The unusual form of dementia described ∼100 years ago by Alois Alzheimer, now known as Alzheimer’s disease (AD), has become a scourge in the 21st century due to the unprecedented increase in human life expectancy since 1900, when AD was an uncommon cause of dementia. Indeed, because the risk of developing AD increases exponentially beyond the seventh decade of life, the prevalence of this neurodegenerative disorder will continue to rise inexorably in the coming decades unless effective interventions can delay its onset or retard its progression. The widespread international recognition of the urgency of this problem has accelerated efforts to translate the remarkable discoveries throughout the past 20 years from research on AD into meaningful therapies for this disorder. For example, advances in understanding mechanisms underlying the conversion of normal soluble tau and Aβ into insoluble fibrils that form neurofibrillary tangles (NFTs) and senile plaques (SPs), respectively, are beginning to clarify how these hallmark lesions of AD contribute to the dysfunction and degeneration of selectively vulnerable neurons in the AD brain. These new insights have led to the identification of multiple novel targets for AD drug discovery to block or reverse Aβ amyloidosis and, more recently, to develop tau-focused therapeutic interventions for the treatment of AD and related tauopathies.
AD and many other aging-related neurodegenerative disorders appear to share common disease mechanisms because they are known to associate with the pathological aggregation of proteins that misfold and accumulate as fibrillar amyloid deposits in selectively vulnerable regions of the central nervous system.1–3 For example, NFTs and SPs, the two diagnostic hallmark lesions of AD that were first described by Alois Alzheimer at the beginning of the 20th century, are formed by intraneuronal accumulations of abnormal tau filaments and extracellular deposits of Aβ fibrils, respectively.1–3 These lesions have long been thought to compromise the function as well as the viability of neurons, and a growing body of evidence supports this, including the discovery of mutations in the genes encoding tau, Aβ precursor proteins (APP), and presenilins that are pathogenic for familial neurodegenerative disorders. Nonetheless, the exact mechanisms whereby brain degeneration results from NFTs and SPs remain incompletely understood.1–3 However, although most AD drug discovery efforts have focused on ameliorating Aβ pathologies, a growing body of evidence supporting the hypothesis that abnormal tau is causally linked to AD neurodegeneration has stimulated increasing interest in mechanisms leading to the formation of tau pathology as tractable targets for developing novel AD therapies.
As reviewed in more detail elsewhere,2,3 the Aβ-centric focus of most current AD drug discovery efforts reflects remarkable progress in understanding the fibrillization and aggregation of Aβ to form SPs as well as how these events contribute to the pathogenesis of AD. The most direct evidence implicating Aβ in the pathogenesis of AD has come from studies of gene mutations that cause autosomal dominantly inherited forms of familial AD. Most but not all of these mutations lead to increased production and accumulation of specific Aβ species (Aβ42), either through effects on APP itself, or through effects on presenilin 1 or 2, which form part of γ-secretase, one of the proteolytic complexes that cleaves APP to generate Aβ.2,3 These and many other observations support the Aβ amyloid cascade hypothesis of AD that was proposed >10 years ago and predict that increased production, aggregation, and accumulation of Aβ initiates a cascade of events leading to neurotoxicity and eventually to clinical symptoms of AD.2,3 Accordingly, there has been great interest in developing therapies and diagnostic tools aimed at Aβ, and most drugs currently in development or clinical trials for AD have been designed to target one or more mechanisms of Aβ amyloidosis such as by inhibiting or reducing the production of amyloidogenic Aβ peptides or by promoting the clearance of SPs and Aβ fibrils.2,3 Thus, inhibitors of γ- and β-secretases, passive and active Aβ vaccines, metal-binding drugs such as clioquinol, statins, nonsteroidal anti-inflammatory drugs such as flurbiprofen, and glycosaminoglycan mimetics are among the compounds that are in various stages of preclinical or clinical testing.2,3
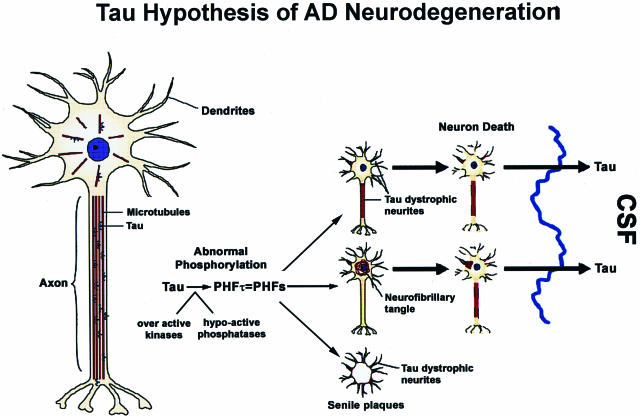
The tau hypothesis of AD neurodegeneration emerged from studies of the pathobiology of NFTs as well as from research into the normal biology and functions of tau.1,2 Six soluble tau isoforms are expressed in the normal adult human brain and are generated from a single gene by alternative splicing. It was known that normal tau binds and stabilizes microtubules (MTs) thereby maintaining the network of MTs that are essential for axonal transport in neurons.1,2 Moreover, the phosphorylation of tau was known to negatively regulate the binding of all six tau isoforms to MTs. Thus, when we identified abnormally phosphorylated tau at the amino acid sequence level as the building blocks or subunit proteins of the paired helical filaments (PHFs) that form AD NFTs,4 we quickly appreciated that the conversion of normal tau into PHFs (known as PHFtau) could result in a loss of tau function.5,6 Specifically, unlike normal tau, PHFtau no longer had the ability to bind MTs. Based on the wealth of information on the normal biology of tau and MTs available at the time, we inferred that the pathological conversion of tau into PHFtau in AD could lead to the depolymerization of MTs, thereby impairing axonal transport followed by neurodegeneration, and we therefore proposed a hypothetical model of PHFtau-mediated neurodegeneration in AD.7 The major predictions of this tau hypothesis of AD neurodegeneration were that by converting normal tau into functionally impaired PHFtau, MTs would be unstable and depolymerize, thereby disrupting MT-based axonal transport and that the attendant failure of affected neurons to export proteins from the cell body to distal processes and to retrieve substances (eg, trophic factors) internalized at axon terminals would compromise the function and viability of these neurons. Thus, as schematically illustrated in Figure 1, we proposed that these events would culminate in neuronal dysfunction and degeneration, leading to the onset/progression of AD.
Figure 1.
The misfolding, fibrillization, and sequestration of tau into filamentous inclusions is schematically depicted and is described in greater detail in the text. Proceeding from the normal neuron on the left, this tau hypothesis of AD neurodegeneration predicts that the cascade of events depicted schematically will compromise the function and viability of neurons (shown to the right) through the pathological conversion of normal tau into PHFtau, which forms NFTs and dystrophic tau neurites thereby depleting levels of functional MT-binding/stabilizing tau below a critical point that results in the depolymerization of MTs and a disruption of axonal transport. As shown on the far right, the dissolution of degenerating tangle and dystrophic neurite-bearing neurons in the AD brain releases abnormal tau into the extracellular space resulting in elevated levels of cerebrospinal fluid (CSF) tau, which is one of the most robust biomarkers of AD in living patients.
Since the most significant predictions of this tau hypothesis of AD neurodegeneration were that tau pathologies would cause brain degeneration by impairing intraneuronal transport thereby compromising the function and viability of affected cells, we subsequently proposed that MT-stabilizing compounds such as the Food and Drug Administration-approved anti-cancer drug paclitaxel could be used for the treatment of AD by offsetting the loss of tau function after its conversion into PHFtau.8 However, the tau hypothesis of AD neurodegeneration did not garner broad support until recently, and a significant catalyst that led to a reassessment of this hypothesis came from a series of remarkable discoveries beginning in 1998. These studies showed that mutations in the gene encoding tau were pathogenic for hereditary frontotemporal dementia (FTD) with parkinsonism linked to chromosome 17 (FTDP-17) syndromes, all of which are characterized by prominent tau pathologies in the absence of significant amounts of other disease-specific amyloid lesions.9–12 Thus, just like the discoveries of genetic mutations pathogenic for familial AD, these findings on the genetic basis of FTDP-17 provided compelling evidence that tau abnormalities were sufficient to cause neurodegenerative disease. Indeed, this notion was supported further by evidence from follow-up studies showing that a number of tau gene mutations pathogenic for FTDP-17 resulted in loss of tau functions or gain of potentially toxic properties, such as an increased amyloidogenic propensity for mutant tau isoforms.13 Other more recent catalysts for dispelling doubt about the critical importance of tau pathologies as mediators of neurodegeneration in AD and other neurodegenerative disorders have been the successful development of worm, fly, and mouse models of tau pathologies that show close similarity to their authentic human counterparts.14 For these reasons, sporadic FTDs characterized by prominent tau lesions, familial FTDP-17 syndromes, and the pathological significance of tau abnormalities for mechanisms of brain degeneration have become increasingly intense focuses of basic and clinical research.
Thus, there is a growing body of evidence from diverse lines of research indicating that brain degeneration in AD is mediated by tau pathologies, and we propose that this could be a major consequence of impaired intraneuronal transport resulting from loss of function defects in tau and/or from toxic gain of functions by pathologically altered tau proteins, including their propensity to misfold, fibrillize, and form AD NFTs in neurons. Indeed, axonal transport failure may be an underlying mechanism of a number of other neurodegenerative disorders, some of which are caused by pathogenic mutations in genes encoding proteins that serve as molecular motors for axonal transport.15
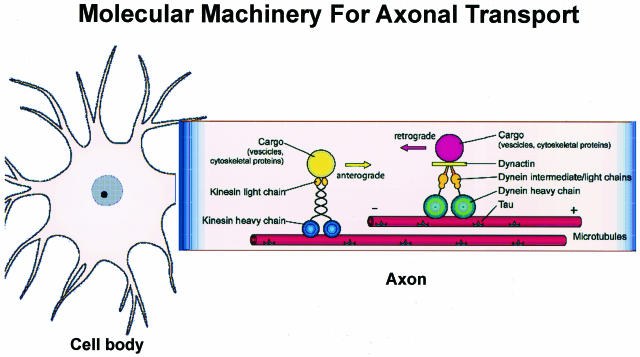
A schematic depiction of the currently understood molecular underpinnings of retrograde and anterograde axonal transport is shown in Figure 2.15,16 This figure illustrates the three major elements of the axonal transport machinery: 1) the transported cargo (ie, vesicles, other organelles, proteins, and so forth); 2) the motor proteins that move the cargo (ie, kinesins, dynein, dynactin, etc); and 3) the rails on which the cargo moves (ie, MTs). However, Figure 2 does not fully illustrate the complexity of the axonal transport system of neurons because this system also includes numerous other components such as the many adaptor/linker and regulatory proteins that are involved in the translocation of cargo away from (anterograde) or toward (retrograde) the cell body of a neuron.15,16 Because nearly all protein synthesis occurs in the perikarya of neurons, defects in any one of the three major components of the axonal transport system described above could impair axonal transport and result in the dysfunction and death of neurons. Thus, axonal transport has been hypothesized to be implicated in mechanisms of several neurodegenerative diseases as summarized in Table 1. Moreover, increasing support for this hypothesis has come from the discovery of mutations in genes encoding motor proteins, contributing to human neurodegenerative diseases, as well as from emerging evidence for axonal transport defects in cell culture and animal models of neurodegenerative disorders, despite controversies on the role of some neurodegenerative disease proteins in modulating axonal transport.15,17–19 Accordingly, because tau is a well characterized molecular component of the axonal transport machinery that has been shown to play a critical role in maintaining the MT network required for axonal transport, it is highly plausible that the tau abnormalities associated with AD and other neurodegenerative tauopathies, including hereditary FTDP-17 syndromes caused by tau gene mutations, could disrupt axonal transport. Thus, by analogy with a railway transportation system, tau functions like the cross ties on railroad tracks (MTs) on which trains (molecular motors) convey cargo (vesicles, other organelles, proteins) to and from nodes on the railway network (destinations in neuronal perikarya and their processes) so that the loss of a critical number of trestle cross ties (the consequence of converting tau into PHFtau) would result in the buckling or splaying of the railroad tracks leading to derailment of trains and the failure to deliver cargo to assigned destinations (impaired axonal transport) with deleterious consequences for the railway network and the communities it serves (the dysfunction and death of affected neurons).
Figure 2.
The molecular machinery underlying mechanisms of anterograde (directed toward the + end of MTs) and retrograde (directed toward the − end of MTs) axonal transport in neurons by kinesin and dynein motor proteins, respectively, is schematically illustrated. Kinesins and dyneins and other motor-associated proteins move on MTs to transport Golgi-derived vesicles, cytosolic protein complexes, cytoskeletal polymers, and so forth (for further details and additional citations, see Roy et al15).
Table 1.
Neurodegenerative Diseases Linked to Impairments in Axonal Transport
| Neurodegenerative disease | Mutant gene/disease protein |
|---|---|
| Hereditary spastic paraplegia | Kinesin heavy chain (Kif 5a) |
| Familial motor neuron disease | Dynactin p150 subunit |
| Charcot-Marie-Tooth disease type 2A | Kinesin Kif 1B β subunit |
| Huntington’s disease | Huntingtin |
| Familial amyotrophic lateral sclerosis | Superoxide dismutase 1 |
| Parkinson’s disease and related synucleinopathies | α-synuclein |
| Alzheimer’s disease | APP, presenilins, tau |
| Neurodegenerative tauopathies | tau |
This table summarizes neurodegenerative diseases potentially linked to impairments in axonal transport as well as their mutant or disease proteins (see Roy et al15 for further details and additional citations).
To test the hypothesis that MT-stabilizing drugs could have therapeutic benefit in AD and related human tauopathies by offsetting the loss of normal tau functions resulting from its hyperphosphorylation and sequestration into tangles, we investigated this possibility using an animal model of filamentous tau pathology.20 Significantly, these studies provided the first proof of concept data validating the use of MT-stabilizing drugs to treat AD and related tauopathies.20 Briefly, transgenic (Tg) mice (PrPT44) that model the neuropathology of human neurodegenerative tauopathies21,22 were treated weekly with intraperitoneal injections of paclitaxel in a micelle vehicle (Paxceed; Angiotech Pharmaceuticals, Inc., Vancouver, British Columbia, Canada) or the micelle vehicle alone for 12 weeks beginning at 9 months of age when disease onset is first detected. Previous studies of these Tg mice showed that they developed filamentous tau inclusions in the spinal cord at disease onset in association with reduced numbers of MTs, reduced fast axonal transport, and impaired motor behavior.21 However, treatment with Paxceed restored fast axonal transport, increased axonal MTs, and ameliorated motor impairments in tau Tg mice, thereby suggesting that MT-stabilizing drugs could have therapeutic benefit for human tauopathies. Notably, the doses of paclitaxel used in the study of Zhang and colleagues20 were far lower than those used to treat cancer, and the treated mice did not show any evidence of toxicity.
Paclitaxel (Taxol) is a complex diterpene obtained from the Pacific yew (Taxus brevifolia), especially from the bark of the yew tree, and paclitaxel is the most well studied MT binding compound for clinical use (for a review of MT binding/stabilizing drugs, see Trojanowski and colleagues23). Indeed, paclitaxel was the first compound derived from a natural product that was shown to stabilize MTs and inhibit cell replication by arresting mitosis, subsequently leading to apoptosis.23 For example, Taxol has been approved by the Food and Drug Administration for the treatment of a number of malignancies including ovarian, breast, and lung cancers. However, the mechanism of action of paclitaxel and its cytotoxic action for cancer chemotherapy are shared by a growing number of natural products derived from microorganisms, plants, and sponges (Table 2).23 For these reasons, extensive research is being conducted to identify synthetic and/or natural product-derived paclitaxel-like MT-binding compounds that show less toxicity than paclitaxel as well as an improved ability to enter the brain and elude the multidrug resistance efflux pump for better efficacy as therapies for a broad range of malignancies.23
Table 2.
MT Binding/Stabilizing Agents from Natural Products
| MT binding/stabilizng agents | Natural product source |
|---|---|
| Paclitaxel (Taxol) | Pacific yew bark |
| Coumarins and dicoumarol | Diverse plant leaves |
| Dictostatins | Marine sponges |
| Discodermolides | Marine sponges |
| Eleutherobin | Soft ocean coral |
| Sarcodictyins A and B | Soft ocean coral |
| Epothilones A and B | Myxobacteria |
| Laulimalides | Marine sponges |
| Peloruside | Marine sponges |
| Taccalonolides | Tiger whisker plant |
| Tubercidin | Marine sponges, bacteria |
This table lists a number of representative MT-binding/stabilizing compounds and the natural products from whence they are derived (see Trojanowski et al23 for further discussion and additional citations).
Thus, convincing experimental evidence supporting the concept that MT-stabilizing drugs could be novel therapeutic interventions in patients with AD or other tauopathies by counteracting the loss of function effects of tau pathology and maintaining the MT network and fast axonal transport in a mouse model of a neurodegenerative tauopathy may stimulate further research on the potential therapeutic utility of MT binding/stabilizing drugs for treating AD. Although further studies of the pharmacobiology of MT-stabilizing drugs in the central nervous system are needed, the data summarized here are significant for designing treatments for AD and other neurodegenerative tauopathies because they suggest that stabilization of MTs is a “drugable” target in tauopathies. However, follow-up studies are needed to determine whether the toxicity of MT-stabilizing drugs can be minimized by using lower, less frequent doses of these compounds throughout long periods of time while still having therapeutic benefit. Nonetheless, recent studies by Michaelis and colleagues24 and Rice and colleagues25 indicate that derivatives of paclitaxel can be generated that cross the blood brain barrier better than the native molecule, and other in vitro studies suggest that Taxol may ameliorate the toxic effects of Aβ amyloidosis in AD. Hence, MT-stabilizing drugs could counteract the neurotoxicity of both hallmark lesions of AD, ie, filamentous Aβ and tau amyloid lesions. Moreover, other strategies to develop novel therapies that target tau abnormalities in AD already are an increasing focus of AD drug discovery research.26–30 For example, high-throughput screening of a 200,000 compound library using tau anti-aggregation assays has yielded hits that appear attractive for further optimization.27 Other high-throughput screening studies suggest that inhibitors of heat shock protein 90 can reduce tau levels, and this could be of potential therapeutic benefit because tau fibrillization is concentration-dependent.30 Additionally, similar high-throughput screening efforts that target inhibition of tau phosphorylation are likely to be informative, and proof-of-concept studies using LiCl to ameliorate tau pathology by inhibiting glycogen synthase kinase-3 (GSK-3) in a mouse model of a neurodegenerative tauopathy suggest that this is indeed a fruitful avenue for drug discovery efforts.26 Notably, like MT-stabilizing drugs, GSK-3 inhibitors may be able to target pathways leading to Aβ and tau amyloid neurotoxicity in AD.31
In summary, the extensive progress in elucidating mechanisms of neurodegeneration in AD since its initial description by Alois Alzheimer 100 years ago has revealed critical aspects of how the AD brain is “broken,” which provides significant clues on how to “fix” it. Thus, there is a growing sense of optimism about the prospects for developing more effective therapies to treat AD and related neurodegenerative disorders in the near future.
Acknowledgments
We thank our colleagues for their contributions to the work summarized in this article and Mary Leonard for providing expert assistance with the graphic artwork.
Footnotes
Address reprint requests to John Q. Trojanowski, M.D., Ph.D., Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Maloney 3, HUP, 3600 Spruce St., Philadelphia, PA 19104-4283. E-mail: trojanow@mail.med.upenn.edu.
Supported by grants from the National Institutes of Health (AG10124, AG14382, AG17586), the Marian S. Ware Alzheimer Program, and Angiotech Pharmaceuticals, Inc. (microtubule stabilizing interventions for Alzheimer’s disease have been licensed to Angiotech from the University of Pennsylvania).
V.M.Y.L. is the John H. Ware Third Professor for Alzheimer’s Disease Research and J.Q.T. is the William Maul Measy-Truman G. Schnabel Jr. M.D. Professor of Geriatric Medicine and Gerontology.
The Rous-Whipple Award was established by the American Society for Investigative Pathology to recognize a career of outstanding scientific contribution. John Q. Trojanowski, the 2005 recipient of the Rous-Whipple Award, delivered a lecture entitled, “The Alzheimer Brain: Finding out What’s Broken Tells Us How to Fix It” after accepting the award on April 4, 2005 at the annual meeting of the American Society for Investigative Pathology in San Diego, California.
References
- Forman MS, Trojanowski JQ, Lee VM-Y. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Lee VM-Y, Trojanowski JQ: Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol (in press) [DOI] [PubMed] [Google Scholar]
- Suh Y-H, Checler F. Amyloid precursor protein, presenilins and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharmacol Rev. 2002;54:469–525. doi: 10.1124/pr.54.3.469. [DOI] [PubMed] [Google Scholar]
- Lee VM-Y, Balin BJ, Otvos L, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM-Y. Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Bramblett GT, Trojanowski JQ, Lee VM-Y. Regions with abundant neurofibrillary pathology in human brain exhibit a selective reduction in levels of binding-competent tau and the accumulation of abnormal tau-isoforms (A68 proteins). Lab Invest. 1992;66:212–222. [PubMed] [Google Scholar]
- Lee VM-Y, Trojanowski JQ. The disordered neuronal cytoskeleton in Alzheimer’s disease. Curr Opin Neurobiol. 1992;2:653–656. doi: 10.1016/0959-4388(92)90034-i. [DOI] [PubMed] [Google Scholar]
- Lee VM-Y, Daughenbaugh R, Trojanowski JQ. Microtubule stabilizing drugs for the treatment of Alzheimer’s disease. Neurobiol Aging. 1994;15:S87–S89. doi: 10.1016/0197-4580(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Clark LN, Poorkaj P, Wszolek Z, Geschwind DH, Nasreddine ZS, Miller B, Li D, Payami H, Awert F, Markopoulou K, Andreadis A, D’Souza I, Lee VM-Y, Reed L, Trojanowski JQ, Zhukareva V, Bird T, Schellenberg G, Wilhelmsen KC. Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurogenerative disorders linked to chromosome 17. Proc Natl Acad Sci USA. 1998;95:13103–13107. doi: 10.1073/pnas.95.22.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski JQ, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd P, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site-mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TE, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell TR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VM-Y. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- Lee VM-Y, Kenyon TK, Trojanowski JQ. Transgenic animal models of tauopathies. Biochem Biophys Acta. 2005;1739:251–259. doi: 10.1016/j.bbadis.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Roy S, Zhang B, Lee VM-Y, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol. 2003;160:817–821. doi: 10.1083/jcb.200212017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena S, Goldstein LS. Cargo-carrying motor vehicles on the neuronal highway: transport pathways and neurodegenerative disease. J Neurobiol. 2004;58:258–271. doi: 10.1002/neu.10319. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Morfini GA, Lee EB, Farah MH, Szodorai A, DeBoer SR, Koliatsos VE, Kins S, Lee VM-Y, Wong PC, Price DL, Brady ST, Sisodia SS. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited. J Neurosci. 2005;25:2386–2395. doi: 10.1523/JNEUROSCI.3089-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee EB, Xie SX, Joyce S, Li C, Toleikis PM, Lee VM-Y, Trojanowski JQ. Microtubule binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a murine neurodegenerative tauopathy model. Proc Natl Acad Sci USA. 2005;102:227–231. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM-Y. Age-dependent emergence and progression of a tauopathy in transgenic mice engineered to overexpress the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Zhang B, Higuchi M, Yoshiyama Y, Trojanowski JQ, Lee VM-Y. Age dependent induction of congophilic neurofibrillary tau inclusions in tau transgenic mice. Am J Pathol. 2001;158:555–561. doi: 10.1016/S0002-9440(10)63997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Smith AB, III, Huryn D, Lee VM-Y. Microtubule-stabilising drugs for therapy of Alzheimer’s disease and other neurodegenerative disorders with axonal transport impairments. Exp Opin Pharmacother. 2005;6:683–686. doi: 10.1517/14656566.6.5.683. [DOI] [PubMed] [Google Scholar]
- Michaelis ML, Ansar S, Chen Y, Reiff ER, Seyb KI, Himes RH, Audus KL, Georg GI. Beta-amyloid-induced neurodegeneration and protection by structurally diverse microtubule-stabilizing agents. J Pharmacol Exp Ther. 2005;312:659–668. doi: 10.1124/jpet.104.074450. [DOI] [PubMed] [Google Scholar]
- Rice A, Liu Y, Michaelis ML, Himes RH, Georg GI, Audus KL. Chemical modification of paclitaxel (Taxol) reduces P-glycoprotein interactions and increases permeation across the blood-brain barrier in vitro and in situ. J Med Chem. 2005;48:832–838. doi: 10.1021/jm040114b. [DOI] [PubMed] [Google Scholar]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, Lafrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt M, Gazova Z, von Bergen M, Khlistunova I, Wang Y, Hascher A, Mandelkow EM, Biernat J, Mandelkow E. Anthraquinones inhibit tau aggregation and dissolve Alzheimer’s paired helical filaments in vitro and in cells. J Biol Chem. 2005;280:3628–3635. doi: 10.1074/jbc.M410984200. [DOI] [PubMed] [Google Scholar]
- Fillit HM, Refolo LM. Advancing drug discovery for Alzheimer’s disease. Curr Alzheimer Res. 2005;2:105–109. doi: 10.2174/1567205053585729. [DOI] [PubMed] [Google Scholar]
- Pickhardt M, von Bergen M, Gazova Z, Hascher A, Biernat J, Mandelkow EM, Mandelkow E. Screening for inhibitors of tau polymerization. Curr Alzheimer Res. 2005;2:219–226. doi: 10.2174/1567205053585891. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Eriksen J, Kamal A, Burrows F, Kasibhatla S, Eckman CB, Hutton M, Petrucelli L. Development of a high throughput drug screening assay for the detection of changes in tau levels: proof of concept with HSP90 inhibitors. Curr Alzheimer Res. 2005;2:231–239. doi: 10.2174/1567205053585927. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM-Y, Klein PS. GSK-3 alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]