Abstract
Alzheimer’s disease (AD) is the most common dementing illness of the elderly and is a mounting public health problem. Pharmacoepidemiological data, analytical data from human tissue and body fluids, and mechanistic data mostly from murine models all have implicated oxidation products of two fatty acids, arachidonic acid (AA) and docosahexaenoic acid (DHA), in the pathogenesis of neurodegeneration. Here we review the biochemistry of AA and DHA oxidation, both enzyme-catalyzed and free radical mediated, and summarize those studies that have investigated these oxidation products as effectors of neurodegeneration and biomarkers of AD. Given the evolving appreciation for toxicity associated with current pharmaceuticals used to block AA and DHA oxidation, we close by speculating on likely areas of future research directed at suppressing this facet of neurodegeneration. If successful, these interventions are unlikely to cure AD, but may check its explosive growth and hopefully reduce its incidence and prevalence in the elderly.
Alzheimer’s disease (AD) is most commonly a disease of late life that derives from pathogenic processes underlying abnormal accumulation of amyloid-β (Aβ) peptides and hyperphosphorylated tau in certain regions of cerebrum. The etiology of late onset AD has been partially illuminated by several associated risk factors but likely is complex and multifactorial. Late onset AD represents a significant and growing public health burden, a silent epidemic currently affecting between 2.5 and 4 million people in the U.S. and more than 10 million people worldwide.1,2 This epidemic is projected to grow significantly throughout the next generation with an estimated 8 to 12 million patients by the year 2050 in the U.S. alone. In addition to the untold suffering by patients and their families, AD is the third most costly medical condition in the U.S.3–5 As the number of patients afflicted continues to mount, the need for safe and effective therapy to delay or avert AD will become imperative.6
Recent data suggest that two partially effective preventative classes of drugs already may have been identified: nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit the cyclooxygenases (COXs), and antioxidants (AOs), which suppress free radical-mediated damage.7–13 Of the AOs, the best studied is α-tocopherol, a lipid radical chain-terminating agent. It is critical to note that the apparent effectiveness for NSAIDs and AOs has been reproducibly observed for these classes of agents in epidemiological studies that measure subsequent risk of developing AD-type dementia.7–12 In contrast, no effect or only modest effect from specific drugs within these classes has been observed in clinical trials of patients with established dementia.13,14 Although there are several possible interpretations of these results, one is that at least some commonly used NSAIDs and AOs are effective at suppressing pathogenic processes of AD during latent or prodromal stages but are ineffective against clinically overt dementia. Although prevention trials for NSAIDs and α-tocopherol are one way to test directly this hypothesis, both recently have been challenged by unexpected toxicity from protracted exposure in the elderly.
In support of a mechanistic role for processes suppressed by NSAIDs or AOs in early phases of AD pathogenesis, transgenic mice that express mutant human amyloid precursor protein and accumulate Aβ deposits in brain with advancing age show significantly less Aβ accumulation when treated with NSAIDs.15 Moreover, a variety of interventions have been reported to increase or decrease Aβ accumulation in transgenic mouse models of cerebral Aβ amyloidogenesis by promoting or suppressing free radical damage to brain.15–18 Using different transgenic mice, others have shown that neuronal overexpression of one COX isozyme, COX-2, in brain leads to neurodegeneration and age-related cognitive deficits.19 The major in vivo activity of the NSAIDs used in these studies is inhibition of both COX isozymes, although several alternatives have been proposed based on in vitro or cell culture data.20–22 It is noteworthy that, despite many proposals for alternative actions of NSAIDs, we are aware of no data demonstrating major therapeutic action in vivo other than through COX suppression. For example, the recent proposal from cell culture data that NSAIDs may act via γ-secretase suppression23 has not been supported by in vivo investigation.24
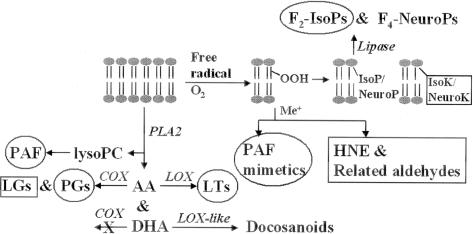
These reproducible and intriguing epidemiological data, in addition to the mechanistic data from animal models, have fueled substantial interest in polyunsaturated fatty acid (PUFA) oxidation, either enzyme-catalyzed or free radical-mediated, in the molecular pathogenesis of AD (Figure 1). Much of this recent investigation has focused on two PUFAs, arachidonic acid (AA, 20:4ω6) whose oxidation products are called eicosanoids, and docosahexaenoic acid (DHA, 22:6ω3) whose oxidation products are termed docosanoids. A critical distinction exists between AA and DHA. AA is evenly distributed in gray matter and white matter and among the different cell types in brain whereas DHA is highly enriched in neuronal membranes.25,26 Thus, eicosanoids reflect oxidation reactions occurring in brain tissue, but not necessarily in neurons, while docosanoid formation is relatively specific for biochemical reactions occurring in neurons.
Figure 1.
Phospholipid is acted on by PLA2 to liberate AA, DHA, and lysoPC that are then converted to a variety of biologically active metabolites via enzyme-catalyzed reactions. Alternatively, free radical-mediated attack on phospholipids followed by oxygen insertion generates lipid hydroperoxides that then may rearrange or fragment to produce a variety of products. Enzymes are listed in italics. Circled molecules are known to activate specific receptors. Squared molecules are chemically reactive and modify cellular nucleophiles.
Enzyme-Catalyzed Oxygenation of AA and DHA
The ester that binds AA or DHA to the glycerol backbone of phospholipids is hydrolyzed by phospholipase (PL) A2 in response to a wide array of physiological and pathophysiological stimuli.27 Although it is clear that products of enzyme-catalyzed oxygenation of hydrolyzed AA function as potent second messengers as well as autocrine and paracrine factors in the central nervous system, the physiological roles of liberated DHA and its docosanoid products are less clearly understood. There are two principal groups of enzymes that catalyze oxygenation of these hydrolyzed PUFAs to yield precursors of potent bioactive products. The first is the COXs that catalyze the formation of prostaglandin (PG) H2 from free AA. The second is the lipoxygenases (LOXs) that catalyze the formation of hydroperoxides from AA and likely also DHA.28 Although cytochrome P450s also may catalyze oxygenation of AA and DHA, the bioactivity of these compounds is less clear.
Products of COXs and Their Bioactivity
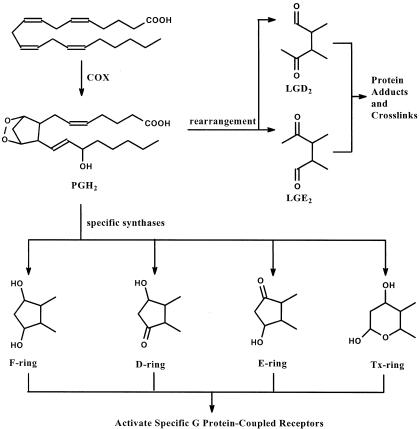
Isozymes of COX catalyze oxygenation of hydrolyzed AA in what is the committed step in PG synthesis (Figure 2);29 DHA is not a substrate for COX isozymes. A bis-oxygenase reaction in which AA plus two O2 are converted to the bicycloendoperoxide intermediate PGG2 is followed by a peroxidase step to generate PGH2 with the release of an oxidizing radical.30 PGH2 exerts biological activity through three different mechanisms. First, PGH2 may be further metabolized by a number of cell-specific synthases or isomerases to PGD2, PGE2, PGF2α, PGI2, and thromboxane (Tx) A2, all of which exert potent autocrine and paracrine activities through stimulation of specific prostanoid cell surface receptors designated DP, EP1--4, FP, IP, and TP, respectively.31 It is through the coordinated cell-specific expression of these synthases or isomerases and receptors that products of COX achieve such a wide variety of actions in different cells and tissue. Second, PGH2 itself is an agonist for TP. Finally, PGH2 may rearrange to form levuglandins (LGs), highly chemically reactive γ-ketoaldehydes that form irreversible adducts with ε-amino groups of protein lysyl residues leading to protein-protein crosslinks;32 indeed, LGs significantly accelerate oligomerization of Aβ peptides in vitro.33 One study has shown a specific approximately fivefold increase in cerebrospinal fluid PGE2 in patients with early AD, none of whom were taking NSAIDs or aspirin.34 We are unaware of any data exploring the time course of cerebrospinal fluid PGs in AD or its prodrome. Given the known and recently discovered toxicities associated with COX inhibitors,35,36 future research in this area likely will concentrate on specific receptor antagonists. For example, microglia derived from mice lacking a specific PGE2 receptor subtype, EP2, display both enhanced phagocytosis of Aβ peptides in AD brain sections and decreased bystander damage to neurons.37
Figure 2.
COX catalyzes the conversion of AA to PGH2. Cell-specific synthases and isomerases catalyze the transformation of PGH2 into other PGs or Tx that exert biological activity by binding to specific cell surface receptors. Alternatively, PGH2 may rearrange to form LGs, γ-ketoaldehydes that irreversibly form adducts with protein lysyl groups and lead to protein-protein crosslinking.
Products of LOXs and Their Bioactivity
LOXs are a family of cytosolic enzymes that catalyze the oxygenation of polyunsaturated fatty acids to form lipid hydroperoxides; for AA, these are called hydroperoxyeicosatetraenoic acids (HPETEs).28 Different LOXs vary in the placement of the hydroperoxy group and are so named. Thus 5-LOX catalyzes the formation of 5-HPETEs, 12-LOX the formation of 12-HPETEs, and so on. HPETEs are unstable intermediates, like PGH2, and are converted to potent autocrine and paracrine factors, their corresponding hydroxyl (HETEs), either nonenzymatically or by peroxidases. Products of 5-LOX may be further metabolized to leukotriene (LT) A4, which can be subsequently hydrolyzed to LTB4 or LTC4. LTC4 is metabolized via the mercapturic acid pathway to the cysteinyl-LTs (CysLTs): LTD4, LTE4, and LTF4. Elements in this arm of LT biosynthesis are well known potent components of local inflammatory response and indeed comprise the slow reacting substance of anaphylaxis. The CysLTs activate two classes of receptors, CysLT1 and CysLT2, that are targets for antagonists being evaluated for efficacy in inflammatory diseases such as asthma.38 In addition to LTs, LOX can catalyze the formation of lipoxins that function in the resolution of inflammatory responses and act via the ALX receptor.38 In contrast to COX, hydrolyzed DHA apparently is a substrate for LOXs, at least in platelets and retina,39,40 and perhaps also cytochrome P450s.41 Although these oxygenated products of DHA have questionable biological significance in platelets, until recently virtually nothing was known about their potential neurobiological activity. One product of DHA oxygenation, 10,17-S-docosatriene, termed neuroprotectin D1 (NPD1), is formed from PLA2-hydrolyzed DHA by a LOX-like enzyme. NPD1 protects retinal pigment epithelial cells in culture from oxidative stress-induced apoptosis.42 It is likely that NPD1 and other products of DHA oxygenation will soon be discovered to have potent neurobiological actions.
Unlike the COX pathway for which there is epidemiological and mechanistic data in support of a pathogenic role in latent or prodromal phases of AD, we are unaware of any epidemiological, pharmacological, or animal model data that yet point to a significant contribution of LOX pathway metabolites in AD pathogenesis; however, inhibitors of LT formation or receptor activation have not been in use for very long, so it is too early to discount this pathway. Nevertheless, 5-LOX is expressed in neurons, including the hippocampus, and its expression may increase with age.43 One autopsy-based study has associated increased activity in 12/15-LOX pathways with late-stage AD.44
Free Radical Damage to AA and DHA
A central hypothesis for the pathogenesis of AD, supported by many experimental, autopsy, and clinical studies, is that increased free radical damage contributes to the initiation and progression of neurodegeneration, although the sources of this free radical stress remain an area of active investigation.45 It is critical to note that unlike enzyme-catalyzed reactions described above, free radical damage is an indiscriminate process that will simultaneously modify multiple targets including nucleic acid, protein, and lipids.46 PUFAs such as AA and DHA are among the most vulnerable targets for free radical damage, a process termed lipid peroxidation.47 This complex process directly damages membranes and generates a number of oxygenated products that can be classified as either chemically reactive or relatively chemically stable products.48
Reactive Products of Lipid Peroxidation and Their Bioactivity
Recently, considerable progress has been made in understanding the potential contribution of chemically reactive products of lipid peroxidation to neurodegeneration. The presumed mechanism of action of all of these electrophilic products is adduction of nucleophilic groups in protein or nucleic acid. For example, adduction of a critical amino acid residue in an enzyme or transporter may lead to its dysfunction.48–50 However, interpreting experiments that investigate the contribution of reactive products of lipid peroxidation to disease pathogenesis is limited by their lack of biochemical specificity.
Despite this limitation to understanding the precise biochemical mechanisms of action, many studies in a variety of model systems and autopsy-derived tissue have implicated reactive products of lipid peroxidation in the pathogenesis of AD.51–57 One class of chemically reactive products of lipid peroxidation that have been studied in great detail is diffusible low-molecular weight aldehydes. By far, the most extensively studied of these are 4-hydroxy-2-nonenal, generated by peroxidation of ω-6 PUFAs like AA, and 4-hydroxy-2-hexenal, a product of peroxidation of ω-3 PUFAs like DHA.58 Although the pathophysiological consequences of overproduction of 4-hydroxy-2-nonenal and 4-hydroxy-2-hexenal have been highlighted in numerous studies, it is noteworthy that these reactive aldehydes also are generated at low levels in all cells and appear to have a role in normal physiological signaling.59 Indeed, several highly polymorphic enzyme systems have evolved apparently to metabolize specifically these lipid peroxidation products and thereby terminate their signaling or detoxify them;60 two of these have been tentatively associated with an increased risk of AD.61,62 Recently, another class of chemically reactive lipid peroxidation products has been identified: γ-ketoaldehyde isoketals (IsoKs) derived from AA and neuroketals (NeuroKs) derived from DHA.63 These γ-ketoaldehydes are much more reactive with cellular nucleophiles than 4-hydroxy-2-nonenal or 4-hydroxy-2-hexenal and, unlike the structurally similar COX-derived LGs, IsoKs and NeuroKs, remain esterified to phospholipids. In light of the ability of LGs to significantly accelerate oligomerization of Aβ peptides in vitro,33 IsoKs and NeuroKs are now being explored for related mechanisms of neurotoxicity.64
Stable Products of Lipid Peroxidation and Their Bioactivity
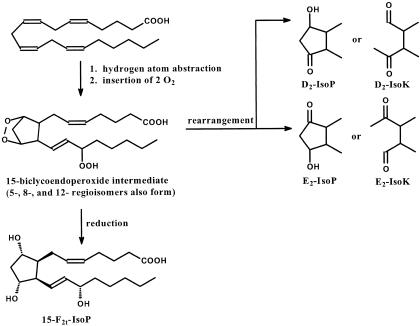
In the early 1990’s Morrow and colleagues65 demonstrated that free radical-mediated damage to AA followed by oxygen insertion and cyclization generated products that were isomeric to PG products of COX. These newly discovered compounds were termed isoprostanes (IsoPs) (Figure 3). There are three important differences between PGs and IsoPs. First, IsoPs are a large class of molecules consisting of 64 enantiomers contained within four regioisomeric families. Second, IsoPs are formed in situ while esterified to phospholipids and may be subsequently released by hydrolysis. Third, although some IsoPs do activate G protein-coupled receptors, the extent of their receptor-mediated activity remains unclear. What is clear is that predicting receptor activity based on similarity to isomeric PGs is limited. Since the discovery of potent renal vasoconstrictor activity for 15-F2t-IsoP, there has been an explosion of interest in the PG receptor-mediated activity of IsoPs, especially effects of 15-F2t-IsoP in vasculature, kidney, lungs, and platelets.66 Much of the receptor-mediated activity of 15-F2t-IsoP occurs via TP.67 The contribution of IsoP-mediated receptor activation to neurodegenerative diseases is not known.
Figure 3.
Free radical-mediated hydrogen atom abstraction from AA followed by insertion of 2 O2 leads to generation of four regioisomeric endoperoxide intermediates, each of which may be reduced to form an F-ring or rearrange to form D-ring, E-ring, Tx-ring (not shown), or LG-like (IsoK) products. Similar reactions occur with DHA to generate NeuroPs and NeuroKs. Nomenclature for the IsoPs and NeuroPs follows conventions for PGs. Prostane rings are denoted D, E, F, or Tx and the subscripted number refers to the number of double bonds. The regioisomers are named by location of the side chain hydroxyl relative to the carboxyl. Default absolute configuration of side chain hydroxyl is S; R configuration is “epi” Side chains may be cis-or trans, denoted “c” or “t” in the subscript, with respect to cyclopentane ring hydroxyls. The structure of the most extensively studied IsoP, 15-F2t-IsoP, is shown.
In addition to liberating diffusible reactive products of lipid peroxidation, fragmentation of lipid hydroperoxides can leave an abnormally shortened fatty acyl group, with or without an additional oxy function, esterified in the sn-2 position.68 When this occurs with phosphatidylcholine (PC), the fragmented alkyl group may yield a mimic of platelet-activating factor, a potent autocoid that is formed by a two-step process: PLA2 hydrolyzes the fatty acid at sn-2 position which is then replaced with an acetyl group from acetyl-CoA. Indeed, more than one of these oxidatively modified PCs activates the platelet-activating factor receptor.68 Other nonplatelet-activating factor-like phospholipids, including lysoPC, also are generated by lipid oxidation and have a variety of biological properties; however, very little is known about their activity in brain parenchyma.69
Quantitative in Vivo Biomarkers of Lipid Peroxidation
A final aspect to consider for lipid oxidation products is their use as quantitative biomarkers of free radical-mediated damage in vivo. Every lipid peroxidation product discussed above has been used as a measure of oxidative damage. Since we have reviewed this topic recently, we will not go into great detail here, however, an important point must be kept in mind.45 The goal of a biomarker is to quantitatively reflect changes in free radical mediated damage. Interpretation of biomarkers that are chemically reactive or that are extensively metabolized has severe, inherent limitations. Consider 4-hydroxy-2-nonenal: although several robust methods exist for its quantification, how do you interpret a change in its concentration? Was it due to a change in lipid peroxidation, a change in the concentration or availability of intracellular nucleophiles like glutathione, a change in the rate of its metabolism by one of several highly efficient enzymes, or some combination of these?
Exquisitely sensitive assays have been developed for IsoPs and, because of the chemical stability and relatively limited metabolism of F2-IsoPs in situ, they have emerged as a leading quantitative biomarker of lipid peroxidation in vivo.70 F2-IsoPs can be measured in tissue samples where this product of lipid peroxidation remains esterified to phospholipids. With respect to neurodegenerative diseases, a limitation of F2-IsoPs is that they derive from oxidation of AA that is relatively uniformly distributed in gray matter and white matter as well as in neurons and glia. In contrast, products formed from DHA by identical chemistry, termed F4-neuroprostanes (NeuroPs), provide a relatively selective window into oxidative damage to neuronal membranes.71 This is an important point for neurodegenerative research using specimens obtained at autopsy. Glia outnumber neurons by ∼10:1 and in neurodegenerative diseases this ratio is even further skewed toward glia. F4-NeuroPs are, as far as we are aware, the only measure of oxidative damage that is relatively selective for neurons.
Several groups have used measurements of hydrolyzed F2-IsoPs in body fluids in an attempt to quantify the magnitude of oxidative damage in vivo. In AD, there is broad agreement that cerebrospinal fluid F2-IsoPs are increased in patients with mild dementia and even in individuals with prodromal dementia.34,72–76 Similar to the attempts to identify peripheral biomarkers of other neurodegenerative diseases, attempts to use plasma or urine F2-IsoPs in AD patients have not yielded reproducible results across centers using a variety of techniques.73–81 This is perhaps not surprising given the small amounts of brain-derived F2-IsoPs relative to peripheral organ-derived F2-IsoPs and the many systemic, dietary, and environmental factors that modulate peripheral F2-IsoPs independent of disease.
Other Diseases
It is important to note that the pathogenic pathways discussed above may contribute to AD pathogenesis but they are by no means specific to AD. Close parallels have been drawn in the pathogenesis of Parkinson’s disease,82 HIV-associated dementia,83 and amyotrophic lateral sclerosis.84 Some pathogenic overlap also exists for ischemic stroke, atherosclerotic vascular disease, diabetes, and arthritis. Although some view this lack of specificity as undermining a central role in AD pathogenesis, an alternative perspective is that these pathways represent fundamental and profoundly important mechanisms of tissue damage in multiple organs.
Summary
Interest in a pathogenic role for AA and DHA oxidation in AD is driven by compelling epidemiological data, associative data from autopsy samples and cerebrospinal fluid obtained early in the course of AD, and mechanistic data mostly from murine models. Given the known limitations of nonselective COX inhibitors,35 the recently discovered limitations of COX-2 inhibitors,36 and the recent (controversial) indication that very high-dose α-tocopherol may carry some toxicity,85 the search is on for agents that suppress these pathways with minimal toxicity. For PGs and LTs, this will likely mean development of selective receptor antagonists. For AOs, this likely will mean a combination of dietary interventions, natural product supplements, and pharmaceuticals to achieve the desired effect with minimal toxicity. Analogous to treatments that suppress hypertension or hypercholesterolemia and thereby reduce vascular disease, these interventions will not cure AD but may check its explosive growth and hopefully reduce its incidence and prevalence in the elderly.
Footnotes
Address reprint requests to Thomas J. Montine, M.D., Ph.D., Department of Pathology, University of Washington, Box 359791, Seattle, WA 98104. E-mail: tmontine@u.washington.edu.
References
- U.S. Congress OTA Washington DC: Government Printing Office,; Losing a Million MindsConfronting the Tragedy of Alzheimer’s Disease and Other Dementias. 1987 [Google Scholar]
- Evans DA. Estimated prevalence of Alzheimer’s disease in the United States. Milbank Q. 1990;68:267–289. [PubMed] [Google Scholar]
- Ernst RL, Hay JW. The US economic and social costs of Alzheimer’s disease revisited. Am J Public Health. 1994;84:1261–1264. doi: 10.2105/ajph.84.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick WC, Hardy J, Kukull WA, Bowen JD, Teri L, Zitzer S, Larson EB. Healthcare utilization and costs in managed care patients with Alzheimer’s disease during the last few years of life. J Am Geriatr Soc. 2001;49:1156–1160. doi: 10.1046/j.1532-5415.2001.49231.x. [DOI] [PubMed] [Google Scholar]
- Welch HG, Walsh JS, Larson EB. The cost of institutional care in Alzheimer’s disease: nursing home and hospital use in a prospective cohort. J Am Geriatr Soc. 1992;40:221–224. doi: 10.1111/j.1532-5415.1992.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Breitner JC, Zandi PP, Meyer MR, Jurasova I, Norton MC, Stone SV. Reduced prevalence of AD in users of NSAIDs and H2 receptor antagonists: the Cache County study. Neurology. 2000;54:2066–2071. doi: 10.1212/wnl.54.11.2066. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, α-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Aβ levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Matsubara E, Bryant-Thomas T, Pacheco Quinto J, Henry TL, Poeggeler B, Herbert D, Cruz-Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke-Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA, Chain DG, Neria E. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J Neurochem. 2003;85:1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- Andreasson KI, Savonenko A, Vidensky S, Goellner JJ, Zhang Y, Shaffer A, Kaufmann WE, Worley PF, Isakson P, Markowska AL. Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J Neurosci. 2001;21:8198–8209. doi: 10.1523/JNEUROSCI.21-20-08198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma M, Nishibayashi-Asanuma S, Miyazaki I, Kohno M. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J Neurochem. 2001;76:1895–1904. doi: 10.1046/j.1471-4159.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- Hamburger SA, McCay PB. Spin trapping of ibuprofen radicals: evidence that ibuprofen is a hydroxyl radical scavenger. Free Radic Res. 1990;9:337–342. doi: 10.3109/10715769009145692. [DOI] [PubMed] [Google Scholar]
- Kennedy TP, Noah RW, Michael JR, Jafri MH, Gurtner GH, Hoidal JR. Ibuprofen prevents oxidant lung injury and in vitro lipid peroxidation by chelating iron. J Clin Invest. 1990;86:1565–1573. doi: 10.1172/JCI114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Lanz TA, Fici GJ, Merchant KM. Lack of specific amyloid-{beta}(1–42) suppression by nonsteroidal anti-inflammatory drugs in young, plaque-free Tg2576 mice and in guinea pig neuronal cultures. J Pharmacol Exp Ther. 2005;312:399–406. doi: 10.1124/jpet.104.073965. [DOI] [PubMed] [Google Scholar]
- Moore SA. Cerebral endothelium and astrocytes cooperate in supplying docosahexaenoic acid to neurons. Adv Exp Med Biol. 1993;331:229–233. doi: 10.1007/978-1-4615-2920-0_36. [DOI] [PubMed] [Google Scholar]
- Salem N, Kim HY, Yergey JA. Docosahexaenoic acid: membrane function and metabolism. Simopoulos AP, Kifer RR, Martin RE, editors. New York: Academic Press,; Health Effects of Polyunsaturated Acids in Seafoods. 1986:pp 263–317. [Google Scholar]
- Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Halushka PV. Lipid-derived autacoids. Hardman JG, Limbird LE, editors. New York: McGraw-Hill,; Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 2001:pp 601–616. [Google Scholar]
- Kaufmann WE, Andreasson KI, Isakson PC, Worley PF. Cyclooxygenases and the central nervous system. Prostaglandins. 1997;54:601–624. doi: 10.1016/s0090-6980(97)00128-7. [DOI] [PubMed] [Google Scholar]
- Siedlik PH, Marnett LJ. Oxidizing radical generation by prostaglandin H synthase. Methods Enzymol. 1984;105:412–416. doi: 10.1016/s0076-6879(84)05057-6. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Ann Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Difranco E, Subbanagounder G, Kim S, Murthi K, Taneda S, Monnier V, Salomon R. Formation and stability of pyrrole adducts in the reaction of levuglandin E2 with proteins. Chem Res Toxicol. 1994;8:61–67. doi: 10.1021/tx00043a008. [DOI] [PubMed] [Google Scholar]
- Boutaud O, Ou JJ, Chaurand P, Caprioli RM, Montine TJ, Oates JA. Prostaglandin H2 (PGH2) accelerates formation of amyloid beta1-42 oligomers. J Neurochem. 2002;82:1003–1006. doi: 10.1046/j.1471-4159.2002.01064.x. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Sidell KS, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ, Morrow JD. Elevated cerebrospinal fluid prostaglandin E2 levels in patients with probable Alzheimer’s disease. Neurology. 1999;53:1495–1498. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- Hooper L, Brown TJ, Elliott R, Payne K, Roberts C, Symmons D. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. BMJ. 2004;329:948–957. doi: 10.1136/bmj.38232.680567.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- Shie FS, Breyer RM, Montine TJ. Microglia lacking E prostanoid receptor subtype 2 have enhanced Aβ phagocytosis yet lack Aβ-activated neurotoxicity. Am J Pathol. 2005;166:1163–1172. doi: 10.1016/s0002-9440(10)62336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norel X, Brink C. The quest for new cysteinyl-leukotriene and lipoxin receptors: recent clues. Pharmacol Ther. 2004;103:81–94. doi: 10.1016/j.pharmthera.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Aveldano MI, Sprecher H. Synthesis of hydroxy fatty acids from 4,7,10,13,16,19-[1–14C] docosahexaenoic acid by human platelets. J Biol Chem. 1983;258:9339–9343. [PubMed] [Google Scholar]
- Bazan NG, Birkle DL, Reddy TS. Docosahexaenoic acid (22: 6, n-3) is metabolized to lipoxygenase reaction products in the retina. Biochem Biophys Res Commun. 1984;125:741–747. doi: 10.1016/0006-291x(84)90601-6. [DOI] [PubMed] [Google Scholar]
- VanRollins M, Baker RC, Sprecher HW, Murphy RC. Oxidation of docosahexaenoic acid by rat liver microsomes. J Biol Chem. 1984;259:5776–5783. [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K, Uz T, Kumar V, Manev H. New anti-inflammatory treatment strategy in Alzheimer’s disease. Jpn J Pharmacol. 2000;82:85–94. doi: 10.1254/jjp.82.85. [DOI] [PubMed] [Google Scholar]
- Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-Lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, II, Morrow JD. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic Biol Med. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- Montine K, Quinn J, Montine T. Membrane lipid peroxidation. Mattson M, editor. Amsterdam: Elsevier,; Membrane Lipid Signaling in Aging and Age-Related Disease. 2003:pp 11–26. [Google Scholar]
- Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Ramos P. Chemistry and pathophysiology of oxidation of LDL. Rev Physiol Biochem Pharmacol. 1995;127:31–64. doi: 10.1007/BFb0048264. [DOI] [PubMed] [Google Scholar]
- Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997;80:685–696. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Ando Y, Brannstrom T, Uchida K, Nyhlin N, Nasman B, Suhr O, Yamashita T, Olsson T, Salhy M, Uchino M, Ando M. Histochemical detection of 4-hydroxynonenal protein in Alzheimer amyloid. J Neurol Sci. 1998;156:172–176. doi: 10.1016/s0022-510x(98)00042-2. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Ehmann WD, Mattson MP, Markesbery WR. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol Aging. 1997;18:457–461. doi: 10.1016/s0197-4580(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- McGrath LT, McGleenon BM, Brennan S, McColl D, McIlroy S, Passmore AP. Increased oxidative stress in Alzheimer’s disease as assessed with 4-hydroxynonenal but not malondialdehyde. QJM. 2001;94:485–490. doi: 10.1093/qjmed/94.9.485. [DOI] [PubMed] [Google Scholar]
- Montine KS, Olson SJ, Amarnath V, Whetsell WOJ, Graham DG, Montine TJ. Immunohistochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer’s disease is associated with inheritance of APOE4. Am J Pathol. 1997;150:437–443. [PMC free article] [PubMed] [Google Scholar]
- Montine KS, Reich E, Olson SJ, Markesbery WR, Montine T. Distribution of reducible 4-hydroxynonenal adduct immunoreactivity in Alzheimer’s disease is associated with APOE genotype. J Neuropathol Exp Neurol. 1998;57:415–425. doi: 10.1097/00005072-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Dickinson DA. Introduction to serial reviews on 4-hydroxy-2-nonenal as a signaling molecule. Free Radic Biol Med. 2004;37:594–596. doi: 10.1016/j.freeradbiomed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Picklo MJ, Montine TJ, Amarnath V, Neely MD. Carbonyl toxicology and Alzheimer’s disease. Toxicol Appl Pharmacol. 2002;184:187–197. doi: 10.1006/taap.2002.9506. [DOI] [PubMed] [Google Scholar]
- Kamino K, Nagasaka K, Imagawa M, Yamamoto H, Yoneda H, Ueki A, Kitamura S, Namekata K, Miki T, Ohta S. Deficiency in mitochondrial aldehyde dehydrogenase increases the risk for late-onset Alzheimer’s disease in the Japanese population. Biochem Biophys Res Commun. 2000;273:192–196. doi: 10.1006/bbrc.2000.2923. [DOI] [PubMed] [Google Scholar]
- Li YJ, Oliveira SA, Xu P, Martin ER, Stenger JE, Hulette C, Scherzer CR, Hauser MA, Scott WK, Small GW, Nance MA, Watts RL, Hubble JP, Koller WC, Pahwa R, Stern MB, Hiner BC, Jankovic J, Goetz CG, Mastaglia F, Middleton LT, Roses AD, Saunders AM, Welsh-Bohmer KA, Schmechel DE, Gullans SR, Haines JL, Gilbert JR, Vance JM, Pericak-Vance MA. Glutathione S-transferase omega-1 modifies age-at-onset of Alzheimer disease and Parkinson disease. Hum Mol Genet. 2003;12:3259–3267. doi: 10.1093/hmg/ddg357. [DOI] [PubMed] [Google Scholar]
- Bernoud-Hubac N, Davies S, Boutaud O, Montine T, Roberts L., II Formation of highly reactive gamma-ketoaldehydes (neuroketals) as products of the neuroprostane pathway. J Biol Chem. 2001;276:30964–30970. doi: 10.1074/jbc.M103768200. [DOI] [PubMed] [Google Scholar]
- Davies SS, Amarnath V, Montine KS, Bernoud-Hubac N, Boutaud O, Montine TJ, Roberts LJ., II Effects of reactive gamma-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J. 2002;16:715–717. doi: 10.1096/fj.01-0696fje. [DOI] [PubMed] [Google Scholar]
- Morrow J, Hill K, Burk R, Nammour T, Badr K, Roberts L. A series of prostaglandin-like compounds produced in vivo in humans by a non-cyclooxygenase, free radical catalyzed mechanism. Proc Natl Acad Sci USA. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- Audoly L, Rocca B, Fabre J, Koller B, Thomas D, Loeb A, Coffman T, FitzGerald G. Cardiovascular responses to the isoprostanes iPF2α-III and iPE2-III are mediated via the thromboxane A2 receptor in vivo. Circulation. 2000;101:2833–2840. doi: 10.1161/01.cir.101.24.2833. [DOI] [PubMed] [Google Scholar]
- Smiley PL, Stremler KE, Prescott SM, Zimmerman GA, McIntyre TM. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. J Biol Chem. 1991;266:11104–11110. [PubMed] [Google Scholar]
- McIntyre TM, Zimmerman GA, Prescott SM. Biologically active oxidized phospholipids. J Biol Chem. 1999;274:25189–25192. doi: 10.1074/jbc.274.36.25189. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ., II Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical catalyzed mechanism. Methods Enzymol. 1994;233:163–174. doi: 10.1016/s0076-6879(94)33019-0. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, II, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Beal MF, Cudkowicz ME, Brown RH, O’Donnell H, Margolin RA, McFarland L, Bachrach AF, Zackert WE, Roberts LJ, Morrow JD. Increased cerebrospinal fluid F2-isoprostane concentration in probable Alzheimer’s disease. Neurology. 1999;52:562–565. doi: 10.1212/wnl.52.3.562. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Beal MF, Robertson D, Cudkowicz ME, Biaggioni I, O’Donnell H, Zackert WE, Roberts LJ, Morrow JD. Cerebrospinal fluid F2-isoprostanes are elevated in Huntington’s disease. Neurology. 1999;52:1104–1105. doi: 10.1212/wnl.52.5.1104. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Kaye JA, Montine KS, McFarland L, Morrow JD, Quinn JF. Cerebrospinal fluid Aβ42, tau, and F2-isoprostane concentrations in patients with Alzheimer disease, other dementias, and in age-matched controls. Arch Pathol Lab Med. 2001;125:510–512. doi: 10.5858/2001-125-0510-CFATAF. [DOI] [PubMed] [Google Scholar]
- Pratico D, Clack CM, Lee VMY, Trojanowski JQ, Rokach J, FitzGerald G. Increased 8,12-iso-iPF2α-IV in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48:809–812. [PubMed] [Google Scholar]
- Pratico D, Clark CM, Liun F, Lee VY-M, Trojanowski JQ. Increase brain oxidative stress in mild cognitive impairment. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Tuppo E, Forman L, Spur B, Chan-Ting R, Chopra A, Cavalieri T. Sign of lipid peroxidation as measured in the urine of patients with probable Alzheimer’s disease. Brain Res Bull. 2001;54:565–568. doi: 10.1016/s0361-9230(01)00450-6. [DOI] [PubMed] [Google Scholar]
- Waddington E, Croft K, Clarnette R, Mori T, Martins R. Plasma F2-isoprostane levels are increased in Alzheimer’s disease: evidence of increased oxidative stress in vivo. Alzheimer’s Rep. 1999;2:277–282. [Google Scholar]
- Bohnstedt K, Karlberg B, Wahlund L, Jonhagen M, Basun H, Schmidt S. Determination of isoprostanes in urine samples from Alzheimer patients using porous graphitic carbon liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:11–19. doi: 10.1016/s1570-0232(03)00600-7. [DOI] [PubMed] [Google Scholar]
- Feillet-Coudray C, Tourtauchaux R, Niculescu M, Rock E, Tauveron I, Alexandre-Gouabau MC, Rayssiguier Y, Jalenques I, Mazur A. Plasma levels of 8-epiPGF2α, an in vivo marker of oxidative stress, are not affected by aging or Alzheimer’s disease. Free Rad Biol Med. 1999;27:463–469. doi: 10.1016/s0891-5849(99)00096-9. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Shinobu L, Montine KS, Roberts LJ, II, Beal MF, Marrow JD. No difference in plasma or urine F2-isoprostanes among patients with Huntington’s disease or Alzheimer’s disease, and controls. Ann Neurol. 2000;48:950. [PubMed] [Google Scholar]
- Hartmann A, Hunot S, Hirsch EC. Inflammation and dopaminergic neuronal loss in Parkinson’s disease: a complex matter. Exp Neurol. 2003;184:561–564. doi: 10.1016/j.expneurol.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Consilvio C, Vincent AM, Feldman EL. Neuroinflammation, COX-2, and ALS—a dual role? Exp Neurol. 2004;187:1–10. doi: 10.1016/j.expneurol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]