Abstract
The cytokine transforming growth factor-β (TGF-β) plays various functions in the control of Trypanosoma cruzi infectivity and in the progression of Chagas’ disease. When we immunostained T. cruzi-infected cardiomyocytes (after either in vivo or in vitro infections) for TGF-β, we observed stronger immunoreactivity in parasites than in host cells. TGF-β immunoreactivity evolved during parasite cycle progression, with intense staining in amastigotes versus very faint staining in trypomastigotes. TGF-β was present on the surface of amastigotes, in the flagellar pocket, and in intraparasitic vesicles as revealed by electron microscopy. However, no ortholog TGF-β gene could be identified in the genome of T. cruzi by in silico analysis or by extensive polymerase chain reaction and reverse transcriptase-polymerase chain reaction studies. Immunoreactive TGF-β was most probably taken up by the parasite from the host cell cytoplasm because such an internalization process of biotinylated TGF-β could be observed in axenic amastigotes in vitro. These observations represent the first example of a novel mechanism by which a primitive unicellular protozoan can use host cell TGF-β to control its own intracellular life cycle.
Chagas’ disease is a human disease caused by infection with the flagellate parasite Trypanosoma cruzi that affects ∼15 million people in Latin America.1 Infective nonreplicative trypomastigote forms of the parasites circulate periodically in the blood of chronic patients whereas proliferative intracellular amastigotes persist in tissues.2 Heart damage and dysfunction are important features in patients with chronic Chagas’ disease and numerous studies are conducted to elucidate the physiopathology of this disease.3 A role for parasite antigens has been proposed to explain the development of extensive fibrosis that is characteristic of the cardiac form of Chagas’ disease.4 We previously reported that circulating levels of transforming growth factor-β1 (TGF-β1) are increased in patients with the cardiac form of Chagas’ disease.5 In addition, we observed a contrasting pattern of fibronectin and phosphorylated Smad 2 (an intracellular signal-transducing protein phosphorylated by activated TGF-β receptors) immunoreactivity in the hearts of patients with chagasic cardiopathy,5 indicating that the TGF-β signaling pathway is highly active in these patients. All these observations point to a functional link between TGF-β1 and the parasite T. cruzi in the etiology of chagasic myocardiopathy.
TGF-β1 is the prototypic member of a family of polypeptidic growth and differentiation factors that play a great variety of biological functions in such diverse processes as inflammation, fibrosis, immunosuppression, cell proliferation, cell differentiation, and cell death.6–8 Virtually all cells synthesize and secrete TGF-β as a biologically inactive protein complex termed latent TGF-β, which is stored in the pericellular environment. Latent TGF-β activation results from different enzymatic and nonenzymatic mechanisms9 and only the active form of TGF-β can interact with the specific transmembrane TGF-β receptors at the cell surface, inducing cell-signaling and biological responses. TGF-β1 has already been implicated in three important processes associated with Chagas’ disease: 1) stimulation of fibrosis,5,10 2) parasitic cell invasion,11,12 3) down-regulation of cellular and immune mechanisms of parasite control.13,14 During the course of our studies on the regulation of fibrosis during T. cruzi infection,10 an interesting observation was made: immunolabeling of infected cardiomyocytes using a polyclonal antiserum against human TGF-β1 revealed immunoreactivity in the intracellular amastigote forms of T. cruzi. In the present study, we further documented this observation and addressed the question of the origin of this intraparasitic TGF-β. Did it result from synthesis by the parasite or was it taken up from the host cell cytoplasm? Our results indicated that the parasite is able to internalize host cell TGF-β, to accumulate it during its intracellular proliferation phase and suggested that it may use it as a signaling mediator to trigger differentiation into trypomastigote. So, like for various other species, TGF-β appears as a regulator of the developmental events driving T. cruzi life cycle.
Materials and Methods
In Situ Immunohistochemical Staining
Paraffin-embedded myocardial sections (5 μm) were obtained from T. cruzi-infected mice as described elsewhere.10 Sections were incubated in 10 mmol/L citrate buffer and microwaved for 2 × 10 minutes, followed by saturation for 1 hour at room temperature with 5% normal goat serum in Tris-buffered saline/1% bovine serum albumin. Sections were double stained with anti-human TGF-β antibody (AB-100-NA; R&D Systems, Oxon, UK) 1:50 and 4,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO) 1:5000. After three washes with Tris-buffered saline/1% bovine serum albumin, secondary fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgGs (Jackson Laboratories, West Grove, PA) were added at 1:100 for 1 hour at room temperature.
In Vitro T. cruzi-Heart Cell Infection
Mouse embryo cardiomyocytes were obtained and grown in primary culture as previously described.15 Briefly, cells were seeded in 24-well plates, incubated for 24 hours at 37°C in a 5% CO2 atmosphere, and cultured in Eagle’s medium supplemented with 0.1% fetal calf serum, 1 mmol/L glutamine, and 2.5 mmol/L CaCl2. To analyze T. cruzi proliferation and differentiation in cardiomyocytes, subconfluent monolayers were incubated at 37°C with T. cruzi trypomastigotes (Y strain) in a parasite/host cell ratio of 10:1, washed out after 24 hours, and monitored for different periods of time (24 to 96 hours). At each time point, the cultures were washed twice in phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for 20 minutes at 4°C, and processed for immunocytochemistry.
Immunocytochemical Staining
Cell monolayers were incubated with PBS-bovine serum albumin 2% for 3 × 10 minutes and then incubated overnight at 4°C with rabbit anti-human TGF-β antibodies (AB-100-NA, R&D Systems) or with nonimmune rabbit serum diluted 1:100 in PBS. The monolayers were further incubated for 1 hour at room temperature with the secondary antibody (goat anti-rabbit IgG-FITC diluted 1:100; Jackson Laboratories), incubated for 30 minutes at room temperature with phalloidin-tetramethyl-rhodamine isothiocyanate (TRITC) (1:500) to stain actin fibers and then with DAPI (1:5000) to stain DNA. The slides were then mounted in CytoFluor AF1 (Agar Scientific, Stansted, UK) and observed under a confocal laser microscope (Leica Microsystems, Wetzlar, Germany). Image processing was performed using Zeiss KS-400 software.
Electron Microscopy Analysis
Cells were fixed for 60 minutes at 4°C in a solution containing 0.2% glutaraldehyde, 4% freshly prepared formaldehyde, 0.8% picric acid in 0.1 mol/L cacodylate buffer, pH 7.2. After a postfixation in 1% OsO4 containing 1.5% potassium ferrocyanide for 30 minutes at 4°C, the samples were dehydrated in graded ethanol series, embedded in lowicryl, and collected on nickel grids coated with formvar and carbon. For immunolabeling, sections were washed in phosphate-buffered saline-3% albumin, quenched in 50 mmol/L NH4Cl for 30 minutes, incubated for 1hour at 37°C in the presence of rabbit anti-TGF-β antibodies (diluted 1:50, R&D Systems), washed three times, and incubated with 5-nm gold particles linked to goat anti-rabbit IgGs (1:100 dilution) for 1 hour. Sections were thinly embedded in a 9:1 mixture of 3% polyvinyl alcohol and uranyl acetate and observed with a transmission electron microscope (EM10C; Zeiss, Oberkochen, Germany) operated at 80 kV. Controls were performed using normal rabbit IgGs or omitting the primary antibody.
Amastigogenesis in Vitro
The infective trypomastigote forms of T. cruzi Y strain were obtained from the blood of infected mice at the peak of parasitemia. In all assays, the living parasites were incubated in serum-free medium. The multiplicative amastigote forms were obtained 24 and 48 hours after acid induction as previously described,16 and counted in a Neubauer chamber.
In Vitro Proliferation of Amastigotes
After 4 hours of acid induction, amastigotes (106/ml) were incubated with 10 ng/ml of recombinant TGF-β1 (Promega, Madison, WI) and/or anti-TGFβ antibodies (AB-100-NA; R&D Systems). 24 hours and 48 hours later, the live parasites were counted in each sample in a Neubauer hematometer.
TGF-β Binding to Amastigotes in Vitro
The multiplicative amastigote forms were obtained 48 hours after acid induction as previously described.16 Axenic amastigotes (0.5 × 106) were incubated for 60 minutes at 4°C with 20 ng of biotinylated human TGF-β1 (R&D Systems) in 45 μl of PBS. Ten μl of streptavidin-FITC (10 μg/ml) were then added and incubation was pursued in the dark for 30 minutes at 4°C. The parasites were washed twice, suspended in 0.2 ml of washing buffer (RDF1, R&D Systems), and analyzed as living organisms in a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA). For confocal fluorescence microscopy observation, the parasites were sequentially incubated with biotinylated TGF-β and streptavidin-FITC as described above and eventually further incubated for 2 hours at 37°C to allow internalization of the TGF-β biotin-avidin-FITC complexes. The labeled parasites were then fixed with 4% paraformaldehyde and seeded onto polylysine-coated slides. Negative controls were incubated with biotin instead of TGF-β-biotin or with streptavidin-FITC alone.
Search for TGF-β Genes in T. cruzi Genome
Genomic DNA was prepared from the T. cruzi II CL-Brenner and T. cruzi I Dm28c strains using standard procedures. Search for TGF-β-like sequences in the T. cruzi genome were performed by BLAST alignments of the TGF-β sequences from various species including Homo sapiens, Mus musculus, Drosophila melanogaster, Brugia malayi, and Caennorhabditis elegans on the T. cruzi genome resource (TcruziDB, release 2.2, http://tcruzidb.org/). The same sequences were aligned between them using Mac Vector Align software and several sets of degenerate oligonucleotide primers were selected from the most conserved protein sequence domains using the CODEHOP software on the Infobiogen website (http://www.infobiogen.fr/). These were used for polymerase chain reaction (PCR) amplification of T. cruzi genomic DNA. The sequences of the obtained amplicons were determined by Genome Express (Meylan, France). Control reverse transcriptase (RT)-PCR amplification of mammalian TGF-β genes was performed using RNAs from human placenta or mouse adrenal glands. Control of T. cruzi DNA quality was achieved by amplifying the parasite actin gene.
Results
Presence of TGF-β Immunoreactivity in Intracellular Amastigotes
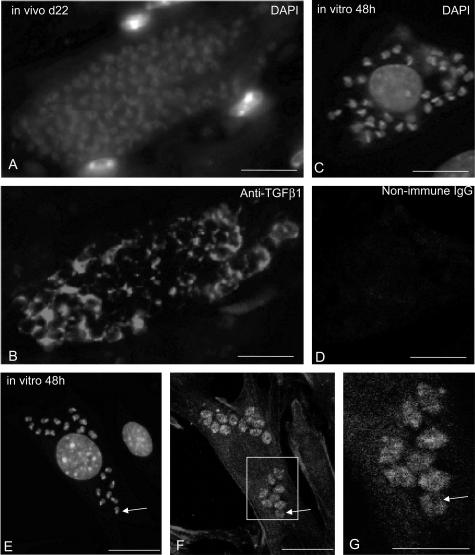
We previously reported that the TGF-β signaling pathway is activated in the infected hearts of human patients with chagasic cardiomyopathy.5 To better understand this mechanism, we wondered whether TGF-β was detectable in the hearts of T. cruzi-infected mice. The analysis was performed on day 22 after infection, when the parasites have infected various tissues including the heart.10 To our surprise, using a pan-specific polyclonal anti-TGF-β antibody that recognizes mammalian TGF-β1 and TGF-β2 as well as Xenopus TGF-β5, we observed that TGF-β immunoreactivity was more intense on intracellular parasites (essentially amastigotes) than in the cytoplasm of cardiomyocytes (Figure 1B). To confirm this observation, we performed an in vitro infection of cultured mouse embryo cardiomyocytes with T. cruzi trypomastigotes and stained the infected cells on day 2 after infection with the same anti-TGF-β antibody. The immunostaining was similar to that observed in infected heart with a stronger staining of the intracellular forms of the parasite (again amastigotes) than of the host cell cytoplasm (Figure 1, F and G). Control staining with nonimmune IgGs only displayed a weak background reactivity (Figure 1D) in regions where DAPI staining (Figure 1C) showed the presence of the parasites. Careful analysis of the pictures clearly indicated that TGF-β immunoreactivity (Figure 1, B and F) was present in every parasite as assessed by DAPI staining of their nuclei and kinetoplast (Figure 1, A and E). Using a monoclonal anti-TGF-β1 antibody (Genzyme), we obtained a similar although slightly less intense immunostaining (data not shown).
Figure 1.
Presence of TGF-β immunoreactivity in intracellular forms of T. cruzi. A and B: Double-immunofluorescent staining for DNA (A) and TGF-β (B) in sections from heart tissue of T. cruzi-infected mice (collected 22 days after infection). Note that TGF-β staining is localized in the cytoplasm of parasites. C to G: Double-immunofluorescent staining for DNA (C, E) and TGF-β (F, G) in cultures of mouse cardiomyocytes fixed 48 hours after T. cruzi infection. In D, control staining was performed with nonimmune rabbit IgGs instead of anti-TGF-β antibodies. The localization of intracellular parasites in F was revealed by DAPI staining of the infected cells immunolabeled for TGF-β in E. Specific TGF-β immunoreactivity is observed in the intracellular forms of the parasites. F corresponds to the stack of serial confocal sections whereas a larger magnification of one single-plane section is shown in G. Note the patchy pattern of staining in parasites in which black holes corresponding to nuclei may be seen (arrows). Scale bars: 20 μm (A–F); 10 μm (G).
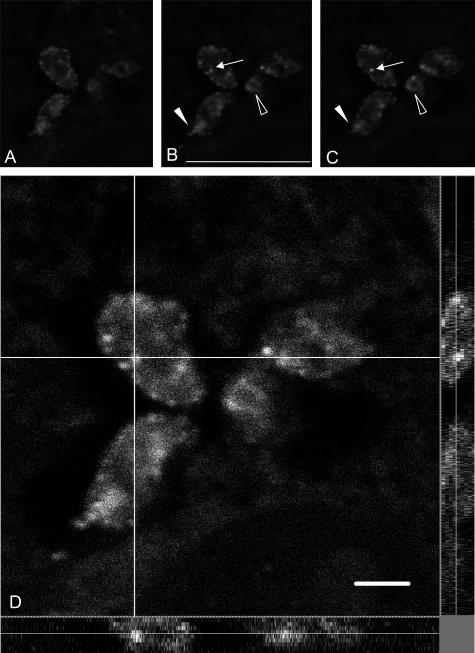
Piled up confocal images showed that TGF-β staining was present in the parasite cytoplasm (Figure 1, F and G), but absent in the nucleus and kinetoplast because DAPI staining of these DNA-containing structures (Figure 1E, arrows) did not overlap with TGF-β immunoreactivity. An enlarged view of amastigotes disclosed a patchy staining (Figure 1G) and serial confocal sections of amastigotes revealed the presence of large fluorescent spots in the parasite cytoplasm that could correspond to internalization or externalization vesicles (Figure 2; A to C). In these latter images, which corresponded to three of nine inner sections in parasites that measure ∼3 to 5 μm in diameter, the labeling was localized in granules (Figure 2, B and C; arrows) and in the area of the flagellar pocket, as seen in longitudinal (Figure 2, B and C; filled arrowhead) or sagittal (Figure 2, B and C; open arrowhead) section planes. To further characterize the intraparasitic TGF-β immunostaining, we deconvoluted the confocal images taken on infected cardiomyocytes along the z axis (Figure 2D). This allowed us to confirm that the stained vesicular structures were inside the parasite rather than on its surface.
Figure 2.
Confocal microscopy observations of intraparasitic TGF-β immunoreactivity. A to C correspond to three of nine successive confocal sections taken around the middle of the z axis. Staining is observed in cytoplasmic granules (arrows) and in the flagellar pocket (arrowheads) both in longitudinal (filled arrowheads) or in sagittal (open arrowheads) sections of the parasites. D: Large magnification of a confocal single-plane image of TGF-β detection in intracellular T. cruzi. The analysis was performed along the x-y axes (central), the x-z axes (bottom), and the y-z axes (right). The white lines indicate the axes along which the deconvolution was performed. Note the fluorescent internal vesicles clearly visible along the z axis. Scale bars, 10 μm.
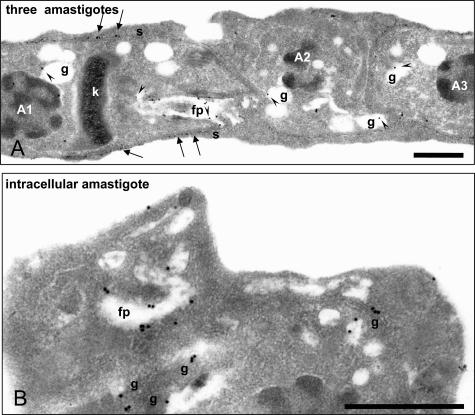
To better explore these aspects, electron microscope immunolabeling was performed on lowicryl-embedded sections of parasitized cardiomyocytes using immunogold particles and anti-TGF-β antibodies (Figure 3, A and B). In all 38 electron microscopic images that were generated, gold particles could be seen on the surface, in the flagellar pocket, and in granules of amastigotes. TGF-β thus appeared to be localized in the endocytic/exocytic parasite machinery.
Figure 3.
Detailed observation of intraparasitic TGF-β immunoreactivity by electron microscopy. A and B: Electron microscopy observations of TGF-β immunogold labeling in intracellular parasites. In A, three contiguous intracellular amastigotes whose nuclei are labeled A1, A2, and A3, show TGF-β labeling (arrowheads) in granules (g), in the flagellar pocket (fp), and at their surface (s, thin arrows). Note the typical structure of the kinetoplast (k) in amastigotes. In B, a larger magnification allows to clearly recognize the presence of immunogold particles in the flagellar pocket and granules of the observed amastigote. Scale bars, 1 μm.
Absence of a TGF-β Ortholog Gene in the T. cruzi Genome
These intriguing observations suggested two possibilities concerning the origin of TGF-β immunoreactivity. Either a TGF-β-like molecule is synthesized by the parasite and its sequence is sufficiently conserved to be recognized by both polyclonal and monoclonal anti-vertebrate TGF-β antibodies or mammalian TGF-β is taken up by the intracellular parasites from the host cells.
We first tried to address the question of the possible existence of a TGF-β gene in the T. cruzi genome. Members of the TGF-β superfamily of growth and differentiation factors have been identified in a wide variety of organisms, ranging from invertebrates to mammals,6,17–19 and the existence of molecular mimicry between T. cruzi and mammalian hosts20 suggested that a TGF-β ortholog might exist in the T. cruzi genome. Conserved peptide motifs throughout TGF-β proteins, spanning species from nematodes to human, were identified. We chose to study four such motifs from the N-terminal part, and four motifs from the C-terminal part, corresponding to the least amount of degenerate codon possibilities. Next, using a T. cruzi-specific codon table, nondegenerate primers were designed corresponding to the selected sequences. In this way, four forward primers and four reverse primers were synthesized and used in different combinations so as to amplify potential TGF-β gene fragments by PCR on T. cruzi genomic DNA because the T. cruzi genes are intronless (Table 1). Under stringent PCR conditions, no amplification was obtained whereas, under less stringent conditions, several bands could be obtained with some combinations of primers. However, after sequencing of the amplification products, none of these fragments proved to have any homology with TGF-β. We then designed conserved and/or degenerate primers from the alignment of human, bovine, murine, and C. elegans TGF-β and performed RT-PCR analyses on RNAs from human placenta and murine adrenal glands and PCR analyses on T. cruzi DNA (Table 1). Although correct amplification could be obtained with mammalian tissues, no amplification of a TGF-β-related gene was obtained from T. cruzi cDNA or genomic DNA.
Table 1.
PCR Primers Used in the Attempts of Amplification of a Putative TGF-β-Related Gene from T. cruzi DNA
| Forward primers | Reverse primers | RT-PCR/human tissue | RT-PCR/mouse tissue | PCR/T.cruzi |
|---|---|---|---|---|
| Primers designed from conserved TGF-β sequences across species using the T. cruzi-specific genetic code: | ||||
| F1:ACGTGCAAGACAATCGACATGGA | R2:CCGTGCTGTGTGCCTCAGGC | Not tested | Not tested | Yes (F1R2; F1R3; F2R2; F2R3; F2R4; F3R2; F3R3; F3R4; F3R5; F4R2; F4R4; and F4R5) but not relevant |
| F2:CAGGGCGAAGTGCCCCCAGGTCC | R3:TACAACCAGCACAATCCAGCAGC | |||
| F3:CCCGAGCCGGAAGCCGACTATTACGCGAAGGAG | R4:GGACCCTGCCCGTACATTTGG | |||
| R5:GGCTGGAAATGGATTCATGAGCCAAAGGGTT | ||||
| F4:TTTGACGTGACAGGAGTGGT | ||||
| No (other combination) | ||||
| Primers designed from human, bovine or murine TGF-β1 sequences: | ||||
| F9:ATTGACTTCCGCAAGGACC Homo sapiens TGF-β1 (M38449) nucleotides 64 to 82 | R9:TCCAGGCTCCAAATGTAGGG Homo sapiens TGF-β1 (M38449) nucleotides 164 to 145 | Yes | No | No |
| F10: CCCTGCCCTTACATCTG Bos taurus TGF-β1 (M36271) nucleotides 750 to 766 | R10: CAACTGCTCCACCTTGG Bos taurus TGF-β1 (M36271) nucleotides 914 to 898 | Yes | No | No |
| F11:TAGGAAGGACCTGGGTTGGAAGTG Mus musculus TGF-β1 (M13177) nucleotides 1258 to 1281 | R11:CGGGTTGTGTTGGTTGTAGAGG Mus musculus TGF-β1 (M13177) nucleotides 1396 to 1375 | Yes | Yes | Yes but not relevant |
| Degenerate primers designed from aligned human, murine, and C. elegans TGF-β1 sequences: | ||||
| F12:CCCCGAGTGGATCGAACTTYGAYGTNAC (NM000660) nucleotides 1442 to 1469 | R12:CCAGTTGTCCTTGCCGAARTCNAYRTA (NM000660) nucleotides 1797 to 1771 | Yes | Yes | Yes but not relevant |
| F13:CCCGAGTGGATCGACTTYGAYGTNACNG (NM000660) nucleotides 1443 to 1470 | R13:GCCCTGGCAGAAGTAGGCRTSRTANCC (NM000660) nucleotides 1843 to 1817 | Yes | Yes | No |
We then performed extensive in silico BLAST searches (BlastP against all putative T. cruzi open reading frames (ORFs) larger than 50 amino acids, or tBlastN with TGF-β1 protein sequences against all T. cruzi sequences translated in six frames) at the T. cruzi genome database (http://tcruzidb.org or http://www.genedb.org). Blast servers using the July 2004 sequence data release for the T. cruzi genome, representing an excess of 16× coverage of the genome were performed without success: none of the potential ORFs presented any significant homology with the known sequence of mature TGF-β1 (C-terminal end of the gene product) from different species. The T. cruzi genome is expressed through polycystronic transcription, followed by RNA processing involving simultaneous trans-splicing and polyadenylation. So far, only one single example of cis-splicing has been detected in T. cruzi involving the poly(A) polymerase gene that contains a single intron.21 We can therefore be very confident that potential cis-splicing cannot be invoked to explain the lack of TGF-β homologous sequences in T. cruzi through either Blast searches or PCR analyses. We therefore concluded that T. cruzi genome was very unlikely to contain any TGF-β-like gene and that the intense TGF-β immunoreactivity observed in amastigotes should derive from host uptake and accumulation inside the amastigotes.
T. cruzi Can Take Up Exogenous TGF-β
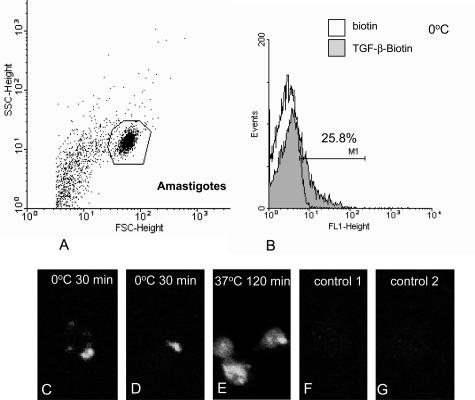
We then tried to check whether T. cruzi amastigotes could bind and internalize exogenous TGF-β. An experimental model allowing to produce T. cruzi amastigotes under host cell-free conditions has been described: acidic pH treatment of trypomastigotes collected either from the supernatant of infected mammalian cells or from the blood of infected mice induces amastigogenesis and yields viable and proliferating amastigotes.16,22 Blood trypomastigotes were acid-induced in vitro to differentiate into amastigotes and these were incubated with biotinylated TGF-β for 1 hour at 4°C. After extensive washes, the parasites were incubated with streptavidin-FITC and analyzed by flow cytometry. In parallel, some preparations were eventually further incubated at 37°C to allow internalization, then fixed, spread on a glass slide, and observed under a confocal microscope. Flow cytometry analysis of amastigotes permitted the design of an unambiguous window containing parasites (polygon in Figure 4A), and the fluorescence analysis inside this window revealed that ∼25% of the parasites had bound biotinylated TGF-β (Figure 4B). Images of the parasites incubated at 4°C with biotinylated TGF-β revealed patches of fluorescence in the region of the cytostome (Figure 4, C and D). If the parasites that had bound biotinylated TGF-β at 4°C were then submitted to a further incubation for 120 minutes at 37°C, microscopic observation of the parasites revealed homogeneous fluorescent labeling of the whole cytoplasm (Figure 4E). Confocal deconvolution of the images confirmed that the fluorescence was intracellular but, due to the extreme flatness of the fixed axenic amastigotes, it was impossible to discern intraparasitic structures. No staining was observed when the parasites were incubated with biotin instead of biotinylated TGF-β (Figure 4F) or with streptavidin-FITC alone (Figure 4G). This prompted us to conclude that axenic amastigotes are able to bind and internalize exogenous TGF-β.
Figure 4.
Uptake of exogenous TGF-β by T. cruzi amastigotes. Axenic amastigotes were obtained in vitro by acidic pH-induced differentiation of trypomastigotes as described in Materials and Methods. A and B: Axenic amastigotes were sequentially incubated at 4°C for 1 hour with biotinylated TGF-β (or with biotin as a negative control) and for 30 minutes with avidin-FITC and subsequently analyzed by FACS. A: Plotting of particle size (forward scatter, FSC) versus granulosity (side scatter, SSC) allowed to define a window (polygon) corresponding to axenic amastigotes. B: The intensity of fluorescence of the parasites within this window was analyzed in both preparations. Approximately 25% of the amastigote population incubated with biotinylated TGF-β presented fluorescence levels higher than those of the control (incubated with biotin) population. C–G: Epifluorescence microscopy of T. cruzi amastigotes after binding with biotinylated TGF-β. Axenic amastigotes were sequentially incubated for 1 hour at 4°C with either biotinylated TGF-β (C–E) or biotin (F) and for 30 minutes at 4°C with avidin-FITC (C–G). The parasites were then incubated for 120 minutes at 37°C, subsequently fixed, and observed under an epifluorescence microscope (E). In C and D, immunofluorescent staining for TGF-β appeared patchy at the parasite surface in the region of the cytostome. In E, the staining was inside the parasite.
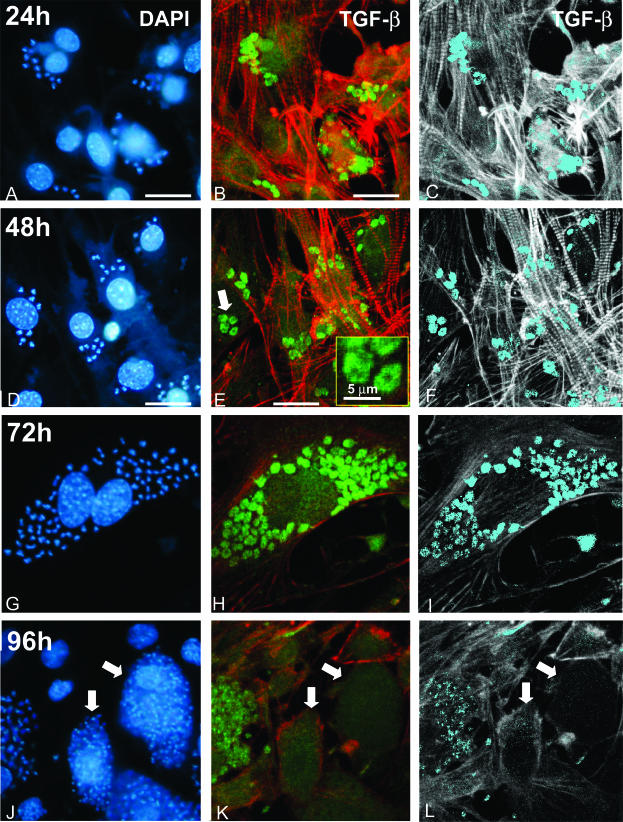
T. cruzi TGF-β Immunoreactivity Is Modulated during the Intracellular Parasite Cycle
We then wondered whether T. cruzi parasites were constantly immunoreactive for TGF-β during the intracellular parasitic cycle. Cultures of infected cardiomyocytes were fixed at various periods of time after infection and TGF-β immunoreactivity (stained with anti-TGF-β-FITC) was imaged by piling up confocal microscopy images. The cells were stained for actin using phalloidin-TRITC to visualize the host cell architecture in red. Image processing using a threshold value for eliminating background FITC fluorescence confirmed the localization in the parasite cytoplasm and the specificity of the TGF-β staining (shown in light blue Figure 5; C, F, and I). At 24 hours and 48 hours, the parasites were mainly amastigotes as characterized by DAPI staining (Figure 5, A and D) and were intensely stained for TGF-β (Figure 5; B, C, E, and F). As shown in the inset of Figure 5E, the pattern of staining appeared cytoplasmic with the location of the nucleus appearing as a black hole (as already noted in Figure 1, F and G). At 72 hours, TGF-β staining was still strong, but more heterogeneous (Figure 5, H and I), indicating a progressive decrease of TGF-β immunoreactivity. This decrease was much more pronounced at 96 hours, when differentiation to trypomastigote was complete as shown by DAPI staining (Figure 5J, arrows) and only a faint staining remained detectable (Figure 5, K and L; arrows). Specificity of the TGF-β labeling was confirmed by absence of staining in negative controls that were treated with nonimmune serum and FITC-labeled secondary antibodies (data not shown). Image processing of the original immunofluorescence micrographies allowed a better view of the changes in TGF-β reactivity in the parasites during the intracellular cycle (Figure 5; C, F, I, and L) and confirmed the dramatic decrease in TGF-β immunoreactivity during the transition of amastigotes toward trypomastigotes.
Figure 5.
Parasite life cycle-dependent immunostaining of TGF-β in intracellular forms of T. cruzi. Cultured cardiomyocytes were infected by T. cruzi trypomastigotes and the localization of TGF-β immunoreactivity was analyzed after 24 hours (A–C), 48 hours (D–F), 72 hours (G–I), or 96 hours (J–L) of infection by triple labeling of DNA with DAPI (A, D, G, J), TGF-β with anti-TGF-β-FITC complexes, and actin fibers with phalloidin-TRITC (green and red, respectively, in B, E, H, K). C, F, I, and L correspond to image-processed views from the original confocal images shown in B, E, H, and K, to stress (in light blue) the localization and the progressive decay of TGF-β immunoreactivity in the parasites. The inset in E shows a larger magnification of the parasites pointed out by the large arrow. In J, K, and L, the arrows show two cells containing a large amount of trypomastigotes that are clearly poorly immunoreactive for TGF-β. Scale bars, 20 μm (unless otherwise indicated).
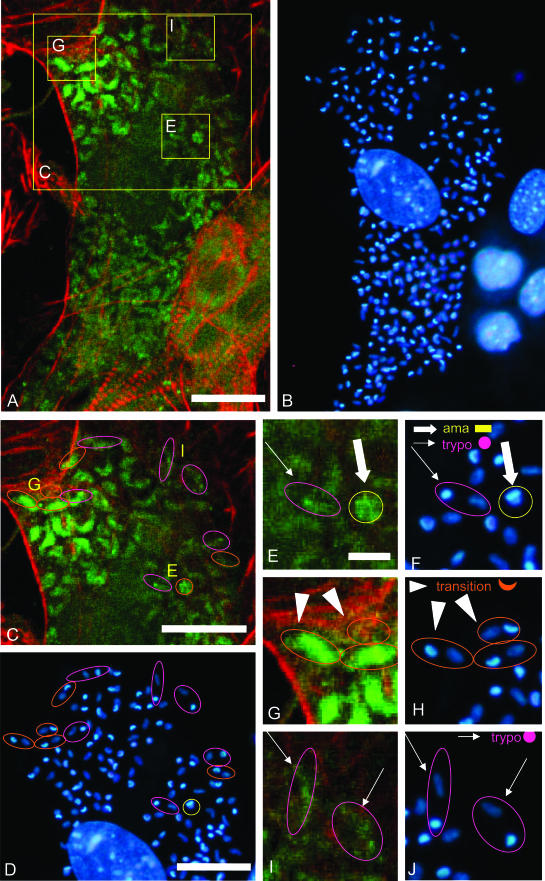
To further illustrate this transition, we analyzed intraparasitic immunoreactivity in cardiomyocytes that were infected for 72 hours and contained parasites at different stages of development. The cell shown in Figure 6A (shown in higher magnification in Figure 6; C, E, G, and I) contained parasites that were heterogeneously immunoreactive for TGF-β. DAPI staining of parasite DNA (Figure 6B, shown at higher magnification in Figure 6; D, F, H, and J) allowed to recognize amastigotes (yellow circles in Figure 6; C, D, E, and F) from transitional forms (orange ellipses in Figure 6; C, D, G, and H) and trypomastigotes (purple ellipses in Figure 6; C, D, I, and J). TGF-β immunoreactivity was strong in all amastigotes, weak in all trypomastigotes, and either strong, mild, or weak in the transitional forms. Because all these forms co-existed in the same cardiomyocyte, it can be concluded that the loss of TGF-β immunoreactivity is not dependent on changes in the host cell cytoplasm but is rather an intrinsic response of the parasite associated with cycle progression from amastigote to trypomastigote.
Figure 6.
Detailed analysis of TGF-β immunoreactivity in the distinct maturation forms of T. cruzi parasites from a unique infected cell. A and B: A cardiomyocyte containing T. cruzi parasites at different stages of maturation was double stained for TGF-β immunoreactivity (green fluorescence, A) and with DAPI (blue fluorescence, B). The areas delineated in A were enlarged in C, E, G, and I. C–J: On the basis of the shape of their DAPI-stained DNA material, amastigotes (rod kinetoplast plus spherical nucleus), trypomastigotes (spherical kinetoplast and elongated nucleus), and transition forms (crescent-like kinetoplast and spherical or elongated nucleus) were identified as shown in the graphic legend (F, H, J), and circled in yellow, purple, and orange, respectively. Magnified pictures of DAPI staining (F, H, J) and immunofluorescent TGF-β staining (E, G, I) of these different forms are shown in E to J. Scale bars: 20 μm (C, D); 5 μm (E–J).
TGF-β Induces Amastigote Growth Inhibition
The progressive decrease of TGF-β immunoreactivity observed during the amastigote-trypomastigote transition is concomitant with the arrest of intracellular parasite proliferation. Because TGF-β is a well established growth inhibitor for a number of mammalian cell types,23 we wondered whether it could have a similar effect on T. cruzi amastigotes. We measured the proliferation of axenic amastigotes for 24 and 48 hours, in the presence or absence of recombinant TGF-β1 (Table 2). The results from three different experiments showed that, under control conditions, the parasite population doubled between 24 hours and 48 hours (ratio 48:24 hours = 1.9 ± 0.2), whereas in the presence of 10 ng/ml TGF-β1, the growth was markedly reduced (ratio 48:24 hours = 1.3 ± 0.1). This difference was statistically significant (P = 0.05) and was emphasized (P = 0.007) by the effect of neutralizing the cytokine with anti-TGF-β. This halt in proliferation was not due to parasite cell death because parasite motility was sustained and vital labeling with propidium iodide did not show any important modification (data not shown).
Table 2.
Effect of TGF-β and Anti-TGF-β on in Vitro Proliferation of Axenic Amastigotes
| Experiment | Condition | Number of amastigotes (×104)
|
Proliferation ratio (throughout 24 hours) | |
|---|---|---|---|---|
| 24 hours | 48 hours | |||
| Exp. no. 1 | PBS | 75.0 | 155.0 | 2.07 |
| Exp. no. 1 | TGF-β* | 245.0 | 295.0 | 1.20 |
| Exp. no. 1 | anti-TGF-β† | 42.5 | 100.0 | 2.35 |
| Exp. no. 2 | PBS | 22.5 | 41.5 | 1.84 |
| Exp. no. 2 | TGF-β* | 34.5 | 45.7 | 1.32 |
| Exp. no. 2 | anti-TGF-β† | 14.0 | 35.0 | 2.50 |
| Exp. no. 3 | PBS | 68.5 | 117.0 | 1.71 |
| Exp. no. 3 | TGF-β* | 61.0 | 80.3 | 1.32 |
| Mean ± SD | PBS | 1.9 ± 0.2 | ||
| Mean ± SD | TGF-β* | 1.3 ± 0.1 | ||
| Mean ± SD | anti-TGF-β† | 2.4 ± 0.1 | ||
| P (PBS versus anti-TGF-β) | 0.23 | |||
| P (PBS versus TGF-β) | 0.05 | |||
| P (anti-TGF-β versus TGF-β) | 0.007 | |||
TGF-β, recombinant TGF-β1 10 ng/ml;
anti-TGF-β (10 ng/ml, R&D).
Discussion
The present results strongly suggest that the protozoan T. cruzi takes up TGF-β from its mammalian host cell, captures it through its cytostome in the flagellar pocket, and concentrates it in intracellular vesicles during specific stages of its intracellular cycle. Maximal accumulation occurs at the amastigote stage and a sudden decay of this storage is observed during the transition from amastigote to trypomastigote. The observation of an anti-proliferative effect of exogenous TGF-β in axenic amastigotes and the fact that TGF-β is captured and accumulated by the parasites during the period of multiplication (amastigote stage) may indicate that the capture of cellular TGF-β might reflect an essential need of the parasite for a host cell molecule that can be used to regulate its own intracellular cycle.
How can the parasite pick up TGF-β inside its host cell? This is an intriguing question because TGF-β, being a secreted protein, possesses a signal peptide and is therefore synthesized in the lumen of the endoplasmic reticulum, glycosylated in the lumen of the Golgi apparatus, and constitutively secreted without being released in the cytoplasm. Also, TGF-β is synthesized under a latent form consisting of a noncovalent association between the dimeric precursor part of the TGF-β gene product (LAP: latency-associated peptide) and the dimeric mature protein (C-terminal peptide). This maturation occurs along the secretion pathway. The antibody that we used for TGF-β immunolocalization has been raised against the mature isoforms TGF-β1, TGF-β2, and TGF-β5 and does not recognize the C-terminal peptide when it is engaged in a latent complex. However, it has been widely shown that the fixation steps necessary for immunofluorescence analyses can activate latent TGF-β and render the C-terminal peptide accessible to the antibody. In other terms, under our experimental conditions, the antibody recognizes both mature and latent TGF-β, and the observed immunoreactivity may correspond to either of these two forms. Three properties of the parasite may explain how it can gain access to cellular TGF-β. First, the parasite has been shown to be in close contact with endoplasmic reticulum membranes of the infected cell (M.N.L. Meirelles, personal communication); second, it has the capacity to engulf membrane vesicles;24 third, as shown in this study, it has the capacity to bind and internalize recombinant TGF-β. Our hypothesis is that amastigotes could take up TGF-β-rich secretory vesicles through their flagellar pocket. In agreement with this hypothesis, electron microscopy showed that TGF-β was present in the flagellar pocket, a major exchange vesicle, as well as in other intracellular granules. Multifunctional endocytic receptors that interact with TGF-β or with other proteins associated with TGF-β could be potential candidates, eg, the LRP (LDL-receptor related protein)/α2M-R(α2-macroglobulin receptor) that we previously described in T. cruzi.25 It was recently shown that the type-V TGF-β receptor, which plays an important role in growth inhibition by TGF-β in responsive cells, is identical to the LRP-1/α2-M receptor.26
The absence of a TGF-β-like gene in the genome of T. cruzi was somehow unexpected because such orthologs (homologous genes in different organisms) have been found in the genomes of nematodes, ascidians, and insects. However, T. cruzi belongs together with other kinetoplastids and with euglenoids to the phylum of Euglenozoa.27 It must be noted that this phylum is more ancestral than those of Craniates, Arthropods, and Nematods in which TGF-β orthologs have been characterized.
The rapid decrease of TGF-β immunoreactivity during the transition from amastigote to trypomastigote also opens a number of questions. This decrease may result either from secretion into the host cell cytoplasm of TGF-β that was accumulated inside the parasite, or from a degradation or modification of the stored TGF-β in a way that masks its immunoreactivity. If this is the result from secretion, then TGF-β would flow into the cell cytoplasm and probably induce parasite proliferation arrest, which is what occurs at this specific stage of the parasite cycle. Then, active TGF-β released during host cell disruption could directly induce extracellular matrix protein synthesis by other infected and/or noninfected cardiomyocytes, thus promoting heart fibrosis by itself, as shown previously.4,5,10,13
Moreover, the present results also suggest the presence of TGF-β receptor(s) on T. cruzi cell surface, as well as the existence of a downstream signaling pathway that would trigger the anti-proliferative effect of TGF-β. Orthologs of the canonical serine-threonine kinase TGF-β receptors (TβRI and TβRII) and Smad proteins have been identified in the helminth parasite Schistosoma mansoni28–30 but could not be found after in silico analysis of the T. cruzi genome. However, several non-Smad signaling pathways are now known to be activated or modulated by TGF-β in eucaryotic cells. These include the Jun-kinase, p38MAP-kinase, Ras/MEK/ERK, Rho-A/p160ROCK, and PP2A/S6kinase.31 Interestingly, homologs of Ras,32 Rho,33 and ERK34,35 have been characterized in T. cruzi and Trypanosma brucei, suggesting that at least some of these alternative TGF-β pathways might be functional in these parasites. A more detailed molecular analysis of T. cruzi TGF-β binding proteins and downstream signaling molecules is under current investigation in our laboratories. It should be remarked that other mammalian growth factors (namely epidermal growth factor and TGF-α) have been shown to induce signal transduction events and cellular proliferation in T. cruzi amastigotes through binding to specific receptors.36,37
The novel role of host cell TGF-β described herein, adds complexity to T. cruzi biology and discloses additional functions for this cytokine in Chagas’ disease. TGF-β thus appears: 1) to be generated at the host cell surface via parasite-mediated activation of latent TGF-β,38 2) to induce downstream signaling along the host cell TGF-β receptor pathway thereby favoring cell invasion,11,12 3) to be taken up intracellularly by the parasites and to control differentiation from amastigotes into trypomastigotes (present study), and 4) to trigger fibrosis in Chagas’ cardiomyopathy.4,5,10,13,14
Acknowledgments
We thank Marcos Meuser for his technical help in parasite preparations, Dr. Didier Grunwald for his advice in confocal microscopy analysis, and Dr. Solange L. Castro and Thais Souto-Padrón for critical reading of the manuscript.
Footnotes
Address reprint requests to Jean-Jacques Feige, INSERM EMI 01-05, DRDC-ANGIO, CEA-Grenoble, 17, Rue des Martyrs, 38054 Grenoble Cedex 9, France. E-mail: jjfeige@cea.fr.
Supported by an Institut National de la Santé et de la Recherche Médicale-Fundação Instituto Oswaldo Cruz (FIOCRUZ) collaborative research program, by grants from Programa de Apoio à Pesquisa Estratégica em Saúde/FIOCRUZ, Instituto Oswaldo Cruz, Conselho Nacional de Desenvolvimento Científico e Techológico, Fundação de Amparo a Perquisa do Estado do Rio de Janeiro to the Brazilian laboratories, and by recurrent funding from Commissariat à l’Energie Atomique and INSERM to the French laboratory.
This work was part of the Ph.D. thesis of M.C.W.
M.C.W. and M.K. equally contributed to this work and should be considered as first co-authors.
T.C.A.-J. and J.-J.F. equally contributed to the scientific direction of this work and should be considered as last co-authors.
References
- Umezawa ES, Stolf AM, Corbett CE, Shikanai-Yasuda MA. Chagas’ disease. Lancet. 2001;357:797–799. doi: 10.1016/S0140-6736(00)04174-X. [DOI] [PubMed] [Google Scholar]
- De Souza W. From the cell biology to the development of new chemotherapeutic approaches against trypanosomatids: dreams and reality. Kinetoplastid Biol Dis. 2002;1:3. doi: 10.1186/1475-9292-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Bestetti RB. The challenge of chagasic cardiomyopathy. The pathologic roles of autonomic abnormalities, autoimmune mechanisms and microvascular changes, and therapeutic implications. Cardiology. 1995;86:1–7. doi: 10.1159/000176822. [DOI] [PubMed] [Google Scholar]
- Higuchi ML, Fukasawa S, De Brito T, Parzianello LC, Bellotti G, Ramires JA. Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy and Chagas’ disease: a three dimensional confocal microscopy study. Heart. 1999;82:279–285. doi: 10.1136/hrt.82.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo-Jorge TC, Waghabi MC, Hasslocher-Moreno AM, Xavier SS, Higuchi ML, Keramidas M, Bailly S, Feige JJ. Implication of transforming growth factor-beta1 in Chagas disease myocardiopathy. J Infect Dis. 2002;186:1823–1828. doi: 10.1086/345882. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. The transforming growth factor-βs. Sporn MB, Roberts AB, editors. Berlin: Springer-Verlag; Peptide Growth Factors and Their Receptors. 1991:pp 419–472. [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Miyazono K. TGF-beta signaling by Smad proteins. Cytokine Growth Factor Rev. 2000;11:15–22. doi: 10.1016/s1359-6101(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I, Rifkin DB. TGF-beta latency: biological significance and mechanisms of activation. Stem Cells. 1997;15:190–197. doi: 10.1002/stem.150190. [DOI] [PubMed] [Google Scholar]
- Waghabi MC, Coutinho CM, Soeiro MN, Pereira MC, Feige JJ, Keramidas M, Cosson A, Minoprio P, Van Leuven F, Araujo-Jorge TC. Increased Trypanosoma cruzi invasion and heart fibrosis associated with high transforming growth factor beta levels in mice deficient in alpha(2)-macroglobulin. Infect Immun. 2002;70:5115–5123. doi: 10.1128/IAI.70.9.5115-5123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming M, Ewen ME, Pereira ME. Trypanosome invasion of mammalian cells requires activation of the TGF beta signaling pathway. Cell. 1995;82:287–296. doi: 10.1016/0092-8674(95)90316-x. [DOI] [PubMed] [Google Scholar]
- Hall BS, Pereira MA. Dual role for transforming growth factor beta-dependent signaling in Trypanosoma cruzi infection of mammalian cells. Infect Immun. 2000;68:2077–2081. doi: 10.1128/iai.68.4.2077-2081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JS, Twardzik DR, Reed SG. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta). J Exp Med. 1991;174:539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tarleton RL. Characterization of cytokine production in murine Trypanosoma cruzi infection by in situ immunocytochemistry: lack of association between susceptibility and type 2 cytokine production. Eur J Immunol. 1996;26:102–109. doi: 10.1002/eji.1830260116. [DOI] [PubMed] [Google Scholar]
- Meirelles MN, de Araujo-Jorge TC, Miranda CF, de Souza W, Barbosa HS. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur J Cell Biol. 1986;41:198–206. [PubMed] [Google Scholar]
- Tomlinson S, Vandekerckhove F, Frevert U, Nussenzweig V. The induction of Trypanosoma cruzi trypomastigote to amastigote transformation by low pH. Parasitology. 1995;110:547–554. doi: 10.1017/s0031182000065264. [DOI] [PubMed] [Google Scholar]
- Padgett RW, St. Johnston RD, Gelbart WM. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987;325:81–84. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Gomez-Escobar N, Gregory WF, Maizels RM. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor beta, expressed in microfilarial and adult stages of Brugia malayi. Infect Immun. 2000;68:6402–6410. doi: 10.1128/iai.68.11.6402-6410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laucella SA, Velazquez E, Dasso M, de Titto E. Trypanosoma cruzi and mammalian heart cross-reactive antigens. Acta Trop. 1996;61:223–238. doi: 10.1016/0001-706x(96)00004-6. [DOI] [PubMed] [Google Scholar]
- Mair G, Shi H, Li H, Djikeng A, Aviles HO, Bishop JR, Falcone FH, Gavrilescu C, Montgomery JL, Santori MI, Stern LS, Wang Z, Ullu E, Tschudi C. A new twist in trypanosome RNA metabolism: cis-splicing of pre-mRNA. Rna. 2000;6:163–169. doi: 10.1017/s135583820099229x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro MC, De Lima AR, Askue J, Contreras VT. Morphological comparison of axenic amastigogenesis of trypomastigotes and metacyclic forms of Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2003;98:83–91. doi: 10.1590/s0074-02762003000100012. [DOI] [PubMed] [Google Scholar]
- Moses HL, Arteaga CL, Alexandrow MG, Dagnino L, Kawabata M, Pierce DF, Jr, Serra R. TGF beta regulation of cell proliferation. Princess Takamatsu Symp. 1994;24:250–263. [PubMed] [Google Scholar]
- Meyer H, De Souza W. On the fine structure of Trypanososma cruzi in tissue cultures of pigment epithelium from the chick embryo. Uptake of melanin granules by the parasite. J Protozool. 1973;20:590–593. doi: 10.1111/j.1550-7408.1973.tb03580.x. [DOI] [PubMed] [Google Scholar]
- Coutinho CM, Cavalcanti GH, van Leuven F, Araujo-Jorge TC. Alpha-2-macroglobulin binds to the surface of Trypanosoma cruzi. Parasitol Res. 1997;83:144–150. doi: 10.1007/s004360050224. [DOI] [PubMed] [Google Scholar]
- Huang SS, Ling TY, Tseng WF, Huang YH, Tang FM, Leal SM, Huang JS. Cellular growth inhibition by IGFBP-3 and TGF-beta1 requires LRP-1. FASEB J. 2003;17:2068–2081. doi: 10.1096/fj.03-0256com. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Kingdom protozoa and its 18 phyla. Microbiol Rev. 1993;57:953–994. doi: 10.1128/mr.57.4.953-994.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall MJ, McGonigle S, Pearce EJ. Functional conservation of Schistosoma mansoni Smads in TGF-beta signaling. Mol Biochem Parasitol. 2000;111:131–142. doi: 10.1016/s0166-6851(00)00307-8. [DOI] [PubMed] [Google Scholar]
- Beall MJ, Pearce EJ. Human transforming growth factor-beta activates a receptor serine/threonine kinase from the intravascular parasite Schistosoma mansoni. J Biol Chem. 2001;276:31613–31619. doi: 10.1074/jbc.M104685200. [DOI] [PubMed] [Google Scholar]
- Beall MJ, Pearce EJ. Transforming growth factor-beta and insulin-like signalling pathways in parasitic helminths. Int J Parasitol. 2002;32:399–404. doi: 10.1016/s0020-7519(01)00348-4. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Sowa MP, Coulter LJ, Tait A, Hide G. A novel gene encoding a ras-like GTP-binding protein from Trypanosoma brucei: an evolutionary ancestor of the ras and rap genes of higher eukaryotes? Gene. 1999;230:155–161. doi: 10.1016/s0378-1119(99)00072-4. [DOI] [PubMed] [Google Scholar]
- Nepomuceno-Silva JL, Yokoyama K, de Mello LD, Mendonca SM, Paixao JC, Baron R, Faye JC, Buckner FS, Van Voorhis WC, Gelb MH, Lopes UG. TcRho1, a farnesylated Rho family homologue from Trypanosoma cruzi: cloning, trans-splicing, and prenylation studies. J Biol Chem. 2001;276:29711–29718. doi: 10.1074/jbc.M102920200. [DOI] [PubMed] [Google Scholar]
- Ellis J, Sarkar M, Hendriks E, Matthews K. A novel ERK-like, CRK-like protein kinase that modulates growth in Trypanosoma brucei via an autoregulatory C-terminal extension. Mol Microbiol. 2004;53:1487–1499. doi: 10.1111/j.1365-2958.2004.04218.x. [DOI] [PubMed] [Google Scholar]
- Muller IB, Domenicali-Pfister D, Roditi I, Vassella E. Stage-specific requirement of a mitogen-activated protein kinase by Trypanosoma brucei. Mol Biol Cell. 2002;13:3787–3799. doi: 10.1091/mbc.E02-02-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghansah TJ, Ager EC, Freeman-Junior P, Villalta F, Lima MF. Epidermal growth factor binds to a receptor on Trypanosoma cruzi amastigotes inducing signal transduction events and cell proliferation. J Eukaryot Microbiol. 2002;49:383–390. doi: 10.1111/j.1550-7408.2002.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Alexander AD, Villalta F, Lima MF. Transforming growth factor alpha binds to Trypanosoma cruzi amastigotes to induce signaling and cellular proliferation. Infect Immun. 2003;71:4201–4205. doi: 10.1128/IAI.71.7.4201-4205.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghabi MC, Keramidas M, Feige JJ, Araujo-Jorge TC, Bailly S. Activation of transforming growth factor β by Trypanosoma cruzi. Cell Microbiol. 2005;7:511–517. doi: 10.1111/j.1462-5822.2004.00481.x. [DOI] [PubMed] [Google Scholar]