Abstract
The enzyme 12/15 lipoxygenase (12/15LO) has been implicated in the oxidative modification of lipoproteins and phospholipids in vivo. In addition, mice deficient in apolipoprotein E (ApoE−/−) are characterized by spontaneous hypercholesterolemia and a systemic increase in oxidative stress. Whereas the absence of 12/15LO reduces lipid peroxidation in the plasma and urine of ApoE−/− mice, the relative contribution of this enzyme to oxidative stress in the central nervous system remains unknown. Here, we provide the first in vivo evidence that 12/15LO modulates brain oxidative stress reactions using ApoE−/− mice crossbred with 12/15LO-deficient (12/15LO−/−) mice (12/15LO−/−/ApoE−/−). In chow-fed 12-month-old 12/15LO−/−/ApoE−/− mice, the amount of brain isoprostane iPF2α-VI, a marker of lipid peroxidation, and carbonyls, markers of protein oxidation, were significantly reduced when com-pared with 12/15LO-expressing controls (12/15LO+/+/ApoE−/−). These results were observed despite the fact that cholesterol, triglyceride, and lipoprotein levels were similar to those of ApoE−/− mice. These data indicate a functional role for 12/15LO in the modulation of oxidative reactions in the central nervous system, supporting the hypothesis that inhibition of this enzymatic pathway may be a novel therapeutic target in clinical settings involving increased brain oxidative stress.
Lipoxygenases (LOs) are members of a large family of non-heme iron-containing dioxygenases that insert molecular oxygen into polyunsaturated fatty acids.1 Human and rabbit 15LO, as well as porcine leukocyte-type 12LO, are all capable of directly oxygenating esterified fatty acids in lipoproteins and phospholipids.2 This group of enzymes is also classified as 12/15LO because they form both 12-hydroxyeicosatetraenoic acid [12(S)-HETE] and 15(S)-HETE from arachidonic acid (in various ratios), and predominantly 13-hydroperoxy-octadecaenoic acid from linoleic acid.3 The 12/15LO is widely distributed in the central nervous system (CNS) in humans as well as animals, and for this reason is also known as the neuronal isoform.4,5
In vitro this enzyme can initiate oxidation of low-density lipoprotein (LDL),6 and LO inhibitors can greatly diminish the ability of macrophages to oxidatively modify phospholipids present in the LDL.7 When LDL preparations are incubated with 12/15LO-transfected fibroblasts, they become seeded with hydroperoxides and develop typical biological properties of oxidized LDL (oxLDL), including the display of oxidation-specific epitopes recognized by antibodies to oxLDL.8 Genetic disruption of 12/15LO gene expression greatly diminished atherosclerosis in apolipoprotein E-deficient (ApoE−/−) mice, a mouse model that spontaneously develops atherosclerotic vascular lesions and a systemic increase in lipid peroxidation.9 This reduction was directly correlated with urinary and plasma isoprostane levels and the titers of antibodies against oxLDL epitopes, two distinct indices of lipid peroxidation.10 Interestingly, ApoE−/− mice are also characterized by signs of oxidative stress in their CNS. Thus, levels of 3-nitrotyrosine, a marker of protein oxidation, are significantly increased in brains of ApoE−/− compared with control mice.11 We also found that another independent marker of oxidative stress, the isoprostane iPF2α-VI, is elevated in the CNS of these mice compared with wild-type animals.12
Based on the pro-oxidant properties, disruption of 12/15LO expression might be expected to reduce oxidative modification also in the CNS. This hypothesis could have important implications for several neurodegenerative diseases and Alzheimer’s disease in particular, in which brain oxidative stress reactions have been shown to be early events in the pathogenesis of the disease.13 Moreover, ApoE allele isoforms can also modulate in vitro and in vivo oxidative stress responses and have been implicated as risk factors for developing Alzheimer’s disease.14 To test the hypothesis that 12/15LO modulates CNS oxidative stress, we crossbred 12/15LO-deficient (12/15LO−/−) mice with ApoE−/− mice and analyzed two distinct oxidative stress responses, protein and lipid oxidation products, in the CNS of these animals.
Materials and Methods
Generation of 12/15LO−/−/ApoE−/− Double-Knockout Mice
12/15LO−/− mice were crossbred with ApoE−/− mice (backcrossed eight and seven times, respectively, to the C57BL/6 background).9 ApoE−/− and C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were genotyped by polymerase chain reaction (PCR) analysis as described below. The animal studies described below comprised three groups of mice: ApoE−/−, 12/15LO−/−, and 12/15LO−/−/ApoE−/− mice; each group had six males and six females. Mice were kept on a 12-hour light/dark cycle and were fed a normal chow and water ad libitum. All animal procedures were performed in accordance with institutional and National Institutes of Health guidelines.
Genotype Determination
Genotype analyses of ApoE−/−, 12/15LO−/− mice were performed by PCR using genomic DNA isolated from mouse tail snip samples as a template with primers specific for ApoE and 12/15LO, as previously published.15,16 PCR analysis was performed for ApoE alleles with the sense primer to ApoE exon 3(5′-GCCTAGCCGAGGGAGAGCCG-3′), and the anti-sense primer to ApoE exon 3 (5′-TGTGACTTGGGAGCTCTGCAGC-3′), and the anti-sense primer corresponding to the region within the vector used to disrupt the ApoE gene (5′-GCCGCCCCGACTGCATCT-3′). For 12/15LO we used a primer within exon 3 (5′-GAGGGCACTGGTGAGCAGA-3′), the anti-sense primer (5′-TCCTGAACAGGCCTTGAGAG-3′), and the anti-sense primer corresponding to the neomycin resistance cassette (5′-ATCGCCTTCTTGACGAGTTC-3′). Reaction mixtures contained PCR buffer, 25 mmol/L MgCl2, 20 mmol/L dNTP, 20 pmol of each primer, and 5 U of TaqDNA polymerase in a total volume of 20 μl. After a denaturation step at 90°C for 5 minutes, 35 cycles of amplification step (94°C for 45 seconds, 60°C for 30 seconds, 72°C for 90 seconds) were performed, followed by a final elongation step of 7 minutes at 72°C.
Primary Neuronal Cultures
Primary neuronal cell cultures were prepared from 14- to 15-day-old embryos of ApoE−/−, 12/15LO−/−/ApoE−/−, and wild-type mice (C57BL/6), following published procedures.17 In brief, embryos were decapitated and the cerebral cortex was dissected out on ice. Trypsin-ethylenediamine tetraacetic acid (EDTA) (0.25%) with DNase-I (10 mg/ml) and trypsin soybean inhibitor (50 mg/ml) was added to the dissected cerebral cortices, and the preparation was gently aspirated with a glass pipette. The cell suspension was plated onto six-well plates precoated with 100 μg/ml poly(d-lysine) and 5 μg/ml laminin. Cultures were performed in Dulbecco’s modified Eagle’s medium supplemented with 2 mmol/L of glutamine in the presence of 5% fetal bovine serum and 5% horse serum. After 6 to 7 days in vitro, proliferation of nonneuronal cells was arrested by adding 10 mmol/L cytosine arabinoside. Experiments were performed on pure neuronal cultures (>98% microtubule-associated protein-2 immunoreactive cells) after 12 to 14 days.
Tissue Preparation
Animal experiments were performed following procedures recommended by the Panel on Euthanasia of the American Veterinary Medical Association. After sacrifice, animals were perfused intracardially with ice-cold 0.9% phosphate-buffered saline (PBS), containing EDTA (2 mmol/L) and butylated hydroxy toluene (BHT) (2 mmol/L), pH 7.4. Brain was removed, gently rinsed in cold 0.9% PBS, and immediately dissected in different anatomical regions (cerebral cortex, which includes frontal and temporal cortex; hippocampus; cerebellum; and basal brain) for total RNA extraction and biochemistry analyses.12
Total RNA Extraction
RNA was extracted using the RNeasy mini kit from Qiagen (Valencia, CA). Briefly, 30 μg of frozen tissue was homogenized with 600 μl of RNeasy Lysis buffer (RLT) buffer. After centrifugation, the supernatant was resuspended in 70% ethanol and loaded onto the RNeasy column, which is a silica-based membrane with selective binding properties. Contaminants were washed away with appropriate wash buffers supplied in the kit. The RNA was finally eluted with nuclease-free water, and its concentration determined by spectrophotometric measurement (absorbance at 260 nm). DNase digestion was performed by treating the RNA with the Amplification Grade DNase 1 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. To remove residual DNase and EDTA from the treated RNA, the solution was passed through the RNeasy system for a second time using the RNA clean-up protocol.
One-Step Reverse Transcriptase (RT) and PCR
Preliminary experiments of RT-PCR were done to optimize the amount of complementary DNA and the reaction conditions. The RT-PCR was performed using the One Step RT-PCR kit (Qiagen). For murine 12/15LO, the primers used were 5′-GCGACGCTGCCCAAT-3′ (forward) and 5′-CATATGGCCACGCTGTTTTCTACC-3′ (reverse), which gives a 256-bp amplified product. Primers for 12/15LO were chosen from the published sequences. The housekeeping gene Hprt (hypoxanthine-guanine phosphoribosyltransferase) was used as internal control. One hundred ng of total RNA was used for RT-PCR reaction according to the manufacturer’s instructions. For 12/15LO, 35 cycles of reaction at 94°C for 30 seconds, 62°C for 1 minute, and 72°C for 1 minute were used. For Hprt, we used the primers forward 5′-GGCCATCTGCCTAGTAAAGCT-3′ and reverse 5′-GTCGGCCTATAGGCTCATAGT-3′, yielding a 111-bp fragment.
Biochemical Analyses
Aliquots of tissue samples were minced, homogenized, and processed as previously described.12,18 Briefly, a fraction of the homogenates was used for total lipid extraction with ice-cold Folch solution (chloroform, methanol, 2:1, v/v). The solution was then vortex-mixed and centrifuged at 3000 rpm for 15 minutes at 4°C. The lipid extracts were subjected to base hydrolysis by adding 15% aqueous KOH and then incubated at 45°C for 1 hour for measurement of total isoprostane iPF2α-VI. In brief, after solid-phase extraction, the samples were derivatized and purified by thin-layer chromatography, and finally analyzed by ion chemical ionization gas chromatography/mass spectrometry as previously described.12,18 An aliquot of the extracted lipids was taken to measure total levels of phospholipids, and arachidonic acid.19 Extracted lipids were also used for measuring total PGE2, and leukotriene B4 (LTB4) by specific and sensitive enzyme-linked immunosorbent assays, following the manufacturer’s protocols (Assay Design, Ann Arbor, MI). Total protein carbonyls in tissues were determined by using the Zenith PC test kit according to the manufacturer’s instructions (Zenith Technology, Dundein, NZ).20 Briefly, tissue homogenates were first reacted with dinitrophenylhydrazine, transferred to a multiwell plate, incubated with blocking reagent, washed, and probed with anti-dinitrophenylhydrazine-biotin antibody solution. After washing, samples were incubated with streptavidin-horseradish peroxidase, washed, and then developed. After 15 minutes, the reaction was stopped and absorbance immediately read at 450 nm. Oxidized protein standards, internal controls, and blanks were always assayed at the same time and in the same way. Total plasma cholesterol, triglycerides, high-density lipoprotein cholesterol, and LDL cholesterol levels were determined as previously described.9,12 Samples were always determined in triplicate and in a blinded manner.
Western Blot Analysis
Total proteins from brain tissues were extracted by RIPA buffer containing 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 2 mmol/L sodium fluoride, 2 mmol/L EDTA, 0.1% sodium dodecyl sulfate, and an EDTA-free protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN). The tissue extracts were centrifuged at 13,000 rpm for 45 minutes at 4°C, and the supernatants used for experiments. The total protein concentrations were determined with a protein assay kit (BCA; Pierce, Rockford, IL). Fifteen μg per lane of proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Richmond, CA) The membranes were blocked with 5% nonfat milk in T-TBS (50 mmol/L Tris, pH 7.5, 0.9% NaCl, and 0.1% Tween-20) overnight at 4°C and then incubated 1 hour at room temperature with rabbit polyclonal antibody raised against recombinant mouse leukocyte-type 12/15LO (1:1000 dilution; Alexis, San Diego, CA). β-Actin was always used as an internal loading control. After three washings with T-TBS, the membranes were incubated for 1 hour at room temperature with the horseradish peroxidase-conjugated secondary antibody goat anti-rabbit IgG (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). After the membranes were washed four times, the signals were detected with an enhanced chemiluminescence reagent (ECL; Amersham, Arlington Heights, IL).5
Statistical Analysis
All data are presented as the mean ± SEM. Data were analyzed by analysis of variance and subsequently by Student’s unpaired two-tailed t-test. Simple regression analysis was performed to analyze the relation among the levels of different parameters that were measured. Significance was set at P < 0.05.
Results
CNS Expression of 12/15LO
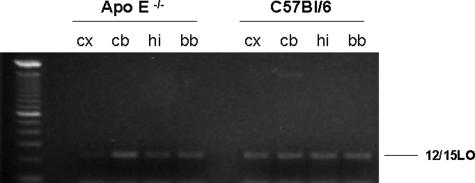
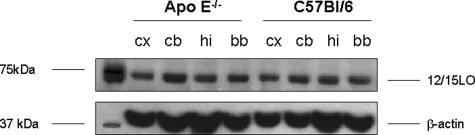
Initial studies were performed to characterize the regional distribution of 12/15LO in ApoE−/− and wild-type littermates, C57BL/6. As shown in Figure 1, we found that the mRNA for this enzyme was widely expressed in the different brain regions considered. However, no difference was observed between the two groups of mice. Similar results were obtained also when brain homogenates from the same brain regions were assayed for 12/15LO protein levels (Figure 2).
Figure 1.
Tissue distribution of 12/15LO mRNA in ApoE−/− and wild-type littermates (C57BL/6). RT-PCR analysis of brain cDNAs with primers derived from 12/15LO (cx, cerebral cortex; cb, cerebellum; hi, hippocampus; bb, basal brain).
Figure 2.
Tissue distribution of 12/15LO protein in ApoE−/− and wild-type littermates (C57BL/6). Representative Western blot analyses of 12/15LO protein in homogenates from ApoE−/− and C57BL/6. Samples were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membrane, and probed with an antibody against 12/15LO (cx, cerebral cortex; cb, cerebellum; hi, hippocampus; bb, basal brain).
In Vitro Experiments
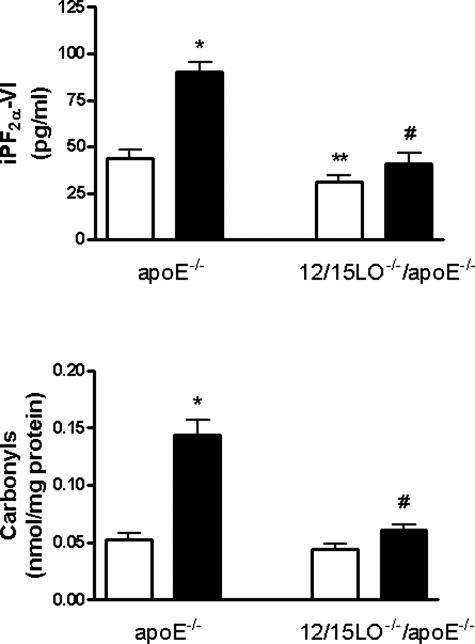
After 12 to 14 days plating ApoE−/− and 12/15LO−/−/ApoE−/− cortical neuronal cultures were used for studying basal oxidative stress parameters by measuring the levels of iPF2α-VI and carbonyls. The results showed that there was a significant increase in basal oxidative stress in primary neurons derived from ApoE−/− mice that express 12/15LO when compared with cells from ApoE−/− mice lacking 12/15LO (double-knockout 12/15LO−/−/ApoE−/−) (Figure 3). Moreover, ApoE−/− primary neurons showed an increased vulnerability to oxidative stress compared with 12/15LO−/−/ApoE−/− cells when challenged with H2O2 (10 μmol/L) (Figure 3).
Figure 3.
Absence of 12/15LO protects primary neuronal cells against oxidative challenge. Primary neurons from ApoE−/− and 12/15LO−/−/ApoE−/− mice were isolated and incubated with vehicle (open bars) or H2O2 (filled bars) (10 μmol/L) for 24 hours. Supernatants were collected and assayed for iPF2α-VI (top) and cell lysates for total carbonyls (bottom) (n = 3 separate experiments; *P < 0.01 versus ApoE−/− vehicle, **P < 0.02 versus ApoE−/− vehicle, #P < 0.001 versus ApoE−/− treated with H2O2).
Cross-Breeding Studies
A total of 36 mice were studied: 12 ApoE−/−, 12 12/15LO−/−, and 12 double-knockout 12/15LO−/−/ApoE−/−. All animals looked healthy and did not show any noticeable difference in eating or drinking habit, and they all gained weight regularly. As previously reported, 12/15LO−/− mice did not differ with the wild-type mice in terms of plasma lipid profile or isoprostane levels (not shown).9,12 Conversely, as shown in Table 1, total plasma cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were higher in ApoE−/− than in 12/15LO−/− mice, but no difference was observed between double-knockout and ApoE−/− mice. No difference in total brain weight and total phospholipids or arachidonic acid content was observed among the three groups (Table 1). Compared with 12/15LO−/−, ApoE−/− mice had higher levels of circulating plasma iPF2α-VI (120 ± 12 pg/ml versus 458 ± 25 pg/ml, P > 0.01), which were significantly reduced by the genetic absence of 12/15LO (220 ± 18 pg/ml) (Table 1).
Table 1.
Age, Gender, Brain Weight, Total Phospholipids and Arachidonic Acid Content, Plasma Lipids, and Isoprostane iPF2α-VI Levels of the Different Groups of Animals Investigated
| 12/15LO−/− | ApoE−/− | 12/15LO−/−/ApoE−/− | |
|---|---|---|---|
| Age (months) | 12 | 12 | 12 |
| Gender (M/F) | 6/6 | 6/6 | 6/6 |
| Brain weight (g) | 0.47 ± 0.05 | 0.51 ± 0.06 | 0.50 ± 0.05 |
| Phospholipids (nmol/g) | 51.2 ± 5.1 | 50 ± 4.8 | 48.4 ± 5.2 |
| Arachidonic acid (μmol/g) | 9.4 ± 1.5 | 9.1 ± 1.4 | 8.9 ± 1.8 |
| Total cholesterol (mg/dl) | 125 ± 18 | 950 ± 45 | 1025 ± 50 |
| Triglycerides (mg/dl) | 80 ± 15 | 120 ± 22 | 115 ± 20 |
| HDL-cholesterol (mg/dl) | 105 ± 15 | 55 ± 8 | 58 ± 6 |
| iPF2α-VI (pg/ml) | 120 ± 12 | 458 ± 25 | 220 ± 18 |
Results are mean ± SEM.
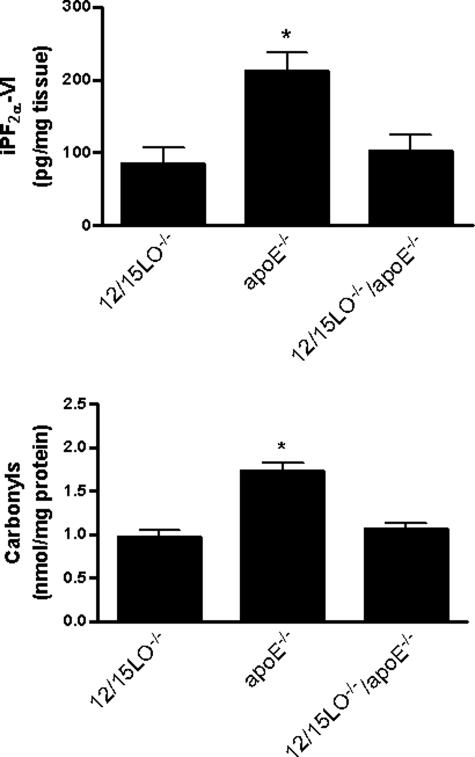
Cortex homogenates from ApoE−/− mice had higher levels of total iPF2α-VI than the ones from 12/15LO−/− mice. In contrast, the double-knockout mice 12/15LO/ApoE−/−, had levels similar to those observed in the 12/15LO−/− mice (Figure 4). Levels of this isoprostane were also elevated in cerebella of ApoE−/− mice, when compared with 12/15LO−/− (185 ± 30 ng/mg versus 103 ± 20 ng/mg tissue, P < 0.01) and 12/15LO−/−/ApoE−/− mice (118 ± 25 ng/mg tissue, P < 0.01).
Figure 4.
Absence of 12/15LO reduces brain oxidative stress. Total iPF2α-VI (top) and carbonyls (bottom) in cortex homogenates from 12/15LO−/−, ApoE−/−, and 12/15LO−/−/ApoE−/− mice at 12 months of age (n = 12, *P < 0.01).
Next, we assessed an independent and unrelated marker of oxidative damage to proteins, total protein carbonyls content. Compared with 12/15LO−/−, ApoE−/− mice had much higher levels of carbonyls in their cortex (1.72 ± 0.09 nmol/mg protein versus 0.97 ± 0.07 nmol/mg protein, P < 0.001) (Figure 4) and cerebellum (1.56 ± 0.07 nmol/mg protein versus 1.02 ± 0.05 nmol/mg protein, P < 0.01) homogenates. However, the mice deficient for ApoE that simultaneously did not express 12/15LO (12/15LO−/−/ApoE−/−), showed significantly reduced levels of this marker in both regions (1.06 ± 0.07 nmol/mg protein and 1.10 ± 0.06 nmol/mg protein, respectively, P < 0.01 for both) (Figure 4).
Finally, to address the hypothesis that the absence of 12/15LO could result in a significant diversion of the arachidonic acid metabolism to the 5LO or cyclooxygenase pathway products, we measured brain levels of the leukotriene (LT) B4 and prostaglandin (PG) E2. Cortices from all three groups of animals did not significantly differ in the levels of both metabolic products (Table 2), suggesting that in the CNS of the double-transgenic mice there is not an enhanced utilization of these pathways when the 12/15LO is disrupted.
Table 2.
Total Prostaglandin PGE2 and Leukotriene LTB4 Levels in Cortex Homogenates of 12-Month-Old Mice (n = 10 for Each Group)
| 12/15LO−/− | ApoE−/− | 12/15LO−/−/ApoE−/− | |
|---|---|---|---|
| PGE2(pg/mg) | 283 ± 57 | 313 ± 45 | 347 ± 67 |
| LTB4(pg/mg) | 29.6 ± 7 | 32.1 ± 5 | 30.3 ± 6 |
Results are mean ± SEM.
Discussion
These studies have addressed the role of 12/15LO in the CNS of ApoE−/− mouse, a well-characterized animal model of spontaneous hypercholesterolemia and systemic oxidative stress.21 Despite the fact that 12/15LO is the principal LO isoform in the CNS of several mammalian species, its expression and regional distribution is not well characterized. First, we showed that this enzyme is widely distributed in the brains of ApoE−/− mice and wild-type littermates, and no significant difference in its regional distribution was observed between these two groups of mice. Second, we demonstrated that primary neuronal cells from ApoE−/− mice have increased levels of basal oxidative stress, and an increased vulnerability to oxidative stress challenges when compared with the double-knockout 12/15LO−/−/ApoE−/− mice. Finally, we provided evidence that genetic disruption of 12/15LO in ApoE−/− mice rescued them from their augmented brain oxidative stress reactions. Our results provide the first evidence showing that the 12/15LO metabolic pathway is involved in the modulation of CNS oxidative stress responses. They support the hypothesis that 12/15LO enzymatic activity is linked to mechanisms of oxidative stress not only in the periphery but also in the brain.
LOs are enzymes that can insert molecular oxygen into molecules of free and esterified polyunsaturated fatty acids and thereby synthesize several different biologically active eicosanoids.1–3 One of the most abundant LO isoform in the CNS is 12/15LO, which has been described mainly in neurons and some glial cells throughout the cerebrum, hippocampus, and basal ganglia. Thus, previous work also showed that 12-HETE and 15-HETE are the principal LO products of the CNS.22,23 Originally, these metabolites had been proposed to play roles as second messengers in synaptic transmission and they were thought to be involved in learning and memory processes.24 Furthermore, 12/15LO mediates γ-aminobutyric acid and glutamate release, two neurotransmitters important for brain function in general and specifically in the regulation of signals mediated by drug abuse.25 However, despite some circumstantial evidence suggesting that this enzyme may also play a role in neurodegeneration and in the apoptotic responses of cortical neurons, a definite biological role for 12/15LO in the CNS has yet to be established.26,27
Previous studies have shown that when LDL is incubated with 12/15LO-transfected fibroblasts they become seeded with hydroperoxides and develop biological properties of oxLDL, including the presence of oxidation-specific epitopes recognized by antibodies to oxLDL.28 The most convincing data to date on a role for 12/15LO in modulating oxidative stress reactions come from our recent studies in which disruption of this gene greatly diminished atherosclerosis and systemic oxidative stress in ApoE−/− mice.9,10 Those studies, however, did not look at the effects of 12/15LO deletion in the CNS of these animals. This is a very important issue because it is known that deficiency for ApoE results in a significant increase not only in peripheral oxidative stress, but also in clear signs of lipid and protein oxidation in the brains of these animals.11,12
Confirming previous reports, we also did not observe any effect on general health nor mortality in mice lacking 12/15LO with and without ApoE.9,15 This double-knockout model is the first to provide specific evidence for a crucial role of a specific LO pathway in the oxidative stress responses of the CNS. Interestingly, we found that the absence of this enzyme activity modulated oxidative stress without any effect on circulating as well as CNS lipid levels. This finding suggests a direct role of 12/15LO in modulating oxidant stress, and supports the hypothesis that this enzyme can initiate chemical oxidative reactions, which by modifying substrates (fatty acids) may render them more susceptible to further oxidation. These biochemical events (propagation reactions) would start a chain reaction, which in turn could lead to the increased generation of specific products of oxidative stress, ie, isoprostanes.29 However, at the present the exact mechanism(s) linking enzymatic pathways with nonenzymatic oxidative reactions are far from being clear.
A possible mechanism that needs to be taken into consideration in studies involving the 12/15LO gene-disrupted mice is the potential compensatory changes in other pathways, ie, 5LO, which subsequently might influence oxidative stress responses. Thus, previous studies found evidence for enhanced 5LO product formation, which was secondary to a diversion of substrate arachidonic acid to this pathway.13 We can rule out this possibility because we found no biochemical sign for an increased metabolic utilization of arachidonic acid via another important LO isoform, the 5LO, or the cyclooxygenase pathways in the CNS of these animals.
In conclusion, we have provided evidence that 12/15LO enzyme plays an important role in modulating oxidative stress responses in the CNS. Its deficiency significantly reduces basal oxidative stress and protects cells from oxidative challenges. Furthermore, its genetic disruption suppresses the formation of lipid- and protein-free radical-dependent oxidation products, ie, iPF2α-VI and carbonyls, in ApoE−/− mice, but does not substantially alter CNS lipid levels. Future studies will determine whether specific inhibitors of this enzyme could prove beneficial in clinical settings in which brain oxidative stress is considered an important player for their pathogenesis.
Footnotes
Address reprint requests to Domenico Praticò, M.D., University of Pennsylvania, 3620 Hamilton Walk, John Morgan Building, Room 124, Philadelphia, PA 19104. E-mail: domenico@spirit.gcrc.upenn.edu.
Supported by the National Institutes of Health (grants AG-22203, AG-22512, HL53558).
C.D.F. holds a Canada Research Chair in Molecular, Cellular, and Physiological Medicine.
References
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrates. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- Khun H, Thiele B. The diversity of the lipoxygenase family. Many sequences data but little information on biological significance. FEBS Lett. 1999;449:7–11. doi: 10.1016/s0014-5793(99)00396-8. [DOI] [PubMed] [Google Scholar]
- Conrad DJ. The arachidonate 12/15lipoxygenase: a review of tissue expression and biological function. Clin Rev Allergy Immunol. 1999;17:71–89. doi: 10.1007/BF02737598. [DOI] [PubMed] [Google Scholar]
- Feinmark SJ, Begum R, Tsvetkov E, Goussakov I, Funk CD, Siegelbaum SA, Bolshakov VY. 12-lipoxygenase metabolites of arachidonic acid mediate metabotropic glutamate receptor-dependent long-term depression at hippocampal CA3-CA1 synapses. J Neurosci. 2003;23:11427–11435. doi: 10.1523/JNEUROSCI.23-36-11427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praticò D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM-Y. 12/15Lipoxygenase is increased in Alzheimer’s disease. Possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H, Belkner J, Suzuki H, Yamamoto S. Oxidative modification of human lipoprotein by lipoxygenases of different positional specificities. J Lipid Res. 1994;35:1749–1759. [PubMed] [Google Scholar]
- Rankin SM, Parthasarathy S, Steinberg D. Evidence for a dominant role of lipoxygenase(s) in the oxidation of LDL by mouse peritoneal macrophages. J Lipid Res. 1991;32:449–456. [PubMed] [Google Scholar]
- Benz DJ, Mol M, Ezaki M, Mori-Ito N, Zelan I, Miyanohara A, Friedmann T, Parthasarathy S, Steinberg D, Witztum JL. Enhanced levels of lipoperoxides in low density lipoprotein incubated with murine fibroblast expressing high levels of human 15-lipoxygenase. J Biol Chem. 1995;270:5191–5197. doi: 10.1074/jbc.270.10.5191. [DOI] [PubMed] [Google Scholar]
- Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of 12/15-lipoxygenase gene diminishes atherosclerosis in Apo E-deficient mice. J Clin Invest. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrus T, Praticò D, Zhao L, Witztum JL, Rader DJ, Rokach J, FitzGerald GA, Funk CD. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein E-deficient mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- Matthew RT, Beal MF. Increased 3-nitrotyrosine in brains of ApoE-deficient mice. Brain Res. 1996;718:181–184. doi: 10.1016/0006-8993(95)01576-0. [DOI] [PubMed] [Google Scholar]
- Praticò D, Rokach J, Tangirala RK. Brains of aged apolipoprotein E-deficient mice have increased levels of F2-isoprostanes, in vivo markers of lipid peroxidation. J Neurochem. 1999;73:736–741. doi: 10.1046/j.1471-4159.1999.0730736.x. [DOI] [PubMed] [Google Scholar]
- Praticò D, Sung S. Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J Alzheimer Dis. 2004;6:171–175. doi: 10.3233/jad-2004-6209. [DOI] [PubMed] [Google Scholar]
- Huang W, Qiu C, von Strauss E, Winblad B, Fratiglioni L. APOE genotype, family history of dementia, and Alzheimer disease risk: a 6-year follow-up study. Arch Neurol. 2004;61:1930–1934. doi: 10.1001/archneur.61.12.1930. [DOI] [PubMed] [Google Scholar]
- Sun D, Funk C. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low-density lipoprotein. J Biol Chem. 1996;271:24055–24062. [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice. Created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Ding Q, Dimayuga E, Markesbery WR, Keller JN. Proteasome inhibition increases DNA and RNA oxidation in astrocytes and neuron cultures. J Neurochem. 2004;91:1211–1218. doi: 10.1111/j.1471-4159.2004.02802.x. [DOI] [PubMed] [Google Scholar]
- Praticò D, Uryu K, Leight S, Trojanowski JQ, Lee VM-Y. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praticò D, Iuliano L, Mauriello A, Spagnoli L, Lawson JA, Maclouf J, Violi F, FitzGerald GA. Localization of distinct F2-isoprostanes in human atherosclerotic plaques. J Clin Invest. 1997;100:2028–2034. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T, Yao Y, Praticò D. Increase in peripheral oxidative stress during hypercholesterolemia is not reflected in the central nervous system; evidence from two mouse models. Neurochem Int. 2005;46:435–439. doi: 10.1016/j.neuint.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Praticò D, Tangirala RK, Horkko S, Witztum JL, Palinski W, Fitzgerald GA. Circulating autoantibodies to oxidized cardiolipin correlate with isoprostane F2α-VI levels and the extent of atherosclerosis in ApoE-deficient mice: modulation by vitamin E. Blood. 2001;97:459–464. doi: 10.1182/blood.v97.2.459. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Medina JF, Haeggstrom JZ, Radmark O, Samuelsson B. Molecular cloning of 12-lipoxygenase cDNA from rat brain. Eur J Biochem. 1993;212:605–612. doi: 10.1111/j.1432-1033.1993.tb17699.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Watanabe T, Ueda N, Tsukamoto H, Watanabe K. Arachidonate 12-lipoxygenase is localized in neurons, glia cells and endothelial cells of canine brain. J Histochem Cytochem. 1993;41:111–117. doi: 10.1177/41.1.8417106. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Volterra A, Dale N, Siegelbaum SA, Kandell ER, Schwartz JH, Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for pre-synaptic inhibition of Aplysia sensory cells. Nature. 1987;328:38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Walters CL, Wang BC, Godfrey M, Sun D, Funk CD, Blendy JA. Augmented responses to morphine and cocaine in mice with a 12-lipoxygenase gene disruption. Psychopharmacology. 2003;170:124–131. doi: 10.1007/s00213-003-1526-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- Lebeau A, Terro F, Rostene W, Pelaprat D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by β-amyloid peptide. Cell Death Diff. 2004;11:875–884. doi: 10.1038/sj.cdd.4401395. [DOI] [PubMed] [Google Scholar]
- Heinecke JW. Mechanisms of oxidative damage of low-density lipoprotein in human atherosclerosis. Curr Opin Lipidol. 1997;8:268–274. doi: 10.1097/00041433-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Funk CD, Cyrus T. 12/15-Lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc Med. 2001;11:116–124. doi: 10.1016/s1050-1738(01)00096-2. [DOI] [PubMed] [Google Scholar]