Abstract
Recent reports have linked mutations in the surfactant protein C gene (SFTPC) to familial forms of pulmonary fibrosis, but it is uncertain whether deficiency of mature SP-C contributes to disease pathogenesis. In this study, we evaluated bleomycin-induced lung fibrosis in mice with genetic deletion of SFTPC. Compared with wild-type (SFTPC+/+) controls, mice lacking surfactant protein C (SFTPC−/−) had greater lung neutrophil influx at 1 week after intratracheal bleomycin, greater weight loss during the first 2 weeks, and increased mortality. At 3 and 6 weeks after bleomycin, lungs from SFTPC−/− mice had increased fibroblast numbers, augmented collagen accumulation, and greater parenchymal distortion. Furthermore, resolution of fibrosis was delayed. Although remodeling was near complete in SFTPC+/+ mice by 6 weeks, SFTPC−/− mice did not return to baseline until 9 weeks after bleomycin. By terminal dUTP nick-end labeling staining, widespread cell injury was observed in SFTPC−/− and SFTPC+/+ mice 1 week after bleomycin; however, ongoing apoptosis of epithelial and interstitial cells occurred in lungs of SFTPC−/− mice, but not SFTPC+/+ mice, 6 weeks after bleomycin. Thus, SP-C functions to limit lung inflammation, inhibit collagen accumulation, and restore normal lung structure after bleomycin.
Idiopathic pulmonary fibrosis is characterized by progressive dyspnea, bilateral interstitial infiltrates, restrictive abnormalities on pulmonary function testing, and a progressive decline in pulmonary function.1,2 Familial forms of pulmonary fibrosis have been reported in more than 68 kindreds.3–6 Recent studies have revealed that some familial forms of pulmonary fibrosis are associated with mutations in the surfactant protein C gene (SFTPC).7–10
Although much is known about the relationship between abnormalities in surfactant proteins, including surfactant protein C (SP-C), and the development of respiratory distress of the newborn, the mechanism by which alterations in SP-C lead to pulmonary fibrosis is unknown. Because SP-C is produced by type II alveolar epithelial cells,11 the existence of familial forms of pulmonary fibrosis associated with defects in SFTPC suggests that the alveolar epithelium can play a pivotal role in the pathogenesis of lung fibrosis. Although some studies have indicated that SFTPC mutations lead to a misfolded or mistargeted mutant protein that results in altered type II cell function,12 the contribution of an absolute or relative deficiency in mature SP-C to the pathobiology of pulmonary fibrosis is uncertain.
In the present study, we investigated the hypothesis that SP-C deficiency predisposes to alveolar epithelial cell injury and abnormal repair, resulting in an enhanced propensity to develop pulmonary fibrosis in response to fibrotic stimuli. In this investigation, we used the murine bleomycin model to induce pulmonary fibrosis in SFTPC-deficient mice.13–16 We evaluated the lung inflammatory response to bleomycin treatment, measured the extent of fibrosis and fibroblast recruitment, and assessed resolution of lung fibrosis. In addition, we performed terminal dUTP nick-end labeling (TUNEL) and active caspase 3 staining on lung sections to determine whether SP-C deficiency influences cell survival after bleomycin treatment.
Materials and Methods
Mouse Model
Generation of mice lacking surfactant protein C (SFTPC−/−), Black Swiss background, has been previously described.17 Wild-type Black Swiss mice were used for controls (Taconic, Germantown, NY). Eight- to ten-week-old mice weighing ∼25 g were used for experiments. Mice were housed in the central animal care facility at Vanderbilt University Medical Center (Nashville, TN) and were given food and water ad libitum. A total of 300 mice were used in these experiments. The experimental protocol was reviewed and approved by the institutional animal care and utilization committee at Vanderbilt University.
Bleomycin was prepared by mixing sterile bleomycin sulfate powder (Blenoxane; Nippon Kayaku, Tokyo, Japan) with normal saline. Bleomycin was injected intratracheally at a dose of 0.01 U (in a total volume of 50 μl). For intratracheal injections, mice were anesthetized with isoflurane by inhalation, placed supine on the operating field, and neck dissection was performed with sterile surgical instruments to obtain exposure of the trachea. The bleomycin solution was delivered by direct injection into the trachea using a syringe with a 25-gauge needle and the neck incision was then closed with 5.0 ethilon sutures. Mice were weighed at baseline and at 2-week intervals after bleomycin.
At designated time points after bleomycin injection, mice were euthanized by exposure to carbon dioxide and lungs were harvested for histological preparations or flash frozen and stored at −70°C. For histology, lung inflation was performed with 1 ml of 10% neutral buffered formalin. After euthanasia some mice had lung lavage or CXR imaging performed as described below.
Histology and Immunohistochemistry
Lungs were fixed in 10% neutral buffered formalin, processed into paraffin blocks, and cut into 5-μm sections. Slides were stained with hematoxylin and eosin (H&E) and trichrome blue. Immunostaining for the fibroblast marker, fibroblast-specific protein 1 (FSP1) was performed using a biotinylated rabbit polyclonal antibody18,19 (generated in the laboratory of Dr. Eric Neilson, Vanderbilt University) followed by a standard immunoperoxidase/avidin-biotin complex protocol using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Immunostains for active transforming growth factor (TGF)-β1 and active caspase 3 were performed using specific polyclonal antibodies (TGF-β1, sc-146, rabbit polyclonal IgG; caspase 3, sc-1226, goat polyclonal IgG; Santa Cruz Biotechnology, Santa Cruz, CA), using a similar protocol. TUNEL assays were performed using a commercially available kit in accordance with manufacturer directions (In Situ Cell Death detection kit; Roche Molecular Biochemicals, Indianapolis, IN). Counterstains for immunohistochemistry preparations were performed with hematoxylin.
Semiquantitative Scores
Quantification of lung fibrosis, FSP1+ cells, and TUNEL+ cells on histological specimens was done by a pathologist blinded to the genotype and treatment group. Slides of lung tissue were prepared by the Vanderbilt Mouse Pathology and Immunostaining Core, randomized, and blindly evaluated by the pathologist on 10 sequential, nonoverlapping fields (magnification, ×300) of lung parenchyma from each specimen. Lung fibrosis was evaluated on trichrome-stained lung sections using a 0 to 4 point scale, with a score of 0, normal lung architecture; 1, increased thickness of some (≤50%) of interalveolar septa; 2, thickening of >50% of interalveolar septa without formation of fibrotic foci; 3, thickening of the interalveolar septa with formation of isolated fibrotic foci; and 4, formation of multiple fibrotic foci with total or subtotal distortion of parenchymal architecture. The mean score for the 10 fields represented the score for each individual specimen. Immunohistochemical staining for FSP1 was evaluated in a similar manner. Unstained slides were provided to the pathologist, who immunostained for FSP1 and evaluated FSP1+ cells on a 0 to 4 point scale, with a score of 0, no positive cells; 1, few (≤3) positive cells; 2, multiple (>3) individual positive cells; 3, multiple positive cells in isolated clumps; and 4, multiple clumps of positive cells. The mean score for the 10 sequential fields represented the score for each individual specimen. For evaluation of TUNEL staining, the percentage of cells with TUNEL-positive nuclei on 10 sequential, nonoverlapping high-power fields from each specimen was recorded. The mean percentage of TUNEL-positive cells on 10 sequential fields represented the score for each individual specimen.
High Performance Liquid Chromatography Hydroxyproline Assay
Frozen lung tissue samples were hydrolyzed in 6 N HCl, and hydroxyproline content quantitated using high performance liquid chromatography as previously described.20 Lung collagen content was calculated from these results because hydroxyproline accounts for ∼13.3% of collagen by weight.
Neutrophil Counts on Lung Lavage
After euthanasia, three 800-μl lavages of sterile saline were performed using a 20-gauge blunt-tipped needle inserted into the trachea. Samples were centrifuged at 400 × g for 10 minutes and the supernatant discarded. Cell counts were performed by manual counting under light microscopy using a hemocytometer. Approximately 30,000 cells from each specimen were loaded onto slides using a Cytospin 2 (Shandon Southern Products, Astmoor, Runcorn, Cheshire, UK). These slide preparations were then stained using a modified Wright stain21 and reviewed under light microscopy for white blood cell differential. The total number of neutrophils for each lavage was reported.
Neutrophil Myeloperoxidase Assay
Myeloperoxidase kinetics assay was performed from lung tissue homogenates as previously reported21 and results normalized to protein content determined using the BCA protein assay (Pierce Biotechnology, Rockford, IL).
Analysis of Surfactant Proteins in Lung Lavage
For evaluation of surfactant proteins, lungs were lavaged in situ with two 750-μl aliquots of sterile phosphate-buffered saline using a 20-gauge blunt tipped needle inserted into the trachea. Samples were centrifuged at 400 × g for 10 minutes and the supernatant collected. Supernatants were then analyzed for the four surfactant proteins, SP-A, SP-B, SP-C, and SP-D. Lavage content of SP-B and SP-D was measured by specific enzyme-linked immunosorbent assay (ELISA) as previously reported.22–24 Western blots were performed for SP-A and the mature form of SP-C using polyclonal antibodies as previously described.17 Densitometry was performed using Gelworks 1D Advanced V3.01 software (Ultra-Violet Products, Cambridge, UK) and the individual band density was normalized to a positive control run on each gel.
TGF-β1 ELISA
Using the same cytoplasmic extracts prepared for use on Western blot, total TGF-β1 was assessed by ELISA as per kit instructions (Quantikine; R&D Systems, Minneapolis, MN). Results were normalized for protein concentration.
CXR Imaging
At baseline and at 3 weeks after bleomycin injection, mice were euthanized by exposure to carbon dioxide. Neck dissection was performed to expose the trachea. The trachea was then cannulated with a 25-gauge angiocath and 750 μl of air instilled to inflate the lungs. The mouse was then positioned in a digital cabinet X-ray system (LX-60; Faxitron, Inc., Wheeling, IL) and anteroposterior and lateral radiographical images were obtained with a source-to-object distance of 10 cm and an object-to-detector distance of 40 cm, resulting in 5× geometric magnification. Imaging was performed using a 0.01-mm focal spot tungsten anode X-ray tube operating at 27 kVp and 0.3 mA with an exposure time of 8 seconds (2.4 mAs). Fourteen-bit digital images with 2048 × 2048 pixels were stored in DICOM (standard medical) format and viewed using ImageJ (public domain software available from the National Institutes of Health).
Statistics
To assess differences among groups, analyses were performed with GraphPad Instat (GraphPad Software, San Diego, CA) using a one-way analysis of variance test (P values <0.05 were considered significant). Mortality differences were evaluated using a Fisher’s exact test. Results are presented as mean ± SEM.
Results
Excessive Weight Loss, Lung Inflammation, and Mortality in Bleomycin-Treated SFTPC−/− Mice
We used bleomycin delivered by intratracheal injection to induce lung fibrosis in SFTPC−/− mice and SFTPC+/+ controls. In initial investigations, doses of bleomycin typically used in mouse pulmonary fibrosis studies produced an extremely high mortality in SFTPC−/− mice by 3 weeks after treatment. Intratracheal bleomycin treatment with 0.04 U resulted in 56% mortality in SFTPC−/− mice compared with no mortality in SFTPC+/+ mice (P < 0.01). After additional dose titration experiments, we found that 0.01 U of bleomycin given intratracheally produced clear histological evidence of fibrosis with an acceptable mortality rate (<15%) in SFTPC−/− mice. Thus, a dose of 0.01 U of bleomycin was used in subsequent experiments.
Three groups of mice were compared in these studies, SFTPC−/−, SFTPC+/−, and SFTPC+/+ mice; however, histological findings in SFTPC+/− mice were similar to those for SFTPC+/+ mice, so only data from SFTPC−/− and SFTPC+/+ mice are shown here. Evaluation of the severity of inflammation was assessed 1 week after bleomycin, and other parameters of fibrosis and remodeling were evaluated at later time points—3, 6, and 9 weeks. Mice were weighed at baseline and at bi-weekly intervals after bleomycin treatment. At baseline, weights were similar between the two groups [SFTPC−/− mice: 25.68 ± 0.74 g; SFTPC+/+ mice: 25.86 ± 0.71 g (n = 24/group)]. Two weeks after bleomycin, SFTPC−/− mice had lost weight, whereas SFTPC+/+ mice had gained weight [SFTPC−/− mice lost 1.44 ± 0.55 g and SFTPC+/+ mice gained 1.71 ± 0.19 g (P < 0.005)]. At 4 weeks, the SFTPC−/− mice had gained weight greater than baseline, but still weighed less than SFTPC mice [SFTPC−/− mice gained 1.28 ± 0.37 g and SFTPC+/+ mice gained 2.61 ± 0.23 g (P < 0.005)]. At 6 weeks, weight gains were not significantly different between the two groups.
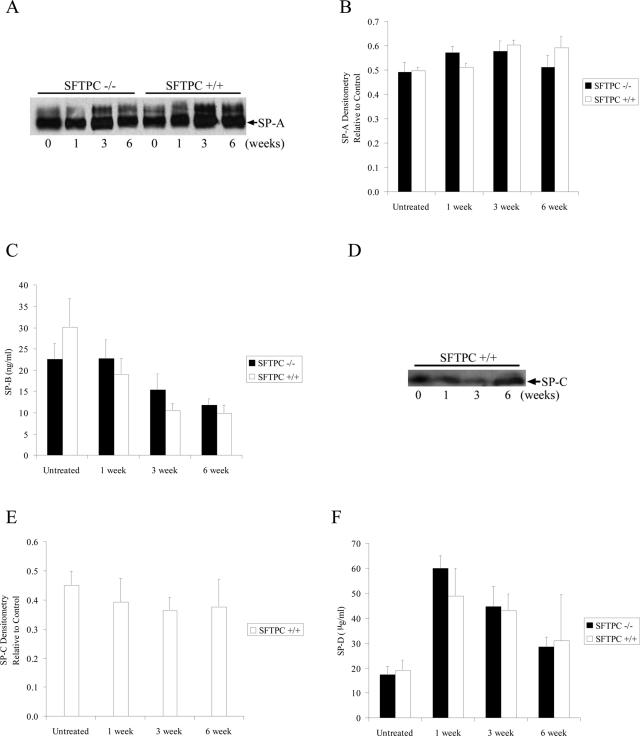
Surfactant protein C is one of four surfactant proteins (A, B, C, and D) present in the alveolar space. To determine the composition of surfactant proteins in the alveolar space from both SFTPC+/+ and SFTPC−/− mice at baseline and after bleomycin treatment, levels of all four surfactant proteins were assessed in lung lavage. By Western blot analysis, SP-A content in lung lavage did not change after bleomycin and was not different between SFTPC−/− and SFTPC+/+ mice (Figure 1, A and B). As expected, SP-C was not detected in lung lavage of SFTPC−/− mice and did not change significantly from baseline after bleomycin in SFTPC+/+ mice (Figure 1, D and E). By ELISA, SP-B decreased after bleomycin treatment in both SFTPC−/− and SFTPC+/+ mice but did not differ between the two groups at any time point (Figure 1C). SP-D levels, also measured by ELISA, increased after bleomycin in both SFTPC−/− and SFTPC+/+ mice but did not differ between the two groups at any time point (Figure 1F).
Figure 1.
Analysis of surfactant proteins in lung lavage. A and B: SP-A levels were similar in SFTPC−/− and SFTPC+/+ mice and were not altered by bleomycin treatment. A representative Western blot (A) and densitometry of SP-A bands (B) compared to a positive control are shown. C: By ELISA, SP-B levels decreased after bleomycin in both SFTPC−/− and SFTPC+/+ mice. D and E: SP-C levels did not change significantly after bleomycin in SFTPC+/+ mice. SP-C was not detectable in SFTPC−/− mice. A representative Western blot from SFTPC+/+ mice (D) and densitometry of SP-C bands (E) compared to a positive control are shown. F: By ELISA, SP-D levels increased after bleomycin in both SFTPC−/− and SFTPC+/+ mice. For ELISA and densitometry, n = 6 at each time point except for the 6-week time point for SFTPC+/+ mice in which n = 3.
By 1 week after bleomycin treatment, increased numbers of inflammatory cells were observed in lung tissue by examination of H&E-stained lung sections from SFTPC−/− mice compared to SFTPC+/+ controls (data not shown). Neutrophil counts in lung lavage increased above baseline at 1 week after bleomycin and were increased in SFTPC−/− mice compared to SFTPC+/+ mice (Figure 2A). Likewise, neutrophil myeloperoxidase activity was higher in SFTPC−/− mice at 1 week after bleomycin treatment (Figure 2B). Baseline myeloperoxidase activity was similar in SFTPC−/− and SFTPC+/+ mice. Together, these studies show that deficiency of SP-C results in increased neutrophilic lung inflammation in response to bleomycin treatment.
Figure 2.
Increased neutrophilic lung inflammation in SFTPC−/− mice after bleomycin. A: Total lung lavage neutrophils were greater in SFTPC−/− mice compared to SFTPC+/+ mice at 1 week after bleomycin. n = 5 to 6 at each time point. *P < 0.05 compared to SFTPC+/+ at 1 week after bleomycin. B: Lung homogenates from SFTPC−/− mice had greater myeloperoxidase activity at 1 week after bleomycin compared to SFTPC+/+ mice. n = 7 for each group. *P < 0.05 compared to SFTPC+/+ 1 week after bleomycin.
Increased Lung Fibrosis and Delayed Remodeling in SFTPC−/− Mice after Bleomycin Treatment
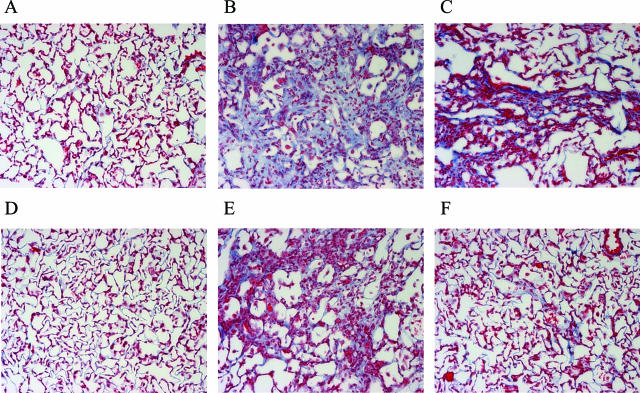
Before bleomycin treatment, lung histology was normal in both SFTPC−/− and SFTPC+/+ mice (Figure 3, A and D). Histological changes were present in both SFTPC−/− and SFTPC+/+ mice after bleomycin treatment, but changes were more marked in SFTPC−/− mice (Figure 3; B, C, E, and F). At 3 and 6 weeks after bleomycin, SFTPC−/− mice showed areas of lung parenchymal distortion characterized by formation of fibrotic foci consisting of fragments of destroyed interalveolar septa, bands of collagen fibrils, and recruited fibroblasts. The fibrotic changes were found throughout the lungs but were most prominent in centriacinar and subpleural regions.
Figure 3.
Increased collagen deposition in lungs of SFTPC−/− mice after bleomycin as assessed by Trichrome staining of lung sections (blue staining). Representative sections are shown for SFTPC−/− mice before bleomycin (A) and 3 weeks (B) and 6 weeks (C), and for SFTPC+/+ mice from the same time points (D–F). Original magnifications, ×200.
After bleomycin, lung collagen deposition was greater in SFTPC−/− mice than SFTPC+/+ mice as assessed by trichrome staining at 3 and 6 weeks (Figure 3; B, C, E, and F). In addition, morphometric analysis of lung fibrotic changes on lung sections indicated that fibrosis was greater in SFTPC−/− mice at 3 and 6 weeks after bleomycin (Figure 4). As can be seen in Figure 4 and in the representative slides in Figure 3, SFTPC−/− mice not only had greater fibrosis compared to SFTPC+/+ mice at 3 weeks, but they also had persistent fibrotic change at 6 weeks. Fibrosis scores from SFTPC+/+ mice were nearly back to baseline at this time point. Resolution of histological changes of fibrosis did not occur until 9 weeks in SFTPC−/− mice. These histological findings were supported by analysis of hydroxyproline content. By this measurement, collagen accumulation was greater in SFTPC−/− mice than SFTPC+/+ mice at 3 and 6 weeks after bleomycin (Figure 5). Consistent with histological scoring, SFTPC−/− mice still had increased lung collagen content at 6 weeks, whereas lung collagen content in SFTPC+/+ mice was similar to baseline.
Figure 4.
Increased lung fibrosis scores in SFTPC−/− mice after bleomycin treatment. In both SFTPC−/− and SFTPC+/+ mice, fibrosis scores increased after bleomycin, but significantly greater fibrotic changes were identified in the SFTPC−/− mice at both 3 and 6 weeks. n = 5 to 7 at each time point. *P < 0.05 compared to SFTPC+/+ at same time point.
Figure 5.
Increased collagen content in lungs of SFTPC−/− mice after bleomycin treatment. Hydroxyproline was assayed by high performance liquid chromatography and found to be greater in SFTPC−/− mice at 3 weeks and 6 weeks after bleomycin treatment. n ≥ 5 per group. *P < 0.05 compared to SFTPC+/+ at same time point.
In addition to the above measures to define differences in degree of lung fibrosis, we performed CXR imaging of SFTPC−/− and SFTPC+/+ mice at baseline and 3 weeks after bleomycin. SFTPC−/− mice had increased interstitial infiltrates by CXR imaging at 3 weeks after bleomycin, consistent with other measures of architectural distortion at this time point (Figure 6).
Figure 6.
Greater evidence of interstitial lung disease in SFTPC−/− mice by CXR imaging after bleomycin treatment. At baseline, SFTPC−/− mice (A) and SFTPC+/+ mice (B) had normal mouse chest radiographs. At 3 weeks after bleomycin SFTPC−/− mice (C) had greater radiographical evidence of interstitial lung disease compared to SFTPC+/+ (D) mice. Arrow points to area of interstitial lung disease in base of right lung. Images representative of three experiments.
To determine whether increased collagen deposi-tion and architectural distortion in bleomycin-treated SFTPC−/− mice was related to an increased number of effector fibroblasts, we immunostained lung sections for fibroblast-specific protein 1 (FSP1) (Figure 7). Recent studies in models of renal fibrosis18,25–28 and lung fibrosis19 have shown FSP1 to be a good marker for identifying tissue fibroblasts. FSP1+ cells were rare at baseline in SFTPC−/− and SFTPC+/+ mouse lungs, but increased significantly after bleomycin (Figure 7). As assessed by a semiquantitative fibroblast scoring, increased numbers of fibroblasts were identified in the lungs of SFTPC−/− mice compared to SFTPC+/+ mice at 3 and 6 weeks after bleomycin (Figure 8). Greater fibroblast numbers in the lungs of SFTPC−/− mice were consistent with the findings of increased fibrosis noted above.
Figure 7.
Immunostaining for FSP1+ fibroblasts (brown staining) in the lungs after bleomycin treatment. Representative sections are shown for SFTPC−/− mice from untreated mouse (A), 3 weeks (B), 6 weeks (C), and for SFTPC+/+ mice from the same time points (D–F). Original magnifications, ×300.
Figure 8.
Assessment of FSP1+ fibroblasts in lungs from SFTPC−/− and SFTPC+/+ mice after bleomycin using a semiquantitative scoring system. FSP1+ fibroblast numbers increased in both groups after bleomycin; however, greater numbers of FSP1+ fibroblasts were observed in the SFTPC−/− mice at 3 and 6 weeks. n = 5 to 7 at each time point. *P < 0.05 compared to SFTPC+/+ at same time point.
Expression of TGF-β1 was evaluated in our studies to determine whether increased levels of TGF-β1 might account for the increased lung fibrosis and increased numbers of fibroblasts seen in the SFTPC−/− mice. By immunohistochemistry, TGF-β1 expression increased to a similar degree after bleomycin in both SFTPC−/− and SFTPC+/+ mice after bleomycin treatment (data not shown). Quantitation of TGF-β1 in the lungs by ELISA revealed that despite having more extensive lung fibrosis, the lungs of SFTPC−/− mice had similar amounts of total TGF-β1 compared to SFTPC+/+ mice at baseline, 1, and 3 weeks after bleomycin treatment. At 1 week after bleomycin treatment, the TGF-β1 concentration in lung homogenate was 0.77 ± 0.04 pg/μg total protein in SFTPC−/− mice compared to 0.98 ± 0.07 pg/μg total protein in SFTPC+/+ mice (n = 7/group). Although bleomycin-treated SFTPC−/− mice did not have greater levels of total lung TGF-β1, we did not rule out the possibility of altered TGF-β1 activation in SFTPC−/− mice.
To investigate whether SFTPC-deficient mice were more susceptible to bleomycin-induced cellular injury, we evaluated the extent of TUNEL staining in lung tissue from SFTPC−/− and SFTPC+/+ mice. In both SFTPC−/− and SFTPC+/+ mice, 40 to 45% of lung parenchymal cells were TUNEL-positive 1 week after intratracheal bleomycin (Figure 9A). This finding is consistent with the widespread cellular injury that occurs in the bleomycin model and indicates that the extent of epithelial cell injury was similar in SFTPC−/− and SFTPC+/+ mice. The percentage of TUNEL-positive cells was similar in both groups of mice at baseline, 1, and 3 weeks after intratracheal bleomycin. However, at 6 weeks after bleomycin, the percentage of TUNEL-positive cells in lung parenchyma of SFTPC−/− mice remained at levels similar to 3 weeks, while the percentage of TUNEL-positive cells returned toward baseline in SFTPC+/+ mice (23.3 ± 2.3% cells TUNEL+ in SFTPC−/− mice, 12.0 ± 3.3% cells TUNEL+ in SFTPC+/+ mice, n = 5/group, P < 0.05) (Figure 9A).
Figure 9.
Analysis of cell injury/death after bleomycin treatment by TUNEL staining and immunostaining for active caspase-3. A: TUNEL staining was performed on lung tissue sections at baseline and 1, 3, 6, and 9 weeks after bleomycin and the percentage of TUNEL-positive cells was determined. At 6 weeks, SPC−/− mice had a greater percentage of TUNEL+ cells than SPC+/+ mice. n = 5 to 7 at each time point. *P < 0.05 compared to SFTPC+/+ at 6-week time point. B and C: Representative photomicrographs of active caspase-3 immunostaining are shown for SFTPC−/− mice (B) and SFTPC+/+ mice (C) at 6 weeks after bleomycin treatment. Increased numbers of cells positive for active caspase-3, including many alveolar epithelial cells, were present in lungs from SFTPC−/− mice. Arrows point to examples of positive cells. Original magnifications, ×400.
To specifically identify cells undergoing apoptosis, immunohistochemistry for active caspase-3 was performed. In contrast to TUNEL staining, cells with positive immunostaining for active caspase-3 were rarely seen at 0, 1, and 3 weeks, but were increased at 6 weeks after bleomycin treatment, particularly in SFTPC−/− mice (Figure 9, B and C). These findings indicate that the initial cellular injury and DNA fragmentation related to bleomycin exposure was followed by apoptotic cell death associated with caspase-3 activation during the period of tissue remodeling. At 6 weeks after bleomycin, active caspase-3 staining was observed in epithelial cells, mesenchymal cells, and alveolar macrophages in lungs of SFTPC−/− mice, whereas active caspase-3 staining was observed predominantly in alveolar macrophages in SFTPC+/+ mice. By 9 weeks, TUNEL and active caspase-3 staining in both SFTPC−/− and SFTPC+/+ mice returned to baseline. Together, these findings indicate that the protracted course of lung fibrosis in SFTPC−/− mice may be related to ongoing activation of proapoptotic pathways with continued turnover of epithelial cells and fibroblasts, potentially limiting the ability to restore normal parenchymal architecture.
Discussion
SFTPC−/− mice demonstrated greater susceptibility to the effects of intratracheal bleomycin than SFTPC+/+ mice, including greater weight loss and a higher mortality rate. In the early period after bleomycin, lung inflammation as defined by neutrophil influx was greater in SFTPC−/− mice. Subsequently, SFTPC−/− mice had more extensive lung fibrosis than SFTPC+/+ mice with increased lung collagen accumulation and increased radiographical changes in the lung parenchyma. Greater numbers of FSP1+ fibroblasts were noted in the lungs of SFTPC−/− mice after bleomycin, whereas levels of TGF-β1 in the lung were similar. In addition, SFTPC−/− mice had delayed resolution of fibrosis with ongoing apoptosis of cells in lung parenchyma. Together, these results indicate that SP-C affects multiple steps in the pathogenesis of bleomycin-induced lung fibrosis.
The hydrophobic surfactant proteins, SP-B and SP-C, contribute to the surface activity of pulmonary surfactant.29 Previous studies with SFTPC−/− mice (Black Swiss background) demonstrated altered stability of pulmonary surfactant in these mice.17 In addition to this primary function, experimental evidence in our studies and others indicate that SP-C has a function in pulmonary homeostasis and plays a role in lung injury, repair, and remodeling.17,30–36 SFTPC−/− mice bred into a congenic 129/SV background develop progressive pulmonary disease with parenchymal remodeling, airspace enlargement, and altered pulmonary mechanics.30 In contrast, SP-C-deficient mice used in our studies (outbred Black Swiss background) show no evidence of lung inflammation or structural abnormalities. However, when combined with partial SP-B deficiency, SFTPC−/− mice (Black Swiss background) developed increased levels of interleukin-6 and interleukin-1β in bronchoalveolar lavage and a more severe impairment of pulmonary function than wild-type mice after exposure to hyperoxia.31 Thus, although deficiency of SP-C can impact lung pathology, the phenotype related to lack of SP-C is strongly influenced by other genetic factors.
Although SP-A and SP-D have been recognized as being important in lung inflammation and host defense,37 studies are now revealing that the hydrophobic surfactant proteins SP-B and SP-C are also important in regulating these processes.31 In our model, neutrophilic inflammation was increased in the lungs of SFTPC−/− mice 1 week after bleomycin compared to SFTPC+/+ controls. The inflammatory response to bleomycin treatment is thought to result from direct injury to epithelial cells;38 however, increased lung inflammation in SFTPC−/− mice after bleomycin does not appear to be related to altered susceptibility to epithelial injury because TUNEL staining demonstrated equivalent numbers of TUNEL+ cells in the lungs of SFTPC−/− and SFTPC+/+ mice at early time points. The results of this study indicate that SP-C plays an important role in limiting inflammation after bleomycin-induced lung injury.
Previous studies have characterized the effect of bleomycin on surfactant protein levels in rats and rabbits.39,40 These studies have shown that levels of alveolar SP-B and SP-C decrease after bleomycin; however, SP-A and SP-D have both been shown to increase after bleomycin.39 In this study, we did not identify an appreciable change in the level of SP-A after bleomycin treatment. SP-D levels increased after bleomycin in both SFTPC−/− and SFTPC+/+ mice, but were not different between the two groups at any time point. We did find a decline in the levels of SP-B in both SFTPC−/− and SFTPC+/+ mice; however, SFTPC+/+ mice did not have an alteration in the level of alveolar SP-C after bleomycin. These findings of lack of alteration in SP-A and SP-C are likely explained by the relatively low dose of bleomycin in our studies compared to the previous studies in rats and rabbits.39,40
Persistent parenchymal remodeling and increased cell apoptosis were observed in the lungs of SFTPC−/− mice 6 weeks after bleomycin. Previous studies have shown that lung fibrosis in the bleomycin model is associated with alveolar epithelial cell apoptosis41 and that mice deficient in Fas are protected from bleomycin-induced fibrosis.42 Two different studies have shown that administration of a caspase inhibitor significantly decreased alveolar epithelial cell apoptosis and lung collagen accumulation after bleomycin treatment.43,44 Another study revealed that alveolar epithelial cell apoptosis was essential for development of TGF-β1-induced lung fibrosis.45 In lung biopsies from patients with idiopathic pulmonary fibrosis, alveolar epithelial cell apoptosis has been found in regions adjacent to areas of heavy myofibroblast activity and collagen deposition,46–48 and it is now thought that ongoing alveolar epithelial cell apoptosis is a key component in the progression of fibrosis in idiopathic pulmonary fibrosis.49 In these studies, the finding of increased apoptosis at 6 weeks in SFTPC−/− mice indicates that ongoing lung remodeling may contribute to the delayed resolution of bleomycin-induced fibrosis in these mice.
SP-C deficiency and genetic mutations in SFTPC have been implicated as a causative factor in familial forms of interstitial lung disease in children and adults. Nogee and colleagues7 first reported a mutation in SFTPC that was associated with interstitial lung disease in a mother and infant child. Mature SP-C was not detected in lung tissue or bronchoalveolar lavage of the affected patient. Subsequently, Amin and colleagues8 reported a mother and two children with interstitial lung disease who had an absence of SP-C on bronchoalveolar lavage and a marked decrease in proSP-C, the precursor protein to mature SP-C, in alveolar epithelial cells. Since these reports, multiple childhood cases of interstitial lung disease associated with mutations in SFTPC have been identified.9 In addition, a large kindred has been reported in which 14 family members had pulmonary fibrosis, including usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis, in association with a mutation in the carboxy-terminal region of proSP-C.10
Previous studies have indicated that expression of mutant SP-C can lead to an abnormal protein product with aberrant intracellular processing and subsequent toxicity to the type II alveolar epithelial cell.12,50 Transcription of the SFTPC gene results in a 197 amino acid precursor protein (proSP-C).11 Within the cytoplasm of the type II alveolar cell, processing of proSP-C results in the functional, highly hydrophobic 35 amino acid SPC protein that is secreted into the alveolar space.11,51 In vitro studies have revealed that mutations in the C-terminal region of the SFTPC gene cause abnormal processing and misfolding of the protein with aggregation in secretory compartments, resulting in endoplasmic reticulum stress.12,52–54 One of the SFTPC mutations associated with human lung disease has been expressed in a transgenic mouse line and resulted in disrupted lung morphogenesis and murine fetal death, with the results from the study supporting the concept of protein misfolding and aberrant surfactant processing.55 Our study supports the concept that deficiency of mature SP-C, in addition to type II alveolar epithelial cell dysfunction, can impact the pathogenesis of pulmonary fibrosis in humans.
Although the degree to which SP-C deficiency may be important in pulmonary fibrosis is not entirely known, our studies demonstrate that the type II epithelial cell derived product SP-C functions to limit lung inflammation, reduce collagen accumulation, and hasten resolution of bleomycin-induced lung fibrosis. These findings highlight the critical nature of the epithelium and epithelial derived products in regulating the course of lung fibrosis.
Acknowledgments
We thank the Mouse Pathology and Immunostaining Core Facility at Vanderbilt University Medical Center for their assistance with the lung tissue slide preparations, Tamara Lasakow for editorial assistance, and Janet Shelton for assistance with manuscript preparation.
Footnotes
Address reprint requests to William E. Lawson, M.D., Instructor of Medicine, Division of Allergy, Pulmonary, and Critical Care, Vanderbilt University School of Medicine, T-1218 MCN, Nashville, TN 37232-2650. E-mail: william.lawson@vanderbilt.edu.
Supported by the National Institutes of Health (grants HL68121, HL66196, HL07123, HL61646), the American Lung Association, the Parker B. Francis Fellowship Program, and the Greek State Scholarship Foundation.
W.E.L. is a Parker B. Francis Fellow in Pulmonary Research.
References
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- Marshall RP, McAnulty RJ, Laurent GJ. The pathogenesis of pulmonary fibrosis: is there a fibrosis gene? Int J Biochem Cell Biol. 1997;29:107–120. doi: 10.1016/s1357-2725(96)00141-0. [DOI] [PubMed] [Google Scholar]
- Marshall RP, Puddicombe A, Cookson WO, Laurent GJ. Adult familial cryptogenic fibrosing alveolitis in the United Kingdom. Thorax. 2000;55:143–146. doi: 10.1136/thorax.55.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman PB, Rennard SI, Keogh BA, Wewers MD, Adelberg S, Crystal RG. Familial idiopathic pulmonary fibrosis. Evidence of lung inflammation in unaffected family members. N Engl J Med. 1986;314:1343–1347. doi: 10.1056/NEJM198605223142103. [DOI] [PubMed] [Google Scholar]
- Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax. 2002;57:338–342. doi: 10.1136/thorax.57.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- Amin RS, Wert SE, Baughman RP, Tomashefski Jr JF, Nogee LM, Brody AS, Hull WM, Whitsett JA. Surfactant protein deficiency in familial interstitial lung disease. J Pediatr. 2001;139:85–92. doi: 10.1067/mpd.2001.114545. [DOI] [PubMed] [Google Scholar]
- Nogee LM, Dunbar AE, III, Wert S, Askin F, Hamvas A, Whitsett JA. Mutations in the surfactant protein C gene associated with interstitial lung disease. Chest. 2002;121:20S–21S. doi: 10.1378/chest.121.3_suppl.20s. [DOI] [PubMed] [Google Scholar]
- Thomas AQ, Lane K, Phillips J, III, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, Roberts R, Haines J, Stahlman M, Loyd JE. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- Weaver TE. Synthesis, processing and secretion of surfactant proteins B and C. Biochim Biophys Acta. 1998;1408:173–179. doi: 10.1016/s0925-4439(98)00066-0. [DOI] [PubMed] [Google Scholar]
- Beers MF, Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol. 2005;67:663–696. doi: 10.1146/annurev.physiol.67.040403.101937. [DOI] [PubMed] [Google Scholar]
- Lazo JS, Hoyt DG, Sebti SM, Pitt BR. Bleomycin: a pharmacologic tool in the study of the pathogenesis of interstitial pulmonary fibrosis. Pharmacol Ther. 1990;47:347–358. doi: 10.1016/0163-7258(90)90061-6. [DOI] [PubMed] [Google Scholar]
- Bowden DH. Unraveling pulmonary fibrosis: the bleomycin model. Lab Invest. 1984;50:487–488. [PubMed] [Google Scholar]
- Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992;18:29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- Glasser SW, Burhans MS, Korfhagen TR, Na CL, Sly PD, Ross GF, Ikegami M, Whitsett JA. Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc Natl Acad Sci USA. 2001;98:6366–6371. doi: 10.1073/pnas.101500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson WE, Polosukhin VV, Zoia O, Stathopoulos GT, Han W, Plieth D, Loyd JE, Neilson EG, Blackwell TS. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:899–907. doi: 10.1164/rccm.200311-1535OC. [DOI] [PubMed] [Google Scholar]
- Hoff CR, Perkins DR, Davidson JM. Elastin gene expression is upregulated during pulmonary fibrosis. Connect Tissue Res. 1999;40:145–153. doi: 10.3109/03008209909029110. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Lancaster LH, Blackwell TR, Venkatakrishnan A, Christman JW. Chemotactic gradients predict neutrophilic alveolitis in endotoxin-treated rats. Am J Respir Crit Care Med. 1999;159:1644–1652. doi: 10.1164/ajrccm.159.5.9806166. [DOI] [PubMed] [Google Scholar]
- Pryhuber GS, Hull WM, Fink I, McMahan MJ, Whitsett JA. Ontogeny of surfactant proteins A and B in human amniotic fluid as indices of fetal lung maturity. Pediatr Res. 1991;30:597–605. doi: 10.1203/00006450-199112000-00023. [DOI] [PubMed] [Google Scholar]
- Akinbi HT, Breslin JS, Ikegami M, Iwamoto HS, Clark JC, Whitsett JA, Jobe AH, Weaver TE. Rescue of SP-B knockout mice with a truncated SP-B proprotein. Function of the C-terminal propeptide. J Biol Chem. 1997;272:9640–9647. doi: 10.1074/jbc.272.15.9640. [DOI] [PubMed] [Google Scholar]
- Noah TL, Murphy PC, Alink JJ, Leigh MW, Hull WM, Stahlman MT, Whitsett JA. Bronchoalveolar lavage fluid surfactant protein-A and surfactant protein-D are inversely related to inflammation in early cystic fibrosis. Am J Respir Crit Care Med. 2003;168:685–691. doi: 10.1164/rccm.200301-005OC. [DOI] [PubMed] [Google Scholar]
- Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- Okada H, Danoff TM, Fischer A, Lopez-Guisa JM, Strutz F, Neilson EG. Identification of a novel cis-acting element for fibroblast-specific transcription of the FSP1 gene. Am J Physiol. 1998;275:F306–F314. doi: 10.1152/ajprenal.1998.275.2.F306. [DOI] [PubMed] [Google Scholar]
- Iwano M, Fischer A, Okada H, Plieth D, Xue C, Danoff TM, Neilson EG. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol Ther. 2001;3:149–159. doi: 10.1006/mthe.2000.0251. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Conkright JJ. Function of surfactant proteins B and C. Annu Rev Physiol. 2001;63:555–578. doi: 10.1146/annurev.physiol.63.1.555. [DOI] [PubMed] [Google Scholar]
- Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J Biol Chem. 2003;278:14291–14298. doi: 10.1074/jbc.M210909200. [DOI] [PubMed] [Google Scholar]
- Ikegami M, Weaver TE, Conkright JJ, Sly PD, Ross GF, Whitsett JA, Glasser SW. Deficiency of SP-B reveals protective role of SP-C during oxygen lung injury. J Appl Physiol. 2002;92:519–526. doi: 10.1152/japplphysiol.00459.2001. [DOI] [PubMed] [Google Scholar]
- Hawgood S, Ogawa A, Yukitake K, Schlueter M, Brown C, White T, Buckley D, Lesikar D, Benson B. Lung function in premature rabbits treated with recombinant human surfactant protein-C. Am J Respir Crit Care Med. 1996;154:484–490. doi: 10.1164/ajrccm.154.2.8756826. [DOI] [PubMed] [Google Scholar]
- Davis AJ, Jobe AH, Hafner D, Ikegami M. Lung function in premature lambs and rabbits treated with a recombinant SP-C surfactant. Am J Respir Crit Care Med. 1998;157:553–559. doi: 10.1164/ajrccm.157.2.97-08019. [DOI] [PubMed] [Google Scholar]
- Hafner D, Germann PG, Hauschke D. Effects of rSP-C surfactant on oxygenation and histology in a rat-lung-lavage model of acute lung injury. Am J Respir Crit Care Med. 1998;158:270–278. doi: 10.1164/ajrccm.158.1.9712061. [DOI] [PubMed] [Google Scholar]
- Lewis J, McCaig L, Hafner D, Spragg R, Veldhuizen R, Kerr C. Dosing and delivery of a recombinant surfactant in lung-injured adult sheep. Am J Respir Crit Care Med. 1999;159:741–747. doi: 10.1164/ajrccm.159.3.9806069. [DOI] [PubMed] [Google Scholar]
- Spragg RG, Smith RM, Harris K, Lewis J, Hafner D, Germann P. Effect of recombinant SP-C surfactant in a porcine lavage model of acute lung injury. J Appl Physiol. 2000;88:674–681. doi: 10.1152/jappl.2000.88.2.674. [DOI] [PubMed] [Google Scholar]
- Floros J, Hoover RR. Genetics of the hydrophilic surfactant proteins A and D. Biochim Biophys Acta. 1998;1408:312–322. doi: 10.1016/s0925-4439(98)00077-5. [DOI] [PubMed] [Google Scholar]
- Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- Savani RC, Godinez RI, Godinez MH, Wentz E, Zaman A, Cui Z, Pooler PM, Guttentag SH, Beers MF, Gonzales LW, Ballard PL. Respiratory distress after intratracheal bleomycin: selective deficiency of surfactant proteins B and C. Am J Physiol. 2001;281:L685–L696. doi: 10.1152/ajplung.2001.281.3.L685. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Ruppert C, Markart P, Lubke N, Ermert L, Weissmann N, Breithecker A, Ermert M, Seeger W, Gunther A. Changes in pulmonary surfactant function and composition in bleomycin-induced pneumonitis and fibrosis. Toxicol Appl Pharmacol. 2004;195:218–231. doi: 10.1016/j.taap.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Hagimoto N, Kuwano K, Nomoto Y, Kunitake R, Hara N. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 1997;16:91–101. doi: 10.1165/ajrcmb.16.1.8998084. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, Hara N. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest. 1999;104:13–19. doi: 10.1172/JCI5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ibarra-Sunga O, Verlinski L, Pick R, Uhal BD. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol. 2000;279:L143–L151. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Kunitake R, Maeyama T, Hagimoto N, Kawasaki M, Matsuba T, Yoshimi M, Inoshima I, Yoshida K, Hara N. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol. 2001;280:L316–L325. doi: 10.1152/ajplung.2001.280.2.L316. [DOI] [PubMed] [Google Scholar]
- Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol. 1998;275:L1192–L1199. doi: 10.1152/ajplung.1998.275.6.L1192. [DOI] [PubMed] [Google Scholar]
- Uhal BD. Apoptosis in lung fibrosis and repair. Chest. 2002;122:293S–298S. doi: 10.1378/chest.122.6_suppl.293s. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, Nakanishi Y, Hara N. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154:477–483. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- Nogee LM. Abnormal expression of surfactant protein C and lung disease. Am J Respir Cell Mol Biol. 2002;26:641–644. doi: 10.1165/ajrcmb.26.6.f241. [DOI] [PubMed] [Google Scholar]
- Beers MF, Lomax CA, Russo SJ. Synthetic processing of surfactant protein C by alevolar epithelial cells. The COOH terminus of proSP-C is required for post-translational targeting and proteolysis. J Biol Chem. 1998;273:15287–15293. doi: 10.1074/jbc.273.24.15287. [DOI] [PubMed] [Google Scholar]
- Keller A, Steinhilber W, Schafer KP, Voss T. The C-terminal domain of the pulmonary surfactant protein C precursor contains signals for intracellular targeting. Am J Respir Cell Mol Biol. 1992;6:601–608. doi: 10.1165/ajrcmb/6.6.601. [DOI] [PubMed] [Google Scholar]
- Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol. 2005;32:521–530. doi: 10.1165/rcmb.2005-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JP, Wert SE, Nogee LM, Weaver TE. Expression of a human surfactant protein C mutation associated with interstitial lung disease disrupts lung development in transgenic mice. J Biol Chem. 2003;278:52739–52746. doi: 10.1074/jbc.M309599200. [DOI] [PubMed] [Google Scholar]