Abstract
Vascular endothelial growth factor (VEGF-A), a potent stimulus for angiogenesis, is up-regulated in the skin after wounding. Although studies have shown that VEGF is important for wound repair, it is unclear whether this is based solely on its ability to promote angiogenesis or if VEGF can also promote healing by acting directly on non-endothelial cell types. By immunohistochemistry and reverse transcriptase-polymerase chain reaction, expression of VEGF receptor-1 (VEGFR-1), but not VEGFR-2, was detected in murine keratinocytes during wound repair and in normal human epidermal keratinocytes (NHEKs). The presence of VEGF receptors on NHEKs was verified by binding studies with 125I-VEGF. In vitro, VEGF stimulated the proliferation of NHEKs, an effect that could be blocked by treatment with neutralizing VEGFR-1 antibodies. A role for VEGFR-1 in keratinocytes was also shown in vivo because treatment of excisional wounds with neutralizing VEGFR-1 antibodies delayed re-epithelialization. Treatment with anti-VEGFR-1 antibodies also reduced the number of proliferating keratinocytes at the leading edge of the wound, suggesting that VEGF sends a proliferative signal to these cells. Together, these data describe a novel role for VEGFR-1 in keratinocytes and suggest that VEGF may play several roles in cutaneous wound repair.
Vascular endothelial growth factor (VEGF)-A is a potent stimulator of proliferation, migration, and survival in endothelial cells.1–3 It is known to be a key regulator of cutaneous angiogenesis, and as such plays a role in several physiological and disease processes in the skin, including hair growth,4–7 cancer development,8–13 psoriasis,14–17 and wound healing.12,18–20 During wound repair, VEGF is produced in abundance by keratinocytes in the skin.18 This supply of VEGF is thought to be critical for proper wound repair by stimulating angiogenesis in the wound bed, which supplies nutrients and oxygen needed for rapid regrowth of the skin.
VEGF binds two high-affinity tyrosine kinase receptors, VEGFR-1 (flt-1) and VEGFR-2 (flk-1/KDR).21 Signaling through these receptors mediates the actions of endothelial cells required for angiogenesis. Expression of the VEGF receptors has been detected in blood vessels within wound granulation tissue,22,23 suggesting that these receptors are involved in the regulation of wound angiogenesis.
Expression of the VEGF receptors was originally thought to be restricted to endothelial cells, but recent reports have demonstrated the presence of one or both of the receptors on various non-endothelial cell types such as uterine24 and vascular smooth muscle cells,25 pericytes,26 and neurons.27 In addition, several non-endothelial cells that express VEGF receptors are known to be involved in wound repair, including neutrophils,28 monocytes,29,30 and stromal cells.31,32 The data presented here demonstrate VEGFR-1 expression in another non-endothelial cell type critical to wound healing, the epidermal keratinocyte. A role for VEGFR-1 in the proliferation of keratinocytes as well as in re-epithelialization after injury was found. These results suggest that VEGFR-1 may play a critical role in processes in the skin requiring significant epidermal cell participation, such as wound healing.
Materials and Methods
Animal Experiments
All animal procedures were approved by the Loyola Institutional Animal Care and Use Committee. Female BALB/c mice (Harlan, Indianapolis, IN), 8 weeks of age, were used for in vivo wound-healing experiments. After anesthetization with isoflurane (IsoFlo; Abbott Laboratories, Abbott Park, IL), animals were shaved and wiped with 70% isopropyl alcohol. Four full-thickness dorsal wounds were made on each mouse using a 3-mm dermal biopsy punch (Acuderm, Inc., Ft. Lauderdale, FL). For analysis of VEGF receptor expression, mice were euthanized and wounds were harvested 7 days after wounding. To analyze VEGFR-1 function in vivo, mice underwent the same wounding procedure as described above. Immediately after wounding, each wound was treated with either 0.5 μg of normal goat IgG (R&D Systems, Minneapolis, MN) as a control or 0.5 μg of neutralizing anti-mouse VEGFR-1 antibody (R&D Systems). The antibodies were mixed in K-Y jelly (Ortho Pharmaceutical Corp., Raritan, NJ), and each wound received a total volume of 25 μl of the mixture. The wounds were then covered with Tegaderm adhesive wound dressing (3M Health Care, St. Paul, MN), which was secured around the edges with tincture benzoin compound (Humco Laboratory, Texarkana, TX). Mice were euthanized and wounds were harvested 3 days after wounding. All wounds were either fixed in formalin or embedded and frozen in TBS tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC) for histological or immunohistochemical analysis.
Immunohistochemical Staining in Skin Sections
Ten-μm sections from frozen embedded tissues were prepared for immunohistochemical analysis of PECAM-1 (platelet endothelial cell adhesion molecule-1), VEGFR-1, and VEGFR-2 expression. All incubations and washes were performed at room temperature. Sections were fixed in acetone for 30 minutes. After washes in phosphate-buffered saline (PBS), pH 7.4, sections were treated with 0.3% H2O2 in methanol for 30 minutes to quench endogenous peroxidase activity. The slides were washed in PBS and blocked with normal mouse serum (1:10; Sigma Chemical Co., St. Louis, MO) in PBS for 30 minutes. For PECAM-1 staining, sections were incubated in 1.0 μg/ml of MEC13.3 rat anti-mouse PECAM-1 antibody (anti-CD31; Pharmingen Int., San Diego, CA) in PBS. After a 30-minute incubation with PECAM-1 primary antibody, the slides were washed in PBS. Sections were then incubated for 30 minutes with 3.0 μg/ml of biotinylated mouse anti-rat IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). For the detection of VEGFR-1 and VEGFR-2, sections were incubated for 2 hours with 50 μg/ml of biotinylated goat anti-mouse VEGFR-1 or 50 μg/ml of biotinylated goat anti-mouse VEGFR-2 (R&D Systems). Incubation with the same concentration of biotinylated isotype-matched nonspecific antibody (R&D Systems) was used as a negative control. After washes in PBS, all slides were incubated with avidin-biotin-horseradish peroxidase complex (Vector Laboratories, Burlingame, CA) for 30 minutes, washed, then incubated with the horseradish peroxidase substrate, 3,3′-diaminobenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD) for 10 minutes. Sections were counterstained with Harris hematoxylin (Sigma Chemical Co.) and coverslips were mounted with Cytoseal 280 (Stephens Scientific, Kalamazoo, MI).
For staining of proliferating cell nuclear antigen (PCNA), 5-μm sections from paraffin-embedded wounds were deparaffinized in xylenes and rehydrated in graded ethanols. For antigen retrieval, the sections were placed in hot citrate buffer (10 mmol/L citrate, pH 6) two times for 3 minutes each, with a 5-minute rinse in water between steps. After washing in PBS, samples were blocked in normal horse serum (1:10, Vector Laboratories, Burlingame, CA) for 30 minutes, then incubated overnight at 4°C in a humid chamber with primary anti-PCNA antibody (1:100; Signet Laboratories, Dedham, MA) or an isotype control. The following day, the sections were washed in PBS, blocked in horse serum for 30 minutes, and incubated with biotinylated secondary antibody (1:200, Vector Laboratories) for 30 minutes. After washing in PBS, the sections were incubated with avidin-biotin-horseradish peroxidase complex (Vector), washed, and incubated with 3,3′-diaminobenzidine (Kirkegaard and Perry Laboratories) for 5 minutes. Sections were counterstained with hematoxylin (Sigma) and coverslips were mounted with Cytoseal 280 (Stephens Scientific).
Cell Culture
Normal human epidermal keratinocytes (NHEKs) (Cambrex BioScience Walkersville, Inc., Walkersville, MD), were grown in KGM-2 (Cambrex) and human umbilical vein endothelial cells (HUVECs) (Cambrex) were grown in EGM-2 (Cambrex). HUVECs were used as a positive control for VEGF receptor expression.33 All cells were grown at 37°C and 5% CO2 and the manufacturer’s guidelines for the culture and maintenance of the cells were followed.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from NHEK or HUVEC cultures grown to 70% confluence using TRIzol reagent (Invitrogen Corp., Carlsbad, CA). RNA samples were then subjected to DNase treatments using the RNase-Free DNase Set as directed by the manufacturer (Qiagen Inc., Valencia, CA). RT-PCR was performed using the following primers: VEGFR-1 sense 5′ CTA GGA TCC GTG ACT TAT TTT TTC TCA ACA AGG 3′, VEGFR-1 anti-sense 5′ CTC GAA TTC AGA TCT TCC ATA GTG ATG GGC TC 3′ (240-bp product); β-actin sense 5′ GTG GGC CGC CCT AGG CAC CA 3′, β-actin anti-sense 5′ CTC TTT GAT GTC ACG CAC GAT TTC 3′ (531-bp product); VEGFR-2 sense 5′ CCT GGG GTA AAG ATT GAT GAA G 3′, VEGFR-2 anti-sense 5′ AGT TGG GGT GTG GAT GCT 3′ (776-bp product). Briefly, 5 μg of total RNA was reverse-transcribed at 42°C for 15 minutes in 100 μl containing 5 mmol/L MgCl2, 1× PCR Buffer II, 1 mmol/L each dGTP, dATP, dTTP, dCTP, 2.5 μmol/L random hexamers, 2.5 U/μl MuLV reverse transcriptase (all Applied Biosystems, Foster City, CA) and 2 U/μl rRNAsin RNase inhibitor (Promega Corp., Madison, WI). Ten μl of the RT reaction was used for PCR in a reaction mixture containing 1.5 mmol/L MgCl2, 1× PCR Buffer II, 0.2 mmol/L each dGTP, dATP, dTTP, dCTP, 0.5 μmol/L sense primer, 0.5 μmol/L anti-sense primer, and 0.05 U/μl AmpliTaq Gold (all Applied Biosystems). After a 10-minute incubation at 95°C to activate the Taq polymerase, 40 cycles of PCR were performed with a 1-minute denaturation at 94°C, 1-minute annealing at 62°C, and a 1-minute extension at 72°C, with a final 7-minute extension at 72°C. Negative controls were performed for each sample and consisted of a RT-PCR reaction that did not contain reverse transcriptase. PCR products were visualized on 1% agarose gels and then pictures of the gels were scanned and digitized.
Immunohistochemical Detection of VEGFR-1 in NHEKs
NHEK cells were trypsinized, counted, and resuspended at a density of 2.5 × 105 cells/ml. Cell suspensions (250 μl) were added to cytofunnels and the cells were spun onto glass slides using a Cytospin 2 cytocentrifuge (Thermo Shandon, Pittsburgh, PA). The slides were stored at −80°C until use. For detection of VEGFR-1, slides were thawed and fixed in cold acetone at 4°C for 7 minutes. After washing in PBS, endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol for 30 minutes. After washing in PBS, the specimens were blocked with normal goat serum (1:10, Sigma Chemical Co.) for 30 minutes, then incubated with 30 μg/ml monoclonal mouse anti-human VEGFR-1 antibody (R&D Systems) or mouse IgG1 (R&D Systems) as a negative control for 1 hour. Samples were washed in PBS, and incubated with biotinylated goat anti-mouse secondary antibody (2 μg/ml, Jackson ImmunoReseach Laboratories) for 1 hour, avidin-biotin-horseradish peroxidase complex (Vector Laboratories) for 30 minutes, and 3,3′-diaminobenzidine (Kirkegaard and Perry Laboratories) for 10 minutes with PBS washes between each step. Samples were dehydrated in ethanols, then coverslipped and mounted with Cytoseal 280 (Stephens Scientific).
Binding of Radiolabeled VEGF to NHEK Cells
NHEKs were seeded in 12-well tissue culture plates at a density of 300,000 cells per well. After 24 hours, cells were washed with PBS and were treated with a range of 125I-VEGF (200 to 20,000 pmol/L; Amersham Biosciences, Piscataway, NJ), with or without 150× molar excess unlabeled VEGF165 (R&D Systems) in HEPES-buffered saline solution (Cambrex) with 0.1% bovine serum albumin (Sigma) for 18 hours at 4°C with gentle agitation. The following day, the cells were rinsed three times with PBS, then lysed with 1% Triton X-100 (Sigma). The levels of 125I in the cell lysates were measured with a gamma counter (Cobra II autogamma; Packard Instrument Co., Meriden, CT) and normalized to protein content as determined by the BCA protein assay (Pierce, Rockford, IL). Specific binding was calculated by subtracting the nonspecific binding from the total binding. Scatchard analysis was used to calculate the KD.
NHEK Proliferation
The effect of VEGF165 on the proliferation of NHEKs was determined using the Alamar Blue assay (Biosource, Camarillo, CA). Cells were seeded in 96-well plates at a density of 500 cells per well, allowed to adhere for 12 hours, then starved in basal KBM-2 (Cambrex) for 12 hours before treatments. At the time of cell starvation, one group of cells received 5 μg/ml of neutralizing anti-VEGFR-1 antibody (R&D Systems) such that these cells were pretreated for 12 hours with neutralizing antibody before exposure to VEGF. Twelve hours after starvation and/or neutralizing antibody treatment, media was removed and cells were treated with 100 ng/ml of VEGF165 (R&D Systems) or without VEGF (control) in KGM-2 containing 10% volume Alamar Blue (Biosource). This dose of VEGF was determined to be optimal for inducing proliferation based on a dose-response curve. Six wells per treatment were used in the analysis and the experiment was repeated three times. The magnitude of the response of the NHEKs to VEGF varied by lot of cells. The plate was read using a SPECTRAmax PLUS 384 spectrophotometer (Molecular Devices, Sunnyvale, CA) 6, 24, 48, and 72 hours after VEGF treatment. The percent reduction of the Alamar Blue dye, which corresponds to cellular proliferation, was calculated based on the A570 and A600 values using the formula supplied by the manufacturer.
Analysis of Re-Epithelialization
Re-epithelialization was measured in mice treated topically with control IgG or anti-VEGFR-1 antibodies using an ocular grid in hematoxylin and eosin (H&E)-stained wound sections as previously described.34 Briefly, the percent re-epithelialization was calculated by determining the distance of the wound bed and the distance covered by neoepidermis. The average of two wounds per mouse (n = 8 mice per treatment group) was used for analysis.
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Statistical differences were determined by two-way analysis of variance with Bonferroni posttest or unpaired t-test, with values of P < 0.05 considered statistically significant.
Results
VEGF Receptor Localization in Cutaneous Wounds and Unwounded Skin
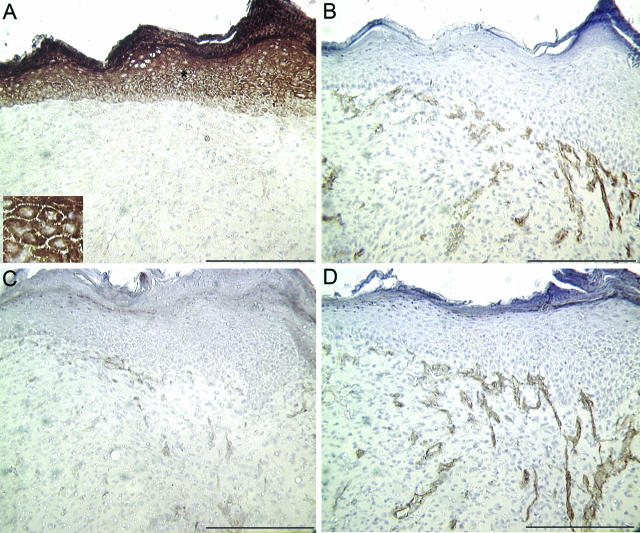
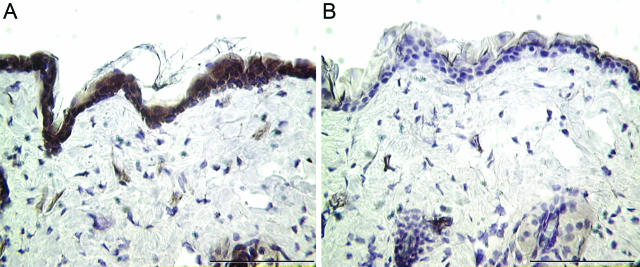
Immunohistochemical analysis was used to examine the expression of VEGFR-1 and VEGFR-2 in healing wounds at time points known to correspond to an increase in vessel density in this excisional wound model. Representative sections shown are from wounds harvested 7 days after injury. Serial sections were stained with PECAM-1, an endothelial cell marker, to delineate the blood vessels within the wound bed. Unexpectedly, little VEGFR-1 (Figure 1A) was detected in capillaries within the wound bed. VEGFR-1 did not co-localize to PECAM-1 (Figure 1B), but instead intense staining was found in epidermal keratinocytes. The inset in Figure 1A highlights the membrane staining of VEGFR-1. In contrast, VEGFR-2 (Figure 1C) was detected in blood vessels within the wound bed as expected, demonstrating a similar staining pattern to PECAM-1 (Figure 1D). Due to the epidermal staining pattern of VEGFR-1 in wounded skin (Figure 1), we examined the expression of this receptor in unwounded skin. Similar to the overlying keratinocytes in wound tissue, intense staining for VEGFR-1 was also found in the epidermal layer of unwounded skin (Figure 2A). Although VEGFR-1 expression has been demonstrated in non-endothelial cell types previously, this is the first report to our knowledge demonstrating VEGR-1 expression in keratinocytes.
Figure 1.
PECAM-1 and VEGF receptor localization in wounds. Histological sections from excisional wounds harvested 7 days after wounding were stained with antibodies specific for VEGFR-1 (A) or VEGFR-2 (C). Corresponding serial sections stained for PECAM-1 are shown for each sample in B and D to highlight the blood vessels in the wound bed. The inset in A is a higher magnification of the area marked with an asterisk. Scale bar, 200 μm.
Figure 2.
VEGFR-1 expression in unwounded skin. Sections of unwounded skin were subjected to immunohistochemistry and stained with antibodies specific for VEGFR-1 (A) or with isotype-matched IgG (B) as a negative control. Scale bar, 100 μm.
Expression of VEGFR-1 on NHEKs
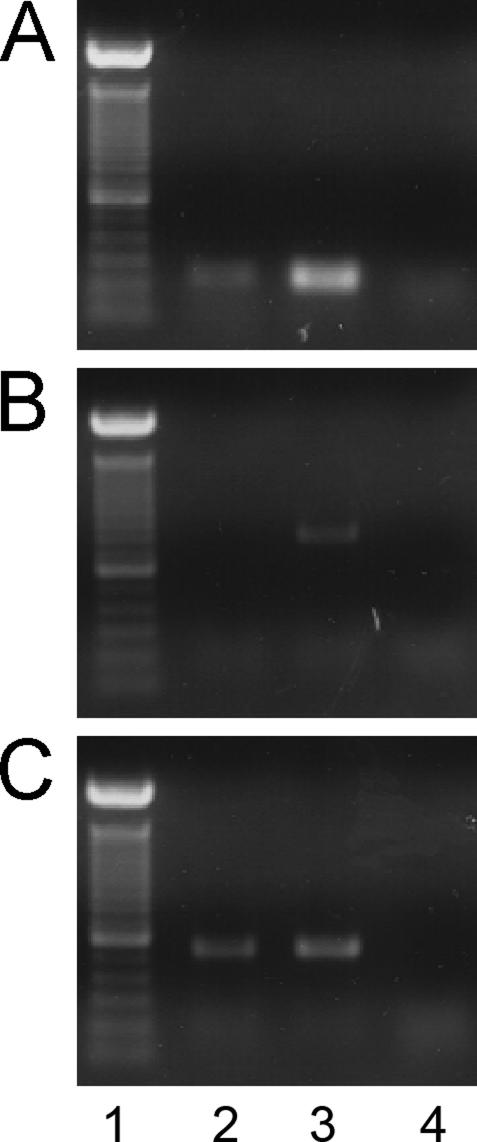
Cultured primary keratinocytes (NHEK cells) were used to confirm the expression of VEGFR-1 in keratinocytes. The expression of VEGFR-1 (Figure 3A), VEGFR-2 (Figure 3B), and the housekeeping gene β-actin (Figure 3C) was examined by RT-PCR. Endothelial cells (HUVECs), known to express both receptors,33 were used as a positive control. As predicted by the staining patterns found in Figure 1, VEGFR-1 mRNA expression was detected in both keratinocytes and endothelial cells, whereas VEGFR-2 transcripts were detected only in endothelial cells.
Figure 3.
VEGF receptor mRNA expression in NHEK cells. Expression of VEGFR-1 (A) and VEGFR-2 (B) mRNA was examined in NHEKs using RT-PCR. C: Expression of the housekeeping gene β-actin is also shown. As a positive control, HUVECs were subjected to the same procedures. The following are shown for each gene: 100-bp ladder (lane 1), NHEK cells (lane 2), HUVECs (lane 3), and a sample containing no reverse transcriptase (RT−) as a negative control (lane 4).
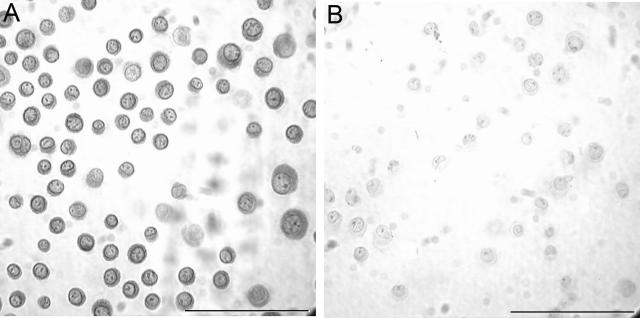
In addition to mRNA expression, the expression of VEGFR-1 protein was detected in NHEKs. Immunohistochemistry was performed on cytospin preparations of cultured keratinocytes to detect VEGFR-1. Intense staining for VEGFR-1 was found in keratinocytes (Figure 4A) compared to the negative control (Figure 4B) that was stained with an isotype-matched, nonspecific antibody. Immunohistochemistry for VEGFR-2 was also performed in keratinocytes, but positive staining was not detected (data not shown). The expression of VEGFR-1 protein by keratinocytes was also confirmed by Western blot (data not shown). Thus, in vitro analysis demonstrated the expression of VEGFR-1 in keratinocytes, confirming the expression pattern found in murine wounds (Figure 1) and in unwounded skin (Figure 2).
Figure 4.
Immunohistochemical detection of VEGFR-1 in NHEK cells. A: After trypsinization, cytospin preparations of NHEK cells were used to examine VEGFR-1 protein expression by immunohistochemistry. B: A negative control slide stained with an isotype control antibody is shown to demonstrate the specificity of the VEGFR-1 staining. Scale bar, 100 μm.
Binding of Radiolabeled VEGF to NHEKs
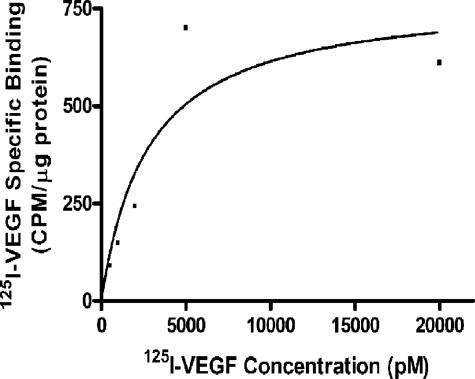
The capacity of NHEK cells to bind VEGF was assessed using 125I-VEGF. Cells were incubated with 125I-VEGF in the presence or absence of unlabeled (cold) VEGF and the specific binding of 125I-VEGF to NHEKs was determined. A representative binding curve is shown in Figure 5. Saturable binding of 125I-VEGF to NHEKs was observed, with a KD of 2.8 nmol/L. Previously, two classes of VEGF binding sites were described in endothelial cells with approximate KDs of 10 pmol/L and 1 nmol/L,35 suggesting that the receptors present on keratinocytes are lower affinity receptors than those found on endothelial cells.
Figure 5.
Specific binding of 125I-VEGF to NHEKs. NHEKs were incubated in the presence of various concentrations of 125I-VEGF with or without a 150-fold molar excess of cold VEGF165. Specific binding was determined and is expressed as cpm per μg protein.
VEGF-Induced Proliferation of NHEKs Is Dependent on VEGFR-1
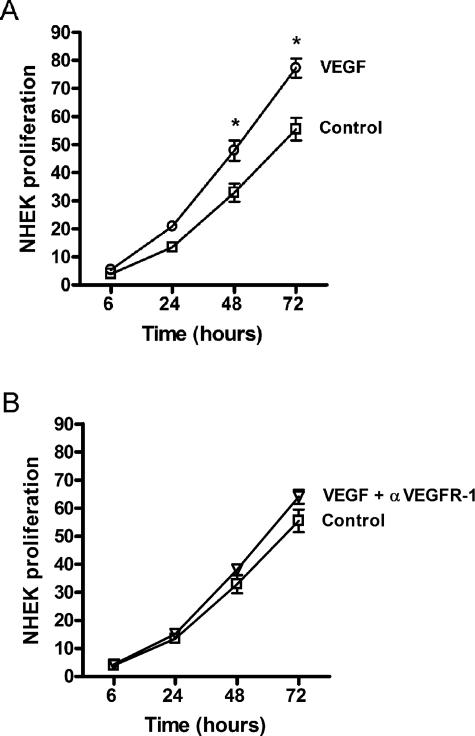
To examine the potential function of VEGFR-1 in keratinocytes, the effect of VEGF165 on NHEK proliferation was assessed using Alamar Blue. Treatment with 100 ng/ml of human VEGF165 resulted in a significant increase in the proliferation of NHEKs (Figure 6A) compared to cells that were not treated with VEGF (control). In addition, pretreatment with neutralizing VEGFR-1 antibodies (5 μg/ml) blocked the increase in proliferation by VEGF (Figure 6B), suggesting the ability of VEGF to promote proliferation in NHEK cells is dependent on VEGF-VEGFR-1 interaction.
Figure 6.
Proliferation of NHEKs in response to VEGF. The effect of VEGF165 on the proliferation of NHEKs was determined using Alamar Blue dye. Cells were treated with 100 ng/ml of VEGF165 (A, ○) or VEGF plus pretreatment with neutralizing VEGFR-1 antibodies (B, ▿). Control cells were not treated with VEGF (A and B, □). The percent reduction of the Alamar Blue dye, which is a measure of proliferation, was determined spectrophotometrically throughout a 72-hour period. *P < 0.05.
Effects of VEGFR-1 on Wound Repair in Vivo
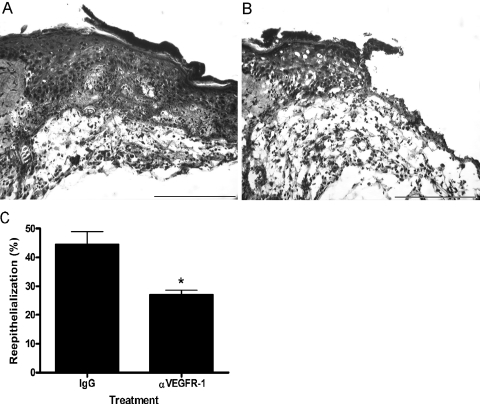
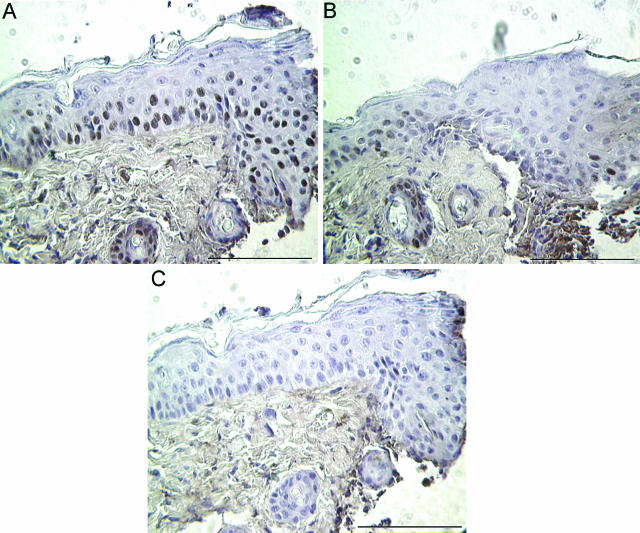
To evaluate the role of VEGFR-1 on keratinocyte function in vivo, neutralizing VEGR-1 antibodies were applied topically to excisional wounds. The wounds were treated topically with either normal goat IgG or neutralizing mouse VEGFR-1 antibody. Three days after injury, wounds treated with blocking antibodies specific for VEGFR-1 exhibited a significant delay in re-epithelialization compared to IgG-treated control wounds. Representative H&E-stained wounds treated with IgG control (Figure 7A) or anti-VEGFR-1 antibodies (Figure 7B) are shown. Note the extension of the epithelium beyond the microscopic field in Figure 7A. Quantification of re-epithelialization demonstrated that the epithelium covered only 27% of the wound bed in wounds treated with anti-VEGFR-1 antibodies, compared to 44% in control wounds (Figure 7C). To assess the effects of anti-VEGFR-1 antibody treatment on keratinocyte proliferation in vivo, immunohistochemical staining for the proliferation marker PCNA was performed. A reduction in the number of PCNA-positive keratinocytes at the wound edge was evident in wounds treated with anti-VEGFR-1 antibodies (Figure 8B) compared to control IgG-treated wounds (Figure 8A). Taken together with the data in Figures 6 and 7, these results suggest that VEGFR-1 signaling may influence the proliferation of keratinocytes that is required for rapid wound re-epithelialization.
Figure 7.
Effect of VEGR-1 antibody treatment on wound repair. Excisional wounds were treated topically with anti-VEGFR-1 antibodies to examine the function of VEGFR-1 on keratinocytes in vivo. Representative photomicrographs of 3-day H&E-stained wounds that had been treated with IgG control (A) or anti-VEGFR-1 antibodies (B) are shown. C: The percent re-epithelialization was measured 3 days after injury and is depicted graphically. Scale bar, 200 μm. *P < 0.05.
Figure 8.
Detection of proliferating wound keratinocytes. PCNA was used to detect proliferating keratinocytes in 3-day wounds treated with either IgG control antibodies (A) or anti-VEGFR-1 antibodies (B). C: A serial section of the wound shown in A was stained with isotype-matched nonspecific antibodies as a negative control. Scale bar, 100 μm.
Discussion
A period of robust angiogenesis occurs during adult wound healing, and is presumed to be essential for appropriate wound repair. New blood vessels within the wound likely supply oxygen and nutrients to support the cellular proliferation involved in tissue restoration. Many studies suggest that VEGF is a critical regulator of angiogenesis in healing wounds.
Since the first description of VEGF production in wound keratinocytes,18 many studies have provided evidence that VEGF can promote cutaneous wound repair.36–39 Although VEGF expression has been reported in macrophages and fibroblasts in response to injury,18,20 keratinocytes are considered the main source of VEGF during wound healing. The VEGF produced by keratinocytes during wound healing is traditionally thought to act in a paracrine manner,40 promoting wound repair by stimulating endothelial cell-mediated angiogenesis. The data presented here, demonstrating the expression of functional VEGFR-1 on keratinocytes, implies that keratinocyte-derived VEGF may be capable of promoting repair through a novel autocrine pathway. Although the expression of functional VEGF receptors in keratinocytes has not been reported before now, several epithelial tumor cells have been shown to express VEGF receptors;41–44 therefore, it is not surprising that epithelial cells in the skin might also express functional VEGF receptors.
Many studies have shown that VEGF is important for wound repair.12,36–39 Until now, the positive effects of VEGF on wound repair were thought to be completely angiogenesis-driven. Although our studies are the first to outline a concrete pathway by which VEGF could encourage healing via the direct stimulation of keratinocytes, several pieces of evidence exist that indirectly support this concept. For example, several angiogenesis inhibitors that act independently of VEGF to reduce wound angiogenesis do not significantly alter wound closure.45–47 In addition, several groups have reported that inhibition of wound angiogenesis by targeting VEGFR-2, which was detected on endothelial cells within the wound bed but could not be detected on keratinocytes, does not change wound healing rates.48–50 Together with our data, this suggests that by directly targeting VEGFR-2 on the endothelium to reduce angiogenesis without reducing available VEGF levels or VEGFR-1 signaling, VEGF is still available to bind VEGFR-1 on keratinocytes and normal closure rates can be attained. All of these results support the theory that VEGF may have a function in wound repair beyond promoting angiogenesis. Additional support for this idea comes from a recent wound-healing study performed on mice that have VEGF-A specifically deleted in keratinocytes.12 These mice, which display an avascular zone just below the epidermis but no difference in overall vascular density, exhibit significant delays in eschar shedding and wound closure. In addition, a time course comparison of VEGF production in excisional murine wounds, which normally peaks between days 2 and 5, versus vascular density patterns, which peak much later (days 10 to 14) and after re-epithelialization is complete, also call into question the hypothesis that VEGF promotes healing solely through angiogenesis stimulation.34,51,52 Taken together with the results presented here, the accumulated data support the notion that the VEGF produced by keratinocytes during wound healing may act, at least in part, in an autocrine manner to promote healing responses in keratinocytes.
The studies presented here demonstrating the presence of functional VEGFR-1 on keratinocytes, as well as other reports of functional VEGF receptor expression on non-endothelial cell types, broadens the understanding of how these receptors may function on a larger level. Our studies also suggest a novel mechanism by which VEGF may act to stimulate wound healing, as an autocrine growth factor in keratinocytes. Our results as well as others indicate that VEGF may play a more diverse role in pathological and physiological processes than previously believed, with the biological activities of VEGF not being restricted solely to the growth and permeability of the vasculature. Future studies will have to be done to dissect the relative contribution of VEGF to the epidermal portion of wound repair versus wound angiogenesis, and which property is more important for healing. A better understanding of exactly how VEGF functions in wound repair could be used to design more effective therapies for nonhealing wounds. Aside from wound healing, several skin disorders with a significant keratinocyte component are linked to a high presence of VEGF, such as skin cancer,9,10 psoriasis,14–17 and a host of other skin conditions.53 Therefore, it will also be important from a clinical standpoint to better define the role of VEGF and whether direct effects of VEGF on keratinocytes may also be a factor in the development of these pathological skin conditions.
Footnotes
Address reprint requests to Luisa A. DiPietro, D.D.S., Ph.D., Loyola University Medical Center, Burn and Shock Trauma Institute, 2160 S. First Ave., Maywood, IL 60153. E-mail: ldipiet@lumc.edu.
Supported by the National Institutes of Health (grant GM50875).
References
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- Spyridopoulos I, Brogi E, Kearney M, Sullivan AB, Cetrulo C, Isner JM, Losordo DW. Vascular endothelial growth factor inhibits endothelial cell apoptosis induced by tumor necrosis factor-alpha: balance between growth and death signals. J Mol Cell Cardiol. 1997;29:1321–1330. doi: 10.1006/jmcc.1996.0365. [DOI] [PubMed] [Google Scholar]
- Goldman CK, Tsai JC, Soroceanu L, Gillespie GY. Loss of vascular endothelial growth factor in human alopecia hair follicles. J Invest Dermatol. 1995;104:18S–20S. doi: 10.1038/jid.1995.40. [DOI] [PubMed] [Google Scholar]
- Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachgar S, Moukadiri H, Jonca F, Charveron M, Bouhaddioui N, Gall Y, Bonafe JL, Plouet J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J Invest Dermatol. 1996;106:17–23. doi: 10.1111/1523-1747.ep12326964. [DOI] [PubMed] [Google Scholar]
- Ozeki M, Tabata Y. Promoted growth of murine hair follicles through controlled release of vascular endothelial growth factor. Biomaterials. 2002;23:2367–2373. doi: 10.1016/s0142-9612(01)00372-6. [DOI] [PubMed] [Google Scholar]
- Brown LF, Tognazzi K, Dvorak HF, Harrist TJ. Strong expression of kinase insert domain-containing receptor, a vascular permeability factor/vascular endothelial growth factor receptor in AIDS-associated Kaposi’s sarcoma and cutaneous angiosarcoma. Am J Pathol. 1996;148:1065–1074. [PMC free article] [PubMed] [Google Scholar]
- Weninger W, Uthman A, Pammer J, Pichler A, Ballaun C, Lang IM, Plettenberg A, Bankl HC, Sturzl M, Tschachler E. Vascular endothelial growth factor production in normal epidermis and in benign and malignant epithelial skin tumors. Lab Invest. 1996;75:647–657. [PubMed] [Google Scholar]
- Larcher F, Robles AI, Duran H, Murillas R, Quintanilla M, Cano A, Conti CJ, Jorcano JL. Up-regulation of vascular endothelial growth factor/vascular permeability factor in mouse skin carcinogenesis correlates with malignant progression state and activated H-ras expression levels. Cancer Res. 1996;56:5391–5396. [PubMed] [Google Scholar]
- Marcoval J, Moreno A, Graells J, Vidal A, Escriba JM, Garcia-Ramirez M, Fabra A. Angiogenesis and malignant melanoma. Angiogenesis is related to the development of vertical (tumorigenic) growth phase. J Cutan Pathol. 1997;24:212–218. doi: 10.1111/j.1600-0560.1997.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Rossiter H, Barresi C, Pammer J, Rendl M, Haigh J, Wagner EF, Tschachler E. Loss of vascular endothelial growth factor a activity in murine epidermal keratinocytes delays wound healing and inhibits tumor formation. Cancer Res. 2004;64:3508–3516. doi: 10.1158/0008-5472.CAN-03-2581. [DOI] [PubMed] [Google Scholar]
- Larcher F, Murillas R, Bolontrade M, Conti CJ, Jorcano JL. VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene. 1998;17:303–311. doi: 10.1038/sj.onc.1201928. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan M, McLaughlin B, Weiss JB, Griffiths CE. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol. 1999;141:1054–1060. doi: 10.1046/j.1365-2133.1999.03205.x. [DOI] [PubMed] [Google Scholar]
- Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- Detmar M. Evidence for vascular endothelial growth factor (VEGF) as a modifier gene in psoriasis. J Invest Dermatol. 2004;122:xiv–xv. doi: 10.1046/j.0022-202X.2003.22140.x. [DOI] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Peters KG, De Vries C, Williams LT. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc Natl Acad Sci USA. 1993;90:8915–8919. doi: 10.1073/pnas.90.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby IA, Bisucci T, Raghoenath S, Olsson J, Muscat GE, Koopman P. Sox18 is transiently expressed during angiogenesis in granulation tissue of skin wounds with an identical expression pattern to Flk-1 mRNA. Lab Invest. 2001;81:937–943. doi: 10.1038/labinvest.3780304. [DOI] [PubMed] [Google Scholar]
- Brown LF, Detmar M, Tognazzi K, Abu-Jawdeh G, Iruela-Arispe ML. Uterine smooth muscle cells express functional receptors (flt-1 and KDR) for vascular permeability factor/vascular endothelial growth factor. Lab Invest. 1997;76:245–255. [PubMed] [Google Scholar]
- Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998;83:832–840. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- Takagi H, King GL, Aiello LP. Identification and characterization of vascular endothelial growth factor receptor (Flt) in bovine retinal pericytes. Diabetes. 1996;45:1016–1023. doi: 10.2337/diab.45.8.1016. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor promotes proliferation of cortical neuron precursors by regulating E2F expression. FASEB J. 2003;17:186–193. doi: 10.1096/fj.02-0515com. [DOI] [PubMed] [Google Scholar]
- Ancelin M, Chollet-Martin S, Herve MA, Legrand C, El Benna J, Perrot-Applanat M. Vascular endothelial growth factor VEGF189 induces human neutrophil chemotaxis in extravascular tissue via an autocrine amplification mechanism. Lab Invest. 2004;84:502–512. doi: 10.1038/labinvest.3700053. [DOI] [PubMed] [Google Scholar]
- Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T, Shibuya M. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- Decaussin M, Sartelet H, Robert C, Moro D, Claraz C, Brambilla C, Brambilla E. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): correlation with angiogenesis and survival. J Pathol. 1999;188:369–377. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Zhang N, Fang Z, Contag PR, Purchio AF, West DB. Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood. 2004;103:617–626. doi: 10.1182/blood-2003-06-1820. [DOI] [PubMed] [Google Scholar]
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479–1487. [PubMed] [Google Scholar]
- Olander JV, Connolly DT, DeLarco JE. Specific binding of vascular permeability factor to endothelial cells. Biochem Biophys Res Commun. 1991;175:68–76. doi: 10.1016/s0006-291x(05)81201-x. [DOI] [PubMed] [Google Scholar]
- Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, Capogrossi MC. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002;9:1271–1277. doi: 10.1038/sj.gt.3301798. [DOI] [PubMed] [Google Scholar]
- Liu PY, Tong W, Liu K, Han SH, Wang XT, Badiavas E, Rieger-Christ K, Summerhayes I. Liposome-mediated transfer of vascular endothelial growth factor cDNA augments survival of random-pattern skin flaps in the rat. Wound Repair Regen. 2004;12:80–85. doi: 10.1111/j.1067-1927.2004.012114.x. [DOI] [PubMed] [Google Scholar]
- Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deodato B, Arsic N, Zentilin L, Galeano M, Santoro D, Torre V, Altavilla D, Valdembri D, Bussolino F, Squadrito F, Giacca M. Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther. 2002;9:777–785. doi: 10.1038/sj.gt.3301697. [DOI] [PubMed] [Google Scholar]
- Detmar M, Yeo KT, Nagy JA, Van de Water L, Brown LF, Berse B, Elicker BM, Ledbetter S, Dvorak HF. Keratinocyte-derived vascular permeability factor (vascular endothelial growth factor) is a potent mitogen for dermal microvascular endothelial cells. J Invest Dermatol. 1995;105:44–50. doi: 10.1111/1523-1747.ep12312542. [DOI] [PubMed] [Google Scholar]
- Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, Twentyman PR, Smith SK. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995;87:506–516. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- Speirs V, Atkin SL. Production of VEGF and expression of the VEGF receptors Flt-1 and KDR in primary cultures of epithelial and stromal cells derived from breast tumours. Br J Cancer. 1999;80:898–903. doi: 10.1038/sj.bjc.6690438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunk C, Ahmed A. Vascular endothelial growth factor receptor-2-mediated mitogenesis is negatively regulated by vascular endothelial growth factor receptor-1 in tumor epithelial cells. Am J Pathol. 2001;158:265–273. doi: 10.1016/S0002-9440(10)63965-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Yoshikawa M, Miyazaki T, Nakajima M, Fukai Y, Masuda N, Fukuchi M, Manda R, Tsukada K, Kuwano H. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) in esophageal squamous cell carcinoma. Anticancer Res. 2002;22:3977–3984. [PubMed] [Google Scholar]
- Jang YC, Arumugam S, Gibran NS, Isik FF. Role of alpha(v) integrins and angiogenesis during wound repair. Wound Repair Regen. 1999;7:375–380. doi: 10.1046/j.1524-475x.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt B, Velasco P, Streit M, Hawighorst T, Pike SE, Tosato G, Detmar M. The angiogenesis inhibitor vasostatin does not impair wound healing at tumor-inhibiting doses. J Invest Dermatol. 2001;117:1036–1041. doi: 10.1046/j.0022-202x.2001.01519.x. [DOI] [PubMed] [Google Scholar]
- Nanney LB, Wamil BD, Whitsitt J, Cardwell NL, Davidson JM, Yan HP, Hellerqvist CG. CM101 stimulates cutaneous wound healing through an anti-angiogenic mechanism. Angiogenesis. 2001;4:61–70. doi: 10.1023/a:1016752925761. [DOI] [PubMed] [Google Scholar]
- Roman CD, Choy H, Nanney L, Riordan C, Parman K, Johnson D, Beauchamp RD. Vascular endothelial growth factor-mediated angiogenesis inhibition and postoperative wound healing in rats. J Surg Res. 2002;105:43–47. doi: 10.1006/jsre.2002.6444. [DOI] [PubMed] [Google Scholar]
- Jacobi J, Tam BY, Sundram U, von Degenfeld G, Blau HM, Kuo CJ, Cooke JP. Discordant effects of a soluble VEGF receptor on wound healing and angiogenesis. Gene Ther. 2004;11:302–309. doi: 10.1038/sj.gt.3302162. [DOI] [PubMed] [Google Scholar]
- Tsou R, Fathke C, Wilson L, Wallace K, Gibran N, Isik F. Retroviral delivery of dominant-negative vascular endothelial growth factor receptor type 2 to murine wounds inhibits wound angiogenesis. Wound Repair Regen. 2002;10:222–229. doi: 10.1046/j.1524-475x.2002.10405.x. [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Egozi EI, Gamelli RL, DiPietro LA. The effect of thrombocytopenia on dermal wound healing. J Invest Dermatol. 2003;120:1130–1137. doi: 10.1046/j.1523-1747.2003.12253.x. [DOI] [PubMed] [Google Scholar]
- Egozi EI, Ferreira AM, Burns AL, Gamelli RL, Dipietro LA. Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair Regen. 2003;11:46–54. doi: 10.1046/j.1524-475x.2003.11108.x. [DOI] [PubMed] [Google Scholar]
- Brown LF, Harrist TJ, Yeo KT, Stahle-Backdahl M, Jackman RW, Berse B, Tognazzi K, Dvorak HF, Detmar M. Increased expression of vascular permeability factor (vascular endothelial growth factor) in bullous pemphigoid, dermatitis herpetiformis, and erythema multiforme. J Invest Dermatol. 1995;104:744–749. doi: 10.1111/1523-1747.ep12606974. [DOI] [PubMed] [Google Scholar]