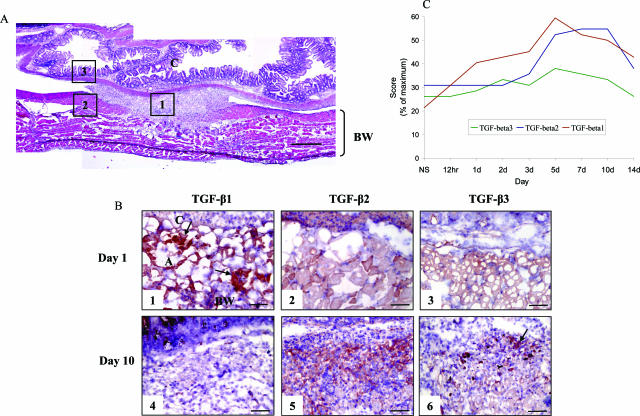
Positive immunostaining intensity and distribution were evaluated by visual examination in a blinded manner. Immunostaining was graded on a scale of (−) representing no staining, to (+++) representing intense immunoreactivity. Tissue sections were divided into three main regions; the trauma site adhesion, adjacent/adjoining body wall, and cecum (Figure 1A). Staining scores for each region were summated to produce a semiquantitative assessment.
Figure 1.
Spatial and temporal expression of TGF-β isoforms during adhesion formation. Animals (n = 6/time point) were surgically induced to form adhesions. From 12 hours to 14 days after surgery, animals were harvested and trauma site adhesion tissue processed for immunocytochemical analysis. A: Tissue sections were divided into three main regions; the trauma site adhesion (1), adjacent body wall (2), and adjacent cecum (3). B: TGF-β isoform expression at days 1 and 10 after surgery. TGF-β1 was the only isoform up-regulated in the first 3 days after injury, associated with the provisional extracellular matrix (arrows). Cellular staining for TGF-β1 was also observed however this was of a transient nature, with negligible immunoreactivity evident at day 10. There was minimal staining for TGF-β2 at day 1 after surgery; however, a marked rise in immunoreactivity was then observed that remained elevated at day 10. TGF-β3 was minimally up-regulated after injury at day 1 and day 10 localized predominantly to degenerating skeletal muscle fibers (arrow). Positive immunostaining was evaluated by visual examination and graded with (−) representing no staining, and (+++) representing intense immunoreactivity. C: Staining scores for each region were summated and displayed as a percentage of the maximum score to produce a semiquantitative assessment. NS, nonsurgical control; C, cecum; BW, body wall; A, adhesion tissue. In all images, cecum is at the top of the image, the body wall at the bottom, with the adhesion in the center. Staining: Nova Red and Gills hematoxylin counterstain. Scale bars: 400 μm (A); 50 μm (B).
Modulation of TGF-β Isoform Profile
In an initial study, animals (n = 6/group) were randomly divided into seven experimental groups; surgical control (group I), vehicle control for TGF-β1 and -β2 (group II), neutralizing antibody to TGF-β1 (group III), neutralizing antibody to TGF-β2 (group IV), combined neutralizing antibodies to TGF-β1 and -β2 (group V), vehicle control for TGF-β3 (group VI), and active TGF-β3 peptide (group VII). Neutralizing antibodies to TGF-β1 (100 μg/direct application or intraperitoneal injection, chicken polyclonal against recombinant human TGF-β1, catalog no: AF-101-NA; R&D Systems Inc., Minneapolis, MN) and TGF-β2 (100 μg/direct application or intraperitoneal injection, goat polyclonal against porcine TGF-β2, catalog no: AF-302-NA; R&D Systems Inc.) were diluted in sterile PBS, pH 7.2. The neutralizing antibodies are stated by the manufacturer (R&D Systems Inc.) to have less than 2% cross-reactivity with other TGF-β isoforms. Exogenous active TGF-β3 peptide (100 ng/topical or intraperitoneal injection; Renovo, Manchester, UK) was diluted in 5% (w/v) mannitol, 20 mmol/L acetic acid in PBS, pH 3.8, and 0.0002% isopropyl alcohol. Injections of all reagents were administered at the time of surgery (50 μl locally infiltrated into the abraded body wall and 100 μl topically applied to the apposed serosa) and then at 4, 24, and 48 hours after surgery (1 ml intraperitoneally). Vehicle control animals in the neutralizing antibody group were treated with sterile PBS, pH 7.2, whereas the vehicle control for the TGF-β3 group contained 5% (w/v) mannitol, 20 mmol/L acetic acid in PBS, pH 3.8, and 0.0002% isopropyl alcohol. In the surgical control group, the abdomen was penetrated with a sterile hypodermic needle at the appropriate time points.
Clearance Kinetics of TGF-β1 Neutralizing Antibody
To determine the optimal administration regime, clearance kinetic studies of TGF-β1 neutralizing antibody were performed. After abrasion of the body wall, neutralizing antibody to TGF-β1 (chicken polyclonal against recombinant human TGF-β1, catalog no: AB-101-NA; R&D Systems) was administered by local infiltration (100 μg in 150 μl of sterile PBS, pH 7.2) or intraperitoneal injection (100 μg in 1 ml of sterile PBS, pH 7.2). Control animals were treated with irrelevant chicken polyclonal IgY antibody (catalog no: AB-101-C; R&D Systems) diluted to the same concentration and volume with sterile PBS, pH 7.2, or PBS alone. Tissue at the wound site and peritoneal fluid were harvested 5, 10, 15, and 60 minutes, and 4, 8, 12, and 24 hours after wounding (n = 2 to 3 at each time point), and processed for immunofluorescent analysis. Frozen cryostat sections (7 μm) and air-dried peritoneal fluid smears were fixed in acetone for 6 minutes and then incubated with biotinylated anti-chicken IgY secondary antibody (catalog no: 303-065-008; Jackson ImmunoResearch, West Grove, PA) at a concentration of 12 μg/ml for 1 hour. After washing with PBS, sections were incubated for 40 minutes with Texas Red-conjugated streptavidin (catalog no: RPN1233; Amersham Int. Plc) at a concentration of 1:100. To label cell nuclei, Hoechst no. 33342 (catalog no: B2261; Sigma-Aldrich Ltd., Dorset, UK) at a concentration of 50 μg/ml was added in the last 10 minutes of incubation, followed by a final wash before mounting. Intensity of immunostaining was evaluated subjectively using a categorical scoring system, ranging from (−) which indicated that staining was absent, to (++++) which indicated strong staining.
A second manipulation (n = 10/group) was performed in which the administration schedule was altered based on a clearance kinetics study of TGF-β1 neutralizing antibody (Table 1). In summary, combined TGF-β1 and -β2 antibody was administered before surgery, at the time of surgery, and then at 4 hourly intervals up to 24 hours, whereas decreasing doses of TGF-β3 peptide were administered before surgery, at the time of surgery, and then at 4, 12, 24, 36, and 48 hours after surgery.
Table 1.
Clearance of Neutralizing Antibodies from the Peritoneal Cavity
| Route | Solution | Site | Time after Injection
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 minutes | 10 minutes | 60 minutes | 4 hours | 8 hours | 12 hours | 24 hours | |||
| DA | PBS | TS | − | − | − | − | − | − | − |
| IgY | TS | ++++ | +++ | ++ | + | +/− | − | − | |
| āTGF-β1 | TS | ++++ | ++++ | +++ | +++ | + | − | − | |
| TS | − | − | − | − | − | − | − | ||
| PBS | PF | − | − | − | − | − | − | − | |
| TS | +++ | ++ | + | − | − | − | − | ||
| IP | Igy | PF | +++ | +++ | + | − | − | − | − |
| TS | +++ | ++ | ++ | + | − | − | − | ||
| āTGF-β1 | PF | ++++ | +++ | +++ | + | +/− | − | − | |
Following abrasion of the body wall anti-TGF-β1 antibodies were administered immediately by direct application (100 μg/150 μl injection; DA) or by intraperitoneal injection (100 μg/1 ml injection; IP) after the abdomen was closed. Control wounds were treated with an irrelevant Ig (chicken IgY) or sterile PBS, pH 7.2, in the same manner as TGF-β1 antibody. Tissue from the trauma site (TS) and peritoneal fluid (PF) were harvested after wounding and antibodies immunolocalized (n = 2 to 3 at each time point). Intensity of immunostaining was evaluated subjectively using a categorical scoring system, ranging from (−) which indicated that staining was absent, to (++++) which indicated strong staining.
Evaluation of Adhesion Tissue, Peritoneal Fluid Collection, and Processing
At 7 days after surgery, mice were killed and peritoneal lavage performed by injecting intraperitoneally 3 ml of ice-cold sterile PBS. After gentle abdominal massage, the lavage was collected and fetal calf serum (PAA Laboratories, Somerset, UK) added (10% w/v). The absolute number of cells recovered was determined using a hemocytometer after trypan blue staining. A differential cell count was performed on cytospin preparations using May-Grunwald Giemsa stain to determine the number of macrophages, lymphocytes, and polymorphonuclear cells per 200 cells.
Adhesions were assessed macroscopically according to the number and site of adhesion formation and then photographed for independent examination. A category 1 adhesion was defined as an adhesion between the two abraded serosal surfaces, ie, cecum to abraded body wall. Category 2 adhesions involved the two abraded surfaces and an uninvolved site, such as the greater omentum and abraded body wall. Category 3 adhesions described adhesions that formed at a distant site to the abraded serosa, for example the fat body to the midline incision. Adhesion tenacity was evaluated by making a subjective assessment of the ease of physical lysis. A categorical system was used, ranging from a score of 1, which indicated lysis with minimal force, up to 3 which indicated lysis only with surgical dissection.
For histological assessment, resected adhesion tissue or intact peritoneum was fixed overnight in 10% buffered formal saline, pH 7.0. After fixation, the cecum was flushed with PBS until clear of all fecal material and processed for routine histology. For immunocytochemical analysis, tissue was snap-frozen in liquid nitrogen-cooled isopentane, embedded in OCT compound (Cell Path), and stored at −80°C. Cryosections (7 μm thick), were collected on 3-aminopropyltriethoxysilane (Sigma-Aldrich Co. Ltd., Poole, UK) coated–glass slides before fixation for 6 minutes in acetone.
Assessment of Adhesion Volume and Cellular Infiltrate
Trauma site adhesions (category 1) were sectioned in their entirety, generating up to 600 sections, 5 μm thick. To calculate the volume of adhesions, the Cavalieri estimator was used.33 This provides an unbiased estimate of volume, deduced from a sum of profile areas on a set of systematically positioned parallel sections separated by a constant distance. Between 5 and 10 sections were analyzed for each adhesion.
To determine the cellular profile in trauma site adhesions, images were captured at ×20 magnification and the adhesion outlined using image analysis software. The cell count was then quantified on the basis of hematoxylin nuclear stain, with data expressed as the mean number of cells per mm2. A minimum of four sections were examined per adhesion.
Both histological and immunostained sections were viewed using a Leica DMRB microscope and images captured with a Spot RT Slider digital camera and Spot RT v3.1 software (Diagnostic Instruments, Inc., Sterling Heights, MI). Cell counts and adhesion volume were quantified using Image Pro-Plus software (Media Cybernetics, Silver Spring, MD). One investigator (D.A.G.) performed all surgery and assessed adhesion formation, blinded to treatment groups. Adhesions were photographed and a second investigator (S.E.H.) validated the scores, again blinded to treatment groups. One investigator (D.A.G.) performed all peritoneal fluid cell counts and demonstrated consistency between repeated counts.
Statistical Analysis
All data were expressed as the mean ± the SEM and analyzed by nonparametric methods using either SimFit (W.G. Bardsley, University of Manchester, Manchester, UK) or Stats Direct (Stats Direct Ltd., Cheshire, UK) statistical software. The Mann-Whitney U-test was used to compare the number of adhesions between two groups. When more than two groups were analyzed, the Kruskall-Wallis test was used initially to identify a difference and if this proved significant, individual groups were further investigated using Conover-Inman, a nonparametric posthoc test. The χ2 test was used to analyze binomial or proportionate data such as the number of trauma site adhesions between groups, whereas the Cuzick’s trend test was used to identify a dose response or temporal effect. In all tests P < 0.05 was considered significant.
Results
TGF-β Isoform Expression
To determine the unique profile of individual TGF-β isoforms during adhesion formation, their temporal and spatial expression after peritoneal injury was investigated through immunolocalization. Mice (n = 6/time point) were wounded, and between 12 hours and 14 days after surgery animals were harvested and adhesion tissue processed for immunocytochemical analysis. All three isoforms showed constitutive, but distinct, staining in uninjured cecum and body wall. Neuronal tissue stained intensely for TGF-β1, whereas TGF-β2 was localized to skeletal muscle fibers of the body wall and smooth muscle of the cecum. Although there was minimal staining for TGF-β3 in the body wall, the tunica media of blood vessels and the muscular propria of the cecum were intensely immunoreactive.
An initial increase in TGF-β1 immunoreactivity occurred 1 day after wounding in association with the fibrin-rich provisional matrix (Figure 1, A and B). Cellular staining for TGF-β1 peaked between 5 and 7 days after injury, however this was significantly reduced by day 10 (Figure 1B). There was negligible staining for TGF-β2 at day 1 after surgery (Figure 1B), however a marked rise in cellular TGF-β2 immunoreactivity was observed at day 5. Unlike the transient cellular expression for TGF-β1, TGF-β2 was up-regulated within the adhesion until day 10 (Figure 1B). In contrast, although TGF-β3 was constitutively present in normal tissue, there was minimal up-regulation after injury localized predominantly to degenerating and necrotic skeletal muscle fibers of the body wall (Figure 1B). Findings from semiquantitative assessment of TGF-β isoform expression are summarized in Figure 1C.
Initial Manipulation of TGF-β Isoforms
The first experimental TGF-β isoform manipulation was performed to assess the effect of administration of TGF-β1 and -β2 neutralizing antibodies and exogenous TGF-β3 peptide on adhesion formation. Mice (n = 6/group) were randomly assigned to treatment (III, IV, V, and VII), surgical control (I), or vehicle control (II, VI) groups, wounded, and injected at the time of surgery and again at 4, 24, and 48 hours. Adhesions were evaluated macroscopically at day 7 after surgery (Figure 2).
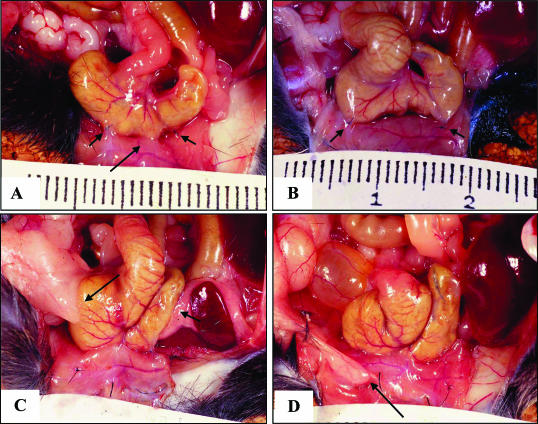
Figure 2.
Macroscopic appearance of adhesions 7 days after surgery. A: Trauma site adhesion (long arrow) from the TGF-β1 and -β2 vehicle control group, showing clear separation from the appositional sutures (short arrows). B: One of three animals from the combined neutralizing TGF-β1 and -β2 antibody group that failed to form a category 1 adhesion between appositional sutures (short arrows).C: Adhesion formed at the trauma site and at a distant site (category 2) in animals treated with TGF-β3 peptide (long arrow, fat body; short arrow, omentum). D: Distant site adhesion (category 3) from the TGF-β1 and -β2 antibody vehicle control group, involving the fat body and the midline incision (arrow).
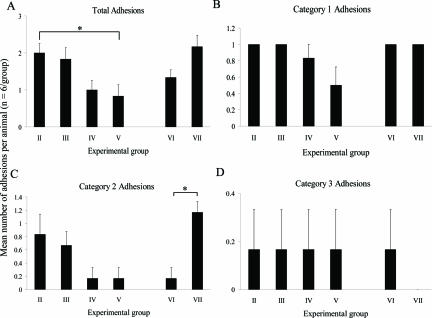
There was no significant difference in the total number of adhesions or any of the categories of adhesions formed between the surgical control group (group I) and vehicle controls (group II and group VI) or between the two vehicle control groups. Therefore, the effects of neutralizing antibodies to TGF-β1 and TGF-β2 or TGF-β3 peptide on adhesion formation were compared with their respective vehicle control group (Figure 3). Exogenous addition of anti-TGF-β1 neutralizing antibody alone did not affect the total number of adhesions formed per animal (Figure 3A), or number of trauma site adhesions (Figure 3B, category 1) compared to the vehicle control group. Injection of anti-TGF-β2 antibody however, led to a reduction in total adhesions (Figure 3A; P = 0.07) compared to the vehicle control, primarily due to a decrease in category 2 adhesions, although this too was not significant (Figure 3C; P = 0.08). A synergistic effect was observed when neutralizing antibodies to TGF-β1 and TGF-β2 were given in combination, resulting in a significant reduction in total adhesion formation (P < 0.05), and a 50% reduction in adhesions at the trauma site (category 1) compared to the vehicle control group. All animals treated with the TGF-β3 vehicle control (group VI) formed category 1 adhesions, thereby allowing only a decrease in adhesions to be observed at this site. Addition of TGF-β3 increased the total number of adhesions formed compared to the vehicle control and this appeared to be due to a significant increase in category 2 adhesions (Figure 3C; P < 0.05), because all animals formed adhesions at the trauma site, similar to that observed in the vehicle control group.
Figure 3.
Effect of initial TGF-β isoform experimental manipulation on adhesion formation 7 days after surgery. Data represents mean number of total (A), category 1 (B), category 2 (C), and category 3 (D) adhesions formed per animal for each experimental group (n = 6/group); vehicle control for TGF-β1 and -β2 antibodies (group II), neutralizing antibody to TGF-β1 (group III), neutralizing antibody to TGF-β2 (group IV), combined neutralizing antibodies to TGF-β1 and -β2 (group V), vehicle control for TGF-β3 (group VI), and TGF-β3 peptide (group VII). No significant difference in adhesion formation was detected between the surgical control and vehicle control groups; therefore the effects of neutralizing antibodies to TGF-β1 and -β2 or TGF-β3 peptide on adhesion formation were compared with their respective vehicle control group. Error bars indicate the SEM. *P < 0.05 compared with the vehicle control (Kruskall-Wallis followed by Conover-Inman posthoc test).
In approximately one third of all operated animals, an appositional suture had pulled through the tissue, thereby allowing damaged surfaces to separate. The loss of a suture clearly had no effect in groups in which all animals formed adhesions at the trauma site. Furthermore, in the anti-TGF-β2 and the combined anti-TGF-β1 and -β2 antibody-treated groups, in which there was a reduction in trauma site adhesions, a Fisher’s exact test showed that suture loss did not influence resultant adhesion formation (anti-TGF-β2, P = 0.33; combined anti-TGF-β1 and -β2, P = 0.99). No difference was observed between treatment and control groups with regards to healing of the mid-line incision.
In summary, the total number of peritoneal adhesions was reduced by the combined addition of neutralizing antibodies to TGF-β1 and TGF-β2, confirming an important role of these two isoforms in adhesion formation. Addition of TGF-β3 either had no significant effect or appeared to increase adhesion formation.
Clearance of Neutralizing Antibodies to TGF-β1
Although these initial results implicated TGF-β1 and -β2 isoforms in adhesion formation, a failure to achieve a significant reduction in trauma site adhesions (category 1) in any of the treatment groups may have been due to inadequate delivery of neutralizing antibodies. Therefore, an additional study was performed investigating the clearance pharmacokinetics of antibodies to TGF-β1 within peritoneal tissue and fluid (Table 1). Neutralizing antibody to TGF-β1 was detected in the abraded body wall and on the surface of uninjured peritoneum up to 4 hours after either direct application or intraperitoneal injection. At 8 hours however, weak staining was observed at the trauma site only after direct application of antibody. By contrast, an irrelevant chicken IgY was detected at the trauma site up to 4 hours after direct application and 1 hour after intraperitoneal injection. At all time points, immunostaining for both the neutralizing antibody and the IgY appeared to be either matrix associated or nonspecifically bound to the surface of both the uninjured and injured body wall.
There was intense immunostaining for both anti-TGF-β1 and the IgY in the peritoneal fluid 10 minutes after intraperitoneal injection (both cells and fluid). After 1 hour, only cellular staining for both the neutralizing antibody and the IgY was present, but at a lower intensity. At 4 hours immunostaining was markedly reduced in the neutralizing antibody-treated group, and was no longer detected for the IgY-treated group. At all times, wounds injected with PBS (negative control) showed no positive staining.
Further Manipulation Using Anti-TGF-β1 and Anti-TGF-β2 Neutralizing Antibodies
Findings from the clearance kinetic study established that the neutralizing antibody to TGF-β1 could be detected in the abraded body wall and on the surface of uninjured peritoneum for up to 4 hours after both direct application and intraperitoneal injection. Therefore in a further study using an altered treatment regime (Table 2), antibodies were administered at 4 hourly intervals up to 24 hours after wounding, as apposed to a 24-hour interval between applications. In addition, it was considered advantageous to administer the antibodies 2 hours before the initial trauma, because TGF-β1 is known to be released immediately at the time of wounding. Adhesions were assessed at 7 days after surgery as previously described.
Table 2.
Dose and Administration Regime of Combined Neutralizing Antibodies to TGF-β1 and -β2 or Exogenous TGF-β3 Peptide in a Second Manipulation
| Time of injection (hours before or after surgery)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −2 | 0 | 4 | 8 | 12 | 16 | 20 | 24 | 36 | 48 | |
| Number of animals at each time point | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Anti-TGF-β1 + β2 (100 μg/injection) | + | + | + | + | + | + | + | + | − | − |
| TGF-β3 (2.5 ng/injection) | + | + | + | − | + | − | − | + | + | + |
| TGF-β3 (5 ng/injection) | + | + | + | − | + | − | − | + | + | + |
| TGF-β3 (10 ng/injection) | + | + | + | − | + | − | − | + | + | + |
| TGF-β3 (50 ng/injection) | + | + | + | − | + | − | − | + | + | + |
| TGF-β3 (100 ng/injection) | + | + | + | − | + | − | − | + | + | + |
Based on the findings of the clearance kinetics study, an altered treatment regime was performed in order to assess the effect of blocking both TGF-β1 and -β2 isoforms on peritoneal adhesion formation. Different doses of TGF-β3 peptide were also administered using a modified treatment regime. + indicates reagent administered, - indicates no administration.
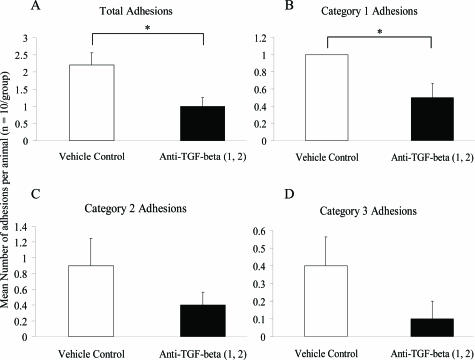
Using this altered treatment regime, all animals in the vehicle control group formed adhesions at the trauma site (category 1), whereas animals treated with neutralizing antibodies to both TGF-β1 and TGF-β2 showed significantly reduced total (Figure 4A; P < 0.05) and trauma site adhesions (Figure 4B; P < 0.05). Furthermore, adhesions involving the abraded surfaces and an uninvolved site (category 2) were reduced in the combined neutralizing antibody group compared to the vehicle control group; however this did not reach significance (Figure 4C; P = 0.4). The majority of these adhesions consisted of the cecum adherent to itself or the fat body. There was also a reduction in distant site adhesions (category 3) in the neutralizing antibody group compared to the vehicle control group, although because this was a rare occurrence in either group statistical significance was not achieved (Figure 4D; P = 0.4).
Figure 4.
Effect of combined neutralizing antibodies to TGF-β1 and TGF-β2 on adhesion formation in second manipulation study. The data represent the mean number of total (A), category 1 (B), category 2 (C), and category 3 (D) adhesions formed per animal for each group (n = 10), with error bars indicating the SEM. *P < 0.05 compared with the vehicle control (Mann-Whitney U-test).
Adhesion tenacity was evaluated by making a subjective assessment of the ease of physical lysis. A categorical system was used, ranging from a score of 1, which indicated lysis with minimal force, up to 3 that indicated lysis only with surgical dissection. Regardless of treatment group, all adhesions that formed at the trauma site (category 1) after 7 days were opaque in appearance and could only be separated by surgical dissection. Although there was a reduction in category 2 adhesion tenacity in the combined neutralizing antibody group compared to the vehicle control group, this was not significant (adhesion tenacity, 2.4 ± 0.4 for anti-TGF-β1 and anti-TGF-β2 versus 3.0 ± 0.0 for vehicle control; P = 0.33). No difference was observed between the groups with regards to healing of the midline incision.
In approximately half of the animals, an appositional suture was absent at harvest. As before, this had no effect on adhesion formation in the vehicle control group in which all animals formed trauma site adhesions. In the combined anti-TGF-β1 and -β2 group, in which there was a reduction in category 1 adhesions, the Fisher’s exact test showed that suture loss did not influence resultant adhesion formation (P = 0.58).
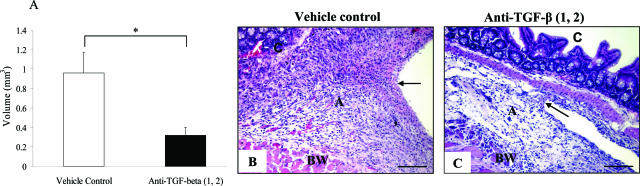
Effect of Neutralizing Antibodies to TGF-β1 and TGF-β2 on Trauma Site Adhesion Volume, Cellularity, and Vascularity
Although the macroscopic appearance of trauma site adhesions (category 1) that formed in the neutralizing antibody and control groups was similar, it was unclear whether inhibiting TGF-β1 and -β2 affected adhesion volume, cellularity, or vascularity. Adhesion volume was determined using the Cavalieri principle, which is an unbiased estimate of volume, deduced from a sum of profile areas on a set of systematically positioned parallel sections separated by a constant distance. This demonstrated that adhesions in the combined anti-TGF-β1 and -β2 antibody group were significantly reduced in volume at 7 days after surgery compared to those in the vehicle control group (Figure 5, A to C; P < 0.05). With regard to cellularity, there was no significant difference (5835 ± 372 nucleated cells/mm2 for anti-TGF-β1 and -β2 versus 6085 ± 280 cells/mm2 for vehicle control; P = 0.42) detected between the antibody-treated and control groups at day 7 after surgery, with minimal intra-adhesion variability. Macrophages and lymphocytes were predominant in both groups, although the vehicle control adhesions appeared to show a higher fibroblast component compared to the neutralizing antibody group. There was no significant difference in the number of blood vessels, as a measure of angiogenic response, between the treatment and control groups at day 7 after surgery (186 ± 17 blood vessels/mm2 for anti-TGF-β1 and -β2 versus 180 ± 16 for vehicle control; P < 0.99).
Figure 5.
Effect of neutralizing antibodies to TGF-β1 and TGF-β2 on adhesion volume and cellularity. A: Trauma site adhesions were processed for routine histology, and adhesion volume evaluated using the Cavalieri principle. Data represent the mean for each group (n = 5), with error bars indicating the SEM. *P < 0.05 compared with the vehicle control (Mann-Whitney U-test). B and C: Typical trauma site (category 1) adhesion in the vehicle control (B) and neutralizing antibody to TGF-β1 and -β2 groups (C). The adhesion in the vehicle control group appeared larger; although there was no significant difference in cellularity (cells/mm2). BW, body wall; A, adhesion; C, cecum. Arrows represent the edge of the adhesion. Staining: H&E. Scale bars, 100 μm.
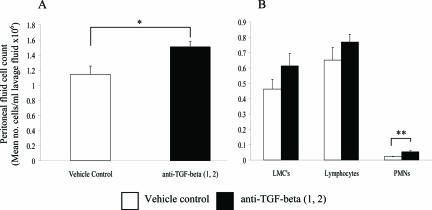
The Effect of Neutralizing Antibodies to TGF-β1 and TGF-β2 on Peritoneal Fluid Cellularity
TGF-β1 is known to play a critical role in immunoregulation; therefore we investigated whether the reduction in adhesion formation after the addition of antibodies to TGF-β1 and TGF-β2 was associated with an altered inflammatory cell profile. At 7 days after surgery, total peritoneal fluid cell count in the neutralizing antibody group was significantly higher compared to the vehicle control group (Figure 6A; P < 0.05). Differential cell counts revealed an increase in all leukocyte subsets assessed; although this was only significant for polymorphonuclear cells (Figure 6B; P < 0.01). An attempt was made to further differentiate mesothelial cells from monocytes/macrophages using BM8, a pan-macrophage marker. This was not satisfactory however, because this antibody fails to detect all monocytes and macrophages. Although a few mast cells were observed, there were similar numbers detected in both groups (less than 1% of the total cell count).
Figure 6.
Total peritoneal fluid cell count 7 days after surgery after the addition of neutralizing antibodies to TGF-β1 and TGF-β2. Peritoneal fluid was collected after lavage with 3 ml of PBS, and the total (A) and differential cell count (B) analyzed. The data represents the mean for each group (n = 10), with error bars indicating the SEM. *P < 0.05 and **P < 0.01 compared with the vehicle control (Mann-Whitney U-test). LMCs represent large mononuclear cells and include monocytes, macrophages, and mesothelial cells.
Further Manipulation Using TGF-β3 Peptide on Adhesion Formation
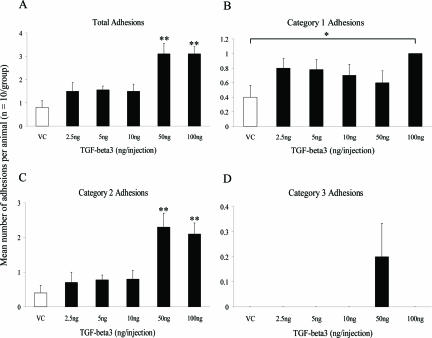
The initial study suggested that TGF-β3 (100 ng/injection) either showed no effect or increased adhesion formation. Therefore, in the second manipulation a dose-response experiment was performed together with an altered treatment regime. Groups of animals (n = 10) were injected intraperitoneally with either vehicle control or exogenous TGF-β3 peptide (2.5, 5, 10, 50, 100 ng/injection) before, at the time of, and after surgery (4, 12, 24, 36, and 48 hours) and adhesions were evaluated 7 days later. Exogenous addition of TGF-β3 produced a significant trend toward increased total adhesion formation with increasing dose (Figure 7A; P < 0.01); with animals treated with 50 and 100 ng of TGF-β3 developing significantly more adhesions compared to the vehicle control group (P < 0.005). A dose response with TGF-β3 was not observed for trauma site adhesions (category 1). However, animals treated with 100 ng of TGF-β3 formed significantly more category 1 adhesions compared to animals in the vehicle control group (Figure 7B; P < 0.05), but not compared to the vehicle control group from the first manipulation. All adhesions that formed at the trauma site whether in treated or untreated groups were opaque in appearance and could only be separated by surgical dissection. At harvest, an appositional suture was absent in a sixth of all animals, however this had no effect on animals treated with 100 ng of TGF-β3 because all formed adhesions at the trauma site. In treatment groups in which there was a reduction in category 1 adhesions, suture loss did not influence resultant adhesion formation, as demonstrated by the Fisher’s exact test (TGF-β3 2.5 ng, P = 0.4; TGF-β3 10 ng, P = 0.3).
Figure 7.
Effect of increasing dose of TGF-β3 peptide adhesion formation in a second manipulation study. The data represent the mean total (A), category 1 (B), category 2 (C), and category 3 (D) adhesions formed in each animal for each group (n = 10), with error bars indicating the SEM. *P < 0.05 and **P < 0.005 compared with the vehicle control (Kruskall-Wallis followed by Conover-Inman posthoc test). VC, vehicle control.
As with total adhesion formation, the addition of TGF-β3 showed a significant trend of increased adhesion formation between the abraded serosal surfaces and a distant site (category 2) with increasing dose of peptide (Figure 7C; P < 0.01, Cuzick’s trend test). In addition, animals treated with 50 and 100 ng of TGF-β3 developed significantly more category 2 adhesions compared to animals in the vehicle control group (P < 0.005). More than 50% of these adhesions consisted of the cecum adherent to itself or the fat body. Animals treated with TGF-β3 also formed adhesions between the cecum and either the omentum or the liver, with 40% of category 2 adhesions involving these sites at the highest doses (50 and 100 ng). In addition, exogenous addition of TGF-β3 led to a dose-dependent increase in category 2 adhesion tenacity (P < 0.001). Adhesions distant to the trauma site (category 3 adhesions) were found only in animals treated with 50 ng of TGF-β3 (Figure 7D), and only 2 of the 10 animals were involved. No difference was observed between experimental groups with regards to healing of the midline incision.
Effect of Exogenous TGF-β3 on Adhesion Volume and Cellular Infiltrate
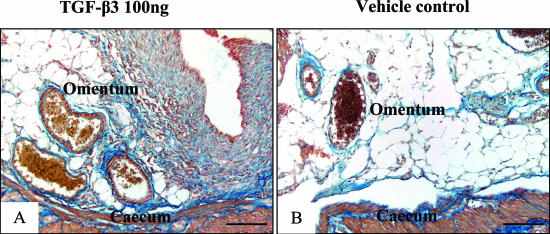
The volume, cellularity, and blood vessel density of trauma site adhesions were evaluated for the group treated with the highest dose of TGF-β3 (100 ng) and the vehicle control group (n = 3/group). In contrast to the TGF-β1 and -β2 neutralizing antibody manipulation, the addition of TGF-β3 (100 ng) led to an increase in adhesion volume compared to the vehicle control, however this failed to reach significance (0.64 ± 0.08 mm3 for 100 ng TGF-β3 versus 0.36 ± 0.07 mm3 for vehicle control; P = 0.1). This was accompanied by an increase in adhesion cellularity (6602 ± 381 cells/mm2 for 100 ng TGF-β3 versus 4811 ± 355 cells/mm2 for vehicle control; P = 0.07) in the TGF-β3 group, that appeared to be predominantly fibroblastic. There appeared to be no difference in the number of blood vessels between treatment groups (166 ± 33 blood vessels/mm2 for 100 ng TGF-β3 versus 117 ± 26 for vehicle control; P = 0.4). Although collagen density was not quantified, based on the Mallory connective tissue stain, adhesions from the 100 ng TGF-β3 group appeared more fibrous compared to the vehicle or surgical control groups. This was particularly striking in adhesions that formed between the abraded serosal surfaces and uninvolved sites such as the cecum and the greater omentum (Figure 8, A and B).
Figure 8.
Effect of TGF-β3 peptide on collagen deposition in category 2 adhesions. Based on the Mallory connective tissue stain, adhesions from animals treated with 100 ng of TGF-β3 (A) appeared more fibrous compared to the vehicle control group (B). This was particularly striking in adhesions that formed between the abraded serosal surfaces and uninvolved sites such as the distant cecum and the omentum. Staining: Mallory’s trichrome. Scale bars, 50 μm.
Effect of Exogenous TGF-β3 on Peritoneal Fluid Cellularity
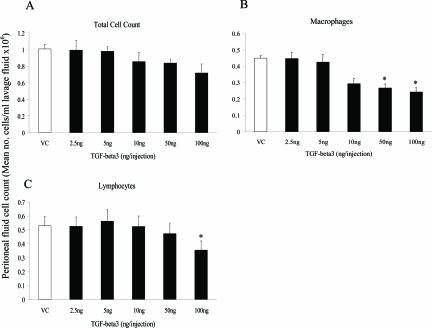
In contrast to the TGF-β1 and -β2 neutralizing antibody results, the addition of TGF-β3 led to a dose-dependent decrease in total peritoneal fluid cell count, reflected by a dose-dependent decrease in macrophages (Figure 9, A and B; P < 0.005, Cuzick’s trend test). In addition, animals treated with 50 and 100 ng of TGF-β3 had a significantly lower number of macrophages compared to the vehicle control (Figure 9B; P < 0.05). Although there was no dose-dependent effect on lymphocyte profile, animals treated with 100 ng of TGF-β3 had a significantly lower lymphocyte number compared to the vehicle control group (Figure 9C; P < 0.05). In contrast to the neutralizing antibody results, the addition of TGF-β3 did not appear to affect the number of polymorphonuclear cells observed and although a few mast cells were detected (less than 0.5% of the total cell count), there was no difference between groups.
Figure 9.
Effect of addition of TGF-β3 peptide on peritoneal fluid cell count. Peritoneal fluid was collected at harvest (7 days after surgery), after lavage with 3 ml of PBS, and the total and differential cell count analyzed as described in the text. The data represent the mean for each group (n = 10), with error bars indicating the SEM. *P < 0.05 compared with the vehicle control (Kruskall-Wallis followed by Conover-Inman posthoc test). VC, vehicle control.
Discussion
Successful adhesion prevention is unlikely to be achieved unless the pathophysiological mechanisms at the cellular and molecular level are understood. Changes in cytokine levels, in particular TGF-β isoforms, are likely to be key molecular mediators of pathological tissue fibrosis. Although TGF-β has been implicated in abdominal adhesion formation through its up-regulation in tissue and peritoneal fluid, manipulation studies in various animal models have led to conflicting results.29–31 In an initial immunolocalization study, we demonstrated that the expression of all three TGF-β isoforms increased after peritoneal wounding confirming the findings of others.28,34 However we also showed that each isoform displayed a unique temporal and spatial pattern, suggesting that each may play a distinct role in adhesion formation. This was subsequently addressed through pharmacological manipulation studies.
In a preliminary manipulation, exogenous addition of neutralizing antibodies to TGF-β1 alone at 0, 4, 24, and 48 hours after surgery had little effect on total adhesion formation compared to either the vehicle or surgical control groups, and no effect on trauma site (category 1) adhesions. However using the same dosing regime, intraperitoneal injection of anti-TGF-β2 antibodies reduced total adhesion formation, primarily due to decreased adhesions between the abraded surfaces and an uninvolved site (category 2 adhesions), although this was not significant. A significant effect was however observed when the neutralizing antibodies were combined (anti-TGF-β1 and -β2), resulting in 50% reduction in the total number of adhesions formed. These data suggest a synergistic effect of TGF-β1 and TGF-β2 in adhesion formation. At the dose and administration schedule used, the addition of TGF-β3 increased the number of category 2 adhesions compared to the vehicle control group.
The failure to achieve a significant reduction in trauma site adhesions (category 1) after the addition of anti-TGF-β1 and TGF-β2 antibodies in the preliminary study could have been due to inadequate delivery of the antibodies to the site of injury. This may also explain contradictory results in several peritoneal adhesion-related TGF-β neutralizing studies, despite the application of antibodies daily for up to 5 days after wounding.29,30,34 A further study was performed to investigate the pharmacokinetic clearance of antibodies in the peritoneum by immunolocalization of anti-TGF-β1 antibody at the trauma site and in the peritoneal fluid. This demonstrated a rapid clearance of both TGF-β1 antibody and an irrelevant control IgY antibody from the peritoneal cavity. These results are in marked contrast to those described for cutaneous wound healing in the rat,22 and highlight the risk of extrapolating clearance kinetic data from one body site to another. The results do however correlate well with a murine study that assessed the efficacy of locally applied polyclonal IgG as a complement to antibiotic therapy for infectious peritonitis.35 Although the initial antibody dose of 10 mg was far higher than that used in the current study (100 μg), the results confirm the rapid clearance of antibodies from the peritoneal cavity, and therefore the need in future studies for frequent administration. Furthermore, it was considered advantageous to administer the antibody before the initial trauma because platelets, a rich source of TGF-β1, release the growth factor on degranulation of α-granules at the site of injury. Because anti-TGF-β1 could be detected on the surface of uninjured peritoneum 4 hours after administration, an intraperitoneal injection 2 hours before surgery was thought appropriate.
In a further manipulation using a modified dosing regime, combined neutralization of TGF-β1 and TGF-β2 led to a significant reduction in the number of trauma site (category 1) adhesions compared to the vehicle control group. This was associated with decreased collagen deposition and lower cellularity. This finding may be due to the pivotal role of TGF-β1 in the reduction of fibrinolytic activity after surgery, through a combined increase in the level of PAI-1 and a decrease in tPA, thus allowing the fibrinous matrix to persist and become organized into a permanent adhesion.36 It is likely that the early application of neutralizing antibodies blocks TGF-β1 released from activated platelets,37 and prevents the subsequent injury-induced expression of immediate-early genes that contribute to prolonged autoinduction and up-regulation of this isoform. The decreased TGF-β1 levels primarily result in an increase in fibrinolytic activity that leads to the complete or partial resolution of the fibrinous matrix. Complete resolution would result in adhesion-free healing, as observed in 50% of the animals treated with anti-TGF-β1 and -β2. If there is only partial resolution however, the fibrinous scaffold will persist, although reduced in volume. Both TGF-β1 and -β2 are likely to play an important role in the fibrous phase of peritoneal adhesion formation.38–42 The effect of blocking TGF-β1 on extracellular matrix production is well recognized and has been demonstrated in a rabbit intra-articular adhesion study, in which the continuous administration of anti-TGF-β1 antibodies led to a reduction in adhesion formation and collagen content, as assessed 4 weeks after wounding.43
TGF-β2 is the most prevalent isoform in body fluids such as the vitreous and aqueous fluid of the eye, tears, and saliva.20 Although TGF-β2 levels have not been measured in peritoneal fluid, we have shown it to be markedly up-regulated after surgery in adhesion tissue. This together with findings from the first manipulation supports a role for TGF-β2 in the pathogenesis of adhesion formation. Whether or not this role is independent of TGF-β1 is unknown. A synergistic effect of neutralizing both TGF-β1 and TGF-β2 was observed in the current study, confirming findings in the skin where a maximal reduction in extracellular matrix deposition and scar formation occurred when both isoforms were blocked in a rat cutaneous wound-healing model.22 TGF-β2 is known to up-regulate the expression and secretion of TGF-β1 by monocytes, macrophages, fibroblasts, and epithelial cells;44–46 therefore the presence of endogenous TGF-β2 may be responsible for some of the up-regulation of TGF-β1 even in the presence of neutralizing antibody to this isoform.
On initial examination, our results contrast with previous TGF-β neutralizing antibody studies in rat peritoneal adhesion models.29,30,34 After surgical injury to the uterine horns in rats, Williams and colleagues29 found that intraperitoneal addition of an antibody against both TGF-β1 and TGF-β2 (10 μg daily for 5 days; R&D Systems Inc.) showed no effect on adhesion formation after uterine horn abrasion. In addition, Lucas and colleagues30 found that the intraperitoneal injection of an antibody directed against TGF-β2 (50 μg daily for 2 days; catalog no: AB-12-NA; R&D Systems Inc.) had little effect on adhesion severity in a rat peritoneal resection model, whereas antibodies to TGF-β1 (67 μg daily for 2 days; catalog no: AB-101; R&D Systems Inc.) reduced adhesion severity. One reason for the discrepancy between their results and ours may be due to the different doses, time of application, and source of neutralizing antibody used. Furthermore, because different species and experimental models were used, it is difficult to make direct comparisons with the findings in our study.
Initial manipulation of TGF-β3 peptide using 100 ng/injection suggested that this isoform either had no effect or increased adhesion formation, in particular those involving one of the abraded surfaces and an uninjured site (category 2 adhesions). In a second manipulation, exogenous addition of TGF-β3 showed a significant trend toward increased total adhesion formation with increasing dose, again predominantly due to an increase in category 2 adhesions. The addition of TGF-β3 at lower doses appeared to have no effect on trauma site adhesion formation (category 1 adhesions). It was only at the highest dose of TGF-β3 (100 ng) that an increase in category 1 adhesions was observed. These adhesions were also larger in volume compared to those of the vehicle control, associated with increased collagen deposition, and increased cellularity due to a predominant fibroblast component. At first sight these findings appear to contrast with the anti-scarring effects of TGF-β3 in the skin.22 However, TGF-β3 is markedly motogenic,47 stimulating filopodia formation and rapid cell migration.48 Therefore, it could be predicted that by increasing fibroblast migration there would be a rapid consolidation of the fibrinous adhesion, with an associated increase in fibroblast number and collagen deposition. This could lead to an increase in adhesion formation, as observed after application of the higher doses of TGF-β3.
There is limited information regarding the biological effects of TGF-β3 in comparison to TGF-β1 and TGF-β2. A reduction in peritoneal fibrinolytic capacity is considered critical in the early stages of the adhesion process6,49 and although a specific role of TGF-β3 in fibrinolysis has not been investigated, all three isoforms have been shown to induce PAI-1 expression in a bioassay using a truncated PAI-1 promoter/luciferase construct.50 TGF-β3 may therefore play a similar role to TGF-β1 in fibrinolytic reduction, through a combined increase in the level of PAI-1 and a decrease in tPA. It is clear that further in vitro studies are required to support this assumption; however, it is tempting to speculate that this mechanism induces the marked increase in adhesions between the abraded serosa and fatty tissues such as the omentum and pelvic fat body. Before a definitive conclusion can be made regarding the role of TGF-β3 in adhesion formation further dose/time response experiments need to be performed, as do experiments using neutralizing antibodies specific to this isoform or a pan-specific neutralizing antibody.
The inflammatory cell infiltrate in peritoneal fluid was assessed to establish a relationship between the manipulation of TGF-β isoforms, corresponding inflammation, and adhesion formation. The combined addition of neutralizing antibodies to TGF-β1 and TGF-β2 led to decreased adhesions but an increased inflammatory cell count in the peritoneal fluid at 7 days after wounding, whereas the addition of TGF-β3 increased adhesions but had an anti-inflammatory effect. Although TGF-β1 plays an important role in promoting the inflammatory process, it is also a powerful immunosuppressant.51 The apparent inverse relationship between inflammatory cell count and adhesion formation at day 7 after wounding has not been reported before, and suggests that the effects of inflammation on adhesiogenesis is complex and that the two may not be causally related. In fact, evidence from studies in the lung suggests that it may be possible to dissociate the inflammatory response from the fibrotic response.52,53 An assessment of inflammatory cells at multiple time points after TGF-β isoform manipulation may elucidate some of the underlying mechanisms; further aided through the use of transgenic mice that underexpress endothelial adhesion molecules such as L-selectin or ICAM-1, resulting in reduced inflammatory cell extravasation.54
In summary, the results from this study have shown that by blocking both TGF-β1 and TGF-β2 using neutralizing antibodies, it is possible to prevent abdominal adhesion formation. Moreover, the few adhesions that did form were decreased in volume compared to those of the vehicle control. These are novel and clinically important findings, because inhibiting TGF-β1 and TGF-β2 activity may reduce both the incidence and the severity of adhesions. Although, the clinical problems associated with adhesions may not always be related to their number or size, decreasing their incidence will result in a beneficial outcome.
Acknowledgments
We thank Mary Birch, Tom Ferreday, Hugh Laverty, Rosie Saleem, and Sylwia Wilkosz for technical assistance.
Footnotes
Address reprint requests to Dr. Sarah E. Herrick, Room 3.239, Stopford Building, Faculty of Life Sciences, University of Manchester, Manchester, UK M13 9PT. E-mail: sarah.herrick@manchester.ac.uk.
Supported by the Wellcome Trust, UK, and the Medical Research Council.
References
- Menzies D, Ellis H. Intestinal obstruction from adhesions—how big is the problem? Ann R Coll Surg Engl. 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg Suppl. 1997;577:5–9. [PubMed] [Google Scholar]
- Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1–9. doi: 10.1016/s1072-7515(97)00127-0. [DOI] [PubMed] [Google Scholar]
- Risberg B. Adhesions: preventive strategies. Eur J Surg Suppl. 1997;577:32–39. [PubMed] [Google Scholar]
- Raftery AT. Regeneration of peritoneum: a fibrinolytic study. J Anat. 1979;129:659–664. [PMC free article] [PubMed] [Google Scholar]
- Vipond MN, Whawell SA, Thompson JN, Dudley HA. Peritoneal fibrinolytic activity and intra-abdominal adhesions. Lancet. 1990;335:1120–1122. doi: 10.1016/0140-6736(90)91125-t. [DOI] [PubMed] [Google Scholar]
- Holmdahl L, Eriksson E, Eriksson BI, Risberg B. Depression of peritoneal fibrinolysis during operation is a local response to trauma. Surgery. 1998;123:539–544. doi: 10.1067/msy.1998.86984. [DOI] [PubMed] [Google Scholar]
- Badia JM, Whawell SA, Scott-Coombes DM, Abel PD, Williamson RC, Thompson JN. Peritoneal and systemic cytokine response to laparotomy. Br J Surg. 1996;83:347–348. doi: 10.1002/bjs.1800830316. [DOI] [PubMed] [Google Scholar]
- Chegini N, Simms J, Williams RS, Masterson BJ. Identification of epidermal growth factor, transforming growth factor-alpha, and epidermal growth factor receptor in surgically induced pelvic adhesions in the rat and intraperitoneal adhesions in the human. Am J Obstet Gynecol. 1994;171:321–328. doi: 10.1016/s0002-9378(94)70030-3. [DOI] [PubMed] [Google Scholar]
- Rong H, Tang X, Zhao Y, Juneja SC, Fay MF, Williams RS, Chegini N. Postsurgical intraperitoneal exposure to glove powders modulates inflammatory and immune-related cytokine production. Wound Repair Regen. 1997;5:89–96. doi: 10.1046/j.1524-475X.1997.50116.x. [DOI] [PubMed] [Google Scholar]
- Tsukada K, Katoh H, Shiojima M, Suzuki T, Takenoshita S, Nagamachi Y. Concentrations of cytokines in peritoneal fluid after abdominal surgery. Eur J Surg. 1993;159:475–479. [PubMed] [Google Scholar]
- Tietze L, Elbrecht A, Schauerte C, Klosterhalfen B, Amo-Takyi B, Gehlen J, Winkeltau G, Mittermayer C, Handt S. Modulation of pro- and antifibrinolytic properties of human peritoneal mesothelial cells by transforming growth factor beta1 (TGF-beta1), tumor necrosis factor alpha (TNF-alpha) and interleukin 1beta (IL-1beta). Thromb Haemost. 1998;79:362–370. [PubMed] [Google Scholar]
- van Hinsbergh VW, Kooistra T, Scheffer MA, Hajo van Bockel J, van Muijen GN. Characterization and fibrinolytic properties of human omental tissue mesothelial cells. Comparison with endothelial cells. Blood. 1990;75:1490–1497. [PubMed] [Google Scholar]
- Whawell SA, Thompson JN. Cytokine-induced release of plasminogen activator inhibitor-1 by human mesothelial cells. Eur J Surg. 1995;161:315–318. [PubMed] [Google Scholar]
- O’Kane S, Ferguson MW. Transforming growth factor betas and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998;11:1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- Okuda S, Languino LR, Ruoslahti E, Border WA. Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest. 1990;86:453–462. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer HU, Friess H, Abou-Shady M, Berberat P, Zimmermann A, Gold LI, Korc M, Buchler MW. Transforming growth factor betas and their receptors in human liver cirrhosis. Eur J Gastroenterol Hepatol. 1998;10:1031–1039. doi: 10.1097/00042737-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang K, Garner W, Cohen L, Rodriguez J, Phan S. Increased types I and III collagen and transforming growth factor-beta 1 mRNA and protein in hypertrophic burn scar. J Invest Dermatol. 1995;104:750–754. doi: 10.1111/1523-1747.ep12606979. [DOI] [PubMed] [Google Scholar]
- Cordeiro MF. Beyond mitomycin: TGF-beta and wound healing. Prog Retin Eye Res. 2002;21:75–89. doi: 10.1016/s1350-9462(01)00021-0. [DOI] [PubMed] [Google Scholar]
- Cox D, McMaster G, Burgi R, Kunz S, Vaxelaire J, O’Reilly T, Schupp J. Experimental wound healing with TGF-beta3. Wound Repair Regen. 1994;2:S208. [Google Scholar]
- Shah M, Foreman DM, Ferguson MWJ. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Wu L, Siddiqui A, Morris DE, Cox DA, Roth SI, Mustoe TA. Transforming growth factor beta 3 (TGF beta 3) accelerates wound healing without alteration of scar prominence. Histologic and competitive reverse-transcription-polymerase chain reaction studies. Arch Surg. 1997;132:753–760. doi: 10.1001/archsurg.1997.01430310067014. [DOI] [PubMed] [Google Scholar]
- Tyrone JW, Marcus JR, Bonomo SR, Mogford JE, Xia Y, Mustoe TA. Transforming growth factor beta3 promotes fascial wound healing in a new animal model. Arch Surg. 2000;135:1154–1159. doi: 10.1001/archsurg.135.10.1154. [DOI] [PubMed] [Google Scholar]
- Coerper S, Sigloch E, Cox D, Starlinger M, Koveker G, Becker HD. Recombinant human transforming growth factor beta 3 accelerates gastric ulcer healing in rats. Scand J Gastroenterol. 1997;32:985–990. doi: 10.3109/00365529709011214. [DOI] [PubMed] [Google Scholar]
- Chegini N, Rong H, Bennett B, Stone IK. Peritoneal fluid cytokine and eicosanoid levels and their relation to the incidence of peritoneal adhesion. J Soc Gynecol Invest. 1999;6:153–157. doi: 10.1016/s1071-5576(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Holmdahl L, Kotseos K, Bergstrom M, Falk P, Ivarsson ML, Chegini N. Overproduction of transforming growth factor-beta1 (TGF-beta1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery. 2001;129:626–632. doi: 10.1067/msy.2001.113039. [DOI] [PubMed] [Google Scholar]
- Ghellai A, Stucchi A, Chegini N, Ma C, Andry C, Kaseta J, Burns J, Skinner K, Becker J. Role of transforming growth factor beta-1 in peritonitis-induced adhesions. J Gastrointest Surg. 2000;4:316–323. doi: 10.1016/s1091-255x(00)80082-7. [DOI] [PubMed] [Google Scholar]
- Williams RS, Rossi AM, Chegini N, Schultz G. Effect of transforming growth factor beta on postoperative adhesion formation and intact peritoneum. J Surg Res. 1992;52:65–70. doi: 10.1016/0022-4804(92)90280-d. [DOI] [PubMed] [Google Scholar]
- Lucas PA, Warejcka DJ, Young HE, Lee BY. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor-beta1. J Surg Res. 1996;65:135–138. doi: 10.1006/jsre.1996.0355. [DOI] [PubMed] [Google Scholar]
- Krause TJ, Katz D, Wheeler CJ, Ebner S, McKinnon RD. Increased levels of surgical adhesions in TGFbeta1 heterozygous mice. J Invest Surg. 1999;12:31–38. doi: 10.1080/089419399272746. [DOI] [PubMed] [Google Scholar]
- Sulaiman H, Gabella G, Davis C, Mutsaers SE, Boulos P, Laurent GJ, Herrick SE. Growth of nerve fibres into murine peritoneal adhesions. J Pathol. 2000;192:396–403. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH710>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Chegini N, Gold LI, Williams RS, Masterson BJ. Localization of transforming growth factor beta isoforms TGF-beta 1, TGF-beta 2, and TGF-beta 3 in surgically induced pelvic adhesions in the rat. Obstet Gynecol. 1994;83:449–454. [PubMed] [Google Scholar]
- Barekzi NA, Poelstra KA, Felts AG, Rojas IA, Slunt JB, Grainger DW. Efficacy of locally delivered polyclonal immunoglobulin against Pseudomonas aeruginosa peritonitis in a murine model. Antimicrob Agents Chemother. 1999;43:1609–1615. doi: 10.1128/aac.43.7.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk P, Ma C, Chegini N, Holmdahl L. Differential regulation of mesothelial cell fibrinolysis by transforming growth factor beta 1. Scand J Clin Lab Invest. 2000;60:439–447. doi: 10.1080/003655100448419. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. [PubMed] [Google Scholar]
- Roberts A, Sporn M. Sporn M, Roberts A, editors. Berlin: Springer Verlag; The Transforming Growth Factors Beta. 1990:pp 419–472. [Google Scholar]
- Quaglino D, Jr, Nanney LB, Ditesheim JA, Davidson JM. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin: incisional wound model. J Invest Dermatol. 1991;97:34–42. [PubMed] [Google Scholar]
- Saed GM, Zhang W, Chegini N, Holmdahl L, Diamond MP. Alteration of type I and III collagen expression in human peritoneal mesothelial cells in response to hypoxia and transforming growth factor-beta1. Wound Repair Regen. 1999;7:504–510. doi: 10.1046/j.1524-475x.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- Ma C, Tarnuzzer RW, Chegini N. Expression of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases in mesothelial cells and their regulation by transforming growth factor-beta1. Wound Repair Regen. 1999;7:477–485. doi: 10.1046/j.1524-475x.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7:423–432. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- Fukui N, Tashiro T, Hiraoka H, Oda H, Nakamura K. Adhesion formation can be reduced by the suppression of transforming growth factor-beta1 activity. J Orthop Res. 2000;18:212–219. doi: 10.1002/jor.1100180208. [DOI] [PubMed] [Google Scholar]
- Bascom CC, Wolfshohl JR, Coffey RJ, Jr, Madisen L, Webb NR, Purchio AR, Derynck R, Moses HL. Complex regulation of transforming growth factor beta 1, beta 2, and beta 3 mRNA expression in mouse fibroblasts and keratinocytes by transforming growth factors beta 1 and beta 2. Mol Cell Biol. 1989;9:5508–5515. doi: 10.1128/mcb.9.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney-Francis N, Mizel D, Wong H, Wahl L, Wahl S. TGF-beta regulates production of growth factors and TGF-beta by human peripheral blood monocytes. Growth Factors. 1990;4:27–35. doi: 10.3109/08977199009011007. [DOI] [PubMed] [Google Scholar]
- O’Reilly MA, Danielpour D, Roberts AB, Sporn MB. Regulation of expression of transforming growth factor-beta 2 by transforming growth factor-beta isoforms is dependent upon cell type. Growth Factors. 1992;6:193–201. doi: 10.3109/08977199209026926. [DOI] [PubMed] [Google Scholar]
- Ferguson MW, O’Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya Y, O’Kane S, Ferguson MW. Pathogenesis of cleft palate in TGF-beta3 knockout mice. Development. 1999;126:3869–3879. doi: 10.1242/dev.126.17.3869. [DOI] [PubMed] [Google Scholar]
- Holmdahl L. The role of fibrinolysis in adhesion formation. Eur J Surg Suppl. 1997;577:24–31. [PubMed] [Google Scholar]
- Yang L, Qiu CX, Ludlow A, Ferguson MW, Brunner G. Active transforming growth factor-beta in wound repair: determination using a new assay. Am J Pathol. 1999;154:105–111. doi: 10.1016/s0002-9440(10)65256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. Gallin J, Snyderman R, editors. Philadelphia: Lippincott Williams & Wilkins; Transforming Growth Factor-Beta (TGF-Beta) in the Resolution and Repair of Inflammation. 1999:pp 883–892. [Google Scholar]
- Wang Q, Wang Y, Hyde DM, Gotwals PJ, Koteliansky VE, Ryan ST, Giri SN. Reduction of bleomycin induced lung fibrosis by transforming growth factor beta soluble receptor in hamsters. Thorax. 1999;54:805–812. doi: 10.1136/thx.54.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, Tedder TF, Sato S. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol. 2000;157:237–247. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]