Abstract
Recent evidence has linked preterm premature rupture of the fetal membranes (PPROM) to placental abruption. Because neutrophils are a rich source of proteases that can degrade extracellular matrix in abruption-associated PPROM, we examined whether decidual neutrophil infiltration complicates abruption-associated PPROM. Accordingly, immunostaining for the neutrophil marker CD15 was performed in placentas obtained after overt abruption (decidual hemorrhage) with or without PPROM and in control placentas. Abruptions were associated with a marked decidual neutrophil infiltration that peaked after PPROM, whereas decidua from gestational age-matched controls were virtually devoid of neutrophils. Neutrophil infiltrates co-localized with fibrin deposition. Because abruptions elicit intense decidua-enhanced thrombin production, we examined the regulation of abruption-induced neutrophil infiltration. Expression of the primary neutrophil chemoattractant interleukin-8 (IL-8) was evaluated in leukocyte-free term decidual cells incubated with estradiol (E2; control) or with E2 + medroxyprogesterone acetate (to mimic pregnancy) ± thrombin. After 24 hours, enzyme-linked immunosorbent assay measurements indicated that thrombin (0.1 to 2.5 U/ml) elicited a dose-dependent elevation in secreted IL-8 (P < 0.05) with 2.5 U/ml of thrombin increasing IL-8 levels by >14-fold in E2 and E2 + medroxyprogesterone incubations. Results were validated by Western blot and quantitative reverse transcriptase-polymerase chain reaction. In summary, thrombin-enhanced IL-8 expression in term decidual cells may explain how abruption-associated PPROM promotes decidual neutrophil infiltration.
Although preterm premature rupture of the membranes (PPROM) is associated with intra-amniotic infections, there is now strong evidence linking PPROM with placental abruption (ie, decidual hemorrhage).1 Moreover, occult decidual hemorrhage and retrochorionic hematoma formation, as reflected by decidual and extraplacental membrane hemosiderin deposition, was reported in 72 of 192 (37.5%) of patients with PPROM compared with only 1 of 108 (0.8%) at term (P < 0.01) suggesting that the association between PPROM and abruption is much stronger than has been generally recognized.2 Preterm membrane rupture is accompanied by increased maternal- and fetal-derived protease activity.3,4 In infection-derived PPROM, proinflammatory cytokines enhance protease expression and neutrophil infiltration.5,6 By contrast, mechanisms underlying abruption-associated PPROM are primarily unknown.
Decidual cells are a rich source of tissue factor,7,8 the primary initiator of hemostasis. After abruption-related hemorrhage, decidual cell (DC) tissue factor is poised to bind to plasma-derived factor VIIa to activate factor X. Factor Xa then complexes with its co-factor, factor Va, to convert prothrombin to thrombin. The latter generates fibrin from fibrinogen and activates platelets. Consistent with this mechanism, consumption of fibrinogen is a standard method of assessing the severity of an abruption. Moreover, elevated circulating thrombin-antithrombin complex levels predict the subsequent occurrence of preterm delivery due to preterm labor and/or PPROM with a high sensitivity and specificity.9–11 Thus, marked decidual thrombin production underlies abruption-associated prematurity and PPROM.
In addition to its hemostatic properties, thrombin induces an array of biological effects via cell surface protease-activated receptors. Previously, we demonstrated that thrombin binds to its protease-activated receptor-1 receptor to reverse progestin inhibition of matrix metalloproteinase-1 (MMP-1) (interstitial collagenase) and MMP-3 (stromelysin-1) expression in unpassaged term DCs as well as in in vitro decidualized endometrial stromal cells derived from cycling endometrium.12,13 Thus, thrombin may play a pivotal role in the generation of proteases required for abruption-associated PPROM. Our finding that thrombin also augments expression of interleukin-8 (IL-8), a potent neutrophil chemoattractant and activator, in cycling endometrium14 prompted speculation that abruption-associated PPROM would lead to thrombin-induced IL-8 expression in term DCs and result in neutrophil infiltration. Because neutrophils are a rich source of extracellular matrix-degrading proteases15–17 such an infiltrate could contribute to the intense extracellular matrix degradation involved in fetal membrane rupture. Thus, the current study sought to determine the extent of decidual neutrophil infiltration in placentas and their fetal membranes complicated by abruption with and without PPROM in the absence of clinical infection by immunohistochemical localization of the neutrophil marker CD15 and to test the hypothesis that thrombin could promote such neutrophil trafficking by enhancing IL-8 expression in a novel leukocyte-free, term DC culture system.
Materials and Methods
Specimens
Fifteen placentas with abruption between 24 and 40 weeks (median, 31 weeks) were randomly selected from the case files of the Department of Human Pathology and Oncology, University of Siena, Siena, Italy. Only cases with clinically obvious abruption documented by the ultrasonographic appearance of a retroplacental hematoma, generally accompanied by vaginal bleeding were chosen as cases. An emergency cesarean delivery was performed in 12 cases (10 live born infants and 2 intrapartum deaths), whereas 3 patients delivered vaginally (1 live born infant at term and 2 intrapartum deaths). Two patients had accompanying PPROM. Eight control placentas were obtained after cesarean delivery between 28 and 40 weeks (median, 36 weeks), due to term breech presentation or from mothers with preterm preeclampsia. Only cases and controls from singleton gestations without clinical diagnosis of chorioamnionitis were included. Approval for this study was granted by the Human Institutional Investigation Committee of the University of Siena. Informed consent was obtained from all women. Placenta sampling for histology included multiple full-thickness placental blocks (10 mm × 10 mm) and sections of the umbilical cord at three different levels. For each specimen, a block that best represented the maternal decidua was selected for immunohistochemistry. The number of neutrophils in the decidua and membranes was evaluated using a semiquantitative method, according to the following scoring system: 1, isolated neutrophils infiltrating the decidua, no polymorphonuclear leukocytes found in the membranes; 2, focal dense neutrophil infiltration in the decidua and isolated polymorphonuclear leukocytes in the membranes; 3, focal dense neutrophil infiltration in the decidua and membranes.
Immunohistochemistry
Sections (4 μm) of paraffin-embedded placenta tissues were cut, deparaffinized, rehydrated, and washed in Tris-buffered saline (20 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.6). Tris-buffered saline was used for all subsequent washes and for dilution of the antibody. Antigen retrieval was performed by incubating sections in sodium citrate buffer (10 mmol/L, pH 6.0) in a microwave oven at 750 W for 5 minutes. Sections were subsequently rinsed in 3% hydrogen peroxide to block endogenous peroxidase and incubated for 1 hour at room temperature with a monoclonal antibody against the neutrophil-specific antigen CD15 (Caltag Laboratories, Burlingame, CA). Staining was visualized using the avidin-biotin peroxidase complex (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) and the 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO) chromogen substrate. Light hematoxylin stain was used for nuclear counterstaining. Negative controls for each tissue section were prepared by substituting the primary antibody with the corresponding preimmune serum. For each specimen, three hematoxylin and eosin-stained slides and at least one immunohistochemical slide were prepared. Co-localization of neutrophils with fibrin was performed by subjecting serial sections to either anti-CD15 or fibrin-specific Picro-Mallory stain using a commercially available kit (Bio-Optica, Milan, Italy). All slides were evaluated and scored by an experienced immunopathologist (P.T.) with >99% concordance in scores on repeat observation.
Isolation of Decidual Cells (DCs)
Placentas and attached fetal membranes were obtained from patients with uncomplicated pregnancies undergoing repeat cesarean deliveries at term either at Bellevue Hospital or Yale-New Haven Hospital under investigational review board and human investigation committee approval, respectively. The decidua was scraped from the maternal surface of the chorion, minced, and digested in a shaking water bath at 37°C for 30 minutes in Ham’s F-10 + 10% charcoal-stripped calf serum (Flow Laboratories, Rockville, MD) plus 25 mg/ml of collagenase (200 U/mg) (Worthington Biochemical Corp., Freehold, NJ); DNase (6.25 U/ml) (Sigma-Aldrich, St. Louis, MO) was then added to the digestate and the incubation was continued for 45 minutes. The digestate was then passed through a 23-gauge needle to dissociate remaining cell clusters and the isolated cells were centrifuged at 1500 rpm for 5 minutes at 4°C then washed in Ham’s F-10. This procedure was repeated three times and the final cell pellet was resuspended (1 g/ml) in 20% Percoll (Sigma-Aldrich), layered on a (60%:50%:40%) discontinuous Percoll gradient, and centrifuged at 22,000 rpm for 20 minutes at 4°C. The top cell layer was washed by resuspension and centrifugation × 2 at 1500 rpm for 5 minutes at 4°C in Ham’s F-10 and the resulting cell pellet was resuspended in 40% Percoll (1 g of tissue/ml), layered on a discontinuous (55%:50%:40%) Percoll gradient, then centrifuged at 22,000 rpm for 20 minutes at 4°C. The top cell layer was washed twice in serum-free Ham’s F-10, and then centrifuged at 500 rpm for 5 minutes at 4°C, resuspended in Ham’s F-10 + 10% stripped calf serum, and DCs counted in a hemocytometer.
Cell Cultures
Isolated DCs (5 × 105 cells/ml) were cultured in basal medium, a phenol red-free 1:1 v/v mix of Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) and Ham’s F-12 (Flow Laboratories) with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml fungizone supplemented with 10% stripped calf serum and seeded onto polystyrene tissue culture dishes coated with 2% type B gelatin (Sigma-Aldrich). Because leukocytes express IL-818 we sought to ensure that the DC cultures were leukocyte-free. Cultures were grown to confluence in a standard 95% air:5% CO2 incubator at 37°C and passaged × 6. Fluorescent antibody cell sorting for the presence of CD45+ was conducted as previously described19 and demonstrated that unpassaged cultures contained 12 to 15% CD45+ cells whereas passaged cultures were >99% free of this common leukocyte marker. The latter were used for experimental cell incubations.
Experimental Cell Incubations
Confluent DCs were incubated for 7 days in basal medium + 10% stripped calf serum with 10−8 mol/L estradiol (E2) or E2 plus 10−7 mol/L medroxyprogesterone acetate (MPA) (Sigma-Aldrich) with one change of medium. Because circulating levels of both E2 and progesterone are high during the third trimester, E2 was used as the control incubation for evaluating the effects of the progestin, MPA. The latter was used because Arici and colleagues20 demonstrated that native progesterone is rapidly metabolized in vitro. The cultures were washed twice with Hanks’ balanced salt solution to remove residual serum components and switched to a serum-free defined medium (DM) consisting of basal medium plus ITS+ (BD Biosciences, Bedford, MA), 5 μm FeSO4, 50 μm ZnSO4, 1 nm CuSO4, 20 nm Na2SeO3, trace elements (Life Technologies, Inc.), 50 μg/ml ascorbic acid (Sigma-Aldrich), and 50 ng/ml epidermal growth factor (BD Biosciences, Bedford, MA) containing the corresponding vehicle or steroid(s) with or without thrombin (American Diagnostica, Greenwich, CT). In some experiments, thrombin and hirudin (Sigma-Aldrich) were mixed and preincubated for 30 minutes at room temperature before incubation with DCs. After experimental treatment, conditioned DM was centrifuged and the supernatant was stored at −20°C. The DC lysates were stored at −20°C. RNA was extracted from parallel cultures with Tri Reagent as recommended by the manufacturer (Sigma-Aldrich).
Protein Assays
Immunoreactive IL-8 was measured in the conditioned media by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). The ELISA assay has a sensitivity of 3.5 pg/ml and intra-assay and inter-assay coefficients of variation of 4.6% and 6.7%, respectively. The protein content of the cell lysates was determined by the Bio-Rad assay (Bio-Rad Laboratories, Hercules, CA), respectively.
Western blot analysis was conducted on supernatants of conditioned medium prepared by diluting the media 1:1 in reducing sample buffer (composed of Laemmli sample buffer and 2-mercaptoethanol, Bio-Rad Laboratories) and then boiling for 3 minutes. Prepared media were centrifuged then electrophoresed on a 10 to 20% sodium dodecyl sulfate polyacrylamide linear gradient gel (Bio-Rad Laboratories). The gel was electroblotted onto a 0.2-μm nitrocellulose membrane (Bio-Rad Laboratories). After transfer, the membrane was blocked overnight in phosphate-buffered saline (PBS) with 1% casein and then incubated for 2 hours with 1.5 μg/ml of a mouse anti-human IL-8 monoclonal antibody (R&D Systems) diluted in 1% casein in PBS. Membranes were rinsed in PBS and 0.2% Tween 20 before and after incubation with horseradish peroxidase-conjugated anti-mouse IgG (ICN Biomedicals, Aurora, OH). Chemiluminescence was detected with enhanced chemiluminescence reagents (Perkin-Elmer Life Sciences, Boston, MA) and audioradiography film (Amersham Pharmacia, Buckinghamshire, UK) according to the manufacturers’ instructions.
Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Assays
To verify that the IL-8 and β-actin probes yielded the correct bands, extracted RNA from experimental cell incubations were subjected to semiquantitative RT-PCR using a kit from Invitrogen (Carlsbad, CA) and performed for 35 cycles with the Eppendorf Mastercycler (Eppendorf, Westbury, NY). To perform quantitative real-time RT-PCR, reverse transcription was initially performed with AMV reverse transcriptase (Invitrogen). A quantitative standard curve was created between 500 pg to 250 ng of cDNA with a Roche Light Cycler (Roche, Indianapolis, IN) by monitoring increasing fluorescence of PCR products during amplification. On establishing the standard curve, quantitation of the unknowns was determined with the Roche Light Cycler and adjusted to the expression of β-actin from the corresponding unknowns. Melting curve analysis determined the specificity of the amplified products and the absence of primer-dimer formation. All products obtained yielded correct melting temperatures. The following primers were synthesized and gel-purified at the Yale DNA Synthesis Laboratory, Critical Technologies: β-actin: sense, 5′-CGTACCACTGGCATCGTGAT-3′ and anti-sense, 5′-GTGTTGGCGTACAGGTCTTTG-3′; size, 452 bp; IL-8: sense, 5′-CACAAGAGCCAGGAAGAAAC-3′ and anti-sense, 5′-CTACAACAGACCCACACAATAC-3′, size 459 bp.
Statistical Analysis
Comparisons of cases and controls were performed using the Kruskall-Wallis analysis of variance on ranks test followed by the Student-Newman-Keuls post hoc test with a P value less than 0.05 representing statistical significance. Normally distributed data were analyzed using a Student’s t-test. For immunohistochemistry, the number of positively stained cells in control and abruption cases were compared using the χ2 test, with significance set at a probability value of <0.05.
Results
Immunohistochemistry
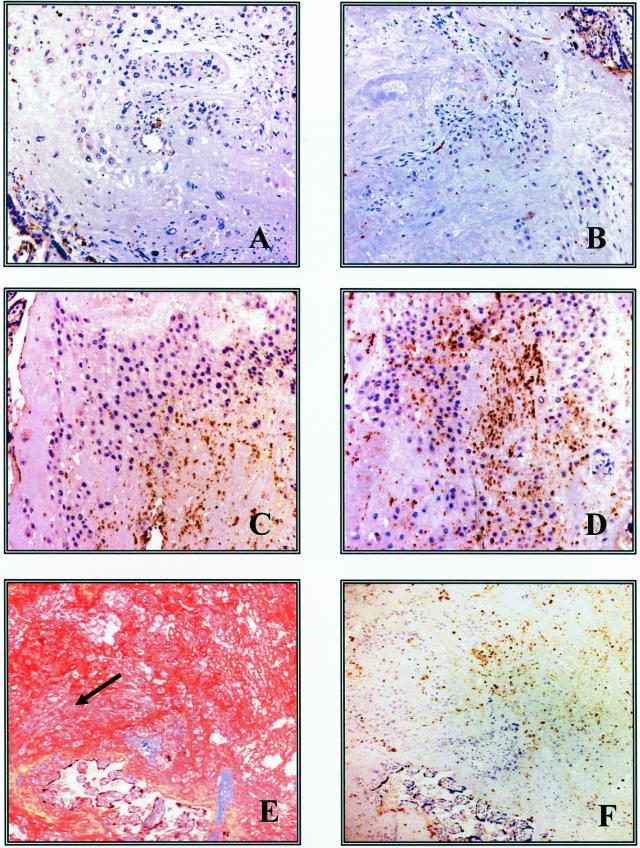
There was no significant age differences between the 8 controls and 15 cases in maternal age (32.1 ± 3.9 years old versus 32.8 ± 5.4 years old; P > 0.7), gestational age at delivery (34.4 ± 4.7 weeks versus 32.2 ± 5.2 weeks; P > 0.3), or mode of delivery (8 of 8 cesarean deliveries versus 12 of 15 cesarean deliveries; P > 0.17). In control cases (Figure 1; n = 8), a minimal inflammatory infiltrate was present in the decidua. Only isolated neutrophils, identified by their morphological appearance and positive immunostaining for CD15, were observed in either term control specimens obtained after an uncomplicated pregnancy and delivered by elective cesarean delivery (Figure 1A) or from preterm control patients undergoing a cesarean delivery because of preeclampsia (Figure 1B). In the placenta, umbilical cord, and amniotic membranes, no infiltration by neutrophils was observed. In contrast, abruption cases (n = 15) consistently displayed variable degrees of inflammatory infiltrate. Four cases presented with isolated neutrophils in the decidua and lack of an inflammatory infiltrate in the amniochorion or intravillous compartments whereas nine cases showed focal dense decidual neutrophil infiltration (Figure 1, C and D). In these cases isolated neutrophils were also present in the amniotic membranes. The two patients with abruption and PPROM had the most extensive infiltration of neutrophils both in the decidua and membranes. Statistical analysis showed that the number of CD15-positive cells was significantly higher in abruption cases compared to control placentas. Thus, although all eight of the control specimens stained at the minimal level, 73% of the case specimens stained at either the intermediate or most intense level (χ2 = 11.244; df = 2; P = 0.004). Figure 1E demonstrates dense fibrin deposition, reflective of local thrombin generation, in decidua from a patient with an abruption. A serial section (Figure 1F) clearly demonstrated co-localization of neutrophils with this fibrin.
Figure 1.
Immunolocalization of CD15-positive cells (brown stain; details in Materials and Methods) in term (A) and preterm (B) control specimens as well as in patients with abruption (C and D). E: Conspicuous deposition of fibrin stained in red by the Picro-Mallory reagent. Decidua cells (arrow) are visible under the fibrin clot. F: CD15 immunostaining on a consecutive section. Note numerous CD15-positive cells in the decidua and in the fibrin clot. Original magnifications: ×200 (A–D); ×40 (E, F).
In Vitro Studies
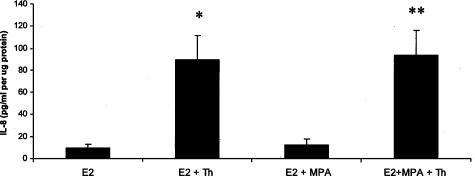
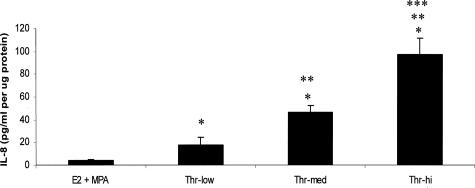
Figure 2 demonstrates that after 24 hours of incubation, thrombin (2.5 U/ml) markedly enhanced levels of immunoreactive IL-8, whereas DC cultures were refractory to MPA. Specifically, thrombin elevated IL-8 output by more than 14-fold (P < 0.05) in the presence of either E2 or E2 + MPA. Figure 3 demonstrates statistically significant dose-dependent effects of adding thrombin between 0.1 and 2.5 U/ml in cultures maintained in E2 + MPA with peak effects evident at the physiological limit of 2.5 U/ml.
Figure 2.
Effects of E2, MPA, and thrombin on IL-8 output by DC monolayers. Confluent passaged, leukocyte-free, term DCs were incubated for 7 days in 10−8 mol/L E2 or E2 + 10−7 mol/L MPA, then switched to DM with corresponding steroid(s) ± 2.5 U/ml thrombin (Th) for 24 hours. IL-8 levels were measured by ELISA in conditioned DM and normalized to cell protein (details in Materials and Methods). *P < 0.05, E2 versus E2 + Th; **P < 0.05 E2 + MPA versus E2 + MPA + Th (n = 10 for all groups, mean ± SEM).
Figure 3.
Dose response effects of thrombin on IL-8 output by DC monolayers maintained in the presence of E2 + MPA. Confluent DCs were incubated for 7 days in 10−8 mol/L E2 + 10−7 mol/L MPA, then switched to DM with corresponding steroid(s) ± 0.1 U/ml (low), 0.5 U/ml (med), and 2.5 U/ml (hi) thrombin (Thr) for 24 hours. IL-8 levels were measured by ELISA in conditioned DM and normalized to cell protein (details in Materials and Methods). *Versus E2 + MPA; **versus E2 + MPA + Th (low); and ***versus E2 + MPA + Th (med) (P < 0.05, mean ± SEM, and n = 10 for all groups).
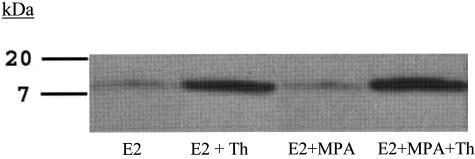
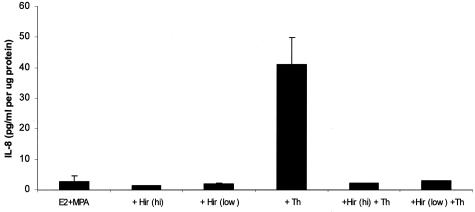
Western blot analysis validated the ELISA results. Thus, the Western blot depicted in Figure 4 reveals the presence of a single band in the DC-conditioned medium that migrated with the molecular weight of authentic IL-8. Moreover, changes in the levels of IL-8 conformed to the same pattern established by the ELISA measurements depicted in Figure 2. As expected from its well-documented thrombin inactivating properties, Figure 5 indicates that hirudin acted as a pure thrombin antagonist, exerting no effects on IL-8 expression when added alone, but completely blocking the thrombin effects.
Figure 4.
Western blot of thrombin effects on IL-8 output by in vitro DCs. Confluent DCs were incubated for 7 days in 10−8 mol/L E2 or E2 + 10−7 mol/L MPA, then switched to DM with corresponding steroid(s) ± 2.5 U/ml thrombin (Th) for 24 hours. The conditioned medium was then subjected to Western blotting (see Materials and Methods). Lane 1: E2; lane 2: E2 + Th; lane 3: E2 + MPA; and lane 4: E2 + MPA + Th.
Figure 5.
Effects of hirudin, thrombin, or both on IL-8 output by DC monolayers maintained in the presence of E2 + MPA. Confluent DCs were incubated for 7 days in 10−8 mol/L E2 + 10−7 mol/L MPA, then switched to DM with corresponding steroid(s) ± 0.5 U/ml thrombin (Th), or 0.5 or 2 U/ml hirudin (Hir), or hirudin plus thrombin for 24 hours. IL-8 levels in conditioned DM were measured by ELISA and normalized to cell protein (mean ± SD, n = 2) (details in Materials and Methods).
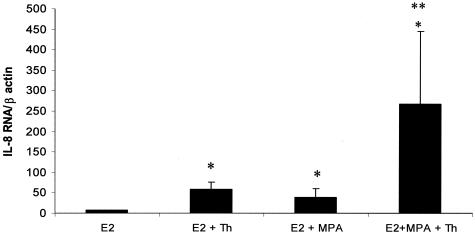
Figure 6 displays the separate and interactive effects of the steroids and thrombin on steady-state IL-8 mRNA levels after 5 hours in DM. Levels of IL-8 mRNA were significantly enhanced in cultures incubated with E2 + MPA compared with E2 alone. Moreover, thrombin significantly up-regulated IL-8 mRNA levels in both E2 and in E2 + MPA-treated cultures (P < 0.05) with the highest steady state values evident in incubations with E2 + MPA. When compared with E2, thrombin increased IL-8 mRNA levels by approximately eightfold, and more than 30-fold when thrombin was added with E2 + MPA. Despite the large increases in IL-8 mRNA after 5 hours of incubation in DM, ELISA results showed no appreciable increase in secreted IL-8 levels by 6 hours (results not shown) and no differential steroid effects as indicated in Figure 2, by 24 hours.
Figure 6.
Quantitative RT-PCR of effects of E2, MPA, and thrombin on IL-8 mRNA levels in leukocyte-free term DC monolayers. Confluent DCs were incubated for 7 days in 10−8 mol/L E2 or E2 + 10−7 mol/L MPA, then switched to DM with corresponding steroid(s) ± 2.5 U/ml thrombin (Thr) for 5 hours. IL-8 mRNA levels were measured by quantitative RT-PCR of thrombin in conditioned DM and normalized to β-actin mRNA levels (details in Materials and Methods). Ordinate: IL-8 mRNA/β actin mRNA. *Versus E2, **versus E2 + MPA (P < 0.05, mean ± SEM, and n = 4 for all groups).
Discussion
IL-8 is a member of the CXC chemokine superfamily that promotes trans-endothelial cell migration of neutrophils by inducing shape change, respiratory burst, enhanced expression of adhesion molecules, chemoattraction, and activation.21–23 Neutrophil infiltration of the cervix and fetal membranes is strongly associated with PPROM and preterm labor accompanying intra-amniotic infection,24 but has not been specifically evaluated in abruptions or abruption-associated PPROM.25 The current study establishes the novel association between the decidual hemorrhage of abruption and neutrophil infiltration. Thus, co-localization of fibrin deposition with neutrophil infiltration was demonstrated and although the number of specimens available was limited, maximum decidual neutrophil infiltration was observed in the setting of abruption-induced PPROM.
That IL-8 is a key mediator of the neutrophil infiltration of the decidua and fetal membranes observed in these pathological states is suggested by a report that IL-8 levels are elevated in the serum, cervicovaginal secretions, and amniotic fluid of women at risk for preterm labor and PPROM.26,27 Although the infection-associated cytokines, IL-1β, and tumor necrosis factor-α induce IL-8 expression in many cell types via enhancement of nuclear factor-κB,22 an increase in IL-8 levels in the amniotic fluid of patients at risk for preterm delivery has also been observed in the absence of infection28 suggesting that noninfectious mediators could also regulate its expression. Our current findings suggest that thrombin may be just such a noninfectious-associated mediator of IL-8 expression in term DCs and help account for the abruption-related influx of neutrophils into the decidua noted in this study. Indeed, we demonstrated clear co-localization of thrombin-induced fibrin with neutrophil infiltrates.
We previously showed that thrombin up-regulates expression of MMP-1 and MMP-3 in term DCs.12,13 We now provide strong evidence that thrombin also initiates the influx of neutrophils into the decidua via enhanced decidual IL-8 expression. Neutrophils are a rich source of elastase and MMP-9.15–17 MMP-1, -3 and -9 display unique substrate specificities, and MMP-3 has the capacity to activate the zymogenic forms of other MMPs.29,30 The potential for these MMPs to interact with each other and with neutrophil-derived elastase suggests that thrombin initiates an extracellular matrix-degrading proteolytic cascade mediating abruption-associated PPROM.
In stromal cell monolayers derived from cycling endometrium, the addition of progestin markedly inhibits expression of IL-814 as well as MMP-1 and MMP-3,31–34 but augments expression of tissue factor35 and plasminogen activator inhibitor-type 1.36,37 Similarly, in cultured term DCs, progestin inhibits basal and thrombin-induced MMP-1 and MMP-3 expression12,13 and enhances the expression of tissue factor and plasminogen activator inhibitor-type 1 (unpublished results), indicating that steroid responsiveness is retained despite three trimesters of continuous exposure to high concentrations of progesterone and estrogen. However, in contrast to the progestational inhibition of basal and thrombin-induced IL-8 in decidualized stromal cell cultures derived from cycling endometrium, the current study found that progestin did not significantly affect either basal or thrombin-induced IL-8 protein expression in term DCs. Indeed, progestin actually augmented both basal and thrombin-induced IL-8 mRNA levels.
Footnotes
Address reprint requests to Charles J. Lockwood, M.D., The Anita O’Keefe Young Professor of Women’s Health and Chair, Department of Obstetrics, Gynecology, and Reproductive Sciences, Yale University School of Medicine, 333 Cedar St., PO Box 208063, Room 334FMB, New Haven, CT 06520-8063. E-mail: chairobgyn@yale.edu.
Supported in part the National Institutes of Health (grant RO1 HL70004 to C.J.L.).
References
- Harger JH, Hsing AW, Tuomala RE, Gibbs RS, Mead PB, Eschenbach DA, Knox GE, Polk BF. Risk factors for preterm rupture of fetal membranes: a multicenter case-control study. Am J Obstet Gynecol. 1990;163:130–137. doi: 10.1016/s0002-9378(11)90686-3. [DOI] [PubMed] [Google Scholar]
- Salafia CM, Lopez-Zeno JA, Sherer DM, Whittington SS, Minior VK, Vintzileos AM. Histologic evidence of old intrauterine bleeding is more frequent in prematurity. Am J Obstet Gynecol. 1995;173:1065–1070. doi: 10.1016/0002-9378(95)91327-0. [DOI] [PubMed] [Google Scholar]
- Darby MJ, Caritis SN, Shen-Schwarz S. Placental abruption in the preterm gestation: an association with chorioamnionitis. Obstet Gynecol. 1989;74:88–92. [PubMed] [Google Scholar]
- Polzin WJ, Brady K. The etiology of premature rupture of the membranes. Clin Obstet Gynecol. 1998;41:810–816. doi: 10.1097/00003081-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, Mazor M, Maymon E, Berry S. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–1130. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- Shobokshi A, Shaarawy M. Maternal serum and amniotic fluid cytokines in patients with preterm premature rupture of membranes with and without intrauterine infection. Int J Gynaecol Obstet. 2002;79:209–215. doi: 10.1016/s0020-7292(02)00238-2. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Nemerson Y, Guller S, Krikun G, Alvarez M, Hausknecht V, Gurpide E, Schatz F. Progestational regulation of human endometrial stromal cell tissue factor expression during decidualization. J Clin Endocrinol Metab. 1993;76:231–236. doi: 10.1210/jcem.76.1.8421090. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Schatz F. Decidual cell-expressed tissue factor maintains hemostasis in human endometrium. Ann NY Acad Sci. 2001;943:77–88. doi: 10.1111/j.1749-6632.2001.tb03793.x. [DOI] [PubMed] [Google Scholar]
- Rosen T, Kuczynski E, O’Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med. 2001;10:297–300. doi: 10.1080/714904361. [DOI] [PubMed] [Google Scholar]
- Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, Yoon BH, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:368–373. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol. 2001;185:1059–1063. doi: 10.1067/mob.2001.117638. [DOI] [PubMed] [Google Scholar]
- Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism lining placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med. 2002;11:11–17. doi: 10.1080/jmf.11.1.11.17. [DOI] [PubMed] [Google Scholar]
- Mackenzie AP, Schatz F, Krikun G, Funai EF, Kadner, Lockwood CJ. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol. 2004;191:1996–2001. doi: 10.1016/j.ajog.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Kumar P, Krikun G, Kadner S, Dubon P, Critchley H, Schatz F. Effects of thrombin, hypoxia, and steroids on interleukin-8 expression in decidualized human endometrial stromal cells: implications for long-term progestin-only contraceptive-induced bleeding. J Clin Endocrinol Metab. 2004;89:1467–1475. doi: 10.1210/jc.2003-030141. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Touat Z, Mtairag el M, Vranckx R, Louedec L, Houard X, Andreassian B, Sebbag U, Palombi T, Jacob MP, Meilhac O, Michel JB. Role of leukocyte elastase in preventing cellular re-colonization of the mural thrombus. Am J Pathol. 2004;164:2077–2087. doi: 10.1016/s0002-9440(10)63766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundall M, Sun Y, Miranda C, Trudeau JB, Barnes S, Wenzel SEJ. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. Allergy Clin Immunol. 2003;2:1064–1071. doi: 10.1016/j.jaci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Lathbury LJ, Salamonsen LA. In-vitro studies of the potential role of neutrophils in the process of menstruation. Mol Hum Reprod. 2000;6:899–906. doi: 10.1093/molehr/6.10.899. [DOI] [PubMed] [Google Scholar]
- Ribeiro FP, Furlaneto CJ, Hatanaka E, Ribeiro WB, Souza GM, Cassatella MA, Campa A. mRNA expression and release of interleukin-8 induced by serum amyloid A in neutrophils and monocytes. Mediators Inflamm. 2003;12:173–178. doi: 10.1080/0962935031000134897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arici A, Marshburn PB, MacDonald PC, Dombrowski RA. Progesterone metabolism in human endometrial stromal and gland cells in culture. Steroids. 1999;64:530–534. doi: 10.1016/s0039-128x(99)00029-x. [DOI] [PubMed] [Google Scholar]
- Wu D, Larosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1988;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, Ohlsson K, Uldbjerg N. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–246. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- Harris BA, Jr, Gore H, Flowers CE., Jr Peripheral placental separation: a possible relationship to premature labor. Obstet Gynecol. 1985;66:774–778. [PubMed] [Google Scholar]
- Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. The diagnostic value of interleukin-8 and fetal fibronectin concentrations in cervical secretions in patients with preterm labor and intact membranes. J Perinat Med. 1997;25:461–468. doi: 10.1515/jpme.1997.25.6.461. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang L, Zhao Y, Kang J. Changes in cytokine (IL-8, IL-6 and TNF-alpha) levels in the amniotic fluid and maternal serum in patients with premature rupture of the membranes. Zhonghua Yi Xue Za Zhi (Taipei) 2000;63:311–315. [PubMed] [Google Scholar]
- Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Nagase H. Parks WC, Mecham R, editors. San Diego: Academic Press,; Stromelysins 1 and 2. Matrix Metalloproteinases. 1998:pp 43–84. [Google Scholar]
- Lockwood CJ, Krikun G, Hausknecht VA, Papp C, Schatz F. Matrix metalloproteinase and matrix metalloproteinase inhibitor expression in endometrial stromal cells during progestin-initiated decidualization and menstruation-related progestin withdrawal. Endocrinology. 1998;139:4607–4613. doi: 10.1210/endo.139.11.6304. [DOI] [PubMed] [Google Scholar]
- Schatz F, Papp C, Toth-Pal E, Lockwood CJ. Ovarian steroid-modulated stromelysin-1 expression in human endometrial stromal and decidual cells. J Clin Endocrinol Metab. 1994;78:1467–1472. doi: 10.1210/jcem.78.6.8200951. [DOI] [PubMed] [Google Scholar]
- Keller NR, Sierra-Rivera E, Eisenberg E, Osteen KG. Progesterone exposure prevents matrix metalloproteinase-3 (MMP-3) stimulation by interleukin-1alpha in human endometrial stromal cells. J Clin Endocrinol Metab. 2000;85:1611–1619. doi: 10.1210/jcem.85.4.6502. [DOI] [PubMed] [Google Scholar]
- Hampton AL, Nie G, Salamonsen LA. Progesterone analogues similarly modulate endometrial matrix metalloproteinase-1 and matrix metalloproteinase-3 and their inhibitor in a model for long-term contraceptive effects. Mol Hum Reprod. 1999;5:365–371. doi: 10.1093/molehr/5.4.365. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Nemerson Y, Guller S, Krikun G, Alvarez M, Hausknecht V, Gurpide E, Schatz F. Progestational regulation of human endometrial stromal cell tissue factor expression during decidualization. J Clin Endocrinol Metab. 1993;76:231–236. doi: 10.1210/jcem.76.1.8421090. [DOI] [PubMed] [Google Scholar]
- Schatz F, Aigner S, Papp C, Toth-Pal E, Hausknecht V, Lockwood CJ. Plasminogen activator activity during decidualization of human endometrial stromal cells is regulated by plasminogen activator inhibitor 1. J Clin Endocrinol Metab. 1995;80:2504–2510. doi: 10.1210/jcem.80.8.7629251. [DOI] [PubMed] [Google Scholar]
- Sandberg T, Eriksson P, Gustavsson B, Casslen B. Differential regulation of the plasminogen activator inhibitor-1 (PAI-1) gene expression by growth factors and progesterone in human endometrial stromal cells. Mol Hum Reprod. 1997;3:781–787. doi: 10.1093/molehr/3.9.781. [DOI] [PubMed] [Google Scholar]