Abstract
Impaired rapid eye movement sleep (REMS) is commonly observed in Alzheimer’s disease, suggesting injury to mesopontine cholinergic neurons. We sought to determine whether abnormal β-amyloid peptides impair REMS and injure mesopontine cholinergic neurons in transgenic (hAPP695.SWE) mice (Tg2576) that model brain amyloid pathologies. Tg2576 mice and wild-type littermates were studied at 2, 6, and 12 months by using sleep recordings, contextual fear conditioning, and immunohistochemistry. At 2 months of age, REMS was indistinguishable by genotype but was reduced in Tg2576 mice at 6 and 12 months. Choline acetyltransferase-positive neurons in the pedunculopontine tegmentum of Tg2576 mice at 2 months evidenced activated caspase-3 immunoreactivity, and at 6 and 12 months the numbers of pedunculopontine tegmentum choline acetyltransferase-positive neurons were reduced in the Tg2576 mice. Other cholinergic groups involved in REMS were unperturbed. At 12 months, Tg2576 mice demonstrated increased 3-nitrotyrosine immunoreactivity in cholinergic projection sites but not in cholinergic soma. We have identified a population of selectively compromised cholinergic neurons in young Tg2576 mice that manifest early onset REMS impairment. The differential vulnerability of these cholinergic neurons to Aβ injury provides an invaluable tool with which to understand mechanisms of sleep/wake perturbations in Alzheimer’s disease.
Disrupted sleep/wake activity in Alzheimer’s disease (AD) significantly impacts the quality of life for AD patients as well as their caregivers and is a major reason for institutionalization.1–3 The mechanisms of disturbed sleep in AD are not known, and consequently there are no therapies directed specifically toward ameliorating AD-mediated perturbations of sleep/wake control mechanisms. One of the more specific changes in sleep/wake activity in AD is rapid eye movement sleep (REMS) impairment that commonly includes reductions in both the total sleep time spent in REMS and the density of phasic activity within REMS, eg, rapid eye movements and muscle twitches.4–6 In addition, spectral analysis reveals more electroencephalographic (EEG) slowing in REMS than in waking among AD patients.7–9 REMS disturbances have been shown to correlate with impairments in cognitive function.6
The initiation and maintenance of REMS, including generation of REMS phasic activity, require a functional cholinergic network involving the pons.10 Mesopontine cholinergic neurons in the pedunculopontine tegmentum (PPT) and laterodorsal tegmentum (LDT) are essential for phasic REMS phenomena.11 The REMS changes in AD, therefore, are consistent with injury to mesopontine cholinergic neurons. Although cholinergic neuron loss is more pronounced in the basal forebrain in AD, loss of mesopontine cholinergic neurons also has been observed.12,13
Overexpression of known mutant human familial AD transgenes in mice has provided models of AD-like Aβ pathologies including age-dependent formation of Aβ plaques.14,15 and cognitive impairment.16–19 One of these transgenic (Tg) mice overexpresses the Swedish human APP mutation and they are known as Tg2576 or hAPP695.SWE.16 This model develops hippocampal-dependent memory impairments at ≥6 months, and increased Aβ oligomers and forebrain plaques at ≥9 months in association with evidence of oxidative damage.16,20,21 As observed in human AD brains,22,23 this model has co-localization of Aβ amyloid plaques at cholinergic terminals.24 Isoprostanes, indicators of lipid peroxidation, rise in the brain in Tg2576 mice beginning at 6 to 8 months of age, but level off by 9 to 10 months of age, just as Aβ levels begin to climb.21 Because of this Aβ-mediated cholinergic injury coupled with evidence of early oxidative injury, the Tg2576 mouse is an ideal model with which to study cholinergic sleep/wake impairments in parallel with oxidative injury.
Two studies have examined sleep in the Tg2576 mice.25,26 The former examined sleep exclusively in 2-month-old mice and found no difference in REM sleep.25 The second study provides a very complete phenotype for sleep and circadian rhythms in Tg2576 mice across ages 5 to 22 months.26 However, the study includes male mice, which are less susceptible to Aβ injury, and with five females/age group was unable to detect a statistical difference in REM sleep time.
Using adequate sample sizes to detect a >20% reduction in REM sleep, Tg2576 mice and nontransgenic wild-type (WT) littermate controls were examined for REM sleep abnormalities, contextual memory, and related neuropathological changes at 2, 6, and 12 months. Our studies show reduction in REMS and in the duration of REMS bout lengths occurring by 6 months. At 2 months of age, we observed increased immunoreactivity to cleaved poly ADP ribose-polymerase (PARP-1 p85), a marker of early caspase-3-dependent apoptosis, present exclusively in the PPT cholinergic neurons. At 6 and 12 months, a decline in the number of choline acetyltransferase (ChAT)-positive neurons in the PPT was observed without changes in the other major REMS neurons including those in the pontine group, LDT, and neurons within the medial septum (MS) that contribute to theta synchronization. Increased nitration was evident only at 12 months and in cholinergic projection sites, rather than in cholinergic soma. Collectively, these studies suggest that PPT cholinergic neurons are uniquely susceptible to early dysfunction in the Tg2576 mouse model of AD-like Aβ amyloidosis. Identification of a select group of cholinergic neurons with early injury, detectable as REMS reductions in the Tg2576 mice will be instrumental in advancing our understanding of mechanisms underlying sleep/wake disturbances linked to neurodegeneration in AD.
Materials and Methods
Experimental Animals
Tg2576 mice engineered to overexpress the FAD Swedish hAPP695 mutation gene (hAPP695.SWE)16 and WT littermate mice with the same background of B6C3 were bred and maintained as hemizygotes (+/−) of Tg2576 and WT littermates for this study as described,21,27 and the genotyping of these mice was performed by established methods.21,27
Retina Degeneration Gene (rd) Screen
Approximately 25% of Tg2576 and WT mice have the retina degeneration gene, which would confound sleep and behavioral studies. Mice in this study were, therefore, screened by polymerase chain reaction with primers described by Practico and colleagues,21 and excluded from study if rd+. All of these animals were bred and reared in a pathogen-free environment, and the experimental procedures used here were approved by the University of Pennsylvania.
Electrode Implantation and Recordings
Surgical implantation of electrodes and electrophysiological recordings were performed using previously described methods28,29 in 2-, 6-, and 12-month-old Tg2576 and WT mice (n = 8 to 12 per group). Briefly, after 5 days of postoperative recovery, electrode implants were connected to recording cables and mice were placed in individual cages, housed inside a sound-attenuated, shielded, and well-ventilated designated sleep recording room with a 12-hour lights-on (7:00 a.m. to 7:00 p.m.) and 12 hours lights-off schedule (7:00 p.m. to 7:00 a.m.; ambient temperature, 25 ± 2°C). EEG and electromyogram signals were amplified and filtered and sent to an A/D board (Converter 4801A; ADAC, Woburn, MA) in standard computers. The behavioral state acquisition and analysis program used for these studies was ACQ 3.4.30 Fast Fourier analysis for sleep state scoring was performed on 10-second epochs of EEG signals (100 Hz digitization). In all mice included in the study, the accuracy of the program relative to human scorer interpretation was >95% for waking and non-REMS (NREMS) and >85% for REMS, as used previously.28 Sleep states were recorded for >5 days to ensure stability in sleep/wake activity.29 For spectral analysis, electrophysiological signals from the same mice were then recorded using Gamma 4.2 recording software (Grass Telefactor, West Warwick, RI) with a sampling rate of 256 Hz, data were converted to EDF for analysis on Science version 3.2 (Somnologica, Reykjavik, Iceland). EEG spectral power was calculated with fast Fourier transformation of 10-second epochs in 0.25 Hz bins. Power across the 0.5 to 25 Hz range for REMS and artifact-free waking for 30 minutes for each state was determined, and mean values were plotted in 0.25 Hz bins. Delta activity was measured for frequencies 0.5 to 4 Hz; theta activity was measured for bins between 5 and 9 Hz. Sleep/wake activity for 10-second epochs across 24 hours was scored by two scorers with 95% agreement. Scorers were blinded to animal age and genotype.
Behavioral State Analysis
Behavioral state parameters were analyzed as previously reported,28 using factorial analysis of variance with Bonferroni corrections. The primary variables were REMS time, REMS bout duration, relative theta power in REMS, and REMS latency. Two-way analysis of variance was used to compare the a priori sleep responses in Tg2576 and WT mice. Secondary analyses included NREMS time, wake bout lengths, delta power in NREMS, arousal index (sleep fragmentation), sleep bout length (sleep consolidation).28,31 Unless otherwise stated, values are reported as mean ± SE. Statistical significance was determined using data analysis software (Graph Pad Prism, San Diego, CA) and defined when probabilities of the null hypothesis were <0.05.
Contextual Fear Conditioning
To test for the impairment of long-term memory formation, Tg2576 and WT mice from 6 and 12 months of age (n = 8 to 12/each age-matched group) were trained in contextual fear conditioning chambers. Fear conditioning experiments were performed in chambers using the methods previously described.32 Mice were handled for 3 consecutive days for 1 minute each day. For contextual fear conditioning, mice were placed into the conditioning chamber and received a 2-second 1.5 mA scrambled footshock 2.5 minutes after placement into the chamber. Mice were removed from the chamber after 3 minutes. During testing, mice received one 5-minute exposure to the same conditioned context in the absence of shock 24 hours after conditioning. Conditioning was assayed by measuring freezing behavior, eg, complete absence of movement.33 Freezing was scored during conditioning as well as testing. The behavior of each mouse was sampled at 5-second intervals and the percentage of those intervals in which the mouse exhibited freezing behavior was calculated.
Immunohistochemical Analyses
Tg2576 and WT littermates from 2, 6, and 12 months of age (n = 3/age/genotype) were perfused with 4% paraformaldehyde in 0.1 mol/L phosphate buffer after being deeply anesthetized by an intraperitoneal injection of ketamine hydrochloride (1 mg/10 g) and xylazine (0.1 mg/10 g). The brains and the spinal cords of mice were then removed and processed. Paraffin-embedded tissue was cut into 6-μm-thick sections and mounted for analysis of five sections/region/mouse (n = 3/age/genotype). Sections were stained with previously described anti-Aβ antibodies,21,27 rabbit anti-nitrotyrosine (3-NT) antibodies (Upstate, Lake Placid, NY) and mouse anti-ChAT (Boehringer Mannheim Biochemica, Mannheim, Germany) using previously reported methods.21,27,34 An additional group of mice at ages 2, 6, and 12 months old (n = 2 to 3/age/strain) were sectioned as dry-mounted serial sections to ensure analysis of the entire nucleus.
To characterize 3-NT staining in cholinergic neurons, in relation to behavioral impairments in REMS and fear conditioning, double-label indirect immunofluorescence was performed with mouse anti-ChAT and rabbit anti-3-NT antibodies and detected by fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG and Texas Red-conjugated donkey anti-mouse IgG (Jackson Laboratories, Bar Harbor, ME), respectively. Light and fluorescent microscopy was performed with the Olympus photomicroscope system. Human tissue blocks from postmortem confirmed AD cases obtained from the Brain Bank of the Center for Neurodegenerative Disease Research were cut and stained as described above as positive controls, during optimization of primary antibody titration. The specificity of 3-NT immunoreactivity was confirmed by using 10 mmol/L nitrotyrosine antigen (Sigma-Aldrich, St. Louis, MO) in solution with the primary 3-NT antibody. There was no positive staining by using this immunoabsorbed antibody solution on brain sections of non-Tg and Tg2576 Tg mice. To identify early caspase-3-dependent apoptosis, anti-rabbit poly (ADP) ribose polymerase cleaved fragment 85 (1:50; Promega, Madison, WI) was used on free-floating sections and detected by Alexa Fluor 594 donkey anti-rabbit IgG (red, 1:500; Molecular Probes, Eugene, OR), and mouse anti-ChAT detected with streptavidin Alexa Flour 350 (blue, 1:100; Molecular Probes). PARP p85 immunoreactivity was quantified by determining the percentage of ChAT-positive neurons with p85 nuclei on average across three sections covering the rostral caudal span of PPT were used in each mouse assayed (n = 3 age group).
Semiquantitative Assessment of Cholinergic and 3-NT-Positive Neurons
Immunolabeled cholinergic cell bodies in Tg2576 and WT mice were quantified by counting the number of ChAT-positive neurons in PPT, LDT, and MS as described.27 This quantitative analysis was performed blinded to age/genotype on every fifth section, total 15 to 20 6-μm or all 20-μm sections/region from each mouse. To control for variance in rostral-caudal sectioning across mice, all sections analyzed for one genotype were paired with a matching section from the other genotype. The similar semiquantitative assessment on the 3-NT-positive neurons was performed on the hippocampus and hypothalamus of 12-month-old Tg2576 and age matched littermates The mean and SD values of the number of neurons in groups of Tg and WT at the same age were analyzed, and the difference in the number of neurons among the groups was examined statistically using the Student’s paired t-test.
Results
Early Development of Disruptions in Sleep/Wake Architecture in Tg2576 Mice
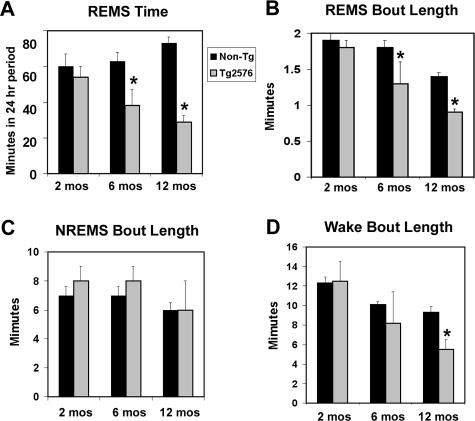
To investigate if the Tg2576 mouse AD-like Aβ brain amyloidosis model develops sleep disorders similar to those found in AD patients, EEG and electromyogram behavioral states were recorded on Tg2576 and WT mice at 2, 6, and 12 months of age after exclusion of rd gene expression. At 2 months, sleep state activity/24 hours (REMS, NREMS, and wake times/24 hours) did not differ with genotype (Tg2576, n = 9; WT, n = 12) (Figure 1). In contrast, at 6 months, REMS time/24 hours in Tg2576 was reduced by 30% of WT REMS time, relative to age-matched WT mice (Tg2576, n = 11; WT, n = 14; P < 0.05) (Figure 1A). A reduction in REMS was also observed in Tg2576 mice at 12 months of age by 50% relative to age-matched WT REMS time, (Tg2576, n = 9 and WT, n = 13; P < 0.05) (Figure 1, A and B). The number of REMS bouts/24 hours period was significantly reduced from 52 ± 5 bouts/24 hours in 12-month-old non-TG mice to 21 ± 3 bouts in 12-month-old Tg, P < 0.01. REMS latency was increased in Tg2576 mice at 12 months of age, from 8 ± 0.4 minutes in WT to 12 ± 1 minutes in Tg2576, P < 0.05. In contrast to changes in REMS time, there were no differences in NREMS total time/24 hours or for the mean duration of bouts (Figure 1C). Although total wake time/24 hours was unchanged, the average duration of wake bouts was shortened in Tg2576 mice at 12 months (Tg2576, 6.0 ± 0.2 minutes versus WT, 9.1 ± 0.2 minutes; P < 0.05) (Figure 1D). The circadian amplitude of wake activity (wake time during light:dark periods) was increased in Tg2576 mice at 12 months (0.92 versus 0.66, P < 0.05) (Figure 1, C and D).
Figure 1.
Early decline in total REMS time for 24-hour period in Tg2576 mice. A: Time spent in REMS/24 hours in Tg2576 and WT non-Tg littermates (nTG) mice at 2 months (n = 9, Tg2576; n = 12, nTG), 6 months (n = 11 Tg2576; n = 14 nTG), and 12 months of age (n = 9, Tg2576; n = 13, nTG). Using the same subjects, data are presented for bout lengths of REMS (B), NREMS (C), and wakefulness (D). Values presented are mean ± SE. *P < 0.05.
Relative theta power across all behavioral states was unchanged. Relative delta declined by 50% (22 ± 5% in WT versus 10 ± 3% in Tg2576 mice) in NREMS, and therefore, delta:theta power in REMS was decreased in Tg2576 mice at 6 and 12 months. Despite the similar theta power in REMS, visually theta synchronization was reduced in Tg2576 mice, particularly by 12 months of age (Figure 2, A and B). The most striking changes in 24-hour spectral analyses in Tg2576 mice were loss of ultradian (<24 hours) rhythm in delta wave activity and a shift from lower frequencies to higher frequencies (Figure 2, C and D). Higher frequency waveforms were present in all behavioral states (Figure 1, E and F). Behavioral state instability (shortened bouts of sleep and wake states with increased frequency in transition) was markedly pronounced in many of the mice by 12 months of age, as shown in Figure 2, E and F).
Figure 2.
Age-dependent sleep/wake disturbances in Tg2576 mice. Representative samples comparing EEG recordings in Tg2576 mice at 2 months (A) and at 12 months (B). Theta synchronization in the EEG tracing is markedly disturbed in REMS in the 12-month-old mouse. Time bar indicates 1 second. Relative spectral power across 9 hours of sleep/wake activity is shown for 9 consecutive hours across the light-dark transition in C and D. C: At 2 months, an ultradian (<24 hours) rhythm in delta (0.5 to 4Hz, red, arrow) activity is apparent; theta (4 to 9 Hz, yellow) shows an ultradian rhythm (periodic cycling with cycle time less than 24 hours). D: In contrast, at 12 months, delta is markedly reduced (red, arrow); theta shows little ultradian variance, and now higher wave frequencies, α (9 to 13 Hz, green) and β (13 to 40 Hz, darker blue), have increased. E: Hypnograms (sleep stages across time) are normal at 2 months with consolidated wake and REMS (paradoxical sleep, PS; red) after many sleep periods. F: By 12 months of age, Tg2576 mice exhibit significant behavioral state instability, resulting in markedly frequent fluctuations between NREMS and waking.
Tg2576 Mice Exhibit Impaired Memory for Contextual Fear Conditioning
To determine whether REMS impairments occurred before hippocampal impairment, memory for contextual fear conditioning was examined in Tg2576 and WT mice at ages 6 and 12 months. At 6 months, WT mice displayed 67 ± 8% (n = 11) freezing in a 24-hour retention test (Figure 3A). By contrast, Tg2576 mice at 6 months, displayed 41 ± 9% (n = 12) freezing, which is 38% less freezing than that observed in the age-matched WT, t = 2.5, P < 0.05 (Figure 3A). At 12 months, WT mice exhibited 76 ± 3% freezing and Tg2576 exhibited 35 ± 6% freezing, t = 2.8, P < 0.05. Thus, Tg2576 have significantly impaired memory for contextual fear conditioning, both at 6 and 12 months of age. To examine the relationship between memory impairments observed in fear conditioning and severity of REMS disturbance, the percent freezing was plotted against percent REM sleep (Figure 3B), for each Tg2567 mouse (n = 15). We found no correlation between the severity of memory impairment and the severity of REMS disturbance (r2 = 0.02, NS).
Figure 3.
Tg2576 mice exhibit impaired memory for contextual fear conditioning. A: WT mice (6 months of age) displayed 67 ± 8% (n = 11) freezing in a 24-hour retention test. By contrast, Tg2576 mice (6 months of age) displayed significantly reduced freezing at 41 ± 9% (n = 12), t = 2.5, P < 0.05. Similarly, at 12 months of age, WT mice (n = 6) exhibited 76 ± 3% freezing whereas Tg2576 mice (n = 12) exhibited significantly reduced freezing at 35 ± 6%, t = 2.8, P < 0.05. B: To examine the relationship between severity of contextual memory impairment and severity of REMS impairment in Tg2576 mice, percent freezing was plotted against percent REMS sleep for each individual mouse (r2 = 0.02, NS). There is no correlation between impaired memory for contextual fear conditioning and impaired REMS sleep. For example, across the spectrum of observed REM impairments, there were Tg2567 mice that exhibited normal freezing (arrow) as well as mice that exhibited very low freezing (arrowhead). *P < 0.05.
Immunoreactive ChAT Is Reduced in PPT Neurons of Tg2576 Mice by 12 Months
To investigate the possible mechanisms related to reduced REMS in AD patients, we examined the numbers of cholinergic neurons in mesopontine cholinergic nuclei of the PTT and LDT. Because the MS nucleus is implicated in theta waveform generation, this cholinergic nucleus was also examined. There was a significant reduction of ChAT positivity in neurons of the PPT nucleus of Tg2576 compared to age-matched WT mice at both 6 and 12 months of age (Figure 4, A to F; P < 0.05). In contrast, we observed no detectable changes in ChAT immunoreactivity in neurons of the LDT and MS in Tg2576 relative to age-matched WT mice (data not shown). Linear regression for REM sleep amount and the total number of ChAT-positive neurons in the PPT was significant (r2 = 0.43, F = 6.1, P < 0.05).
Figure 4.
Reduction of ChAT immunoreactivity in cholinergic PPT neurons of TG2576 compared to WT mice. ChAT-immunoreactive (reddish brown) neurons in the PPT of Tg2576 and age-matched WT mice at 6 (A and B) and 12 months (C and D) at low (A–D, left)- and high (A–D, right)-power magnifications. The higher magnification views more clearly reveal differences in morphology and intensity of ChAT immunoreactivity (arrow) (A–D) in WT versus TG mice. The average number of ChAT-positive neurons/matched slice/genotype in the PPT of Tg2576 mice at 6 and 12 months of age compared to age-matched WT littermate controls are presented in a histogram (E) and a scatter graph (F). *P < 0.05.
Nitrotyrosine Immunoreactivity Is Significantly Increased in Aging Tg2576 Mice
Oxidative/nitrative injury has been implicated in the pathogenesis of neurodegenerative diseases, especially in AD and Parkinson’s disease patients. To examine if nitrative changes in REMS cholinergic neurons or their projection targets might play a role in the impairment of sleep and memory in the brain regions related to sleep and memory formation in Tg2576 mice, immunohistochemical studies with a 3-NT-specific antibody were conducted in the Tg2576 and age-matched control WT mice. These studies demonstrate that 3-NT immunoactivity is increased in neurons of hippocampus (Figure 5, B and D) and hypothalamus (Figure 5F) in Tg2576 mice at 12 months of age compared to the same regions in the WT littermate control mice (Figure 5, A and C). Semiquantitative assessment showed a significant increase in number of 3-NT-positive neurons (Figure 5G) and percentage of 3-NT-positive neurons (Tg/non-Tg; Figure 5G) in the hippocampus and hypothalamus of Tg2576 mice compared to age-matched non-Tg WT mice at 12 months of age (Figure 5, G and H; P < 0.0001) but not increased in the 6-month-old Tg2576 mice compared to non-Tg WT littermates (data not shown).
Figure 5.
3-NT is strongly expressed in mesopontine and forebrain cholinergic terminal projection sites in Tg2576 mice. There is minimal 3-NT immunoactivity in the hippocampus of 12-month-old WT mice (A and C). In contrast, there is intense 3-NT immunolabeling in the hippocampus of 12-month-old Tg2576 (B and D, which are shown at higher power than the images in A and C to illustrate the nature of the increased 3-NT immunolabeling). Similarly, there is minimal 3-NT immunolabeling in the lateral hypothalamus of 12-month-old WT mice (E), but intense 3-NT immunolabeling in the lateral hypothalamus of 12-month-old Tg2576 mice (F). Average number of 3-NT-positive neurons/matched slice/genotype in the hippocampus and hypothalamus of 12-month-old Tg2576 mice compared to age-matched WT littermate controls (G). Percentage of 3-NT-positive neurons in Tg2576 mice is compared to non-Tg mice (H). ***P < 0.0001.
PPT Cholinergic Neurons Show Evidence of an Early Caspase-3-Dependent Apoptotic Marker
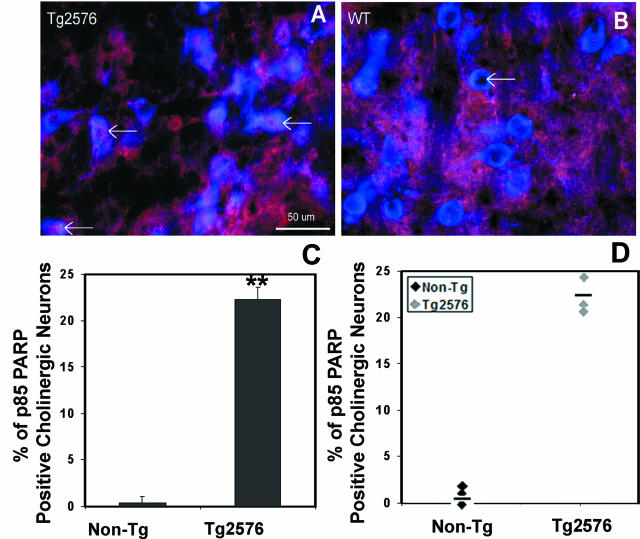
Cleaved fragment p85 of PARP was observed in cholinergic neurons in the PPT of Tg2576 mice. Tg2576 mice at 2.5 months (after sleep studies were performed on these same mice) had impressive increased cholinergic nuclear immunoreactivity to cleaved PARP p85 fragment (n = 3/geneotype) in the PPT. All Tg2576 mice showed increased PARP p85 in 22 ± 1% of cholinergic nuclei throughout the PPT nucleus (Figure 6A) and no PARP p85-positive neurons in the LDT or MS. In contrast, WT littermates showed 0.3 ± 0.3 p85-immunoreactive ChAT nuclei (t = 14.7, P < 0.001). Moreover, there were no PARP p85+ nuclei in WT ChAT neurons in any of the cholinergic groups examined (Figure 6B). PARP p85 immunoreactivity was not detectable in either Tg2576 or WT mice at 12 months of age (show n = 3/group).
Figure 6.
PARP-cleaved fragment p85 is expressed in cholinergic neurons in the PPT of Tg2576 mice. A: Cholinergic neurons in PPT of Tg2576 mouse at 2.5 months of age evidenced immunoreactivity for the PARP p85 (arrows pointing to purple in nuclei) within ChAT-positive (blue in cytoplasm) nuclei. B: In contrast, age-matched WT littermates show no PARP p85 immunoreactivity in any nuclei (arrow). Histogram (C) and scatter (D) graphs for percentage of cholinergic neurons immunoreactive for caspase-3-cleaved PARP. Individual data are presented for Tg2576 female mice at 2.5 months and WT age- and sex-matched controls (n = 3/group). Asterisk denotes a difference across strains, P < 0.001.
Discussion
Overexpression of the hAPPswe mutant gene in the Tg2576 mouse, results in progressive accumulation of Aβ peptide aggregates and ultimately plaque formation.16 In proximity to Aβ plaques in the cerebral cortex, Tg2576 mice show cholinergic synapse and fiber injury24 as well as reduced levels of ChAT in the cortex and hippocampus,35 and they also develop hippocampal memory impairments after 6 months of age.36 Remarkably, however, these deficits develop in the absence of overt hippocampal neuron loss, although there are other AD-like pathologies in the brains of these mice.37 We now add important information to the phenotype of this Tg murine model of AD-like Aβ brain amyloidosis by showing that REMS disturbances also occur in these mice. Specifically, there is reduction in the total REMS/24 hours and in the number of REMS bouts/24 hours, and the reduced REMS is associated with injury to cholinergic neurons in a subgroup of REMS neurons, ie, PPT cholinergic neurons. The injury involves expression of caspase-3-dependent PARP cleavage (p85) immunoreactivity occurring as early as 2 to 3 months in the PPT, without affecting other cholinergic groups in these mice. Moreover, nitrative injury occurred later and was more pronounced in projection sites than in cholinergic soma. Specifically, this injury was evident after REMS alterations and reduced ChAT immunoreactivity were identified, while the REMS impairments appeared to precede contextual memory deficits. This study, therefore, identifies a group of cholinergic neurons with heightened vulnerability to early dysfunction in a Tg model of AD-like Aβ brain amyloidosis. REMS monitoring provides an invaluable model with which to explore the mechanisms of early neural Aβ injury to select cholinergic neurons.
The impairments in contextual fear-freezing responses in Tg2576 mice, identified in the present study, are consistent with previous reports.35,36,38 We extend these findings by showing that there is no significant relationship between the severity of contextual learning impairment and reductions in REMS. Absence of PARP p85 immunoreactivity and reduced ChAT staining in the MS, a major cholinergic input to the hippocampus, suggests that medial septal injury is not necessary for reduced REMS.
An age-dependent reduction in REMS and later impaired maintenance of wakefulness, and disturbed ultradian and circadian amplitudes in the Tg2576 mouse observed in the present study recapitulate sleep disturbances observed in persons with AD. Two other groups have recently examined sleep/wake activity in the Tg2576 mice. One group found no reductions in REMS in mice at 2 months of age,25 in agreement with our findings, but did not examine older mice. The second group, studying males and females together, did not find reduced REMS in Tg2576 mice until 22 months, and only in females.26 We believe larger sample sizes and separate analysis of females will enable early detection of reductions in REMS in the Tg2576 mice. To achieve 80% power to detect a 30% reduction in REMS, samples sizes more than nine females are needed for each group. Alternatively, the background strains may contribute to the REMS phenotype in Tg2576 mice.
Oxidative and nitrosative injury have been implicated in the pathogenesis of neurodegenerative diseases, especially in AD and Parkinson’s disease patients.39 Differences in nitration were not detectable until 12 months of age when Tg2576 mice had substantially more nitration in cholinergic projecting targets, eg, hippocampus (a MS target), lateral hypothalamus, and anterior thalamus (both PPT and LDT targets). The lack of nitrosative injury within the soma despite substantial nitration in target regions suggests that the nitration develops in the terminals or postsynaptically as a relatively late biochemical modification.
In summary, our findings in the Tg2576 mice suggest that Swedish hAPP695 mutation gene in these Tg mice promotes early dysfunction of and injury to a select group of cholinergic neurons, ie, those in the PPT. Before REMS impairment, activated caspase-3 is evidenced in these neurons with PARP p85 immunoreactivity in cholinergic PPT neurons, whereas evidence of nitrosative injury, involving the terminal regions to a greater extent than the cholinergic soma, occurs after the appearance of apoptotic markers, impaired REMS and loss of ChAT. Therefore, the early REMS impairments and cholinergic PPT neuronal injury in the Tg2576 mouse provide insight into the relative timing of various toxic consequences mediated by rising levels of Aβ, thereby advancing understanding of the mechanisms underlying sleep/wake disturbances in AD and the sequelae of early injury in Aβ neurodegeneration.
Acknowledgments
We thank Jennifer Bruce, Sonali Joyce, Kristen Hilburger, and Theresa Schuck, Department of Pathology and Laboratory Medicine, Center for Neurodegenerative Disease Research, the University of Pennsylvania, for technical assistance.
Footnotes
Address reprint requests to John Q. Trojanowski M.D., Ph.D., Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Maloney 3, HUP, 3600 Spruce St., Philadelphia, PA 19104-4283. E-mail: trojanow@mail.med.upenn.edu.
Supported by the National Institute on Aging (grants AG-14382, AG-11542, AG-17628) and the Marina S. Ware Alzheimer Drug Discovery Program.
B.Z. and S.C.V. contributed equally to this article.
V.M.-Y.L. is the John H. Ware Third Professor for Alzheimer’s Disease Research and J.Q.T. is the William Maul Measey and Truman G. Schnabel Jr., M.D., Professor of Geriatric Medicine and Gerontology.
References
- Pollack CP, Perlick D, Linsner JP. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health. 1990;15:123–135. doi: 10.1007/BF01321316. [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Brooks JO, III, Bliwise D, Leader J, Yesavage JA. The relations among caregiver stress, “sundowning” symptoms, and cognitive decline in Alzheimer’s disease. J Am Geriatr Soc. 1992;40:807–810. doi: 10.1111/j.1532-5415.1992.tb01853.x. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Davey A, Pearlin LI, Zarit SH. Modeling caregiver adaptation over time: the longitudinal impact of behavior problems. Psychol Aging. 2000;15:437–450. doi: 10.1037//0882-7974.15.3.437. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, Zemcuznikov N, Gerber CJ. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30:86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging. 1982;3:361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone. 2004;6(Suppl 1A):S16–S28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- Petit D, Montplaisir J, Lorrain D, Gauthier S. Spectral analysis of the rapid eye movement sleep electroencephalogram in right and left temporal regions: a biological marker of Alzheimer’s disease. Ann Neurol. 1992;32:172–176. doi: 10.1002/ana.410320208. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Larsen LH, Moe KE, Vitiello MV. EEG markers of early Alzheimer’s disease in computer selected tonic REM sleep. Electroenceph Clin Neurophys. 1992;83:36–43. doi: 10.1016/0013-4694(92)90130-a. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Petit D, Lorrain D, Gauthier S, Nielsen T. Sleep in Alzheimer’s disease: further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. Sleep. 1995;18:145–148. doi: 10.1093/sleep/18.3.145. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Jones BE. Paradoxical sleep and its chemical/structural substrates in the brain. Neuroscience. 1991;40:637–656. doi: 10.1016/0306-4522(91)90002-6. [DOI] [PubMed] [Google Scholar]
- Terry RD, Katzman R. Senile dementia of the Alzheimer type. Ann Neurol. 1983;14:497–506. doi: 10.1002/ana.410140502. [DOI] [PubMed] [Google Scholar]
- Xuereb JH, Perry EK, Candy JM, Bonham JR, Perry RH, Marshall E. Parameters of cholinergic neurotransmission in the thalamus in Parkinson’s disease and Alzheimer’s disease. J Neurol Sci. 1990;99:185–197. doi: 10.1016/0022-510x(90)90155-g. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Kurt MA, Davies DC, Kidd M, Duff K, Rolph SC, Jennings KH, Howlett DR. Neurodegenerative changes associated with beta-amyloid deposition in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Exp Neurol. 2001;171:59–71. doi: 10.1006/exnr.2001.7717. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckma C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–103. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet. 1999;29:177–185. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol Behav. 2002;75:627–642. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Bondolfi L, Hunziker D, Schlecht HP, Carver K, Maguire E, Abramowski D, Wiederhold KH, Sturchler-Pierrat C, Jucker M, Bergmann R, Staufenbiel M, Sommer B. Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol Aging. 2003;24:365–378. doi: 10.1016/s0197-4580(02)00098-2. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp Neurol. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM-Y. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Alzheimer plaques and cortical cholinergic innervation. Neuroscience. 1986;17:275–276. doi: 10.1016/0306-4522(86)90242-3. [DOI] [PubMed] [Google Scholar]
- Moran MA, Mufson EJ, Gomez-Ramos P. Colocalization of cholinesterases with beta amyloid protein in aged and Alzheimer’s brains. Acta Neuropathol. 1993;85:362–369. doi: 10.1007/BF00334445. [DOI] [PubMed] [Google Scholar]
- Luth HJ, Apelt J, Ihunwo AO, Arendt T, Schliebs R. Degeneration of β-amyloid-associated cholinergic structures in transgenic APP SW mice. Brain Res. 2003;977:16–22. doi: 10.1016/s0006-8993(03)02658-1. [DOI] [PubMed] [Google Scholar]
- Colas D, London J, Gharib A, Cespuglio R, Sarda N. Sleep-wake architecture in mouse models for Down syndrome. Neurobiol Disease. 2004;16:291–299. doi: 10.1016/j.nbd.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Edgar DM, Yesavage J, Ryan HS, McCormick CM, Lapustea N, Murphy GM., Jr Sleep and circadian abnormalities in a transgenic mouse model of Alzheimer’s disease: a role for cholinergic transmission. Neuroscience. 2005;131:375–385. doi: 10.1016/j.neuroscience.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Uryu K, Laurer K, McIntosh T, Praticò D, Martinez D, Leight S, Lee VM-Y, Trojanowski JQ. Repetitive mild brain trauma accelerates Aβ deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci. 2002;22:446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Valladares O, Fenik P, Kapfhamer D, Sanford L, Benington J, Bucan M. An automated system for recording and analysis of sleep in mice. Sleep. 2000;23:1025–1040. [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- Benington JH, Kodali SK, Heller HC. Scoring transitions to REM sleep in rats based on the EEG phenomena of pre-REM sleep: an improved analysis of sleep structure. Sleep. 1994;17:28–36. doi: 10.1093/sleep/17.1.28. [DOI] [PubMed] [Google Scholar]
- Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2004;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Abel T. An immediate-shock freezing deficit with discrete cues: a possible role for unconditioned stimulus processing mechanisms. J Exp Psychol Animal Behav Proc. 2001;27:394–406. [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlovian J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM-Y. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Chang Y, Cheng Y, Zhang BL, Qu ZW, Qin C, Zhang JT. melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J Pineal Res. 2004;37:129–136. doi: 10.1111/j.1600-079X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin L, Carlson GA, Younkin SG, Ashe KH. The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Lu Y, Turner RS, Maren S. Overexpression of hAPPswe impairs rewarded alternation and contextual fear conditioning in a transgenic mouse model of Alzheimer’s disease. Learning Memory. 2002;9:243–252. doi: 10.1101/lm.51002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]