Abstract
Little is known about adoptive transfer of allogeneic ex vivo expanded dendritic cells (eDCs). We investigated the trafficking pattern of eDCs in mice after allogeneic bone marrow transplantation by using bioluminescence imaging. eDCs were expanded from bone marrow precursors in the presence of GM-CSF, interleukin-4, and Flt3L and retrovirally transduced to express luciferase (luc) and green fluorescence protein (gfp). Flow cytometry showed polyclonal DC populations after expansion that consisted of CD11c+CD11b+ and CD11c−CD11b+ cells that co-expressed CD40, CD80, CD86, and MHCII. eDCs were functional in mixed lymphocyte reactions and produced tumor necrosis factor-α on phytohemagglutinin stimulation. The eDCs were then injected intravenously into BALB/c recipient mice that had received allogeneic bone marrow transplantation 6 weeks previously. On day 1 after transfer, eDCs were detected by bioluminescence imaging throughout the lungs and spleen. In the later course, signals were observed throughout thymus, lower abdomen, and spleen throughout a period of more than 42 days. Immunofluorescence microscopy confirmed CD11c positivity on the gfp+ donor cells, which localized in T-cell zones of mesenteric lymph nodes, Peyer’s patches, spleen, and thymus. These findings are important for adoptive immunotherapies because they indicate that eDCs migrate efficiently in vivo and are capable of surviving long term.
Allogeneic bone marrow transplantation (BMT) has been shown to be effective in the treatment of many hematological malignancies.1 In the case of tumor relapse adoptive immunotransfer using donor lymphocytes can reinduce remission.2 However this strategy bears the risk of severe graft-versus-host disease (GvHD) and prolonged aplasia.3 Therefore additional strategies are warranted. Little is known about adoptive immunotransfer of allogeneic ex vivo expanded dendritic cells (eDCs) after an allogeneic BMT.
Dendritic cells (DCs) are antigen-presenting cells that induce primary immune responses by capturing foreign antigens and presenting them to effector cells of the adaptive immune system.4 DCs express co-stimulatory molecules that are required for induction of an effective T-cell immune response.5 Furthermore, DCs are also involved in the maintenance of immunological tolerance and regulation of T-cell-mediated immune responses.6–8 These capacities make DCs a key cell population for adoptive immunotherapy. Although the understanding of DC biology is still incomplete, DCs are already used in adoptive immunotherapies to elicit immunity against cancer and infectious diseases.9,10
Mature, autologous DCs pulsed with tumor antigens, specific proteins, RNA from tumor cells, or tumor lysates have been successfully used for the treatment of a variety of malignancies. Specific immune responses have been observed and in some patients the clinical outcome has been improved.11,12 In addition, administration of immature DCs inhibited proliferation of alloreactive T cells and could induce prolongation of graft survival in mice receiving solid organ transplantation.13–17
A major limitation for the clinical use of DCs is the availability of sufficient cell numbers. In humans, DCs represent ∼0.5% of peripheral blood mononuclear cells.18 Therefore, various groups have explored culture conditions to improve expansion of DCs ex vivo. Culturing of bone marrow (BM)-derived cells in the presence of GM-CSF, interleukin (IL)-4, and Flt3 ligand has been shown to result in a sufficient expansion of DCs.19,20 These cells are fully capable of antigen presentation and other DC functions. Despite effective enrichment protocols little is known about the survival, trafficking, and function of eDCs after in vivo transfer into allogeneic recipients. This information is essential for the design of future cellular therapies using eDCs in patients.
To explore the in vivo survival and trafficking of eDCs we used in vivo bioluminescence imaging (BLI). This method has proven to be a sensitive technique for visualizing the trafficking and survival of cellular populations in living animals.21 It allows the serial investigation of a single animal throughout an extended period of time and represents a sensitive and specific guidance for histopathological tissue sampling.22 BLI is based on the introduction of a reporter gene encoding for the bioluminescent protein luciferase (luc). Dual function reporter genes, that express luc in addition to a fluorescent reporter (as for instance gfp), can link in vivo and ex vivo assays. This offers an opportunity to refine and accelerate studies of cell fates and function after transfer to unlabeled recipient animals.21
To explore the in vivo survival and trafficking of eDCs we used in vivo BLI. This method has proven to be a sensitive technique for visualizing the trafficking and survival of cellular populations in living animals.21 It allows the serial investigation of a single animal throughout a long period of time and especially in combination with ex vivo imaging represents a sensitive and specific guidance for histopathological tissue sampling.22 BLI is based on the introduction of a reporter gene encoding for the bioluminescent protein luciferase (luc). Dual function reporter genes, that express luc in addition to a fluorescent reporter (as for instance gfp), can link in vivo and ex vivo assays. This offers an opportunity to refine and accelerate studies of cell fates and function after transfer to unlabeled recipient animals.21
In the present study we demonstrate that eDCs generated from BM precursors can be transduced with a retroviral vector encoding for luc and gfp and can be monitored in vivo in allogeneic recipients with BLI. The gfp positivity of eDCs enabled immunofluorescence microscopy on excised tissues that were carefully sampled based on the findings of in vivo BLI. On day 1 after transfer eDCs were detected in the lungs and spleen. During the later course eDCs were found in lymph nodes, Peyer’s patches, spleen, and thymus for a period of up to 6 weeks after transfer. Nonmyeloablative or myeloablative conditioning did not affect migration patterns and in both settings intravenous injection of eDCs did not induce GvHD.
Materials and Methods
Animals
Male BALB/c (H2d) and C57BL/6 (H2b) mice were obtained from the breeding facility of the Department of Comparative Medicine, Stanford, CA. Male OSB mice (gfp transgenic) were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were used between 8 to 10 weeks of age and were housed under sterile conditions. Care of animals was in accordance with guidelines on protocols approved by the animal care Committee of Stanford University. Animals were examined at least once a day for clinical signs of GvHD including changes in fur, hunched back, weight loss, and diarrhea.
BMT Conditioning Regimens
BALB/c recipient mice were conditioned with either myeloablative or nonmyeloablative regimens. Chemokine responsiveness and tissue inflammation are critical parameters for DC migration.23–27 To eliminate any toxic influence due to the conditioning regimen we administered eDCs at least 6 weeks after allogeneic BMT. The myeloablative conditioning regimen consisted of 800 cGy total body irradiation administered in two dosages of 400 cGy within an interval of 12 hours, followed by the infusion of 5 × 106 C56BL/6 BM cells to re-establish hematopoiesis. Nonmyeloablative conditioning was performed with 10 doses of 240 cGy total lymphoid irradiation plus 200 μl of anti-thymocyte serum given for five doses, followed by the injection of 5 × 107 C57BL/6 BM cells.28
Generation, Transduction, and Injection of eDCs
BM cells of C57BL/6 mice were harvested from bilateral femura and tibiae. B and T cells and granulocytes were removed with anti-CD3-PE and anti-Gr1-PE monoclonal antibodies (mAb; BD Pharmingen, San Jose, CA) for 30 minutes at 4°C followed by anti-B220-magnetic beads and anti-phycoerythrin (PE) magnetic beads (Miltenyi, Auburn, CA) for 20 minutes at 4°C. The remaining cells were counted and cultured for 1 week in complete RPMI containing 10% fetal calf serum, 10 ng/ml GM-CSF (Peprotech, Rocky Hill, NJ), 10 ng/ml IL-4 (Peprotech), and 5 ng/ml Flt3 L (R&D Systems, Minneapolis, MN).
For detection of eDCs by BLI, cells were transduced with the retroviral p3-luc plasmid (kind gift of Dr. G. Fathman, Stanford University). P3-luc is a retroviral dual-reporter gene construct encoding for green fluorescent protein (gfp) and the firefly luciferase (luc). Retroviral transduction of eDCs was performed as described previously.29 Briefly, ecotropic p3-luc viral particles were generated by transient transfection using the calcium phosphate precipitation technique and the PhoenixEco packaging cell line.30,31 Phoenix cells were a kind gift of Dr. G. Nolan, Stanford University. Retrovirus-containing supernatant was harvested and cryopreserved at −80°C. For transduction of eDCs, half of the media was removed and the same amount of freshly thawed virus-containing supernatant was added to the culture in the presence of 16 μg/ml protamine sulfate and cytokines. EDCs were then centrifuged at 2000 rpm and 32°C for 90 minutes. After centrifugation, the plates were transferred to a 32°C incubator for 90 minutes. Subsequently the supernatant was removed from each well and replaced with fresh complete media containing IL-4 and GM-CSF. Transduction was performed on days 3, 4, and 5 of culture. On day 7 of culture, adherent and nonadherent cells were harvested and 4 × 106 cells were injected intravenously (via the tail vein) into allogeneic recipient mice, that had received allogeneic BMT 6 to 8 weeks before cell transfer.
Antibodies and Flow Cytometry
The following monoclonal antibodies were used for staining of DCs (clone no.): CD80-PE (16−10A1), Gr1-PE (RB6−8C5), CD8a-PE (53−6.7), CD19-PE (1D3), CD11b-PE and CD11b-Cy5 APC (M1/70), CD3mc-PE (17A2), I-Ab-PE (AF6−120.1), CD86-PE (GL1), CD40-PE (3/23), H2Kb-PE (AF6−88.5), CD11c-APC (HL3), all purchased from BD Pharmingen. CD11b-TxR was a gift of Dr. J. Brown (Stanford University). Staining was performed in phosphate-buffered saline (PBS) supplemented with 1% calf serum in the presence of purified anti-CD16/32 to block nonspecific staining. Dead cells were excluded with propidium iodide. Flow cytometric analyses were performed on either a FACScan, a modified dual-laser FACS Vantage, or a LSR flow cytometer. Fluorescence-activated cell sorting data were analyzed using FlowJo software (Tree Star, San Carlos, CA). At least 50,000 cells were analyzed. Cells analyzed for gfp expression were examined using the fluorescein isothiocyanate (FL1) channel on the flow cytometer.
Mixed Lymphocyte Reaction (MLR)
The ability of the eDCs to present antigen and to elicit an immune response assessed by induction of T-cell proliferation was studied as previously described.32 Cultures were set up in triplicates in 96-well flat-bottom plates (Falcon BD, San Jose, CA) in a total volume of 200 μl. Cells were cultured in RPMI 1640 medium (Life Technologies, Inc., Gaithersburg, MD) with 10% fetal calf serum (Sigma Chemical Co., St. Louis, MO), 2 mmol/L l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (Life Technologies, Inc.) and 5 × 105 mol/L 2-mercaptoethanol (Sigma Aldrich, St. Louis, MO). Fixed numbers of responders’ cells either from C57BL/6 or BALB/c spleen and irradiated eDCs from C57BL/6 (allogeneic or syngeneic stimulator cells) were mixed in specific ratios to achieve ratios of responders to stimulators (R:S) of 1:0, 1:1, 1:2, and 1:4. After lysis of red blood cells from the spleens of the two mouse strains the cells were plated at 2 × 105 cells/well. The eDCs at day 7 were harvested, washed twice, irradiated with 3000 cGy and mixed at 2 × 105, 4 × 105, and 8 × 105 to get the above-mentioned ratios of R:S. After 5 days at 37°C in 5% CO2 the proliferation of responder cells was assessed. The cells were pulsed with 1 μCi/well [3H] thymidine in the last 16 to 18 hours of the assay. Cells were harvested and the amount of incorporated [3H] thymidine was measured with a harvester and read with a Betaplate counter (1205 betaplate; Wallac/Perkin Elmer, Torrance, CA).
Cytometric Bead Array Immunoassay
eDCs (2 × 105) in culture at day 7 and freshly isolated DCs at day 0 were harvested, washed twice, and plated in triplicates in a flat-bottom 96-well plate. The cells were incubated with 5 μg/ml phytohemagglutinin (PHA; Sigma Biochemicals, St. Louis, MO) or media alone RPMI 1640 in a total volume of 200 μl/well. The cells were incubated for 48 hours in a humidified incubator at 37°C with 5% CO2. After 48 hours 100 μl of cell-free supernatant was collected and frozen at −80°C until cytokine analysis. Cytokines were detected with the cytometric bead array kit (BD Pharmingen) according to the manufacturer’s protocol and as otherwise described.33,34 Briefly, particles (polystyrene beads, 7.5 μm) are dyed to six different fluorescence intensities. Each particle is coupled with a mAb against one of five cytokines [interferon-γ, tumor necrosis factor (TNF)-α, IL-2, IL-4, IL-5]. The Ab particle serves as capture for a given cytokine in an immunoassay panel and can be detected simultaneously in a mixture. The mixture of beads detects five cytokines in one sample. A secondary PE-conjugated mAb stains the beads proportionally to the amount of bound cytokine, which emits at ∼585 nm. The calibrators (standards ranging from 0 to 5000 pg/ml) for the assay system are mixtures of all five cytokines. Five standard curves are thus obtained from one set of calibrators. After fluorescence intensity calibration and electronic color compensation procedures, standard, and test samples were collected and analyzed with FACS Scan and Cell Quest software (BD Biosciences).
In Vivo BLI
Mice were sedated using an intraperitoneal injection of ketamine (100 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (10 mg/kg; Butler, Columbus, OH). An aqueous solution of luciferin (150 mg/kg; Xenogen, Alameda, CA) was injected intraperitoneally 5 minutes before imaging. Animals were placed into the light-tight chamber of the charge-coupled device camera imaging system (IVIS, Xenogen), and a grayscale reference image (digital photograph) was taken under weak illumination.35 After switching off the light source, images of DC distribution in the body were generated using the photons emitted from luc-expressing cells within the animal’s body that were transmitted through the tissue. Integration times ranging up to 5 minutes (indicated in legends to figures) were used to collect the data. The Living Image software program (Xenogen), an overlay on Igor (WaveMetrics, Lake Oswego, OR), was used for data acquisition and analysis, and to create a pseudocolor image representing light intensity (blue, least intense; red, most intense) and the overlay of this image on the reference image to enable anatomical localization. Animals were imaged from the left lateral, ventral, and dorsal positions.
Immunofluorescence Microscopy
Tissues of abdominal and thoracic organs were sampled and cryopreserved (−80°C) corresponding to time points and regions of interest defined by BLI. Fresh frozen sections of 6 to 8 μm thickness were mounted on positively charged precleaned microscope slides (Superfrost/Plus; Fisher Scientific, Pittsburgh, PA) and stored at −80°C. To retain the cytoplasmatic gfp signal, the frozen sections were pretreated with formaldehyde vapor overnight at −20°C.36 Immunofluorescent staining was performed according to standard protocols (Current Protocols of Immunology, articles 2 to 3). Briefly, after acetone fixation (7 minutes at room temperature) and drying in air (2 minutes) the sections were incubated with blocking solution (2% fetal calf serum in PBS) for 15 minutes. Incubation with primary antibodies was performed for 1 hour at room temperature in a humidified chamber. To detect the fluorescent signal from gfp, we used a polyclonal anti-green fluorescent protein antibody (rabbit IgG fraction, anti-gfp; Molecular Probes, Eugene, OR) as primary antibody in a 1:100 dilution in PBS. The secondary detection was performed using a goat anti-rabbit antibody conjugated to the green fluorochrome Alexa 488 (Molecular Probes), in a 1:300 dilution. In addition we also applied a primary anti-CD11c antibody conjugated with phycoerythrin (CD11c-PE, BD Pharmingen), in a 1:100 dilution. Nuclear staining was performed with 4,6-diamidino-2-phenylindole. Washing steps after antibody incubations and 4,6-diamidino-2-phenylindole staining were done with 1× PBS (two times for 5 minutes each). For mounting we used 50% glycerol in H2O and coverslips (no. 1, Fisher Scientific). Fluorescence microscopic evaluation of immunostained frozen sections was performed on a Nikon microscope (Eclipse, TE 300; Technical Instrument San Francisco, Burlingame, CA). Microscopic photos were documented using a digital camera system (Spot; Diagnostic Instruments, Sterling Heights, MI).
Results
Ex Vivo Expansion Generates DC Population with Mature Phenotype and a CD11c−CD11b+ Population of Potential DC Precursor Type
BM of donor (C57BL/6, OSB) mice was harvested and depleted of CD3+ T cells, B220+ B cells, and GR1+ granulocytes to enrich for DC precursors. An average yield of 44.5 × 106 (±18.7) mononuclear cells was obtained from each mouse. After depletion the total number of remaining cells was 2.9 × 106 (±1.7) cells per mouse, equaling 6.9% (±2.5) of the initial cell number. Expansion of depleted BM cells in the presence of GM-CSF, IL-4, and Flt3L throughout a period of 7 days led to a 7.3 (±2.6)-fold increase in total cell number. Cells that were transduced with p3-luc could be expanded 4.9-fold (±3.5).
The ex vivo expansion resulted in generation of two cell populations, a CD11c+CD11b+ population and a CD11c−CD11b+ cell population (Figure 1A). The proportion of CD11c+CD11b+ was 50.7% (range, 40.5 to 65.5%) and the proportion of CD11c−CD11b+ was 48.2% (range, 24.7 to 50.3%). CD86, CD80, and CD40 were expressed on both populations; however, the CD11c+CD11b+ population showed a higher level of expression of these co-stimulatory molecules (Figure 1B). Furthermore, both cell populations expressed high levels of MHC class I and II molecules (IA and H2K), while T-cell and NK-cell markers were negative (CD3, CD4, CD8, and NK1.1; Figure 1C). Transduction of cultured eDCs with the p3-luc plasmid yielded a median transduction efficiency of 19.3% (range, 2 to 36.6%). CD11c+CD11b+ cells were transduced slightly more efficiently than CD11c−CD11b+ cells (Figure 1D). Transduction did not affect the expression of co-stimulatory markers or the expression of MHC I and II (data not shown).
Figure 1.
Phenotype of ex vivo eDCs. A:Ex vivo expansion of depleted BM for 7 days (by GM-CSF, IL-4, and Flt3L) resulted in development of two major cell populations, CD11c+CD11b+ and CD11c−CD11b+ cell populations. B: CD11c+ CD11b+ eDCs (bold black line) expressed higher levels of CD80, CD86, and CD40 as compared to the CD11c−CD11b+ cell population (thin black line). All cell populations expressed MHC class I and II. The isotype control (thin gray line) was negative. C:Ex vivo expansion of depleted BM did not lead to generation of T cells (CD3+, CD4+, CD8+), B cells (CD19+), or NK cells (NK1.1+). D: Transduction of the eDCs with the gfp-luc dual function reporter, during the 1-week expansion resulted in a transduction efficiency of up to 37%. The majority of transduced gfp+ cells were CD11c+.
eDCs Induce Alloreactive Proliferation in Vitro and Produce TNF-α on PHA Stimulation
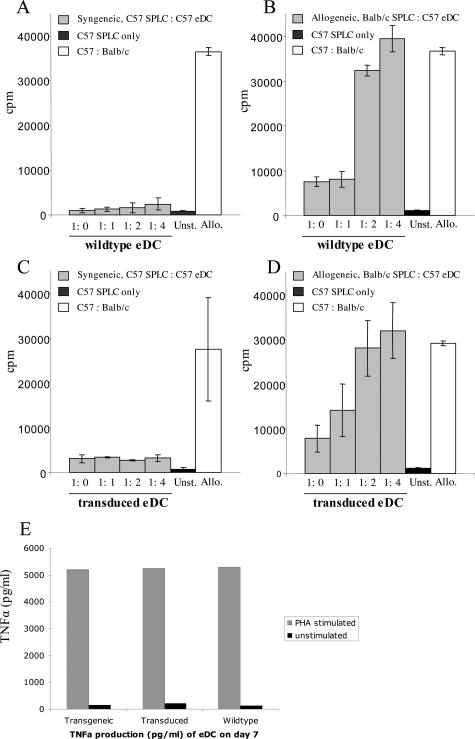
We have compared the capacities of eDCs derived from C57BL/6 wild-type (WT) mice, transduced luc+gfp+ eDCs, and transgenic gfp+ eDCs to present antigen and to induce proliferation in a MLR. Splenocytes (2 × 105) from C57BL/6 were co-cultured with irradiated eDCs at variable numbers to achieve the indicated ratios of responder (R) to stimulator (S) cells. In the syngeneic setting C57BL/6 splenocytes showed minimal proliferation when exposed to irradiated eDCs of the same origin (Figure 2A). In contrast, responder cells (C57BL/6 splenocytes) proliferated vigorously against irradiated eDCs of allogeneic origin (BALB/c) in a dose-dependent manner (Figure 2B). We also conducted the MLR with syngeneic transduced eDCs, which only induced a minimal proliferation (Figure 2C), similar to the one induced by syngeneic WT eDCs. In contrast allogeneic transduced eDCs induced a vigorous proliferation (Figure 2D), which was similar to the one induced by allogeneic WT eDCs. Compared with transduced eDCs, the controls showed a low level of proliferation in unstimulated splenocytes (C57BL/6 origin) in contrast to a high level of proliferation (∼50,000 cpm) in the allogeneic control (C57BL/6:irradiated BALB/c splenocytes, at a ratio of 1:1; Figure 2, A and B).
Figure 2.
Expanded DCs are capable of inducing alloreactive proliferation and TNF-α production after PHA stimulation. We analyzed the capacity of eDCs to induce proliferation in a MLR. Splenocytes (2 × 105) from C57BL/6 mice were co-cultured with irradiated eDCs at variable numbers at the indicated ratios of responder (R) to stimulator (S) cells. A: In the syngeneic setting C57BL/6 splenocytes showed minimal proliferation when exposed to irradiated eDCs of the same origin. B: In contrast, responder cells (C57BL/6 splenocytes) proliferated vigorously against irradiated eDCs of allogeneic origin (BALB/c) in a dose-dependent manner. C: Syngeneic transduced eDCs induced only minimal proliferation and were therefore similar to syngeneic WT eDCs. D: In contrast allogeneic transduced eDCs induced a vigorous proliferation that was similar to the one induced by allogeneic WT eDCs. The controls demonstrated only a low level of proliferation of unstimulated splenocytes (C57BL/6 origin), in contrast to a high level of proliferation (∼30,000 cpm) in the allogeneic control (C57BL/6:irradiated BALB/c splenocytes, at a ratio of 1:1). E: Cytokine production of eDCs from C57BL/6 WT mice, transduced gfp+luc+ eDCs, and eDCs from gfp+ transgenic mice (OSB) was assessed for TNF-α production by cytometric bead array immunoassays. After stimulation with PHA the three groups displayed a comparable high level of TNF-α production of more than 5000 pg/ml. The unstimulated control samples of each group were also comparable, in showing only a low basal level of TNF-α secretion.
Cytokine production of eDCs from C57BL/6 WT mice, transduced gfp+luc+ eDCs, and eDCs from the gfp+ transgenic mice (OSB) was assessed for interferon-γ, TNF-α, IL-2, IL-4, and IL-5 by cytometric bead array immunoassays. To perform these studies, freshly isolated DCs and eDCs (at day 7 after expansion) were evaluated for their cytokine production with and without PHA stimulation in vitro for 48 hours. The major cytokine produced by the eDCs after 7 days of expansion was TNF-α. After stimulation with PHA the three groups (transgenic versus transduced versus WT eDCs) displayed a comparable high level of TNF-α production (Figure 2C). The unstimulated control samples of each group were also comparable, in showing only low levels of basal TNF-α secretion. Furthermore, we found low levels of TNF-α production in freshly isolated DCs (day 0) ranging between 73 to 100 pg/ml. No significant production of interferon-γ, IL-2, IL-4, or IL-5 was detected in the samples with and without PHA stimulation.
Ex Vivo eDCs Home to T Zones of Lymphoid Organs and Survive in Vivo for up to 42 Days
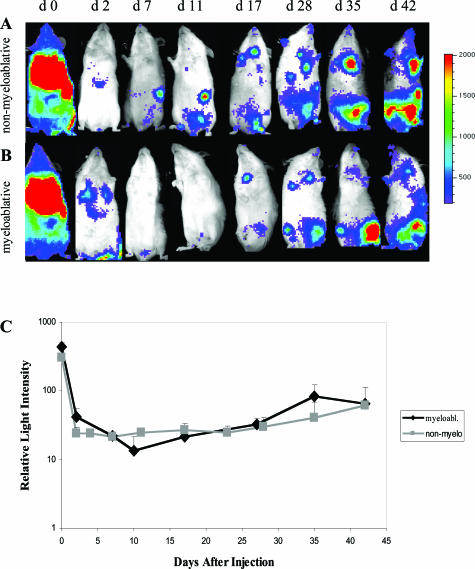
Transduced C57BL/6 gfp+luc+ eDCs were injected intravenously into BALB/C recipient mice that had received an allogeneic BMT 6 to 8 weeks previously. This time point was chosen to minimize effects of preconditioning on the trafficking of eDCs. Myeloablative versus nonmyeloablative conditioning regimens were used to investigate the influence of mixed chimerism versus complete chimerism on the trafficking patterns and survival of eDCs. Luc+ eDCs were tracked in vivo by using BLI. In both treatment groups, 30 minutes after injection of eDCs, bioluminescent signals were detectable from the whole body of the animal with highest intensities appearing throughout the lungs and spleen (Figure 3, A and B). Signal intensities decreased significantly during the next 48 hours and light emission was detectable only from throughout the lungs and liver. In animals that had received a nonmyeloablative BMT, bioluminescent signals reappeared throughout the spleen on day 7 after transfer. From weeks 2 to 6 signal intensities increased in both groups throughout the spleen, thymus, and lower abdomen, suggesting proliferation or accumulation of the eDCs in vivo (Figure 3, A and B). Light intensities were quantitated in both groups, showing no significant differences in the myeloablative group as compared to the nonmyeloablative group (Figure 3C). Of note, none of the animals developed acute or chronic GvHD during the observation period (100 days).
Figure 3.
In vivo BLI of recipients after adoptive transfer of allogeneic eDCs. Displayed are bioluminescent images of a representative animal throughout time (of a group of 10 animals) that had received nonmyeloablative (A) versus myeloablative (B) conditioning followed by injection of gfp+luc+ eDCs. The animals were monitored throughout a time course of 42 days. In vivo BLI was performed on eight different time points (A, B) as indicated. C: Quantification of the in vivo BLI signals (total body emission of photons per animal) showed a similar course of signal strength throughout time, comparing the myeloablative (myeloabl.) to the nonmyeloablative (non-myelo.) conditioning.
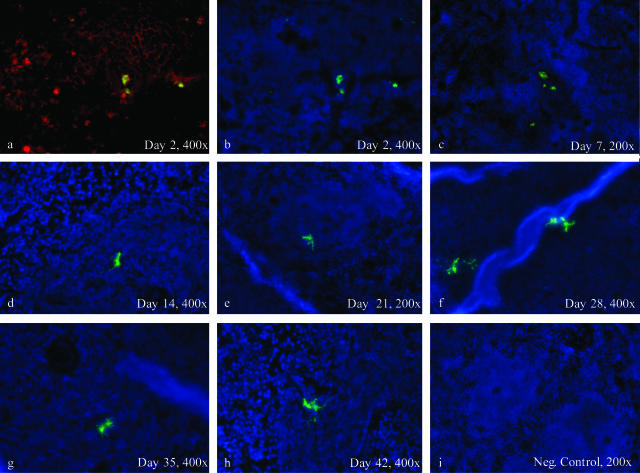
To test if these results were an effect of the retroviral transduction of eDCs with p3-luc, we performed identical experiments with eDCs derived from gfp-transgenic donor mice. In organs harvested at day 42 after injection of transduced gfp+luc+ eDCs or gfp-transgenic eDCs, gfp+ DCs were found in Peyer’s patches, spleen, lymph nodes, and thymus by immunofluorescence microscopy (Figure 4; a to h). In the Peyer’s patches we observed gfp-transgenic and gfp+luc+ eDCs in parafollicular and subepithelial dome regions. The gfp+ eDCs co-stained with anti-CD11c (Figure 4, a and b). Occasionally, we observed gfp+ eDCs interacting with gfp−DCs. In contrast the B cell follicles of Peyer’s patches were clearly spared. In mesenteric lymph nodes and thymus we found that transduced eDCs were localized more frequently in subcapsular regions, whereas transgenic eDCs demonstrated a more dispersed distribution in cortical and subcortical regions of these organs (Figure 4; c, d, g, h). Tissues from liver and skin of all experimental mice remained negative for eDCs throughout the observation period (shown are representative samples of day 42 after transfer; Figure 4, m and n). All of the control tissues from irradiated BALB/c mice were completely negative for gfp (Figure 4; i to l).
Figure 4.
Detection of gfp-positive transduced and transgenic eDCs in allogeneic recipients by immunofluorescence microscopy at day 42 after adoptive transfer. eDCs could be observed for up to 6 weeks (42 days) after transplantation in different lymphatic organs such as the spleen, the peripheral lymph nodes, and the Peyer’s patches (PPs). EDCs were found in locations typical for myeloid DCs as for instance the parafollicular T-cell zones of PPs (a, e) and the periarteriolar lymphoid sheaths (PALS) of the spleen (b, f). Co-stainings with a phycoerythrin-labeled antibody conjugate (red) directed against the DC-specific murine surface marker CD11c showed membrane-bound positivity of eDCs (a, b). Furthermore, eDCs at many spots were found in close proximity to CD11c-positive but gfp-negative DCs, while B-cell follicles were clearly spared (a, b). Morphology and localization of eDCs were similar, comparing samples of mice that had received transduced gfp+luc+ eDCs (a–d) to mice that had received transgenic gfp+ eDCs (e–h). Control samples of BALB/c mice, which had received the same preconditioning regimen as the other groups without adoptive transfer, did not show any gfp+ cells, while the autofluorescence background in general was rather low (i–l). Insets show digitally amplified magnifications of gfp+ eDCs (e–h). Samples of skin and liver were negative for transduced and transgenic eDCs throughout the observation period. Shown are representative samples on day 42 after transfer of transgenic eDCs (m, n).
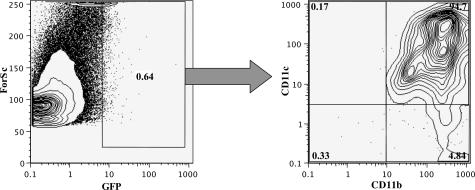
The proportion of gfp+ eDCs in the spleen of representative transplanted animals on day 42 was quantified by flow cytometry. Although the percentage of gfp+ eDCs was rather low (∼1%), specific gating on this gfp+ population revealed a clear population of CD11c+ and CD11b+ cells (Figure 5). Furthermore, gfp+ eDCs were detected by immunostaining and fluorescence microscopy, in frozen tissues of the spleen throughout a time course of 42 days (Figure 6). On days 2 and 7 most of the eDCs were located in perivascular regions, showing a morphology of oval- to polygonal-shaped cells with bean-like nuclei (Figure 6, b and c). In addition to their positivity for gfp these cells also displayed membrane-bound CD11c (Figure 6a). From day 14 until day 42, the eDCs displayed long extended dendrons and were found within the periarteriolar lymphoid sheaths of the spleen (Figure 6; d to h). Irradiation controls, as well as tissues of WT BALB/c and C57BL/6 mice did not show any gfp+ cells (Figure 6i).
Figure 5.
The gfp+ donor cells show typical DC markers at day 42 after adoptive transfer. Spleens of recipient animals were harvested on day 42 after adoptive transfer of gfp+ transgenic eDCs and analyzed for their gfp expression by fluorescence-activated cell sorting. Gating on the gfp+ cells (left) showed that the majority (94%) were consisting of CD11c+ and CD11b+ cells (right).
Figure 6.
Time course of eDC migration in the spleens of allogeneic recipients. b to h:gfp+ eDCs (shown in green) could be observed in the periarteriolar lymphatic sheaths (PALS) of the spleen, at all seven time points analyzed in this study (day 2 until day 42). a to c: Although the morphology of eDCs on day 2 and day 7 (b, c) appears somehow immature, by showing a rather macrophage-like phenotype (oval to polygonal shape), immunofluorescence stainings showed a clear positivity (red) for CD11c (a). d: From day 14 on, gfp+ eDCs showed a more mature phenotype with long extended dendrons. e–h: This phenotype could be observed throughout day 42 (6 weeks), while eDCs were still located in the PALS. i: The control tissue of an untreated BALB/c mouse, which had received the same conditioning regimen, without transfer of eDCs, was completely negative in the green channel. Overall the signal-to-noise (background) ratio was successfully enhanced by applying an additional immunofluorescence staining, using an anti-gfp-Alexa-488 conjugate. b to i: Nuclei were counterstained with 4,6-diamidino-2-phenylindole, displayed in blue.
Discussion
Autologous ex vivo expanded DCs are capable of generating potent immunological reactions and are being used in a broad range of immunotherapeutic protocols. Due to the low numbers of DCs in the peripheral blood in humans these cells have been expanded with cytokines for adoptive immunotherapy.9 Despite the use of autologous eDCs for vaccination little is known about the biological activity, survival, and trafficking of eDCs after injection into allogeneic hosts. This knowledge is clearly needed for future adoptive immune therapies using eDCs.
In the present study we investigated the survival and trafficking of allogeneic murine eDCs in recipients that had received allogeneic BMT. To visualize migration, homing, and survival we used DCs that were expanded in the presence of GM-CSF, IL-4, and FLT3L and transduced with a dual retroviral reporter gene encoding gfp and luc. This approach allowed us to detect transduced gfp+luc+ eDCs in living animals by applying BLI. Furthermore it helped to guide specific tissue sampling for flow cytometric analysis and immunofluorescence microscopy.37–40 Because myeloablative or a nonmyeloablative conditioning before allogeneic BMT influences the kinetics of chimerism and the development of tolerance, the type of conditioning might affect survival and trafficking of allogeneic eDCs.41,42 For this reason the experiments were performed under both conditions.
Depleted BM cells expanded ex vivo with GM-CSF, IL-4, and Flt3L differentiated mainly into two cell populations of CD11c+CD11b+ or CD11c−CD11b+ cells. These DC populations expressed co-stimulatory molecules such as CD80, CD86, and CD40, did not express CD8 or B220 and were potent stimulators of allogeneic T cells in MLR experiments. These data indicate that the expanded DCs in a large part consisted of mature DCs, capable of mounting a strong immune response. This was also supported by the findings of cytokine analyses, showing that eDCs produced large amounts of TNF-α after stimulation with PHA. The phenotype and the type of cytokine production were not altered by retroviral transduction when compared to eDCs of WT or transgenic origin. Furthermore, transduction did not alter the capacity of eDCs to induce alloreactive proliferation in an MLR, comparing allogeneic transduced eDCs to allogeneic WT eDCs.
Signals from gfp+luc+ eDCs initially localized to regions throughout the lungs, and later in the course to abdominal sites, thymus, and spleen. Initially after injection the total light emission decreased significantly, followed by an increase in signal intensity indicative of cellular proliferation in vivo. Analysis of BLI-positive organs with immunostaining and fluorescence microscopy confirmed infiltration of transduced eDCs in lymphoid tissues including Peyer’s patches, lymph nodes, spleen, and thymus. These donor-derived gfp+ transgenic and transduced eDCs were found in typical localizations as the periarteriolar lymphoid sheaths of the spleen and subcortical T-cell zones of lymph nodes, indicating that their migration capacities were conserved. Only slight differences were observed for mesenteric lymph nodes and thymus in which transduced eDCs were found more often in subcapsular regions whereas transgenic eDCs showed a more dispersed distribution in cortex and paracortex. We did not observe differences in the migration pattern of eDCs comparing mice preconditioned with either myeloablative or nonmyeloablative conditioning.
Previous studies have suggested that DCs have a relatively short half life in the range of 2 to 9 days.43,44 In contrast, transduced gfp+luc+ eDCs in our study were detectable with BLI for at least 42 days and in some animals for up to 100 days. However, there have also been reports in the literature, that donor DCs can be detected in recipients for a significantly longer period of time.45,46 Rastellini and co-workers47 expanded liver-derived DCs with GM-CSF and injected these cells into allogeneic recipients 7 days before transplantation of pancreatic islets from the same donor strain. Donor MHC class II+ cells with distinct DC morphology were first observed in the spleen 24 hours after the injection and maximal numbers were seen on day 5. Thereafter the mean number declined but donor-derived MHC II+ cells were detectable for at least 2 months. These cells were detected in close proximity to arterioles in T-cell-dependent zones and were not observed in islet graft recipients pretreated with syngeneic, liver-derived DC progenitors.47 Furthermore a recent report by Zhang and co-workers48 described, that ex vivo expanded mature DCs can proliferate in co-culture with splenic stroma cells and differentiate into a regulatory subset. This report underlines that the dogma of mature DCs as being end-stage cells that always die within a few days should be modified. It furthermore supports our findings that eDCs home specifically to the periarteriolar lymphatic sheaths suggesting that these lymphoid compartments might have a special capacity in enabling long-term DC survival.
Bioluminescence is an ATP-dependent process and therefore linked to the metabolic activity of the cells. The decrease in signal shortly after injection of transduced eDCs followed by a modest increase in signal intensity suggests that a majority of transferred cells might have died during the first 48 hours after injection and that only a small fraction of eDCs survived and potentially proliferated. Stimulation of BM using GM-CSF, IL-4, and FLT3L induced polyclonal expansion of eDCs leading mainly to CD11c+CD11b+ and CD11c−CD11b+ cells. However, we also saw a small population of cells that expressed CD80, CD86, and CD40 but were negative for CD11c and CD11b (data not shown). These cells might represent a population of DC precursors, still existent after the expansion of DCs that could be capable to mature and survive long term. The question which compartment of polyclonal eDCs is capable of long-term engraftment and survival is of significant interest for adoptive immuno-therapy. This question can only be answered using highly purified CD11c+CD11b+, CD11c−CD11b+, and CD11c−CD11b− cells, which is the focus of ongoing studies.
After BMT very little is known about the effects and interactions of adoptively transferred donor-derived DCs in the host. Shlomchik and co-workers49 and Zhang and colleagues50 showed that the balance of donor- and host-derived DCs is crucial for the development of GvHD and that depletion of host-derived DCs leads to a reduction in GvHD risk. Recently, Merad and colleagues51 showed that depletion of host Langerhans cells before transplantation of alloreactive donor T-cells prevents skin GvHD. However, it has also been reported that host-derived DCs expanded in the presence of IL-10 protect animals from GvHD and leukemic relapse in an allogeneic BMT model.49,50,52 Interestingly, in our experiments administration of donor-derived eDCs did not induce clinical or subclinical signs of GvHD as measured by survival, weight loss, changes of fur tone, diarrhea, or histological alterations. Furthermore the lack of signal of in vivo BLI throughout the ears combined with negative findings of immunofluorescence on corresponding skin samples supported the view, that eDCs did not exhibit skin homing.22 These observations correspond well with the recent findings of Matte and colleagues,53 who showed that allogeneic donor DCs do not cause GvHD on their own, but are capable of intensifying the severity of GvHD, once it is initiated by host antigen-presenting cells.
To our knowledge, this is the first report investigating the serial trafficking patterns and survival of adoptively transferred allogeneic donor eDCs in mice that had received an allogeneic BMT. We demonstrated that ex vivo expanded DCs maintain their capability of homing to lymphoid organs and are detectable for at least 6 weeks in allogeneic transplant recipients. The type of conditioning did not appear to influence migration patterns and survival. Furthermore, injection of allogeneic eDCs did not induce GvHD. In summary, the findings of this study show that eDCs home to lymphatic organs and in part survive long term. This is of importance for adoptive immunotherapies because it indicates that eDCs could sustain desired clinical effects in a long-term manner.
Acknowledgments
We thank Louis Soares, Ph.D., for providing the p3-luc vector and his support in all technical details; Andreas Beilhack, M.D., for generously supporting us with transgenic mice; Pia Bjorck for helpful suggestions and critical review; and Marie Joe Mont Renault for excellent technical assistance.
Footnotes
Address reprint requests to Robert S. Negrin, M.D., Center for Clinical Science Building, Room 2205, 269 West Campus Dr., Stanford, CA 94305. E-mail: negrs@stanford.edu.
Supported in parts by grants from the Deutsche Krebshilfe (to C.H.S.), the Akademie der Naturforscher Leopoldina (to S.S.), and the National Institutes of Health (grant R24 CA 92862 to C.H.C. and R.S.N.).
C.H.S. and S.S. contributed equally to this study.
References
- Horrowitz M. Uses and growth of hematopoietic cell transplantation. Thomas ED, Blume KG, Forman SJ, Appelbaum FR, editors. Malden: Blackwell Publishers; Thomas’ Hematopoietic Cell Transplantation. 2004:pp 9–15. [Google Scholar]
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, van Rhee F, Mittermueller J, de Witte T, Holler E, Ansari H. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- Kolb HJ, Holler E. Adoptive immunotherapy with donor lymphocyte transfusions. Curr Opin Oncol. 1997;9:139–145. doi: 10.1097/00001622-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- Moser M. Dendritic cells. Paul WE, editor. Philadelphia: Lippincott Williams & Wilkins; Fundamental Immunology. 2003:pp 455–480. [Google Scholar]
- O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- Stift A, Friedl J, Dubsky P, Bachleitner-Hofmann T, Schueller G, Zontsich T, Benkoe T, Radelbauer K, Brostjan C, Jakesz R, Gnant M. Dendritic cell-based vaccination in solid cancer. J Clin Oncol. 2003;21:135–142. doi: 10.1200/JCO.2003.02.135. [DOI] [PubMed] [Google Scholar]
- Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21:873–886. doi: 10.1081/cnv-120025091. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell PJ, Li W, Wang Z, Specht SM, Logar AJ, Thomson AW. Immature and mature CD8{alpha}+ dendritic cells prolong the survival of vascularized heart allografts. J Immunol. 2002;168:143–154. doi: 10.4049/jimmunol.168.1.143. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Bonham CA, Qian S, Zhou Z, Peng L, Harnaha J, Li W, Thomson AW, Fung JJ, Robbins PD, Lu L. Prolongation of cardiac allograft survival using dendritic cells treated with NF-kB decoy oligodeoxyribonucleotides. Mol Ther. 2000;1:430–437. doi: 10.1006/mthe.2000.0060. [DOI] [PubMed] [Google Scholar]
- Bonham CA, Peng L, Liang X, Chen Z, Wang L, Ma L, Hackstein H, Robbins PD, Thomson AW, Fung JJ, Qian S, Lu L. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J Immunol. 2002;169:3382–3391. doi: 10.4049/jimmunol.169.6.3382. [DOI] [PubMed] [Google Scholar]
- Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, Fung JJ, Thomson AW. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–665. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley DB, Whyte LF, Carnoutsos SA, Cook AH, Hart DNJ. Monitoring human blood dendritic cell numbers in normal individuals and in stem cell transplantation. Blood. 1999;93:728–736. [PubMed] [Google Scholar]
- Traver D, Akashi K, Manz M, Merad M, Miyamoto T, Engleman EG, Weissman IL. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–2154. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- Leon B, Martinez del Hoyo G, Parrillas V, Hernandez Vargas H, Sanchez-Mateos P, Longo N, Lopez-Bravo M, Ardavin C. Dendritic cell differentiation potential of mouse monocytes: monocytes represent immediate precursors of CD8− and CD8+ splenic dendritic cells. Blood. 2004;103:2001–0286. doi: 10.1182/blood-2003-01-0286. [DOI] [PubMed] [Google Scholar]
- Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH, Negrin RS. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C, Ait-Yahia S, Chemin K, de Bouteiller O, Dieu-Nosjean MC, Homey B, Massacrier C, Vanbervliet B, Zlotnik A, Vicari A. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–369. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin BL, Morelli AE, Logar AJ, Lau AH, Thomson AW. Comparative evaluation of CC chemokine-induced migration of murine CD8alpha+ and CD8alpha− dendritic cells and their in vivo trafficking. J Leukoc Biol. 2004;75:275–285. doi: 10.1189/jlb.1202613. [DOI] [PubMed] [Google Scholar]
- Serafini B, Columba-Cabezas S, Di Rosa F, Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol. 2000;157:1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middel P, Thelen P, Blaschke S, Polzien F, Reich K, Blaschke V, Wrede A, Hummel KM, Gunawan B, Radzun HJ. Expression of the T-cell chemoattractant chemokine lymphotactin in Crohn’s disease. Am J Pathol. 2001;159:1751–1761. doi: 10.1016/S0002-9440(10)63022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Zeng D, Shizuru J, Gworek J, Dejbakhsh-Jones S, Taniguchi M, Strober S. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169:5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- Takayama T, Tahara H, Thomson AW. Transduction of dendritic cell progenitors with a retroviral vector encoding viral interleukin-10 and enhanced green fluorescent protein allows purification of poten-tially tolerogenic antigen-presenting cells. Transplantation. 1999;68:1903–1909. doi: 10.1097/00007890-199912270-00015. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa GL, Benson JM, Seroogy CM, Achacoso P, Fathman CG, Nolan GP. Targeting rare populations of murine antigen-specific T lymphocytes by retroviral transduction for potential application in gene therapy for autoimmune disease. J Immunol. 2000;164:3581–3590. doi: 10.4049/jimmunol.164.7.3581. [DOI] [PubMed] [Google Scholar]
- Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- Cook EB, Stahl JL, Lowe L, Chen R, Morgan E, Wilson J, Varro R, Chan A, Graziano FM, Barney NP. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non-allergics. J Immunol Methods. 2001;254:109–118. doi: 10.1016/s0022-1759(01)00407-0. [DOI] [PubMed] [Google Scholar]
- Khanna A, Plummer M, Bromberek K, Woodliff J, Hariharan S. Immunomodulation in stable renal transplant recipients with concomitant tacrolimus and sirolimus therapy. Med Immunol. 2002;1:3. doi: 10.1186/1476-9433-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice BW, Cable MD, Nelson MB. In vivo imaging of light-emitting probes. J Biomed Opt. 2001;6:432–440. doi: 10.1117/1.1413210. [DOI] [PubMed] [Google Scholar]
- Jockusch H, Voigt S, Eberhard D. Localization of GFP in frozen sections from unfixed mouse tissues: immobilization of a highly soluble marker protein by formaldehyde vapor. J Histochem Cytochem. 2003;51:401–404. doi: 10.1177/002215540305100315. [DOI] [PubMed] [Google Scholar]
- Mandl S, Schimmelpfennig C, Edinger M, Negrin RS, Contag CH. Understanding immune cell trafficking patterns via in vivo bioluminescence imaging. J Cell Biochem Suppl. 2002;39:239–248. doi: 10.1002/jcb.10454. [DOI] [PubMed] [Google Scholar]
- Edinger M, Cao YA, Hornig YS, Jenkins DE, Verneris MR, Bachmann MH, Negrin RS, Contag CH. Advancing animal models of neoplasia through in vivo bioluminescence imaging. Eur J Cancer. 2002;38:2128–2136. doi: 10.1016/s0959-8049(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Sweeney TJ, Mailander V, Tucker AA, Olomu AB, Zhang W, Cao Y, Negrin RS, Contag CH. Visualizing the kinetics of tumor-cell clearance in living animals. Proc Natl Acad Sci USA. 1999;96:12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger M, Sweeney TJ, Tucker AA, Olomu AB, Negrin RS, Contag CH. Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia. 1999;1:303–310. doi: 10.1038/sj.neo.7900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004;13:117–130. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Claas F. Chimerism as a tool to induce clinical transplantation tolerance. Curr Opin Immunol. 2004;16:578–583. doi: 10.1016/j.coi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- Kamath AT, Pooley J, O’Keeffe MA, Vremec D, Zhan Y, Lew AM, D’Amico A, Wu L, Tough DF, Shortman K. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165:6762–6770. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- Garg S, Oran A, Wajchman J, Sasaki S, Maris CH, Kapp JA, Jacob J. Genetic tagging shows increased frequency and longevity of antigen-presenting, skin-derived dendritic cells in vivo. Nat Immunol. 2003;4:907–912. doi: 10.1038/ni962. [DOI] [PubMed] [Google Scholar]
- Hon H, Rucker EB, III, Hennighausen L, Jacob J. bcl-xL is critical for dendritic cell survival in vivo. J Immunol. 2004;173:4425–4432. doi: 10.4049/jimmunol.173.7.4425. [DOI] [PubMed] [Google Scholar]
- Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation. 1995;60:1366–1370. [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Tang H, Guo Z, An H, Zhu X, Song W, Guo J, Huang X, Chen T, Wang J, Cao X. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shlomchik WD, Joe G, Louboutin J-P, Zhu J, Rivera A, Giannola D, Emerson SG. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J Immunol. 2002;169:7111–7118. doi: 10.4049/jimmunol.169.12.7111. [DOI] [PubMed] [Google Scholar]
- Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–379. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]