Abstract
It has traditionally been believed that only the human collagenases (matrix metalloproteinase-1, -8, and -13) are capable of initiating the degradation of collagens. Here, we show that human trypsin-2 is also capable of cleaving the triple helix of human cartilage collagen type II. We purified human trypsin-2 and tumor-associated trypsin inhibitor by affinity chromatography whereas collagen type II was purified from cartilage extracts using pepsin digestion and salt precipitation. Degradation of type II collagen and gelatin by trypsin-2 was demonstrated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis, zymography, and mass spectrometry, and tumor-associated trypsin inhibitor specifically inhibited this degradation. Although human trypsin-2 efficiently digested type II collagen, bovine trypsin did not. Furthermore, immunohistochemical staining detected trypsin-2 in the fibroblast-like synovial lining and in stromal cells of human rheumatoid arthritis synovial membrane. These findings were confirmed by reverse transcriptase-polymerase chain reaction and nucleotide sequencing. Trypsin-2 alone and complexed with α1-proteinase inhibitor were also detected in the synovial fluid of affected joints by time-resolved immunofluorometric assay, suggesting that trypsin-2 is activated locally. These results are the first to assess the ability of human trypsin to cleave human type II collagen. Thus, trypsin-2 and its regulators should be further studied for use as markers of prognosis and disease activity in rheumatoid arthritis.
Rheumatoid arthritis (RA) is a chronic inflammatory tissue-destructive disease. Synovial membrane in RA is heavily infiltrated by mononuclear inflammatory cells and the synovial lining cell layer is hypertrophic with greatly increased numbers of fibroblast-like type B and macrophage-like type A lining cells. This aggressive tumor-like tissue1–4 produces various proteinases that solubilize and degrade the underlying collagen and proteoglycan cartilaginous matrix. Conventionally, collagenase-1, formerly known as fibroblast collagenase or matrix metalloproteinase (MMP)-1, has been considered responsible.5 In addition, collagenase-2 or MMP-8 produced and released by neutrophils, but also by mesenchymal cells,6 is also envisioned to play a role. More recently, collagenase-3 or MMP-13 has been found to be particularly effective in degradation of type II collagen7 in RA.8 A non-MMP, cysteine endoproteinase cathepsin K, has been described as collagenolytic, in particular in osteoarthritis, in which it is produced by chondrocytes and released into their territorial matrix.9
Since the early days, a hypothesis of mesenchymal transformation of synovial membrane in RA has been entertained. This is based on the aggressive growth of synovial membrane and its pannus-like extension into hard tissue, cartilage, and bone.1,2,4 Synovial tissue does not respect normal tissue barriers, but instead causes the most typical radiological findings of RA, ie, peripheral erosions. This process starts as cartilage destruction followed by erosive osteolysis. The former process is probably driven by neutral endoproteinases, whereas bony erosions are thought to be formed by receptor activator of nuclear factor-κB ligand-driven osteoclastogenesis and activation maintained by local production of proinflammatory cytokines classically associated with type 1 macrophage activation.10 We have been interested in the potential role of trypsin-2 (TRY-2), which has been found to be the most efficient activator described this far for proMMP-9.11 By activating proMMP-9 TRY-2 could play an indirect role in tissue destruction. Recent studies have furthermore shown that TRY-2 activates collagenases (MMP-1, -8, -13) and was tentatively shown to degrade type I collagen.12 We therefore decided to assess if TRY-2 is active against the main structural matrix protein of hyaline articular cartilage, type II collagen. Since this was found to be the case, we also studied expression of TRY-2 in RA synovial membrane, fluid, and serum.
Materials and Methods
Materials
Trypsinogen-2 and tumor-associated trypsin inhibitor (TATI) were purified from urine from a patient with pancreatitis.13,14 Human collagenase-2 (MMP-8) and bovine trypsin were purchased from Sigma Chemicals (St. Louis, MO). The broad-spectrum MMP-inhibitor GM6001 (Ilomastat) was purchased from Chemicon Int., Temecula, CA. Native type II collagen was purified from human cartilage using pepsin extraction and selective salt precipitations at acidic and neutral pH and analyzed for purity by cyanogen bromide cleavage peptide analysis.15
Patient Samples
Synovial membrane and synovial fluid samples were collected with the permission of the local ethical committee and with the informed patient consent during joint replacement surgery or arthroscopic procedures from 26 patients (21 females, 5 males; median age, 59 years; range, 19 to 82 years) in the Satalinna Hospital, Rauma, Finland. Synovial membrane samples were snap-frozen in liquid nitrogen. Synovial fluid was pretreated with 10 μg/ml of bovine testicular hyaluronidase for 15 minutes at +37°C. Both synovial fluid and serum samples were centrifuged at 2200 rpm for 10 minutes at room temperature. Cell-free synovial fluid supernatant and serum were aliquoted. All samples were stored at −70°C. Patient records were reviewed to ensure the diagnosis based on the American College of Rheumatology criteria of rheumatoid arthritis.16
Cell Culture
For fibroblast culture, capsular (n = 3) or synovial membrane-like interface (n = 3) tissue samples were obtained from RA patients undergoing primary or revision total hip replacement surgery, respectively. There were no clinical or laboratory signs of infection. Fat and loose connective tissue were removed and the tissue was minced to pieces with a sterile scalpel in a laminar flow hood. The pieces were left overnight in RPMI medium (Biomedicum Helsinki, Helsinki, Finland) containing 10% fetal bovine serum (BioWhittaker, Liege, Belgium) and 10% penicillin/streptomycin. On the second day the media was changed to same media but the concentration of penicillin/streptomycin was reduced to 1%. The medium was changed twice a week, and when ∼80% of the dish area was covered with cells the tissue pieces were removed and the cells were allowed to grow to confluence. The cells were split 1:5 and allowed to grow to confluence before being frozen in liquid nitrogen at passage 1. When all samples had been collected they were retrieved from liquid nitrogen and maintained in RPMI supplemented with 10% fetal bovine serum and antibiotics. Cells were grown in six-well plates to confluence and analyzed at passages 2 to 4.
Measurement of Collagenolytic Activity
TRY-2 (55 nmol/L enzyme protein) was incubated for up to 48 hours with 1.5 μmol/L type II collagen substrate at 22°C to ensure that temperature did not cause denaturation of the native triple-helical configuration of the collagen. Human MMP-8 was used for comparison because it is collagenolytic but not gelatinolytic. The other control was human MMP-9, which is gelatinolytic but not collagenolytic. These MMPs were activated using 1 mmol/L aminophenylmercuric acetate (Sigma). When indicated TRY-2 and MMP-8 were pretreated with 200 nmol/L TATI and/or 30 μmol/L GM 6001. After incubation samples containing 20 to 30 μg of collagen were mixed with nonreducing sample buffer and heated at 100°C for 5 minutes. Intact collagen α chains and their degradation products were separated using 10% (T%) sodium dodecyl sulfate-polyacrylamide gel electrophoresis.17
Mass Spectrometry
The human TRY-2-treated human collagen type II sample was desalted using Millipore μC18 and C4 ZipTips (Bedford, MA) and eluted with 0.1% trifluoroacetic acid in 60% acetonitrile. The eluates were analyzed on a Biflex matrix-assisted laser desorption ionization/time of flight (MALDI-TOF) instrument (Bruker-Daltonics, Bremen, Germany) equipped with a nitrogen laser operating at 337 nm. The eluate from the μC18 ZipTip was analyzed in a positive ion reflector mode using α-cyano-4-hydroxycinnamic acid as the matrix. The MALDI-TOF instrument was externally calibrated with the standard peptides, angiotensin II and adrenocorticotropin-18-39. Eluate from the C4 ZipTip was analyzed in a positive ion linear mode using α-cyano-4-hydroxycinnamic acid as the matrix and the instrument was externally calibrated with insulin. For mass spectrometric analysis, the peptides were directly injected using a syringe pump into a Q-TOF (quadrupole/time-of-flight) hybrid mass spectrometer (Micromass, Manchester, UK) equipped with an electrospray ionization source. The mass spectrometer was calibrated using (Glu1)-fibrinopeptide B as a standard. MS/MS spectra were acquired by colliding the doubly and triply charged precursor ions with argon collision gas with accelerating voltages of 30 to 75 V. Analysis of the data and sequencing of the peptides was performed using the MassLynx 3.5 program (Micromass).
Time-Resolved Immunofluorometric Assays
Measurement of the concentrations of TRY-2, TATI, and TRY-2-α1-proteinase inhibitor (API) complex was based on the use of monoclonal catcher antibodies and europium (Eu)-labeled monoclonal tracer antibodies. For TRY-2 14D4 and 14F10 (Eu) were used as catcher and tracer antibodies, respectively.18 For TATI 6E8 and 11B3 (Eu) were used as catcher and tracer antibodies, respectively.19 For TRY-2-API complex 14F10 and polyclonal rabbit anti-API (Eu) were used as catcher and tracer antibodies, respectively.20 Fluorescence was measured in a 1234 DELFIA research fluorometer (Wallac, Turku, Finland). Detection limits and intra- and interassay coefficients of variations have been described.18–20
Alkaline Phosphatase-Anti-Alkaline Phosphatase Staining
Formalin-fixed, paraffin-embedded sections were mount-ed on Superfrost Plus slides and 1) deparaffinized and rehydrated and 2) pretreated with 0.4% pepsin before incubations in 3) monoclonal mouse anti-human TRY-2 IgG (6D11, 1:500) overnight, 4) rabbit anti-mouse IgG (1:25), 5) soluble complexes of calf intestinal alkaline phosphatase and mouse monoclonal anti-alkaline phosphatase antibody complexes (1:25), 6) 0.3 mg/ml naphtol AS-MX phosphate in dimethylformamide, 1.5 mg/ml Fast Red, and 5 mmol/L levamisole.21 As a negative staining control, adjacent sections were incubated with nonimmune mouse IgG1 used at the same concentration as the primary monoclonal anti-TRY-2 antibodies. Sections of pancreas were used as positive controls.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from ∼80 mg of frozen synovial membrane tissue using TRIzol reagent (Invitrogen, Paisley, UK). RNA was quantitated spectrophotometrically and the quality was determined with ethidium bromide-stained 1% agarose gel. mRNA was isolated using magnetic (dT)25-polystyrene beads (Dynal, Oslo, Norway). One hundred ng of mRNA was reverse-transcribed to cDNA with SuperScript preamplification system using oligo(dT)12-18 for priming and RNase H for removal of mRNA (Invitrogen). cDNA synthesis without enzyme and without sample were used for negative controls. PCR amplification was performed using 0.4 μmol/L of target-specific primers for TRY-2 (accession no. M27602): sense 5′-TGCTGTTGCTGCCCCCTTTG-3′ and anti-sense 5′-GCACAGCCATAGCCCCAGGAG-3′ producing a 627-bp band and for internal control 0.1 μmol/L of primers for β-actin (accession no. X00351): sense 5′-TCACCCACACTGTGCCCATCTACGA-3′ and anti-sense 5′-CAGCGGAACCGCTCATTGCCAATGG-3′ producing a 295-bp band. The primers were mixed with 5 ng of sample cDNA, 200 μmol/L of dNTP mix, and 5 U of the AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA) in 50 μl of PCR buffer. For primers the corresponding sequences were searched with the NCBI Entrez search system and sequence similarity search was done using NCBI Blastn program. The reaction was run in a thermal cycler (RoboCycler 40 temperature cycler; Stratagene, La Jolla, CA) for 40 cycles of 1-minute denaturation at + 95°C, 1 minute annealing at + 64°C, 1-minute extension at + 72°C with a 10-minute extra extension used for the last cycle. The identity of the product was verified by sequencing 50 ng of isolated amplicon (QIAquick; Qiagen Inc., Chatsworth, CA) using the automated Applied Biosystems 373 A sequencer (Applied Biosystems).
Results
Collagenolytic Activity of TRY-2
When human TRY-2 was incubated with native type II collagen at + 22°C for 24 hours, it degraded the collagen efficiently producing multiple cleavage fragments. The gelatinolytic, but not collagenolytic, human neutrophil gelatinase B (MMP-9) used as a control did not degrade native type II collagen. The collagenolytic but not gelatinolytic human neutrophil collagenase-2 (MMP-8) produced the characteristic 3/4;- or αA cleavage fragment. Degradation of type II collagen by TRY-2 was completely prevented by preincubation with its specific inhibition TATI but not by GM6001, an efficient synthetic MMP-inhibitor. TRY-2 and MMP-9, but not MMP-8, degraded gelatin as evidenced by gelatin zymography. Bovine trypsin did not cleave type II collagen (Figure 1).
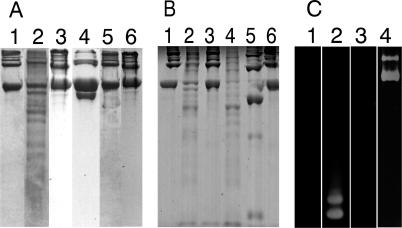
Figure 1.
A: Sodium dodecyl sulfate-polyacrylamide gel electrophoresis demonstrates that TRY-2 is an interstitial collagenase able to cleave type II collagen at multiple sites to small molecular weight fragments. Lane 1: Human homotrimeric collagen II substrate only, intact α2 chains. Lane 2: TRY-2 cleaves intact, triple helical human type II collagen monomers at multiple sites to small molecular weight collagen fragments. Lane 3: TRY-2-mediated collagenolysis inhibited by the presence of its specific inhibitor TATI. Lane 4: MMP-8 (neutrophil collagenase, collagenase-2), a positive collagenase control, produces classical ¾ and ¼ fragments as a result of action on the single initial cleavage site for mammalian collagenases. Lanes 5 and 6: MMP-9 (gelatinase B, 92-kd type IV collagenase; lane 5) and bovine trypsin (lane 6) not able to cleave type II collagen. B: Lane 1: Intact type II collagen. Lane 2: TRY-2 cleaves type II collagen. Lane 3: TRY-2 collagenolysis inhibited by TATI. Lane 4: MMP-inhibitor GM 6001 (Ilomastat) does not inhibit TRY-2-mediated collagenolysis of type II collagen. Lanes 5 and 6: MMP-8 produces classic ¾ and ¼ fragments of type II collagen (lane 5) that is inhibited by GM 6001 (Ilomastat) (lane 6). C: Lane 1: Zymography gels demonstrate gelatin substrate only. Lane 2: TRY-2 is also a gelatinase able to cleave gelatin (denatured collagen). Lanes 3 and 4: MMP-8 is not gelatinolytic (lane 3), whereas MMP-9 is gelatinolytic (lane 4). It is not possible to demonstrate inhibition of TRY-2 by TATI using zymography because TRY-2-TATI complexes dissociate in this assay.
Mass Spectrometry
Mass spectrometry showed that TRY-2 cleaved human collagen type II exclusively after basic arginine and lysine residues as is typical for trypsin proteolysis. The masses and sequences of the fragments analyzed by mass spectrometry were identical to those predicted from the known amino acid sequence (Table 1).
Table 1.
Comparison of the Sequences and Molecular Weights of the Experimentally Produced and Theoretical Human Type II Collagen Fragments Demonstrates the Collagenolytic Activity of TRY-2 Enzyme
| Collagen type II alpha 1* | Determined peptide sequences (L = I) | Mw exp. | Mw theor. |
|---|---|---|---|
| (R)717GLTGPIGPPGPAGANGEKGEVGPPGPAGSAGAR749 | GLTGPLGP(hyP)GPAGANGE(hyK)GEVGP(hyP)GPAGTAGAR | 2911, 23 | 2911, 456 |
| (R)819GAQGPPGATGFPGAAGR835 | GAQGP(hyP)GATGF(hyP)GAAGR | 1499, 62 | 1499, 721 |
| (K)1106DGANGIPGPIGPPGPR1121 | DGANGL(hyP)GPLG(hyP)(hyP)GPR | 1518, 68 | 1518, 756 |
As expected, TRY-2 cleaves only after arginine and lysine residues.
Swiss-Prot P02458.
hyP, hydroxyproline; hyK, hydroxylysine.
TRY-1, TRY-2, and TATI Levels in Synovial Fluid and Serum
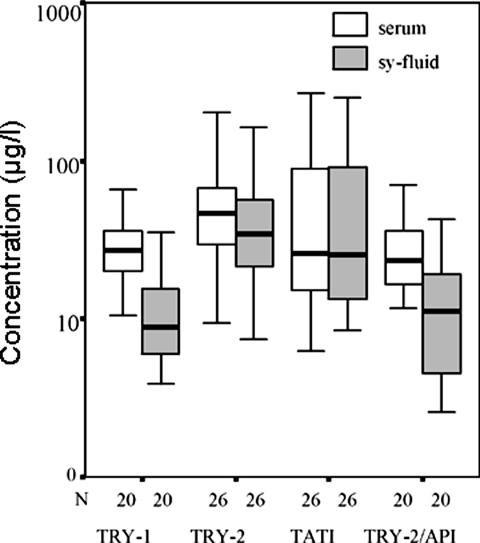
Concentrations of TRY-1, TRY-2, TATI, and TRY-2-API in serum and in synovial fluid samples of 26 patients with RA are shown in Figure 2. Part of both TRY-1 (not shown) and TRY-2 were found to be complexed with αPI indicating local conversion to active trypsin. Synovial fluid-to-serum ratio of TAT-2 was significantly higher than that for TAT-1 (P < 0.001, Wilcoxon’s test) indicating local production of TRY-2.
Figure 2.
Time-resolved immunofluorometric assay results of parallel serum and synovial fluid samples from 26 RA patients. The concentrations of TRY-1, TRY-2, TATI, and TRY-2-API are shown on a logarithmic scale. Boxes indicate median values and quartiles.
TRY-2 Protein and mRNA Expression in Human Rheumatoid Synovial Tissue
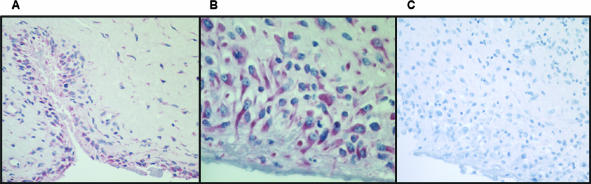
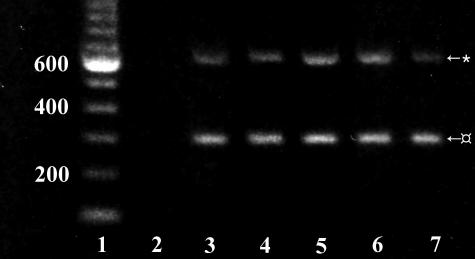
Immunohistochemically, TRY-2 was detected in all rheumatoid synovitis tissue samples (Figure 3). In particular, synovial lining cell layer and stromal fibroblast-like cells showed staining for TRY-2 (Figure 3). RA synovial membrane always contained TRY-2 mRNA as shown by RT-PCR (Figure 4).
Figure 3.
Alkaline phosphatase-anti-alkaline phosphatase staining of human TRY-2 in RA synovial membrane. A and B: The synovial lining cell layer and stromal sublining fibroblast-like cells stain for TRY-2. C: Staining control in which normal rabbit IgG was used instead of and at the same concentration as the primary rabbit anti-human TRY-2 IgG. Hematoxylin counterstaining. Original magnifications: ×20 (A and C); ×40 (B).
Figure 4.
RT-PCR of RA synovial membrane samples showing amplified 627-bp TRY-2 bands and internal controls of 295-bp β-actin bands. Lane 1: DNA ladder, lane 2: negative control without sample, lanes 3–7: RA samples.
Discussion
Many human cancers express trypsinogen isoenzymes.22,23 The two tumor-associated trypsinogen isoenzymes TAT-1 and TAT-2 are encoded by the same genes as pancreatic trypsinogen-1 and -2 and display the same specificity but differ with respect to isoelectric point, apparently because of differences in posttranslational modification. TRY-2 is an efficient activator of prourokinase22 suggesting a role in the urokinase-type plasminogen activator-plasmin system in tumor invasion.24 We recently described that TRY-2 effectively activates proMMP-9 at a molar ratio of 1:1000, the lowest reported so far for any proMMP-9 activator.11 Furthermore, TRY-2 was shown to activate collagenases MMP-1, -8, and -13.12 Thus, TRY-2 may play an important but indirect function in tissue destruction as a key enzyme for activation of proenzymes such as prourokinase and type IV procollagenase.
We now demonstrate that TRY-2 is able to cleave across the type II collagen triple helix at multiple sites. Mass spectrometric analysis confirmed that the cleavage had taken place after Arg and Lys, as expected for trypsin.25 Furthermore, TRY-2 was gelatinolytic. Thus, after the initial collagenolytic cleavage, gelatinolytic proteolysis proceeds and several small molecular collagen and gelatin fragments are produced. This collagenolysis and gelatinolysis is TRY-2-specific because it was shown to be inhibited by its specific inhibitor TATI. To exclude that the collagenolytic activity was caused by activation of a procollagenase contamination of the type II collagen preparation we studied the effect of bovine trypsin, which did not induce degradation. Contamination of the TRY-2 preparation by other proteases is highly unlikely because TRY-2 was purified from urine by immunoaffinity chromatography using a monoclonal antibody. Furthermore, the collagenolytic activity of TRY-2 was abolished by TATI, which is a very specific trypsin inhibitor, which excludes contamination of the TRY-2 preparation. We excluded the possibility that a collagenase contamination (MMP-1, -8, or -13) was responsible for the initial collagenolysis by showing that the MMP-inhibitor GM 6001 (Ilomastat) does not inhibit the collagenolytic activity of TRY-2. It is therefore concluded that unlike bovine trypsin, human TRY-2 degrades human type II collagen and thus may participate in the degradation of type II collagen-rich cartilage matrix.
After the above-mentioned in vitro observation on the collagenolytic and gelatinolytic catalytic competence of TRY-2, we looked for evidence for its eventual presence in affected joints of RA patients. Using a time-resolved immunofluorometric assay, TRY-1 and TRY-2 were shown to be present in rheumatoid synovial fluid. This may partially be due to diffusion from serum, but because TRY-1 and TRY-2 are of identical size diffusion it is expected to be equal. Because synovial fluid-to-serum ratio of TRY-2 was significantly higher than that of TRY-1 it is most likely that TRY-2 is locally produced in RA-affected joints.
Local production of TRY-2 in synovitis tissue was also demonstrated by immunohistochemistry, which showed clear cell-associated cytoplasmic staining, in particular in the lining cell layer and in stromal fibroblast-like cells. RT-PCR further confirmed that rheumatoid fibroblasts express TRY-2 mRNA because TRY-2 mRNA was found in all RA synovial membrane samples analyzed. Sequencing of the PCR amplicon confirmed the identity of the TRY-2 bands. It is therefore concluded that TRY-2 is expressed in rheumatoid synovitis tissue.
TRY-2 is secreted as a proenzyme zymogen, thus the presence of the TRY-2-API complex in rheumatoid synovial fluid indicates that TRY-2 has been locally activated. Thus it is able to exert its full potential as an activator of prourokinase, proMMP-9 and procollagenase, and as an interstitial collagenase. The association with fibroblast-like synoviocytes with an activated and aggressive phenotype26–28 is interesting also from another point of view. TRY-2 activates proteinase-activated receptor-2 enhancing the classical fibronectin RGD receptor (integrin α5β1)-dependent adhesion to fibronectin.29 This might play a role in the early fibroblast-like cell adhesion to and migration along the fibronectin precipitates at the leading edge of the advancing pannus.3
A collagenolytic activity of trypsin or any other serine protease has not been described before. We attribute this to the fact that, to the best of our knowledge, the present study is the first in which both human TRY-2 enzyme and collagen type II substrate have been used. Human TRY-2 is a far more effective activator of proMMP-9 than bovine trypsin,11 which shows that the substrate specificity of human TRY-2 differs from that of bovine trypsin.30 We now show that human but not bovine trypsin cleaves native human type II collagen.
In conclusion TRY-2 is expressed in the synovial cells of patients with RA and can be detected in the synovial lining cells and the synovial fluid. This trypsin is capable of degrading the major structural collagen of articular cartilage, type II collagen, and activates several collagenases and the PAR-2 receptor; all mechanisms that contribute to the characteristic tissue degeneration of RA. Thus, factors regulating synthesis and activation of TRY-2 deserve to be further studied as well as its role as a potential marker of prognosis and disease activity. Because of its many roles TRY-2 is also a potential target for drug therapy.
Footnotes
Address reprint requests to Mathias Stenman, Biomedicum Helsinki, P.O. Box 63, FIN-00014, University of Helsinki, Helsinki, Finland. E-mail: mathias.stenman@helsinki.fi.
Supoorted by the National Center of Excellence (the Academy of Finland) and Graduate School (the Ministry of Education) programs, the Academy of Finland; National Technology Agency of Finland; Invalid Foundation; University of Helsinki; the Sigrid Juselius Foundation; Helsinki University Research Funds; the K. Albin Johansson Foundation; the Wilhelm and Else Stockmann Foundation; Finska Läkaresällskapet; and Helsinki University Central Hospital (EVO projects TYH3306, TYH5306, TI020 Y0002).
References
- Fassbender HG. Morphologisches substrat und pathogenese der rheumatischen erkrankungen. Therapiewoche. 1973;23:611–614. [Google Scholar]
- Fassbender HG. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983;3:141–155. doi: 10.1016/s0174-173x(83)80040-5. [DOI] [PubMed] [Google Scholar]
- Shiozawa S, Ziff M. Immunoelectron microscopic demonstration of fibronectin in rheumatoid pannus and at the cartilage-pannus junction. Ann Rheum Dis. 1983;42:254–263. doi: 10.1136/ard.42.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender HG, Gay S. Synovial processes in rheumatoid arthritis. Sci J Rheumatol Suppl. 1988;76:S1–S7. doi: 10.3109/03009748809102945. [DOI] [PubMed] [Google Scholar]
- Evanson JM, Jeffrey JJ, Krane SM. Human collagenase: identification and characterization of an enzyme from rheumatoid synovium in culture. Science. 1967;158:2104–2113. doi: 10.1126/science.158.3800.499. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R, Sorsa T, Konttinen YT, Ding Y, Sutinen M, Visser H, van Hinsbergh VW, Helaakoski T, Kainulainen T, Ronka H, Tschesche H, Salo T. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem. 1997;272:31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- Knäuper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R. Tumor targeting with a selective gelatinase inhibitor. Nature Biotechnol. 1999;17:768–774. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Mandelin J, Li TF, Salo J, Lassus J, Liljestrom M, Hukkanen M, Takagi M, Virtanen I, Santavirta S. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 2002;46:953–960. doi: 10.1002/art.10185. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–269. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Salo T, Koivunen E, Tyynela J, Konttinen YT, Bergmann U, Tuuttila A, Niemi E, Teronen O, Heikkila P, Tschesche H, Leinonen J, Osman S, Stenman U-H. Activation of type IV procollagenases by human tumor-associated trypsin-2. J Biol Chem. 1997;272:21067–21074. doi: 10.1074/jbc.272.34.21067. [DOI] [PubMed] [Google Scholar]
- Moilanen M, Sorsa T, Stenman M, Nyberg P, Lindy O, Vesterinen J, Paju A, Konttinen YT, Stenman U-H, Salo T. Tumor-associated trypsinogen-2 (trypsinogen-2) activates procollagenases (MMP-1, -8, -13) and stromelysin-1 (MMP-3) and degrades type I collagen. Biochemistry. 2003;42:5414–5420. doi: 10.1021/bi020582s. [DOI] [PubMed] [Google Scholar]
- Koivunen E, Itkonen O, Halila H, Stenman U-H. Cyst fluid of ovarian cancer patients contains high concentrations of trypsinogen-2. Cancer Res. 1990;50:2375–2378. [PubMed] [Google Scholar]
- Hedstrom J, Sainio V, Kemppainen E, Puolakkainen P, Haapiainen R, Kivilaakso E, Schauman KO, Stenman U-H. Urine trypsinogen-2 as marker of acute pancreatitis. Clin Chem. 1996;42:685–690. [PubMed] [Google Scholar]
- Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82:33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Jr, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Turto H, Lindy S, Uitto VJ, Wegelius O, Uitto J. Human leukocyte collagenase: characterization of enzyme kinetics by a new method. Anal Biochem. 1977;83:557–569. doi: 10.1016/0003-2697(77)90059-8. [DOI] [PubMed] [Google Scholar]
- Itkonen O, Koivunen E, Hurme M, Alfthan H, Schroder T, Stenman U-H. Time-resolved immunofluorometric assays for trypsinogen-1 and 2 in serum reveal preferential elevation of trypsinogen-2 in pancreatitis. J Lab Clin Med. 1990;115:712–718. [PubMed] [Google Scholar]
- Osman S, Turpeinen U, Itkonen O, Stenman UH. Optimization of a time-resolved immunofluorometric assay for tumor-associated trypsin inhibitor (TATI) using the streptavidin-biotin system. J Immunol Methods. 1993;161:97–106. doi: 10.1016/0022-1759(93)90201-h. [DOI] [PubMed] [Google Scholar]
- Hedström J, Leinonen J, Sainio V, Stenman U-H. Time-resolved immunofluorometric assay of trypsin-2 complexed with alpha 1-antitrypsin in serum. Clin Chem. 1994;40:1761–1765. [PubMed] [Google Scholar]
- Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S, Pulford KA, Stein H, Mason DY. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984;32:219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Koivunen E, Huhtala ML, Stenman U-H. Human ovarian tumor-associated trypsin. Its purification and characterization from mucinous cyst fluid and identification as an activator of pro-urokinase. J Biol Chem. 1989;264:14095–14099. [PubMed] [Google Scholar]
- Terada T, Ohta T, Minato H, Nakanuma Y. Expression of pancreatic trypsinogen/trypsin and cathepsin B in human cholangiocarcinomas and hepatocellular carcinomas. Hum Pathol. 1995;26:746–752. doi: 10.1016/0046-8177(95)90222-8. [DOI] [PubMed] [Google Scholar]
- Rabbani SA, Mazar AP. The role of the plasminogen activation system in angiogenesis and metastasis. Surg Oncol Clin N Am. 2001;10:393–415. [PubMed] [Google Scholar]
- Craik CS, Largman C, Fletcher T, Roczniak S, Barr PJ, Fletterick R, Rutter WJ. Redesigning trypsin: alteration of substrate specificity. Science. 1985;228:291–297. doi: 10.1126/science.3838593. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Nykanen P, Nordstrom D, Saari H, Selin J, Santavirta S, Kouri T. DNA synthesis in prolyl 4-hydroxylase positive fibroblasts in situ in synovial tissue. An autoradiography-immunoperoxidase double labeling study. J Rheumatol. 1989;16:339–345. [PubMed] [Google Scholar]
- Konttinen YT, Von Essen R, Gronblad M, Vahvanen V, Santavirta S, Antti-Poika I, Hamalainen M. Fibroblasts in synovitis in rheumatoid arthritis but not in noninflammatory synovial tissue in meniscus lesions express the carboxyterminal domain of procollagen type I. Am J Clin Pathol. 1990;93:340–346. doi: 10.1093/ajcp/93.3.340. [DOI] [PubMed] [Google Scholar]
- Sen M, Chamorro M, Reifert J, Corr M, Carson DA. Blockade of Wnt-5A/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001;44:772–781. doi: 10.1002/1529-0131(200104)44:4<772::AID-ANR133>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Miyata S, Koshikawa N, Yasumitsu H, Miyazaki K. Trypsin stimulates integrin alpha(5)beta(1)-dependent adhesion to fibronectin and proliferation of human gastric carcinoma cells through activation of proteinase-activated receptor-2. J Biol Chem. 2000;275:4592–4598. doi: 10.1074/jbc.275.7.4592. [DOI] [PubMed] [Google Scholar]
- Turpeinen U, Koivunen E, Stenman U-H. Reaction of a tumour-associated trypsin inhibitor with serine proteinases associated with coagulation and tumour invasion. Biochem J. 1988;254:911–914. doi: 10.1042/bj2540911. [DOI] [PMC free article] [PubMed] [Google Scholar]