Abstract
Increased angiotensin II (Ang II), matrix metalloproteinase type II (MMP2), and sympathetic activity accompany age-associated arterial remodeling. To analyze this relationship, we infused a low subpressor dose of Ang II into young (8 months old) rats. This increased carotid arterial MMP2 transcription, translation, and activation, as well as transforming growth factor-β1 activity and collagen deposition. A higher Ang II concentration, which increased arterial pressure to that of old (30 months old) untreated rats, produced carotid media thickening and intima infiltration by vascular smooth muscle cells (VSMCs). Ex vivo, Ang II increased MMP2 activity in carotid rings from young rats to that of untreated old rats. Ang II also increased the ability of early passage VSMCs from young rats to invade a synthetic basement membrane, similar to that of untreated VSMCs from old rats. The MMP inhibitor GM6001 and the AT1 receptor antagonist Losartan inhibited these effects. The α-adrenoreceptor agonist phenylephrine increased arterial Ang II protein, causing MMP2 activation and intima and media thickening. Exposure of young VSMCs to phenylephrine in vitro increased Ang II protein and MMP2 activity to the levels of old VSMCs; Losartan abolished these effects. Thus, Ang II-induced effects on MMP2, transforming growth factor-β1, collagen, and VSMCs are central to the arterial remodeling that accompanies advancing age.
Advancing age is the most potent risk factor for the development of hypertension and atherosclerosis, and this risk is linked, in part, to a diffuse arterial intimal thickening.1 This occurs with aging not only in humans but also in most mammalian species.2–5 The thickened intima is infiltrated with vascular smooth muscle cells (VSMCs) in the absence of lipid deposition, macrophages, or leukocytes.4 Other molecules that become altered during aging within the arterial wall include increased angiotensin-converting enzyme (ACE), angiotensin II (Ang II), and its receptor type-1 (AT1), α-adrenoreceptor (α-AR), and endothelin-1 (ET-1).4,6–11
Previous studies from our laboratory demonstrate that matrix metalloproteinase type 2 (MMP2) activity in situ is increased within the aortic intima and inner media of older rodents and nonhuman primates.2,4,12 Further, early passage VSMCs isolated from the aortic media of old rats show an increased ability to invade a matrix-coated membrane, compared to cells from arteries of younger rats,13,14 and this requires the presence of activated MMP2.13 Previous studies suggest that the increased number of VSMCs within the thickened, aged arterial intima might be attributable, in part, to an enhanced invasion potential of VSMCs, mediated by MMP2.2,4,12 Subsequently, we observed that enhanced MMP2 staining co-localizes with that of increased Ang II in VSMCs within the thickened, aged, intima,4 suggesting that Ang II-AT1 signaling may be involved in age-associated arterial remodeling, as it is in hypertension and atherosclerosis.15,16
In the present study, we used an in vivo approach to determine whether chronic administration of Ang II to young rats can induce arterial remodeling with features similar to that which accompanies advancing age. We also studied whether exposure of arterial rings or VSMCs from young rats to Ang II administered ex vivo or in vitro, respectively, induces MMP2 activation and endows these cells with the enhanced invasion capacity characteristic of cells from old rats. Additionally, in an attempt to verify whether the effect of Ang II was specific, and because the sympathetic nervous discharge, which activates Ang II signaling, increases with aging,9,10 we determined whether a chronic infusion of an α-AR agonist, phenylephrine hydrochloride (PE), mimics the effects of Ang II infusion. We also studied whether exposure of young VSMCs to PE in vitro induces MMP2 activation and whether this involves Ang II signaling.
Materials and Methods
Animals
Male Fisher344 × Brown Norway rats (F344XBN) were obtained from the National Institute on Aging Contract Colonies (Harlan Sprague Dawley, Inc., Indianapolis, IN). All procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee and complied with the guide for the care and use of laboratory animals (National Institutes of Health publication No. 3040-2, revised 1999).
Drug Infusion and Blood Pressure Measurement
Thirty-five young rats (8 months old) were randomly allocated into four groups and implanted subcutaneously with osmotic minipumps (model 2004; Alzet Corp., Palo Alto, CA) in the midscapular region, according to methods described previously.17 Briefly, rats were infused with Ang II (Sigma, St. Louis, MO) at a rate of 50 ng/kg/min (Y-50, n = 10), at a rate of 200 ng/kg/min (Y-200, n = 10), or with PE (Sigma) at a rate of 15 mg/kg/day (Y-PE, n = 5) for 28 days. Rats infused with saline served as untreated young controls (Y, n = 10), 30-month rats infused with saline solution served as an untreated old group (O, n = 10).
Systolic blood pressure (SBP) was measured via a tail-cuff method according to the manufacturer’s guidelines (RTBP 1007; Kent Scientific Co., Torrington, CT). Owing to peripheral vascular constriction, blood pressure was not acquired directly by a tail-cuff approach in rats infused with PE. Rather, intra-aortic pressure was measured via a 2.0-F Millar pressure conductance catheter (model SPR-838; Millar Instruments, Houston, TX) at the endpoint of the experiment.
Tissue Harvesting
Rats were sacrificed with an overdose of sodium pentobarbital, and carotid tissues were harvested and processed according to the protocol described previously.18,19 Diameter measurements were performed using an automatic image processing system (MetaMorph Imaging System; Universal Imaging Corp., Downington, PA). To determine the type of carotid arterial remodeling we calculated the ratio of wall to lumen, W/L, based on the intima-media thickness (IMT) as follows: W/L = 2 × carotid IMT/carotid diameter; and vascular mass, VM, calculated as: ρ π IMT2, where ρ is the arterial wall density.18,20
Organ Culture
Carotid artery rings were explanted and cultured as previously described.21 Organ cultures were used to examine the effect of Ang II on intact carotid arteries in the absence of neurohumoral adaptations and changes in blood pressure.21 This method also permitted the analysis of potential signaling mechanisms responsible for Ang II modulation of MMP2 expression. After dissection of adventitial tissue, carotid segments were placed in six-well dishes containing Dulbecco’s modified Eagle’s medium (DMEM) + F12 (1:1) (Life Technologies, Inc., Grand Island, NY) and antibiotics (penicillin 100 U/ml, streptomycin 100 mg/ml) and supplemented with 5 μg/ml of transferrin and 5 μg/ml of insulin. The vessel segments were then maintained in a tissue culture incubator at 37°C and exposed to various experimental conditions for the times indicated. The vascular segments were frozen in LN2 and the supernatants were stored at −80°C until use. Some of the vascular segments were embedded in OCT medium for in situ zymography and immunohistochemistry.
VSMC Isolation and Culture
Smooth muscle cells were enzymatically isolated as previously described.22 Briefly, F344XBN rat thoracic aortae were rinsed in Hanks’ balanced salt solution containing 50 μg/ml penicillin, 50 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Life Technologies, Inc.). After digestion for 30 minutes in 2 mg/ml of collagenase I solution (Worthington Biomedical, Freehold, NJ) at 37°C, the adventitia and intima were removed from the vessel media layer, which was placed overnight in complete medium (DMEM plus 10% fetal calf serum). On day 2 the vascular media was further digested with 2 mg ml of collagenase II/0.5 mg/ml elastase (Sigma) for 1 hour at 37°C, and the isolated cells were washed and plated in complete medium. In all cases, >95% of cells stained positive for α-smooth muscle actin (α-SMA).
VSMC Invasion
VSMC invasion was performed as described previously in modified Boyden chambers (Neuroprobe, Cabin John, MD) with 8-μm pore-size polycarbonate filters (Costar, Cambridge, MA) coated with Matrigel (BD Biosciences, Palo Alto, CA).21 Bovine serum albumin (control) or platelet-derived growth factor (PDGF)-BB (10 ng/ml, Upstate Biotechnology, Inc., Lake Placid, NY) was added to the lower chamber in 220 μl of migration medium: DMEM, 0.1% bovine serum albumin. Early passage (p3 to p5) VSMCs were cultured with or without Ang II (100 nmol/L), GM6001 (15 μmol/L), and Losartan (100 nmol/L) for 24 hours in 2.5% fetal bovine serum and DMEM and added to the upper chamber at 106 cells/ml in 200 μl of migration medium. After 4 hours at 37°C, 5% CO2, the cells were fixed on the lower side of the filter and stained using the HEMA3 system (Curtin Matheson Scientific, Inc., Houston, TX). Four random fields were counted at ×400 magnification. Data are expressed as the average number of cells/field.
Early passage (p3 to p5) VSMCs were cultured with or without Ang II (100 nmol/L), PE (100 nmol/L), and Losartan (100 nmol/L; Merck Research Laboratories, Rahway, NJ) for 24 hours in 2.5% fetal bovine serum and DMEM. In addition, VSMCs were treated with or without ET-1 for 24 hours. The activated MMP-2 was assessed by both Western blot and polyacrylamide gel electrophoresis gelatin zymography. The average of activated MMP-2 was from three independent experiments.
In Situ Hybridization, Immunohistochemistry, and Immunofluorescence
In situ hybridization, immunohistochemistry, and immunofluorescence, as well as their positive signal quantifi-cation, were performed according to the methods described previously.2,4 Immunohistochemistry was performed according to the protocols provided by the manufacturer (DAKO, Carpinteria, CA). The source and characteristics of primary antibodies used are listed in Table 1.
Table 1.
Primary Antibodies Used for Immunostaining and Western Blotting
| Antibody | Species | Titer for IHC | Titer for WB | Source |
|---|---|---|---|---|
| MMP-2 | Rabbit | 1:100 | 1:1250 | Chemicon Int., Inc., Temecula, CA |
| TIMP-2 | Rabbit | 1:100 | Chemicon Int. Inc. | |
| Mouse | 1:100 | Chemicon Int. Inc. | ||
| MT1-MMP | Rabbit | 1:250 | Chemicon Int. Inc. | |
| Mouse | 1:250 | Chemicon Int. Inc. | ||
| α-SMA | Mouse | 1:80 | DAKO, Glostrup, Denmark | |
| CD4 | Mouse | 1:50 | DAKO | |
| CD14 | Mouse | 1:50 | DAKO | |
| CD19 | Mouse | 1:50 | DAKO | |
| Collagen type I | Rabbit | 1:50 | Rockland, Gilbertsville, PA | |
| Collagen type III | Rabbit | 1:50 | Rockland | |
| TGF-β1 | Rabbit | 1:50 | Santa Cruz Biotechnology, Santa Cruz, CA | |
| Ang II | Rabbit | 1:50 | Peninsula Laboratories, Inc., San Carlos, CA |
Western Blot
Immunoblotting was performed according to the modified method previously described.4 Primary antibodies for Western blot analyses are listed in Table 1.
In Situ Zymography
To localize net in situ gelatinolytic activity of MMPs by zymography, fluorescein isothiocyanate-labeled DQ gelatin intramolecularly quenched (Molecular Probes, Inc., Eugene, OR) was used as a substrate. Fresh carotids were collected and rinsed in cold phosphate-buffered saline to remove outside connective tissue. The carotids were then immersed in ornithine carbamyl transferase (OCT) (Tissue-Tek, Torrance, CA) and quick-frozen into a block of dry ice. The carotids in the OCT blocks were cut into 10-μm sections using a cryostat (Leica, Wetzler, Germany) and collected sequentially. In situ zymography was performed by modification of a combination of two methods previously described.19,23 Fluorescein isothiocyanate-labeled DQ gelatin (1 mg/ml; Molecular Probes, Inc.) was mixed (1:1) with 1% agarose and melted in reaction buffer (0.05 mol/L Tris-HCl, 0.15 mol/L NaCl, 5 mmol/L CaCl2, and 0.2 mmol/L NaN3, pH 7.6). The liquid mixture was spread on prewarmed glass slides by a maneuver similar to that used to produce blood smears, and the film was allowed to gel at room temperature. Frozen sections of unfixed tissue were cut and applied to the top of the substrate film. A drop of the reaction buffer was added over each tissue section, and a silated coverslip was applied. Slides were incubated in light-protected humidified chambers at 37°C for 48 hours. At the end of the incubation period and without fixation or washes, lysis of the substrate was assessed by examination under fluorescence microscopy. As a negative control for in situ zymography, the frozen sections were processed as above but without the fluorescein isothiocyanate-labeled DQ gelatin, and 3.8 μg/ml of an antibody against MMP2 was added to the reaction to inhibit metalloproteinase.
Statistical Analysis
All results are expressed as the mean ± SEM. Statistical comparisons for group differences were made via an analysis of variance, followed by Bonferroni post hoc tests. A P value of <0.05 was taken as statistically significant.
Results
Systolic Blood Pressure and Intimal Remodeling of Carotid Arteries
In F344XBN rats, systolic blood pressure (SBP) increased with age, from 108 ± 6 mmHg at 8 months of age to 158 ± 6 mmHg at 30 months of age (Figure 1; online, see http://ajp.amjpathol.org). A 28 days infusion of Ang II (200 ng/kg/minute) to young rats increased SBP from day 2 onward. At day 28, SBP was 144 ± 14 mmHg, similar to that level observed in old untreated rats. SBP did not increase significantly in young rats infused with Ang II (50 ng/kg/minute) (Figure 1; online supplement, see http://ajp.amjpathol.org).
To characterize the carotid arterial remodeling that accompanies aging or that is affected by Ang II infusion, we measured the vessel diameter and the ratio of the wall-lumen, W/L, and vascular mass, VM. With aging W/L and VM were increased 1.3-fold and 2-fold, respectively. Compared to young untreated rats the high dose of Ang II infusion (200 ng/kg/minute) induced hypertrophic remodeling of the carotid wall (1.2-fold increase in W/L and 1.7-fold in VM), similar to that of the old, untreated animals (Figure 2; online supplement, see http://ajp.amjpathol.org). The low dose of Ang II (50 ng/kg/minute), while increasing collagen content (see below), did not produce wall thickening of young arteries.
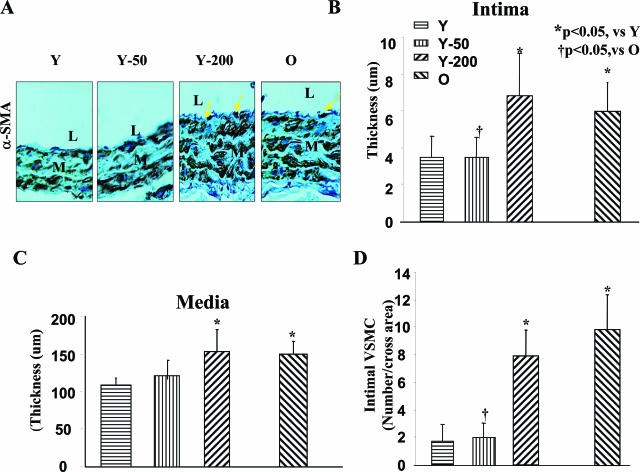
In untreated rats, the age-associated increase in intimal thickness was 77% (Figure 1). Ang II infusion into young rats (200 ng/kg/minute) increased their intimal thickness to that of the old untreated rats (Figure 1, A and B). The medial thickness increased 1.4-fold with age in untreated rats, and after Ang II, the medial thickness of young rats was increased to the level of the old rats (Figure 1C). Immunostaining for α-smooth muscle actin, a marker for VSMCs, indicated that disoriented cells were located within the thickened intima of untreated old rats, and in young rats infused with Ang II (200 ng/kg/minute) (Figure 1A). The number of intimal VSMCs increased by 6.3-fold with aging (Figure 1D), and by 4.7-fold in young rats infused with Ang II (200 ng/kg/minute). Intimal collagen type I and type III increased with aging, 3.5- and 3.8-fold, respectively (Figure 3; online, see http://ajp.amjpathol.org). Ang II infusion (200 ng/kg/minute) in young rats increased intimal collagen type I expression by 3.4-fold, and type III by 4.0-fold (Figure 3; online supplement, see http://ajp.amjpathol.org). Both types of collagen increased within the intima and media, but the relative increases were greater in the intima.
Figure 1.
Carotid arterial remodeling. A: α-Smooth muscle actin immunostaining of paraffin sections of carotid from untreated young (Y) and old (O) rats and young rats infused with Ang II (200 ng/kg/min, Y-200; or 50 ng/kg/min, Y-50). Yellow arrows indicate intimal VSMCs. B and C: Average carotid artery intima (B) and media (C) thickness. D: The average number of VSMCs per cross area. L, Lumen; M, media. Original magnifications, ×400.
It is noteworthy that, although Ang II at the lower rate (50 ng/kg/minute) did not induce an increase in arterial wall thickness or an infiltration of the intima with VSMCs (Figure 1), it did, however, significantly increase the deposition of collagen type I and type III to a level equaling that of the high infusion rate (Figure 3; online supplement, see http://ajp.amjpathol.org). Because transforming growth factor-β1 (TGF-β1) is a potent profibrotic cytokine, and a downstream target of Ang II signaling,15,24 we analyzed its protein expression during aging, and after Ang II infusion. TGF-β1 staining was increased 3.1-fold within the old rat carotid wall compared to that of untreated young rats (Figure 3; online, see http://ajp.amjpathol.org). Furthermore, a 1.6- and a 1.9-fold increase of TGF-β1 staining was observed within the carotid wall of young rats infused with Ang II at either a low rate (50 ng/kg/minute) or high rate (200 ng/kg/minute), similar to old untreated rats (Figure 3; online supplement, see http://ajp.amjpathol.org).
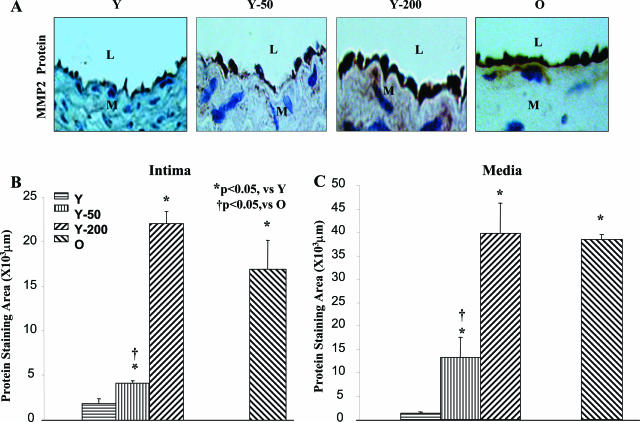
Figure 3.
MMP2 protein staining in situ. A: Representative photomicrographs of paraffin carotid sections stained with an anti-MMP2 antibody (brown color). B and C: Average staining for MMP2 within the intima (B) and media (C). L, Lumen, M, media. Original magnifications, ×400.
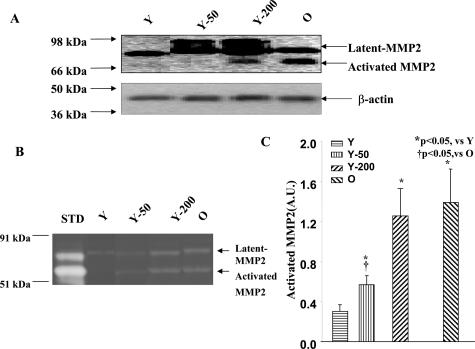
Total Vascular MMP2 Protein and Activity
Total MMP2 protein in the carotid artery significantly increased with age in untreated rats and in young rats infused with Ang II (Figure 2A). Polyacrylamide gel electrophoresis zymography demonstrated that bands corresponding to activated MMP2 were only detectable in immunoblots of young rats infused with Ang II (200 ng/kg/minute) and untreated old rats (Figure 2A). The 50 ng/kg/minute Ang II infusion had no measurable effect (Figure 2B). On average, activated MMP2 was increased by 4.6-fold in old rats compared to young rats. Ang II infusion (200 ng/kg/minute) into young rats increased activated MMP2 to the level of untreated old rats (Figure 2C). Of note, activated MMP2 was also significantly increased after infusion with Ang II 50 ng/kg/minute, but to a level less that of untreated old rats (Figure 2, B and C).
Figure 2.
Carotid artery MMP2 total protein and activity. A: Representative immunoblots of protein lysates from carotid arteries with an antibody against MMP2. Total and activated vascular MMP2 increased in young rats infused with Ang II (Y-200) and untreated old rats. B: A representative polyacrylamide gel electrophoresis gel zymogram of protein lysates from carotid arteries, showing two clear proteolytic bands corresponding to latent and activated MMP-2 in young rats infused with Ang II (Y-200) and in untreated old rats. C: Average carotid MMP-2 activity. STD, Recombinant MMP2 (50 ng).
MMP2 Protein Localization in Situ
Immunohistochemistry showed that intimal MMP2 staining in carotids of old untreated rats increased by 8.8-fold (Figure 3B). Ang II (200 ng/kg/minute) infusion increased intimal MMP2 staining of young rats by 11.6-fold, to the level of untreated old rats. The subpressor dose of Ang II also significantly increased MMP2 immunostaining, but to a level less that in untreated old rats. MMP2 staining patterns in the media were similar to that in the intima (Figure 3C). In the carotid, either from old or from young rats infused with Ang II, MMP2 co-localized with the VSMC marker, α-smooth muscle actin, (Figure 4, top right; online supplement, see http://ajp.amjpathol.org), and with the endothelial cell marker, CD31, (Figure 4, bottom right; online supplement, see http://ajp.amjpathol.org). There was no evidence of T cells (CD4), B cells (CD19), monocytes (CD14), or macrophages (CD68), within the intima or media. These cells, however, were occasionally observed within the adventitia of old rats and Ang II-treated young rats, and also exhibited increased MMP-2 staining (data not shown).
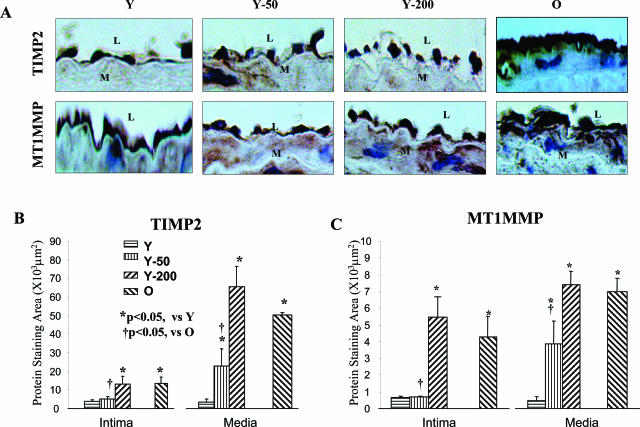
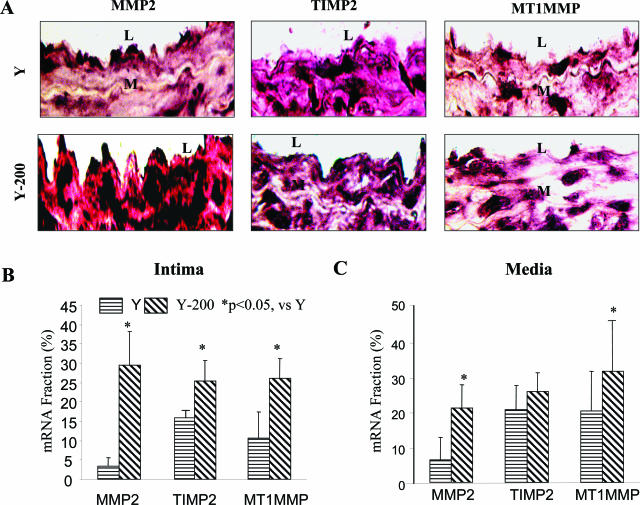
In Situ TIMP2 and MT1MMP Protein Expression
Intimal MT1MMP staining in old, untreated rats was increased by 4.8-fold, and TIMP2 staining was increased by 3.5-fold compared to untreated young rats (Figure 4, A and B). Moreover, the ratios of intimal MMP2 and MT1MMP staining to TIMP2 staining were enhanced by 3.1-fold and by 1.3-fold, respectively, in old versus young rats (Figure 5; online supplement, see http://ajp.amjpathol.org). Ang II infusion (200 ng/kg/minute) into young rats increased intimal MT1MMP staining by 6.3-fold and intimal TIMP2 by 3.4-fold, ie, to the level of the old rats (Figure 4, B and C). The ratios of intimal MMP2 or MT1MMP to TIMP2 staining in young rats infused with Ang II (200 ng/kg/minute) were increased by 3.0-fold and by 1.8-fold, respectively, similar to untreated old rats (Figure 5; online supplement, see http://ajp.amjpathol.org).
Figure 4.
TIMP2 and MT1MMP protein staining in situ. A: Representative photomicrographs of paraffin carotid sections stained with anti-TIMP2 or MT1MMP antibodies (brown color). B and C: Average staining for TIMP2 (B) or MT1MMP (C). L, Lumen; M, media. Original magnifications, ×400.
Intimal MT1MMP and TIMP2 staining as well as the ratios of MMP2 and MT1MMP to TIMP2 in young rats infused with Ang II (50 ng/kg/minute) were not significantly increased. In contrast, a significant increase in both TIMP2 and MT1MMP staining by this Ang II infusion rate was observed within the carotid media. The TIMP2 and MT1MMP staining patterns within the media were similar to that in the intima (Figure 4, B and C).
In Situ MMP2, TIMP2, and MT1MMP mRNA
Arterial MMP2, TIMP2, and MT1MMP mRNA all increase with aging.2,4 We used in situ hybridization to determine whether increased transcription could account for the increased protein levels of MMP2, TIMP2, or MT1MMP after Ang II infusion. The mRNA staining for MMP2 within the intima was markedly increased (ninefold) in young rats infused with Ang II (200 ng/kg/minute) (Figure 5A). MT1MMP staining increased by 3-fold, and that for TIMP2 by 1.5-fold (Figure 5). The patterns of altered staining for mRNA in the media are similar to that in the intima (Figure 5C). The ratio of MMP2 toTIMP2 mRNA within the intima of young rats infused with Ang II (200 ng/kg/minute) increased by 7.0-fold, and that of MT1MMP to TIMP2 mRNA by 1.7-fold (Figure 6; online supplement, see http://ajp.amjpathol.org).
Figure 5.
MMP2, TIMP2, and MT1MMP in situ mRNA hybridization. A: Representative photomicrographs of paraffin carotid sections stained with anti-sense MMP2, TIMP2, and MT1MMP RNA probes (purple color). B and C: Average staining for MMP2, TIMP2, and MT1MMP mRNA within the intima (B) or media (C). L, Lumen; M, media. Original magnifications, ×400.
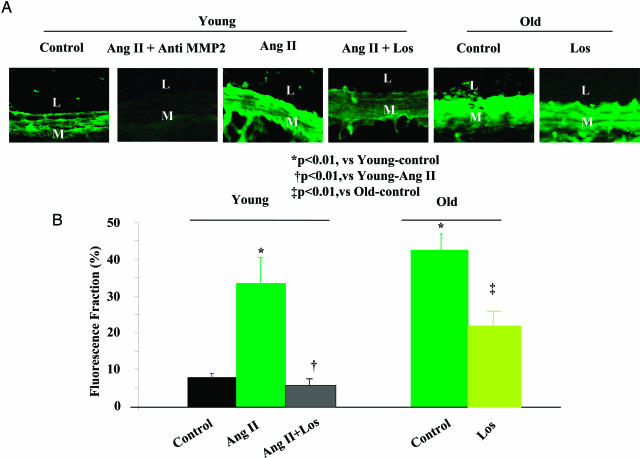
In Situ MMP2 Activity of Carotid Arterial Rings ex Vivo
In vivo Ang II infusion is accompanied by hemodynamic effects, ie, altered shear stress, and circumferential wall stress, and at higher doses, by increased SBP (Figure 1, online supplement, see http://ajp.amjpathol.org).25 To determine the Ang II-dependent effects on MMP2 in the absence of any Ang II hemodynamic effects, we assessed MMP2 activity by in situ zymography in isolated carotid rings under zero pressure, and no flow. The in situ fluorescence of activated MMP2 in rings from young rats was weak, and homogeneously distributed throughout the wall. In contrast, treatment with Ang II (100 nmol/L) increased activated MMP2 in young rats within the intima and inner media, similar to untreated old rings (Figure 6A). On average, the in situ activated MMP2 fluorescence fraction in rings from old untreated rats was increased by 4.8-fold, and after Ang II treatment of young rings, MMP2 increased by 4.4-fold, to the level of old rings (Figure 6B). The increase of in situ MMP2 in the young carotid rings treated with Ang II was completely inhibited by the AT1 receptor antagonist Losartan (100 nmol/L) (Figure 6). Importantly, the elevated MMP-2 activity in untreated rings from old rats in the absence of exogenously added Ang II was also reduced by Losartan (Figure 6).
Figure 6.
In situ gelatinase zymogram in explanted carotid rings of young (n = 4) and old (n = 4) rats, untreated or treated with Ang II (100 nmol/L) and/or Losartan (Los, 100 nmol/L) for 72 hours. A: Gelatinase activity (green fluorescence) in carotid sections from young and treated with Ang II and Los. B: Morphometric quantification of in situ gelatinase activity. L, Lumen; M, media. Original magnifications, ×400.
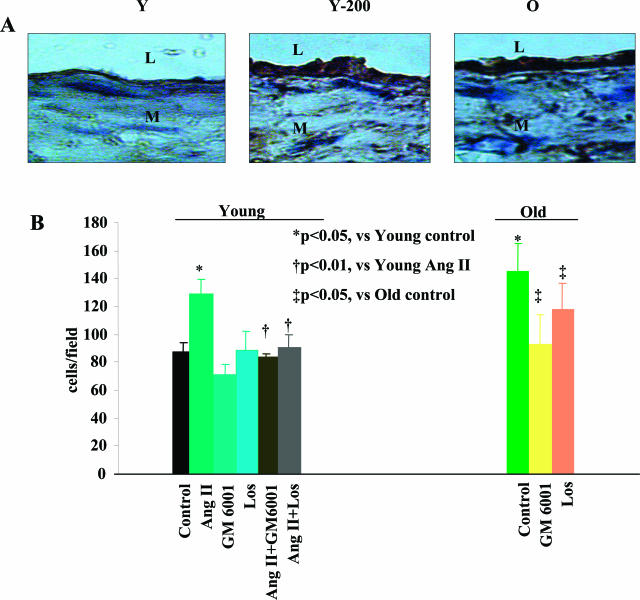
VSMC Invasion in Vitro
Because MMP2 activation is required for VSMC invasion of basement membranes,10 an increased VSMC invasion potential is an expected functional consequence of the Ang II-induced increase in MMP2 activity. To determine whether this was indeed the case, we used a modified Boyden chamber with a filter coated with Matrigel, mimicking the basement membrane, to assay aortic VSMC invasion. PDGF-BB was chosen as the chemoattractant, since we observed this to be increased in the thickened aortic intima of old rats, and of young rats infused with Ang II (Figure 7A). On average, the intimal PDGF-BB-positive staining areas in aortae from old untreated rats increased by 1.8-fold. Ang II infusion (200 ng/kg/minute) to young rats increased the PDGF-BB staining by 1.8-fold, as in old untreated arteries (Figure 7; online supplement, see http://ajp.amjpathol.org).
Figure 7.
A: Representative photomicrographs of aortic paraffin sections stained with an anti-PDGF-BB antibody (brown color) from a young untreated rat, a young rat infused with Ang II, and an old untreated rat. B: VSMC invasion assay using a modified Boyden chamber with a Matrigel-coated membrane. The chemoattractant was PDGF-BB (10 ng/ml). Early passage (p3 to p5) rat VSMCs were exposed to Ang II (100 nmol/L) for 24 hours with or without Losartan (100 nmol/L) (Los) or GM6001 (15 μmol/L). The average of three independent experiments is shown. L, Lumen; M, media. Original magnifications, ×400.
The invasion ability of early passage (p3 to p5) medial VSMCs from old rats was increased by 40% compared to that of cells from untreated young animals (Figure 7B). Exposure of young aortic medial VSMCs to Ang II (100 nmol/L) induced a 30% increase in invasion ability, similar to the level of untreated, old medial VSMCs (Figure 7B). The enhanced invasion ability of young Ang II-treated VSMCs was inhibited by Losartan (100 nmol/L) and GM6001 (15 μmol/L), an MMP inhibitor. Importantly, the enhanced invasion ability of untreated medial VSMCs from old rats was also reduced by GM6001 or Losartan (Figure 7B).
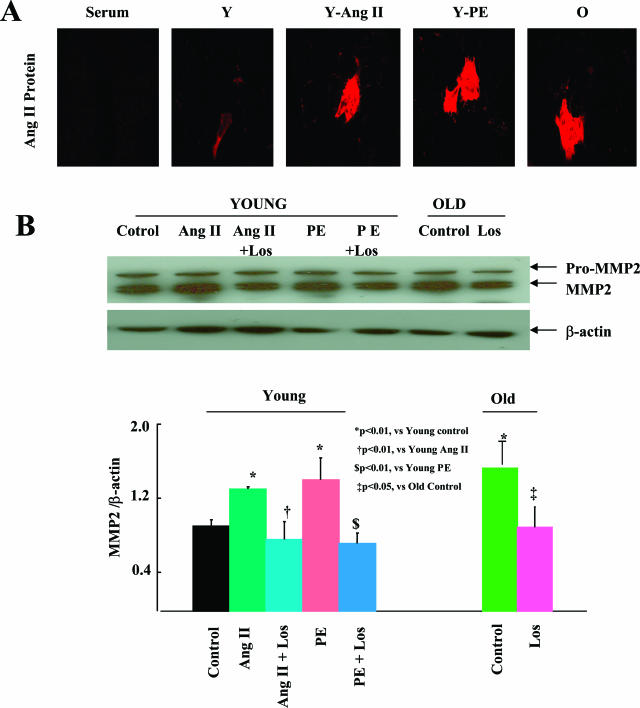
Phenylephrine Increases Arterial Ang II and Reproduces Ang II Effects in Young Rat Aorta and VSMCs
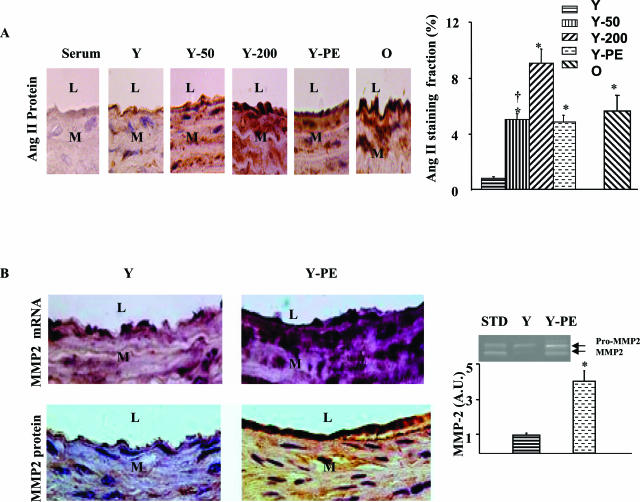
Because sympathetic activity activates Ang II signaling and because arterial sympathetic activity increases with aging,9,10 we determined whether PE, an α-AR agonist, induces arterial Ang II expression and MMP2 activation, and effects arterial remodeling similar to that obtained after Ang II (200 ng/kg/minute) infusion. PE infusion, at 15 mg/kg/day, into young rats increased aortic SBP to the same level as Ang II infusion (162 ± 1 versus 150 ± 7 mmHg, P > 0.05). PE infusion increased intimal thickness by 1.6-fold, and media thickness by 1.4-fold (both P < 0.05), similar to Ang II infusion. PE infusion increased the Ang II protein staining fraction by sixfold (P < 0.01), reaching the level of old untreated rats (Figure 8A). In PE-treated young rats, MMP2 mRNA and protein were abundantly expressed within the carotid wall, particularly within the thickened intima, (Figure 8B, middle), and activated MMP2 was elevated by fourfold (P < 0.01) (Figure 8B, right).
Figure 8.
Phenylephrine reproduces Ang II effects in young rat aorta. A: Representative photomicrographs of carotid sections stained with an anti-Ang II antibody (brown color). Average Ang II staining fractions (right). B: Representative photomicrographs of carotid sections stained with an anti-sense MMP2 RNA probe (purple color; top, right, and middle) and an anti-MMP2 antibody (brown color; bottom, right, and middle). Representative polyacrylamide gel electrophoresis gelatin zymogram of carotid (inset), and average data of activated MMP2 (right). L, Lumen; M, media. Original magnifications, ×400.
In vitro, in VSMCs from young rats, PE also induced Ang II protein expression (Figure 9A) and MMP2 activity (Figure 9B), similar to that after Ang II treatment. Both PE and Ang II induced similar increases in activated MMP-2 in young VSMCs, reaching the level of untreated VSMCs from old rats. Importantly, this effect of both PE and Ang II, was reduced by Losartan (Figure 9B).
Figure 9.
Phenylephrine reproduces Ang II effects in young VSMCs. A: Representative photomicrographs of VSMCs with anti-Ang II antibody staining. B: Representative immunoblots for MMP2 (top) and for β-actin (middle) using protein lysates from VSMCs. Average activated MMP2 quantification (bottom).
Discussion
The present results indicate that the remodeling of the common carotid artery with advancing age in Ang II untreated rats resembles that of the aorta.2,12 This remodeling includes intimal-medial thickening; an increased number of VSMCs within the intima (without evidence of macrophage or lymphocyte infiltration); increased collagen deposition; MMP2 mRNA, protein, and in situ activity; and TGF-β1 expression throughout the wall. The staining for MMP2 co-localizes with that of endothelial cell and VSMC markers. The increase in MMP2 activity in situ is due to an increase in the total protein amount, and also to an imbalance in the ratio of its activator, MT1MMP, and its inhibitor, TIMP2. This study also shows that explanted carotid rings, or isolated early passage VSMCs from old rats in vitro produce active MMP2, and that Ang administered in vitro to arterial rings or VSMCs of young rats increases MMP2 activity. Previous studies have demonstrated that links between the sympathetic system and the Ang II system, eg, immunoreactive renin is found in sympathetic nerve terminals of the heart;26 increased α-AR signaling enhances angiotensinogen gene expression in kidney cells;27 and Ang II outflow increases from skeletal muscle and accumulates in the arterial wall with sympathetic nerve stimulation.28 Our results demonstrate that in vivo, a chronic infusion of PE reproduced the effects of Ang II infusion in arteries, including up-regulation of Ang II expression and MMP2 activation. Similarly, exposure of young VSMCs to either PE or Ang II in vitro increases Ang II protein and MMP2 activity to the level of old untreated VSMCs and this effect is reduced by Losartan.
Previous studies have demonstrated an age-associated increase in arterial ACE, Ang II, and AT1 receptor.4,6,7 In addition, Ang II and MMP2 are co-localized with endothelial cells and intimal VSMCs within the aged aortic wall.4 In the present study, Ang II infusion induces an increase in MMP2 transcription, translation, and activity, and an imbalance of the MMP2 activator, MT1MMP and inhibitor, TIMP2, contributing to MMP2 activation, similar to that which accompanies age in arterial walls.2,12 Further, the AT1 receptor antagonist, Losartan, inhibited MMP2 activation in freshly explanted carotid rings and VSMCs from old rats. These results suggest that Ang II-induced MMP2 activation occurs within the carotid artery remodeled in the context of aging.
The present study also demonstrates that Ang II infusion at a high dose into young rats mimicked the age-associated increase in arterial pressure in this rat strain and reproduced nearly all of the aforementioned structural and molecular features of the arterial wall that have been observed previously in older rats of this strain and hypertensive rats.2,12,29,30 This includes intimal and medial thickening, with increased collagen deposition, VSMC infiltration in the thickened intima. In contrast, Ang II infusion at a lower rate, which did not result in an increased arterial pressure, still induced collagen deposition and increased MMP2 activity. However this increase in MMP2 did not reach that observed with the higher rate of Ang II infusion into young rats or of untreated old rats. Previous studies show that ACE, Ang II, and AT1 are all up-regulated within the aortic wall of hypertensive rats,31 and transmural pressure increases MMP2 activity within the arterial wall in a pressure-dependent manner.32 Other studies have shown that stretch, per se, induces Ang II secretion and activation of AT1 receptor in the absence of an increase in arterial pressure.33 Thus, arterial stretch that accompanies an increase in SBP with aging in this rat strain may be a factor for age-associated increase in Ang II and MMP2. However, our results clearly demonstrate that Ang II administered to arterial rings ex vivo and early passage medial VSMCs in vitro from young rats increased MMP2 activity to the level observed in old rats in the absence of hemodynamic changes, including increased SBP. Thus, arterial pressure and Ang II may have synergistic roles in activation of arterial MMP2 and structural remodeling.
Several components of the diverse and complex signaling pathway downstream of Ang II/AT1 receptor may be linked to MMP2 activation, collagen expression, and VSMC invasion within the arterial wall after Ang II infusion or aging. Ang II infusion increases arterial NAD(P)H oxidase,34 reactive oxygen species,34 tumor necrosis factor-α,35 TGF-β1,15,24,36 intracellular adhesion molecule-1,37,38 nuclear factor-κB,38,39 activator protein-1,39 monocyte chemoattractant protein-1 (MCP-1),40 fibronectin, and collagen expression.17 These are also known to increase in the arterial wall with aging in rats.12,41–47 MCP-1, a potent chemoattractant cytokine, increases within the aged arterial wall, and is co-localized with intimal VSMC and endothelial cell markers.46,47 MCP-1 is also abundantly expressed in arteries of rodents treated with Ang II, which has a critical role in arterial fibrosis.40 Previous studies show that treatment of medial VSMCs from young rats with MCP-1 enhances MMP2 activity and increases migration and invasion ability to the levels observed in VSMCs of old, untreated rats.46,47 Notably, these effects are abolished by an MMP2 inhibitor, GM6001.47
The present results show, for the first time, that an increase in arterial ET-1 staining with age accompanies age-associated arterial structural remodeling. Our results also demonstrate that infusion of Ang II to young rats increases arterial ET-1 reaching the level of old untreated rats (Figure 8; online supplement, see http://ajp.amjpathol.org). Furthermore, in vitro treatment with ET-1 in VSMCs from young rats increases the activation of MMP2. Previous studies show that ET-1 also decreases the binding of Ang II to the AT2 receptor, consequentially magnifying the action of AT1.48 Thus, there are multiple, interacting mechanisms to produce age-associated arterial remodeling, but the present study demonstrates that they may converge on AngII-MMP2 signaling
PDGF-BB staining was increased in the thickened intima containing infiltrated VSMCs, resembling the old artery. Ang II also up-regulates the expression of the chemoattractant PDGF-BB, and the PDGF-β receptor.49–51 The arterial PDGF-β receptor also increases with aging.52 The present results show, for the first time, that the chemoattractant PDGF-BB protein is markedly increased within the thickened carotid intima of old rats, and that early passage medial VSMCs of old rats, which still retain in vivo VSMC characteristics, have an increased ability to invade basement membrane in response to PDGF-BB. This increased VSMC invasiveness is reduced by MMP2 or AT1 receptor inhibition, suggesting that Ang II may be implicated in age-associated changes in VSMC properties in vivo (Figure 7). Thus, interactions of Ang II, MMP2, and PDGF-BB within the arterial wall likely favor medial VSMC invasion to the intima.13,14,37,39,53,54
In summary, in vivo chronic infusion of Ang II at concentrations sufficient to elicit an increase in arterial pressure in this strain similar to that associated with age, increases MMP2 activity and imparts to their central arteries structural and molecular characteristics of arteries of old, untreated rats. A subpressor infusion of Ang II also increased MMP2 expression and activity, and increased collagen production within the arterial wall. Ex vivo or in vitro exposure of carotid rings or medial VSMCs from young rats to Ang II increases the expression and activation of MMP2, and imparts medial VSMCs with an MMP2-dependent increase in their invasion capacity, to a similar level of untreated rings or VSMCs from old rats. Additionally, infusion of PE to young rats increases arterial Ang II levels and reproduces the Ang II effects. PE administered to VSMCs of young rats increases Ang II and MMP2 activity. Both Ang II and PE effects on VSMC MMP2 activity or invasion are blocked by Losartan. These results demonstrate that Ang II via the AT1 receptor can indeed mediate structural, biochemical, and functional features of the arterial wall and of VSMCs that occur with aging, and that similar effects of α1-AR signaling are mediated, in part at least, by Ang II. In this regard, the present results complement those of previous studies that have demonstrated that chronic ACE inhibition and AT1 receptor blockade, beginning at an early age, markedly delays the progression of age-associated arterial remodeling.55,56 Finally, as evidence for Ang II signaling in the pathophysiology of hypertension and atherosclerosis continues to evolve,15,16 the present results support the idea that Ang II-MMP2 signaling is a central pathway, which mediates cellular and molecular mechanisms that underlie arterial aging and confer on aging, per se, the status of the major risk factor for the development or exacerbation of these diseases.1,3,15,16 In other words, exaggerated Ang II signaling via the AT1 receptor appears to be a central feature of the long recognized and well-documented interactions among aging, hypertension, and atherosclerosis.
Supplementary Material
Footnotes
Address reprint requests to Edward G. Lakatta, M.D., Laboratory of Cardiovascular Sciences, Gerontology Research Center, National Institute on Aging, National Institutes of Health, 5600 Nathan Shock Dr., 3-B-03, Baltimore, MD 21224-6825. E-mail: lakattae@grc.nia.nih.gov.
Supported by the National Institutes of Health (National Institute on Aging intramural research program).
References
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- Orlandi A, Marcellini M, Spagnoli LG. Aging influences development and progression of early aortic atherosclerotic lesions in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 2000;20:1123–1136. doi: 10.1161/01.atv.20.4.1123. [DOI] [PubMed] [Google Scholar]
- Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- Virmani R, Avolio AP, Mergner WJ, Robinowitz M, Herderick EE, Cornhill JF, Guo SY, Liu TH, Ou DY, O’Rourke M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139:1119–1129. [PMC free article] [PubMed] [Google Scholar]
- Challah M, Nadaud S, Philippe M, Battle T, Soubrier F, Corman B, Michel JB. Circulating and cellular markers of endothelial dysfunction with aging in rats. Am J Physiol. 1997;273:H1941–H1948. doi: 10.1152/ajpheart.1997.273.4.H1941. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Endemann D, He G, Li JS, Schiffrin EL. Role of AT2 receptors in angiotensin II-stimulated contraction of small mesenteric arteries in young SHR. Hypertension. 1999;33:366–372. doi: 10.1161/01.hyp.33.1.366. [DOI] [PubMed] [Google Scholar]
- Akasu M, Urata H, Kinoshita A, Sasaguri M, Ideishi M, Arakawa K. Differences in tissue angiotensin II-forming pathways by species and organs in vitro. Hypertension. 1998;32:514–520. doi: 10.1161/01.hyp.32.3.514. [DOI] [PubMed] [Google Scholar]
- Benetos A, Huguet F, Albaladejo P, Brisac AM, Pappo M, Safar ME, Levy BI. Role of adrenergic tone in mechanical and functional properties of carotid artery during aging. Am J Physiol. 1993;265:H1132–H1138. doi: 10.1152/ajpheart.1993.265.4.H1132. [DOI] [PubMed] [Google Scholar]
- Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D’Amico EB, El-Moalem H, Page SO, Richardson CD, Winters B, Marucci L, Schwinn DA. Subtype specific regulation of human vascular alpha (1)-adrenergic receptors by vessel bed and age. Circulation. 1999;100:2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- Goettsch W, Lattmann T, Amann K, Szibor M, Morawietz H, Munter K, Muller SP, Shaw S, Barton M. Increased expression of endothelin-1 and inducible nitric oxide synthase isoform II in aging arteries in vivo: implications for atherosclerosis. Biochem Biophys Res Commun. 2001;280:908–913. doi: 10.1006/bbrc.2000.4180. [DOI] [PubMed] [Google Scholar]
- Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999;33:116–123. doi: 10.1161/01.hyp.33.1.116. [DOI] [PubMed] [Google Scholar]
- Pauly RR, Passaniti A, Bilato C, Monticone R, Cheng L, Papadopoulos N, Gluzband YA, Smith L, Weinstein C, Lakatta EG, Crow MT. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res. 1994;75:41–54. doi: 10.1161/01.res.75.1.41. [DOI] [PubMed] [Google Scholar]
- Lundberg MS, Crow MT. Age-related changes in the signaling and function of vascular smooth muscle cells. Exp Gerontol. 1999;34:549–557. doi: 10.1016/s0531-5565(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Williams B. Angiotensin II and the pathophysiology of cardiovascular remodeling. Am J Cardiol. 2001;87:10C–17C. doi: 10.1016/s0002-9149(01)01507-7. [DOI] [PubMed] [Google Scholar]
- Strawn WB, Ferrario CM. Mechanisms linking angiotensin II and atherogenesis. Curr Opin Lipidol. 2002;13:505–512. doi: 10.1097/00041433-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Levy BI, Benessiano J, Henrion D, Caputo L, Heymes C, Duriez M, Poitevin P, Samuel JL. Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J Clin Invest. 1996;98:418–425. doi: 10.1172/JCI118807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girerd X, Mourad JJ, Copie X, Moulin C, Acar C, Safar M, Laurent S. Noninvasive detection of an increased vascular mass in untreated hypertensive patients. Am J Hypertens. 1994;7:1076–1084. doi: 10.1093/ajh/7.12.1076. [DOI] [PubMed] [Google Scholar]
- Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri A, Chen CH, Yin FC, Chih-Tai T, Spurgeon HA, Lakatta EG. Functional correlates of central arterial geometric phenotypes. Hypertension. 2001;38:1471–1475. doi: 10.1161/hy1201.099291. [DOI] [PubMed] [Google Scholar]
- Brogelli L, Parenti A, Ledda F. Inhibition of vascular smooth muscle cell growth by angiotensin type 2 receptor stimulation for in vitro organ culture model. J Cardiovasc Pharmacol. 2002;39:739–745. doi: 10.1097/00005344-200205000-00015. [DOI] [PubMed] [Google Scholar]
- Pauly RR, Bilato C, Cheng L, Monticone R, Crow MT. Vascular smooth muscle cell cultures. Methods Cell Biol. 1997;52:133–154. doi: 10.1016/s0091-679x(08)60377-5. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Suknova GK, Libby P. Microscopic localization of active proteases by in situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J. 1995;9:974–980. doi: 10.1096/fasebj.9.10.7615167. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Morishita R, Tomita N, Shimozato T, Nakagami H, Kikuchi A, Aoki M, Higaki J, Kaneda Y, Ogihara T. Ribozyme oligonucleotides against transforming growth factor-beta inhibited neointimal formation after vascular injury in rat model: potential application of ribozyme strategy to treat cardiovascular disease. Circulation. 2000;102:1308–1314. doi: 10.1161/01.cir.102.11.1308. [DOI] [PubMed] [Google Scholar]
- Henrion D, Kubis N, Levy BI. Physiological and pathophysiological functions of the AT(2) subtype receptor of angiotensin II: from large arteries to the microcirculation. Hypertension. 2001;38:1150–1157. doi: 10.1161/hy1101.096109. [DOI] [PubMed] [Google Scholar]
- Seyedi N, Koyama M, Mackins CJ, Levi R. Ischemia promotes renin activation and angiotensin formation in sympathetic nerve terminals isolated from the human heart: contribution to carrier-mediated norepinephrine release. J Pharmacol Exp Ther. 2002;302:539–544. doi: 10.1124/jpet.302.2.539. [DOI] [PubMed] [Google Scholar]
- Wang TT, Wu XH, Zhang SL, Chan JS. Molecular mechanism(s) of action of norepinephrine on the expression of the angiotensinogen gene in opossum kidney cells. Kidney Int. 1998;54:785–795. doi: 10.1046/j.1523-1755.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- Jonsson JR, Smid SD, Frewin DB, Head RJ. Angiotensin II-mediated facilitation of sympathetic neurotransmission in the spontaneously hypertensive rat is not associated with neuronal uptake of the peptide. J Cardiovasc Pharmacol. 1993;22:750–753. doi: 10.1097/00005344-199311000-00012. [DOI] [PubMed] [Google Scholar]
- Haudenschild CC, Prescott MF, Chobanian AV. Aortic endothelial and subendothelial cells in experimental hypertension and aging. Hypertension. 1981;3:I148–I153. doi: 10.1161/01.hyp.3.3_pt_2.i148. [DOI] [PubMed] [Google Scholar]
- Haudenschild CC, Chobanian AV. Blood pressure lowering diminishes age-related changes in the rat aortic intima. Hypertension. 1984;6:I62–I68. doi: 10.1161/01.hyp.6.2_pt_2.i62. [DOI] [PubMed] [Google Scholar]
- Otsuka S, Sugano M, Makino N, Sawada S, Hata T, Niho Y. Interaction of mRNAs for angiotensin II type 1 and type 2 receptors to vascular remodeling in spontaneously hypertensive rats. Hypertension. 1998;32:467–472. doi: 10.1161/01.hyp.32.3.467. [DOI] [PubMed] [Google Scholar]
- Chesler NC, Ku DN, Galis ZS. Transmural pressure induces matrix-degrading activity in porcine arteries ex vivo. Am J Physiol. 1999;277:H2002–H2009. doi: 10.1152/ajpheart.1999.277.5.H2002. [DOI] [PubMed] [Google Scholar]
- Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47 (phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;82:S12–S22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- Pawlowski JE, Taylor DS, Valentine M, Hail ME, Ferrer P, Kowala MC, Molloy CJ. Stimulation of activin A expression in rat aortic smooth muscle cells by thrombin and angiotensin II correlates with neointimal formation in vivo. J Clin Invest. 1997;100:639–648. doi: 10.1172/JCI119575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore L, Tessitore A, Martinotti S, Toniato E, Alesse E, Bravi MC, Ferri C, Desideri G, Gulino A, Santucci A. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation. 1999;100:1646–1652. doi: 10.1161/01.cir.100.15.1646. [DOI] [PubMed] [Google Scholar]
- Tham DM, Martin-McNulty B, Wang YX, Wilson DW, Vergona R, Sullivan ME, Dole W, Rutledge JC. Angiotensin II is associated with activation of NF-kappaB-mediated genes and downregulation of PPARs. Physiol Genomics. 2002;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- Muller DN, Mervaala EM, Dechend R, Fiebeler A, Park JK, Schmidt F, Theuer J, Breu V, Mackman N, Luther T, Schneider W, Gulba D, Ganten D, Haller H, Luft FC. Angiotensin II (AT(1)) receptor blockade reduces vascular tissue factor in angiotensin II-induced cardiac vasculopathy. Am J Pathol. 2000;157:111–122. doi: 10.1016/S0002-9440(10)64523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, Tsuchihashi M, Sugaya T, Charo IF, Kura S, Tsuzuki T, Ishibashi T, Takeshita A, Egashira K. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004;94:1203–1210. doi: 10.1161/01.RES.0000126924.23467.A3. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;12:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Yan ZQ, Sirsjo A, Bochaton-Piallat ML, Gabbiani G, Hansson GK. Augmented expression of inducible NO synthase in vascular smooth muscle cells during aging is associated with enhanced NF-kappaB activation. Arterioscler Thromb Vasc Biol. 1999;19:2854–2862. doi: 10.1161/01.atv.19.12.2854. [DOI] [PubMed] [Google Scholar]
- Moon SK, Cha BY, Lee YC, Nam KS, Runge MS, Patterson C, Kim CH. Age-related changes in matrix metalloproteinase-9 regulation in cultured mouse aortic smooth muscle cells. Exp Gerontol. 2004;39:123–131. doi: 10.1016/j.exger.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Spinetti G, Wang M, Monticone RE, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- Spinetti G, Wang MY, Monticone RE, Zhang J, Di Z, Lakatta EG. The chemokine, MCP-1, activates metalloproteinase-2 production: a novel mechanism for vascular smooth muscle cell invasion. Circulation. 2003;108:S299. [Google Scholar]
- Kambayashi Y, Bardhan S, Inagami T. Peptide growth factors markedly decrease the ligand binding of angiotensin II type 2 receptor in rat cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1993;194:478–482. doi: 10.1006/bbrc.1993.1844. [DOI] [PubMed] [Google Scholar]
- Abe J, Deguchi J, Matsumoto T, Takuwa N, Noda M, Ohno M, Makuuchi M, Kurokawa K, Takuwa Y. Stimulated activation of platelet-derived growth factor receptor in vivo in balloon-injured arteries: a link between angiotensin II and intimal thickening. Circulation. 1997;96:1906–1913. doi: 10.1161/01.cir.96.6.1906. [DOI] [PubMed] [Google Scholar]
- Kim S, Zhan Y, Kim S, Izumi Y, Yasumoto H, Yano M, Iwao H. In vivo activation of rat aortic platelet-derived growth factor and epidermal growth factor receptors by angiotensin II and hypertension. Arterioscler Thromb Vasc Biol. 2000;20:2539–2545. doi: 10.1161/01.atv.20.12.2539. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Cox AJ, Gow RM, Zhang Y, Kemp BE, Gilbert RE. Platelet-derived growth factor receptor transactivation mediates the trophic effects of angiotensin II in vivo. Hypertension. 2004;44:195–202. doi: 10.1161/01.HYP.0000132883.20764.12. [DOI] [PubMed] [Google Scholar]
- Sarzani R, Arnaldi G, Takasaki I, Brecher P, Chobanian AV. Effects of hypertension and aging on platelet-derived growth factor and platelet-derived growth factor receptor expression in rat aorta and heart. Hypertension. 1991;18:III93–III99. doi: 10.1161/01.hyp.18.5_suppl.iii93. [DOI] [PubMed] [Google Scholar]
- Kopp CW, Holzenbein T, Steiner S, Marculescu R, Bergmeister H, Seidinger D, Mosberger I, Kaun C, Cejna M, Horvat R, Wojta J, Maurer G, Binder BR, Breuss JM, Ecker RC, de Martin R, Minar E. Inhibition of restenosis by tissue factor pathway inhibitor: in vivo and in vitro evidence for suppressed monocyte chemoattraction and reduced gelatinolytic activity. Blood. 2004;103:1653–1661. doi: 10.1182/blood-2003-04-1148. [DOI] [PubMed] [Google Scholar]
- Saito S, Frank GD, Motley ED, Dempsey PJ, Utsunomiya H, Inagami T, Eguchi S. Metalloprotease inhibitor blocks angiotensin II-induced migration through inhibition of epidermal growth factor receptor transactivation. Biochem Biophys Res Commun. 2002;294:1023–1029. doi: 10.1016/S0006-291X(02)00595-8. [DOI] [PubMed] [Google Scholar]
- Michel JB, Heudes D, Michel O, Poitevin P, Philippe M, Scalbert E, Corman B, Levy BI. Effect of chronic ANG I-converting enzyme inhibition on aging processes. II. Large arteries. Am J Physiol. 1994;267:R124–R135. doi: 10.1152/ajpregu.1994.267.1.R124. [DOI] [PubMed] [Google Scholar]
- Huang W, Alhenc Gelas F, Osborne-Pellegrin MJ. Protection of the arterial internal elastic lamina by inhibition of the renin-angiotensin system in the rat. Circ Res. 1998;82:879–890. doi: 10.1161/01.res.82.8.879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.