Abstract
Mature and immature myeloid dendritic cells (DCs) are thought to differentially modulate T-cell responses in secondary lymphoid tissues. Although mature DCs are believed to induce T-cell activation under proinflammatory conditions, immature DCs are believed to maintain a state of T-cell tolerance under steady state conditions. However, little is known about the actual activation state of human DCs under these different conditions. Here, we compare the frequency and activation state of human DCs between matched skin and sentinel lymph node (SLN) samples, after intradermal administration of either granulocyte/macrophage colony-stimulating factor (GM-CSF) or saline, at the excision site of stage I primary melanoma. Although DCs remained immature (CD1a+CD83−) and mostly situated in the epidermis of the saline-injected skin (fully consistent with a quiescent steady state), mature (CD1a+CD83+) DC frequencies significantly increased in the GM-CSF-injected skin and correlated with the number of mature DCs in the SLN, indicative of increased DC migration. Interestingly, irrespective of GM-CSF or saline administration, all CD1a+ myeloid DCs in the SLN were phenotypically mature (ie, CD83+). These data are indicative of migration of small numbers of phenotypically mature DCs to lymph nodes under steady state conditions.
Antigen-specific cutaneous immune responses are initiated by epidermal and dermal dendritic cells (DCs).1 The majority of DCs in the skin are Langerhans cells (LCs), residing in the epidermis in an immature state. These immature LCs (iLCs) derive from CD34+ hematopoietic progenitor cells.2 LC precursors home from the bone marrow to the skin, where they differentiate to actively phagocytic LCs. When an antigen is encountered under proinflammatory conditions, LCs are activated and start migrating to regional lymph nodes (LNs), synthesizing new MHC molecules and up-regulating CD80, CD86, and other co-stimulatory and adhesion molecules. On arrival in the LN they have become mature DCs (mDCs). Expression of the chemokine receptor CCR7 facilitates their migration to the paracortical areas where T cells reside and may then be primed. Recently it has been suggested that immature DCs (iDCs) also migrate to the LN to induce peripheral T-cell tolerance in the steady state, this way preventing autoimmunity.3,4 Data that support iDCs migrating to the LN to induce peripheral T-cell tolerance, originate mainly from murine studies.5,6 In humans iLCs were reported in skin-draining LNs only under chronic inflammatory conditions.7 So far, evidence for the presence of iDCs in LNs under normal steady state conditions is lacking. In melanoma, LCs take up and transport tumor-associated antigens to tumor-draining lymph nodes (TDLNs).8,9 To subsequently activate melanoma-specific T cells, the migrated LCs need to become activated.10 DC development and activation can both be frustrated by inhibitory factors commonly associated with melanoma, such as IL-10 or gangliosides.9,11,12 iDCs with ready access to tumor-associated antigens from the tumor may induce specific tolerance through inappropriate or abortive T-cell activation.13,14 DCs in TDLNs were similarly reported to display immature characteristics.15 The degree of immunosuppression in TDLNs is directly related to their distance to the primary tumor, indicating the causative agents to be tumor-derived. The first LN to directly drain the primary tumor, the so-called sentinel lymph node (SLN), is the preferential site of early metastasis16–18 and shows the most pronounced immunosuppression.19,20 Clearly, this crippling of DC functions in the first line of immunological defense will frustrate specific T-cell activation and increase the chance of tumor immune escape and metastatic spread.20,21 To overcome this suppression, we recently administered intradermal injections of granulocyte/macrophage colony-stimulating factor (GM-CSF) around the excision site of primary melanoma tumors and found increased numbers and activation state of DCs in the paracortical areas of the SLN.22 In the same study, the absence of iDCs in the TDLNs of the saline control group seemed to contrast with the currently dominant school of thought that holds iDCs in the TDLN to be primarily responsible for cancer-associated immune tolerance. Patients included in this study underwent re-excision of the scar of the primary melanoma excision at the same time as the SLN procedure. This gave us the unique opportunity to compare the phenotype of DCs in the skin to their phenotype in the exactly corresponding SLN after local administration of either recombinant human GM-CSF or saline.
The results reported here are consistent with increased migration of large numbers of mature CD83+ LCs through the dermis of GM-CSF-injected skin to the corresponding SLN. In contrast, a quiescent steady state prevails in the control group with iLCs scattered throughout the epidermis and only small numbers of isolated CD83+ LCs in the dermis. Nevertheless, iDCs (CD1a+CD83−) are completely absent in the SLN under both these conditions. We conclude that small numbers of mDCs migrate to LN under steady state conditions and that these are apparently responsible for a maintained state of tolerance under these conditions.
Materials and Methods
Patients
Twelve patients with stage I melanoma according to criteria of the American Joint Committee on Cancer (Breslow thickness, ≤1.5 mm; patient age, 18 to 70 years) were included in this single-blinded phase II study. All patients were scheduled to undergo a SLN procedure and re-excision of the scar of the primary melanoma excision. Re-excision of the scar of the primary tumor in all cases took place subsequent to SLN excision, during the same operative procedure. An excision margin of 1 cm was applied, as routine for melanoma with a Breslow thickness ≤2 mm in our hospital. They were randomly assigned to preoperative local administration of either recombinant human GM-CSF or saline. Patients who had undergone previous immunotherapy or chemotherapy were excluded as well as patients receiving immunosuppressive medication or suffering from any autoimmune disorder. The medical ethical committee of the VU University Medical Center approved the study and written informed consent was obtained from each patient before treatment. There were no significant differences between the patient groups in terms of gender, age (average, 57 ± 12 years), or Breslow thickness (average, 0.94 ± 0.30 mm). None of the patients had a tumor-positive SLN. In the saline and GM-CSF groups, respectively, four and five of the primary tumors were located on the trunk and, respectively, two and one were located on the extremities. For the saline group this resulted in the excision of the SLN from the groin area in two patients and from the axilla in four patients. In the GM-CSF group all SLNs were located in the axilla.
GM-CSF Administration
Both patient groups received daily intradermal injections, with either 3 μg per kg body weight rhGM-CSF (Leucomax; Schering Plough, Maarssen, The Netherlands), dissolved in 1.0 ml of saline or 1.0 ml of plain saline. These injections were given directly adjacent to the scar of the primary melanoma excision, from day −3 until day 0 (just before surgery).
Triple-Technique SLN Procedure and Isolation of Viable SLN Cells
On day 0, patients underwent a triple-technique SLN procedure as described previously.18,23 Immediately after removal, SLNs were collected in sterile ice-cold complete medium, comprising IMDM supplemented with 25 mmol/L Hepes buffer (BioWhittaker, Verviers, Belgium) with 10% fetal calf serum, 50 IU/ml penicillin-streptomycin, 1.6 mmol/L l-glutamine, and 0.05 mmol/L β-mercaptoethanol. Before routine histopathological examination of the SLN, viable cells were isolated using a previously described cytological scraping method.24 In short, after measuring the size of the SLN, it was bisected crosswise with a surgical scalpel and the cutting surface of the SLN was scraped 10 times with a surgical blade (size no. 22; Swann Morton Ltd., UK). SLN cells were rinsed from the blade with medium containing 0.1% DNase I, 0.14% collagenase A (Boehringer-Mannheim, Mannheim, Germany), and 5% fetal calf serum, incubated for 45 minutes at 37°C, and subsequently in phosphate-buffered saline with 5 mmol/L ethylenediamine tetraacetic acid for 10 minutes on ice. Finally, the SLN cells were washed twice in complete medium, counted, and further processed. After isolation of viable SLN cells, the bisected SLN was examined meticulously by the pathologist according to routine diagnostic procedures.25
Flow Cytometry
Freshly isolated SLN cells were directly stained with antibodies labeled with either phycoerythrin or fluorescein isothiocyanate, and analyzed by flow cytometry at 100,000 events per measurement, as previously described.24 Monoclonal antibodies against CD1a, CD86 (Pharmingen, San Diego, CA), and CD83 (Immunotech, Marseille, France) were used.
Immunocytochemistry
Cytospin preparations of SLN cells were acetone-fixed and stained immunocytochemically as described previously.24 Monoclonal antibodies against CD1a, CD3, CD86 (Becton Dickinson, San Jose, CA), CD83 (Immunotech), and S100 (DAKO A/S, Glostrup, Denmark) were used. The number of positively stained DCs was determined using an interactive video morphometry system (Q-PRODIT; Leica, Cambridge, UK). The outer border of each cytospot was demarcated at a 100-fold magnification and 40 fields of vision were randomly selected in an automated manner for subsequent evaluation.26 The total number of CD3+ T cells was counted in these 40 fields of vision and used to correct for cell density of the cytospots of each patient. In each field of vision the number of DCs was counted on the basis of positive staining of specific markers and DC morphology. Results are listed as total number of DCs normalized per 600 CD3+ T cells (ie, the mean number of T cells detected per 40 fields of vision).
Immunohistochemistry of the Skin
Paraffin-embedded re-excision skin biopsies were available for 11 of the 12 patients. Paraffin sections were mounted on Superfrost Plus glass slides and dried overnight at 37°C. After deparaffination, the tissue sections were hydrated through decreasing (v/v) percentages of ethanol and endogenous peroxidase was blocked with 0.1% hydrogen peroxide in methanol. Tissue sections were pretreated with 10 mmol/L citrate (pH 6) in an autoclave for 21 minutes at 121°C (for Langerin, CD83, CD14, and an isotype-matched IgG1 control antibody, MOPC21) or in a microwave at 100°C for 10 minutes (CD1a and CD68). Other tissue sections were pretreated with 10 mmol/L Tris/1 mmol/L ethylenediamine tetraacetic acid (pH 9) in a microwave at 100°C for 10 minutes (CD3), or with protease (S100; Ventana, Tucson, AZ). All antibodies (except CD68 and S100) were applied and incubated at room temperature for 1 hour. Detection and visualization (with diaminobenzidine/hydrogen peroxide) were performed with the DAKO Chemmate Envision detection kit (Dakopatts, Glostrup, Denmark) as described for CD1a, CD83, CD14, and CD3 or with NeoMarkers Labvision kit (NeoMarkers, Fremont, CA) for Langerin. For the CD68 antibody and the S100 antibody an automated immunostainer (Ventana) was used for pretreatment (protease, S100), incubation, detection, and visualization steps according to standard procedures. Sections were counterstained with hematoxylin, dehydrated, and mounted. The used antibodies and pretreatments are summarized in Table 1. Tonsillar tissue sections were used as positive control samples.
Table 1.
Markers Tested and Immunohistochemical Detection Methods
| Marker | Marker description | Dilution | Pretreatment | Buffer | Detection |
|---|---|---|---|---|---|
| CD1a* | MHC-like molecule, expressed on immature and mature myeloid DCs | 1:5 | Microwave | Citrate (pH 6) | Envision |
| Langerin* | C-type lectin, immature Langerhans cell marker | 1:50 | Autoclave | Citrate (pH 6) | Labvision |
| CD83* | Immunoglobulin superfamily member, DC maturation marker | 1:25 | Autoclave | Citrate (pH 6) | Envision |
| CD14* | Lipopolysaccharide co-receptor, expressed on myelomonocytic cells | 1:25 | Autoclave | Citrate (pH 6) | Envision |
| CD68† | Lysosome-associated molecule, macrophage marker | 1:400 | Microwave | Citrate (pH 6) | ABC method |
| S100† | Intracellular calcium-binding protein, expressed on activated Langerhans cells and myeloid DCs in lymph nodes | 1:400 | Protease§ | ABC method | |
| CD3† | T-cell receptor-associated signaling complex | 1:100 | Microwave | Tris/EDTA (pH 9) | Envision |
| MOPC21‡ | Isotype control antibody | 1:100 | Autoclave | Citrate (pH 6) | Envision |
Novocastra, Newcastle upon Tyne, UK.
Dakopatts, Glostrup, Denmark.
Organon Teknika-Cappel, Boxtel, The Netherlands.
Ventana, Tuscon, AZ.
Quantitation
All slides were coded and counted by two independent observers. The number of CD1a-, Langerin-, CD83-, CD14-, CD68-, S100-, and CD3-positive cells in the epidermis, the superficial papillary dermis, and the deep reticular dermis were evaluated by direct counting of stained nucleated cell bodies per ×400 magnification microscopic field [ie, high-power field (HPF)]. Each observer counted 10 HPFs in the epidermis, in the superficial dermis, defined as the HPF adjacent to the epidermis, and in the deep dermis for each slide. For CD3, five adjacent HPFs were counted, in the epidermis, superficial, and deep dermis. Counts were expressed as mean number (averaged between the independent observers) of positive cells per HPF.
Statistical Analysis
Differences between patient study groups were analyzed using the two-sample Mann-Whitney U-test and considered significant when P was <0.05. Correlations were calculated using Spearman’s ρ test and also considered significant when P was <0.05.
Results and Discussion
Intradermal Injection of GM-CSF Induces Maturation and Migration of Skin DCs
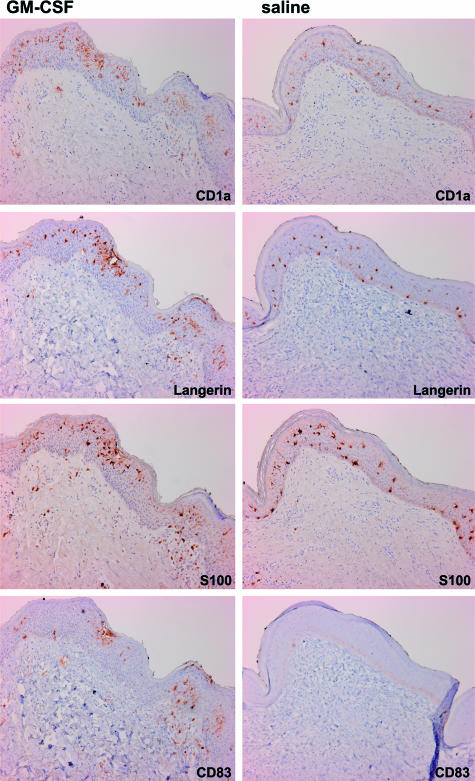
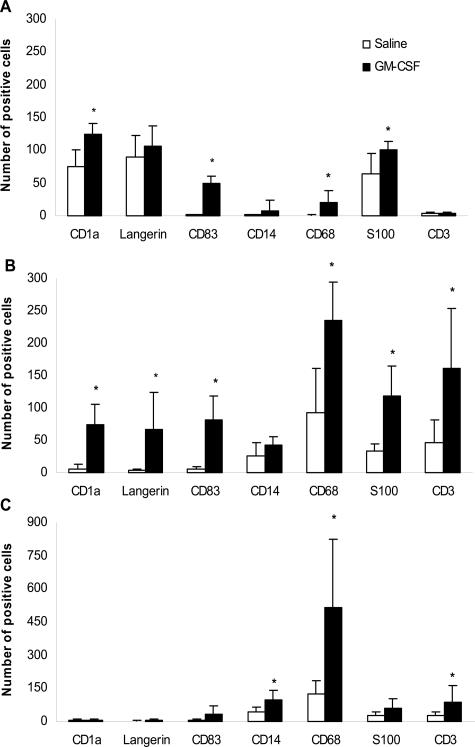
Immunohistochemical analysis of the skin was performed to study the effects of preoperative intradermal injections of GM-CSF and saline on the DCs of the epidermis, superficial dermis, and deep dermis (Figure 1). We found significantly more CD1a+, CD83+, and S100+ DCs in the epidermis of the GM-CSF-administered patients (Figure 2A). Increased numbers of CD1a+ epidermal DCs and the presence of CD83+ and S100+ DCs, scattered throughout the epidermis, is highly suggestive of an ongoing recruitment of LCs (precursors) and a simultaneous migration of maturing LCs from the epidermis, both under the influence of preoperative GM-CSF administration for 4 consecutive days. Our results differ from those reported by Smith and colleagues1 who studied the effect of GM-CSF on LCs in normal and healthy atopic volunteers and found that intradermal injections of GM-CSF led to a reduction of CD1a+ DCs in the epidermis in parallel with increased numbers in the dermis. This may be explained by the difference in administered GM-CSF dosages: ie, 0.05 μg in their study versus 3 μg/kg body weight in our study. The relatively high doses in our study may have led to significantly more CD1a+ cells in the epidermis resulting from an ongoing recruitment of new LCs (precursors).
Figure 1.
DC phenotype and localization in primary tumor re-excision skin samples after intradermal administration of saline or GM-CSF. Immunohistochemical analysis for the indicated DC markers from two representative melanoma patients (stage I) after intradermal injections of either saline or GM-CSF. Original magnifications, ×400.
Figure 2.
Cutaneous DC, macrophage, and T-cell frequencies in relation to saline or GM-CSF administration. Numbers of DCs and T cells in the epidermis (A), superficial (B), and deep dermis (C), counted in paraffin-embedded slides after immunohistochemical analysis of the indicated markers. All tissue samples were obtained through re-excision of the scar of the primary melanoma excision. Five patients were given GM-CSF around the scar tissue and six patients were given saline. Results are shown as mean number per 10 HPFs (HPF at ×400 magnification), except for CD3: mean per five HPFs. *Significant in a Mann-Whitney U-test at P < 0.05.
A significant increase in the amount of CD1a+, Langerin+, CD83+, and S100+ DCs was found in the superficial dermis of the GM-CSF-injected skin (Figure 2B). The equal amount of cells in the superficial dermis expressing CD1a, Langerin, and CD83, as well as their co-localization in consecutive sections (Figure 1), suggests that these are all CD83+ mature LCs in the process of GM-CSF-induced migration.
In the deep dermis of both the saline- and the GM-CSF-administered patients, only low amounts of CD1a+, Langerin+, CD83+, and S100+ DCs were found (Figure 2C). This is consistent with DCs entering and migrating via lymph vessels, which are mainly situated in the superficial dermis. Significantly increased numbers of CD68+ macrophages were found throughout the skin, including the deep dermis, under the influence of intradermal injections of GM-CSF. In line with the recruitment of immune effector cells by the activated DCs and by macrophages, we also found significantly more CD3+ T cells infiltrating the superficial and the deep dermis on GM-CSF administration (Figure 2, B and C).
Strong Correlation between Frequencies of Mature DCs in the Superficial Dermis and the SLN
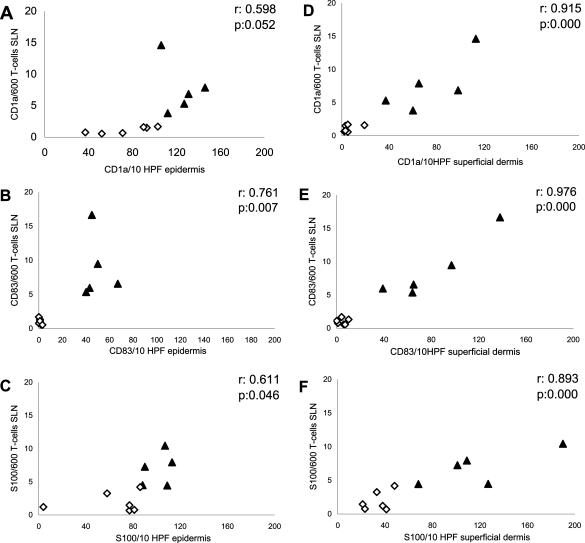
The numbers of CD1a-, CD83-, and S100-positive DCs correlated strongly and significantly between the superficial dermis and the SLN (Figure 3; D to F). A less perfect correlation was observed between the numbers of CD1a-, CD83-, and S100-positive DCs in the epidermis and the SLN (Figure 3; A to C). These observations are consistent with the contiguous nature of the superficial dermis and the SLN, with mature DCs migrating between these compartments through the lymph vessels. The higher numbers of mature DCs in both compartments after GM-CSF administration (Figure 3; A to F) is consistent with DC maturation induction and an increased DC migration rate.
Figure 3.
Correlation between DC numbers in the epidermis or superficial dermis and in the SLN. The numbers of DCs positive for CD1a, CD83, and S100 in the SLN (expressed as number per 600 T cells: quantified on cytospins from SLN cell suspensions), correlated to the number of DCs positive for CD1a (A), CD83 (B), and S100 (C) in the epidermis and CD1a (D), CD83 (E), and S100 (F) in the superficial dermis (all shown as mean numbers per 10 HPFs, HPF at ×400 magnification). ⋄Patients treated with saline; ▴patients treated with GM-CSF. Correlations were calculated using Spearman’s ρ test: r and P values are displayed.
Only Mature DCs Migrate to the SLN
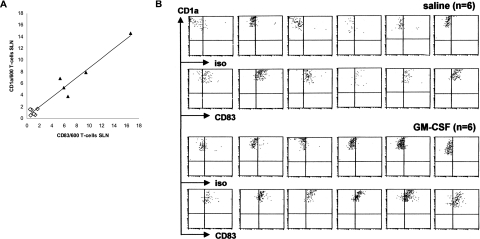
The correlation between the number of CD1a+ DCs and of CD83+ DCs in the SLN is very strong and near linear (r = 0.836, P = 0.001; Figure 4A). Flow cytometric analysis of SLN single cell suspensions supports these findings (Figure 4B), revealing all CD1a+ DCs to be CD83+, irrespective of GM-CSF administration. This shows a lack of CD1a+, CD83− immature DCs in the SLN, which implies that only CD83+ mDCs migrate to the skin-draining LN. This is contrary to the currently prevailing idea that iDCs in the LN induce peripheral T-cell tolerance in a steady state.3,4 It is also in contrast with findings by Geissmann and colleagues7 who reported that DCs in the human skin-draining LN were primarily immature Langerin+ cells. An explanation for this apparent discrepancy might be that all patients included in the study of Geissmann and colleagues,7 were suffering from dermatopathic lymphadenitis. Chronic inflammation of the skin may have led to a high local turnover rate of DCs, leading to abnormal iDC migration. Human lymph from normal skin (steady state) was sampled by Brand and colleagues,27 by means of microsurgical lymph cannulation. It was found that virtually all migrating CD1a+ cells under these conditions co-expressed CD80. Although the expression of CD83 was not tested, these data nevertheless indicate that skin-originating DCs in afferent lymph have a mature phenotype, which is in line with our own observation that the expression of CD80 is closely linked to that of CD83 and CCR7 on LCs migrating from ex vivo cultured skin explants (unpublished data). In a recent murine study by Stoitzner and colleagues,28 it was also shown that LCs trafficking into the skin-draining LN in the steady state express the maturation marker 2A1, the co-stimulatory molecules CD86 and CD40, and high levels of MHC class II. After application of contact allergen, a small but consistent increase in the expression of CD86 and CD40 was seen. This study thus confirms our findings that even in the steady state, LCs migrate from the epidermis in a mature state. Further activation (eg, by GM-CSF) only leads to higher numbers of mature LCs that migrate to the SLN with a coinciding up-regulation of co-stimulatory markers such as CD86 and CD40.22
Figure 4.
Myeloid DCs in skin-draining SLNs have a CD83+ mature phenotype, irrespective of intradermal GM-CSF or saline administration. A: Correlation between the number of CD1a+ DCs and CD83+ DCs in the SLN (expressed as number per 600 T cells) of patients treated with saline (⋄) and patients treated with GM-CSF (▴) reveals a linear relationship (Spearman’s ρ r = 0.836, P = 0.001). B: Flow cytometric analysis of CD83 expression on myeloid DCs in SLN single cell suspensions from all saline- and GM-CSF-treated melanoma patients. Myeloid DCs were gated by CD1a positivity and high side scatter levels.
Small Numbers of Mature DCs May Maintain Tolerance in the Steady State LN
In conclusion, our findings do not support the hypothesis that iDCs induce peripheral T-cell tolerance in the steady state. On the contrary, we have found evidence to suggest that small numbers of mDCs that migrate to the LN are responsible for the maintenance of tolerance. We do realize that our data are from stage I melanoma patients and as such may not qualify as normal steady state. However, the primary melanoma was previously resected (on average 89 days before the re-excision and SLN procedure) and none of the tested patients harbored metastases in the SLN, minimizing any tumor-associated effects at the time of testing.
The observed mature phenotype of DCs in SLN under these quiescent conditions (as established in the corresponding skin samples) is in keeping with a previous study by Albert and colleagues,29 who reported the necessity of DC maturation for the cross-tolerance of cytotoxic T lymphocytes. Cytotoxic T-lymphocyte activation required further CD40-mediated activation of the DCs by Th cells, which is accompanied by the release of cytokines essential for cytotoxic T-lymphocyte activation, such as IL-12. Thus, as previously suggested by Shortman and Heath,30 small numbers of short-lived, phenotypically mature but quiescent DCs may induce tolerance, whereas activated mature DCs induce immunity. Our findings from matched melanoma skin and SLN samples certainly seem to underline this notion.
Acknowledgments
We thank Schering-Plough (Maarssen, The Netherlands) for the kind provision of recombinant human GM-CSF.
Footnotes
Address reprint requests to P.A.M. van Leeuwen, M.D., Ph.D., VU University Medical Center, Department of Surgical Oncology, PO Box 7057, 1007 MB Amsterdam, The Netherlands. E-mail: pam.vleeuwen@vumc.nl.
This work was supported by The Fritz Ahlquist Foundation and by a grant from Nederlandse Organisatie voor Wetenschappelijk Onderzoek/Zorg Onderzoek Nederland Medische Wetenschappen (VIDI 917.56.32 to T.D.d.G.).
References
- Smith CH, Allen MH, Groves RW, Barker JN. Effect of granulocyte macrophage-colony stimulating factor on Langerhans cells in normal and healthy atopic subjects. Br J Dermatol. 1998;139:239–246. doi: 10.1046/j.1365-2133.1998.02360.x. [DOI] [PubMed] [Google Scholar]
- Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- Ruedl C, Koebel P, Bachmann M, Hess M, Karjalainen K. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J Immunol. 2000;165:4910–4916. doi: 10.4049/jimmunol.165.9.4910. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Dieu-Nosjean MC, Dezutter C, Valladeau J, Kayal S, Leborgne M, Brousse N, Saeland S, Davoust J. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196:417–430. doi: 10.1084/jem.20020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Toriyama K, Wen DR, Paul E, Cochran AJ. Variations in the distribution, frequency, and phenotype of Langerhans cells during the evolution of malignant melanoma of the skin. J Invest Dermatol. 1993;100:269S–273S. doi: 10.1111/1523-1747.ep12470135. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–140. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309–316. doi: 10.1002/(sici)1097-0215(19971104)73:3<309::aid-ijc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Peguet-Navarro J, Sportouch M, Popa I, Berthier O, Schmitt D, Portoukalian J. Gangliosides from human melanoma tumors impair dendritic cell differentiation from monocytes and induce their apoptosis. J Immunol. 2003;170:3488–3494. doi: 10.4049/jimmunol.170.7.3488. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RR, Wen DR, Guo J, Giuliano AE, Nguyen M, Offodile R, Stern S, Turner R, Cochran AJ. Selective modulation of paracortical dendritic cells and T-lymphocytes in breast cancer sentinel lymph nodes. Breast J. 2000;6:225–232. doi: 10.1046/j.1524-4741.2000.98114.x. [DOI] [PubMed] [Google Scholar]
- Bostick PJ, Morton DL, Turner RR, Huynh KT, Wang HJ, Elashoff R, Essner R, Hoon DS. Prognostic significance of occult metastases detected by sentinel lymphadenectomy and reverse transcriptase-polymerase chain reaction in early-stage melanoma patients. J Clin Oncol. 1999;17:3238–3244. doi: 10.1200/JCO.1999.17.10.3238. [DOI] [PubMed] [Google Scholar]
- Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng CH, Lee JJ, Balch CM, Reintgen DS, Ross MI. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–983. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]
- Veen HVD, Hoekstra OS, Paul MA, Cuesta MA, Meijer S. Gamma probe-guided sentinel node biopsy to select patients with melanoma for lymphadenectomy. Br J Surg. 1994;81:1769–1770. doi: 10.1002/bjs.1800811220. [DOI] [PubMed] [Google Scholar]
- Essner R, Kojima M. Dendritic cell function in sentinel nodes. Oncology (Huntingt) 2002;16:27–31. [PubMed] [Google Scholar]
- Cochran AJ, Morton DL, Stern S, Lana AM, Essner R, Wen DR. Sentinel lymph nodes show profound downregulation of antigen-presenting cells of the paracortex: implications for tumor biology and treatment. Mod Pathol. 2001;14:604–608. doi: 10.1038/modpathol.3880358. [DOI] [PubMed] [Google Scholar]
- Lana AM, Wen DR, Cochran AJ. The morphology, immunophenotype and distribution of paracortical dendritic leucocytes in lymph nodes regional to cutaneous melanoma. Melanoma Res. 2001;11:401–410. doi: 10.1097/00008390-200108000-00011. [DOI] [PubMed] [Google Scholar]
- Vuylsteke RJCLM, Molenkamp BG, Gietema HA, van Leeuwen PAM, Wijnands PGJTB, Vos W, van Diest PJ, Scheper RJ, Meijer S, de Gruijl TD. Local Administration of granulocyte/macrophage colony-stimulating factor increases the number and activation state of dendritic cells in the sentinel lymph node of early-stage melanoma. Cancer Res. 2004;64:8456–8460. doi: 10.1158/0008-5472.CAN-03-3251. [DOI] [PubMed] [Google Scholar]
- Vuylsteke RJCLM, van Leeuwen PAM, Statius Muller MG, Gietema HA, Kragt DR, Meijer S. Clinical outcome of stage I/II melanoma patients after selective sentinel lymph node dissection: long-term follow-up results. J Clin Oncol. 2003;21:1057–1065. doi: 10.1200/JCO.2003.07.170. [DOI] [PubMed] [Google Scholar]
- Vuylsteke RJCLM, van Leeuwen PAM, Meijer S, Wijnands PGJTB, Statius Muller MG, Busch DH, Scheper RJ, de Gruijl TD. Sampling tumor-draining lymph nodes for phenotypic and functional analysis of dendritic cells and T cells. Am J Pathol. 2002;161:19–26. doi: 10.1016/S0002-9440(10)64152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietema HA, Vuylsteke RJ, de Jonge IA, van Leeuwen PA, Molenkamp BG, van der Sijp JR, Meijer S, van Diest PJ. Sentinel lymph node investigation in melanoma: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol. 2004;57:618–620. doi: 10.1136/jcp.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hensbergen Y, Luykx-de Bakker SA, Heideman DA, Meijer GA, Pinedo HM, van Diest PJ. Rapid stereology based quantitative immunohistochemistry of dendritic cells in lymph nodes: a methodological study. Anal Cell Pathol. 2001;22:143–149. doi: 10.1155/2001/483019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand CU, Hunger RE, Yawalkar N, Gerber HA, Schaffner T, Braathen LR. Characterization of human skin-derived CD1a-positive lymph cells. Arch Dermatol Res. 1999;291:65–72. doi: 10.1007/s004030050385. [DOI] [PubMed] [Google Scholar]
- Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stossel H, Kapp M, Kammerer U, Douillard P, Kampgen E, Koch F, Saeland S, Romani N. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- Shortman K, Heath WR. Immunity or tolerance? That is the question for dendritic cells. Nat Immunol. 2001;2:988–989. doi: 10.1038/ni1101-988. [DOI] [PubMed] [Google Scholar]