Abstract
Pulmonary fibrosis is a progressive illness characterized by interstitial fibrosis. Although the precise mechanism for pulmonary fibrosis is not completely understood, an immune response involving interferon (IFN)-γ appears to play a role. Therefore, we examined the functional roles of natural killer T (NKT) cells, which produce IFN-γ and interleukin-4 on activation, in bleomycin-induced pulmonary fibrosis. In NKT cell-deficient mice, pulmonary fibrosis was worse in terms of histology, hydroxyproline levels, and mortality than in control mice. The transforming growth factor (TGF)-β1 levels were higher in the lung after injecting bleomycin, and blockade of TGF-β1 by neutralizing monoclonal antibody attenuated the pulmonary fibrosis in CD1d−/− mice. In contrast, the production of IFN-γ was reduced in lungs from CD1d−/− mice. Moreover, the adoptive transfer of NKT cells into CD1d−/− mice increased IFN-γ and reduced TGF-β1 production, attenuating pulmonary fibrosis. An in vitro assay demonstrated that IFN-γ was involved in suppressing TGF-β1 production in cells collected from bronchoalveolar lavage. The adoptive transfer of NKT cells from IFN-γ−/− mice did not reverse pulmonary fibrosis or TGF-β1 production in lungs of CD1d−/− mice whereas NKT cells from B6 control mice attenuated fibrosis and reduced TGF-β1 production. In conclusion, IFN-γ-producing NKT cells play a novel anti-fibrotic role in pulmonary fibrosis by regulating TGF-β1 production.
Pulmonary fibrosis is a consequence of diverse insults to the alveolar epithelial cells of the lung.1 This fibrotic process is initiated by inflammation followed by the massive production of fibrous connective tissue in the alveolar septa, which result in an excessive number of fibroblasts, an increase in the lung collagen content, an abnormal spatial distribution of the extracellular matrix proteins, and an ultimately deteriorated pulmonary function.2–5 During the development of bleomycin-induced pulmonary fibrosis, various immune cells infiltrate the lung parenchyma, and these cells are involved in the pathogenesis of pulmonary fibrosis.6–8 The cytokine milieu in the microenvironment of the lung is critical in sustaining the activation of the pulmonary fibroblast.9 It has been proposed that an immune response dominated by interferon (IFN)-γ and other Th1-type cytokines such as interleukin (IL)-12 and IL-18 prevents overt fibroblast activation and pulmonary fibrosis.10–12 In contrast, an immune response dominated by Th2-type cytokines such as IL-4 and IL-13 influence fibroblast activity.13–17 Therefore, the cytokine milieu in the microenvironment of the lung may be important for inducing pulmonary fibrosis.
Natural killer T (NKT) cells express intermediate levels of a semi-invariant Vα14-Jα18 TCR in mice or an invariant Vα24-Jα15 TCR in humans,18 which recognize the glycolipid antigens presented by nonpolymorphic MHC class I-like protein CD1d.19 α-Galactosyl ceramide (α-GalCer) binds to CD1d in humans and mice, and these complexes are recognized by TCR of NKT cells.20,21 On activation, NKT cells rapidly produce a large amount of IL-4 and IFN-γ.22 These cytokines have been shown to play an important role in regulating the innate and adoptive immune responses by NKT cells.23,24 A recent report demonstrated that an injection of α-GalCer into mice attenuated the bleomycin-induced pulmonary fibrosis and its protective effects were associated with an increase in the IFN-γ level and a decrease in the transforming growth factor (TGF)-β level in the lung, suggesting that α-GalCer might be a useful therapeutic reagent to reduce fibrosis.25 Therefore, it was proposed that NKT cells play critical roles in the bleomycin-induced pulmonary fibrosis. However, the mechanism by which NKT cells play this critical role in the development of bleomycin-induced pulmonary fibrosis is unclear.
This study examined the functional roles of NKT cells in bleomycin-induced pulmonary fibrosis. The results show that NKT cells play a novel anti-fibrotic role by producing IFN-γ at approximately day 7 rather than an earlier time point in bleomycin-induced pulmonary fibrosis, which contributes to the suppression of TGF-β production in the lung.
Materials and Methods
Mice and Bleomycin-Induced Pulmonary Fibrosis Model
C57BL/6 mice were purchased from DaeHan Biolink (Seoul, Korea). CD1d−/− mice (C57BL/6 background) were a generous gift from Dr. Hua Gu (Columbia University, New York, NY). Jα18−/− (C57BL/6 background) and RAG−/− Vα14tg Vβ8.2tg mice were a generous gift from Dr. M. Taniguchi (Chiba University, Chiba, Japan). IFN-γ−/− and IL-4−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME).
Male mice, 8 to 10 weeks of age, were used in all of the experiments. These mice were bred and maintained under specific pathogen-free conditions at the Clinical Research Institute, Seoul National University Hospital. All animal experiments were performed after receiving approval of the Institutional Animal Care and Use Committee of Clinical Research Institute, Seoul National University Hospital. To induce pulmonary fibrosis, the mice were anesthetized with 2,2,2-tribromomethanol (Sigma-Aldrich, St. Louis, MO) and injected with 2 mg/kg of bleomycin (Nippon Kayaku, Tokyo, Japan) in phos-phate-buffered saline (PBS) (50 μl) via intratracheal instillation in all experiments except for those experiments evaluating the survival rates of the mice. The mice were injected with 3 mg/kg of bleomycin to evaluate clinical outcomes.
Hydroxyproline Assay
The total hydroxyproline level of the lung was measured on day 21 after the bleomycin injection. The whole lung was excised, homogenized in PBS (2 ml), and dried for 6 hours by vacuum dryer. The samples was added to 1 ml of 6 N HCl for 12 hours at 110°C and then filtered. Aliquots (50 μl) of the samples were then examined by adding 50 μl of citrate acetate buffer and 1 ml of chloramines T solution followed by a reaction with Erlich’s solution at 65°C for 15 minutes. The absorbance was measured at 550 nm using a spectrophotometer (DU650; Beckman). The increased hydroxyproline contents in the lungs of experimental groups were compared with the amount of hydroxyproline of the lung from untreated B6 mice. The value of the B6 control group was set to 1 and the relative increased values of hydroxyproline in each group were calculated. The results were statistically analyzed using analysis of variance and presented as percentage.
Bronchoalveolar Lavage (BAL) and Fluorescence-Activated Cell Sorting Analysis
The mice were euthanized via CO2 asphyxiation. The trachea was cannulated, and the lung was lavaged five times with 0.7 ml of cold PBS. The BAL fluid was centrifuged at 1500 rpm for 10 minutes at 4°C, and the supernatant was removed. The total BAL cells were counted using a hemocytometer, and incubated for 15 minutes on ice in FcγRII/III blockade. After washing, the cells were stained in a 200-μl total volume with a 1-μg combination of the following monoclonal antibodies (mAbs): anti-CD8, CD4, CD25, CD69, NK1.1, F4/80, TCR-β, which were purchased from BD Pharmingen (San Diego, CA). The cells were collected using cytospin (10 minutes at 55 × g) and stained with hematoxylin and eosin (H&E) to evaluate the number of polymorphonuclear leukocytes in the BAL. For identification of CD1d-restricted NKT cells, the total BAL cells were preincubated with mAb (24G2, BD Pharmingen) against the Fcγ receptors, washed, and incubated with anti-TCR-β mAb (BD Pharmingen) and α-GalCer-loaded dimer (dimer X I: recombinant soluble mouse CD1d:Ig fusion protein; BD Pharmingen) conjugated with phycoerythrin-streptavidin for 1 hour at 37°C. The cells were washed and analyzed on a FACSCalibur (Becton Dickinson, San Diego, CA).
Adoptive Transfer Experiment
After sacrifice, the livers were homogenized and resuspended in a loading buffer (PBS plus 10% fetal bovine serum and 1 mmol/L ethylenediamine tetraacetic acid) and overlaid onto lympholyte-M (Cedarlane, Ontario, Canada). After centrifugation for 20 minutes at 900 × g at 25°C, the liver mononuclear cells were isolated from the interface and washed with PBS. The cells obtained were stained with phycoerythrin-conjugated anti-NK1.1 (BD Pharmingen) and Cy-conjugated anti-TCR-β (BD Pharmingen). The stained cells were then sorted by FACStar Plus using CellQuest software, and the purity of the sorted cells was >95%. The splenocyte suspensions of RAG−/− Vα14tg Vβ8.2tg mice and Jα18−/− mice were prepared and depleted of red blood cells using a RBC lysis buffer (Sigma-Aldrich, St. Louis, MO) and washed with PBS. NKT cells (3 × 105 per mouse) or splenocytes (1 × 107 per mouse) were adoptively transferred via an intravenous injection.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) and Real-Time PCR Analysis
For quantitative real-time PCR, the total RNA was isolated form the whole lung homogenates using an RNeasy kit (Qiagen, Courtaboeuf, France) according to the manufacturer’s instructions. The contaminated genomic DNA was digested with DNase I (Qiagen). RNA was reverse-transcribed with MMLV-RT Taq polymerase (Koschem, Seoul, Korea) and PCR was then performed. Briefly, 1 μg of cDNA was amplified in the presence of a TaqMan universal master mix (Perkin-Elmer Biosystems), gene-specific TaqMan probe, the forward and reverse primers, and water. The GAPDH-specific TaqMan probe and forward and reverse primers were used as the endogenous control. The gene-specific PCR products were measured using an Applied Biosystems 7500 sequence detection system (Perkin-Elmer Biosystems). The results for each cytokine were normalized with respect to the GAPDH expression level. The reactions in each group were performed in triplicate. For real-time PCR, the following primers were synthesized by Applied Biosystems (Foster City, CA): GAPDH: TGCACCACCAACTGCTTA (forward), GGA-TGCAGGGATGATGTT (reverse), and CAGAAGACTGTGGATGGCCCCTC-VIC; IFN-γ: AGCAACAGCAAGGCGA-AAA (forward), CTGGACCTGTGGGTTGTTGA (reverse), and CTCAAACTTGGCAATACTCATGAATGCATC-TAM-RA; and TGF-β1: GCAACATGTGGAACTCTACCAGAA (forward), GACGTCAAAAGACAGCCACTCA (reverse), and ACCTTGGTAACCGGCTGCTGACCC-TAM-RA; IL-4: FAM-CTC CGT GCA TGG GGT CCC TTC-Black Hole Quencher (Biosource, Camarillo, CA).
For RT-PCR, the following primers were synthesized by Genotech Corp. (Daejeon, Korea) β-actin: CC-CAACTTGATGTATGAAGG (forward) and TTTGTGTAAGGTAAGGTGTGC (reverse); IFN-γ: AGCGGCTGACTGAACTCAGATTGT (forward) and GTCACAG-TTTTCAGCTGTATAGGG (reverse); TGF-β1: TCGACAT-GGAGCTGGTGAAA (forward) and GAGCCTTAGTTT-GGACAGGATCTG (reverse); tumor necrosis factor-α: GGCAGGTCTACTTTGGAGTCATTG (forward) and AC-ATTCGACCCAGTG (reverse); Fas: TTGCTGTCAACCATGCCAAC (forward) and ACGTGAACCATAAGACCCAG (reverse); FasL: ATCCCTCTGGAATGGGAAGA (forward) and CCATATCTGTCCAGTAGTGC (reverse).
Enzyme-Linked Immunosorbent Assay (ELISA)
Bronchoalveolar lavage fluid cells were taken from the lungs of C57BL/6 mice administrated bleomycin 7 days after the bleomycin injection and cultured with ConA (Sigma-Aldrich) and α-GalCer or α-GalCer only. α-GalCer was synthesized using the method developed by Kim and colleagues.26 Anti-IL-4 (R&D, Minneapolis, MN), anti-IL-10 (R&D), anti-IL-13 (R&D), and anti-IFN-γ (clone R4-6A2; American Type Culture Collection, Manassas, VA) mAbs were used to neutralize the respective cytokines at a concentration of 50 μg/ml. CD1d was blocked using 5 μg of blocking mAbs (BD Pharmingen) during activation of BAL fluid cells. TGF-β1 or IFN-γ (BD Pharmingen) was coated on the high-affinity 96-well ELISA plates (Costar, Cambridge, MA) at 10 μg/ml in PBS. The plates were then blocked with PBS/1% BSA. TGF-β1 or IFN-γ was detected using biotinylated rat anti-TGF-β1 or IFN-γ (BD Pharmingen) and streptavidin-peroxidase (BD Pharmingen) followed by adding TMB substrate (BD Pharmingen). The reaction was quenched with 3 N hydroxy acid and the absorbance was read at 450 nm and 570 nm. The data were analyzed using SOFTmaxPro version 4.3LS (Molecular Devices, Berkeley, CA).
Cytokine Neutralization in Vivo Using Blocking mAb
The hybridomas for anti-TGF-β mAb (clone 1D11.16.8) and anti-IFN-γ mAb (clone R4-6A2) were purchased from the American Tissue Cell Culture. The mAbs were purified using a protein G column (Amersham Bioscience, Sweden). Five hundred μg of each mAb was injected via intravenously 1 day before, and 4, 8, 12, and 16 days after the administration of bleomycin.
Histology and Pathological Scoring
The mice were euthanized and perfused with saline via the right ventricle. The lungs were inflated with 1 ml of 10% neutral buffered formalin and fixed for 24 hours. Routine histological techniques were used to paraffin-embed the entire lung. The sections were cut, mounted on slides, and stained with H&E. A morphological evaluation of the bleomycin-induced lung fibrosis was performed using a scoring method as previously described.27 The pathological scores were defined as follows: 0, no lung abnormality; 1, the presence of inflammation and fibrosis involving <25% of the lung; 2, lesions involving 25 to 50% of the lung; and 3, lesions involving >50% of the lung. The mean of the pathological scores for five mice was determined for the individual groups.
Statistics
Statistical significance was analyzed using the Prism 3.0 program. Student’s t-tests were run to determine the P value when comparing two groups. The survival data were analyzed using the log-rank test. P values <0.05 were considered significant. In terms of increased contents of hydroxyproline in the lungs, the one-way analysis of variance statistical analysis was performed. The results are expressed as a mean ± SEM.
Results
NKT Cell-Deficient Mice Aggravate Bleomycin-Induced Pulmonary Fibrosis
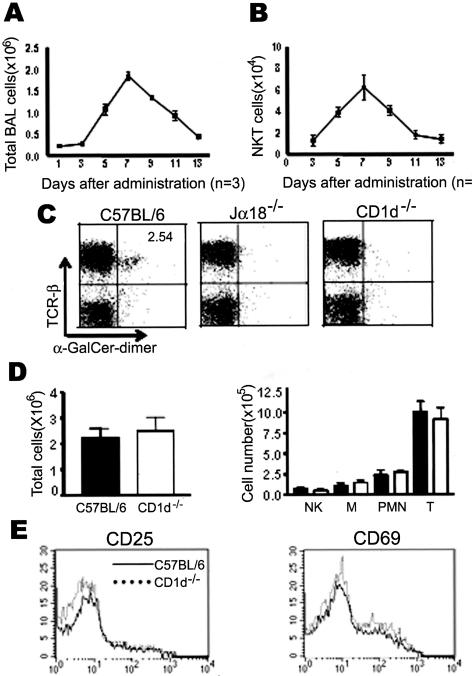
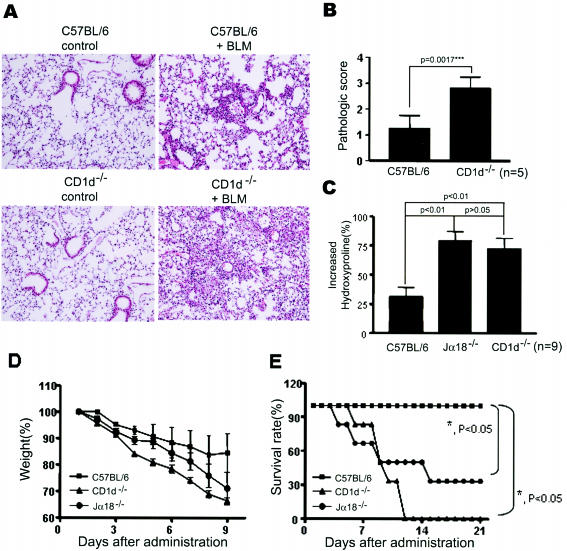
To investigate the functional roles of NKT cells during the development of bleomycin-induced pulmonary fibrosis, we examined the kinetics of infiltrated total immune cells and NKT cells in the BAL fluid. In wild-type B6 mice, the number of immune cells in the BAL fluids increased 4 days after the intratracheal administration of the bleomycin and reached a peak level on day 7 (Figure 1A). NKT cells, recognized by the α-GalCer/CD1d dimer, were detected in BAL fluid whereas NKT cells were rarely found in the two NKT cell-deficient mouse strains CD1d−/− and Jα18−/− mice (Figure 1C). CD1d−/− mice lack CD1d-dependent NKT cells, and Jα18−/− mice lack NKT cells (iNKT) expressing invariant Vα14Jα18 TCR in vivo.28–30 The kinetics of NKT cell infiltration in the lung was similar to that of the total inflammatory cells (Figure 1B). The total cell numbers were counted and subset analysis for the NK cells (NK), T cells (T), macrophages (M), and polymorphonuclear leukocytes (PMN) was performed in the BAL fluids from the wild-type B6 and CD1d−/− mice 7 days after the bleomycin injection. The total number of cells and the number of each subset of immune cells in the BAL fluid were similar in B6 and CD1d−/− mice of bleomycin-induced pulmonary fibrosis model (Figure 1D). A recent study suggested that NKT cells might contribute to activating NK1.1−TCR-β+ conventional T cells in lung during bleomycin-induced pulmonary fibrosis.25 Therefore, the activation status of conventional T cells in the BAL was analyzed by evaluating the expression level of activation markers such as CD25 and CD69. However, the expression levels of CD25 and CD69 on the conventional T cells of the BAL from B6 mice were similar to those from CD1d−/− mice in bleomycin-induced pulmonary fibrosis (Figure 1E). To determine whether the depletion of NKT cells in vivo contributes to the development of pulmonary fibrosis, pulmonary fibrosis was induced in CD1d−/− and Jα18−/− mice by an intratracheal injection of bleomycin. Bleomycin induced multifocal fibrotic pulmonary lesions with thickened alveolar septa, collapses of alveolar spaces, and massive infiltrations of lymphocytes in the interstitium of wild-type mice as estimated 21 days after the injection of bleomycin (Figure 2A). The histological scorings of the fibrotic lesions revealed significant aggravation of bleomycin-induced pulmonary fibrosis in CD1d−/− mice compared with wild-type B6 mice (Figure 2B). Moreover, the content of hydroxyproline, a collagen component, in the lung was significantly higher in CD1d−/− and Jα18−/− mice than in wild-type mice, as estimated 21 days after administrating bleomycin (Figure 2C). These findings indicate that the bleomycin-induced pulmonary fibrosis is significantly increased in NKT cell-deficient mice as compared with wild-type B6 mice.
Figure 1.
NKT cells infiltrate the lung during bleomycin-induced pulmonary fibrosis. A and B: The BAL fluids were taken from B6 mice intratracheally injected with bleomycin and analyzed for the total cell and NKT cell (NK1.1+TCRβ+) numbers on days 1, 3, 5, 7, 9, 11, and 13. The results are indicated as a mean ± SEM of three mice in each group. BAL cells were taken from B6, Jα18−/−, and CD1d−/− mice intratracheally administrated with bleomycin and a subset of NKT cells (C) was analyzed using anti-TCR-β and α-GalCer loaded CD1d dimer by flow cytometry. D: In addition, the total cell numbers and subset analysis of the BAL cells were performed in B6 (black bar) and CD1d−/− (white bar) mice 7 days after bleomycin injection. M indicates macrophages. E: The BAL cells were taken from the lungs of B6 and CD1d−/− mice and stained using either anti-CD25 or anti-CD69 mAbs, anti-NK1.1, and anti-TCR-β mAbs. The CD25 and CD69 expression levels were plotted on the gated NK1.1−TCR-β+ T cells from the BAL cells of B6 (solid line) and CD1d−/− (dotted line) mice. The results from a representative of five repeated experiments are shown.
Figure 2.
NKT cell-deficient mice aggravate bleomycin-induced pulmonary fibrosis. A: The lungs were removed from B6 and CD1d−/− mice on day 21 after injecting bleomycin (right) or PBS (left), and paraffin sections were stained with H&E. The pictures represent 1 of 10 mice in each group. B: Fibrotic responses in the lungs of B6 and CD1d−/− mice were graded according to the criteria described in Materials and Methods. The results are indicated as a mean ± SEM of five mice in each group. C: The lungs were taken from B6, Jα18−/−, and CD1d−/− mice with or without an intratracheal injection of bleomycin (2 mg/kg) on day 21, and the hydroxyproline content was determined. The increased hydroxyproline contents in the lungs of experimental groups were presented as percentage. The results are indicated as a mean ± SEM of nine mice in each group. D and E: B6, Jα18−/−, and CD1d−/− mice were administrated with a high dose of bleomycin (3 mg/kg), and the body weight and survival rate were measured. The results are indicated as a mean ± SEM of six mice in each group. The results from a representative of three repeated experiments are shown. Original magnifications, ×200 (A).
The biological significance of bleomycin-induced pulmonary fibrosis was assessed by measuring the body weight and estimating the survival rate of CD1d−/−, Jα18−/−, and wild-type B6 mice. As shown in Figure 2D, the weight losses accelerated significantly in CD1d−/− and Jα18−/− mice compared with wild-type B6 mice. All CD1d−/− mice and 70% of Jα18−/− mice died within 2 weeks after administrating the high dose (3 mg/kg) of bleomycin whereas wild-type B6 mice were still alive for up to 3 weeks (Figure 2E). Overall, these findings suggest that NKT cells in mice play a suppressive role in the bleomycin-induced acute lung injury and fibrosis.
Adoptive Transfer of NKT Cells Attenuates Pulmonary Fibrosis in NKT Cell-Deficient Mice
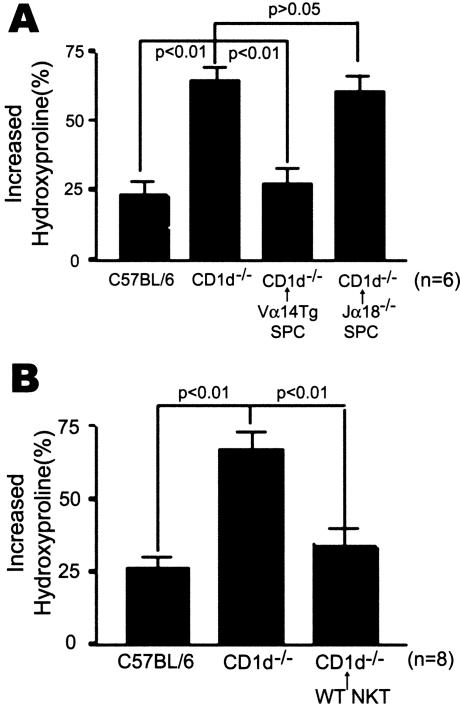
To demonstrate that the lack of NKT cells specifically caused an increase in pulmonary fibrosis in CD1d−/− mice, either splenocytes of RAG−/− Vα14tg Vβ8.2tg mice (Figure 3A) or NKT cells from normal B6 mice (Figure 3B) were adoptively transferred into CD1d−/− mice and the contents of hydroxyproline were measured in these mice. Splenocytes of Jα18−/− mice were adoptively transferred as the control. RAG−/− Vα14tg Vβ8.2tg mice contain NKT cells expressing Vα14Jα18 TCR in the absence of conventional T cells and B cells. Therefore, RAG−/− Vα14tg Vβ8.2tg mice contains a higher number of iNKT cells in the spleen than B6 mice. The adoptive transfer of NKT cells into CD1d−/− mice reduced the hydroxyproline contents in the lung of CD1d−/− mice administrated intratracheally with bleomycin (Figure 3, A and B). These findings indicate that adoptively transferred NKT cells in CD1d−/− mice attenuated the bleomycin-induced pulmonary fibrosis.
Figure 3.
Adoptive transfer of NKT cells attenuates the bleomycin-induced pulmonary fibrosis in CD1d−/− mice. Splenocytes (1 × 107 cells/mice) from RAG−/− Vα14tg Vβ8.2tg (Vα14 Tg SPC) or Jα18−/− mice (Jα18−/− SPC) as a control (A) or sorted NKT cells (WT NKT, 3 × 105 cells/mice) from the normal C57BL/6 mice (B) were adoptively transferred by intravenous injection into CD1d−/− mice 1 day before administering bleomycin. The lungs were taken from mice with or without an intratracheal injection of bleomycin (2 mg/kg) on day 21, and the hydroxyproline content was determined. The increased hydroxyproline contents of the lungs in each group were presented as percentage. The data are as a mean ± SEM of six mice in A and eight mice in B in each group. The results from a representative of three repeated experiments are shown.
NKT Cells Attenuate Pulmonary Fibrosis by Suppression of the Production of TGF-β1
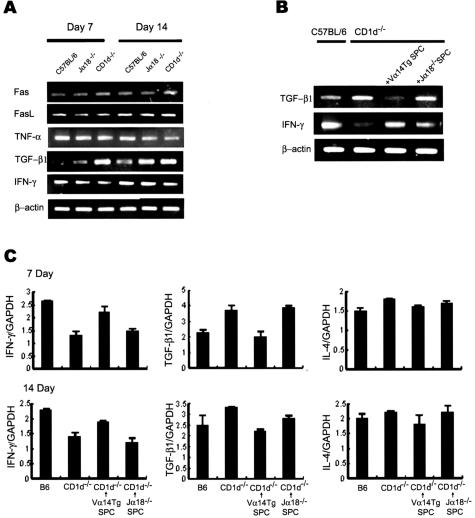
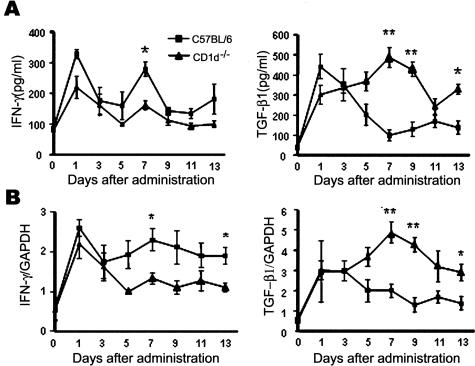
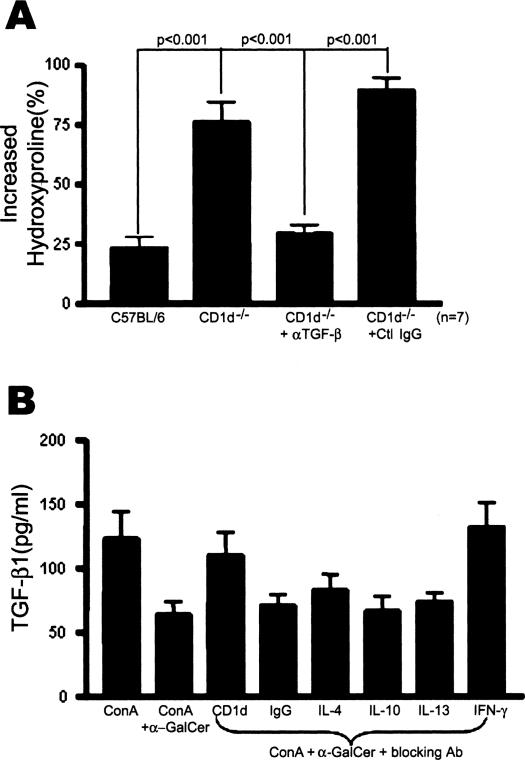
To investigate the mechanism by which an absence of NKT cells aggravated bleomycin-induced pulmonary fibrosis in CD1d−/− mice, the expression pattern of various cytokines in the lungs of B6, Jα18−/−, and CD1d−/− mice was examined using RT-PCR (Figure 4A). Among the cytokines tested, the significantly higher TGF-β1 transcription was detected in CD1d−/− mice 7 and 14 days after bleomycin injection whereas it was barely detectable in wild-type B6. In contrast, wild-type B6 mice produced a significant level of IFN-γ transcription in the bleomycin-treated lung whereas there was little IFN-γ transcription in the lung of CD1d−/− mice treated with bleomycin. Furthermore, real-time PCR showed that the induction of TGF-β1 mRNA increased significantly and the induction of IFN-γ mRNAs were reduced in the lung of CD1d−/− mice treated with bleomycin (Figure 4C). Kinetic analysis of IFN-γ and TGF-β1 production in the BAL fluid and lung tissues revealed that the amounts of IFN-γ were high on days 1 and 7 after the bleomycin injection into B6 mice whereas CD1d−/− mice maintained low concentrations of IFN-γ in the BAL fluid and lung tissues. In contrast, the amounts of TGF-β1 in the lung tissues and BAL fluids from CD1d−/− mice were higher than those of wild-type B6 mice in the bleomycin-induced pulmonary fibrosis model (Figure 5, A and B). The subsequent experiment was designed to examine whether or not the production of these cytokines in the lung is restored by the reconstitution of NKT cells in CD1d−/− mice treated with bleomycin. The TGF-β1 transcript in the lung was restored in CD1d−/− mice and was comparable to that of wild-type B6 by the adoptive transfer of NKT cells during bleomycin-induced pulmonary fibrosis (Figure 4, B and C). These findings suggest that the significant production of TGF-β1 in the absence of NKT cells might be involved in the aggravated pulmonary fibrosis in CD1d−/− mice. Although IL-13 and IL-4 are known to be involved in the induction of pulmonary fibrosis,14,15,31 the amount of these cytokines produced during pulmonary fibrosis was similar in wild-type B6 and CD1d−/− mice (data for IL-13 not shown). To establish a functional link between the TGF-β1 activity and pulmonary fibrosis, a blocking anti-TGF-β1 mAb was injected intravenously into CD1d−/− mice, as described in Materials and Methods. The TGF-β1 blockade in CD1d−/− mice induced a significant decrease in the bleomycin-induced pulmonary fibrosis when compared with the control antibody (Figure 6A). These results indicate that the TGF-β1 blockade in CD1d−/− mice results in a similar phenotype that is found in normal B6 mice with bleomycin-induced pulmonary fibrosis. These findings show that the regulatory effects of NKT cells on pulmonary fibrosis depended on the amount of TGF-β1 produced in the lung.
Figure 4.
TGF-β1 production is increased and IFN-γ production is reduced in the lung of CD1d−/− mice treated with bleomycin. A: The transcription levels of the various proinflammatory cytokines were measured by a RT-PCR assay in the lungs of B6, CD1d−/−, and Jα18−/− mice on days 7 and 14 after the bleomycin injection. B and C: Splenocytes (1 × 107 cells/mice) from RAG−/− Vα14tg Vβ8.2tg mice (Vα14Tg SPC) or Jα18−/− mice (Jα18−/− SPC) were adoptively transferred into CD1d−/− mice. B: The TGF-β1 and IFN-γ transcript levels were measured by RT-PCR. C: The transcript levels of TGF-β1, IFN-γ, and IL-4 were examined for quantitative analysis relative to the housekeeping transcript GAPDH by real-time PCR in the lungs. These results are taken from a representative one of three repeated experiments.
Figure 5.
Kinetic analysis of IFN-γ and TGF-β1 production in the BAL fluid and lung tissues during bleomycin-induced pulmonary fibrosis. The lungs and BAL fluids were taken from B6 and CD1d−/− mice on days 0, 1, 3, 5, 7, 9, 11, and 13 of intratracheal injection of bleomycin (2 mg/kg). A: The amounts of IFN-γ and TGF-β1 in the BAL fluid were measured using ELISA. B: The IFN-γ and TGF-β1 expression levels in the lung tissues were determined using real-time PCR. The results from a representative of three repeated experiments are shown (*P < 0.05, **P < 0.01, B6 versus CD1d−/− mice).
Figure 6.
Anti-fibrotic effects of NKT cells on pulmonary fibrosis mediated by TGF-β1. A: TGF-β1 blockade reduced pulmonary fibrosis in CD1d−/− mice. Five hundred μg of anti-TGF-β1 mAb or isotype-matched IgG was injected intravenously into CD1d−/− mice twice per week before and after administering bleomycin. The hydroxyproline contents were measured on day 21. The increased hydroxyproline contents of the lungs in each group are presented as percentage. The data are a mean ± SEM of seven mice in each group. B: The suppressive effects of NKT cells on TGF-β1 production in the lungs are dependent on IFN-γ. The BAL cells were taken from C57BL/6 mice treated with bleomycin and cultured with ConA and α-GalCer. IL-4, IL-10, IL-13, and IFN-γ were neutralized or CD1d was blocked using mAbs during BAL cell activation. The TGF-β1 produced by the BAL cells was determined using ELISA. These results are taken from a representative one of three repeated experiments.
The Production of TGF-β1 in the Bleomycin-Treated Lung Is Regulated by NKT Cells Producing IFN-γ
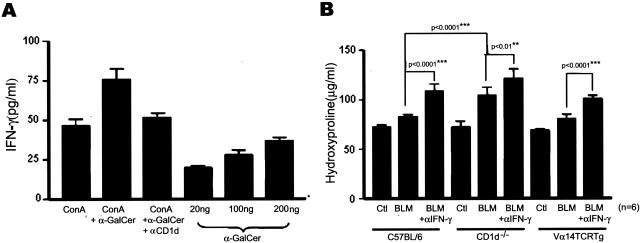
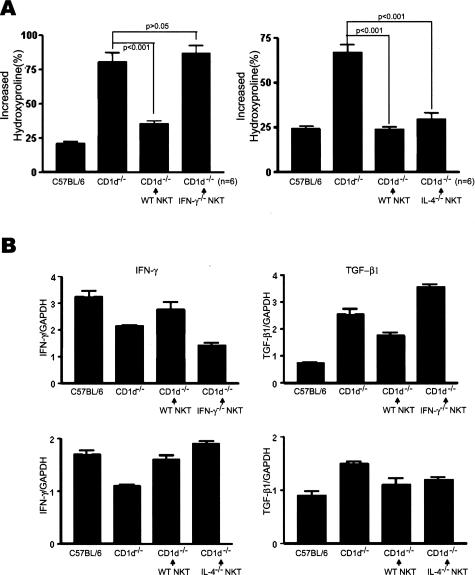
To determine how NKT cells regulated the production of TGF-β1 in the bleomycin-treated lung, the BAL cells were taken from wild-type B6 mice injected with bleomycin, and cultured with ConA. On activation of NKT cells using α-GalCer, the production of TGF-β1 was suppressed and restored by blocking the interaction between CD1d and TCR of NKT cells using anti-CD1d mAb (Figure 6B). This indicates that activation of NKT cells specifically suppressed the production of TGF-β1 production. It was reported that NKT cells rapidly secret a large amount of IL-4, IFN-γ, and IL-13, and enhance IL-10 production on activation by α-GalCer in vitro.22,32–35 Therefore, it was proposed that these cytokines produced by NKT cells play an important role in regulating the immune responses by NKT cells. This study next examined which cytokines were involved in the suppression on TGF-β1 production by NKT cells. IL-4, IL-10, IL-13, and IFN-γ were neutralized using the mAbs during the activation of BAL cells and NKT cells in in vitro culture. Among these blocking mAbs tested, mAbs against IFN-γ partially restored production of TGF-β1 whereas mAbs against IL-4, IL-10, and IL-13 as well as control IgG could not affect production of TGF-β1 in culture (Figure 6B). This suggests that NKT cells produce IFN-γ, which is involved in the suppression of TGF-β1 produced by BAL cells. To address this hypothesis, the amount of IFN-γ produced by BAL cells was measured after treating BAL cells with α-GalCer. α-GalCer induced IFN-γ production by the BAL cells in a dose-dependent manner, which was blocked by anti-CD1d mAbs (Figure 7A). The aim of the subsequent experiment was to determine whether the IFN-γ secreted by NKT cells play an anti-fibrotic role in the development of pulmonary fibrosis and the suppression of TGF-β1 production in vivo. The blockade of IFN-γ in RAG−/− Vα14tg Vβ8.2tg, B6, and CD1d−/− mice by the anti-IFN-γ mAb injection increased the amount of hydroxyproline in the lung during the development of pulmonary fibrosis (Figure 7B). These results suggest that either NKT cells or NK cells contribute to the production of IFN-γ during pulmonary fibrosis because RAG−/− Vα14tg Vβ8.2tg mice contain iNKT cells and NK cells in the absence of T and B cells. In addition, the blockade of IFN-γ in CD1d−/− mice partially increased pulmonary fibrosis, suggesting NK cells or T cells partially reduce pulmonary fibrosis by producing IFN-γ in the absence of CD1d-dependent NKT cells. To demonstrate whether or not NKT cells producing IFN-γ contribute to the regulation of pulmonary fibrosis, NKT cells from either IFN-γ−/− or IL-4−/− mice were transferred into CD1d−/− mice injected with bleomycin and the amount of hydroxyproline and the transcriptional level for TGF-β1 in the lung were measured. NKT cells from normal B6 mice were transferred as a control. The hydroxyproline content in CD1d−/− mice transferred with NKT cells from IFN-γ−/− mice was still high and comparable to that in CD1d−/− control mice, whereas CD1d−/− mice transferred with NKT cells from normal B6 mice produced a small amount of hydroxyproline as in the control B6 (Figure 8A). Furthermore, the TGF-β1 transcript level was higher in CD1d−/− mice adoptively transferred with NKT cells from IFN-g−/− mice (Figure 8B) than in CD1d−/− mice transferred with NKT cells from B6 mice. In contrast, CD1d−/− mice transferred with NKT cells from IL-4−/− mice restored the pulmonary fibrosis and TGF-β1 transcript level in the lung. As observed in the in vitro assay, IFN-γ secreted from NKT cells down-regulated TGF-β1 production during the development of pulmonary fibrosis. Altogether, these results demonstrated that IFN-γ produced by NKT cells played important roles in attenuating bleomycin-induced pulmonary fibrosis by suppressing TGF-β1 production in the lung.
Figure 7.
NKT cells infiltrating lung tissues produce IFN-γ during the bleomycin-induced pulmonary fibrosis. A: BAL cells were taken from B6 mice treated with bleomycin and cultured with ConA or ConA + α-GalCer or α-GalCer only. CD1d was blocked using mAbs during BAL cell activation. IFN-γ produced by BAL cells was determined using ELISA. B: Five hundred μg of the anti-IFN-γ mAb was injected intravenously into B6, CD1d−/−, and RAG−/− Vα14tg Vβ8.2tg (Vα14 TCR Tg) mice twice per week before and after administering bleomycin. The hydroxyproline content was measured on day 21. The data are a mean ± SEM of five mice in each group and results from a representative of three repeated experiments are shown.
Figure 8.
Bleomycin-induced pulmonary fibrosis was attenuated by IFN-γ secreted by NKT cells. A: The sorted NKT cells from IFN-γ−/− (IFN-g−/− NKT) or IL-4−/− (IL-4−/− NKT) or B6 (WT NKT) mice were adoptively transferred into the CD1d−/− mice. The hydroxyproline contents were measured on day 21. The increased hydroxyproline contents of the lungs in each group were presented as percentage. The data are represented as a mean ± SEM of six mice in each group. B: The TGF-β1 and IFN-γ mRNA levels were measured in the lung from B6, CD1d−/−, and CD1d−/− mice adoptively transferred with sorted NKT cells from B6 or IFN-g−/− or IL-4−/− mice by real-time PCR 7 days after the administration of bleomycin. The results from a representative of three repeated experiments are shown.
Discussion
During the development of bleomycin-induced pulmonary fibrosis, NKT cells, recognized by α-GalCer/CD1d dimer, infiltrated the lung tissue of B6 mice whereas NKT cells were not detected in CD1d−/− mice. Subset analysis for the BAL fluids revealed that NKT cells infiltrated the lung 5 to 9 days after administering the bleomycin. The deficiency of NKT cells in B6 mice aggravates the bleomycin-induced pulmonary fibrosis, as estimated by the hydroxyproline content and histological analysis. The aggravated pulmonary fibrosis to bleomycin in NKT cell-deficient mice was also associated with a higher degree of weight loss and mortality when compared with wild-type B6 mice (Figure 2, D and E). In contrary, Kimura and colleagues25 reported that mortality in wild-type B6 mice was ∼50% by 28 days after administrating the bleomycin, which was similar to the 65% mortality in Jα18−/− mice. In their experiments, they administrated high bleomycin concentration (0.5 mg per mouse) into the mice for pulmonary fibrosis, which is in stark contrast to the dose used in our experiments (0.04 or 0.06 mg per mouse). It appears that the large amounts of bleomycin used in their experiments were inappropriate for evaluating the differences of mortality between wild-type B6 and Jα18−/− mice in the bleomycin-induced pulmonary fibrosis model because high bleomycin concentrations used in their experiments caused severe acute injury to the lung tissues that even wild-type B6 mice could not overcome. Therefore, it is likely that a large discrepancy in the dose of the bleomycin administrated affects the mortality rate of the mice in two independent experiments. In addition, some NKT cell-deficient mice died before day 7 of intratracheal bleomycin injection, which appears to be an early time for the induction of fibrosis in the lung tissues. It is possible that the poor clinical outcome of the NKT cell-deficient mice in the early phase may be due to a bleomycin-induced acute lung injury rather than to pulmonary fibrosis. Therefore, it is likely that both bleomycin-induced acute lung injury and pulmonary fibrosis may contribute to the poor overall survival rate of CD1d−/− and Jα18−/− mice in the bleomycin-induced pulmonary fibrosis model. The critical role of NKT cells during the development of bleomycin-induced pulmonary fibrosis was further substantiated by the fact that adoptive transfer of NKT cells from normal B6 or RAG−/− Vα14tg Vβ8.2tg mice restored the impaired fibrosis in CD1d−/− mice. Altogether, these findings suggest that NKT cells play important roles in the development of bleomycin-induced pulmonary fibrosis.
Among the cytokines tested, TGF-β1 production was prominent in the lungs of CD1d−/− mice compared with that in control B6 mice, and the amount of TGF-β1 produced in the lung closely correlated with the bleomycin-induced pulmonary fibrosis in CD1d−/− mice. Moreover, the adoptive transfer of either NKT cells from B6 or splenocytes from RAG−/− Vα14tg Vβ8.2tg mice into CD1d−/− mice decreased the level of TGF-β1 transcription in the lung. The TGF-β1 blockade in CD1d−/− mice resulted in reducing the level of pulmonary fibrosis and increasing the survival rate in the bleomycin-induced pulmonary fibrosis. Taken together, these findings suggest that TGF-β1 is involved in the bleomycin-induced pulmonary fibrosis of CD1d−/− mice, which is tightly regulated by NKT cells in the lung.
In animal models of pulmonary fibrosis, the TGF-β1 mRNA and protein levels are increased in the lung tissues.36 TGF-β1 is both mitogenic and chemotactic for the fibroblasts, monocytes, and macrophages, resulting in the promoting accumulation of the extracellular matrix proteins by increasing their synthesis while inhibiting matrix degradation.37 Although the precise mechanism of the pulmonary fibrosis remains enigmatic, TGF-β1 signaling appears to be one of the important mediators of pulmonary fibrosis in both animals and humans.38 Therefore, regulating the endogenous TGF-β1 production and signaling in the pulmonary fibrosis has been regarded as the main issue for therapeutic purposes.37 Among the cytokine network, IFN-γ has been shown to suppress TGF-β1 expression39 and directly suppress fibroblast collagen production in vitro.40 Recently, Jiang and colleagues41 demonstrated that IFN-γ produced by NK cells in the lungs regulate bleomycin-induced pulmonary fibrosis in CXCR3-deficient mice, suggesting that innate burst of IFN-γ production in the lungs might play important roles for attenuating pulmonary fibrosis in vivo system.
In this study it was shown that a NKT cell/IFN-γ regulatory circuit represses TGF-β1 production in bleomycin-induced pulmonary fibrosis. Segal and colleagues10 reported that the majority (54%) of the IFN-γ-producing lymphocytes during the development of bleomycin-induced pulmonary fibrosis in mice were CD4+, and 27%, 11%, and 5% were NK1.1+, CD8+, and B220+, respectively. Although the proportion of NKT cells in the IFN-γ-producing lymphocytes was not clarified in this study, NKT cells were considered to be included in NK1.1+ cells or CD4+ T cells. These results suggested that NKT cells might be a subset of T cells that produce IFN-γ and suppress TGF-β1 production during the development of bleomycin-induced pulmonary fibrosis. However, Kimura and colleagues25 demonstrated that the injection of α-GalCer into B6 mice induced the production of IFN-γ, which attenuated pulmonary fibrosis. IFN-γ induced by injection of α-GalCer was mainly produced by conventional T cells and NK cells rather than NKT cells, which suggests that NKT cells participate in the activation of conventional T cells rather than be a major source of IFN-γ in attenuating the bleomycin-induced pulmonary fibrosis by α-GalCer treatment. In contrast, our results revealed that the activation status, determined by expression levels of CD25 or CD69 on cell surface, of conventional NK1.1−TCRβ+ T cells in BAL fluid from B6 mice administered bleomycin was similar to that in CD1d−/− mice (Figure 1E). Moreover, the intratracheal administration of bleomycin induced pulmonary fibrosis in RAG−/− Vα14tg Vβ8.2tg mice, which contain iNKT cells and NK cells in the absence of conventional T and B cells, suggesting that conventional T cells do not play essential roles in the development of bleomycin-induced pulmonary fibrosis. Overall, it is unlikely that NKT cells contribute to activation of conventional T cells during the development of pulmonary fibrosis. In the present study, our results demonstrated that NKT cells produced IFN-γ during bleomycin-induced pulmonary fibrosis, and IFN-γ secreted by NKT cells contributed to the attenuation of pulmonary fibrosis. Several lines of evidence in this study suggest the critical roles of IFN-γ secreted by NKT cells on the anti-fibrotic effects in the bleomycin-induced pulmonary fibrosis. First, the level of IFN-γ transcription was lower in the lungs of CD1d−/− than in the B6 mice in the bleomycin-induced pulmonary fibrosis. Second, α-GalCer induced the production of IFN-γ by BAL cells in a dose-dependent manner in the in vitro culture experiment. Third, the adoptive transfer of NKT cells into CD1d−/− mice restored the amount of hydroxyproline and the transcriptional level of IFN-γ, and reduced the levels of TGF-β1 transcription in the lung, which were comparable to those of B6 mice in the bleomycin-induced pulmonary fibrosis model. In contrast, the adoptive transfer of IFN-γ-deficient NKT cells did not restore the amount of hydroxyproline and level of TGF-β1 transcription. Fourth, a blockade of IFN-γ using neutralizing mAb aggravated the pulmonary fibrosis in RAG−/− Vα14tg Vβ8.2tg mice as well as in B6 and CD1d−/− mice, suggesting that conventional T cells are not the major source of IFN-γ in attenuating pulmonary fibrosis by NKT cells. Taken together, these findings indicate that NKT cells are a major subset of immune cells producing IFN-γ in the bleomycin-induced pulmonary fibrosis model. In vitro experiments demonstrated that the priming condition favoring a high level of IL-4 production and/or a low level of IFN-γ production greatly enhanced the TGF-β1 production in the secondary cultures.39,42 It appears that IFN-γ inhibits the differentiation of TGF-β1-producing CD4+ T cells. IFN-γ also inhibits the TGFβ-induced phosphorylation of Smad3 and the activation of the TGFβ-responsive genes.43,44 Thus, it has been proposed that IFN-γ contributes to the anti-fibrotic effects on pulmonary fibrosis by suppressing TGF-β1 production in the lung. Consistent with this hypothesis, IFN-γ has been shown to have anti-fibrotic effects by directly suppressing fibroblast type I and III collagen synthesis as well as TGF-β1 production during pulmonary fibrosis.37,38,40,45 Therefore, NKT cells contribute to the anti-fibrotic effects on bleomycin-induced pulmonary fibrosis by producing IFN-γ, which suppresses the production and signaling of TGF-β1 in the lung.
Kinetic analysis for IFN-γ production in the BAL and lung tissues of B6 mice revealed two time points (days 1 and 7) showing peak in amounts of IFN-γ. In contrast, the amounts of IFN-γ in the BAL and lung tissues of CD1d−/− mice were low at day 7 whereas they were high on day 1 like in the B6 mice. This suggests that NKT cells might contribute to the production of large amounts of IFN-γ in the BAL and lung tissues approximately at day 7. Kinetic analysis of the NKT cell influx in the lungs of the wild-type B6 mice showed that the influx of NKT cells peaked at approximately day 7 and a small number of NKT cells infiltrated lung tissues at day 1 in the bleomycin-induced pulmonary fibrosis model. It is possible that the infiltrated NKT cells in the lung regulate bleomycin-induced pulmonary fibrosis by producing large amounts of IFN-γ at approximately day 7. However, it is also possible that NK cells rather than NKT cells produce IFN-γ in the BAL and lung tissues during the early stage of injury because a small number of NKT cells infiltrated the lung tissues and the concentration of IFN-γ in the lung of CD1d−/− mice were similar to those in B6 mice in early phase of bleomycin-induced pulmonary fibrosis. Therefore, it is likely that NK cells contribute to producing large amounts of IFN-γ during the early phase (at day 1) and that NKT cells regulate collagen synthesis during the later phase (approximately day 7) in the bleomycin-induced pulmonary model by producing IFN-γ in the lung tissues. In a human system, therapeutic approaches using IFN-γ has been attempted on the patients with idiopathic pulmonary fibrosis (IPF). However, in a well-defined population of patients with idiopathic pulmonary fibrosis, IFN-γ-1b did not affect the progression-free survival, pulmonary function, or the quality of life.46 Therefore, IFN-γ does not appear to work well in human IPF despite its effect on mice. It has been argued that bleomycin-induced pulmonary fibrosis in a mouse system does not reflect all of the immunological mechanisms involved in human IPF, which might explain the discrepancy regarding the IFN-γ effects in modulating pulmonary fibrosis between the human and mouse system. Alternatively, it is also possible that the bleomycin-induced pulmonary fibrosis in mice reflects a more acute process in the balance between collagen synthesis and degradation in the lung rather than the long-term processes of pulmonary fibrosis involved in human IPF.
In summary, during the bleomycin-induced pulmonary fibrosis, NKT cells infiltrate the lung parenchyma and secret a large amount of IFN-γ, which suppresses TGF-β1 production in the lung. Therefore, NKT cells attenuate bleomycin-induced pulmonary fibrosis by producing IFN-γ in the lung tissues.
Acknowledgments
We thank Dr. Seong Hoe Park for his support of this project; Dr. Youngmee Bae for helpful comments on the manuscript; Dr. Sung-Soo Yoon for providing reagent for this project; all members of our laboratory for their enthusiasm; and all members of the Department of Experimental Animal Research, Clinical Research Institute, Seoul National University Hospital for animal management.
Footnotes
Address reprint requests to Doo Hyun Chung, Department of Pathology and Laboratory of Immune Regulation in Graduate Program for Immunology, Seoul National University College of Medicine, 28 Yongon-dong, Chongno-gu, Seoul, 110-799, Korea. E-mail: doohyun@plaza.snu.ac.kr.
Supported by the Ministry of Science and Technology (grant M10422010004-04N2201-00410) and the Seoul National University Hospital Research Fund (grant 03-2003-011-0).
References
- Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP., III Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs. 2004;64:405–430. doi: 10.2165/00003495-200464040-00005. [DOI] [PubMed] [Google Scholar]
- Corbel M, Caulet-Maugendre S, Germain N, Molet S, Lagente V, Boichot E. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J Pathol. 2001;193:538–545. doi: 10.1002/path.826. [DOI] [PubMed] [Google Scholar]
- Hagimoto N, Kuwano K, Nomoto Y, Kunitake R, Hara N. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 1997;16:91–101. doi: 10.1165/ajrcmb.16.1.8998084. [DOI] [PubMed] [Google Scholar]
- Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaisgood CM, French EL, Noga C, Simon RH, Ploplis VA. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol. 2000;157:177–187. doi: 10.1016/S0002-9440(10)64529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Nakao A, Nakano H, Takahashi F, Takahashi K, Shimozato O, Takeda K, Yagita H, Okumura K. Impairment of bleomycin-induced lung fibrosis in CD28-deficient mice. J Immunol. 2001;167:1977–1981. doi: 10.4049/jimmunol.167.4.1977. [DOI] [PubMed] [Google Scholar]
- Doherty DE, Hirose N, Zagarella L, Cherniack RM. Prolonged monocyte accumulation in the lung during bleomycin-induced pulmonary fibrosis. A noninvasive assessment of monocyte kinetics by scintigraphy. Lab Invest. 1992;66:231–242. [PubMed] [Google Scholar]
- Zhu J, Cohen DA, Goud SN, Kaplan AM. Contribution of T lymphocytes to the development of bleomycin-induced pulmonary fibrosis. Ann NY Acad Sci. 1996;796:194–202. doi: 10.1111/j.1749-6632.1996.tb32581.x. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Hogaboam C, Chensue SW, Blease K, Kunkel SL. Type 1/type 2 cytokine paradigm and the progression of pulmonary fibrosis. Chest. 2001;120:5S–8S. doi: 10.1378/chest.120.1_suppl.s5. [DOI] [PubMed] [Google Scholar]
- Segel MJ, Izbicki G, Cohen PY, Or R, Christensen TG, Wallach-Dayan SB, Breuer R. Role of interferon-γ in the evolution of murine bleomycin lung fibrosis. Am J Physiol. 2003;285:L1255–L1262. doi: 10.1152/ajplung.00303.2002. [DOI] [PubMed] [Google Scholar]
- Keane MP, Belperio JA, Burdick MD, Strieter RM. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am J Physiol. 2001;281:L92–L97. doi: 10.1152/ajplung.2001.281.1.L92. [DOI] [PubMed] [Google Scholar]
- Kitasato Y, Hoshino T, Okamoto M, Kato S, Koda Y, Nagata N, Kinoshita M, Koga H, Yoon DY, Asao H, Ohmoto H, Koga T, Rikimaru T, Aizawa H. Enhanced expression of IL-18 and its receptor in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2004;31:619–625. doi: 10.1165/rcmb.2003-0306OC. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Nozaki Y, Hatano K, Phan SH. Lung interleukin-4 gene expression in a murine model of bleomycin-induced pulmonary fibrosis. Cytokine. 2001;15:138–147. doi: 10.1006/cyto.2001.0903. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, Keane MP. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27:419–427. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]
- Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol. 2003;170:2083–2092. doi: 10.4049/jimmunol.170.4.2083. [DOI] [PubMed] [Google Scholar]
- Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-β independent. J Immunol. 2004;173:4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, McKenzie A, Lukacs NW, Phan SH. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J Immunol. 2004;173:3425–3431. doi: 10.4049/jimmunol.173.5.3425. [DOI] [PubMed] [Google Scholar]
- Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A. CD1: presenting unusual antigens to unusual T lymphocytes. Science. 1995;269:185–186. doi: 10.1126/science.7542402. [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Paul WE. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- Kim HY, Kim HJ, Min HS, Kim S, Park WS, Park SH, Chung DH. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor β1 production. J Exp Med. 2005;201:41–47. doi: 10.1084/jem.20041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Ishii Y, Morishima Y, Shibuya A, Shibuya K, Taniguchi M, Mochizuki M, Hegab AE, Sakamoto T, Nomura A, Sekizawa K. Treatment with α-galactosylceramide attenuates the development of bleomycin-induced pulmonary fibrosis. J Immunol. 2004;172:5782–5789. doi: 10.4049/jimmunol.172.9.5782. [DOI] [PubMed] [Google Scholar]
- Kim SLS, Lee T, Jung S, Kim D. Practical synthesis of KRN7000 form phytosphingosine. Synthesis. 2004;22:847–850. [Google Scholar]
- Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, Hara N. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest. 1999;104:13–19. doi: 10.1172/JCI5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- Hammond KJ, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. Alpha/beta-T cell receptor (TCR)+CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992;18:29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Venkatesan N, Roughley PJ, Ludwig MS. Proteoglycan expression in bleomycin lung fibroblasts: role of transforming growth factor-β1 and interferon-γ. Am J Physiol. 2002;283:L806–L814. doi: 10.1152/ajplung.00061.2002. [DOI] [PubMed] [Google Scholar]
- Wen FQ, Liu X, Kobayashi T, Abe S, Fang Q, Kohyama T, Ertl R, Terasaki Y, Manouilova L, Rennard SI. Interferon-γ inhibits transforming growth factor-β production in human airway epithelial cells by targeting Smads. Am J Respir Cell Mol Biol. 2004;30:816–822. doi: 10.1165/rcmb.2002-0249OC. [DOI] [PubMed] [Google Scholar]
- Seder RA, Marth T, Sieve MC, Strober W, Letterio JJ, Roberts AB, Kelsall B. Factors involved in the differentiation of TGF-β-producing cells from naive CD4+ T cells: IL-4 and IFN-γ have opposing effects, while TGF-β positively regulates its own production. J Immunol. 1998;160:5719–5728. [PubMed] [Google Scholar]
- Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon γ in bleomycin-mouse model of lung fibrosis: downregulation of TGF-β and procollagen I and III gene expression. Exp Lung Res. 1995;21:791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, Gerard C, Noble PW. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth T, Strober W, Seder RA, Kelsall BL. Regulation of transforming growth factor-β production by interleukin-12. Eur J Immunol. 1997;27:1213–1220. doi: 10.1002/eji.1830270524. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-β/SMAD signalling by the interferon-γ/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol. 2002;282:L585–L593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- Sempowski GD, Derdak S, Phipps RP. Interleukin-4 and interferon-γ discordantly regulate collagen biosynthesis by functionally distinct lung fibroblast subsets. J Cell Physiol. 1996;167:290–296. doi: 10.1002/(SICI)1097-4652(199605)167:2<290::AID-JCP13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE., Jr A placebo-controlled trial of interferon γ-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]