Abstract
Chronic limb-threatening ischemia is a devastating disease with limited surgical options. However, inducing controlled angiogenesis and enhancing reperfusion holds therapeutic promise. To gain a better understanding of the mechanisms that contribute to limb reperfusion, we examined the temporal biochemical and structural changes occurring within the extracellular matrix of ischemic skeletal muscle. Both the latent and active forms of MMP-2 and -9 significantly increased during the active phase of limb reperfusion. Moreover, small but significant alterations in tissue inhibitors of metalloproteinase levels also occurred during a similar time course, consistent with a net increase in extracellular matrix remodeling. This temporal increase in MMP activity coincided with enhanced exposure of the unique HU177 cryptic collagen epitope. Although the HUIV26 cryptic collagen epitope has been implicated in angiogenesis, little is known concerning such epitopes within ischemic muscle tissue. Here, we provide the first evidence that a functionally distinct cryptic collagen epitope (HU177) is temporally exposed in ischemic muscle tissue during the active phase of reperfusion. Interestingly, the exposure of the HU177 epitope was greatly diminished in MMP-9 null mice, corresponding with significantly reduced limb reperfusion. Therefore, the regulated exposure of a unique cryptic collagen epitope within ischemic muscle suggests an important role for collagen remodeling during the active phase of ischemic limb reperfusion.
Severe peripheral arterial occlusive disease is a devastating condition that results in gangrene, chronic ulceration, rest pain, and amputation.1 Current therapeutic options are limited for successful treatment of peripheral arterial occlusive disease-induced limb-threatening arterial insufficiency. Therapeutic angiogenesis remains a promising alternative, although current approaches using growth factors have failed to conclusively yield long-term, clinically significant improvements in blood flow. Therefore, a better understanding of the cellular and molecular mechanisms regulating revascularization and reperfusion after ischemic injury is of great importance.
Angiogenesis is the proliferation of new capillaries via sprouting from existing vessels or through bridging and intussusception of existing capillaries.2,3 This complex physiological process likely contributes, in part, to ischemic limb reperfusion.4–6 A second, distinct process that also contributes to enhanced blood flow following ischemia is arteriogenesis. Arteriogenesis is the process whereby latent but existing bypasses, known as collateral vessels, are activated in response to limb ischemia caused by occlusion of a main axial artery.7,8 Although a number of stimulators of both angiogenesis and arteriogenesis have been evaluated clinically for the treatment of ischemic diseases, failed human trials have emphasized the shortcomings in our understanding of spontaneous revascularization.9,10 Thus, a more fundamental understanding of the molecular mechanisms controlling reperfusion is of paramount importance for overcoming this clinical problem.
Previous studies have documented the critical importance of growth factors, growth factor receptors, and various proteolytic enzymes in creating a permissive microenvironment for blood vessel growth.2,11–13 Interestingly, studies have provided evidence that the extracellular matrix (ECM) plays an important role in regulating angiogenesis.13–16 In particular, proteolytic remodeling of genetically distinct forms of collagen can expose cryptic regulatory elements that are normally inaccessible to cells.14,17–19 Cellular interaction with these cryptic elements may initiate unique signaling cascades required for new blood vessel growth.14,18 In fact, a monoclonal antibody (mAb) directed to a cryptic regulatory site within collagen type IV (HUIV26) potently inhibits new blood vessel growth in multiple in vivo models. Interestingly, recent studies have defined a second cryptic site (HUI77) shown to be present within a variety of distinct forms of collagen, including collagen types I to V.19 Exposure of this unique cryptic epitope has been detected within the basement membrane of angiogenic blood vessels and within the interstitial matrix of malignant tumors. Little, if any, HU177 has been detected within normal tissues.19 Importantly, exposure of these cryptic sites can be modulated by proteolysis as well as radiation.20 In this regard, members of the matrix metalloproteinase (MMP) family such as MMP-2 and MMP-9 have been shown to specifically cleave triple helical collagen-IV, the predominant form of collagen found in vascular basement membranes.21,22 Because MMP-mediated remodeling of the collagenous microenvironment contributes to angiogenesis, it is likely that ECM remodeling also contributes to revascularization and reperfusion of ischemic tissues. Although studies have examined the expression and functional significance of MMPs in ischemic tissues,23,24 little is known concerning the exposure of cryptic epitopes during this process. Previous studies have established that successful murine limb revascularization occurs over a time course of 30 to 35 days after induction of severe ischemia in a murine hindlimb model.25 Collateral vessel enlargement (arteriogenesis) and angiogenesis are thought to contribute to the revascularization observed within this model.4,25 Therefore, to gain a more complete understanding of the mechanisms that regulate reperfusion of ischemic limbs, an analysis of the biochemical and structural changes occurring within the ECM of ischemic skeletal muscle was carried out. In particular, the functional and temporal expression of specific MMPs and their respective endogenous inhibitors, tissue inhibitors of metalloproteinases (TIMPs), were examined within the ischemic hindlimb. Here, we provide evidence, for the first time, that the unique cryptic collagen epitope, HU177, is specifically exposed within ischemic muscle tissue in a time-dependent manner and may be dependent, in part, on the availability of MMP-9.
Materials and Methods
Antibodies and Reagents
MMP-2 and MMP-9 levels (active and total) in muscle lysates were measured using Biotrak Activity assay systems (Amersham Biosciences, Piscataway, NJ). Purified MMP-2 and MMP-9 (Amersham Biosciences) were used as controls. Enzyme-linked immunosorbent assay (ELISA) kits (Amersham Biosciences) were used to measure TIMP-1 and TIMP-2 levels in muscle lysates. Purified TIMP-1 and TIMP-2 (Amersham Biosciences) were used as controls and to derive standard curves. Muscle fragments were embedded in OCT compound (Tissue Tek; Sakura Finetek USA, Inc., Torrance, CA) for the preparation of frozen sections. Development of the mAb HU177 through subtractive immunization has been previously described.19 mAb QH2b is a humanized version of the mAb HU177 and was provided as a gift by Cancervax (Carlsbad, CA). mAb QH2B was used to stain for the HU177 cryptic collagen epitope on frozen sections of muscle. A goat anti-human IgG horseradish peroxidase (HRP) conjugate (BioSource, Camarillo, CA) was the secondary antibody used in the mAb QH2B staining experiments. Peroxidase Substrate kit (Vector Laboratories, Inc., Burlingame, CA) was used to develop the HRP in the immunohistochemical staining experiments. Harris hematoxylin (EMD Biosciences, Inc., La Jolla, CA) was used to counterstain the frozen sections to better define histology. Permount (Fischer Scientific, Fair Lawn, NJ) was used to fix slips on the frozen sections. Rat anti-mouse monoclonal CD31 antibody (Pharmingen, Franklin Lakes, NJ) was used for the costaining experiments with mAb QH2B. Goat anti-rat biotin-conjugated antibody (Pharmingen) and alkaline phosphatase-labeled streptavidin (Ventana Medical Systems, Tucson, AZ) were used to label the mAb CD31 on tissue sections. HRP-labeled goat anti-human antibody (Sigma-Aldrich, St. Louis, MO) was used to label the mAb QH2B in the costaining experiments.
Animals
Several different mouse strains were used in this study. FVB/N mice (Taconic Farms, Germantown, NY) 6 to 8 weeks of age and weighing 20 to 30 g were used for all experiments involving quantification of MMP-2, MMP-9, TIMP-1, TIMP-2, and serial measurement of HU177 cryptic site exposure. MMP-9 null mice in a 129SvEv background were kindly provided by Drs. Robert Senior and Michael Shipley (Washington University, St. Louis, MO).26 Backbreeding of the MMP-9 null mice was performed periodically to prevent genetic drift. 129SvEv mice (Taconic Farms) were used as background-matched control animals in all experiments involving MMP-9 null mice. Protocols were approved by New York University School of Medicine’s Institutional Animal Care and Use Committee. The animals were anesthetized with 0.12 to 0.15 ml of an anesthesia cocktail [1.5 ml of ketamine (100 mg/ml; Fort Dodge Animal Health, Fort Dodge, IA); 0.5 ml of acepromazine (10 mg/ml; Boehringer Ingelheim, St. Joseph, MO); and 1.5 ml of xylazine (20 mg/ml; Ben Venue Laboratories, Inc., Bedford, OH) in 8.5 ml of phosphate buffered saline (PBS)] intraperitoneally before the surgical procedure. Postoperatively, the animals were warmed, administered 3.0 ml of sterile 0.9% normal saline solution (Abbott Laboratories, Chicago, IL) to correct iatrogenic volume depletion, and closely monitored.
Hindlimb Ischemia Model
Operative intervention was performed to create unilateral hindlimb ischemia in the mice. Exposure was obtained by performing a skin incision in the right groin. The neurovascular bundle was located, and the femoral artery was then identified and dissected free. The femoral artery, in the most proximal portion of the hindlimb, was ligated and then divided. The skin was closed using interrupted 6-0 nylon sutures. To document changes in blood flow preoperatively, postoperatively, and during the recovery period, laser Doppler imaging was used.
Laser Doppler Imaging
Laser Doppler imaging (LDI) to determine limb blood flow has been described previously.25 Briefly, the lower torso and both hindlimbs were scanned with a Moor LDI VR laser Doppler (Moor Instruments Limited, Wilmington, DE) in a raster pattern for detection of a Doppler shift from the moving blood in the microvasculature of the skin and the underlying arterioles and venules. Blood flow was represented in a color-coded digital image and reported numerically, using proprietary Moor LDI Perfusion Measurement software (v3.09; Moor Instruments Limited). A black-and-white digital image was simultaneously obtained, and both color-coded and black-and-white images were stored on a laptop computer (Winbook Z1; Winbook Computer Corporation, Hilliard, OH). Whole-limb blood flow was determined using Moor LDI Image Processing software (v3.09; Moor Instruments Limited). To compute whole-limb blood flow, the entire hindlimb was digitally outlined based on anatomical landmarks in the image. The Image Processing software was then used to calculate the mean flow of blood within the designated area. Blood flow in the ischemic limb was indexed against that within the nonischemic limb. The nonischemic limb served as an internal control to account for any variability in blood flow due to extraneous factors such as systemic hemodynamic factors, animal surface vasoreactivity, and external environmental changes such as ambient temperature. As part of the measurement procedure, animals were anesthetized, as described above, before LDI. LDI was performed before and immediately after femoral artery ligation to document the level of arterial insufficiency rendered. Reperfusion studies were performed through serial LDI measurements at defined time points.
Muscle Lysate Preparation
Immediately after harvesting, 30- to 50-mg fragments of hindlimb calf muscle were snap-frozen on dry ice and then stored at −80°C until the protein was extracted. Muscle fragments were placed in 1 ml of extraction buffer on ice containing 50 mmol/L Tris-HCl (pH 7.5), 300 mmol/L NaCl, and 1% Triton X-100. The tissue was minced and homogenized on ice, using a PCU 11 Polytron (Kinematica Ag, Littau-Lucerne, Switzerland). The specimens were centrifuged at 10,000 × g at 4°C for 20 minutes, and the supernatant was decanted and filtered using a 50-μm filter column (Fisher Scientific, Pittsburgh, PA). All muscle lysate specimens were then stored at −80°C until assayed.
Bioactivity Assays for MMP-2 and -9
Bioactivity assays were performed using Biotrak’s activity assay system (Amersham Biosciences) according to the manufacturer’s protocol. Briefly, muscle lysate samples were placed in 96-microtitre well plates coated with anti-MMP-2 (100 μl/well). The plates were incubated overnight at 4°C. The following day, the plates were washed four times with washing buffer. P-Aminophenylmercuric acetate was added to samples from which the “total” MMP-2 (ie, total latent and active MMP-2) was measured. Buffer alone was added to samples prepared for measuring “active” (ie, endogenous active MMP-2) levels of MMP-2. Detection agent was then added to all wells (50 μl/well), and the plate was read at 405 nm (t = 0 minutes) and again after a 3-hour incubation at 37°C. The same procedure was followed to evaluate the amount of endogenous active MMP-9 and the total MMP-9. Purified MMP-2 and MMP-9 (Amersham Biosciences) were used, as positive and negative controls. Standard curves generated from known quantities of MMP-2 and MMP-9 were used to calculate the concentration of active MMP-2 or MMP-9 in these samples.
ELISA Measurement of TIMP-1 and TIMP-2
Commercially available ELISA kits (Amersham Biosciences) were used to measure TIMP-1 and TIMP-2 levels in muscle lysates according to the manufacturer’s instructions. Briefly, standards and samples were incubated in microtitre wells coated with anti-TIMP-1 and anti-TIMP-2 antibody, respectively. Peroxidase-labeled antibody directed to the respective TIMPs was added to each well. The amount of peroxidase was determined by the addition of a TMB substrate followed by addition of 100 μl of 1 mol/L sulfuric acid. The plates were read at 450 nm. Standard curves were generated from known quantities of TIMP-1 and TIMP-2 (Amersham Biosciences) to calculate the concentration of TIMP-1 and TIMP-2 in the samples.
Immunohistochemical Staining for Cryptic Collagen Epitope, HU177
Calf muscle from ischemic and nonischemic limbs was harvested, and 3 × 3 mm2 muscle sections were imbedded in OCT compound (Tissue Tek; Sakura Finetek USA, Inc.). The blocks were snap frozen and stored at −80°C until sectioned. Four-micrometer sections were cut on a Microtome Cryostat (Microm HM 505E; Microm International GmbH Robert/Boschster, Walldorf, Germany) and fixed in a 50:50 solution of methanol (VWR International, West Chester, PA) and acetone (Fischer Scientific, Fair Lawn, NJ) for 3 minutes at room temperature. The sections were then dried for 90 to 120 minutes at room temperature and washed with a 0.05 mol/L ethylenediamine tetraacetic acid/PBS solution. Each section was then treated with 3% hydrogen peroxide for 10 minutes to reduce endogenous peroxidase activity, washed as above, and then blocked with 1% bovine serum albumin (BSA) (Sigma-Aldrich, Inc.) for 1 hour at room temperature. The sections were then washed twice with the same ethylenediamine tetraacetic acid/PBS solution and incubated with mAb QH2B (100 μg/ml in 1% BSA) (Cancervax) at 4°C overnight. The sections were then washed with PBS, blocked with 1% BSA for 30 minutes, and then washed twice with PBS all at room temperature. Goat anti-human IgG HRP conjugate (BioSource) was diluted in 1% BSA (1:1000) and was applied to each section for 30 minutes at room temperature. The slides were washed twice with PBS, and then DAB was applied for 1 to 3 minutes (Peroxidase Substrate kit, Vector Laboratories, Inc.). The slides were washed with PBS and counterstained with Harris hematoxylin (EMD Biosciences, Inc.). The sections were then dehydrated through graded alcohols (ie, 70 to 100% ethanol), preserved with xylene, and then mounted with Permount (Fischer Scientific).
Quantitation of mAb QH2B Staining of HU177 Cryptic Sites in Hindlimb Muscle Sections
Stained sections were analyzed on an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan), and representative areas were digitally photographed with a Sony DXC-S500 (Sony Corporation, Tokyo, Japan) high-resolution digital camera. The images were stored on a Power Macintosh G4 computer (Apple Computer, Cupertino, CA). The images were analyzed and scored for staining intensity with Adobe Photoshop 6.0 (Adobe Systems, Inc., San Jose, CA) by two blinded observers. Briefly, total pixel number for each image was measured, and the background pixels (eg, white and non-muscle) were then subtracted from the total pixel number to determine the pixel count representing total muscle tissue. Staining was then measured, the number of pixels representing positively stained tissue was compared with the pixel count representing total muscle tissue, and a percentage was calculated (www.musc.edu/cando/earthkam/measure/Measuring.html). Muscle sections from three animals at each time point were examined, and three to five representative microscopic fields per animal were analyzed.
Costaining for Cryptic Collagen Epitope, HU177, and Endothelial Cells (CD31)
Frozen sections were cut and dried as described above. Each section was treated with 3% hydrogen peroxide for 10 minutes to reduce endogenous peroxidase activity and then washed with Tris-HCl (Ventana Medical Systems). Using an automated immunohistochemical slide-staining system (NexES, Ventana Medical Systems) co-staining for CD31 and HU177 was accomplished. Staining for CD31 was carried out using rat anti-mouse monoclonal CD31 antibody (Pharmingen) followed by incubation with goat anti-rat biotin-conjugated antibody (Pharmingen) and alkaline phosphatase-labeled streptavidin (Ventana Medical Systems). For detection of the HU177 cryptic epitope, tissue was incubated with mAb QH2B followed by incubation with HRP-labeled goat anti-human antibody (Sigma-Aldrich). All slides were then manually counterstained with Harris hematoxylin and mounted as described above.
Statistics
Unpaired t-test with Welch correction was performed using GraphPad InStat version 3.0b for Macintosh (GraphPad Software, San Diego, CA). P < 0.05 was considered significant. Error bars in all figures represent the SEM.
Results
Laser Doppler Analysis of Spontaneous Hindlimb Reperfusion after Ischemia
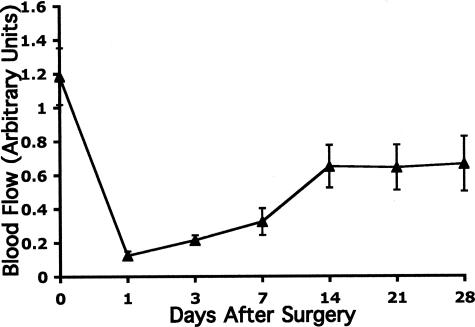
A more in-depth understanding of the molecular alterations occurring within the vascular microenvironment of ischemic skeletal muscle is of great importance to the development of more effective approaches for treating arterial insufficiency. To study specific molecular changes in the ECM of ischemic skeletal muscle, a murine model of hindlimb ischemia was used.25 As shown in Figure 1, proximal ligation of the femoral artery in the murine hindlimb leads to an acute 80% decrease in arterial blood flow within 24 hours of surgery compared with the normal contralateral hindlimb. Beginning approximately 48 hours later, an active phase of recovery begins with an increase in blood flow observed in the ischemic limb that ultimately plateaus with blood flow stable at approximately 66% of the contralateral non-ischemic limb. Blood flow in the calf of the ischemic limb remains relatively stable from 14 to 28 days after injury (Figure 1). Importantly, little if any change in this stabilized blood flow was observed throughout an 8-week time period after arterial ligation (data not shown). These findings are consistent with previously reported results25 and suggest that while blood flow returns, complete recovery to the level observed before the injury fails to occur.
Figure 1.
Laser Doppler imaging of ischemic hindlimb after surgical ligation of the femoral artery (ie, hindlimb ischemia). Wild-type mice 6 to 8 weeks of age underwent surgical ligation of the femoral artery. Blood flow was assessed by laser Doppler imaging before ligation (day 0) and at days 1, 3, 7, 14, 21, and 28 after surgery. Blood flow in the ischemic calf is expressed as a ratio of the blood flow in the contralateral nonischemic limb. Data represent an average value for all animals at each time point (n = 7). Blood flow decreases to 20% after arterial ligation and then recovers to 66%. Error bars represent the SEM.
Altered Levels and Functional Activity of MMPs within Ischemic Skeletal Muscle
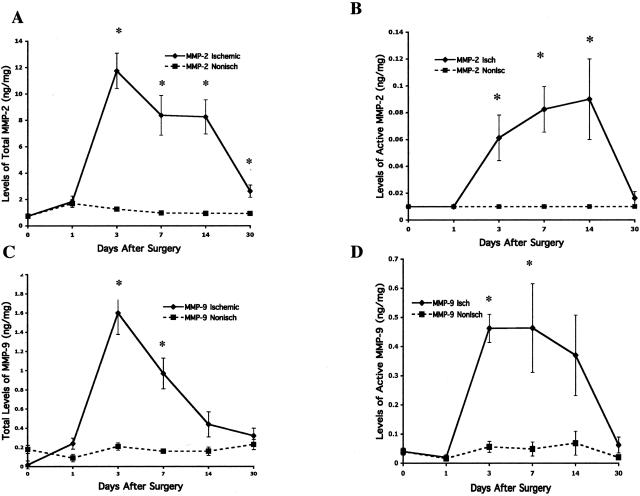
Previous studies have suggested that angiogenesis and arteriogenesis may contribute to the reperfusion observed in ischemic limb models.4 Moreover, studies also suggest that MMPs play crucial roles in angiogenesis and arteriogenesis.22,27 To this end, we analyzed the temporal levels and functional activity of MMP-2 and MMP-9 within ischemic skeletal muscle. After proximal ligation of the femoral artery, tissue samples of calf muscle were collected over a 30-day time course, and tissue extracts were prepared. The combined level of latent and active MMP-2 or MMP-9 (total MMP) as well as the level of endogenously active MMP (active MMP) was quantified within both ischemic and nonischemic skeletal muscle. As shown in Figure 2A, a rapid increase in levels of total MMP-2 in ischemic muscle was observed beginning 24 hours after arterial ligation with peak levels detected at 3 days. In fact, total MMP-2 levels were significantly (P < 0.001) increased by approximately four- to sixfold from 3 to 14 days after arterial ligation compared with nonischemic muscle. Interestingly, this elevated level of total MMP-2 returned to levels approaching those observed in nonischemic muscle by day 30. A time-dependent increase in proteolytically active MMP-2 was also observed over the same time course exhibiting a significant (P < 0.001) four- to eightfold increase compared with that within the control nonischemic muscle (Figure 2B). In similar experiments, the levels of both total and active MMP-9 were also quantified within these tissues. As was observed with MMP-2, a rapid increase in total and proteolytically active MMP-9 was observed in ischemic muscle beginning approximately 24 hours after arterial ligation (Figure 2, C and D). Levels of both total and active MMP-9 were significantly (P < 0.002) increased at its peak by approximately eight- to ninefold (3 days) compared with nonischemic muscle. Importantly, these elevated levels of total and active MMP-9 returned to levels observed in the nonischemic muscle by day 30.
Figure 2.
Effect of ischemia over time on MMP-2 and MMP-9 levels both total and active for 30 days after onset of hindlimb ischemia. MMP-2 and -9 levels were assessed by quantitative bioactivity assays of muscle tissue taken from the ischemic and nonischemic limbs of wild-type mice on days 0 (pre-op), 1, 3, 7, 14, and 30. For both MMP-9 and MMP-2, total levels increased markedly within 3 days and then diminished by 30 days after surgery compared with nonischemic calf muscle. Active MMP-9 and MMP-2 remain markedly elevated in the ischemic calf muscle compared with the nonischemic muscle between 3 and 14 days after surgery when reperfusion by LDI was most marked. A: Total levels of MMP-2 including both the latent and the active forms in the ischemic and nonischemic muscle tissue (n = 8) at each time point. The error bars in the nonischemic data are narrow and obscured by the associated data point. B: Level of active MMP-2 present in the ischemic and nonischemic muscle tissue (n = 8) at each time point. The error bars for the nonischemic data do not exist because active MMP-2 was not detectable in the nonischemic calf muscle. C: Represents the total level of MMP-9, including both the latent form and the active forms, in the ischemic and nonischemic muscle tissue (n = 8) at each time point. D: Represents the level of active MMP-9 present in the ischemic and nonischemic muscle tissue (n = 8) at each time point. For A through D the asterisk denotes a significant difference (P < 0.05) between the ischemic and the contralateral nonischemic limb at the same time point. Error bars represent the SEM.
Temporal Changes in Levels of Tissue Inhibitors of Metalloproteinase in Ischemic Muscle
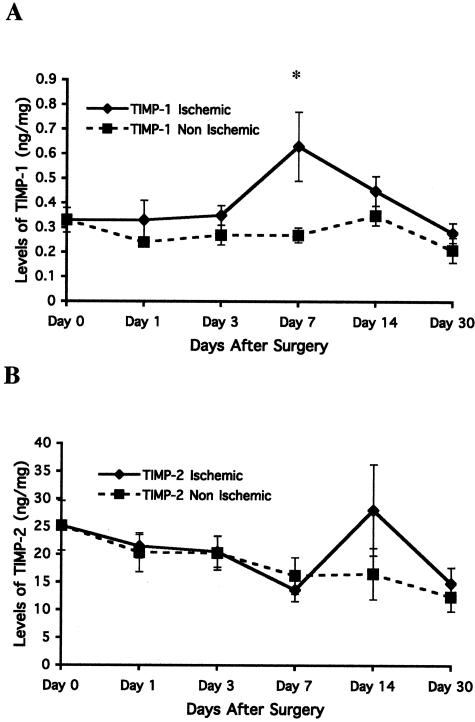
The functional activity of proteolytic enzymes such as MMPs is thought to be controlled, in part, by endogenously available inhibitors.28 Interestingly, TIMPs, endogenous tissue inhibitors of MMPs, have been suggested to regulate ECM remodeling as well as angiogenesis.22 In particular, TIMP-1 is a preferential regulator of MMP-9 whereas TIMP-2 is known to regulate MMP-2 activity.28 Therefore, to gain additional insight into the temporal regulation of both MMP-2 and 9 within ischemic muscle, we examined the levels of both TIMP-1 and -2 within skeletal muscle over a time course of 30 days. After proximal ligation of the femoral artery, tissue samples of calf muscle were collected, and tissue extracts were prepared. Levels of both TIMP-1 and -2 were quantified within both ischemic and nonischemic skeletal muscle by solid phase ELISA.29 As shown in Figure 3A, little if any significant change in the levels of TIMP-1 was observed within the first 3 days after arterial ligation. Interestingly, a significant (P < 0.05) 2-fold increase in TIMP-1 levels was detected at day 7 after arterial ligation that returned to baseline levels by 30 days. In contrast, no significant change in TIMP-2 levels was detected over the entire 30-day time course (Figure 3B). These findings, taken together with the time-dependent increase in functionally active MMPs is consistent with the possibility that elevated levels of ECM remodeling may occur during recovery of blood flow to the ischemic limb.
Figure 3.
Effect of ischemia over time on TIMP-1 and TIMP-2 levels for 30 days after onset of hindlimb ischemia. ELISAs were used to measure total TIMP levels in the muscle tissue of ischemic and nonischemic limbs of wild-type mice on days 0 (pre-op), 1, 3, 7, 14, and 30 after ischemia. A: TIMP-1 levels measured by ELISAs. Each time point represents an average value (n = 8). The asterisk indicates a significant difference (P < 0.05) between the ischemic and the contralateral nonischemic muscle tissue at the same time point. TIMP-1 levels increased at day 7 at the same time that total MMP-9 levels decreased. B: TIMP-2 levels measured by ELISA. Each time point represents an average value (n = 8). There was no significant difference in levels between ischemic and nonischemic limbs at any time point.
Temporal Exposure of the HU177 Cryptic Collagen Epitope within Ischemic Muscle
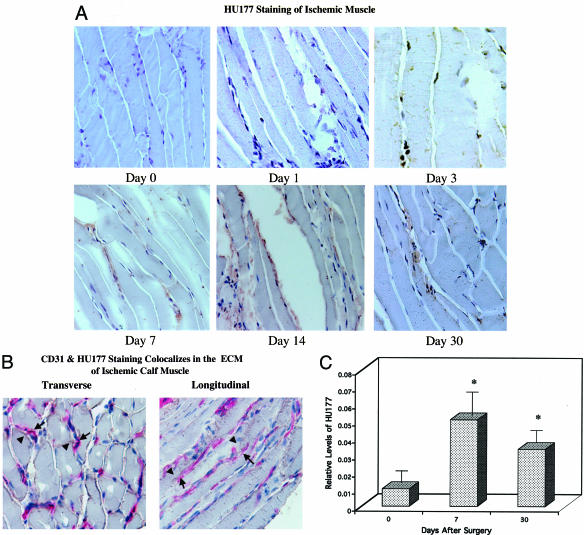
It is well documented that proteolytically active MMPs such as MMP-2 and MMP-9 can cleave basement membrane-associated triple helical collagen type IV.21,28 Interestingly, previous studies have suggested that proteolytic exposure of the HUIV26 cryptic epitope within collagen type IV may be facilitated by MMPs such as MMP-2 and MMP-9 in vitro and in vivo.14 Although previous studies have documented the exposure and functional relevance of the HUIV26 cryptic epitope14,18 during angiogenesis in vivo, little is known concerning the exposure of the HU177 cryptic epitope in vivo and more specifically, during reperfusion of ischemic skeletal muscle. To this end, we studied the exposure of the HUI77 cryptic collagen epitope within skeletal muscle over a time course of 30 days. After proximal ligation of the femoral artery, tissue samples of calf muscle were collected, snap frozen, sectioned, and analyzed by immunohistochemistry using mAb QH2B, a humanized mAb directed to the HUI77 cryptic epitope. Although some muscle necrosis was evident in the ischemic calf after arterial ligation, muscle integrity was largely preserved at all time points. As shown in Figure 4A, little if any exposure of the HUI77 cryptic epitope is present within the nonischemic calf muscle (day 0). Importantly, a time-dependent increase in exposure of the HUI77 cryptic epitope was detected within the ECM of ischemic muscle tissue (Figure 4A). Exposure of the HUI77 epitope was predominately localized within the ECM between muscle cells as well as around blood vessels, as indicated by co-localization with CD31+ vessels. (Figure 4B). To quantify the relative exposure levels of the HUI77 epitope over the 30-day time course, stained (mAb QH2B) tissue samples were scanned by digital image analysis and the pixel density of positively stained specimens was quantified. As shown in Figure 4C, exposure of the HUI77 epitope was elevated by approximately sixfold by day 7 after arterial ligation compared with the contralateral non-ischemic muscle. Interestingly, increased exposure of the HUI77 epitope occurred during the same period as MMP levels were elevated (days 3 to 14). Although exposure of the HUI77 epitope was still evident by 30 days following arterial ligation, the relative levels were reduced compared with earlier time points (Figure 4C). Although not direct proof, these findings are consistent with a possible role for MMPs in the exposure of the HUI77 cryptic collagen epitope within ischemic muscle tissue.
Figure 4.
Location and time course of HU177 cryptic collagen epitope exposure after onset of hindlimb ischemia. A: Frozen sections (×40) of ischemic muscle harvested at days 0 (pre-op), 1, 3, 7, 14, and 30 days were stained for the HU177 cryptic collagen epitope using mAb QH2B. Sections were counterstained with horseradish peroxidase for detection of antibody. B: Frozen sections (×40) of ischemic muscle harvested at day 7 were costained for the cryptic collagen epitope, HU177, and the endothelial cell marker, CD31. HU177 is denoted with the brown horseradish peroxidase staining, whereas CD31 is indicated by the red alkaline phosphatase staining. The solid arrows indicate CD31 staining of blood vessel endothelial cells in the ECM between ischemic skeletal muscle cells. The arrowheads indicate HU177 staining in the ECM, which colocalizes with and surrounds CD31+ endothelial cells. C: Quantification of HU177 staining was performed using digital images of stained sections. Sections were analyzed by blinded observers using Adobe Photoshop. The number of mAb QH2B-stained pixels in each image was determined and expressed as a ratio of the total number of pixels of muscle tissue present in each slide. Each time point is the average value obtained from analyzing three to five representative microscopic fields from muscle sections from animals at each time point (n = 3) for HU177 staining. HU177 exposure parallels increased MMP-9 and MMP-2 activity and limb reperfusion. The asterisk denotes a significant difference (P < 0.05) between nonischemic tissue at day 0 (preoperative) and ischemic tissue at days 7 and 30.
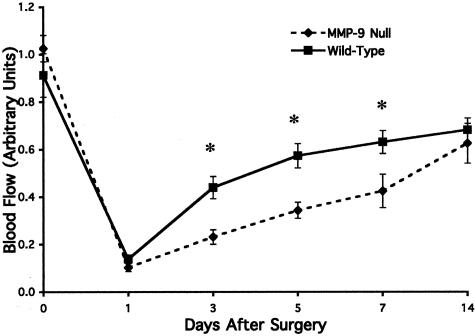
Reduction in Spontaneous Ischemic Hindlimb Reperfusion in MMP-9 Null Mice
Our temporal and quantitative studies of active MMPs within the ischemic muscle during recovery of blood flow, as well as previously published findings, suggest a potential role for MMPs in ischemic limb revascularization and reperfusion.23,24 In particular, previous studies have suggested that MMP-9 plays an important role in the initiation of the angiogenic switch as well as facilitating exposure of cryptic collagen epitopes in vivo.18,24,26 Because angiogenesis and MMPs likely contribute to the return of blood flow within ischemic muscle, we examined the rates of blood flow recovery within MMP-9 null and control wild-type mice. Limb reperfusion was monitored over a 30-day time course following femoral artery ligation. As shown in Figure 5, proximal ligation of the femoral artery in the murine hindlimb leads to an approximate 80% decrease in arterial blood flow compared with the normal contralateral hindlimb within 24 hours of injury in both wild-type and MMP-9 null mice. Consistent with our previous studies, blood flow within the skeletal calf muscle recovers to approximately 70% of that observed in the control nonischemic muscle by 14 days and remains stable thereafter. Interestingly, during the active recovery period from days 3 through 7, a significant (P < 0.05) 2-fold reduction in blood flow was detected within the ischemic limbs of MMP-9 null mice compared with the ischemic muscle of wild-type control mice (Figure 5). These data are consistent with a role for MMP-9 within the active reperfusion recovery phase of the ischemic limb.
Figure 5.
Comparison of blood flow between MMP-9 null and wild-type mice for 14 days after onset of hindlimb ischemia. 129SvEv mice (wild-type) and MMP-9 null mice underwent surgical ligation of the femoral artery. Blood flow in the calf/foot was assessed by laser Doppler imaging before ligation (day 0) and at days 1, 3, 5, and 14 after surgery. Blood flow in the ischemic limb was expressed as a ratio of the blood flow in the contralateral nonischemic limb. Blood flow in the distal limb of the MMP-9 null mice was significantly diminished, compared with the wild-type controls, during the first 2 weeks after induction of ischemia. Each time point represents the average value for all animals at each time point (n ≥ 9). The asterisk denotes a significant difference (P < 0.05) between ischemic limb reperfusion in wild-type versus MMP-9 null mice.
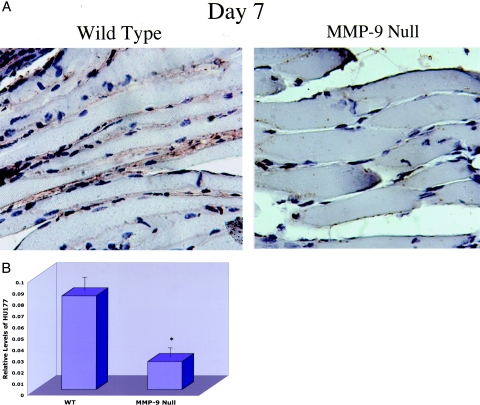
Reduced Exposure of the HU177 Cryptic Collagen Epitope within Ischemic Muscle of MMP-9 Null Mice
Previous studies have suggested that MMP-mediated proteolysis of collagen results in exposure of the cryptic collagen epitopes both in vitro and in vivo.14,18 Given these findings, in conjunction with our current results indicating a temporal increase in both MMPs and the HUI77 cryptic epitope in ischemic muscle, we analyzed the relative exposure of the HUI77 cryptic epitope in ischemic muscle during the active blood flow recovery phase within wild-type and MMP-9 null mice. As shown in Figure 6A, the HUI77 cryptic epitope was readily detected within the ECM of ischemic skeletal muscle from wild-type mice. Consistent with our previous studies, elevated levels of the HUI77 cryptic epitope were detected within the ischemic muscle of wild-type mice at days 3 and 7 after arterial ligation compared with nonischemic muscle (data not shown). In contrast, the exposure of the HUI77 cryptic epitope was markedly decreased in ischemic muscle from MMP-9 null mice compared with wild-type controls (Figure 6A). In fact, quantification of the HU177 epitope in these ischemic muscle samples (day 7) from MMP-9 null mice indicated an approximate fourfold reduction in relative exposure of the HUI77 epitope compared with ischemic wild-type controls (Figure 6B). By day 14 when stabilized blood flow had returned, little change in exposure of the HU177 epitope was observed between wild-type and MMP-9 null mice (data not shown). These data are consistent with a possible role for MMP-9 in the proteolytic exposure of the HUI77 cryptic collagen epitope within ischemic muscle.
Figure 6.
A comparison of HU177 staining of ischemic calf muscle tissue in MMP-9 null mice versus wild-type mice. A: Frozen sections (×40) of ischemic muscle tissue harvested 7 days after onset of ischemia were stained with mAb QH2B for the cryptic collagen epitope HU177. Sections were counterstained with horseradish peroxidase for detection of antibody. The ECM of the ischemic skeletal muscle of the MMP-9 null mice exhibit markedly less HU177 cryptic site exposure. B: Digital images were analyzed by a blinded observer using Adobe Photoshop. The number of pixels stained was determined and expressed as a ratio of the total number of pixels of muscle tissue present in each slide. Muscle sections from the ischemic calf of wild-type (WT) (n = 3) and MMP-9 null (n = 3) mice were examined, and four representative microscopic fields per animal were analyzed. Each data point is the average value obtained from analyzing these representative microscopic fields. Error bars represent the SEM.
Discussion
Critical limb ischemia, if unresolved, results in amputation in most patients. Unfortunately, many patients with critical limb ischemia are unable to undergo reperfusion through percutaneous angioplasty or arterial bypass surgery. The ability to induce controlled and localized angiogenesis may significantly improve the treatment of patients suffering from lower extremity arterial occlusive disease. Not only could critical, limb-threatening ischemia, be addressed, but impaired walking due to intermittent claudication might also be treated. However, the promise of effective therapeutic angiogenesis has yet to be realized.
It is likely that to accommodate local physiological reperfusion and revascularization within ischemic muscle, endothelial cells may acquire specialized characteristics. For example, distinct endothelial cell types have been found in liver, kidney, and lung tissue.30,31 Studies aimed at determining whether this diversity is genetically preprogrammed or a result of the tissue microenvironment suggest that there are novel tissue-specific pathways that regulate endothelial cell function in response to signals from the local microenvironment.32 In fact, numerous molecules have been identified that effect endothelial cells and angiogenesis associated with distinct vascular beds.33,34 For example, in αv-null mice, most vascular beds appear normal while the brain and intestine exhibit some vascular defects. These findings emphasize the concept that different regulatory mechanisms may function to control angiogenesis in distinct tissue microenvironments. Given this diversity, a better understanding of the microenvironment and potential mechanisms governing reperfusion in ischemic limb skeletal muscle is of critical importance.
Previous studies have documented the importance of MMPs in the control of angiogenesis and tissue remodeling.22,27 In this regard, cleavage of triple helical collagen enables migration and invasion of endothelial cells within the ECM. This proteolytic remodeling not only removes physical barriers to migration but also exposes unique regulatory elements essential for cellular migration and angiogenesis to occur.14,18,20 Moreover, MMPs may also release matrix bound growth factors including vascular endothelial growth factor, basic fibroblast growth factor, and insulin-like growth factor.21,35
Given the multiple functions of MMPs during angiogenesis, understanding the unique temporal patterns and activity of these enzymes, along with the response of their endogenous inhibitors during the active phase of ischemic limb reperfusion is critical for developing novel approaches for the treatment of tissue ischemia. To this end, we23 and others24,36 have shown that MMP levels significantly increase after induction of hindlimb ischemia. However, only limited information is available on the temporal regulation of proteolytically active MMPs within the ischemic limb milieu during active reperfusion. In this report, we have shown that levels of both the latent and active forms of MMP-9 are immediately increased in the calf muscle of the ischemic murine hindlimb. This marked increase in MMP-9 levels may be due in part to increased production from muscle and/or inflammatory cells.27 Consistent with this possibility, we have previously shown that MMP-9 levels are diminished after hindlimb ischemia if anti-neutrophil antibodies are administered before femoral artery ligation.37 Additionally, others have shown that macrophages are also an important source of MMP-9 after hindlimb ischemia.24 Importantly, MMP-9 likely contributes to the activation of satellite cells, which are essential for the regeneration of injured muscle.38 Limited muscle injury and regenerative skeletal muscle satellite cells are consistently observed within histological sections of ischemic calf muscle during reperfusion.
MMP-2 levels were also increased significantly after hindlimb ischemia, although in a somewhat delayed fashion when compared with total MMP-9 levels. Interestingly, MMP-2 has been previously shown to play an important role in regulating the integrity and composition of the ECM in skeletal muscle.27 MMP-2 is also necessary for myofiber proliferation, differentiation, fiber healing after injury, and maintenance of the surrounding connective tissue.27,28 Thus, it is not surprising that a prolonged increase in MMP-2 levels was observed in ischemic skeletal muscle.
Previous studies have provided evidence that the proteolytic activity of MMPs can be controlled in part by TIMPs.22,27,28 In fact, TIMPs not only play an important role in the inhibition of proteolytic activity but also may facilitate cell surface activation of MMP-2 by a mechanism involving formation of a trimolecular complex.28,39 Therefore, a balance between the expression of TIMPs and MMPs is likely crucial for regulating ECM remodeling in vivo. In this regard, we provide evidence for a small but significant increase in TIMP-1 expression but not TIMP-2 within ischemic skeletal muscle during the active phase of reperfusion. Taken together with the significant increase in both MMP-2 and MMP-9, it is possible that the net proteolytic balance within the ischemic muscle favors controlled ECM remodeling. Consistent with this possibility, elevated exposure of the unique HUI77 cryptic collagen epitope was observed during the active phase of reperfusion.
Interestingly, previous studies have implicated MMP-2 and MMP-9 in facilitating exposure of the HUIV26 cryptic epitope present in collagen type IV.14,18 Although MMP-2 was able to facilitate exposure of the HUIV26 cryptic collagen epitope in vitro, exposure of this regulatory element during retinal angiogenesis in vivo appeared to depend primarily on MMP-9.18 Here, we provide evidence that MMP-9 may also play a role in the exposure of the HU177 cryptic epitope in vivo, because the relative levels of this epitope were significantly reduced in MMP-9 null mice during the active phase of reperfusion. Furthermore, MMP-9 null mice also had significantly diminished reperfusion during the same time period in which there was reduced HU177 exposure, suggesting a possible role for type IV collagen remodeling for ischemic limb reperfusion.
Reperfusion within ischemic muscle tissue is clearly a complex process that likely involves angiogenesis as well as arteriogenesis. Previous attempts at improving blood flow have focused largely on growth factors known to induce endothelial cell proliferation and blood vessel sprouting.40,41 However, because ECM remodeling plays a crucial role in angiogenesis, it is likely that ECM remodeling also contributes to efficient ischemic limb reperfusion. Consistent with this possibility, our studies indicate a temporal increase in the exposure of the HUI77 cryptic collagen epitope during the active phase of ischemic muscle reperfusion. Moreover, exposure of this cryptic epitope, paralleling MMP activity, decreased as reperfusion stabilized. The absence of MMP-9 was associated with both diminished cryptic collagen epitope exposure and reperfusion. Although only correlative in nature, these findings are consistent with a possible functional role for the HU177 cryptic epitope in ischemic limb reperfusion. In fact, current studies are now underway to examine this possibility.
Footnotes
Address reprint requests to Paul J. Gagne M.D., New York University School of Medicine, Department of Surgery, 530 First Avenue, Suite 6F, New York, NY 10016. E-mail: paul.gagne@med.nyu.edu.
Supported by National Institutes of Health grants KO8 HL073378–01A1 (to P.J.G.) and R01CA91645 (to P.B.), the W.L. Gore Foundation (to P.J.G.), and Cancervax (to P.B.).
References
- Lewis CD. Peripheral arterial disease of the lower extremity. J Cardiovasc Nurs. 2001:45–63. doi: 10.1097/00005082-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Hershey JC, Baskin EP, Glass JD, Hartman HA, Gilberto DB, Rogers IT, Cook JJ. Revascularization in the rabbit hindlimb: dissociation between capillary sprouting and arteriogenesis. Cardiovasc Res. 2001;49:618–625. doi: 10.1016/s0008-6363(00)00232-7. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Beyersdorf F, Lutter G. Collateral growth: cells arrive at the construction site. Cardiovasc Surg. 2002;10:570–578. doi: 10.1016/s0967-2109(02)00108-4. [DOI] [PubMed] [Google Scholar]
- Wahlberg E. Angiogenesis and arteriogenesis in limb ischemia. J Vasc Surg. 2003;38:198–203. doi: 10.1016/s0741-5214(03)00151-4. [DOI] [PubMed] [Google Scholar]
- Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- Schaper W, Buschmann I. Arteriogenesis, the good and bad of it. Cardiovasc Res. 1999;43:835–837. doi: 10.1016/s0008-6363(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Isner JM, Baumgartner I, Rauh G, Schainfeld R, Blair R, Manor O, Razvi S, Symes JF. Treatment of thromboangiitis obliterans (Buerger’s disease) by intramuscular gene transfer of vascular endothelial growth factor: preliminary clinical results. J Vasc Surg. 1998:964–973. doi: 10.1016/s0741-5214(98)70022-9. [DOI] [PubMed] [Google Scholar]
- Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Mallat Z, Tamarat R, Duriez M, Tedgui A, Levy BI. Regulation of matrix metalloproteinase activity in ischemic tissue by interleukin-10: role in ischemia-induced angiogenesis in mice hindlimb. Circ Res. 2000;89:259–264. doi: 10.1161/hh1501.094269. [DOI] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Yuen SM, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco CD, Hou G, Bendeck MP. Collagens, integrins, and the discoidin domain receptors in arterial occlusive disease. Trends Cardiovasc Med. 2002;12:143–148. doi: 10.1016/s1050-1738(01)00165-7. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Integrin signaling revisited. Trends Cell Biol. 2001;11:466–470. doi: 10.1016/s0962-8924(01)02152-3. [DOI] [PubMed] [Google Scholar]
- Davis GE. Affinity of integrins for damaged extracellular matrix: avb3 binds to denatured collagen type 1 through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- Hangai M, Kitaya N, Xu J, Chan CK, Kim JJ, Werb Z, Ryan SJ, Brooks PC. Matrix metalloproteinase-9-dependent exposure of a cryptic migratory control site in collagen is required before retinal angiogenesis. Am J Pathol. 2002;161:1429–1437. doi: 10.1016/S0002-9440(10)64418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Rodriguez D, Kim JJ, Brooks PC. Generation of monoclonal antibodies to cryptic collagen sites by using subtractive immunization. Hybridoma. 2000:375–385. doi: 10.1089/02724570050198893. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Roth JM, Lymberis SC, DeWyngaert K, Broek D, Formenti SC. Ionizing radiation modulates the exposure of the HUIV26 cryptic epitope within collagen type IV during angiogenesis. Int J Radiat Oncol Biol Phys. 2002:1194–1201. doi: 10.1016/s0360-3016(02)03748-3. [DOI] [PubMed] [Google Scholar]
- Mignatti P, Rifkin D. Plasminogen activators and matrix metalloproteinases in angiogenesis. Enzyme Protein. 1996;49:117–137. doi: 10.1159/000468621. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- Muhs BE, Plitas G, Delgado Y, Ianus I, Shaw JP, Adelman MA, Lamparello P, Shamamian P, Gagne P. Temporal expression and activation of matrix metalloproteinases-2, -9, and membrane type 1-matrix metalloproteinase following acute hindlimb ischemia. J Surg Res. 2003;111:8–15. doi: 10.1016/s0022-4804(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Johnson C, Sung H, Lessner S, Fini M, Galis ZS. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues. Circ Res. 2004;94:262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli E, Moas M, Reznick A, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. 2004;29:191–197. doi: 10.1002/mus.10529. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Zhang J, Iwata K, Shinya T, Okada Y, Hayakawa T. A one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases-2 using monoclonal antibodies. Clin Chim Acta. 1993:31–45. doi: 10.1016/0009-8981(93)90004-n. [DOI] [PubMed] [Google Scholar]
- DeFouw DO. Structural heterogeneity within the pulmonary microcirculation of the normal rat. Anat Rec. 1988;221:645–654. doi: 10.1002/ar.1092210210. [DOI] [PubMed] [Google Scholar]
- Fleming S, Jones DB. Antigenic heterogeneity of renal endothelium. J Pathol. 1989;158:319–323. doi: 10.1002/path.1711580409. [DOI] [PubMed] [Google Scholar]
- Aird WC, Edelber JM, Wiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol. 1997;138:1117–1124. doi: 10.1083/jcb.138.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Croix B, Ragom C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Bogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Rajotte D, Arap W, Hagedorn M, Koivunem E, Pasqualini T, Rouslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest. 1998;102:430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisdal E, Teiger E, Lefaucheur JP, Adnot S, Planus E, Lafuma C, D’Ortho MP. Increased expression of gelatinases and alteration of basement membrane in rat soleus muscle following femoral artery ligation. Neuropathol Appl Neurobiol. 2000;26:11–21. doi: 10.1046/j.1365-2990.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- Muhs BE, Gagne P, Plitas G, Shaw JP, Shamamian P. Experimental hindlimb ischemia leads to neutrophil-mediated increases in gastrocnemius MMP-2 and -9 activity: a potential mechanism for ischemia induced MMP activation. J Surg Res. 2004;117:249–254. doi: 10.1016/j.jss.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, Fardeau M, Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol. 1999;205:158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Janssen M, Foidart JM, Noel A. Membrane type-1 matrix metalloproteinase and TIMP-2 in tumor angiogenesis. Matrix Biol. 2003:55–61. doi: 10.1016/s0945-053x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Chang H, Isner JM. Synergistic effect of angiopoietin-1 and vascular endothelial growth factor on neoangiogenesis in hypercholesterolemic rabbit model with acute hindlimb ischemia. Life Sci. 2003:563–579. doi: 10.1016/s0024-3205(03)00318-7. [DOI] [PubMed] [Google Scholar]
- Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]